Open Access Article
Open Access ArticleA reversible and fast-responsive humidity sensor based on a lead-free Cs2TeCl6 double perovskite†
Chaojie
Pi
ab,
Xue
Yu
 a,
Weiqing
Chen
a,
Liuli
Yang
a,
Chao
Wang
a,
Zhichao
Liu
a,
Yiya
Wang
b,
Jianbei
Qiu
a,
Weiqing
Chen
a,
Liuli
Yang
a,
Chao
Wang
a,
Zhichao
Liu
a,
Yiya
Wang
b,
Jianbei
Qiu
 a,
Bitao
Liu
a,
Bitao
Liu
 *ab and
Xuhui
Xu
*ab and
Xuhui
Xu
 *a
*a
aCollege of Materials Science and Engineering, Kunming University of Science and Technology, Kunming 650093, P. R. China. E-mail: xuxuh07@126.com
bResearch Institute for New Materials Technology, Chongqing University of Arts and Sciences, Chongqing 402160, P. R. China. E-mail: liubitao007@163.com
First published on 22nd January 2021
Abstract
Owing to their high conductivity and carrier mobility, the outstanding achievements of lead halide perovskites have been demonstrated in humidity sensor applications. However, the rapid degradation of perovskites upon over-exposure to high humidity as well as the toxicity of lead hinder their large-scale commercial applications. Herein, a lead-free Cs2TeCl6 double perovskite is explored to detect humidity owing to its extraordinary humidity-dependent electrical properties. The Cs2TeCl6 thin film explored as a perovskite-based humidity sensor shows excellent reliability and stability in relative humidity (RH) range from 5% to 90% with a super-fast response (13.8 s) and recovery time (1.39 s). Moreover, the outstanding reversible ability of the developed sensor was achieved when the humidity detector was repaired using HCl gas, thus overcoming the inevitable degradation issue of the perovskite-based sensors, even working in a long-term humidity environment (RH > 90%). Furthermore, the integrated Cs2TeCl6 perovskite-based face mask was used for the real-time monitoring of human respiration, giving a basic human assessment of health status directly and rapidly.
Introduction
Humidity sensing devices have attracted tremendous attention for their potential applications covering health monitoring, weather forecast, agricultural fields, and aerospace exploration.1–4 Metal oxides, photonic crystals, polymer materials, and graphene composites have been employed as detection materials;5–12 unfortunately, the complicated synthesis procedures, low sensitivity, and limited operating range contribute to an urgent demand for exploring more reliable, responsive and commercial humidity-sensing materials. Compared with available candidates, organic/inorganic perovskites greatly appear to be attractive and competitive potential materials due to their high sensitivity, simple fabrication process, and excellent physical properties (e.g., high conductivity, high carrier mobility, and tenable bandgap). Hu et al.13 reported CH3NH3PbI3−xClx films as a humidity sensor for detecting relative humidity (RH) range from 32 to 97% with a recovery time of 74 s. Furthermore, a CH3NH3PbBr3 humidity sensor with a wider humidity detection range (7–98%), high sensitivity and fast response (250 s), and recovery time (30–70 s) were investigated.14 Subsequently, Ren et al.15 demonstrated the excellent moisture sensitivity of the vertically aligned nanosheet arrays of CH3NH3PbI3−xClx, which show strong stability against decomposition by water molecules. Moreover, the correspondingly developed device exhibits a faster response time than the commercially available psychrometer, reaching the first RH peak in 21 s, compared to 51 s by the commercial counterpart. Although considerable efforts have been made, lead toxicity and the inevitable device degradation in the humidity environment (RH > 90%) of the involved perovskite-based sensor remains a daunting challenge.16–21 The degradation of organic/inorganic perovskites is determined by the low formation energy and inherent structural instability, which can easily cause crystal structure damage, phase separation, and precipitation of the heavy metal element lead.22,23Herein, a full inorganic lead-free and stable Cs2TeCl6 perovskite, which is a vacancy-ordered structure with isolated [TeCl6]2− octahedral, was explored via a wet-chemical process. Moreover, the Cs2TeCl6 thin film, as a perovskite-based humidity sensor, exhibits a promising humidity detection ability with an outstanding reversible property due to its special structure. A fast response time could be achieved for the designed humidity sensor causing a facile repair process and overcoming the degradation issue of the perovskite-based sensor, even working in a long-term high humidity environment (RH > 90%). Owing to its good stability, superfast response and recovery ability, the explored Cs2TeCl6-based humidity sensor is integrated into a portable mask, providing the real-time assessment of human respiration with high-performance.
