Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Conformable on-skin devices for thermo-electro-tactile stimulation: materials, design, and fabrication
Arianna
Mazzotta
 ,
Marco
Carlotti
,
Marco
Carlotti
 * and
Virgilio
Mattoli
* and
Virgilio
Mattoli
 *
*
Istituto Italiano di Tecnologia, Center for Materials Interfaces, Viale Rinaldo Piaggio 34, 56025 Pontedera, Pisa, Italy. E-mail: marco.carlotti@iit.it; virgilio.mattoli@iit.it
First published on 15th February 2021
Abstract
Conformable electronics is an emerging and innovative research field investigating functional materials and electronic devices capable of adhering and conforming to non-planar surfaces such as human skin. Conformable devices find applications of high economical and scientific interest in healthcare, human-machine interfaces, wearable electronics, robotics, and the internet of things. Compared to the more widely used rigid and bulky electrodes often employed in wearable platforms, the former offers multiple advantages such as imperceptibility, low cost, and the possibility of continuous use, being able to follow the multiple deformations of curvilinear living tissues. In recent years, much attention has been paid to the development of soft and skin-wearable sensors, but an additional area of great interest is certainly that of conformable electrodes capable of providing some kind of stimulation. This review provides an overview of the most attractive and innovative conformable devices developed to stimulate the human body through thermal, electrical, mechanical, and optical stimuli. In particular, it focuses on the functional materials employed, the fabrication techniques involved, and the design solutions proposed to improve the performance of conformable stimulation devices.
1. Introduction
The growing demand in recent years for miniaturized and portable electronics has led to the development of numerous conformable and flexible technologies. This was (and still is) driven by the revolutionary opportunities offered by a class of materials and devices able to adhere and conform to nonplanar surfaces.1 These devices find applications of high economical and scientific interest, such as in healthcare, human-machine interfaces, wearable electronics, robotics, and the internet of things. Technological advancements like new lithographic and microfabrication techniques allow us to reach complex design and unprecedented resolutions,2–5 and the implementation of innovative functional materials combined the necessary mechanical properties with appealing electronic characteristics.6Compared to the former, most of the electronics employed in the development of wearable platforms are planar and rigid, they are incompatible with the curvilinear and deformable living tissues, they lack conformability, and thus they result in high-impedance, unreliability, and uncomfortable contacts.1 Instead, conformable electronic skin devices, which have the intrinsic capability to perfectly adhere to surfaces, can follow the multiple deformations of the human skin and have unparalleled advantages like imperceptibility, low cost, and continuous use.7
In the medical field, the use of conformable electronics allows the replacement of stiff and bulky devices, which contrast with the soft body tissues and impose severe restrictions to the user comfort and mobility, limiting clinical applications and efficacy. The field of bioelectronics investigates electronic devices that operate as transducers between the signals and functions of biological systems and those of electronic processing systems. Through a myriad of options offered by chemistry, one can design organic electronic materials and devices that show the desired flexibility and conformability, the desired optical properties, and surface characteristics that promote biocompatibility and stability for long periods of time.8
So, in recent years, particular attention has been devoted to the development of devices able to monitor human physiological signals, through recording of the electrical activities of the human body (such as electrocardiography, ECG,9 electromyography, EMG,1,9 electroencephalography, and EEG,10), and measurements of biological parameters (such as pH, pressure, temperature and sweat composition).11–16
However, electronic skin devices can accomplish more than just sensing. Another class of conformable devices consists of active platforms, which find use in stimulation, instead of recording. These kinds of devices can apply a specific stimulus on the skin – which could be thermal, electrical, luminous, or mechanical – in order to activate the numerous receptors included in the dermis and epidermis. Such platforms can find diverse applications in different therapies, rehabilitation, personal thermal management technologies, bone remodeling, and human-robot interaction.17–21 Because of their design and characteristics, these devices are particularly promising, in particular considering that one of their paramount characteristics is the limited invasiveness. Their use as smart and self-care treatments is particularly appealing, providing patients that require frequent treatments or suffer chronic disorders with a platform they can use at home or in their daily activities without the need to physically go to the hospital.
As well as this, they also open new channels of communication between machines and the human body. This concept is central in applications such as gaining control of prosthetic limbs: the stimulation of the users is a means to restore tactile feedback and give them the ability to better control the device and to react faster to external stimuli coming from the prosthetic limb, overall resulting in an improvement of the embodiment and acceptability of the prosthesis itself.
Finally, apart from biomedical applications, the capability to provide specific environmental sensation (e.g. tactile, kinesthetic, and temperature feedback), while still maintaining a high level of comfort, is of paramount importance in applications, which require virtual reality (VR), like for example remote control, immersive experiences, and gaming.22
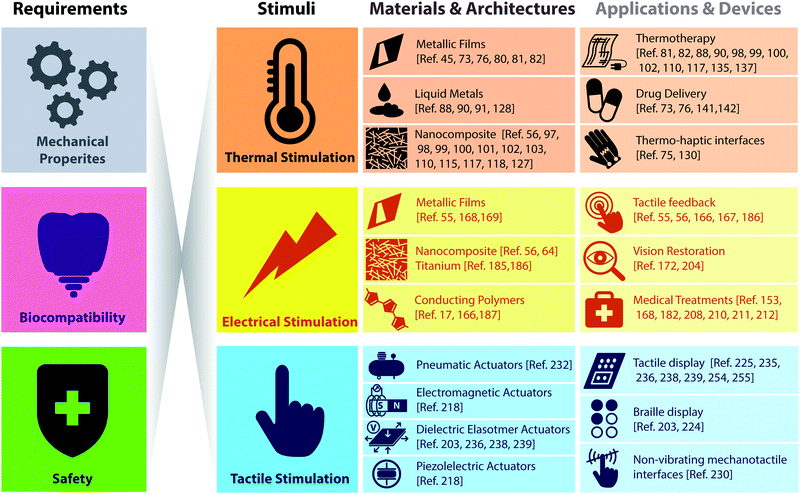
While applications of recording physiological signals have already been widely reviewed,23–26 less attention has been paid to devices able to generate a signal and interact directly with the human body. In this review we intend to report the conformable devices, which, according to us, are the most attractive and innovative in stimulating the human body, highlighting the functional materials that are employed, the fabrication techniques involved, and the design solutions that were proposed to improve their performance. In particular, we decided to focus our review on three main groups of stimulation devices, synthesized as follows: (i) devices able to provide thermal stimuli to the human body, (ii) devices providing electrical stimulation, and (iii) haptic displays aiming to restore tactile feedback. For completeness, in the last section, we also briefly examine innovative conformable photostimulation devices, a novel technological platform with a very promising future in healthcare and consumer electronics.
1.1. Requirements for conformable devices
Conformable electronics need to match these mechanical properties to ensure mechanical compatibility between the skin (or an internal tissue) and the device.26,30–33 A failure to do so may result in a misfunction of the device, a shortening of its lifetime, or, even worse, tissue damage and infections.33 This last point is particularly relevant in the case of stimulation. Hence, when designing a new device, one must bear in mind what its purpose is and how it is going to be applied to the user.25 The limitations posed by the skin in terms of stiffness and stretchability may not apply to other kinds of tissues and organs and implantable devices for the latter may need different materials (e.g. because of their limited strain, brain and bone tissues can be addressed with metal electrodes).34
In recent years, researchers have come up with many ways to match the mechanical properties of the substrate with those of the electrical components and connectors.24,31 A straightforward method is to use intrinsically stretchable polymers and composites.35,36 These offer many combinations of softness and stretchability to meet the device needs and can perform as stretchable substrates for printing and deposition, encapsulation materials for more rigid stuff (e.g. regular silicon-based electronic components), or – in the case of opportune functional composites – as themselves.32,33,37 Among this class of materials, silicone-based polymers (like polydimethylsiloxane (PDMS), Ecoflex, Dragon Skin, and Moldmax; Young's modulus: 1–7 MPa), poly(styrene-butadiene-styrene) (SBS; 2–33 MPa), poly(styrene-ethylene-butylene-styrene) (SEBS; 10–35 MPa), polyacrylates (7–35 MPa), and polyurethanes (PU, 3–51 MPa) are the more frequently used.32,35 It is worth mentioning that, just like for the current stimulation technology available on the market – which relies on bulky instrumentation and wired, stand-alone electrodes38 –devices obtained by encapsulation are often thick and require an additional adhesive to conform to the skin.39
Careful geometrical engineering can help in obtaining stretchable structures even from tough materials.40,41 2D (but also 3D) stretchable structures of metallic wires (as also rigid conductive polymers)42 are common in epidermal electronics as they allow stretching of the structure as a whole without straining the material much. Serpentines and spring structures, often with fractal elements, are the most common, especially to prepare connectors.24,37 The downside of this approach is that it is seldom applicable to the active components, limiting its scope. For the latter, a common methodology consists in preparing the desired element on pre-stressed substrates: if the former is thin enough (i.e. less than 5 μm), upon releasing it will buckle (without sensing much strain), and it can stretch again without compromising its performance.41,43,44
Just the limited thickness of thin structures allows them to bend without suffering the strain originating from the differences in length between the inner and the outer bending arches (which scales with the third power of the thickness for a film). Such principles can also support the fabrication of flexible devices from rigid materials that can work as conformable systems if they are not subjected to strain. This is the reason why it is common to find strong and tough polymers like polyimide (PI) and poly(ethylene naphtalate) (PEN) as substrates for thin skin electronics.29,45 Moreover, these devices can perfectly conform and adhere to the skin thanks to van der Waals forces, without the need of any adhesive or contact medium. Among these systems, ultrathin-film devices (<100 nm thick) are particularly interesting. Because of the negligible difference in bending radii as we discussed above, and thanks to an incredibly large aspect ratio which favors the adhesion, such systems can perfectly adhere to any substrate, even rough ones.5,46 This is very appealing for systems like the human skin whose elasticity is predominantly due to its fractal wrinkled structure. In particular, the ultra-thin film devices can elongate with the skin they are adhered to without cracking, no matter the Young's moduli of the materials, thus allowing the use of functional materials that would not be otherwise available because of their mechanical properties.1,9
Finally, among the ensemble of biocompatible materials, one may also find some which are biodegradable. This definition brings together all those materials, which our body is capable of decomposing without any harmful consequence or side effect. Many flexible and stretchable polymers are considered biodegradable, like polysaccharides, poly(lactic acid) (PLA), poly(ethylene glycol) (PEG), and poly(vinyl alcohol) (PVA),52 but also metals including iron, magnesium, zinc, and gold.53 Devices obtained from these materials find applications as conformable implants or practical disposable electronics, which do not need to be removed.
Commercial skin-mountable devices, widely used in the clinical practice, generally consist of a metal electrode that can interface with the skin by different contact methods. In some cases, the electrode can directly adhere to the skin without any interposed layer (dry electrodes), while another possibility is to interpose a gel layer (wet electrode) or a dielectric layer (capacitive contact electrode) between the electrode and the skin.54 However, the presence of interposed layers can avoid skin irritations and air gap formation, thus making the first configuration not as safe as the others. Air gaps indeed can degrade the electrical signal recorded54 or, in the case of stimulation devices, they can generate hot spots and burn the user as well as damaging the electrode, especially in electrical stimulations where the current reaches several mA.55,56 Since a non-uniform and inconsistent adhesion between the device and the skin during stimulation could damage human tissues, another key factor is the perfect contact between the former and the latter and therefore the ability of the device to conform well to non-planar surfaces.57 Ultra-thin devices, such as dry electrodes transferred from tattoo paper, can perfectly adhere to all the imperfections of human skin, safely recording electrophysiological signals replacing standard electrodes.
Moreover, attention should be paid to the conditioning of electronic components connected to the skin-mountable device. An important issue is to remove, orreduce, the possibility of electrical leakage from the electronics to the body: leakage currents can pose a health risk to the user by causing severe burns, muscle spasm, or may damage the nervous system to the extent that they may provoke cardiac arrests and amnesia.58 One should consider that the general standard (IEC 60601-1) for medical electrical equipment imposes a maximum leakage current of 0.5 mA for patient safety.59 Electrically safe devices could consist of capacitive structures made of an insulation layer that encapsulates the metal electrodes. This design ensures electrical safety in mounting the device directly on the skin but also for implantable electronics where the device has to be mounted on internal organ surfaces. In addition, the device so developed facilitates its own sterilization and cleaning operations, making possible its fast and safe reuse also for different subjects or in different trials.59 The insulation layer also encapsulates the active parts of the system in order to prevent degradation of the electrodes and, next to this, to prevent possible consequences of malfunctions that could damage the user's tissues.
In recent years, the interest in wearable biochemical sensing has increased, leading to the development of multiple bioelectronic systems that allow epidermal electrochemical monitoring of a variety of metabolites and electrolytes. Enzyme-based devices, with applications like continuous glucose monitoring in diabetic patients, alarm against dangerous chemical threats, or even the detection of alcohol in sweat are just some of the multiple examples with such applications.60,61 Concerning this latter application, different skin-worn alcohol monitoring platforms are integrated with an iontophoretic-biosensing system so as to locally stimulate sweat secretion by loading the iontophoretic electrodes with a sweat stimulant (for example, pilocarpine and carbachol). The Iontophoresis process consists of applying short-timed and localized current pulses to improve the drug diffusion through the skin. Hence, particular attention should be paid during the design and fabrication of the overall system so as to avoid possible skin burning and/or irritation caused by too high currents.62
Finally, another prerequisite for user safety is that wearable devices should work at a low driving voltage.35 Some applications still need deeper analysis of this factor. For example, mechanical stimulation systems require a high voltage supply to work properly. This aspect makes it difficult to safely integrate them into skin mountable devices.21 Other examples concern thermal stimulation: the heating module of heaters must not reach excessive temperatures. The designer should pay attention to developing a system capable of continuously remaining within a comfortable heating range for the user, in order to avoid sudden increases in temperature with consequent pain perception.63 Heaters must also have uniform electrical and thermal properties on their active parts, maintaining electrical stability despite the deformations of the skin once mounted on it: heating inhomogeneity can lead to burns on the user's skin. Similarly, conformable electrodes used in electrical stimulation devices must have a homogeneous current density over the entire electrode; larger electrodes allow more conformable sensations.64
The purpose of this review is to provide an overview of conformable stimulation devices (Fig. 1), here classified as (i) devices capable of delivering thermal stimuli to the human body, (ii) devices that provide electrical stimulation, and (iii) tactile displays that aim to restore tactile feedback. In the last section, we also report recent studies about conformable photostimulation devices. In particular, we have only considered “conformable to the body” technologies, emphasizing both the enabling materials exploited for their construction and their working principles.
2. Conformable devices for thermal stimulation
Conformable thermal stimulation devices consist of conductive and often transparent sheets capable of heating or, in some cases, cooling the surface they are in contact with. Their main applications concern mainly transport and building industrial sectors where they find application, for example, as vehicle defrosters and smart heat-retaining windows or solar panels.65–67 In recent years heaters have attracted increasing attention also in the medical field: the use of new materials and new fabrication techniques allowed the development of numerous conformable and stretchable heaters to directly mount onto human skin. Thermal stimulation is important in personal thermal management (PTM) technologies,68 where one of the most important applications in this sense is in physical treatments and, in particular, in thermotherapy. The latter can be defined as the therapeutic application of heat to specific regions of the body resulting in increased temperature in the tissue,69 often useful for the treatment of musculoskeletal disorders. The heating of body surfaces provides pain relief in wrist, shoulder, or lower back injuries,69,70 and alleviates disturbances from arthritis, stiff muscles, injuries to deep skin tissue, and joint injuries due to aging or obesity.17,71 Heat also increases the distensibility and flexibility of soft tissue: physiotherapy can exploit heaters to reduce injuries in sports.72 Furthermore, heaters find their use also in controlled drug delivery: in this sense, one can control the transdermal diffusion rate of therapeutic drugs – loaded on micro/nano-particles and embedded in conformable devices – by controlling the temperature.73 Since thermal stimulation allows discrimination between materials with different thermal properties, heaters integrated into haptic interfaces can activate thermoreceptors of the user, transferring cold and hot sensations useful to recognize different virtual objects (e.g. touching wood will give a different thermal sensation than touching glass). This impacts the sense of immersion in applications concerning VR.74 Surely, thermal displays could help blind people to avoid obstacles while walking or interacting with objects in their surroundings, increasing their level of independence.752.1 Materials
Conformable heaters usually take advantage of Joule heating, thus making the relatively high resistance of many flexible conductors (usually between 10−1 and 102 Ω)11,35 a strength point. One should pay attention to heat dissipation and the working voltage, as uncontrolled overheating might damage both the component and the host matrix.36,45 As we will discuss in this section, many materials have been proposed as active elements in heating solutions. However, here we decided to focus only on those studies, which made explicit reference to the use of their devices as body-conformable heaters (see Table 1), and therefore also addressed (at least partially) issues like biocompatibility and stability.36 As the reader will see, the latter only covers a small sub-group of all the flexible heaters, leaving a lot of ideas for improvements in future research.| System | Resistance/resistivitya | Resistance/resistivity at max. strain | Max. strain | Application | Ref. |
|---|---|---|---|---|---|
| a Resistance is used where the studies did not specify any form factors of the resistive elements. | |||||
| PEDOT:PSS/rGO in PU | 0.05 Ω cm | 530% | Heating pad | 17 | |
| Evaporated Au film (100 nm) on polyethylene naphtalate | 188 Ωsq | — | E-skin heater and temperature sensor | 45 | |
| 3D Au@AgNW network in SBS | 2.4 × 10−5 Ω cm | ∼3 × 10−5 Ω cm | 265% | E-skin heater, monitoring and stimulating mesh for hearts (implantable) | 56 |
| Evaporated Au (70 nm) on PI | 550 Ω | 630 Ω | Up to 30% | E-skin heater | 73 |
| P- and n-type Bi2Te3 thermoelectrics between electrodes | Peltier effect | Haptic device based on heating and cooling | 75 | ||
| Sputtered Zn/Mg/Ag (500 nm) on poly(glycerol sebacate)–poly(caprolactone) nanomesh | 20% | E-skin heater for drug delivery | 76 | ||
| Electrodeposited metal films (Au, and Pt) on PI | 1.2 Ω | — | E-skin heater and temperature sensor | 80 | |
| Al(9 μm) on PET | 17.2 Ω | E-skin heater and temperature sensor (simultaneous) | 81 | ||
| R2R continuous plasma-sputtered SiNx/Cu(12 nm)/[CNTs/polytetrafluor-oethylene] | 5 Ωsq | 30 Ωsq | Bending to 2 mm radius | Wearable heater | 82 |
| Galinstan/PDMS composite | 8.7 Ω | 9.1 Ω | >100% | Wearable heater, knee pad | 88 |
| EGaIn-Ni composite | 2.2 × 10−2 Ω | 2.4 × 10−2 Ω | 5 mm bend | Tattoo heater | 90 |
| Oxidated EGaIn | 2.9 × 10−5 Ω cm | 180° bending angle | Hypertermia element (wireless inductive heating) | 91 | |
| 2D AgNW network (vacuum filtration) in PDMS | 30 Ωsq | 180 Ωsq | 50% | Heating pad | 97 |
| 2D AgNW network (vacuum filtration) on PI | <5 Ωsq | <5 Ωsq | 450% | Kirigami heater for articulations | 98 |
| 2D AgNF network (electrospun) in PDMS (or PET) | 0.05 Ωsq | 0.06 Ωsq | 90% (PDMS) 10 mm bend radius (PET) | Wireless heating pad | 99 |
| 2D CuZr network (evaporated on template) in PDMS | 3.8 Ωsq | 4.9 Ωsq | 70% | Heating pad | 100 |
| 2D AgNW network in PDMS | 5.4 Ωsq | ∼40 Ωsq | 200% | Heating pad | 101 |
| 3D AgNW network in SBS | 8.3 × 10−5 Ω cm | ∼10−4 Ω cm | 100% | E-skin for articular thermotherapy | 102 |
| 2D AgNW network (spun coat) between PDMS/PVA | 3.7 Ωsq | 3.7 Ωsq | 1 mm bend radius | Heating pad | 103 |
| 2D CuNW (spray coated) between PET/PMMA | 17 Ωsq | 17 Ωsq | 3 mm bending radius | Heating pad | 110 |
| SWCNT in PVA | 475 Ωsq | 475 Ωsq | 20 mm bend radius | >10 μm-thin heating pad | 115 |
| Sintered AgNP on medical tape | 12 × 10−6 Ωsq | 13 × 10−6 Ωsq | 8 mm bend radius | 3D-printed heating pad | 117 |
| AgNW on PU sponge | 0.05 Ω cm | 3D compression-extension | Heating pad | 118 | |
| Au@SiO2 in alginate-chitosan matrix | Photothermal | Ultraconformable | Photothermal treatment of tumors | 127 | |
| Mg in GaIn alloy | Photothermal | Bendable | Photothermal treatment of skin tumors | 128 | |
| p- And n-type Bi2Te3 thermoelectrics between Cu electrodes | Peltier effect | 230% | Heating and cooling pad | 130 | |
The simplest, go-to solution for many e-skin systems consists of metallic wires.29 Usually gold and other noble metals are employed (see Table 1), yet for many applications it would be possible to even use bioabsorbable metals that have higher resistivity and that can be safely degraded by our bodies, like magnesium and zinc.76–78 Masked evaporation of thin layers of metal on a flexible substrate (e.g. polyimide (PI), polyurethane (PU), and polydimethylsiloxane (PDMS) are the most common) yields precise features that can work as connectors, and electrodes, as well as a part of a large selection of components.45,79 The possibility of obtaining entire circuits with few evaporation steps is very appealing, especially if the aim is to converge many functions in the same device. Next to metal evaporation, electrodeposition, cut-and-paste methodologies, sputtering, and roll-to-roll printing are also widely used techniques, but the level of resolution is much lower.4,80–82
Since the resistance increases as the metallic feature shrinks in size, it is no surprise that serpentines realized with thin lines of metal have been widely used from the beginning of epidermal electronics to act as local heaters.29,80,81 However, such elements are usually small and not able to heat large parts of the human body as often required in thermotherapy. This is because the mechanical properties of the metals – which do not match with those of the human skin or the flexible substrates – allow only limited freedom of movement and thus an increment of the area would result in an increased chance of failure.20 As the lines in a serpentine need to be dense and tightly packed (in order to provide uniform heat), one can only adopt limited geometric solutions to improve their stress resistance (e.g. an array of elements can be a solution to cover larger areas).73
Liquid metal (LM) alloys have similar conductive characteristics to metals, but are extremely more flexible thanks to their liquid-like nature.83 In this regard, the most used materials are Ga-based alloys, like Ga–In at their eutectic composition (EGaIn) or ternary alloys of Ga–In–Sn (Galinstan), as they offer advantages like low toxicity,84 negligible vapor tension, and can be printed even in 3D architectures, thanks to their intrinsic non-Newtonian behavior.83,85 Because of these reasons, LM can have a large number of applications in soft electronics.86,87 Anyhow, one can only find limited use of such materials in fabricating conformable heaters, perhaps as a result of the limited resolutions achievable compared to the previously mentioned lithographic techniques, or because their high conductance makes them more useful to prepare other circuitry elements.86 We will discuss LM again in this review (Sections 3.1 and 5). Concerning conformable heaters, Wang et al. prepared a Galinstan-uncured PDMS composite ink that they could use to write thin lines by direct ink writing (Fig. 2a).88 By successive encapsulation and polymerization, they printed serpentines that could act as heaters with a minimal change in performance even when stretched to more than 100% of their original length. Compared to pure LM, the composite limited the flow of the LM thus increasing drastically the stability of the device.89 Guo et al. took a different approach for the fabrication of their devices and realized electronic tattoos based on the selective adhesion on polymethylacrylate (PMA) glue of a semi-liquid EGaIn–Ni composite:90 in their study, they used a skin-friendly PMA glue to draw different designs on skin and then applied the conductive composite on them with a roller (Fig. 2b). Conversely, Wang et al. modified the adhesion properties of EGaIn by thorough oxidation.91 After stirring in air, the adhesion between the LM and the skin increased drastically and the authors were able to write circuits directly on skin using a brush. They also demonstrated that these structures could be used for wireless thermotherapy by inducing a current with an alternating magnetic field.
