Open Access Article
Open Access ArticleTransition metal oxynitride catalysts for electrochemical reduction of nitrogen to ammonia†
Damilola
Ologunagba
and
Shyam
Kattel
 *
*
Department of Physics, Florida A&M University Tallahassee, FL 32307, USA. E-mail: shyam.kattel@famu.edu
First published on 23rd December 2020
Abstract
Electrochemical nitrogen reduction reaction (ENRR) under ambient conditions is beneficial compared to the energy intensive thermochemical Haber–Bosch process for NH3 production. Here, periodic density functional theory (DFT) calculations are carried out to study the ENRR on transition metal oxynitride (TMNO) catalysts. Our calculations show that the ENRR occurs at thermodynamically more favorable surface nitrogen vacancy (N-vac) sites compared to surface oxygen vacancy (O-vac) sites. The DFT results show that TiNO efficiently catalyzes the ENRR at a low applied potential (U) and its ENRR activity is predicted to be similar to that of VNO, a previously identified excellent ENRR catalyst. We observed a volcano like relationship between the DFT calculated nitrogen binding energy (NBE) and the limiting potential (UL), which suggests that the NBE can be used as a descriptor of the ENRR activity on TMNO based catalysts.
Introduction
Ammonia (NH3), a key precursor used to produce nitrogen-containing fertilizers, is currently produced by an energy intensive Haber–Bosch process.1–8 The Haber–Bosch process is typically carried out at high temperatures (400–500 °C) and pressures (200–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) atm) where nitrogen from the atmosphere reacts with the hydrogen produced from non-renewable resources to produce NH39–11 and consumes ∼1–2% of global energy.12–17 Electrochemical nitrogen reduction reaction (ENRR) to produce NH3 on a small scale near agricultural fields under ambient conditions using the electricity generated from renewable resources (e.g. solar and wind energy) is a promising alternative to the carbon/energy intensive Haber–Bosch process.18–26
atm) where nitrogen from the atmosphere reacts with the hydrogen produced from non-renewable resources to produce NH39–11 and consumes ∼1–2% of global energy.12–17 Electrochemical nitrogen reduction reaction (ENRR) to produce NH3 on a small scale near agricultural fields under ambient conditions using the electricity generated from renewable resources (e.g. solar and wind energy) is a promising alternative to the carbon/energy intensive Haber–Bosch process.18–26
N2 is a stable molecule and its activation and transformation under ambient conditions are challenging.27,28 Different classes of heterogeneous catalysts such as transition metals,29 transition metal carbides and nitrides30–33 and single atom catalysts anchored on graphene,34–36 boron nitrides,37 and Mxenes38,39 have been explored for the ENRR. Among them, transition metal nitride-based materials have emerged as promising catalysts for electrochemical N2 conversion to NH3. For example, using density functional theory (DFT) based calculations, Abghoui et al. investigated various transition metal nitrides (TiN, YN, ScN, HfN, ZrN, CrN, VN, TaN, OsN, NbN, MoN, RuN, MnN, IrN, RhN, FeN, CoN, AgN, PdN, NiN, and CuN) and reported that the (100) facets of the rocksalt structures of VN and ZrN are the most promising candidates that catalyze the electrochemical conversion of N2 to NH3.1,18,31 Unlike transition metals, transition metal nitride surfaces are less likely to be covered by hydrogen at the onset potentials needed for the ENRR, and hence the HER is predicted to be less competitive resulting in an enhanced ENRR activity on transition metal nitride based catalysts.1,18 Furthermore, recent combined experimental and DFT studies have shown that O modified VN (i.e. VNO) is an excellent ENRR catalyst suggesting that transition metal oxynitrides (TMNOs) may serve as a new class of materials that facilitate the ENRR at low potentials.40,41 The ENRR on VNO was shown to proceed on nitrogen vacancy via the Mars–van Krevelen (MvK) mechanism. Along the MvK mechanism, N2 adsorption at the N-vacancy is followed by (H+ + e−) transfer to form *N2H which further undergoes (H+ + e−) transfer to form *N2H2. Additional (H+ + e−) transfer to *N2H2 facilitates N–N bond scission, a key step of the ENRR. In contrast to the promoting role of the surface nitrogen vacancy, the surface oxygen vacancies on VNO have been shown to bind the ENRR reaction intermediates too strongly and potentially poison the catalyst surface. On VNO(111), the rate-limiting step of the ENRR facilitated by the nitrogen vacancy was predicted to be the reduction of *N2 to N2H.41 Similarly, Kang et al. reported that titanium nitride (TiN) manufactured by plasma-etching as an excellent catalyst for the ENRR.42
In this study, we employed first-principles DFT calculations to study the ENRR on TMNOs. DFT results show that the ENRR follows the MvK mechanism on the surface nitrogen vacancy, whose formation is found to be thermodynamically more favorable compared to the formation of the oxygen vacancy. Our computations predict that TiNO is the best ENRR catalysts with a performance similar to that of VNO which has been experimentally shown to be an excellent ENRR catalyst. Furthermore, the DFT calculations identified the nitrogen binding energy as a potential descriptor of the ENRR activity on TMNO based catalysts.