Results and discussion
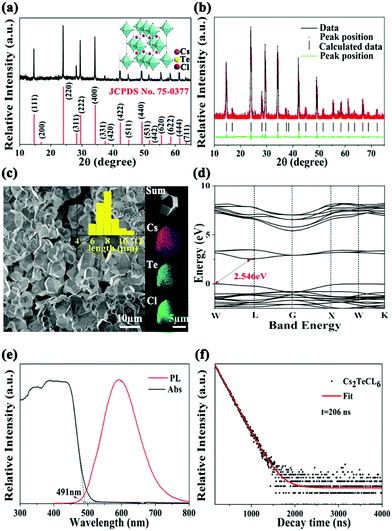
We investigated the structural properties of the Cs2TeCl6 perovskite particles via X-ray diffraction (XRD). All the diffraction patterns match well with the standard patterns of Cs2TeCl6 (JCPDS No. 75-0377) shown in Fig. 1a, and the XRD refinement shown in Fig. 1b ensured that the pure phase was derived for the Cs2TeCl6 perovskite. The inset is the crystal structure of Cs2TeCl6, which belongs to the Fm3m space group (a = 10.449 Å) and adopts a vacancy-ordered structure with isolated [TeCl6]2− octahedra.24 The scanning electron microscopy (SEM) image (Fig. 1c) shows that the mean particle size is 7–10 μm, and the energy dispersive spectroscopy (EDS) mapping demonstrates the uniform distribution of the Cs, Te, and Cl elements, with the atomic ratio of Cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Te
Te![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl being close to 2
Cl being close to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (Fig. S1, ESI†), which is consistent with the XRD results. As shown in Fig. 1d, the density functional theory (DFT) calculation results show that Cs2TeCl6 has an indirect bandgap with a bandgap of 2.546 eV, and the electronic structure of the valence band originates predominantly from Cs 2p states, whereas the conduction band is composed mostly of Te 4s and Cl 2p states as inferred from the partial density of state (PDOS) information shown in Fig. S2 (ESI†). Cs2TeCl6 exhibits yellow emission centered at 595 nm with a long lifetime of 206 ns at room temperature (Fig. 1e and f). Its absorption edge is located at 495 nm, which is consistent with the DFT calculation.
6 (Fig. S1, ESI†), which is consistent with the XRD results. As shown in Fig. 1d, the density functional theory (DFT) calculation results show that Cs2TeCl6 has an indirect bandgap with a bandgap of 2.546 eV, and the electronic structure of the valence band originates predominantly from Cs 2p states, whereas the conduction band is composed mostly of Te 4s and Cl 2p states as inferred from the partial density of state (PDOS) information shown in Fig. S2 (ESI†). Cs2TeCl6 exhibits yellow emission centered at 595 nm with a long lifetime of 206 ns at room temperature (Fig. 1e and f). Its absorption edge is located at 495 nm, which is consistent with the DFT calculation.