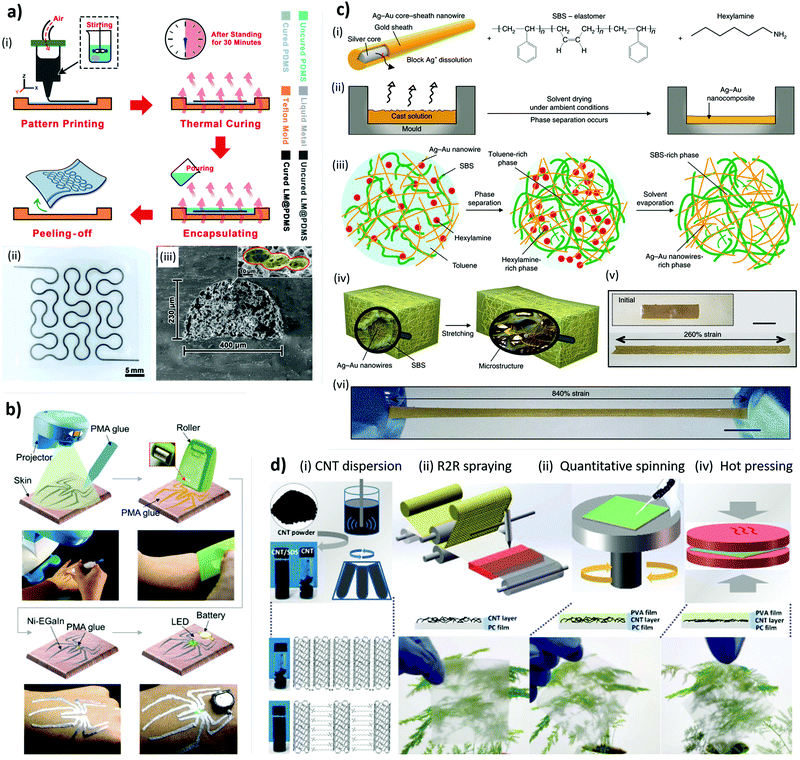 | ||
Fig. 2 Materials and fabrication methods employed for the development of conformable heaters. (a) (i) Fabrication procedure for preparing LM@PDMS stretchable, and wearable heaters and (ii) a prepared sample with a complex fractal structure. (iii) Cross-sectional SEM image of LM@PDMS with its internal microstructure. Reproduced from ref. 88 with permission from John Wiley & Sons, DOI: http://10.1002/admt.201800435. (b) Fabrication of the Ni-EGaIn electronic tattoo: schematic diagrams and corresponding optical photographs of the Ni-EGaIn electronic tattoo printing procedure. Reproduced from ref. 90 with permission from John Wiley & Sons, DOI: http://10.1002/admt.201900183. (c) (i) Ag–Au nanocomposite made by combining a mixture of Ag@Au NWs, SBS, and hexylamine in toluene. (ii) Solvent-drying process under ambient conditions. (iii) Schematic illustration of the initial solution (left) separated into a Ag@Au NW-rich phase and a SBS-rich phase during dry-casting (middle). Subsequent solvent evaporation (right) forms the microstructured Ag–Au nanowire nanocomposite. Red dots, hexylamine; yellow wires, Ag@Au nanowires; green wires, SBS. (iv) Schematic illustration of the microstructured NWs-SBS nanocomposite before and after stretching. (v and vi) Optical camera images of the nanocomposite before (v, inset shows before stretching) and after (vi) the heat rolling-pressed nanocomposite sandwiched between the elastomeric substrates (VHB film). Scale bars, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mm. Reproduced from ref. 56 with permission from Springer Nature, DOI: http://10.1038/s41565-018-0226-8. (d) Illustration of the fabrication process for PVA/CNTs-HP film: (i) CNT dispersion and corresponding optical image; (ii) R2R spraying method for pre-constructing CNT network on PC surface; (iii) quantitative spinning process to introduce ultrathin PVA layer; (iv) hot pressing treatment to prepare PVA/CNTs-HP film. Reproduced from ref. 115 with permission of Elsevier, DOI: http://10.1016/j.compscitech.2019.107796. mm. Reproduced from ref. 56 with permission from Springer Nature, DOI: http://10.1038/s41565-018-0226-8. (d) Illustration of the fabrication process for PVA/CNTs-HP film: (i) CNT dispersion and corresponding optical image; (ii) R2R spraying method for pre-constructing CNT network on PC surface; (iii) quantitative spinning process to introduce ultrathin PVA layer; (iv) hot pressing treatment to prepare PVA/CNTs-HP film. Reproduced from ref. 115 with permission of Elsevier, DOI: http://10.1016/j.compscitech.2019.107796. | ||
As well as the opportunities offered by bare metals, (nano-)composite materials also combine an adequate compromise between conductivity and flexibility by relying on percolation networks of conductive fillers in polymer matrices.33,92 Their resistivity is usually higher than that of the aforementioned systems93 and thus, as introduced before, it is no surprise that in the literature we can find countless examples of their use as heaters, utilizing both metallic and carbon-based nanomaterials (e.g. nanoparticles, nanowires, nanofibers, and flakes). For application as conformable electronics, it is worth mentioning that the devices do not necessarily need to have characteristics like ultra-high strain and high transmittance which are appealing for other applications as soft-robotics and smart-architecture.21,94–96
One of the most common methodologies to prepare flexible conductive composites is to make use of percolation networks of metallic nanowires (NWs) or nanofibers (NFs) on flexible, and biocompatible substrates. Compared to nanoparticles, the use of these nanostructures results in a lower percolation threshold and thus they influence to a smaller extent the mechanical properties of the matrix.92 There are several methodologies to achieve such networks: (i) vacuum filtration of dilute NW solutions;97,98 (ii) electrospinning;99 (iii) deposition on a sacrificial 2D-network;100 (iv) drop casting;101 (v) casting of composite solutions.56,102 In particular in this last case, the ligands on the NW surface covers an extremely important role to achieve a good dispersion: Choi et al., for example, by carefully tuning the amount of hexylamine to function as the ligand of Au-coated AgNWs in poly(styrene-butadiene-styrene) (SBS), their composite could reach a remarkable 840% stretch strain without sacrificing the conductance of their composite (Fig. 2c).56 As we show in the entries in Table 2, the most used metal for this kind of device is silver because it combines good electrical properties with simple procedures to prepare long NWs.56,102 Yet, the resulting networks are prone to the formation of a surface oxide, which hinders the contact between different wires upon oxidation.97 One may overcome this issue by coating the NWs with Au (which does not oxidize),56,98 by embedding them in a suitable material,103 or by adding carbon-based nanomaterials (e.g. graphene or carbon nanotubes (CNT), which are reported to increase the stability to mechanical stress and oxidation, in addition to being better heat conductors).104–106 Coating the Ag NW network with a conductive polymer was reported to increase the thermal properties of a heater (but not the resistivity).107,108 A valid alternative to silver to prepare similar structures is copper,109 which has a lower price but is also a worse conductor.110 Another possibility is to use nanostructures of a different material altogether.95,100,111–114 For example, recently Zhou et al. realized a conformable heating pad by hot pressing a dispersion of CNT in PVA formed with the help of sodium dodecylsulfate as a surfactant (Fig. 2d).115 Remarkably, the preparation of this composite involved only water-processable materials.
| Electrode materials | Circuit materials | Impedance | Applications | Ref. |
|---|---|---|---|---|
| Au (evaporated) | Au evaporated on PI | 205 kΩ (20 Hz) | Electrotactile stim., electrical muscular stimulation | 55 |
| Au@AgNW network in SBS | Au@AgNW-SBS composite in SBS | 1.6 kΩ (1 Hz) 0.7 kΩ (105 Hz) | ECG, EMG. FES of cardiac muscles | 56 |
| Graphene-SEBS composite | Graphene-SEBS composite | 12 kΩ (103 Hz) 4 kΩ (106 Hz) | ECG, EMG. Electrical stimulation | 64 |
| Ag/AgCl conductive ink | PEDOT:PSS | — | Electrotactile stimulation | 166 |
| Au (evaporated) | AgNW-SBS composite | 200 Ω (1 Hz) 20 Ω (105 Hz) | FES of cardiac muscles | 168 |
| Au (evaporated) | Au evaporated on LCP (Vecstar) | — | Stimulation of bone cell regrowth | 169 |
| Ti microneedle | Ti | Eelctrotactile display, data transfer | 185 and 186 | |
| PEDOT:PSS-b-PPEGMEA | PEDOT:PSS-b-PPEGMEA | 2 × 102 kΩ (0.1 Hz) 0.1 kΩ (105 Hz) | Electrotactile element (surface roughness sensations) | 187 |
While many groups employ NWs because the resulting composites are highly transparent,116 Moon et al. used a conductive ink containing Ag nanoparticles that they sintered by adding NaCl to prepare a bendable composite that they could print on different substrates;117 with a different approach, Weng et al. realized a conductive 3D sponge by self-assembly of Ag NWs after surface treatment with dopamine: the resulting material could not only stretch and bend but also be compressed and could work as a heater, as well among many other applications.118
Other interesting candidates for conformable heaters are intrinsically conductive polymers (CPs).96,119 Polymers like polypyrrole, polyaniline, and PEDOT:PSS possess good conductivity,1 are considered biocompatible,42 and can be patterned easily to make circuits.5,120,121 The latter polymer in particular attracted much attention because it is water-processable and for its chemical and thermal stability. The main drawback of these materials is their intrinsic rigidity. Pristine PEDOT:PSS, for example, can stretch only about 5% before cracking, limiting its appeal for highly stretchable electronics applications. However, as mentioned before, conformable electronic devices do not need to sustain high strains, and thus one may adopt many solutions to modify both its mechanical and electric properties.122–124 Conversely, our group showed the use of thin films of PEDOT:PSS with a thickness of a few hundred nanometers as transferable ultra-conformable electrodes for electrophysiology;1,9 ultra-thin films of rigid materials can show extreme flexibility as bending motions produce only negligible strain (i.e. the difference between the inner and outer bending radii). Such devices can adhere perfectly to the skin thanks to van der Waals forces, therefore making them an attractive platform for other skin electronics applications.125 In any case, despite the known Joule heating properties of PEDOT:PSS,96,124,126 and its use as a coating for fabrics to be used in heated clothing (we will not address these devices here as Lu et al. covered them in detail in a recent review,68), one can only find limited examples of CP-based conformable heaters in the literature. Zhou et al. developed a conductive composite of PEDOT:PSS and waterborne PU with remarkable stretchability; the addition of in situ generated rGO (GO reduction with HI), while improving only slightly the electrical conductivity, increased dramatically the thermal conductivity of the films.17
While all the examples introduced so far have to do with Joule heating, there are more mechanisms capable of generating heat. Our group, for example, investigated the photothermal performance of a submicrometric ultraconformal polysaccharide film containing Au@SiO2 particles, which produced a dramatic temperature increase when exposed to an NIR laser.127 Wang et al. used a composite of magnesium and Ga–In alloy to produce LM patches with similar properties.128 Remarkably, Lee et al. used p- and n-doped Bi2Te3 samples sandwiched between Cu electrodes incorporated in Ecoflex to prepare a series of Peltier elements,129 which are not only capable of acting as heaters but also as cooling pads just by reversing the applied bias.130 Kim et al. fabricated a device based on the same technology and showed its possible use as a haptic display for guidance of blind users.75 While these devices are still relatively thick compared to those presented above, the possibility of generating both hot and cold sensation on the skin by the same device is incredibly fascinating. A possible solution to make thinner devices could arrive from organic materials, which, despite having the worst thermoelectric performance achieved so far, possess several advantages concerning fabrication procedures (e.g. they can be printed, they are flexible, and they are obtained from abundant resources).131
2.2 Devices and applications
Most of the thermal stimulation devices find use in industrial applications, but in recent years they have also become relevant in the medical field. One of the main characteristics required from electrical heaters both for industrial and medical applications is low sheet resistivity in order to obtain low-voltage and power-efficient devices, according to Joule's law. However, medical heaters require some additional attention. For these specific applications (i) biocompatibility of the materials is fundamental so that the device mounted on human skin does not cause damage to the user or skin irritations also in long-term treatment perspectives; (ii) the heating module must not reach too high temperatures: the temperature of the human skin should remain between the safety thresholds of 15 and 45 °C to have comfortable sensations, otherwise nociceptors respond to thermal stimuli with consequent pain for the user;132 (iii) heaters should be flexible and stretchable in order to follow dynamic strains of the skin, and stable from a mechanical point of view: the inability to conform well leads to a non-uniform and inconsistent adhesion between the heater and the skin. This aspect could cause air voids that generate hot spots57 and could burn the user if the heater is operating near the 45 °C safety threshold; (iv) heaters must have uniform electrical and thermal properties: heating inhomogeneity can lead to burns on the user's skin;101 and, finally, to reach the objective of using heaters in healthcare and in thermotherapy two main key issues are (v) their portability and (vi) the possibility to adjust heating temperature in real-time. The latter is important also to avoid the aforementioned overshoots.However, most of the studies rely only on the – experimentally obtained – relationship between the applied voltage and the corresponding reached temperature to control the heating level of the device.82,103–105,110,121,133 However, this method does not take into account the inter-subject variability of heat transfer conditions and provoked sensations, an issue of primary importance for the healthcare market. For example, changes in blood flow can cause changes in skin temperature differently from person to person. Hence, different studies aimed to achieve more precise control over the heat generated and the temperature reached. In some stretchable heaters, which also included temperature sensors, the sensing element was the same as the heating element (usually a metallic serpentine).73,134 Such a design does not allow one to measure the temperature and to generate heat simultaneously; also, it risks measuring its own temperature instead of the actual skin one. Stier et al. proposed instead a stretchable aluminum tattoo-like heater on a PET substrate, which also included a gold resistance temperature sensor, separated from the heater by an insulation layer in order to record skin temperature.81 The overall device included a proportional-integrative-derivative (PID) control so as to have a real-time temperature feedback. PID control allowed us to maintain a target temperature on human skin over a specific time interval, 38.5 °C for 30 minutes in their experiment, automatically adjusting the DC power applied on the heater (Fig. 3a).