Computational methods
Density functional theory (DFT)43 calculations were performed using the Vienna Ab Initio Simulation Package (VASP) code.44,45 All the DFT calculations were spin-polarized and were performed at the GGA level within the PAW-PW91 formalism.46,47The bulk structure of transition metal oxynitrides (TMNOs) in the present study was modeled using the rocksalt structure with an oxygen (O) concentration of 25% consistent with the experimental O concentration in VNO, a previously identified efficient catalyst for the ENRR.41 The TMNO(111) surfaces were modeled using 4 layer 2 × 2 surface slabs. Our modeled TMNO(111) surfaces contain N and O atoms on the topmost surface layer, which allowed us to investigate the ENRR on the surface N and O vacancies. A vacuum of approximately 15 Å was added to the surface in the z-direction to reduce the artificial interplay between the surface and its periodic images.
During calculations, the atoms in the top two layers were allowed to be relaxed, while the atoms in the bottom two layers were fixed until the Hellman–Feynman force on each ion was less than 0.02 eV Å−1. The total energy was calculated using a plane wave basis set at a cut-off energy of 400 eV and a 3 × 3 × 1 Monkhorst–Pack grid was used to carry out the Brillion zone integration.48
The binding energy (BE) of the adsorbate was calculated as:
| BE(adsorbate) = E(slab + adsorbate) − E(slab) − E(adsorbate) |
The free energy changes were calculated using the computational hydrogen electrode (CHE) model developed by Norskov and co-workers.49 In this model, the chemical potential of a proton–electron pair (H+ + e−) is equal to half of the chemical potential of the hydrogen gas molecule (1/2μ(H2)) at zero applied potential (U).
Thus, the total chemical potential of the proton–electron pair as a function of applied potential (U), at all temperatures and pH values, can be calculated as μ(H+ + e−) = 1/2μ(H2(g)) – eU.
The CHE model is employed at U = 0 V to construct free energy diagrams (ΔG vs. reaction coordinates) of the ENRR. The Gibbs free energy (G) of a species was calculated as49
| G = E + ZPE − TS |
Results and discussion
Previous DFT studies have shown that the ENRR via associative or dissociative mechanisms is thermodynamically unfavorable on perfect transition metal (TM) nitride surfaces. Along the associative pathway, the formation of *N2H is found to be thermodynamically unfavorable while the dissociative pathway is predicted to be unfavorable due to the highly activated N2 dissociation. In contrast, the presence of the surface N-vacancy (N-vac) on TM nitrides is found to facilitate the ENRR via the MvK mechanism by strengthening the binding affinity of the reaction intermediates and significantly reducing the energy barrier for N2 dissociation.40,41 Along the MvK mechanism, the first NH3 molecule is formed by the protonation of a surface N atom creating an N vacancy (N-vac) on the catalyst surface.1,18,41 The created N-vac then facilitates adsorption of N2 and its conversion to NH3. Therefore, firstly, we computed the formation energies of the surface oxygen vacancy (O-vac) and the nitrogen-vacancy (N-vac) on transition metal oxynitride surfaces using the following scheme:| Surface + H2(g) → O-vac + H2O(g) |
| Surface + 3/2H2(g) → N-vac + NH3(g) |
The DFT calculated formation energies in Table 1 show that except MnNO, the N-vac formation is thermodynamically more favorable compared to the formation of O-vac indicating that the ENRR via the MvK mechanism likely occurs at the N-vac sites. Subsequently, the N-vac promoted ENRR is discussed in detail in the following sections and the results for the O-vac mediated ENRR are summarized at the end of the results and discussion section.