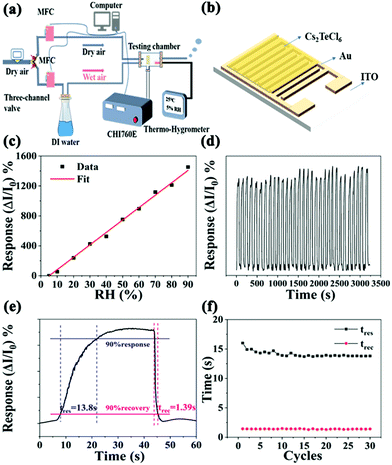
We investigated the performance of the humidity detection ability by building a sensing setup, where the relative data were acquired in a clean-room with an ambient temperature of 30 °C (Fig. 2a). The as-developed Cs2TeCl6 thin film as a sensor (Fig. 2b) was placed into a hermetic metallic conduit with three through-holes for the dry and wet air inlets and outlets. The RH in the metallic conduit was controlled by a mass flow controller (MFC) to flow the wet or dry air gas into the testing chamber, and the RH value in the tube could be read from a commercial humidity sensor. Notably, we observe the reversible operation of the Cs2TeCl6-based device when exposed in the RH range from 5 to 90% (Fig. 2c). The developed humidity sensor device exhibits extraordinary humidity-dependent electrical properties in the RH ranging from 5 to 90%. Moreover, the dynamic response and recovery curve of the Cs2TeCl6 humidity sensor for 30 cycles under a bias of 0.1 V at 90% RH are shown in Fig. 2d. The results confirm that the developed humidity sensor possesses superior repeatability and reliability in a continuous measurement (RH: 90%). The response and recovery times from the sensing curves are extrapolated, as illustrated in Fig. 2e. The response (tres) and recovery time (trec) are defined as the time needed to attain 90% of ΔI(I − I0), where I0 is the initial current, which in the present case is taken as the value at 5% RH, and I is the current measured at different RH levels.25 Hence, the humidity sensor exhibits an ultra-fast response and recovery time of 13.8 and 1.39 s, respectively, which are mainly due to the planar structure, where the water molecules can be easily volatilized during the desorption process.26 In addition, the bond between the Cs2TeCl6 thin film surface and water molecules is relatively weaker than that on the metal oxide ceramics humidity sensor since the hydrogen bond H–Cl is weaker than H–O. As a result, it could be easier for the first-layer water molecules to volatilize from the Cs2TeCl6 film surface than from the metal oxide ceramic surface. The performance of which is superior to that of the reported available humidity sensors listed in Table 1 to the best of our knowledge. Particularly, the recovery speed is extremely noticeable surpassing that of the reported perovskite-based humidity sensors.14,15,27–33 In fact, the stability of the humidity sensor is determined by the performance of the explored Cs2TeCl6 perovskite. The long-term performance of the Cs2TeCl6-based humidity sensor in the RH range of 5 to 90% was investigated. As shown in the PL spectra in Fig. S3 (ESI†), Cs2TeCl6 particles still maintain 85% of the initial luminescence intensity after being placed in an ambient environment at room temperature with an RH of 50% for 45 days. In addition, XRD results (Fig. S4, ESI†) confirmed the stability of the as-obtained Cs2TeCl6 humidity sensor, as seen from the original XRD patterns of Cs2TeCl6 exhibited insignificant changes after being placed at room temperature for 45 days. The TG and DSC curves in Fig. S5 (ESI†) characterize its thermodynamic properties, which suggests that Cs2TeCl6 could be stabilized below 400 °C. The above results prove the unique and stable crystal structure of the explored Cs2TeCl6 in the humidity range (5–90%). As shown in Fig. 2f, the response and recovery time hardly changed with the increased cycles, which proves the excellent stability of the explored Cs2TeCl6-based sensor.