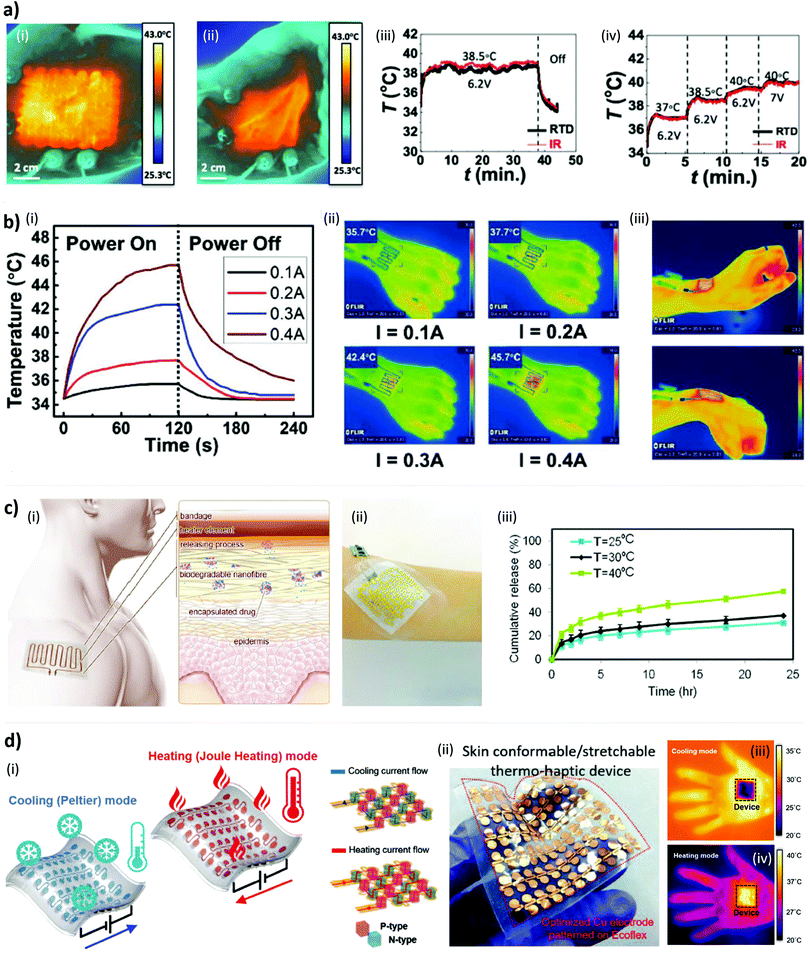 | ||
| Fig. 3 Functioning principles and performance of conformable heaters. (a) Heater conforms to the hand during (i) opening and (ii) closing without changing its temperature. (iii) Temperature stability with time and (iv) voltage steps. Reproduced from ref. 81 under Open Access CC BY Licence, DOI: http://10.3390/mi9040170. (b) Characterization of Ni-EGaIn Joule heating electronic tattoo directly printed on the skin at (i and ii) different voltages and (iii) hand movements. Reproduced from ref. 90 with permission from John Wiley & Sons, DOI: http://10.1002/admt.201900183. (c) Thermo-responsive drug nanocarriers released upon temperature increase induced by the integrated flexible heater. (i) Schematic of working principle. (ii) Photograph of the thermal patch. (iii) Temperature response at different voltages. Reproduced from ref. 76 under Open Access CC BY 4.0 Licence, DOI: http://10.1038/s41598-017-04749-8. (d) Skin-mountable Peltier-based thermo-haptic device with cooling and heating modes. (i) Working principles. (ii) Photograph of the device. (iii) Thermal characterization. Reproduced from ref. 130 with permission from John Wiley & Sons, DOI: http://10.1002/adfm.201909171. | ||
The integration of the heater with electronic circuitries has been another step forward in reaching both the objective of portability and real-time wireless temperature control. An et al., for example, reported an electronic band accompanying the heater, which involved different electronic components such as a Bluetooth module to communicate wirelessly with a smartphone from which the user could both decide the heating mode and control temperature up to 50 °C.100 The microcontroller unit (MCU) embedded in the band received the signal and adjusted the voltage, which determined the amount of electric current flowing through the heater according to Joule's law. With such a device, however, the user shall rely only on their own thermal cues to regulate the heating mode. Cheng et al. proposed to use a digital temperature sensor in addition to the electrical components considered in the previous example.135 The MCU transmitted wirelessly the value recorded by the sensor to a smartphone so as to show the real value on the display. In this way, the user could decide to adjust the output DC voltage, consequently regulating the temperature as needed. In this study, the heater, MCU, and the temperature sensor were integrated into the heating fabric and clothes so as to be almost not visible but still in contact with the skin. Jang et al. demonstrated that the same set-up was used by Cheng et al. (same components, with a thermistor as a temperature sensor) can be integrated on a band instead of clothes, depending on the needs.99
Another interesting field of research in medical treatments is personalized care, which aims to develop targeted therapies for each individual patient.136 In order to obtain custom devices, Moon et al. proposed to use a 3D scanner to acquire body information, which enabled them to print very personalized heaters.117 In particular, data acquired from a 3D scan could help in deciding the dimension and shape of the custom heater. The user could select different points on the scan surface and the software calculated the length and width of the single 2D heaters but also how to stitch them together in order to apply the entire film on the interested body surface. In this way, the final heater can have various shapes and sizes depending on the injury or medical condition of the patient. However, low-cost fabrication techniques are necessary to prepare heaters for disposable use. Guo et al. exploited a very low-cost fabrication technique in producing their tattoo-like heater: the device could be directly fabricated on the user skin with any shape and size, as already explained in Section 2.1, and as shown in Fig. 3b.90 Another cost-effective method for heater fabrication is the kirigami technique – the oriental art of cutting and folding paper, used to cut the considered substrates in order to obtain more complex geometries, often with higher stretchability.98,137
3. Conformable devices for electrical stimulation
Nowadays different fields such as rehabilitation or physiotherapy38,145 but also virtual reality (VR)-based applications22 often exploit electrical stimulation of the peripheral or central nervous system. However, electrical stimulation and the effects it provides on humans are still a wide field of research. Some examples concern how an electric field can modify cell behavior, including orientation, proliferation, cell migration, and differentiation,146,147 which are the best stimulation parameters in terms of current amplitude, pulse width, and frequency of the applied electrical signal and the best electrode placement to achieve optimal results in electrical stimulation therapy (ETS);148 how haptic perception evoked, for example, on the hand by applying transcutaneous electrical nerve stimulation (TENS) can vary in terms of the region perceived and amplitude of the sensation, varying stimulation parameters;149 the optimal current or voltage waveform to apply. Currently, the most used waveforms for the different applications of electrical stimulation are the sinusoidal, triangular, and mono/biphasic square waveform.64,150,151 Moreover, in recent years, different studies aimed to restore tactile and pain feedback in amputees by electrically stimulating the subject by applying short current pulses, developing a so-called neuromorphic model, which attempts to mimic the behavior of the nervous system, with the aim of providing more realistic sensations.152Conformability of the device is a central issue in medical applications: different studies propose conformable electrodes that perfectly adhere to soft tissues of the body and are capable of injecting a small amplitude of current flow. In the medical field, such devices find their use in the regeneration of neural networks, in the electrically induced differentiation of osteoblasts and therefore in bone remodeling, or in the electrical stimulation of the heart for smart defibrillation.20,153 Moreover, as already mentioned, electrical stimulating devices are used in rehabilitation and sports medicine for neuromuscular electrical stimulation (NMES) and functional electrical stimulation (FES). NMES consists of the repeated application of current to produce contraction of innervated muscle by depolarizing local motor nerves producing effects that enhance functions such as muscle strengthening but that do not provide a functional activity. FES, instead, exploits electrical currents to enable a functional activity in order to replace a completely lost movement in patients with neurological impairments, activating the sensory-motor system, allowing the control of walking or grasping in stroke patients, or, also, for tremor suppression.18,148 Furthermore, experiments have been conducted to demonstrate how targeted spinal cord stimulation could re-establish adaptive control of paralyzed muscles during overground walking in patients with spinal cord injuries.19 Other applications for stimulating devices regarding human-robot interaction, where artificial components and the user have to form interface with each other.21 In particular, if a robot is equipped with some kind of sensor, it will “sense” different stimuli from the environment. The sensed signal could then be transferred to the user, stimulated by the wearable conformal device. This concept is central in applications such as prosthetic limbs, where stimulating the user is a means to restore tactile feedback. In this way the users will better control the prosthetic device and they will react faster to external stimuli coming toward the artificial limb, as well as enhancing the embodiment and acceptability of the prosthesis itself. Furthermore, TENS is a treatment generally used against painful conditions, including chronic neuropathic pain,154 as well as a method that is being explored to restore tactile feedback on amputees.152 When designing devices and, in particular, electrodes for superficial – i.e. non-invasive electrical stimulation of the human body, several factors must be considered, such as skin impedance, perfect contact between the electrode and the skin, and the particular frequencies and current amplitude to apply in order to deliver comfortable stimulation.
3.1 Materials
Electro-dermal stimulation requires the electrodes to be in intimate contact with the skin in order to deliver a reliable and effective signal. At the same time, the circuitry must be isolated from it so that the signals can reach only the desired points. For these reasons, the majority of electrostimulation devices available today consist of a set of wired electrodes that one can apply on the interested areas via adhesive pads and gels designed to lower the impedance of the contact.155–161For conformable devices, these limitations translate to a higher complexity in the fabrication processes when compared to the heaters we discussed earlier, requiring distribution of the conductive medium on multiple levels so that only the electrodes are in contact with the skin. On top of this, the proposed devices must also possess a low impedance at the skin electrode interface in order to deliver a reliable signal at low currents and thus limit the discomfort associated with electrostimulation, an issue that often requires an accurate choice of material.162,163 Finally, all of these characteristics must be suitable to stimulate the target receptors, which respond only to certain electric fields and frequencies.164 As a result, most of the research and medical applications concern wearable devices,38,162 which are usually powerful enough to overcome the high impedance of the stratum corneum (i.e. the outer layer of the epidermis, with an impedance higher than 105 Ω).165 We will now examine some of the materials and architectures recently proposed in the literature to work as conformable neuromuscular and/or electrotactile stimulators, with their strength points and shortcomings (the most relevant devices are summarized in Table 2).
One fabrication solution to prepare these devices, in which the electrodes require to be in direct contact with the skin, is to deposit a layer of conductive material on a layer of insulator bearing holes in places of the electrodes55,166 as shown in Fig. 4a (or etch the holes in a sequent step).167 The difference in height obtained is not an issue for the contact if the size of the electrode is large compared to the thickness of the insulating layer on the skin.55,167
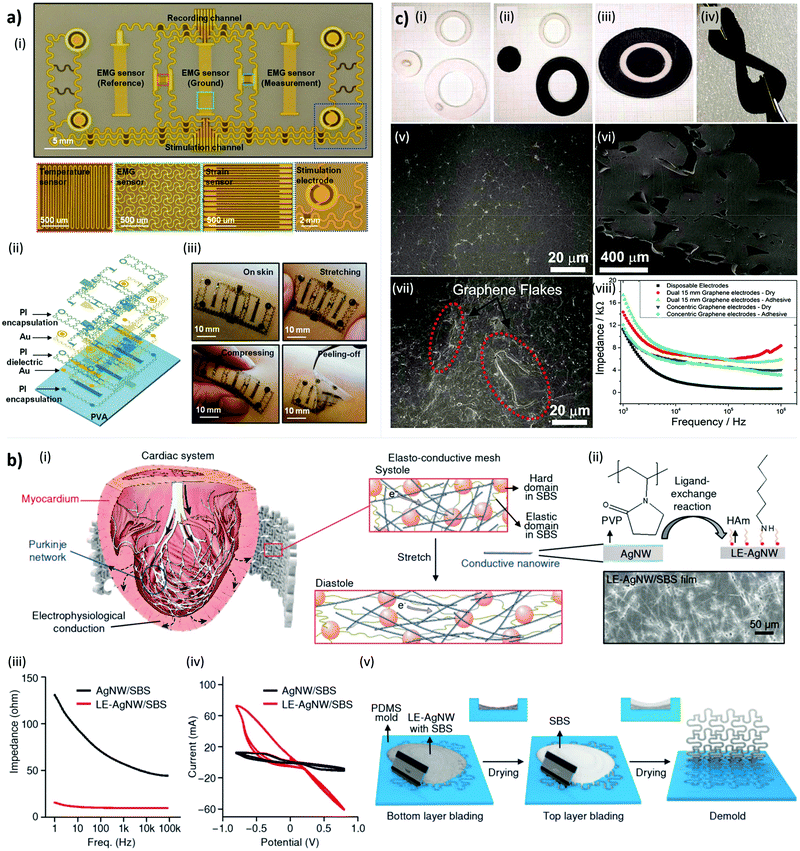 | ||
| Fig. 4 Materials and design strategies of conformable systems for electrical stimulation. (a) E-skin device capable of both sensing and stimulation. (i) Planar view of the different features of a representative device with insets for the active regions. (ii) Exploded-view of the multilayer construction. (iii) Pictures of the device mounted on the forearm with examples under different deformations. Reproduced from ref. 55 with permission from John Wiley & Sons, DOI: http://10.1002/adma.201504155. (b) Elasto-conductive epicardial mesh based on a LE-Ag NWs/SBS composite. (i) Design strategy of the mesh for electromechanical cardioplasty and composite composition. (ii) Scheme of the reaction for LE-AgNW. The ligand of AgNW was exchanged from PVP to Ham; a scanning electron microscopy image of LEAg NW/SBS nanocomposite is shown. (iii) Impedance and (iv) CV (with conductivity measurement) of the initial and LE. (v) Schematic illustration of the epicardial mesh fabrication. Reproduced from ref. 168 with permission from the American Association for the Advancement of Science, DOI: http://10.1126/scitranslmed.aad8568. (c) 3D printed, reusable, flexible electrode coated with conductive graphene ink for electrotactile stimulation. Electrode preparation: (i) the electrode components are 3D printed, (ii) coated with graphene ink, and (iii) assembled. (iv) Demonstration of coated electrode's flexibility. (v) Surface and (vi) cross-sectional morphology of the pristine 3D printed component; (vii) surface of the spray coated layer. (viii) Impedeance measurement. Reproduced from ref. 64 under Open Access CC BY Licence, DOI: http://10.3389/fbioe.2018.00179. | ||
An example of this approach is the work of Park et al. where the authors obtained a stretchable and conformable Ag NW and SBS composite by mold casting and then covered it with a thin layer of evaporated gold to make it more biocompatible (Fig. 4b).168 Choi et al. used a similar system comprising a network of Au-coated Ag-NWs in SBS encapsulated between SBS sheets.56 Thanks to a hot pressing step, the thickness of the device was much lower, uniform, and the composite electrodes could already form reliable conformal contact with the skin. Since both the encapsulation and the conductor shared the SBS, the resulting device was intrinsically stretchable and less prone to failure by Young modulus mismatch at the interfaces.36
Kim et al. realized Au electrodes on a liquid crystal polymer (LCP) substrate (Vecstar) that they could thermo-mold in conformable devices to follow bone structures.169 LCPs are a class of polymeric material comprising rigid and flexible monomers, which provides them with mechanical toughness and simple processability at high temperatures (even to realize curvilinear manufacts).170,171 Moreover, they are chemically inert and provide an extremely low gas permeation and humidity uptake (lower than those of polyimmides or parylene), making them an ideal substrate for implantable devices.171–173 Although our review focuses on external applications, it is worth mentioning that electrical stimulation of cells in the human body with soft and conformable devices can promote regeneration, proliferation, and differentiation, and thus may find many potential applications in therapy.20,78,87,169,174,175 Notably, the design of some of these devices included the implementation of implantable generators relying on triboelectric effects,20,87,176 piezoelectricity,177 or wireless powering78,178 so as to be energetically self-sufficient, since they would need constant activation. In the future, such solutions could efficiently be applied also to skin-mounted stimulation devices.179
Stepping away from gold, Wang et al. proposed the use of a liquid Ga–n alloy to prepare an electrode for both muscular stimulation and recording of physiological signals.153 Ga-Based LMs have the advantage of being non-toxic (even for implantable applications)180,181 and inherently produce conformable contacts even with the skin;182 however, their properties have a significant dependence on the thin Ga oxide skin that covers their surface (less than 1 nm thick)83 and rapidly forms in contact with oxygen.153
To reduce the impedance of the contact with the external layer of the skin (the stratum corneum), most of the electrostimulation electrodes require the use of gels or special conductive glues. However, there are solutions to lower the impedance (and thus the working voltage), while avoiding the use of these latter altogether:183 one may find a lot of research concerning novel dry electrode materials, and also more extensive systems could take advantage of some of these ideas to realize functional conformable devices.
For example, Stephens-Fripp et al. realized a 3D printed electrode spray-coated with a conductive graphene-SEBS composite ink (Fig. 4c).64 The authors claimed it was possible to reuse indefinitely these devices and that they had an impedance similar to that of commercial gel-based electrodes. Wei et al. also reported the functional properties of carbon-based materials in lowering the skin impedance.184
With a different approach, Tezuka et al. avoided the stratum corneum high impedance by implementing microneedles for their tactile displays for electrotactile stimulation.185,186 The authors used chemical etching of titanium wires with the tip protruding from a soft embedding matrix (PI or Ecoflex) to prepare a matrix of 600 μm tall sharp cones (with a 300 μm diameter at the base). The design of the tips ensured their insertion into the skin without generating pain responses. Moreover, bypassing the stratum corneum lowers drastically the voltage needed to surpass the sensation threshold.
To summarize this section, the main issue conformable electronics must overcome to become a reliable platform for portable, easy-to-use electrostimulation devices is finding solutions to lower the high impedance of the outer skin. The high voltages needed by the wired systems comprising metallic plate electrodes are not compatible with the more delicate flexible electronics. However, materials science still has a lot to offer in this respect: materials like polymer composites and conductive polymers offer a plethora of options – thanks to chemical functionalization and material engineering – to optimize material properties and the way they can interact with the human skin. A recent example of this is the work of Keef et al. in which the authors prepared a PEDOT blend with a PSS and poly(polyethylene glycol methyl ether acrylate) block copolymer (PSS-b-PPEGMEA) to be used as an electrotactile element in a VR glove.187 The blend showed high flexibility and low impedance, two ideal qualities for conformable stimulation devices. However, at the moment, only a limited amount of research moves in this direction despite its possible technological and economical values.