| TMNO | N-vac | O-vac |
|---|---|---|
| CrNO | 1.43 | 3.37 |
| MnNO | −1.00 | −1.46 |
| TaNO | 0.34 | 2.41 |
| HfNO | 1.92 | 3.93 |
| ScNO | −3.72 | 0.81 |
| TiNO | 0.31 | 2.80 |
| YNO | −3.08 | 1.38 |
| ZrNO | 1.21 | 3.51 |
| VNO | −0.57 | 2.05 |
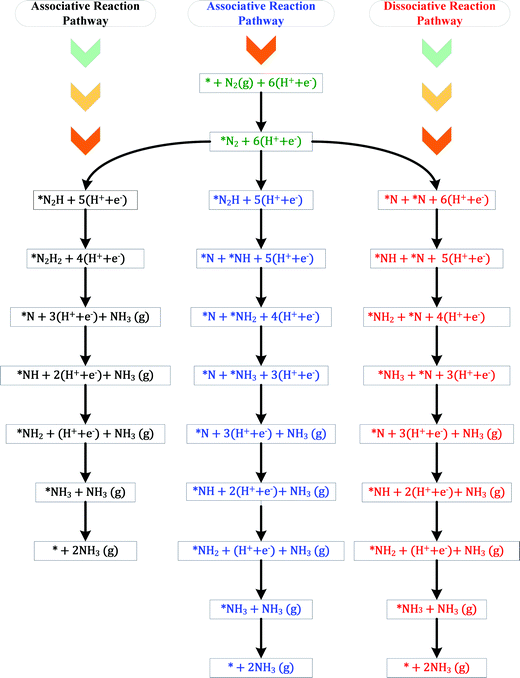
The ENRR can proceed via three main pathways: (i) distal, (ii) alternating, and (iii) enzymatic (Fig. S1 in the ESI†).50 Our calculations show that N2 binds at N/O-vac in an endon configuration which prohibits the ENRR from occurring via the enzymatic pathway. As shown in Fig. S1 (ESI†), the formation of the *N2H2 intermediate promotes the distal pathway, while the formation of the *HNNH intermediate promotes the alternating pathway. Our DFT calculated results in Fig. 1 show that the *N2H2 formation is energetically more favorable than the formation of *HNNH on all TMNO catalysts considered in the present study. This suggests that the distal pathway is favorable over the alternating pathway. Therefore, the dissociative and the two commonly used associative pathways (along the distal channel) shown in Fig. 2 are discussed in detail in the subsequent sections.
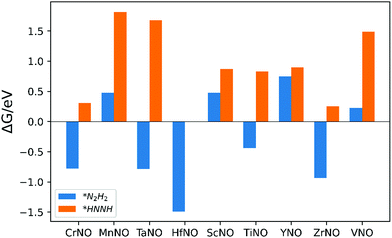 | ||
| Fig. 1 Free energy change for the formation of *N2H2 (i.e. step: *N2H + (H+ + e−) → *N2H2) and *HNNH (i.e. step: *N2H + (H+ + e−) → *HNNH) intermediates. | ||
H assisted N–N bond scission prevails in the associative pathways of the ENRR (shown in black and blue in Fig. 2). Along the associative pathway shown in black in Fig. 2 (associative N2H2 pathway hereafter) *N2 (* = active site) is first reduced to *N2H2, which subsequently undergoes (H+ + e−) transfer and dissociation reactions to form *N + NH3(g). *N then undergoes three sequential (H+ + e−) transfer reactions to form *NH3, which desorbs as a product. Along the pathway shown in blue in Fig. 2 (associative N2H pathway hereafter), N–N bond scission occurs when *N2H is formed to produce *N + *NH. *N and *NH then undergo (H+ + e−) transfer reactions to form *NH3. Along the dissociative pathway (shown in red in Fig. 2), *N2 adsorbed at N-vac dissociates to 2*N (vacancy dimer). *N then undergoes three subsequent electrochemical (H+ + e− transfer) reactions to form *NH3. *NH3 finally leaves the active site as NH3(g), making the site available for the next reaction cycle.
DFT calculations are performed to calculate the binding energies of the reaction intermediates (*H, *N, *N2, *N2H, *N2H2, *NH, *NH2, and *NH3) associated with the reaction channels shown in Fig. 2. The DFT optimized geometries of the reaction intermediates on the N-vac of the TMNO(111) surfaces in Fig. 3 show that *N2, *N2H, and *N2H2 bind in end on configurations in which only one N forms chemical bonds to the surface sites. The adsorption of *N heals the surface N-vac and the binding of *NH, *NH2, *NH3 at N-vac occurs via the N atoms. Similar binding configurations are observed at O-vac on the TMNO(111) surfaces (Fig. S2 in the ESI†).
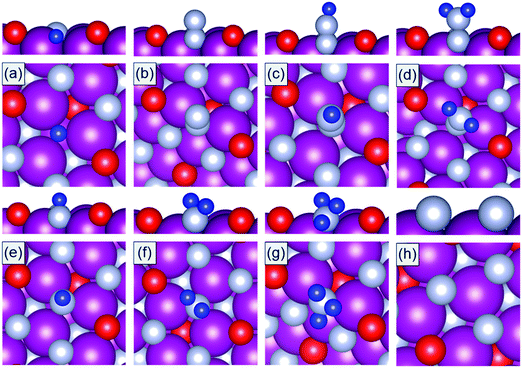 | ||
| Fig. 3 DFT optimized geometries of (a) *H, (b) *N2, (c) *N2H, (d) *N2H2, (e) *NH, (f) *NH2, (g) *NH3, and (h) *N adsorbed on N-vac of the MnNO(111) surface. Mn: purple, N: gray, O: red, H: blue. | ||
The DFT calculated binding energies in Fig. S3(a)–(i) (ESI†) show that most of the reaction intermediates bind more strongly on O-vac compared to N-vac except for MnNO where most of the reaction intermediates bind more strongly on N-vac. The stronger binding of the ENRR intermediates observed on O-vac is most likely due to the more electron density of states available near the Fermi level (for bonding) as shown in the density of state plots in Fig. S4 (ESI†). On MnNO(111), N-vac is predicted to be less favorable compared to O-vac (Table 1) and stronger binding of the ENRR intermediates on N-vac is observed due to the availability of a larger electron density of states near the Fermi level (Fig. S4, ESI†) as expected.