| Type | Fabrication | Measure range (%) | t res (s) | t rec (s) | Ref. |
|---|---|---|---|---|---|
| CH3NH3PbBr3 | Grinding | 7–98 | 250 | 30–70 | Xu et al.14 |
| CH3NH3PbI0.2Cl2.8 | Solution growth | 35–65 | 24 | 24 | Ren et al.15 |
| Cs2BiAgBr6 film | Spin-coating | 5–75 | 1.78 | 0.45 | Weng et al.26 |
| CdTiO3 nanofibers | Electrospinning | 40–90 | 4 | 6 | Imran et al.27 |
| ZnSnO3 | Hydrothermal | 11–97 | 7 | 16 | Bauskar et al.28 |
| K0.5Na0.5NbO3 | MOD | 11–95 | 8 | 18 | Yuan et al.29 |
| CdS nanoparticles | Chemical bath deposition | 17–85 | 60 | 30 | Demir et al.30 |
| BaTiO3 nanofiber | Electrospinning | 11–95 | 30 | 9 | Wang et al.31 |
| Al2O3 nanotubes | Precipitation | 11–95 | 10 | 20 | Cheng et al.32 |
| SnO2 nanowire | Chemical vapor deposition | 30–85 | 120 | 20 | Kuang et al.33 |
| Cs2TeCl6 | Solution growth | 5–90 | 13.8 | 1.39 | Our work |
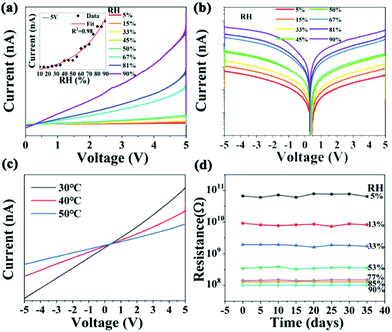
Normalized sensitivity (S) is a significant parameter for a humidity sensor, which is defined as S = |Rx − R0|/R0. Rx and R0 refer to resistances at x% and 5% RH, respectively.34 The resistance of the sensor can be obtained from the linear fitting of the I–V curves. Hence, the I–V (current–voltage) curves of the sensor are measured in a climatic chamber, and the result shows a continuous increase in the current response with the increase in the RH value, as shown in Fig. 3a. Moreover, the relationship between the current and the RH values is recorded when the sensor is biased at the voltage of 5 V, and the result of which is plotted in the inset of Fig. 3a. It shows that the current in the humidity sensor grows exponentially with the increased RH value. As the humidity increases from 5 to 90%, the resistance of the sensors decreases from 1011 to 108 Ω. The sensitivity of the humidity sensor is determined to be 984. Besides, there is an abrupt increase in current when the humidity increases from 50 to 67% RH, as shown in the logarithmic representation shown in Fig. 3b, which indicates that moisture absorption is enhanced in this humidity range. These results demonstrate that the resistance shows an excellent logarithmic linearity toward RH. It has been reported that heat treatment can facilitate the degradation process of the perovskite under humidity condition.35–37 Hence, we monitored the sensor resistance at 30 °C, 40 °C, and 50 °C, as shown in Fig. 3c. It can be seen that the resistance of the humidity sensor decreases with the increase in temperature from 30 to 50 °C, which can be attributed to the larger saturated water vapor pressure at 50 °C. Fig. 3d shows that the resistance of the device decreases significantly with the increase in humidity, which is consistent with the result of the CV curve shown in Fig. 3a. Notably, the resistance value of the humidity sensor hardly changed, which indicates the long-term stability of the device. However, after Cs2TeCl6 was treated at ultra-high humidity (RH = 100%), as shown in Fig. S6 (ESI†), it was found that a small impurity peak of H2Te2O6 appeared, although the main diffraction peak of XRD still existed. Moreover, Cs2TeCl6 and a very small amount of CsCl raw material phase of the above-involved perovskite appears after treatment at 70 °C, suggesting that the damaged Cs2TeCl6 in a high humidity environment (RH > 90%) could be repaired to some extent. The PL emission spectra shown in Fig. S8 (ESI†) further confirmed the above conclusion. The sample that was immersed in water exhibited characteristic yellow-green luminescence again, with a much weaker luminous intensity compared to the original luminous intensity. Fig. S9 (ESI†) illustrates that the performance of the repaired Cs2TeCl6 sensor is far from satisfactory. It is speculated that there is a continuous loss of Cl1− during heat treatment. XPS results shown in Fig. S10 (ESI†) indicate that the peaks located at 573.2 and 576.4 eV are ascribed to TeCl4 and TeO2, respectively.38,39 Fig. S11 (ESI†) indicates that during the oxidation process on the surface, the TeCl4 peak was still present on the surface of the sensor when the RH was below 90%. In contrast, once the RH is higher than 90%, only the TeO2 information could be detected, it will return to the original position after heat treatment, which can be proved from the offset of the binding energy before and after O 1s and Cl 2p repair. The above-mentioned results confirm that a post-heat treatment benefits the recovery of the device performance. Unfortunately, the continuous loss of Cl1− and the destruction of the structure during the heat treatment contribute to an unsatisfied outcome.