3.2 Devices and applications
One of the most innovative objectives in research is to use electrical stimulation to restore lost senses, for example, touch or vision. In recent years numerous studies demonstrated that tactile stimulation increases tactile acuity and manual dexterity in patients suffering from sensory loss, maladaptive plasticity or certain forms of motor impairment, relying on the induction of plasticity in the somatosensory cortex.188 The user can receive a controlled electrotactile stimulation signal properly modulated in terms of electrical current injected into the skin, which can excite cutaneous mechanoreceptors because of the generation of a potential gradient21,164 resulting in a tingle, itch, vibration, pinch, or touch sensations.189 The demand for such systems is high also for human-robot interaction, as required by prosthetic users or remote slave-robot control: adding this feature to a prosthesis can, of course, increase the sense of embodiment of the device190 as well as allowing the amputee to better manipulate and grasp objects.191 Moreover, electrotactile stimulation could also substitute the sense of vision in blind people (tactile vision substitution) using tactile displays. Particularly, a two-dimensional matrix of stimulators could give spatial information similarly to the way eyes present spatial information to the retina.192Transcutaneous electrical stimulation (TES) consists of activating excitable tissues in the human body from the skin surface, placing the electrodes over the area of the skin where one intends to activate underlying tissue (see Fig. 5a).164 Electrodes used in TES shall provide optimal performance without causing permanent skin damage such as burns or irritation and without producing discomfort. To avoid this latter aspect, one should consider designing electrodes that do not allow high current to reach deep into the tissues. An important issue in TES is skin impedance (i.e. opposition of the skin to AC flow). It is possible to study physiological operation of the skin thanks to models that show its electrical characteristics, going from a simplified R-C circuit model to more complex models considering the three layers that compose skin – i.e. stratum corneum, dermis and hypodermis, as shown in Fig. 5a.165 Since the skin is an insulator and it stores charges on its outer surface, it acts as a capacitor, whose resistance is inversely proportional to frequency: an increase in the AC frequency results in a decrease of pulse duration, allowing less time to store charges on the skin and, consequently, effectively lowering impedance. Hence, when medium- (1000 Hz) or high-frequency currents are applied, they pass more easily through the skin layer (than low-frequency ones), allowing more current to reach the motor nerves.148,193 Furthermore, the voltage-current dependence is linear if low currents (in the order of μA) and low voltages (lower than 1 V) are considered, while for higher currents the impedance results in a non-linear function of the current density.164 The skin presents local inhomogeneities due to the presence of pores or different water content, leading to resistance changes also in the same subject, causing higher or lower current densities.164 Measurements and models concerning bioimpedance detection could be of help as a feedback reference for adjusting stimulation parameters such as current amplitude, pulse width, and frequency, promoting methods to reduce impedance differences in the human body or between subjects.165 Moreover, the electrodes should have good contact with the skin allowing the largest surface at the interface. The current density should be homogeneous over the entire electrode. The size of the electrodes depends on the size of the region to stimulate, for example the size of the muscle.164 Smaller electrodes allow higher selectivity, but the case of using electrical stimulation for restoring sensory feedback requires larger electrodes to produce a comfortable sensation.64
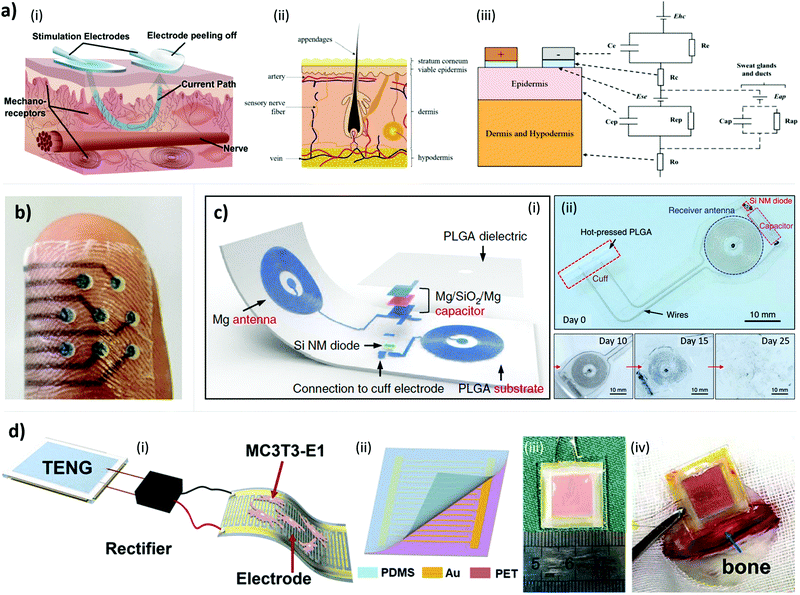 | ||
| Fig. 5 Functioning principles, schematics, and performance of conformable devices for electrical stimulation. (a) Schematic illustrations of (i) transcutaneous electrical stimulation (TES) (reproduced from ref. 200 with permission from the American Association for the Advancement of Science) DOI: http://10.1126/SCIROBOTICS.AAP9770 and (ii) the skin structure with (iii) an equivalent circuit of human skin impedance measurement. Reproduced from ref. 165 under Open Access CC BY Licence, DOI: http://10.3390/bios8020031. (b) Picture of a temporary tattoo electrode interface, named Tacttoo, providing a feel-through interface for electro-tactile output on the user's skin. The Tacttoo can be applied on complex body geometries, including the fingertip. Overall thickness is less than 35 μm. Reproduced from ref. 166 with permission from the authors, DOI: http://10.1145/3242587.3242645. (c) Example of a conformable wireless and bioresorbable implantable device for electrical-stimulation-assisted tissue regeneration: (i) schematic illustration of the device and (ii) images of the fabricated bioresorbable stimulator, with its dissolution over several days once immersed in PBS, and its implantation. Reproduced from ref. 78 with permission of Springer Nature, DOI: http://10.1038/s41591-018-0196-2. (d) Example of conformable self-powering implantable device for enhancing osteoblasts proliferation and differentiation. (i) Schematic illustration of the device and (ii) of the interdigitated electrode. (iii) The size of the implantable TENG and (iv) the surface of the mouse femur region where the flexible TENG was implanted. Reproduced from ref. 20 with permission from Elsevier, DOI: http://10.1016/j.nanoen.2019.02.073. | ||
Regarding muscular activation, especially in FES, multi-electrode arrays have some advantages with respect to a single pair of electrodes. Arrays allow the user to try many different electrode sizes and positions so as to find the best ones for stimulation, without removing the electrode from the skin.194
For all the applications mentioned above, conformable devices could be particularly practical as they inherently provide good contact with the substrate, they are less bulky and more comfortable than applied electrodes, their application is less invasive, and the user could wear them when they prefer, even when not in use, thus making self-use at home possible.11,23,35,36
Implantable electrodes for electrical stimulation often rely on conformable technology so as to adapt to the human body tissues. Such devices can be implanted (i) in the brain: deep brain stimulation (DBS) helps patients with epilepsy or Parkinson's disease,173 and neuroprosthesis implants (such as auditory brainstem implants) can provide sound sensations stimulating neurons;195 (ii) in muscles: functional electrical stimulation (FES) can restore sensory or motor functionality if applied on a targeted muscle, against atrophy or for generating limb movements like walking or grasping;164,196 (iii) in the spinal cord: epidural electrical stimulation of the spinal cord enables the use of functionally silent descending neural pathways in order to produce movements of paralyzed limbs and also improves the ability of the spinal cord to translate sensory information into muscle activities that control standing and walking.19,197,198
In recent years, the interest in non-invasive techniques has grown, with the objective of avoiding surgical implantation of electrodes, thus renouncing the high selectivity provided by invasive methods. However, studies on the use of conformable electrodes to mount on the skin are still very few. This could be due to the difficulty in reaching low impedances in conformable devices compared to the disposable electrodes generally used in TES – which usually make use of a suitable gel,64 thus resulting in a higher voltage to maintain the desired current and higher power consumption. Usually, the conductive gel enhances contact between the electrode and the skin; however, conformable electrodes cannot use it.166 Although we want to remind the reader that the stimulation parameters used in tactile feedback are very subjective and can widely vary inter-subject, rarely the sensation threshold in terms of current amplitude is less than 1 mA, reaching tens of mAs in FES applications.199,200 Because the current injected could have significant values to electrically stimulate the skin, having a consistent and firm electrical contact with the skin is a central issue in order to avoid sparks that can damage the electrodes and can burn the skin. Another factor is the increased complexity characterizing the manufacturing of these systems. Some examples of conformable electrical stimulating devices will follow.
Withana et al. developed a very thin tactile interface (35 μm thick) consisting of an electronic temporary tattoo able to provide safe electrical operation of skin-worn electrotactile devices through a three-layer architecture.166 To overcome the challenge of achieving perfect contact with the skin, the authors selected a combination of elastic and rigid materials with different thicknesses in order to create a micro-spring mechanism that pushes electrodes toward the skin. Moreover, the design of the three layers allowed control of the current flowing through the skin so as to avoid and prevent over-currents and leakage currents. Screen printing of PEDOT:PSS and Ag/AgCl on a substrate of commercially available temporary tattoo paper allowed fabrication of the tattoos. Thus designed, the tattoos could be easily used by the subjects themselves without the intervention of specialized-personal to attach such devices on the skin (see Fig. 5b).
Another innovative field of research is retinal prosthetic devices that aim to partially restore vision in patients suffering from retinal degeneration pathologies. Flexible and conformable materials are perfectly suitable with such application, since the available space between the eye and orbital rim is limited. Furthermore, the continuous movement of the eyeball may impose mechanical stress also on the device, leading to a higher risk of device failure and discomfort to patients. Jeong et al. proposed an eye conformable structure that can fit the curvature of the eyeball made of a liquid crystal polymer (LCP). LCPs are compatible with microfabrication techniques and different LCP films can be thermally bonded together because of their thermoplasticity. The authors used a monolithic fabrication process consisting of a multilayered integration of electrical components, thermal deformation of the layers, LCP-power packaging, and laser-machining. The implanted device so constructed could generate biphasic current pulses delivering electrical stimulation.172
Both the two studies involved in vivo experiments only on animals: the possibility to restore vision in humans using a conformable stimulation device, and restoring tactile feedback in patients suffering from the loss of tactile sensations are still a broad and innovative field of research.
All the internal mechanisms of the human body base their working principle on bioelectricity, which is present also in almost all cell events. Hence, numerous cell types respond to electrical stimulation: this aspect can help in tissue repairment (with cellular proliferation) and in cell differentiation.146 Some examples are electrical-stimulation-assisted tissue regeneration (see Fig. 5c),78 cell cultivation on scaffolds made of conductive and high biocompatible materials creating substrates for the attachment, proliferation and neural differentiation of stem cells,174,206 and enhancing osteoblast proliferation and differentiation against osteoporosis and its related fractures (Fig. 5d).20
Iontophoresis is a process that makes use of short-timed and localized current pulses (0.1–1.0 mA cm−2) to improve the drug diffusion through the skin. One of its major applications consists of inducing sweat by stimulating sweat glands infusing pilocarpine. In particular, the measurement of the levels of sodium and chloride in sweat is useful in the diagnosis of cystic fibrosis.207,208 For example, Heikenfeld et al. fabricated a sweat-sensing patch that took advantage of iontophoresis to stimulate sweat production (by releasing pilocarpine) and analyze its composition, also transmitting wirelessly this information to a smartphone.208 In particular, the measurement of the levels of sodium and chloride in sweat is useful in the diagnosis of cystic fibrosis.207,208 Since sweat can give additional information related to glucose monitoring, iontophoresis could also help in diabetes management: reverse iontophoresis is the passage of a current over the skin to drive ions from the interstitial fluid and onto the surface of the skin, where they can be analyzed.209 Bandodkar et al. proposed an iontophoretic-biosensing tattoo-based platform targeting non-invasive glucose extraction and monitoring:210 thanks to the application of a mild current to the epidermis, ions move across the skin and toward the electrodes. The device consisted of a pair of reverse iontophoresis electrodes (Ag/AgCl ink), a pseudo reference/counter (Ag/AgCl ink), and working electrodes (Prussian blue ink). In particular, each tattoo consisted of anodic and cathodic contingents and an amperometric biosensing module using a glucose oxidase (GOx)-modified Prussian blue transducer able to work at a low voltage. Once the tattoos were mounted on subjects, a 0.2 mA cm−2 current applied between the two iontophoretic electrodes for 10 minutes allowed extraction of the interstitial fluid. Then a voltage of −0.1 V applied for 5 minutes at the GOx level allowed the amperometric glucose response to be recorded. Kim et al. reported a conformable device able both to sample and analyze in a non-invasive way epidermal biofluids such as glucose and sweat, thanks to the parallel operation of reverse iontophoresis for fluid extraction and iontophoretic delivery of the sweat-inducing drug pilocarpine into the skin.211 In particular, they developed an epidermal temporary tattoo integrated with a flexible circuit to drive the iontophoretic electrodes, control the biosensors, and wirelessly transmit the sensed information to a smartphone. Thanks to electrorepulsion, the tattoo delivers the positive charged policarpine from the anode so as to generate sweat, while glucose moves toward the cathode because of electro-osmosis and the consequent conductive flow.
In addition, the application of an electric field finds use in tumor treatments. One of the techniques used in electrochemotherapy is the electroporation, i.e. the application of short, millisecond and high-voltage (5–500 V) electrical pulses on the skin through closely spaced electrodes, so as to help diffuse the drug into cancer cells by enhancing permeability as a result of the formation of small pores.207 To date, most of the electrodes used in this application are rigid and do not adapt to soft body tissues. Hence, because of the possibility to completely wrap the tumoral tissue with the electrode, conformable electrodes could be of central importance to increase the effectiveness of electrochemotherapy in tumor treatments. Such electrodes should deliver an electric field in the range of 20 and 30 kV m−1 so as to obtain effective electroporation. Nenzi et al. proposed a polymer-metal-hybrid conformable flexible electrode realized thanks to the conversion of porous silicon into nano-porous metals filled by a biocompatible thermoplastic polymer, able to treat large areas of the human body (16 cm2).212 They demonstrated that the most effective configuration for this application is the one where an electrode at a specific potential is encircled by electrodes with opposite polarity: they developed an electrode made of 40 concentric square loops 250 μm wide, with the outermost 14 loops set at 0 V, and the other 25 inner loops at 1000 V. The authors showed in simulation that this configuration could generate an effective electric field of 27 kV m−1 up to 1 cm depth in biological tissues.
Another non-invasive regional treatment against tumors consists of low-intensity, intermediate-frequency, and alternating electric fields delivered through transducer arrays on the skin around the region of the body containing the tumor in order to kill cancer cells. This particular therapy relies on tuning the frequency of the electric field according to specific types of cancer.182 Indeed, the applied pulsed electric fields induce the formation of nanopores in cell membranes that play a significant role in destroying cancer cells: cell death occurs by immediate membrane rupture or by programmed cell death mechanisms like apoptosis or autophagy.213 In this application, rigid circuits present disadvantages such as poor coupling performance, discomfort or hurt on the human body. Conformal electrodes could instead increase the contact area with the skin and enhance therapeutic effects. To this aim, LM electrodes could be optimal candidates for flexible electronics in tumor treatments, directly printing them on the skin in correspondence to the tumor or injecting them in the body.91,214 Li et al. conducted in-vivo experiments on mice and demonstrated how this method slowed down malignant melanoma growth after 6 days of electrical stimulation treatment.182 A combination of such treatment with chemotherapy could increase killed cell numbers.
Next to tumor treatment, also other medical applications use LM because of their capability to perfectly conform to soft tissues. The study conducted by Wang et al. demonstrated, through finite element method simulations, the effectiveness of using conformable LM electrodes for smart defibrillation.153 They modelled a simplified human trunk applying the electrode on the chest. The results showed that conformal LM electrodes reached more uniform electric potential and electric field than rigid electrodes, probably because of their direct and immediate skin contact, high conformability, and good conductivity. The growing interest in conformable and flexible devices led to the development of heart-conformable electrodes, aiming at an integration of the devices with the epicardial surface. Heart activity relies on pumping blood thanks its muscle contraction (myocardium) induced by electrical impulses. A failure in its conductive system leads to heart impairments. Park et al. introduced a soft, conductive and elastic epicardial mesh designed to mechanically adapt to the heart thanks to its elasticity, almost identical to the epicardium one, enabling global pacing to stimulate the whole ventricle.168 The mesh could detect abnormal activities such as spontaneously occurring ventricular tachycardia and ventricular fibrillation and could deliver an electrical shock to terminate ventricular fibrillation, demonstrating that the device could serve both for pacing and defibrillation in the clinical setting.
4. Conformable devices for mechanical stimulation
Haptic interaction refers to the process by which humans touch, explore, and manipulate objects, but it is also an important means for humans to perceive the external world, receiving stimulation from the environment.215 The sense of touch is one the most important senses of the human body, allowing humans to interact with the world. In particular, two sub-senses build the sense of touch: kinesthetic and cutaneous feedback. The first refers to receptors located in muscles, tendons, and joints, which provide proprioception (i.e. limb position) and muscle tension. Cutaneous sensing consists of mechanoreceptors embedded in the skin and is responsible for pressure and touch sensations: the latter have become the primary means for haptic interfaces.216Despite most of the modern technologies relying on visual and audio feedback,217 the significance and potential of the tactile sense led to the development of numerous applications concerning virtual or augmented reality that requires tactile feedback to recreate the mechanical properties of objects – i.e. mass, stiffness, and temperature – for virtual interaction. The development of such technology will have a strong impact on many different aspects of our life, from social media and communications, to gaming and entertainment, to clinical medicine, rehabilitation and recovery (Fig. 7c).218 Haptic feedback is becoming progressively important to increase immersion in such applications. Different researchers focus on non-rigid interfaces between the user and the device and, also, in using soft materials for interactive applications.219
One of the most investigated methods to provide sensory feedback to users are vibrations,220 although in recent years the interest related to the possibility to generate displacements and morphological changes of the haptic device has also grown.221 In particular, in the following sections, the term ‘actuator’ refers to specific devices piloted with different driving forces developed just to exert some pressure on the human skin. Given that the different types of mechanoreceptors distributed in the glabrous skin respond to mechanical pressure, vibration, or deformation,216 tactile sensations are provided by mechanically stimulating the skin through pressures/forces applied by actuators, thus reproducing the true nature of the sense of touch.186 The request for soft materials has led to focus on polymer-based dielectric elastomers because of their cost-effective fabrication techniques and their flexibility and elastic properties being similar to those of human skin, allowing adhesion on body surfaces.222 Also shape memory alloy (SMA) platforms could be of interest for the development of haptic devices because of their capability to stretch skin once attached, creating shear forces similar to light pinches.223 Numerous tactile displays have been produced, consisting of tactile stimulators that stimulate human skin.203 Tactile displays are especially a means for communication for the visually impaired: new fabrication techniques allowed very thin, flexible, and lightweight sheet-type Braille displays to be created.203,224 In rehabilitation and, in particular, neurorehabilitation, studies demonstrated how robotic therapy provided with haptic interaction with the user improved performance of subjects following stroke or paralysis.225,226 In the medical field, haptic feedback has relevant importance in minimally invasive surgery, in particular in laparoscopic surgery, where the surgeon loses his own tactile feedback due to the interposition of long instruments between his hand and the interested tissue. Haptic feedback devices could help the surgeon in identifying tissue properties and in handling tissues in a safe manner.215,227
4.1 Materials and architectures
Devices capable of providing a tactile sensation to a human being has to actively perform a displacement in the range between microns and millimeters. On top of this, by matching the frequencies of the different mechanoreceptors in our skin, they can more efficiently provoke a sensation, while limiting the required power.30,228 To achieve this, many diverse solutions have been brought up relying on different actuation technologies, some of which have been successfully employed in conformable devices. However, we must make a distinction between wearable and actually conformable devices. The former – as in many examples of gloves for VR applications – can just serve as a substrate on which one can mount pneumatic and mechanical actuators. They are very efficient in providing a sensation, but are also generally bulky, they require external moduli, and limit the freedom of movement of the user.217,223,229,230 On the other hand, the design of conformable tactile stimulators allow one to maximize the comfort of the user and simplify the utilization procedures.231 However, it is worth mentioning, that there are many technologies from these formers to be applied to conformable devices with few modifications (Table 3). Song et al., for instance, realized a soft-pneumatic actuator, which did not require the use of an external compressor.232 To do so, they encapsulated an air pocket between two silicone sheets, which bared two concentric electrodes (Fig. 6a): the electrostatic attraction between the latter would squeeze the side of the pocket thus pushing up the central part of the element not covered by the electrodes to keep the volume constant. In another recent example, Yu et al. prepared an array of Lorentz-type actuators embedded in a silicone rubber thus resulting in a flexible tactile display (Fig. 6b).218 Such actuators work based on the electromagnetic force generated from a current flowing in a magnetic field. Remarkably, their device could power wirelessly thanks to a flat antenna embedded in the same matrix. These two studies point out that, compared to the thermal and electro stimulation, in this case the skin contact does not need to be as intimate, and therefore the devices can be less compliant. However, the latter are still quite thick. Thinner systems would be preferable to facilitate their use. | ||
| Fig. 6 Fabrication strategies and materials employed for the development of conformable haptic interfaces. (a) Soft pneumatic actuator with internal air pressure generated by an electrostatic force for providing tactile feedback. (i) Structure schematic and (ii) operation mechanism. Adapted from ref. 232 under Open Access Attribution License CC BY, DOI: http://10.1038/s41598-019-45422-6. (b) Wireless and battery-free platform composed of electronic systems and haptic interfaces capable of communicating information via programmable patterns using mechanical vibrations. (i) Exploded-view of the device and (ii) schematic illustration of the electronics and circuit. (iii and iv) Optical images of an NFC coil; (iii) before and (iv) after integrating the electronic components. (v) Exploded-view schematic diagram of a haptic actuator with the schematic diagram of an actuator viewed from (vi) above and (vii) below, and (viii–ix) their respective optical images. (x–xii) Qualitative mechanical tests of the device under (x) bending, (xi) folding, and (xii) twisting. Reproduced from ref. 218 with permission from Springer Nature, DOI: http://10.1038/s41586-019-1687-0. (c) Soft actuator for tactile stimulation interfaces prepared by multilayered accumulation of thin electro-active polymer (EAP) films. (i) Cross-sectional view of the multilayered DE and (ii) a picture of the fabricated device. (iii) Schematic illustration of the operating principle of the DE actuator. (iv) Induced displacement profile as a function of the input voltage at 1 Hz. Reproduced from ref. 236 with permission from IEEE, DOI: http://10.1109/TOH.2018.2805901. (d) Preparation of a visuo-haptic interface composed of a touch-sensitive visual display based on polymer waveguides and a dielectric elastomer microactuators (DEMA) array. (i) Schematic illustration of the fabrication process. (ii) SEM images of PDMS protrusions peeled off from a photoresist mold on a Si wafer and (iii) AgNWs patterns on the 3-D structure, respectively. (iv) Optical microscope images of the AgNW-coated protrusions (top) and its change after eliminating the AgNW coating eliminated from the top surface of the protrusions (bottom). (v) Picture of tactile-actuator array with the AgNW electrode patterns and their 3-D geometry (inset). (vi) Transparency and flexibility of the tactile-actuator array. Reproduced from ref. 238 with permission form IEEE, DOI: http://10.1109/TIE.2019.2898620. | ||
A particularly studied solution involves the use of dielectric elastomer actuators (DEAs).30,233 They consist of a layer of insulating elastomer sandwiched between two electrodes: once charged, a Coulomb force pushes them together, deforming the elastomer (which will try to retain its volume).222 Since the deformation is rapid and reversible, they are capable of producing a large force and reaching high frequencies. They, however, require high working voltages (in the order of kV) to obtain a significant displacement. To improve the performance of these devices, the elastomers employed should have low Young and shear moduli (allowing a large strain with a high frequency), a large dielectric constant, and a high breakdown threshold (larger than 10−6 V m−1).234 The most frequently employed polymers are polyacrylate elastomers, polyurethanes, and silicones. By rational design of the device architecture and choice of the materials, these systems can be very versatile with respect to the type of motion they can provide and their dimensions, allowing the preparation of wearable and conformable devices.203,235 Mun et al. fabricated a compliant, 500 μm-thick DEA device comprising a flat multilayered PDMS/AgNW-electrode structure (Fig. 6c).236 Thanks to a slight curvature in the active element in the OFF state that induced asymmetry, the authors could obtain a vertical displacement from an otherwise flat geometry.