The DFT calculated binding energies are then used to compute the free energy change at an applied potential (U) = 0 V using the CHE model developed by Norskov et al.49Fig. 4 and 5 show the DFT computed free energy diagrams of the ENRR along the associative N2H2 and N2H pathways at U = 0 V. It is seen from Fig. 4 and 5 that the overall ENRR activity of ScNO and YNO is limited by the formation of *N from the elementary step: *N2H2 + (H+ + e−) → *N + NH3(g) with free energy barriers (ΔG) = 2.08 eV and 1.16 eV along the associative N2H2 pathway and *N2H + * → *N + *NH with ΔG = 3.57 eV and 1.34 eV along the associative N2H pathway, respectively, due to the weaker binding energy of N on N-vac. On the other hand, HfNO, TaNO, CrNO, and ZrNO bind the ENRR intermediates strongly. The most difficult steps on HfNO, TaNO, CrNO, and ZrNO along the associative N2H2 pathway are predicted to be *NH2 + (H+ + e−) → *NH3, *NH + (H+ + e−) → *NH2, *N + (H+ + e−) → *NH, and *NH3 → * + NH3(g) and have ΔG values of 1.11 eV, 1.27 eV, 1.18 eV, and 1.12 eV, respectively. Similarly, the most difficult steps along the associative N2H pathway on HfNO, TaNO, CrNO, and ZrNO are *NH2 + (H+ + e−) → *NH3, *NH + (H+ + e−) → *NH2, *N + (H+ + e−) → *NH, and *NH3 → * + NH3(g) and have ΔG values of 1.11 eV, 1.27 eV, 2.10 eV, and 1.12 eV, respectively. Interestingly, we find a relatively mild change in the free energy between the steps in both associative pathways for MnNO and TiNO, and the overall features of the free energy diagrams are very similar when compared to VNO. The most difficult steps on MnNO, TiNO, and VNO along the associative N2H2 as well as N2H pathways are *N2 + (H+ + e−) → *N2H, *NH2 + (H+ + e−) → *NH3 and *N2 + (H+ + e−) → *N2H with ΔG values of 0.82 eV, 0.42 eV and 0.68 eV, respectively. The relatively smaller ΔG values of the most difficult steps on MnNO, TiNO, and VNO suggest an efficient ENRR along the associative N2H2 and N2H pathways on these candidates.
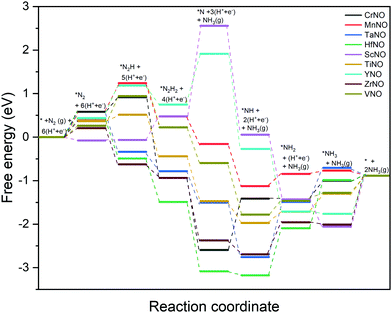 | ||
| Fig. 4 DFT calculated free energy diagrams of the ENRR at U = 0 V along the associative N2H2 pathway on N-vac. | ||
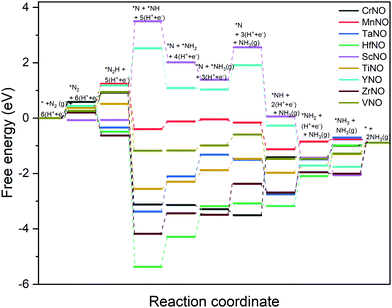 | ||
| Fig. 5 DFT calculated free energy diagrams of the ENRR at U = 0 V along the associative N2H pathway on N-vac. | ||
Fig. 6 shows the DFT calculated free energy change of the elementary steps of the N-vac mediated ENRR along the dissociative pathway. N2 dissociation (to 2*N on a vacancy dimer), a non-electrochemical step, is predicted to be the rate-limiting step on ScNO and YNO with an uphill energy barrier of 6.08 eV and 4.27 eV, respectively. Thus, the weaker binding of *N severely limits the ENRR along the dissociative pathway on ScNO and YNO. In contrast, N2 dissociation is exothermic on all other catalysts (CrNO, MnNO, TaNO, HfNO, TiNO, ZrNO, and VNO). HfNO, CrNO, ZrNO and TaNO bind N/NHx species too strongly, and their further reduction requires a large energy. The most difficult steps on HfNO, CrNO, ZrNO and TaNO are predicted to be *NH2 + (H+ + e−) → *NH3, *N + (H+ + e−) → *NH, *NH3 → * + NH3(g), and *NH + (H+ + e−) → *NH2 with ΔG values of 1.11 eV, 1.18 eV, 1.12 eV and 1.27 eV, respectively. Interestingly, the free energy change between the elementary steps on MnNO, TiNO, and VNO is small and these candidates are predicted to show enhanced performance for the ENRR. On MnNO, TiNO and VNO, the most difficult steps are * + N2 → *N2, *NH2 + (H+ + e−) → *NH3, and *NH3 → * + NH3(g) and have ΔG values of 0.42 eV, 0.42 eV, and 0.39 eV respectively.