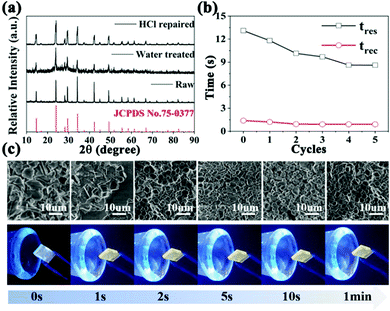
To solve the problem of the continuous loss of Cl1− caused by the heat treatment, HCl gas was employed to repair the as-explored humidity sensor (Movie S1, ESI†). As shown in Fig. 4a, the sample returned to its original pure phase of Cs2TeCl6 with a broad diffraction peak after the HCl gas repair, which suggests that finer Cs2TeCl6 particles are obtained after the HCl gas treatment, as shown in SEM images (Fig. 4c). The characteristic luminescence properties of the HCl gas treated sample presented in Fig. S11 (ESI†) indicate that the PL intensity increases with the prolonged HCl gas treatment, and after 5 s, finally it reaches the initial luminescence intensity. Moreover, we measured the response and recovery time of the device before and after multiple cycles of the HCl gas repair, and the results are shown in Fig. 4b. After the HCl gas repair, the response and recovery time of the humidity-sensitive detector could be recovered. With the increase in the number of HCl gas repair, the response time and recovery time of the detector become less, which could be related to the continuous supply of Cl1− and larger surface over bulk area owing to grain refinement. Furthermore, we compared the morphology of the samples before and after the repair and found that the samples after the HCl gas repair appeared to show grain refinement. The significantly-increased surface of Cs2TeCl6 could be the reason for the decrease in the response time and recovery time of the humidity sensor. The XPS spectra illustrated in Fig. S12 and S13 (ESI†) show a raw representation of the original sample without any processing. The main peaks of Cl 2p located at 197.5 and 200.3 eV have binding energy positions moved towards a low binding energy after the device surface contacted with water in air. Moreover, the peaks of O 1s located at 532.5 eV moved to 531.7 eV after water treatment. It is worth noting that the main peaks of Cl 2p and O 1s completely returned to their original peak location after a simple HCl gas repair, demonstrating that HCl gas can completely repair the performance of the humidity sensor and solve the problem of device degradation that cannot be solved by simple heat treatment. This also proves the particularity of the HCl gas repair method.
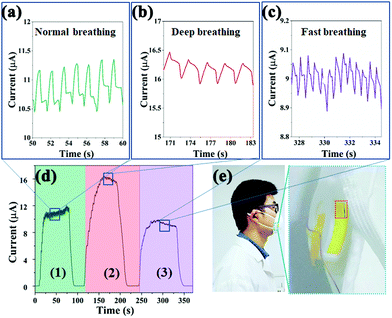
Herein, the reversible lead-free Cs2TeCl6 double perovskite-based sensor was integrated with a commercial face mask to realize the real-time monitor of the respiratory profiles of individuals (Movie S2, ESI†). Normal, deep, and fast breathing can be easily differentiated from the recorded current (Fig. 5d). It was found that the peak currents of all respiration modes gradually increase during the first few exhalation-inhalation cycles. The phenomenon could be explained by the gradual formation of a stable water molecule film and the warming of the sensing layer.40Fig. 5a, b and c are the enlarged maps corresponding to normal, deep, and fast breathing. In comparison with the normal one, deep breathing induces a series of much stronger electrical signals with a relatively low frequency, while along with the deep respiration process, water molecules are exhaled out, leading to a thick water film on the sensing layer. Thus, the resistance of the device remarkably reduces and strong current signals can be detected. Hence, weak electrical responses with a very high frequency are displayed in Fig. 5c. The weak signals could be attributed to two reasons: less moisture from the exhaled air as well as insufficient removal of the adsorbed water layer due to the rapid and shallow breathing.41 Herein, human respiration could be monitored in real-time by the as-developed sensor, promising wearable electronic devices for clinical respiratory monitoring (Fig. 5e).