Not only the fabrication of these devices can result in thin, conformable systems, but it can also incorporate other functionalities.237 Yun et al. realized a flexible and transparent tactile display capable of both delivering haptic stimuli and sense touch.238 Using an ingenious fabrication involving masked evaporation on vertical PDMS patterns, they obtained the Ag/PDMS/Ag actuators perpendicular to the device surface and thus capable of exerting their force directly on the skin (Fig. 6d). Yoon et al. used a multistack of PVDF layers between Ag electrodes to produce tactile sensations, while at the same time working as a pressure sensor taking advantage of the piezoelectric properties of PVDF.239 Piezoelectric materials also find applications as stimulators; however, despite them reaching very high frequencies (kHz), the displacement they can achieve is very limited.240,241
Electro-active systems, such as those introduced so far, have the advantage to reach high frequencies that can improve the interaction with the skin without increasing the power. Other electro-responding actuators based on functional materials – such as ionic conductive polymer metal composites or electroadhesive displays – have been employed for tactile stimulation but never implemented in conformable devices.224 A recent review specifically about haptics by Biswas et al. introduces them nicely.30 However, there are many more materials that one could use instead, whose actuating principles do not rely on electromagnetic forces.
SMAs are deformable alloys which are capable of reforming their original shape upon heating.242 A current that flows in a SMA wire can generate enough Joule heating to trigger the response in many alloys of specific compositions, making them suitable for skin-contact applications. SMA-based actuators make use of the force that accompanies this transformation, which can be of several N for a mm of wire, and enhanced even further with shape engineering.243 In the literature, one can find recent examples of devices relying on this technology for tactile stimulation.220,223,230 Compared to previous examples, such systems have longer actuation time (1–2 s) but can provide a much more realistic sensation when compared to vibro- and elctro-tactile stimulation by actually stretching and pinching the skin.
| Working principle | Material/design | Force or displacement | Freq. | Application | Ref. |
|---|---|---|---|---|---|
| Note: in this table we reported only conformable devices as discussed in the text, leaving out the wearable options. | |||||
| DEA | 8 Elastomer layers between carbon electrodes of alternating polarity (200 μm in total) | Up to 0.9 mm | 0–150 Hz | Tactile display | 203 |
| Lorentz force | Moving magnet over metal coil | Up to 0.3 mm | 0–300 Hz | Wireless tactile display | 218 |
| Soft pneumatic act | Air pocket squeeze by electrostatic attraction between electrodes | 0.10–0.13 mm | 0.2–1 Hz | Elements for VR glove | 232 |
| DEA | 5 PDMS layers between AgNW electrodes of alternating polarities (500 μm in total) | Up to 0.33 mm | 1–330 Hz | Tactile display | 236 |
| DEA | PDMS between AgNW electrode perpendicular to the device (total thickness 50 μm) | Up to 12 μm | 20–240 Hz | Transparent tactile display and sensor | 238 |
| DEA | 25 PVDF layers between Ag electrodes of alternating polarity (700 μm in total) | Up to 4 μm | 250 Hz | Haptic stimulation and sensing | 239 |
| Piezo | 500 nm lead zirconate titanate nanoribbon between Au and Pt electrodes | 1 kHz | Measurement of mechanical properties of biological tissues | 240 | |
A different type of functional material often used as actuators in soft robotics are liquid crystal elastomers (LCEs). These are polymeric materials obtained via the polymerization of molecules characterized by liquid crystalline behavior (i.e. comprising a flat, rigid, and long region and flexible side chains).244,245 While at low temperature most of the molecules are aligned and stacked together in a well ordered nematic phase, upon application of suitable stimuli, the order is lost and the material changes shape, shortening along what was the alignment direction and broadening in the other directions. Such movement is reversible and can be triggered by different stimuli such as heat, light, and electric fields, thus providing actuation in different systems, often with tensile strengths of several kPa.246–249 While LCEs have found application in soft electronics, deeper studies should better explore their potential in conformable devices. Similar remarks can be made on conductive polymers (CPs), one of the go-to choices for actuators in soft robotics, as they require low voltages to work (but relatively high currents).221,224 However, both CPs and LCEs, usually require longer actuation time than DEAs.49,146
4.2. Devices and applications
Mechanical stimulation is the most commonly used method to restore tactile sensations of the user: mechanoreceptors are very sensitive to vibrations, forces, deformations, and skin stretching. Numerous mechanoreceptors are distributed across the skin, in particular in the dermis. In general, the speed of their adaptation to changes in the pressure applied to some points on the skin classify the type of tactile receptors.192 Hence, mechanoreceptors respond to stimuli coming from the world generating an electrical signal, transmitted to the brain. This incoming signal defines the mechanical sense of our surrounding.218 In particular, the changes in the firing rate of action potentials on the corresponding afferent nerve could be a way for measuring mechanoreceptor response. Four types of mechanoreceptors exist in the glabrous skin (Merkel cells, Ruffini endings, Meissner corpuscles, and Pacinian corpuscles), which respond to mechanical pressure, vibration or deformation.250 Since mechanoreceptors are sensitive to mechanical stimuli, the tactile sensation can arise stimulating the skin in a mechanical way, reproducing the real nature of the sense of touch.192 However, each of the four aforementioned mechanoreceptors respond to different stimuli and can therefore be classified on the basis of the different frequencies and displacements of the stimuli to which they respond: (i) Merkel Cells are stimulated at 0.3–3 Hz and need 8 μm displacements; (ii) Meissner Corpuscles respond to 3–40 Hz frequency and 3 μm displacements; (iii) Pacinian corpuscles 40–500 Hz with lower displacements, 0.01 μm; (iv) Ruffini Corpuscles (located deeply) 40–500 Hz and 40 μm.237 Hence, a mechanotactile device should have high bandwidth and generate relatively high displacements, as we will discuss later. | ||
| Fig. 7 Examples of conformable devices for haptic interfaces and their applications. (a) Piezoelectric haptic conformable devices. (i) Exploded view of the device. (ii) Optical image of the fabricated device (front and back). (iii) Schematic of the closed-loop control of the actuation mechanism for adjusting vibration amplitude. Adapted from ref. 225 under Open Access Attribution License CC BY, DOI: http://10.3389/frobt.2015.00038. (b) Haptic devices based on DEA embedded into a patched display. Reproduced from ref. 236 with permission from IEEE, DOI: http://10.1109/TOH.2018.2805901. (c) Examples of VR applications of a battery-free epidermal system used as a haptic interface capable of communicating information via mechanical vibrations. Reproduced from ref. 218 with permission from Springer Nature, DOI: http://10.1038/s41586-019-1687-0. | ||
Multiple studies used polymer-based systems that respond to electrical stimulation with shape or size change, called electroactive polymers, which are flexible, lightweight, fracture-tolerant, and easy to mould.30 Their mechanical properties are comparable to those of the human skin, making these actuators attractive for haptic devices.238
Conformable and wearable platforms for haptics can efficiently employ DEAs. Thanks to their lightweight, flexibility, cost-effectiveness, and fast response, DEAs have opened many opportunities in applications such as tactile or Braille displays (Section 4.2.2).222 The aim is to provide tactile sensations using a localized tuneable mechanical pressure applied to the skin through a soft interface. For example, Carpi et al. demonstrated the possibility to employ an acrylic elastomeric film to create a VHB-based DEA wearable tactile interface for use in portable and more cost-effective virtual reality (VR) or augmented reality (AR) systems.254,255 Some other recent studies demonstrated the possibility of using DEAs for developing conformable devices. Mun et al. designed a tactile display to embed into clothes, integrating flexible soft actuators onto the inside fingertip surface of a rubber glove or a patched display that could give vibrotactile feedback to the forearm (Fig. 7b).236 The soft actuator consisted of a multilayer dielectric elastomer with compliant electrodes made of AgNWs, stacked to obtain six layers. They demonstrated that the device could exert up to 255 mN at the maximum driving voltage of 4 kV when working at the resonance frequency of the membrane structure, considering, however, that the force perception threshold at the human fingertip is much lower (∼2.2 mN at 240 Hz frequency).228 Such a high range of forces could be useful to provide different sensations, for example distinguishing “hard” touch from “soft” touch. The device presented a response delay of actuation below 1 ms and the actuators produced outputs that covered a wide frequency range.
Fingertips have a very high sensitivity; hence, most of the studies concerning haptic feedback aim at designing devices that fit this region of the body. However, this configuration leads to difficulties in using the hands for everyday activities, such as grasping or manipulating objects.256 For this reason, Zhao et al. proposed a soft device to wear on the arm instead of fingertips, with the drawback that the arm is less sensitive than fingers, requiring higher forces and displacements for stimulation.235 The devices consisted of a 2-by-2 array of actuators: the authors demonstrated how an array of such components could create an illusion of motion, allowing them to reproduce a moving touch sensation through sequential activation of two actuators. This latter aspect could be of importance in applications concerning VR or AR for more realistic feedback of tactile sensations, as well as for prosthetic users who could detect movements of the grasped object avoiding its slippage. Yun et al. designed a perfectly skin-mountable interface based on DEAs arranged in arrays with AgNW-compliant electrodes. The device was able to reach an output force of 130 mN with, however, a very high driving electric field (20 MV m−1).238
The great issue related to the devices that base their operating principle on DEAs is the charge and discharge cycle time. In particular, slow charging time led to the slow response of the device because of the limited current flowing into the actuators. This aspect affected frequency response, resulting in high driving voltage requirement and low bandwidth. The devices needed driving voltages of several kVs (electric fields in the order of tens of MV m−1)238 resulting in severe limitations for wearable purposes. A step forward in this sense could be the development of flexible, stretchable, lightweight, and wearable devices consisting in wirelessly powered actuators so as to use haptic display in numerous applications. Moreover, the device should include a proper insulation layer because of high-operating voltage, thus leading to higher fabrication costs and output force degradation.222 Another technical challenge of DEA is the relatively low vertical force they are able to provide and small displacements, despite high driving voltages:203,236 an ideal tactile display should provide a 4 mm stroke.257 By causing the device to work at its resonant frequency, it is possible to reach higher forces and displacements.
In general, all the interfaces considered above still present some challenges related to adaptation (loss of sensitiveness) and habituation (getting used to certain stimuli) of the user to the provided sensations due to the continuous sensory stimuli. These aspects could result in not completely effective transmission of sensory information in the long-term use of haptic interfaces.258
5. Photostimulation devices
In modern medicine, light and optical techniques are profoundly impacting clinical practice in the health assessment and disease treatments.259 When photons interact with biological tissues, they can either scatter the photons (used to monitor diverse body parameters)259,260 or absorb them, activating photochemical and biological reactions of the tissues themselves, as well as generating heat.261–263 New therapeutic strategies exploit these phenomena, leading to an increasing interest in light-based therapies, such as (i) photodynamic therapy, in which a light source with a specific wavelength activates a photosensitizing agent located in a tumor, which causes irreversible photodamage to its tissues;261 (ii) photothermal therapy, that is a minimally-invasive therapy in which photon energy produces heat (also used in cancer treatment);262 (iii) optogenetic therapies, which provide initial genetic modifications of cells in order to express photosensitive traits, subsequently utilized for directly irradiating cells with visible light enabling users to photocontrol molecules, proteins, and cells in vitro and in vivo.264,265 Most of these kinds of therapies base their working principle on synthetic chromophores that undergo light-dependent structural or electronic changes and can target unique cellular traits, notably proteins. They have been proven useful in the treatment of diabetes, peripheral nerves, pain, and bladder diseases, as well as in regulating cardiac pacing when applied to cardiomyocytes;259,260,266 (iv) photobiomodulation exploits visible and near-infrared portions of the light spectrum in order to induce chemical changes within the cells, provoking biological reactions that benefit the body in triggering neuroprotective responses, improvements in metabolism, blood flow, and neurogenesis. The relatively low power density ensures no burn damage to the tissues;263 (v) photochemotherapy depends on the connection of drug and light: the drug exerts locally an antiproliferative effect only after interaction with suitable light, being otherwise ineffective and unable to target more cells than desired;267 (vi) in photopharmacology light influences the biological activity of synthetic molecules by changing their pharmacokinetic or pharmacodynamic properties: light precisely controls the bioactivity of smart drugs with incorporated molecular photoswitches, which change the molecular structure.268All these therapies can benefit from the use of dermal and transdermal opto-devices as the skin can let through several wavelengths. For example, light with a wavelength of 400–500 nm will stop at the epidermal layer, while light with a wavelength longer than 700 nm can reach deeper tissues beyond the dermis.260 Hence, one must select the light with a proper wavelength depending on the specific objective of the application (Fig. 8a).
 | ||
| Fig. 8 Examples of photostimulation devices. (a) Schematic depicting how light can interact with human skin. Reproduced from ref. 260 with permission from Springer Nature, DOI: http://10.1038/s41578-019-0167-3. (b) Implantable μLEDs embedded in PDMS provided with wireless communication for cancer treatments. Reproduced from ref. 275 with permission from Springer Nature, DOI: http://10.1038/s41551-018-0261-7. (c) Flexible OLED for wound treatments. (i) Schematic of device. Optical images of the (ii) device as prepared, (iii) mounted on skin, and (iv) working upon wrinkling. Reproduced from ref. 277 with permission from John Wiley & Sons, DOI: http://10.1002/jsid.882. (d) Schematic illustration of a wirelessly powered wearable μLED. (i) Schematic of preparation and (ii) exploded device. (iii) Working principle and circuit schematics. Reproduced from ref. 270 with permission from Elsevier, DOI: http://10.1016/j.nanoen.2018.11.017. (e) Schematic illustration of monolithic fabrication process of red μLEDs on a polymer substrate and application of the wearable device on a mouse to test treatments against alopecia. Adapted with permission from ref. 279. Copyright 2018 American Chemical Society, DOI: http://10.1021/acsnano.8b05568. | ||
Recent research led to the development of new materials and new techniques to manufacture conformable and stretchable devices. In particular, the possibility of fabricating LEDs with micrometric dimensions (μLEDs) and preparing inherently flexible OLEDs based on polymeric emitters propelled this field forward.44,269–271 Bioelectronic medicine aims to develop engineered systems that can relieve clinical conditions by photostimulating the body of the patient.272,273 Hence, while in past years pain relief relied largely on electrical stimulation, now bio-optoelectronic devices can overcome some limits of the former. Some of these limits are possible discomfort and pain caused by a continuous stimulation protocol, direct physical contact between the electrodes and the nerve, which can lead to injuries and inflammations, and lack of organ specificity.273
Most of the conformable photonic healthcare devices found in the literature consist of implantable devices, developed in particular for optogenetic and photodynamic therapies.260 The most attractive systems consist of μLEDs transferred on a flexible substrate so as to be directly implanted in the body, without the necessity of using an external light source for the specifically considered therapy. Moreover, studies developed devices with LEDs mounted on microneedles, with the ability to operate at wavelengths ranging from UV to blue, green-yellow, and red.260,274 Their use is particularly growing in biomedical applications that include biosensors, healthcare devices, and optogenetic stimulators, because of their high biocompatibility, low heating property, and excellent stability.27,271 Indeed, implantable photonic devices must be highly biocompatible and fixed inside the body for continuous local light delivery without causing any damage to other tissues or organs.260 For this reason, photostimulation implantable devices widely use PDMS as substrates or encapsulation material. Moreover, many are also provided with a wireless power supply system. For example, Yamagishi et al. used μLEDs embedded in PDMS for developing an implantable photodynamic device against cancer (Fig. 8b).275 Similarly, Mickle et al. used μLEDs on a PDMS substrate to fabricate a closed-loop device that exploited biophysical sensors for continuously monitoring and controlling bladder function through a smart device in order to eliminate pathological behaviours as they occur in real-time.273 Some other applications in which implantable photonic devices can offer benefits concern the stimulation of the spinal cord and peripheral nerves, against chronic pain, itch, and other neurological disorders.276
Wearable photonic healthcare devices are currently part of several applications including skin rejuvenation, wound treatment, mental healthcare and brain-disease therapy.260 Current rigid and bulky conventional light sources are, however, difficult to transport and use for regular irradiation treatments.277 Skin-mountable conformable devices – comprising μLEDs, flexible OLEDs, quantum dot displays and other thin technologies – can overcome these limitations, paving the way for developing lightweight, portable, and comfortable devices capable of providing on-demand, and continuous treatment.43,260,277,278
However, to the best of our knowledge, only a few examples of skin-mountable photonic devices exist. One application concerns the management of surgical wounds in order to ensure quick skin regeneration, pain relief, and scar prevention. Surgical wounds typically require periodic light treatment: Jeon et al. recently proposed a very thin flexible photonic skin, comprising a iridium(III) OLED, having a total thickness of 6 μm, that they developed for skin attachable phototherapy (Fig. 8c).277 The authors studied the regeneration effects on an artificial skin model composed of fibroblasts and keratinocytes: OLED light could have beneficial effects in cell metabolism, hence regeneration improved control of the peak wavelengths (600–700 nm) and irradiation interval of the flexible OLED. The authors designed the OLED with a bottom-emitting structure so that light could safely spread out through the substrate without exposing organic materials to the skin, so avoiding possible harm to the user. Moreover, they demonstrated that it is possible to mount the photonic skin on commercial dressing film and successfully applied it to patients with surgical wounds. Stretchability is surely important in wearable devices, so that the platforms can follow all the skin movements and deformations, without degrading. Yin et al. proposed another example of a wearable and flexible device composed of highly stretchable iridium(III) OLEDs that could exhibit 100% strain without a decrease in performance.44 In particular, the authors proposed a laser-programmable buckling process in order to create ordered buckles on the OLEDs, which permitted controllable stretch-release cycles and enhanced stretchability and mechanical robustness of the devices. In general, such platforms work through wireless power-transfer systems.272 Lee et al. proposed a method to supply electrical power to μLEDs using a wearable wireless power transmission/reception system consisting of flexible antennas, a peak rectifier, and a voltage regulator (Fig. 8d).270 An AC power supply source supplied radio frequency power to the wearable transmitter antenna wirelessly, which was collected by the wearable receiver antenna and then transmitted to the peak rectifier for converting AC to DC power. The converted DC power hence powered μLEDs through the voltage regulator. Another interesting application of the skin-mountable photostimulation devices concerns the acceleration of hair growth against alopecia. Lee et al. developed a methodology to prepare flexible microscale red LEDs fabricated through a simple monolithic fabrication process, and successfully used these elements to stimulate hair follicles in a rat, which are located 2 mm under the epidermis (Fig. 8e).279 The 650 nm radiation could penetrate deep into the skin compared to green and blue ones. This method aims to replace the use of laser stimulation, generally used in the clinical practice against alopecia, which has numerous drawbacks (e.g. high-power consumption, large size, and restrictive use in daily life due to its low controllability).