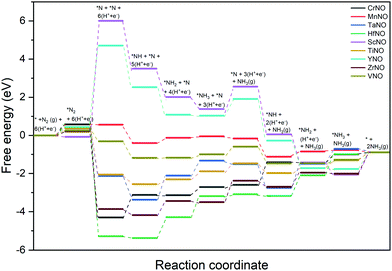 | ||
| Fig. 6 DFT calculated free energy diagrams of the ENRR at U = 0 V along the dissociative pathway on N-vac. | ||
A comparison of the free energy profiles (Fig. 4–6) shows that the ENRR occurs via the associative N2H2 pathway on ScNO and YNO and have ΔG values of the rate limiting step as 2.08 eV and 1.16 eV, respectively. In contrast, the dissociative pathway is predicted to be the most favorable pathway on CrNO, MnNO, TaNO, HfNO, TiNO, ZrNO, and VNO. The ΔG values of the rate limiting step are 1.18 eV, 0.42 eV, 1.27 eV, 1.11 eV, 0.42 eV, 1.12 eV and 0.39 eV on CrNO, MnNO, TaNO, HfNO, TiNO, ZrNO and VNO, respectively. The predicted large ΔG values on ScNO, YNO, HfNO, CrNO, ZrNO, and TaNO indicate that the ENRR is sluggish on these candidates. However, we find relatively small and similar ΔG values on MnNO, TiNO, and VNO. As a result, a similar ENRR activity of MnNO and TiNO compared to VNO is expected. Previous experimental and theoretical studies41 have shown an efficient ENRR catalyzed by the N-vac of VNO at a low potential. Therefore, our DFT calculations predict MnNO and TiNO as potential candidates for N2 reduction to NH3 under ambient conditions based on the calculated free energy diagrams of the ENRR.
The overall activity of a catalyst for the ENRR along a reaction pathway is determined by the most difficult step (i.e. a step with the largest uphill energy in free energy diagrams). The limiting potential (UL), a metric often used in electrochemistry, is defined as a potential at which all the electrochemical steps along the reaction channel are thermodynamically downhill in energy. Thus the most difficult electrochemical step, also called a potential limiting step, would dictate the UL values along the reaction channel on a catalyst surface. The free energy diagrams shown in Fig. 4–6 are used to compute the UL values on TMNOs.
Along the most favorable associative N2H2 pathway, our calculations predict UL = −2.08 V and −1.16 V on ScNO and YNO, respectively. The UL values calculated on CrNO, MnNO, TaNO, HfNO, TiNO, ZrNO, and VNO along the most favorable dissociative pathway are −1.18 V, −0.28 V, −1.27 V, −1.11 V, −0.42 V, −0.74 V, and −0.18 V, respectively. Interestingly we find that the non-electrochemical steps have larger ΔG barriers than the most difficult electrochemical steps on MnNO, ScNO, YNO, ZrNO, and VNO and are predicted to determine the overall ENRR activity on these candidates. In contrast, the electrochemical steps are the overall rate controlling steps on CrNO, TaNO, HfNO, and TiNO. Thus, the ENRR activity of CrNO, TaNO, HfNO, and TiNO can be tuned by applying an external potential.
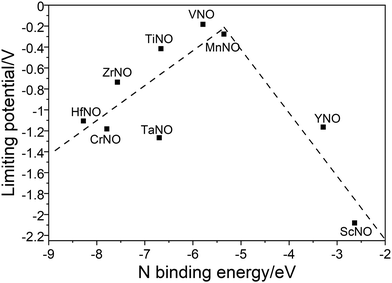
Along the most favorable reaction pathways, the calculated UL values were plotted against the N binding energies (NBEs). The calculated UL values show a volcano-like relationship with the NBE as shown in Fig. 7. The UL calculated on VNO is the lowest and lies close to the top of the volcano. Importantly, the DFT predicted UL on MnNO and TiNO are smaller than those calculated on some of the previously predicted efficient ENRR catalysts (e.g. VN(100): −0.51 V, ZrN(100): −0.76 V, CrN(100): −0.76 V, NbN(100): −0.65 V, and −0.69 V on the graphene single atom catalyst).1,34,41 This observation suggests that MnNO and TiNO should show excellent activity for N2 reduction to NH3. The volcano-like plots in Fig. 7 also show that YNO and ScNO bind N too weakly and the UL values of these candidates lie to the right of the volcano. In contrast, HfNO, ZrNO, TaNO, and CrNO bind N too strongly and lie on the left of the volcano. Importantly, our results identified the NBE as a descriptor of the ENRR activity on TMNO based catalysts. Thus, a future catalyst development strategy should focus on finding TM oxynitrides that have an NBE similar to that of VNO.