Conclusions
In summary, we synthesized a lead-free Cs2TeCl6 double perovskite-based humidity sensor. The humidity sensor shows a fast response (13.8 s) and recovery times (1.39 s) in the RH range from 5% to 90%. Moreover, the resistance of the sensor suffered from relatively insignificant change during 35 days of repeated measurements in a relative humidity range of 5% to 90%. Furthermore, the device degradation problem in a high humidity environment (RH > 90%) was fundamentally solved via HCl gas repair. Finally, the humidity sensor shows the ability to detect the human respiration rate in real-time, which could be a promising electronic device for clinical respiration monitoring.Experimental
Materials
Cesium chloride (CsCl, 99.99%) and tellurium dioxide (TeO2, 99.999%) were purchased from Sigma-Aldrich. Hydrochloric acid (HCl, 37%) and ethanol were purchased from Sinopharm Chemical Reagent Company. All materials were used as received without further purification.Synthesis of double-perovskite materials
Cs2TeCl6 was synthesized according to the procedure reported by Slavney et al.42 In brief, 610 mg CsCl was dissolved in 10 ml of hydrochloric acid in a flask at 60 °C and stirred for 30 min to get precursor A. In another flask, 280 mg TeO2 was dissolved in 15 ml hydrochloric acid at 90 °C and stirred for 3 h until all solids were dissolved to get the precursor solution B. Then precursor solution A was swiftly injected into the flask containing the precursor solution B and stirred in a sealed glass bottle at 110 °C for 6 h. The solution was centrifuged at 4000 rpm for 8 min. The precipitants were collected and washed with ethanol and dried in a furnace at 60 °C overnight.Humidity sensor fabrication
Cs2TeCl6 were dispersed in absolute ethanol, and 80 μL of the Cs2TeCl6 solution was added to the ITO substrate and was spin-coated at 2000 rpm for 30 s to the completion of the spin-coating process. The substrates were subsequently annealed at 150 °C for 30 min to allow the complete formation of the desired double perovskite phase. Finally, the device was completed by evaporating two gold electrodes (50 nm) in a vacuum evaporation-coating equipment.Material characterization
The powder X-ray diffraction (XRD) patterns of the samples were recorded on a Dandong TD-3500 X-ray diffractometer using Cu Kα radiation, operating at 30 kV and 20 mA. The surface morphology and microstructure of as-obtained products were analyzed on a scanning electron microscope (Hitachi, SU-8010). X-ray photoelectron spectroscopy (XPS) was performed on an ESCA Lab MKII instrument to analyze the chemical composition of the obtained Ni foam. The spectra were recorded using a HITACHI U-4100 UV-vis-NIR spectrophotometer with an integrated sphere in the diffuse-reflectance mode. Samples were prepared by diluting the solution in ethanol in 1 cm path length quartz cuvettes. The PL spectra were taken on a Hitachi F-7000 fluorescence spectrophotometer.Humidity sensing measurement
The real-time sensing plots and I–V curve of Cs2TeCl6 thin film humidity sensors were obtained using an electrochemical workstation (CHI 660E, CH Instrument Company, Shanghai, China), which was employed to collect electrical signals by testing amperometric curves of the devices. For the real-time sensing plots, a homemade test system equipped with three channel-valve to switch on the dry air or wet air was fabricated. The gas flow rate was controlled at 15 L min−1 and the RH of gas flow was read using a commercial hygrometer (Shandong Renke Co. Ltd, China, Cos-03). HCl gas was used to repair the degraded humidity sensors in a simple and effective way. The details of the HCl gas repair method are shown in the Movie S1 (ESI†). Moreover, we monitored different respiration modes of a volunteer, including normal, deep, and fast breathing. The detailed test process is shown in the Movie S2 (ESI†).Theoretical calculation
In this study, the CASTEP module in the Materials Studio software is used to make geometric optimization of Cs2TeCl6, and Perdew–Burke–Ernzerhof (PBE) method in General gradient approximation (GGA) was used to calculate its electronic structure. In the calculation, the plane wave truncation energy was selected to be 550 eV, and the Brillouin region integral was adopted to be an inverse lattice space of 2 × 2 × 2 k points, and the convergence value of the total energy of each atom was 1.0 × 10−5 eV.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by National Nature Science Foundation of China (NSFC) (61965012, 11664022), the Reserve talents project of Yunnan Province (2017HB011), Yunnan Ten Thousand Talents Plan Young & Elite Talents Project (YNWR-QNBJ-2018-295, YNWR-QNBJ-2018-325), the Excellent Youth Project of Yunnan Province Applied Basic Research Project (2019FI001), foundation of Yunnan Province (2019HC016), National Science Funds of China (Grant No. 12064022), and China Postdoctoral Science Foundation (2019M663575).Notes and references
- C. Zhou, X. Zhang, N. Tang, Y. Fang, H. Zhang and X. Duan, Nanotechnology, 2020, 31, 125302 CrossRef CAS.