In conclusion, photostimulation conformable devices can surely bring benefits in numerous health areas. However, this field has been developed only in recent years, when technological breakthrough allowed the preparation of microscale and flexible light sources, and the research on wearable opto-stimulating devices is still in its infancy.
6. Conclusions and future outlook
The skin has a fundamental role in the human body: it is not only the largest organ of the human body, but it also allows humans to interact with the external world, thanks to the continuous exchange of information with the environment. In recent years, skin has been the base to develop new platforms and devices, creating new generations of technologies now recognized as conformable devices. Such devices perfectly adhere to non-planar surfaces such as the human body, paving the way for substituting the rigid and bulk devices now used especially in clinical practice with flexible and stretchable platforms. Such devices have advantages concerning: the possibility to follow strains and movements of the human body because of their similar mechanical properties to those of the skin; their imperceptibility; low cost due to innovative fabrication technologies; the possibility of a continuous use of such devices. Hence, many of them found their use in healthcare or as human-machine interfaces. In particular, we extensively reviewed conformable devices able to provide some kind of stimulation to the human body, their working principles, the materials they comprise, and their fabrication. We decided to focus on three categories based on the method used to generate feedback – thermal, electrical, or mechanical stimulation – since these three categories require different design approaches due to their different purposes. In the last section, we also reviewed the most innovative conformable photostimulation devices and discussed their promising application in healthcare. We highlighted the recent steps forward research has made in new material explorations, fabrication techniques for the production of increasingly cost-effective devices, and innovative powering systems: all these factors contribute to creating more and more wearable, comfortable, conformable, and disposable devices. We also outlined the most important parameters (Tables) to consider and to assess during the investigations concerning new materials for developing more and more effective devices.What has emerged from the reviewed devices is that for the three categories the number of conformable devices already developed is high and, in particular, we have found that the demand for such devices is particularly growing in the medical field. Thermal stimulation provided by conformable heaters find use in personal treatment management and, so, in thermotherapy applications against muscular disorders or in controlled therapeutic drug delivery. At the same time, electrical stimulation can help patients with neurological impairments or for tremor suppression. Also, haptic feedback provided through mechanical stimulation could help in developing tactile displays for blind people. In addition, in this review, we discussed many other applications of conformable stimulating devices. Conformable devices capable of providing transcutaneous electrical stimulation through electrodes applied on the surface of the skin, which are the ones that present more difficulties in their development. So far, such conformable devices are mainly implantable. We think this aspect correlates with the difficulties concerning the development of conformable devices that have low impedance at the interface but also concerning the generation of a uniform electric field. Conformable devices used in electrical stimulation must have only the electrodes in contact with the skin in order to stimulate just the interested point: this translates in a complex fabrication process to manufacture multilevel interconnected layers.
In particular for the medical field, we have noticed the importance of developing portable, disposable, but also customizable devices. One of the main problems is that most of the devices considered required high driving voltage and, so, high power-consumption. This leads to the addition of multiple electronic components on the platform, necessary for the operating principle of such devices, but also brings severe limitations for wearability. Hence, further investigations are necessary aiming to obtain less bulky platforms: basing device power supply on wireless technologies could be a disruptive strategy, as already proposed by some of the studies here reviewed. Also, one can investigate and exploit technologies and materials concerning energy storage and energy harvesting: triboelectric generators added to the platform can constitute self-powered devices, transducing mechanical signals such as movements of the human body into electrical energy. However, conformable stimulation devices could benefit from wireless communication, in addition to miniaturization of electronic components, also because it could guarantee a continuous exchange of data between the device mounted on the skin, and the user. The latter could indeed directly control the temperature reached on the skin using the heating module or the stimulation parameters for transcutaneous electrical stimulation, simply from his smartphone. A more complete vision of such platforms could be to develop a closed-loop control based on feedback signals provided directly from the mounted device, in order to have a system capable of auto-regulating thermal, electrical, or mechanical stimulation parameters on the basis of the data transmitted.
The long-term perspective concerns the use of these devices in clinical practice, in order to replace rigid and bulk electrodes and to reach a level of complexity in controlling these devices, which also suits the capabilities of non-specialized users. More in-depth studies concerning both the materials and fabrication techniques, which allow simplifying the application of the device on the skin, are necessary. Materials investigation and materials engineering are of paramount importance to achieve thinner stimulating systems (in the order of microns), and thus develop bendable and flexible devices that perfectly adhere to all the pores and imperfections of the human skin. Moreover, the miniaturization of different components such as sensors or communication modules for wireless connections and power supply is of crucial importance. This would allow all the electronic components in the stimulating device to be directly embeded so as to transfer all the electronics to the device. This aspect is important for such platforms to be used by individuals themselves at home, making their use in a non-clinical environment also possible.
In conclusion, we presented numerous systems capable of delivering thermal, electrical, mechanical, or optical stimulation, which could be valid platforms to exploit in practical usage. However, all the considered studies were conducted on bench-scale experiments: their use by individuals at home can only be achieved after careful clinical tests, in order to assess the efficacy and effectiveness of the stimulating systems. In doing so, collaborations between specialists like engineers, medical specialists, and chemists will be fundamental to improve the field of soft and conformable electronics.
Conflicts of interest
The authors declare no conflict of interests.References
- A. Zucca, C. Cipriani, Sudha, S. Tarantino, D. Ricci, V. Mattoli and F. Greco, Adv. Healthcare Mater., 2015, 4, 983–990 CrossRef CAS.
- S. C. Chang, J. Bharathan, Y. Yang, R. Helgeson, F. Wudl, M. B. Ramey and J. R. Reynolds, Appl. Phys. Lett., 1998, 73, 2561–2563 CrossRef CAS.
- Z. Bao, Y. Feng, A. Dodabalapur, V. R. Raju and A. J. Lovinger, Chem. Mater., 1997, 9, 1299–1301 CrossRef CAS.
- S. Chun, D. Grudinin, D. Lee, S. H. Kim, G. R. Yi and I. Hwang, Chem. Mater., 2009, 21, 343–350 CrossRef CAS.
- A. Zucca, K. Yamagishi, T. Fujie, S. Takeoka, V. Mattoli and F. Greco, J. Mater. Chem. C, 2015, 3, 6539–6548 RSC.
- Z. Fan, J. C. Ho, T. Takahashi, R. Yerushalmi, K. Takei, A. C. Ford, Y. L. Chueh and A. Javey, Adv. Mater., 2009, 21, 3730–3743 CrossRef CAS.
- J. A. Rogers, T. Someya and Y. Huang, Science, 2010, 327, 1603–1607 CrossRef CAS.
- D. T. Simon, E. O. Gabrielsson, K. Tybrandt and M. Berggren, Chem. Rev., 2016, 116, 13009–13041 CrossRef CAS.
- L. M. Ferrari, S. Sudha, S. Tarantino, R. Esposti, F. Bolzoni, P. Cavallari, C. Cipriani, V. Mattoli and F. Greco, Adv. Sci., 2018, 5, 1–11 Search PubMed.
- L. M. Ferrari, U. Ismailov, J. M. Badier, F. Greco and E. Ismailova, npj Flex. Electron., 2020, 4, 1–9 CrossRef.
- M. Wang, P. Baek, A. Akbarinejad, D. Barker and J. Travas-Sejdic, J. Mater. Chem. C, 2019, 7, 5534–5552 RSC.
- D. A. May-Arrioja, V. I. Ruiz-Perez, Y. Bustos-Terrones and M. A. Basurto-Pensado, IEEE Sens. J., 2016, 16, 1956–1961 CAS.
- J. Kim, M. Lee, H. J. Shim, R. Ghaffari, H. R. Cho, D. Son, Y. H. Jung, M. Soh, C. Choi, S. Jung, K. Chu, D. Jeon, S. T. Lee, J. H. Kim, S. H. Choi, T. Hyeon and D. H. Kim, Nat. Commun., 2014, 5, 1–11 Search PubMed.
- A. J. Bandodkar, V. W. S. Hung, W. Jia, G. Valdés-Ramírez, J. R. Windmiller, A. G. Martinez, J. Ramírez, G. Chan, K. Kerman and J. Wang, Analyst, 2013, 138, 123–128 RSC.
- Y. Y. Hsu, H. James, R. Ghaffari, B. Ives, P. Wei, L. Klinker, B. Morey, B. Elolampi, D. Davis, C. Rafferty and K. Dowling, Proc. Tech. Pap. - Int. Microsystems, Packag. Assem. Circuits Technol. Conf. IMPACT, 2012, 228–231.
- S. Taccola, F. Greco, A. Zucca, C. Innocenti, C. De Julián Fernández, G. Campo, C. Sangregorio, B. Mazzolai and V. Mattoli, ACS Appl. Mater. Interfaces, 2013, 5, 6324–6332 CrossRef CAS.
- R. Zhou, P. Li, Z. Fan, D. Du and J. Ouyang, J. Mater. Chem. C, 2017, 5, 1544–1551 RSC.
- A. D. Koutsou, J. C. Moreno, A. J. Del Ama, E. Rocon and J. L. Pons, J. Neuroeng. Rehabil., 2016, 13, 56 CrossRef.
- F. B. Wagner, J. B. Mignardot, C. G. Le Goff-Mignardot, R. Demesmaeker, S. Komi, M. Capogrosso, A. Rowald, I. Seáñez, M. Caban, E. Pirondini, M. Vat, L. A. McCracken, R. Heimgartner, I. Fodor, A. Watrin, P. Seguin, E. Paoles, K. Van Den Keybus, G. Eberle, B. Schurch, E. Pralong, F. Becce, J. Prior, N. Buse, R. Buschman, E. Neufeld, N. Kuster, S. Carda, J. von Zitzewitz, V. Delattre, T. Denison, H. Lambert, K. Minassian, J. Bloch and G. Courtine, Nature, 2018, 563, 65–93 CrossRef CAS.
- J. Tian, R. Shi, Z. Liu, H. Ouyang, M. Yu, C. Zhao, Y. Zou, D. Jiang, J. Zhang and Z. Li, Nano Energy, 2019, 59, 705–714 CrossRef CAS.
- N. Lu and D. H. Kim, Soft Robot., 2014, 1, 53–62 CrossRef.
- P. Lopes, S. You, L. P. Cheng, S. Marwecki and P. Baudisch, Conf. Hum. Factors Comput. Syst. - Proc., 2017, 2017-May, 1471–1482.
- D. Gao, K. Parida and P. S. Lee, Adv. Funct. Mater., 2020, 30, 1–30 Search PubMed.
- W. Dong, Y. Wang, Y. Zhou, Y. Bai, Z. Ju, J. Guo, G. Gu, K. Bai, G. Ouyang, S. Chen, Q. Zhang and Y. A. Huang, Int. J. Intell. Robot. Appl., 2018, 2, 313–338 CrossRef.
- A. Nag, S. C. Mukhopadhyay and J. Kosel, IEEE Sens. J., 2017, 17, 3949–3960 CAS.
- M. Amjadi, K. U. Kyung, I. Park and M. Sitti, Adv. Funct. Mater., 2016, 26, 1678–1698 CrossRef CAS.
- Z. W. K. Low, Z. Li, C. Owh, P. L. Chee, E. Ye, D. Kai, D. P. Yang and X. J. Loh, Small, 2019, 15, 1–23 CrossRef.
- M. L. Cohen, J. Invest. Dermatol., 1977, 69, 333–338 CrossRef CAS.
- D. H. Kim, N. Lu, R. Ma, Y. S. Kim, R. H. Kim, S. Wang, J. Wu, S. M. Won, H. Tao, A. Islam, K. J. Yu, T. Il Kim, R. Chowdhury, M. Ying, L. Xu, M. Li, H. J. Chung, H. Keum, M. McCormick, P. Liu, Y. W. Zhang, F. G. Omenetto, Y. Huang, T. Coleman and J. A. Rogers, Science, 2011, 333, 838–843 CrossRef CAS.
- S. Biswas and Y. Visell, Adv. Mater. Technol., 2019, 4, 1–30 Search PubMed.
- K. D. Harris, A. L. Elias and H. J. Chung, J. Mater. Sci., 2016, 51, 2771–2805 CrossRef CAS.
- Y. Liu, M. Pharr and G. A. Salvatore, ACS Nano, 2017, 11, 9614–9635 CrossRef CAS.
- X. Chen, Small Methods, 2017, 1, 1600029 CrossRef.
- Y. H. Jung, J. U. Kim, J. S. Lee, J. H. Shin, W. Jung, J. Ok and T. il Kim, Adv. Mater., 2020, 32, 1–25 Search PubMed.
- B. Wang and A. Facchetti, Adv. Mater., 2019, 31, 1–53 Search PubMed.
- J. C. Yang, J. Mun, S. Y. Kwon, S. Park, Z. Bao and S. Park, Adv. Mater., 2019, 31, 1–50 Search PubMed.
- M. L. Hammock, A. Chortos, B. C. K. Tee, J. B. H. Tok and Z. Bao, Adv. Mater., 2013, 25, 5997–6038 CrossRef CAS.
- J. S. Knutson, N. S. Makowski, K. L. Kilgore and J. Chae, Neuromuscular Electrical Stimulation Applications, Elsevier Inc., Fifth Edn, 2019 Search PubMed.
- J. Park, Y. Lee, H. Lee and H. Ko, ACS Nano, 2020, 14, 12–20 CrossRef CAS.
- D. Bin Moon, J. Lee, E. Roh and N. E. Lee, Thin Solid Films, 2019, 688, 137435 CrossRef.
- D. F. Fernandes, C. Majidi and M. Tavakoli, J. Mater. Chem. C, 2019, 7, 14035–14068 RSC.
- S. H. Park, Y. J. Kang and S. Majd, Adv. Mater., 2015, 27, 7583–7619 CrossRef CAS.
- T. Yokota, P. Zalar, M. Kaltenbrunner, H. Jinno, N. Matsuhisa, H. Kitanosako, Y. Tachibana, W. Yukita, M. Koizumi and T. Someya, Sci. Adv., 2016, 2, 1–9 Search PubMed.
- D. Yin, J. Feng, R. Ma, Y. F. Liu, Y. L. Zhang, X. L. Zhang, Y. G. Bi, Q. D. Chen and H. B. Sun, Nat. Commun., 2016, 7, 1–7 Search PubMed.
- M. Kaltenbrunner, T. Sekitani, J. Reeder, T. Yokota, K. Kuribara, T. Tokuhara, M. Drack, R. Schwödiauer, I. Graz, S. Bauer-Gogonea, S. Bauer and T. Someya, Nature, 2013, 499, 458–463 CrossRef CAS.
- J. Barsotti, I. Hirata, F. Pignatelli, M. Caironi, F. Greco and V. Mattoli, Adv. Electron. Mater., 2018, 4, 1–9 Search PubMed.
- J. C. McDonald and G. M. Whitesides, Acc. Chem. Res., 2002, 35, 491–499 CrossRef CAS.
- C. P. Constantin, M. Aflori, R. F. Damian and R. D. Rusu, Materials, 2019, 12, 3166 CrossRef CAS.
- R. Balint, N. J. Cassidy and S. H. Cartmell, Acta Biomater., 2014, 10, 2341–2353 CrossRef CAS.
- N. Hadrup, A. K. Sharma and K. Loeschner, Regul. Toxicol. Pharmacol., 2018, 98, 257–267 CrossRef CAS.
- M. Fedel, C—J. Carbon Res., 2020, 6, 12 CrossRef CAS.
- T. P. Haider, C. Völker, J. Kramm, K. Landfester and F. R. Wurm, Angew. Chem., Int. Ed., 2019, 58, 50–62 CrossRef CAS.
- E. Aghion, Metals, 2018, 8, 10–13 CrossRef.
- E. K. Lee, M. K. Kim and C. H. Lee, Annu. Rev. Biomed. Eng., 2019, 21, 299–323 CrossRef CAS.
- B. Xu, A. Akhtar, Y. Liu, H. Chen, W. H. Yeo, S. Park, B. Boyce, H. Kim, J. Yu, H. Y. Lai, S. Jung, Y. Zhou, J. Kim, S. Cho, Y. Huang, T. Bretl and J. A. Rogers, Adv. Mater., 2016, 28, 4462–4471 CrossRef CAS.
- S. Choi, S. I. Han, D. Jung, H. J. Hwang, C. Lim, S. Bae, O. K. Park, C. M. Tschabrunn, M. Lee, S. Y. Bae, J. W. Yu, J. H. Ryu, S. W. Lee, K. Park, P. M. Kang, W. B. Lee, R. Nezafat, T. Hyeon and D. H. Kim, Nat. Nanotechnol., 2018, 13, 1048–1056 CrossRef CAS.
- M. L. Cohen, J. Invest. Dermatol., 1977, 69, 333–338 CrossRef CAS.
- J. Kasper, D. L. Fauci, A. S. Hauser, S. L. Longo, D. L. 1. Jameson and J. L. Loscalzo, Harrison's principles of internal medicine, 19th edn, New York, McGraw Hill Education, 2015 Search PubMed.
- J. W. Jeong, M. K. Kim, H. Cheng, W. H. Yeo, X. Huang, Y. Liu, Y. Zhang, Y. Huang and J. A. Rogers, Adv. Healthcare Mater., 2014, 3, 642–648 CrossRef CAS.