The HER is a competing reaction with the ENRR and most likely competes for the same reaction sites. Thus, the overall ENRR activity of a catalyst is influenced by the HER. DFT calculations are performed to calculate the binding energy of *H (Fig. S3(f), ESI†), a key reaction intermediate of the HER, on all the TMNO(111) surfaces studied in the present study. The calculated *H binding energy is then used to construct free energy diagrams shown in Fig. 8a. Fig. 8a shows that the HER efficiently occurs on MnNO with ΔG close to 0 eV. On CrNO, TaNO, HfNO, ScNO, YNO, TiNO, ZrNO, and VNO, *H + (H+ + e−) to H2(g) is predicted to be the rate limiting step. We observed a correlation between the HER limiting potential and the DFT calculated H binding energy (Fig. 8b). The HER activity is predicted to follow the order: MnNO > YNO > CrNO > ScNO > VNO> TaNO > TiNO > HfNO > ZrNO. MnNO lies close to the top of the volcano indicating that it efficiently promotes the HER. We find that the HER activity of TiNO, which has been predicted to be one of the best candidates for the ENRR in our above analysis, is not optimal, and more importantly, the calculated UL value for the ENRR is smaller than the UL for the HER. Thus, TiNO is more selective to the ENRR compared to the HER and thus selectively transforms N2 to NH3 under electrochemical conditions. Hence, TiNO is a potential candidate for selectively promoting the ENRR among all the TMNO catalysts studied here.
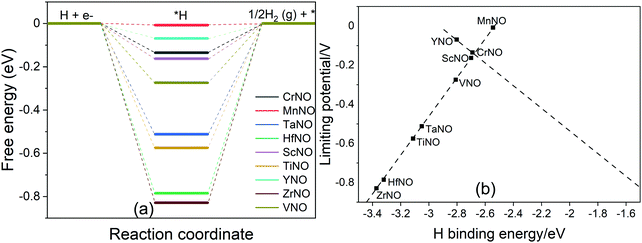 | ||
| Fig. 8 (a) DFT calculated free energy diagram of the HER at U = 0 V on N-vac. (b) Plot between the H binding energy and UL. | ||
We performed a similar investigation of the ENRR activity on O-vac, whose formation has been predicted to be less favorable compared to N-vac. The calculated free energy diagrams are included in Fig. S5–S7 in the ESI.† On O-vac, the ENRR proceeds along the associative N2H2 pathway on MnNO and ScNO. All other candidates (YNO, TaNO, TiNO, ZrNO, HfNO, CrNO, and VNO) promote the ENRR along the dissociative pathway. The most difficult steps along the associative N2H2 pathway on MnNO and ScNO are *NH2 + (H+ + e−) → *NH3 and *N2 + (H+ + e−) → *N2H with ΔG values of 1.70 eV and 2.73 eV, respectively. Similarly, the most difficult steps along the most favorable dissociative pathway on CrNO, TaNO, TiNO, YNO, VNO, ZrNO, and HfNO are *NH + (H+ + e−) → *NH2, *NH3 → * + NH3(g), *NH2 + (H+ + e−) → *NH3, *NH3 → * + NH3(g), *NH3 → * + NH3(g), *NH2 + (H+ + e−), and *NH + (H+ + e−) → *NH2 with ΔG values of 1.68 eV, 1.11 eV, 0.63 eV, 1.11 eV, 0.59 eV, 1.09 eV and 1.38 eV, respectively. The calculated UL values along the most favorable pathways on TiNO and VNO are −0.63 V and −0.56 V, respectively. We find that the ΔG values of the most difficult steps and UL on TiNO are similar to those on VNO. Thus the DFT calculations predict the ENRR activity of TiNO comparable to VNO, which has been experimentally shown to be an excellent ENRR catalyst.