- J. Zhao, B. Cai, Z. Luo, Y. Dong, Y. Zhang, H. Xu, B. Hong, Y. Yang, L. Li and W. Zhang, Sci. Rep., 2016, 6, 21976 CrossRef CAS.
- Y.-Y. Zhang, S. Chen, P. Xu, H. Xiang, X.-G. Gong, A. Walsh and S.-H. Wei, Chin. Phys. Lett., 2018, 35, 036104 CrossRef.
- D. Zhang, H. Chang, P. Li, R. Liu and Q. Xue, Sens. Actuators, B, 2016, 225, 233–240 CrossRef CAS.
- J. S. Yun, J. Kim, T. Young, R. J. Patterson, D. Kim, J. Seidel, S. Lim, M. A. Green, S. Huang and A. Ho-Baillie, Adv. Funct. Mater., 2018, 28, 1705363 CrossRef.
- M. Yuan, Y. Zhang, X. Zheng, B. Jiang, P. Li and S. Deng, Sens. Actuators, B, 2015, 209, 252–257 CrossRef CAS.
- P. Yasaei, A. Behranginia, T. Foroozan, M. Asadi, K. Kim, F. Khalili-Araghi and A. Salehi-Khojin, ACS Nano, 2015, 9, 9898–9905 CrossRef CAS.
- J. Yang, B. D. Siempelkamp, D. Liu and T. L. Kelly, ACS Nano, 2015, 9, 1955–1963 CrossRef CAS.
- W. Xu, F. Li, Z. Cai, Y. Wang, F. Luo and X. Chen, J. Mater. Chem. C, 2016, 4, 9651–9655 RSC.
- Z. Weng, J. Qin, A. A. Umar, J. Wang, X. Zhang, H. Wang, X. Cui, X. Li, L. Zheng and Y. Zhan, Adv. Funct. Mater., 2019, 29, 1902234 CrossRef.
- Y. Wang, R. W. Besant, C. J. Simonson and W. Shang, Sens. Actuators, B, 2006, 115, 93–101 CrossRef CAS.
- L. Wang, Y. He, J. Hu, Q. Qi and T. Zhang, Sens. Actuators, B, 2011, 153, 460–464 CrossRef CAS.
- M. A. Squillaci, L. Ferlauto, Y. Zagranyarski, S. Milita, K. Müllen and P. Samorì, Adv. Mater., 2015, 27, 3170–3174 CrossRef CAS.
- A. H. Slavney, T. Hu, A. M. Lindenberg and H. I. Karunadasa, J. Am. Chem. Soc., 2016, 138, 2138–2141 CrossRef CAS.
- J. A. Schwenzer, L. Rakocevic, R. Gehlhaar, T. Abzieher, S. Gharibzadeh, S. Moghadamzadeh, A. Quintilla, B. S. Richards, U. Lemmer and U. W. Paetzold, ACS Appl. Mater. Interfaces, 2018, 10, 16390–16399 CrossRef CAS.
- C. Rubinger, C. Martins, M.-A. De Paoli and R. Rubinger, Sens. Actuators, B, 2007, 123, 42–49 CrossRef CAS.
- K. Ren, L. Huang, S. Yue, S. Lu, K. Liu, M. Azam, Z. Wang, Z. Wei, S. Qu and Z. Wang, J. Mater. Chem. C, 2017, 5, 2504–2508 RSC.
- B. W. Park and S. I. Seok, Adv. Mater., 2019, 31, 1805337 CrossRef.
- Y. Pang, J. Jian, T. Tu, Z. Yang, J. Ling, Y. Li, X. Wang, Y. Qiao, H. Tian and Y. Yang, Biosens. Bioelectron., 2018, 116, 123–129 CrossRef CAS.