- J. Kim, I. Jeerapan, J. R. Sempionatto, A. Barfidokht, R. K. Mishra, A. S. Campbell, L. J. Hubble and J. Wang, Acc. Chem. Res., 2018, 51, 2820–2828 CrossRef CAS.
- A. S. Campbell, J. Kim and J. Wang, Curr. Opin. Electrochem., 2018, 10, 126–135 CrossRef CAS.
- J. Kim, I. Jeerapan, S. Imani, T. N. Cho, A. Bandodkar, S. Cinti, P. P. Mercier and J. Wang, ACS Sens., 2016, 1, 1011–1019 CrossRef CAS.
- E. R. Lee, T. R. Wilsey, P. Tarczy-Homoch, D. S. Kapp, P. Fessenden, A. Lohrbach and S. D. Prionas, IEEE Trans. Biomed. Eng., 1992, 39, 470–483 CrossRef CAS.
- B. Stephens-Fripp, V. Sencadas, R. Mutlu and G. Alici, Front. Bioeng. Biotechnol., 2018, 6, 1–9 CrossRef.
- R. G. Gordon, MRS Bull., 2000, 25, 52–57 CrossRef CAS.
- H. S. Jo, S. An, J. G. Lee, H. G. Park, S. S. Al-Deyab, A. L. Yarin and S. S. Yoon, NPG Asia Mater., 2017, 9, e347 CrossRef CAS.
- S. Selkowitz, Transparent heat mirrors for passive solar heating applications, California Univ., Lawrence Berkeley Lab., Berkeley, USA, 1978 Search PubMed.
- R. Hu, Y. Liu, S. Shin, S. Huang, X. Ren, W. Shu, J. Cheng, G. Tao, W. Xu, R. Chen and X. Luo, Adv. Energy Mater., 2020, 10, 1–23 Search PubMed.
- S. F. Nadler, K. Weingand and R. J. Kruse, Pain Physician, 2004, 7, 395–399 Search PubMed.
- S. Michlovitz, L. Hun, G. N. Erasala, D. A. Hengehold and K. W. Weingand, Arch. Phys. Med. Rehabil., 2004, 85, 1409–1416 CrossRef.
- R. B. Roemer, Annu. Rev. Biomed. Eng., 1999, 347–376 CrossRef CAS.
- J. S. Petrofsky, M. Laymon and H. Lee, Med. Sci. Monit., 2013, 19, 661–667 CrossRef.
- D. Son, J. Lee, S. Qiao, R. Ghaffari, J. Kim, J. E. Lee, C. Song, S. J. Kim, D. J. Lee, S. W. Jun, S. Yang, M. Park, J. Shin, K. Do, M. Lee, K. Kang, C. S. Hwang, N. Lu, T. Hyeon and D. H. Kim, Nat. Nanotechnol., 2014, 9, 397–404 CrossRef CAS.
- G. Chernyshov, K. Ragozin, C. Caremel and K. Kunze, Proc. ACM Symp. Virtual Real. Softw. Technol. VRST, 2018, 52 Search PubMed.
- S. Kim, T. Kim, C. S. Kim, H. Choi, Y. J. Kim, G. S. Lee, O. Oh and B. J. Cho, Soft Robot., 2020, 00, 1–7 Search PubMed.
- A. Tamayol, A. Hassani Najafabadi, P. Mostafalu, A. K. Yetisen, M. Commotto, M. Aldhahri, M. S. Abdel-Wahab, Z. I. Najafabadi, S. Latifi, M. Akbari, N. Annabi, S. H. Yun, A. Memic, M. R. Dokmeci and A. Khademhosseini, Sci. Rep., 2017, 7, 1–10 CrossRef CAS.
- S. W. Hwang, H. Tao, D. H. Kim, H. Cheng, J. K. Song, E. Rill, M. A. Brenckle, B. Panilaitis, S. M. Won, Y. S. Kim, Y. M. Song, K. J. Yu, A. A. Ameen, R. Li, Y. Su, M. Yang, D. L. Kaplan, M. R. Zakin, M. J. Slepian, Y. Huang, F. G. Omenetto and J. A. Rogers, Science, 2012, 337, 1640–1644 CrossRef CAS.
- J. Koo, M. R. MacEwan, S. K. Kang, S. M. Won, M. Stephen, P. Gamble, Z. Xie, Y. Yan, Y. Y. Chen, J. Shin, N. Birenbaum, S. Chung, S. B. Kim, J. Khalifeh, D. V. Harburg, K. Bean, M. Paskett, J. Kim, Z. S. Zohny, S. M. Lee, R. Zhang, K. Luo, B. Ji, A. Banks, H. M. Lee, Y. Huang, W. Z. Ray and J. A. Rogers, Nat. Med., 2018, 24, 1830–1836 CrossRef CAS.
- J. M. Kim, D. R. Oh, J. Sanchez, S. H. Kim and J. M. Seo, Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS, 2013, 1716–1719 CAS.
- P. Kassanos, F. Seichepine, D. Wales and G. Z. Yang, BioCAS 2019 - Biomed. Circuits Syst. Conf. Proc., 2019, 1–4 Search PubMed.
- A. Stier, E. Halekote, A. Mark, S. Qiao, S. Yang, K. Diller and N. Lu, Micromachines, 2018, 9, 1–14 CrossRef.
- T. W. Kang, S. H. Kim, M. Kim, E. Cho and S. J. Lee, Plasma Process. Polym., 2020, 17, e1900188 CrossRef.
- M. D. Dickey, R. C. Chiechi, R. J. Larsen, E. A. Weiss, D. A. Weitz and G. M. Whitesides, Adv. Funct. Mater., 2008, 18, 1097–1104 CrossRef CAS.
- L. Jing and Y. Liting, Liquid Metal Biomaterials, Springer, Singapore, 2018, pp. 149–186 Search PubMed.
- Y. G. Park, H. S. An, J. Y. Kim and J. U. Park, Sci. Adv., 2019, 5, 1–10 Search PubMed.
- J. Yang, W. Cheng and K. Kalantar-Zadeh, Proc. IEEE, 2019, 107, 2168–2184 CAS.
- J. Wang, H. Wang, N. V. Thakor and C. Lee, ACS Nano, 2019, 13, 3589–3599 CrossRef CAS.
- Y. Wang, Z. Yu, G. Mao, Y. Liu, G. Liu, J. Shang, S. Qu, Q. Chen and R. W. Li, Adv. Mater. Technol., 2019, 4, 1–9 CAS.
- M. G. Kim, H. Alrowais, S. Pavlidis and O. Brand, Adv. Funct. Mater., 2017, 27, 1604466 CrossRef.
- R. Guo, X. Sun, S. Yao, M. Duan, H. Wang, J. Liu and Z. Deng, Adv. Mater. Technol., 2019, 4, 1–11 Search PubMed.
- X. Wang, L. Fan, J. Zhang, X. Sun, H. Chang, B. Yuan, R. Guo, M. Duan and J. Liu, Adv. Funct. Mater., 2019, 29, 1–13 Search PubMed.
- D. X. Song, Y. Z. Du, W. G. Ma and X. Zhang, Int. J. Heat Mass Transf., 2020, 147, 119014 CrossRef.
- S. Sorel, D. Bellet and J. N. Coleman, ACS Nano, 2014, 8, 4805–4814 CrossRef CAS.
- L. Veeramuthu, B. Y. Chen, C. Y. Tsai, F. C. Liang, M. Venkatesan, D. H. Jiang, C. W. Chen, X. Cai and C. C. Kuo, RSC Adv., 2019, 9, 35786–35796 RSC.
- J. Kang, H. Kim, K. S. Kim, S. K. Lee, S. Bae, J. H. Ahn, Y. J. Kim, J. B. Choi and B. H. Hong, Nano Lett., 2011, 11, 5154–5158 CrossRef CAS.
- D. T. Papanastasiou, A. Schultheiss, D. Muñoz-Rojas, C. Celle, A. Carella, J. P. Simonato and D. Bellet, Adv. Funct. Mater., 2020, 30, 1–33 CrossRef.
- S. Hong, H. Lee, J. Lee, J. Kwon, S. Han, Y. D. Suh, H. Cho, J. Shin, J. Yeo and S. H. Ko, Adv. Mater., 2015, 27, 4744–4751 CrossRef CAS.
- P. Won, J. J. Park, T. Lee, I. Ha, S. Han, M. Choi, J. Lee, S. Hong, K. J. Cho and S. H. Ko, Nano Lett., 2019, 19, 6087–6096 CrossRef CAS.
- J. Jang, B. G. Hyun, S. Ji, E. Cho, B. W. An, W. H. Cheong and J.-U. Park, NPG Asia Mater., 2017, 9, e432–e432 CrossRef CAS.
- B. W. An, E. J. Gwak, K. Kim, Y. C. Kim, J. Jang, J. Y. Kim and J. U. Park, Nano Lett., 2016, 16, 471–478 CrossRef CAS.
- D. Han, Y. Li, X. Jiang, W. Zhao, F. Wang, W. Lan, E. Xie and W. Han, Compos. Sci. Technol., 2018, 168, 460–466 CrossRef CAS.
- S. Choi, J. Park, W. Hyun, J. Kim, J. Kim, Y. B. Lee, C. Song, H. J. Hwang, J. H. Kim, T. Hyeon and D. H. Kim, ACS Nano, 2015, 9, 6626–6633 CrossRef CAS.
- W. Lan, Y. Chen, Z. Yang, W. Han, J. Zhou, Y. Zhang, J. Wang, G. Tang, Y. Wei, W. Dou, Q. Su and E. Xie, ACS Appl. Mater. Interfaces, 2017, 9, 6644–6651 CrossRef CAS.
- M. Cao, M. Wang, L. Li, H. Qiu and Z. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 1077–1083 CrossRef CAS.
- J. C. Goak, T. Y. Kim, D. U. Kim, K. S. Chang, C. S. Lee and N. Lee, Appl. Surf. Sci., 2020, 510, 145445 CrossRef CAS.
- J. Ahn, J. Gu, Y. Jeong, K. Kim, J. Jeong and I. Park, IEEE 32nd Int. Conf. Micro Electro Mech. Syst., 2019, 303–306 Search PubMed.
- K. Hee Pyo and J. W. Kim, Compos. Sci. Technol., 2016, 133, 7–14 CrossRef.
- J. Park, D. Han, S. Choi, Y. Kim and J. Kwak, RSC Adv., 2019, 9, 5731–5737 RSC.
- X. Chen, S. Nie, W. Guo, F. Fei, W. Su, W. Gu and Z. Cui, Adv. Electron. Mater., 2019, 5, 1–8 Search PubMed.
- H. Zhai, R. Wang, X. Wang, Y. Cheng, L. Shi and J. Sun, Nano Res., 2016, 9, 3924–3936 CrossRef CAS.
- J. Koo, C. Lee, C. R. Chu, S. K. Kang and H. M. Lee, Adv. Mater. Technol., 2020, 5, 1–7 Search PubMed.
- L. Yang, W. Weng, J. Yang, Y. Zhang, Y. Liang, X. Luo and M. Zhu, ACS Appl. Nano Mater., 2018, 1, 4781–4787 CrossRef CAS.
- R. Kolisnyk, M. Korab, M. Iurzhenko, O. Masiuchok, A. Shadrin, Y. Mamunya, S. Pruvost and V. Demchenko, Advances in Thin Films, Nanostructured Materials, and Coatings, Springer, Singapore, 2019, pp. 215–224 Search PubMed.
- S. Kim, W. Oh, J. S. Bae, S. B. Yang, J. H. Yeum, J. Park, C. Sung, J. Kim and J. C. Shin, Int. J. Polym. Sci., 2019, 2019, 3478325 Search PubMed.
- B. Zhou, X. Han, L. Li, Y. Feng, T. Fang, G. Zheng, B. Wang, K. Dai, C. Liu and C. Shen, Compos. Sci. Technol., 2019, 183, 107796 CrossRef CAS.
- N. M. Abbasi, H. Yu, L. Wang, Z. Ul-Abdin, W. A. Amer, M. Akram, H. Khalid, Y. Chen, M. Saleem, R. Sun and J. Shan, Mater. Chem. Phys., 2015, 166, 1–15 CrossRef CAS.
- D. Il Moon, G. Plečkaitytė, T. Choi, M. L. Seol, B. Kim, D. Lee, J. W. Han and M. Meyyappan, Adv. Healthcare Mater., 2020, 9, 1–7 Search PubMed.
- C. Weng, Z. Dai, G. Wang, L. Liu and Z. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 6541–6549 CrossRef CAS.
- L. J. Romasanta, P. Schäfer and J. Leng, Sci. Rep., 2018, 8, 1–7 CrossRef CAS.
- F. Greco, A. Zucca, S. Taccola, B. Mazzolai and V. Mattoli, ACS Appl. Mater. Interfaces, 2013, 5, 9461–9469 CrossRef CAS.
- J. Chang, J. He, Q. Lei and D. Li, ACS Appl. Mater. Interfaces, 2018, 10, 19116–19122 CrossRef CAS.
- C. Wang, K. Sun, J. Fu, R. Chen, M. Li, Z. Zang, X. Liu, B. Li, H. Gong and J. Ouyang, Adv. Sustainable Syst., 2018, 2, 1800085 CrossRef.
- H. He, L. Zhang, X. Guan, H. Cheng, X. Liu, S. Yu, J. Wei and J. Ouyang, ACS Appl. Mater. Interfaces, 2019, 11, 26185–26193 CrossRef CAS.
- M. N. Gueye, A. Carella, R. Demadrille and J. P. Simonato, ACS Appl. Mater. Interfaces, 2017, 9, 27250–27256 CrossRef CAS.
- F. Greco, A. Zucca, S. Taccola, A. Menciassi, T. Fujie, H. Haniuda, S. Takeoka, P. Dario and V. Mattoli, Soft Matter, 2011, 7, 10642–10650 RSC.
- S. Taccola, F. Greco, E. Sinibaldi, A. Mondini, B. Mazzolai and V. Mattoli, Adv. Mater., 2015, 27, 1668–1675 CrossRef CAS.
- E. Ridolfi Riva, A. Desii, E. Sinibaldi, G. Ciofani, V. Piazza, B. Mazzolai and V. Mattoli, ACS Nano, 2014, 8, 5552–5563 CrossRef.
- X. Wang, W. Yao, R. Guo, X. Yang, J. Tang, J. Zhang, W. Gao, V. Timchenko and J. Liu, Adv. Healthcare Mater., 2018, 7, 1–10 Search PubMed.
- J. H. Bahk, H. Fang, K. Yazawa and A. Shakouri, J. Mater. Chem. C, 2015, 3, 10362–10374 RSC.
- J. Lee, H. Sul, W. Lee, K. R. Pyun, I. Ha, D. Kim, H. Park, H. Eom, Y. Yoon, J. Jung, D. Lee and S. H. Ko, Adv. Funct. Mater., 2020, 30, 1–11 Search PubMed.
- D. Beretta, A. J. Barker, I. Maqueira-Albo, A. Calloni, G. Bussetti, G. Dell’Erba, A. Luzio, L. Duò, A. Petrozza, G. Lanzani and M. Caironi, ACS Appl. Mater. Interfaces, 2017, 9, 18151–18160 CrossRef CAS.
- H. N. Ho, Temperature, 2018, 5, 36–55 CrossRef.
- Z. Ma, S. Kang, J. Ma, L. Shao, A. Wei, C. Liang, J. Gu, B. Yang, D. Dong, L. Wei and Z. Ji, ACS Nano, 2019, 13, 7578–7590 CrossRef CAS.
- R. C. Webb, A. P. Bonifas, A. Behnaz, Y. Zhang, K. J. Yu, H. Cheng, M. Shi, Z. Bian, Z. Liu, Y. S. Kim, W. H. Yeo, J. S. Park, J. Song, Y. Li, Y. Huang, A. M. Gorbach and J. A. Rogers, Nat. Mater., 2013, 12, 938–944 CrossRef CAS.
- Y. Cheng, H. Zhang, R. Wang, X. Wang, H. Zhai, T. Wang, Q. Jin and J. Sun, ACS Appl. Mater. Interfaces, 2016, 8, 32925–32933 CrossRef CAS.
- A. Margaret, M. D. Hamburg and F. S. Collins, N. Engl. J. Med., 2010, 363, 301–304 CrossRef.
- N. S. Jang, K. H. Kim, S. H. Ha, S. H. Jung, H. M. Lee and J. M. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 19612–19621 CrossRef CAS.
- S. S. Gharaie, S. M. H. Dabiri and M. Akbari, Polymers, 2018, 10, 1317 CrossRef.
- J. H. Park, J. W. Lee, Y. C. Kim and M. R. Prausnitz, Int. J. Pharm., 2008, 359, 94–103 CrossRef CAS.
- M. R. Prausnitz, S. Mitragotri and R. Langer, Nat. Rev. Drug Discovery, 2004, 3, 115–124 CrossRef CAS.
- S. Bagherifard, A. Tamayol, P. Mostafalu, M. Akbari, M. Comotto, N. Annabi, M. Ghaderi, S. Sonkusale, M. R. Dokmeci and A. Khademhosseini, Adv. Healthcare Mater., 2016, 5, 175–184 CrossRef CAS.
- H. Lee, C. Song, Y. S. Hong, M. S. Kim, H. R. Cho, T. Kang, K. Shin, S. H. Choi, T. Hyeon and D. H. Kim, Sci. Adv., 2017, 3, 1–9 CAS.
- L. A. Jones and H. N. Ho, IEEE Trans. Haptics, 2008, 1, 53–70 CAS.
- D. Beretta, A. Perego, G. Lanzani and M. Caironi, Sustainable Energy Fuels, 2017, 1, 174–190 RSC.
- A. V. Roy, J. Camchong and K. O. Lim, Principles and applications of transcranial electrical stimulation, Elsevier Inc., 2018 Search PubMed.
- H. Palza, P. A. Zapata and C. Angulo-Pineda, Materials, 2019, 12, 277 CrossRef CAS.
- C. Galli, G. Pedrazzi and S. Guizzardi, Bioelectromagnetics, 2019, 40, 211–233 CrossRef.
- E. L. Nussbaum, P. Houghton, J. Anthony, S. Rennie, B. L. Shay and A. M. Hoens, Physiother. Canada, 2017, 69, 1–76 CrossRef.
- E. D’Anna, F. M. Petrini, F. Artoni, I. Popovic, I. Simanić, S. Raspopovic and S. Micera, Sci. Rep., 2017, 7, 1–15 CrossRef.
- P. E. Houghton, Chronic Wound Care Manag. Res., 2017, 4, 25–44 CrossRef.
- S. D. Bennie, J. S. Petrofsky, J. Nisperos, M. Tsurudome and M. Laymon, Eur. J. Appl. Physiol., 2002, 88, 13–19 CrossRef.
- L. E. Osborn, A. Dragomir, J. L. Betthauser, C. L. Hunt, H. H. Nguyen, R. R. Kaliki and N. V. Thakor, Sci. Robot., 2018, 3, eaat3818 CrossRef.
- X. Wang, Y. Zhang, R. Guo, H. Wang, B. Yuan and J. Liu, J. Micromechanics Microengineering, 2018, 28, aaa80f Search PubMed.
- W. Navaraj, C. Smith and R. Dahiya, E-skin and wearable systems for health care, Elsevier Ltd., 2020 Search PubMed.
- J. C. Loitz, A. Reinert, A. K. Neumann, F. Quandt, D. Schroeder and W. H. Krautschneider, Curr. Dir. Biomed. Eng, 2016, 2, 391–394 Search PubMed.