Conclusions
DFT calculations are performed to study the ENRR on transition metal oxynitrides (TMNOs: TM = Sc, Ti, V, Cr, Mn, Y, Zr, Hf, and Ta). The results show a vacancy mediated ENRR via the MvK mechanism. Our thermodynamic analysis predicted a favorable formation surface N-vac compared to O-vac on all TMNOs except MnNO, and thus N-vacs are the active sites for the adsorption of the reaction intermediates involved in the ENRR. Among all TMNOs in the present study, our DFT calculations identified TiNO as a promising candidate with UL values similar to those of VNO, which has been shown to be an excellent ENRR catalyst experimentally. Furthermore, a volcano like feature is observed between the calculated UL values and the NBE suggesting that the NBE is a potential descriptor of the ENRR activity on TMNO based catalysts and the future catalyst development strategy should focus on finding TM oxynitrides that have NBEs similar to those of VNO.Conflicts of interest
There are no conflicts to declare.Acknowledgements
DFT calculations were performed using computational resources at the Extreme Science and Engineering Discovery Environment, which is supported by the National Science Foundation Grant number ACI-1548562 and at the National Energy Research Scientific Computing Center (NERSC), a U.S. Department of Energy Office of Science User Facility operated under Contract No. DE-AC02-05CH11231. S. K. acknowledges the faculty start-up fund from Florida A&M University.References
- Y. Abghoui, A. L. Garden, V. F. Hlynsson, S. Björgvinsdóttir, H. Ólafsdóttir and E. Skúlason, Phys. Chem. Chem. Phys., 2015, 17, 4909–4918 RSC.
- I. A. Amar, R. Lan, C. T. G. Petit and S. Tao, J. Solid State Electrochem., 2011, 15, 1845–1860 CrossRef CAS.
- S. Giddey, S. P. S. Badwal and A. Kulkarni, Int. J. Hydrogen Energy, 2013, 38, 14576–14594 CrossRef CAS.
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS.
- R. Zhang, X. Ren, X. Shi, F. Xie, B. Zheng, X. Guo and X. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 28251–28255 CrossRef CAS.
- I. Garagounis, A. Vourros, D. Stoukides, D. Dasopoulos and M. Stoukides, Membranes, 2019, 9, 112 CrossRef CAS.
- I. Garagounis, V. Kyriakou, A. Skodra, E. Vasileiou and M. Stoukides, Front. Energy Res., 2014, 2, 1 Search PubMed.
- D. G. H. Ham, C. J. M. Koper and M. T. M. Hetterscheid, Chem. Soc. Rev., 2014, 43, 5183–5191 RSC.
- V. Kyriakou, I. Garagounis, A. Vourros, E. Vasileiou and M. Stoukides, Joule, 2020, 4, 142–158 CrossRef CAS.
- E. Cussler, A. McCormick, M. Reese and M. Malmali, J. Visualized Exp., 2017, 126, 55691 Search PubMed.
- J. Yu, C. Li, B. Li, X. Zhu, R. Zhang, L. Ji, D. Tang, A. M. Asiri, X. Sun, Q. Li, S. Liu and Y. Luo, Chem. Commun., 2019, 55, 6401–6404 RSC.
- A. Hellman, E. J. Baerends, M. Biczysko, T. Bligaard, C. H. Christensen, D. C. Clary, S. Dahl, R. Van Harrevelt, K. Honkala, H. Jonsson, G. J. Kroes, M. Luppi, U. Manthe, J. K. Nørskov, R. A. Olsen, J. Rossmeisl, E. Skúlason, C. S. Tautermann, A. J. C. Varandas and J. K. Vincent, J. Phys. Chem. B, 2006, 110, 17719–17735 CrossRef CAS.
- B. H. R. Suryanto, D. Wang, L. M. Azofra, M. Harb, L. Cavallo, R. Jalili, D. R. G. Mitchell, M. Chatti and D. R. MacFarlane, ACS Energy Lett., 2019, 4, 430–435 CrossRef CAS.
- V. Smil, Sci. Am., 1997, 277, 76–81 CrossRef CAS.
- Y. Tanabe and Y. Nishibayashi, Coord. Chem. Rev., 2013, 257, 2551–2564 CrossRef CAS.
- S. Licht, B. Cui, B. Wang, F. F. Li, J. Lau and S. Liu, Science, 2014, 345, 637–640 CrossRef CAS.
- J. M. Modak, Resonance, 2011, 16, 1159–1167 CrossRef.
- Y. Abghoui and E. Skúlasson, Proc. Comput. Sci., 2015, 51, 1897–1906 CrossRef.
- M. Yuan, H. Zhang, D. Gao, H. He, Y. Sun, P. Lu, S. Dipazir, Q. Li, L. Zhou, S. Li, Z. Liu, J. Yang, Y. Xie, H. Zhao and G. Zhang, J. Mater. Chem. A, 2020, 8, 2691–2700 RSC.