- U. Mogera, A. A. Sagade, S. J. George and G. U. Kulkarni, Sci. Rep., 2014, 4, 4103 CrossRef CAS.
- W. Ming, S. Chen and M.-H. Du, J. Mater. Chem. A, 2016, 4, 16975–16981 RSC.
- B. Li, G. Xiao, F. Liu, Y. Qiao, C. M. Li and Z. Lu, J. Mater. Chem. C, 2018, 6, 4549–4554 RSC.
- C.-W. Lee, H.-S. Park, J.-G. Kim, B.-K. Choi, S.-W. Joo and M.-S. Gong, Sens. Actuators, B, 2005, 109, 315–322 CrossRef CAS.
- Q. Kuang, C. Lao, Z. L. Wang, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2007, 129, 6070–6071 CrossRef CAS.
- D. Kou, W. Ma, S. Zhang, J. L. Lutkenhaus and B. Tang, ACS Appl. Mater. Interfaces, 2018, 10, 41645–41654 CrossRef CAS.
- H. Konno and Y. Yamamoto, Bull. Chem. Soc. Jpn., 1988, 61, 2990–2992 CrossRef CAS.
- A. Jia, B. Liu, H. Liu, Q. Li and Y. Yun, Front. Chem., 2020, 8, 383 CrossRef CAS.
- Z. Imran, S. Batool, H. Jamil, M. Usman, M. Israr-Qadir, S. Shah, S. Jamil-Rana, M. A. Rafiq, M. Hasan and M. Willander, Ceram. Int., 2013, 39, 457–462 CrossRef CAS.
- L. Hu, G. Shao, T. Jiang, D. Li, X. Lv, H. Wang, X. Liu, H. Song, J. Tang and H. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 25113–25120 CrossRef CAS.
- Y. Han, S. Meyer, Y. Dkhissi, K. Weber, J. M. Pringle, U. Bach, L. Spiccia and Y.-B. Cheng, J. Mater. Chem. A, 2015, 3, 8139–8147 RSC.
- C. D. Flint and A. G. Paulusz, Inorg. Chem., 1981, 20, 1768–1771 CrossRef CAS.
- R. H. Fang, A. V. Kroll, W. Gao and L. Zhang, Adv. Mater., 2018, 30, 1706759 CrossRef.
- G. Divitini, S. Cacovich, F. Matteocci, L. Cinà, A. Di Carlo and C. Ducati, Nat. Energy, 2016, 1, 1–6 Search PubMed.
- M. R. Detty, W. C. Lenhart, P. G. Gassman and M. R. Callstrom, Organometallics, 1989, 8, 861–865 CrossRef CAS.
- R. Demir, S. Okur and M. E. Şeker, Ind. Eng. Chem. Res., 2012, 51, 3309–3313 CrossRef CAS.
- B. Conings, J. Drijkoningen, N. Gauquelin, A. Babayigit, J. D'Haen and L. D'Olieslaeger, Adv. Energy Mater., 2015, 5, 1 Search PubMed.
- B. Cheng, B. Tian, C. Xie, Y. Xiao and S. Lei, J. Mater. Chem., 2011, 21, 1907–1912 RSC.
- Y. Chen, X. Ma, C. Li, Q. Wu, Y. Wang, M. Huang, B. Liu and L. Li, Int. J. Mod. Phys. B, 2020, 34, 2040003 CrossRef CAS.
- Y. Chen, C. Li, X. Ma, Q. Qiang, B. Liu, S. Cao, L. Peng, L. Li and T. Lang, Appl. Surf. Sci., 2020, 506, 144816 CrossRef CAS.
- J. Bisquert and E. J. Juarez-Perez, The Causes of Degradation of Perovskite Solar Cells, ACS Publications, 2019 Search PubMed.
- K. P. Biju and M. K. Jain, Sens. Actuators, B, 2008, 128, 407–413 CrossRef CAS.
- D. Bauskar, B. Kale and P. Patil, Sens. Actuators, B, 2012, 161, 396–400 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ma00835d |
| This journal is © The Royal Society of Chemistry 2021 |