- Y. Song, D. Johnson, R. Peng, D. K. Hensley, P. V. Bonnesen, L. Liang, J. Huang, F. Yang, F. Zhang, R. Qiao, A. P. Baddorf, T. J. Tschaplinski, N. L. Engle, M. C. Hatzell, Z. Wu, D. A. Cullen, H. M. Meyer, B. G. Sumpter and A. J. Rondinone, Sci. Adv., 2018, 4, 1700336 CrossRef.
- L. Hollevoet, M. De Ras, M. Roeffaers, J. Hofkens and J. A. Martens, ACS Energy Lett., 2020, 5, 1124–1127 CrossRef CAS.
- M. J. Palys, A. McCormick, E. L. Cussler and P. Daoutidis, Processes, 2018, 6, 91 CrossRef.
- J. M. McEnaney, A. R. Singh, J. A. Schwalbe, J. Kibsgaard, J. C. Lin, M. Cargnello, T. F. Jaramillo and J. K. Nørskov, Energy Environ. Sci., 2017, 10, 1621–1630 RSC.
- A. Klerke, C. H. Christensen, J. K. Nørskov and T. Vegge, J. Mater. Chem., 2008, 18, 2304–2310 RSC.
- J. H. Montoya, C. Tsai, A. Vojvodic and J. K. Nørskov, ChemSusChem, 2015, 8, 2180–2186 CrossRef CAS.
- Y. Yao, Q. Feng, S. Zhu, J. Li, Y. Yao, Y. Wang, Q. Wang, M. Gu, H. Wang, H. Li, X. Z. Yuan and M. Shao, Small Methods, 2018, 3, 1800324 CrossRef.
- C. Guo, J. Ran, A. Vasileff and S. Z. Qiao, Energy Environ. Sci., 2018, 11, 45–56 RSC.
- Z. Wang, F. Gong, L. Zhang, R. Wang, L. Ji, Q. Liu, Y. Luo, H. Guo, Y. Li, P. Gao, X. Shi, B. Li, B. Tang and X. Sun, Adv. Sci., 2018, 6, 1801182 CrossRef.
- M. M. Shi, D. Bao, S. J. Li, B. R. Wulan, J. M. Yan and Q. Jiang, Adv. Energy Mater., 2018, 8, 1800124 CrossRef.
- Y. Abghoui and E. Skúlason, J. Phys. Chem. C, 2017, 121, 6141–6151 CrossRef CAS.
- Y. Abghoui, A. L. Garden, J. G. Howalt, T. Vegge and E. Skúlason, ACS Catal., 2016, 6, 635–646 CrossRef CAS.
- Y. Abghoui and E. Skúlason, Catal. Today, 2017, 286, 78–84 CrossRef CAS.
- Q. Li, L. He, C. Sun and X. Zhang, J. Phys. Chem. C, 2017, 121, 27563–27568 CrossRef CAS.
- C. Choi, S. Back, N. Y. Kim, J. Lim, Y. H. Kim and Y. Jung, ACS Catal., 2018, 8, 7517–7525 CrossRef CAS.
- C. Ling, X. Niu, Q. Li, A. Du and J. Wang, J. Am. Chem. Soc., 2018, 140, 14161–14168 CrossRef CAS.
- C. Hering-Junghans, Angew. Chem., Int. Ed., 2018, 57, 6738–6740 Search PubMed.
- J. Zhao and Z. Chen, J. Am. Chem. Soc., 2017, 139, 12480–12487 CrossRef CAS.
- L. M. Azofra, N. Li, D. R. Macfarlane and C. Sun, Energy Environ. Sci., 2016, 9, 2545–2549 RSC.
- S. Zheng, S. Li, Z. Mei, Z. Hu, M. Chu, J. Liu, X. Chen and F. Pan, J. Phys. Chem. Lett., 2019, 10, 6984–6989 CrossRef CAS.
- X. Yang, J. Nash, J. Anibal, M. Dunwell, S. Kattel, E. Stavitski, K. Attenkofer, J. G. Chen, Y. Yan and B. Xu, J. Am. Chem. Soc., 2018, 140, 13387–13391 CrossRef CAS.
- X. Yang, S. Kattel, J. Nash, X. Chang, J. H. Lee, Y. Yan, J. G. Chen and B. Xu, Angew. Chem., Int. Ed., 2019, 58, 13768–13772 CrossRef CAS.
- S. Kang, J. Wang, S. Zhang, C. Zhao, G. Wang, W. Cai and H. Zhang, Electrochem. Commun., 2019, 100, 90–95 CrossRef CAS.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 145, 561 Search PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 13115–13118 CrossRef CAS.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- J. P. Perdew and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 12947–12954 CrossRef.
- J. D. Pack and H. J. Monkhorst, Phys. Rev. B: Solid State, 1977, 16, 1748–1749 CrossRef.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- Z. Wei, Y. Feng and J. Ma, J. Energy Chem., 2020, 48, 322–327 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00849d |
| This journal is © The Royal Society of Chemistry 2021 |