- H. P. Wang, A. W. Guo, Z. Y. Bi, F. Li, X. Y. Lu and Z. G. Wang, Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS, 2017, 714–717 Search PubMed.
- L. Z. Popović and N. M. Malešević, Proc. 31st Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. Eng. Futur. Biomed. EMBC 2009, 2009, 6785–6788 Search PubMed.
- C. De Marchis, T. S. Monteiro, C. Simon-Martinez, S. Conforto and A. Gharabaghi, J. Neuroeng. Rehabil., 2016, 13, 1–9 CrossRef.
- M. Perovic, M. Stevanovic, T. Jevtic, M. Strbac, G. Bijelic, C. Vucetic, L. Popovic-Maneski and D. Popovic, J. Autom. Control, 2013, 21, 13–18 CrossRef.
- S. Cheng and D. Zhang, Proc. 10th Int. Conf. on Human System Interactions (HSI), 2017, 120–124 Search PubMed.
- T. Štrbac, M. Belić, M. Isaković, M. Kojić, V. Bijelić, G. Popović and I. Keller, J. Neural Eng., 2016, 13, 046014 CrossRef.
- H. Zhou, Y. Lu, W. Chen, Z. Wu, H. Zou, L. Krundel and G. Li, Sensors, 2015, 15, 17241–17257 CrossRef.
- M. Rahimi, F. Jiang and Y. Shen, IEEE Access, 2019, 7, 169844–169852 Search PubMed.
- T. Keller and A. Kuhn, J. Autom. Control, 2008, 18, 35–45 CrossRef.
- F. Lu, C. Wang, R. Zhao, L. Du, Z. Fang, X. Guo and Z. Zhao, Biosensors, 2018, 8, 31 CrossRef.
- A. Withana, D. Groeger and J. Steimle, UIST 2018 - Proc. 31st Annu. ACM Symp. User Interface Softw. Technol., 2018, 365–378 CrossRef.
- M. Ying, A. P. Bonifas, N. Lu, Y. Su, R. Li, H. Cheng, A. Ameen, Y. Huang and J. A. Rogers, Nanotechnology, 2012, 23, 344004 CrossRef.
- J. Park, S. Choi, A. H. Janardhan, S. Y. Lee, S. Raut, J. Soares, K. Shin, S. Yang, C. Lee, K. W. Kang, H. R. Cho, S. J. Kim, P. Seo, W. Hyun, S. Jung, H. J. Lee, N. Lee, S. H. Choi, M. Sacks, N. Lu, M. E. Josephson, T. Hyeon, D. H. Kim and H. J. Hwang, Sci. Transl. Med., 2016, 8, 344ra86 CrossRef.
- C. Kim, H. J. Yang, T. H. Cho, B. S. Lee, T. M. Gwon, S. Shin, I. S. Kim, S. J. Kim and S. J. Hwang, Med. Biol. Eng. Comput., 2020, 58, 383–399 CrossRef.
- J. K. Fink, High Performance Polymers, Second Edition, Elsevier Inc., 2014, pp. 381–400 Search PubMed.
- J. Jeong, K. S. Min and S. J. Kim, Microelectron. Eng., 2019, 216, 111096 CrossRef CAS.
- J. Jeong, S. H. Bae, K. S. Min, J. M. Seo, H. Chung and S. J. Kim, IEEE Trans. Biomed. Eng., 2015, 62, 982–989 Search PubMed.
- N. Obidin, F. Tasnim and C. Dagdeviren, Adv. Mater., 2020, 32, 1–26 CrossRef.
- O. Akhavan, E. Ghaderi, S. A. Shirazian and R. Rahighi, Carbon N. Y., 2016, 97, 71–77 CrossRef CAS.
- J. Pfau, D. Ganatra, A. Weltin, G. Urban, J. Kieninger and T. Stieglitz, Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS, 2019, 3762–3765 CAS.
- H. Ouyang, Z. Liu, N. Li, B. Shi, Y. Zou, F. Xie, Y. Ma, Z. Li, H. Li, Q. Zheng, X. Qu, Y. Fan, Z. L. Wang, H. Zhang and Z. Li, Nat. Commun., 2019, 10, 1–10 CrossRef CAS.
- D. Y. Park, D. J. Joe, D. H. Kim, H. Park, J. H. Han, C. K. Jeong, H. Park, J. G. Park, B. Joung and K. J. Lee, Adv. Mater., 2017, 29, 1–9 Search PubMed.
- R. Guo, X. Wang, H. Chang, W. Yu, S. Liang, W. Rao and J. Liu, Adv. Eng. Mater., 2018, 20, 1–9 Search PubMed.
- S. Park, S. W. Heo, W. Lee, D. Inoue, Z. Jiang, K. Yu, H. Jinno, D. Hashizume, M. Sekino, T. Yokota, K. Fukuda, K. Tajima and T. Someya, Nature, 2018, 561, 516–521 CrossRef CAS.
- R. David and N. Miki, Proc. IEEE Int. Conf. Micro Electro Mech. Syst., 2018, 2018-Janua, 373–375 Search PubMed.
- J. Liu and L. Yi, Liquid Metal Enabled Skin Electronics, 2018, 255–323 CAS.
- J. Li, C. Guo, Z. Wang, K. Gao, X. Shi and J. Liu, Clin. Transl. Med., 2016, 5, 21 CrossRef.
- M. Leandri, L. Marinelli, A. Siri and L. Pellegrino, J. Neurosci. Methods, 2018, 293, 17–26 CrossRef.
- Y. Wei, K. Yang, M. Browne, L. Bostan and P. Worsley, IEEE Sensors Lett., 2019, 3, 2017–2020 Search PubMed.
- M. Tezuka, N. Kitamura and N. Miki, Jpn. J. Appl. Phys., 2016, 55, 6S1 Search PubMed.
- M. Tezuka, K. Ishimaru and N. Miki, Sens. Actuators, A, 2017, 258, 32–38 CrossRef CAS.
- C. V. Keef, L. V. Kayser, S. Tronboll, C. W. Carpenter, N. B. Root, M. Finn, T. F. O’Connor, S. N. Abuhamdieh, D. M. Davies, R. Runser, Y. S. Meng, V. S. Ramachandran and D. J. Lipomi, Adv. Intell. Syst., 2020, 2, 2000018 CrossRef.
- F. H. Parianen Lesemann, E. M. Reuter and B. Godde, Neurosci. Biobehav. Rev., 2015, 51, 126–137 CrossRef.
- C. Antfolk, M. D’alonzo, B. Rosén, G. Lundborg, F. Sebelius and C. Cipriani, Expert Rev. Med. Devices, 2013, 10, 45–54 CrossRef CAS.
- M. R. Mulvey, H. J. Fawkner, H. E. Radford and M. I. Johnson, Neuromodulation, 2012, 15, 42–47 CrossRef.
- E. D’Anna, G. Valle, A. Mazzoni, I. Strauss, F. Iberite, J. Patton, F. M. Petrini, S. Raspopovic, G. Granata, R. Di Iorio, M. Controzzi, C. Cipriani, T. Stieglitz, P. M. Rossini and S. Micera, Sci. Robot., 2019, 4, eaau8892 CrossRef.
- K. A. Kaczmarek, J. G. Webster, P. Bach-y-Rita and W. J. Tompkins, IEEE Trans. Biomed. Eng., 1991, 38, 1–16 CrossRef CAS.
- D. A. Lake, Sport. Med. An Int. J. Appl. Med. Sci. Sport Exerc., 1992, 13, 320–336 CAS.
- A. Popović-Bijelić, G. Bijelić, N. Jorgovanović, D. Bojanić, M. B. Popović and D. B. Popović, Artif. Organs, 2005, 29, 448–452 CrossRef.
- A. A. Guex, A. E. Hight, S. Narasimhan, N. Vachicouras, D. J. Lee, S. P. Lacour and M. C. Brown, Hear. Res., 2019, 377, 339–352 CrossRef.
- L. Guo and S. P. Deweerth, Proc. 31st Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. Eng. Futur. Biomed. EMBC 2009, 2009, 1623–1626 Search PubMed.
- K. W. Meacham, L. Guo, S. P. DeWeerth and S. Hochman, Front. Neuroeng., 2011, 4, 1–12 Search PubMed.
- I. R. Minev, P. Musienko, A. Hirsch, Q. Barraud, N. Wenger, E. M. Moraud, J. Gandar, M. Capogrosso, T. Milekovic, L. Asboth, R. F. Torres, N. Vachicouras, Q. Liu, N. Pavlova, S. Duis, A. Larmagnac, J. Vörös, S. Micera, Z. Suo, G. Courtine and S. P. Lacour, Science, 2015, 347, 159–163 CrossRef CAS.
- G. M. Graham, T. A. Thrasher and M. R. Popovic, IEEE Trans. Neural Syst. Rehabil. Eng, 2006, 14, 38–45 CrossRef.
- A. Akhtar, J. Sombeck, B. Boyce and T. Bretl, Sci. Robot., 2018, 3, eaap9770 CrossRef.
- P. Bach-y-Rita, M. E. Tyler and K. A. Kaczmarek, Int. J. Hum. Comput. Interact., 2003, 15, 285–295 CrossRef.
- H. Tang and D. J. Beebe, J. Microelectromechanical Syst., 2003, 12, 29–36 CrossRef.
- I. M. Koo, K. Jung, J. C. Koo, J. Do Nam, Y. K. Lee and H. R. Choi, IEEE Trans. Robot., 2008, 24, 549–558 Search PubMed.
- Y. Zhang, Y. Chen, B. Zhao, Y. Hu and Z. Li, Proc. IEEE Int. Conf. Micro Electro Mech. Syst., 2018, 415–418 Search PubMed.
- Y. Song, J. Min and W. Gao, ACS Nano, 2019, 13, 12280–12286 CrossRef CAS.
- J. Li, X. Liu, E. Tomaskovic-Crook, J. M. Crook and G. G. Wallace, Colloids Surf., B, 2019, 176, 87–95 CrossRef CAS.
- N. Akhtar, V. Singh, M. Yusuf, R. A. Khan and R. A. Khan, Biomed. Tech., 2020, 65, 243–272 CAS.
- J. Heikenfeld, IEEE Spectr, 2014, 51, 46–63 Search PubMed.
- D. Bruen, C. Delaney, L. Florea and D. Diamond, Sensors, 2017, 17, 1–21 CrossRef.
- A. J. Bandodkar, W. Jia, C. Yard, X. Wang, J. Ramirez and J. Wang, Anal. Chem., 2015, 87, 394–398 CrossRef CAS.
- J. Kim, J. R. Sempionatto, S. Imani, M. C. Hartel, A. Barfidokht, G. Tang, A. S. Campbell, P. P. Mercier and J. Wang, Adv. Sci., 2018, 5, 1800880 CrossRef.
- P. Nenzi, A. Denzi, K. Kholostov, R. Crescenzi, F. Apollonio, M. Liberti, P. Marracino, A. Ongaro, R. Cadossi and M. Balucani, Proc. - Electron. Components Technol. Conf., 2013, 486–493 CAS.
- W. E. Ford, W. Ren, P. F. Blackmore, K. H. Schoenbach and S. J. Beebe, Arch. Biochem. Biophys., 2010, 497, 82–89 CrossRef CAS.
- X. Sun, B. Yuan, W. Rao and J. Liu, Biomaterials, 2017, 146, 156–167 CrossRef CAS.
- K. Salisbury and C. Tarr, Am. Soc. Mech. Eng. Dyn. Syst. Control Div. DSC, 1997, 61, 61–67 Search PubMed.
- A. U. Alahakone and S. M. N. A. Senanayake, IEEE/ASME Int. Conf. Adv. Intell. Mechatronics, AIM, 2009, 1148–1153 Search PubMed.
- S. Jadhav, V. Kannanda, B. Kang, M. T. Tolley and J. P. Schulze, IST Int. Symp. Electron. Imaging Sci. Technol., 2017, 19–24 Search PubMed.
- X. Yu, Z. Xie, Y. Yu, J. Lee, A. Vazquez-Guardado, H. Luan, J. Ruban, X. Ning, A. Akhtar, D. Li, B. Ji, Y. Liu, R. Sun, J. Cao, Q. Huo, Y. Zhong, C. M. Lee, S. Y. Kim, P. Gutruf, C. Zhang, Y. Xue, Q. Guo, A. Chempakasseril, P. Tian, W. Lu, J. Y. Jeong, Y. J. Yu, J. Cornman, C. S. Tan, B. H. Kim, K. H. Lee, X. Feng, Y. Huang and J. A. Rogers, Nature, 2019, 575, 473–479 CrossRef CAS.
- A. Boem and G. M. Troiano, DIS 2019 - Proc. 2019 ACM Des. Interact. Syst. Conf., 2019, 885–906 Search PubMed.
- A. Haynes, M. F. Simons, T. Helps, Y. Nakamura and J. Rossiter, IEEE Robot. Autom. Lett., 2019, 4, 1641–1646 Search PubMed.
- M. Watanabe, H. Shirai and T. Hirai, J. Appl. Phys., 2002, 92, 4631–4637 CrossRef CAS.
- J. H. Youn, S. M. Jeong, G. Hwang, H. Kim, K. Hyeon, J. Park and K. U. Kyung, Appl. Sci., 2020, 10, 640 CrossRef CAS.
- S. Muthukumarana, D. S. Elvitigala, J. P. F. Cortes, D. J. C. Matthies and S. Nanayakkara, SIGGRAPH Asia 2019 XR, SA, 2019, 2019, 29–30 Search PubMed.
- Y. Kato, T. Sekitani, M. Takamiya, M. Doi, K. Asaka, T. Sakurai and T. Someya, IEEE Trans. Electron Devices, 2007, 54, 202–209 CAS.
- H. A. Sonar and J. Paik, Front. Robot. AI, 2016, 2, 1–11 Search PubMed.
- P. Morasso, M. Casadio, V. Sanguineti, V. Squeri, S. Member and E. Vergaro, 2007 Virtual Rehabilitation, 2007, 70–77 Search PubMed.
- S. Kim, P. Kim, C. Y. Park and S. B. Choi, Sens. Actuators, A, 2016, 239, 61–69 CrossRef CAS.
- C. Hatzfeld and R. Werthschützky, Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics), 2010, 6191 LNCS, 99–104 Search PubMed.
- M. Raitor, J. M. Walker, A. M. Okamura and H. Culbertson, Proc. - IEEE Int. Conf. Robot. Autom., 2017, 427–432 Search PubMed.
- N. Al-huda Hamdan, A. Wagner, S. Voelker, J. Steimle and J. Borchers, Conf. Hum. Factors Comput. Syst. - Proc., 2019, 488 Search PubMed.
- C. Larson, B. Peele, S. Li, S. Robinson, M. Totaro, L. Beccai, B. Mazzolai and R. Shepherd, Science, 2016, 351, 1071–1074 CrossRef CAS.
- K. Song, S. H. Kim, S. Jin, S. Kim, S. Lee, J. S. Kim, J. M. Park and Y. Cha, Sci. Rep., 2019, 9, 1–8 CrossRef.
- D. J. Lipomi, C. Dhong, C. W. Carpenter, N. B. Root and V. S. Ramachandran, Adv. Funct. Mater., 2020, 30, 1–15 CrossRef.
- P. Le Floch, N. Molinari, K. Nan, S. Zhang, B. Kozinsky, Z. Suo and J. Liu, Nano Lett., 2020, 20, 224–233 CrossRef CAS.
- H. Zhao, A. M. Hussain, A. Israr, D. M. Vogt, M. Duduta, D. R. Clarke and R. J. Wood, Soft Robot., 2020, 7, 451–461 CrossRef.
- S. Mun, S. Yun, S. Nam, S. K. Park, S. Park, B. J. Park, J. M. Lim and K. U. Kyung, IEEE Trans. Haptics, 2018, 11, 15–21 Search PubMed.
- H. Phung, P. T. Hoang, C. T. Nguyen, T. Dat Nguyen, H. Jung, U. Kim and H. R. Choi, IEEE Int. Conf. Intell. Robot. Syst., 2017, 2017, 886–891 Search PubMed.
- S. Yun, S. Park, B. Park, S. Ryu, S. M. Jeong and K. U. Kyung, IEEE Trans. Ind. Electron., 2020, 67, 717–724 Search PubMed.
- S. H. Yoon, J. D. Ma, L. Siyuan, T. Woo Suk, S. Shantanu, R. Di and F. P. Holbery, Proceedings of the 32nd Annual ACM Symposium on User Interface Software and Technology, 2019, pp. 949–961.
- C. Dagdeviren, Y. Shi, P. Joe, R. Ghaffari, G. Balooch, K. Usgaonkar, O. Gur, P. L. Tran, J. R. Crosby, M. Meyer, Y. Su, R. C. Webb, A. S. Tedesco, M. J. Slepian, Y. Huang and J. A. Rogers, Nat. Mater., 2015, 14, 728–736 CrossRef CAS.
- D. G. Seo and Y. H. Cho, J. Mech. Sci. Technol., 2018, 32, 631–636 CrossRef.
- J. Mohd Jani, M. Leary, A. Subic and M. A. Gibson, Mater. Des., 2014, 56, 1078–1113 CrossRef CAS.
- J. Mohd Jani, M. Leary and A. Subic, J. Intell. Mater. Syst. Struct, 2017, 28, 1699–1718 CrossRef.
- R. S. Kularatne, H. Kim, J. M. Boothby and T. H. Ware, J. Polym. Sci., Part B: Polym. Phys., 2017, 55, 395–411 CrossRef CAS.
- M. E. Prévôt, S. Ustunel and E. Hegmann, Materials, 2018, 11, 377 CrossRef.
- N. Torras, K. E. Zinoviev, C. J. Camargo, E. M. Campo, H. Campanella, J. Esteve, J. E. Marshall, E. M. Terentjev, M. Omastová, I. Krupa, P. Teplický, B. Mamojka, P. Bruns, B. Roeder, M. Vallribera, R. Malet, S. Zuffanelli, V. Soler, J. Roig, N. Walker, D. Wenn, F. Vossen and F. M. H. Crompvoets, Sens. Actuators, A, 2014, 208, 104–112 CrossRef CAS.
- C. Wang, K. Sim, J. Chen, H. Kim, Z. Rao, Y. Li, W. Chen, J. Song, R. Verduzco and C. Yu, Adv. Mater., 2018, 30, 1–9 Search PubMed.
- N. Torras, K. E. Zinoviev, J. Esteve and A. Sánchez-Ferrer, J. Mater. Chem. C, 2013, 1, 5183–5190 RSC.
- V. S. R. Jampani, R. H. Volpe, K. R. De Sousa, J. F. Machado, C. M. Yakacki and J. P. F. Lagerwall, Sci. Adv., 2019, 5, eaaw2476 CrossRef CAS.
- K. O. Johnson, Curr. Opin. Neurobiol., 2001, 11, 455–461 CrossRef CAS.
- E. Van Breda, S. Verwulgen, W. Saeys, K. Wuyts, T. Peeters and S. Truijen, BMJ Open Sport Exerc. Med, 2017, 3, 1–12 Search PubMed.
- A. Manuscript, J. Neurol., 2011, 34, 98–104 Search PubMed.
- C. Cipriani, M. Dalonzo and M. C. Carrozza, IEEE Trans. Biomed. Eng., 2012, 59, 400–408 Search PubMed.
- G. Frediani, D. Mazzei, D. E. De Rossi and F. Carpi, Front. Bioeng. Biotechnol., 2014, 2, 1–7 Search PubMed.
- H. Boys, G. Frediani, M. Ghilardi, S. Poslad, J. C. Busfield and F. Carpi, 2018 IEEE Int. Conf. Soft Robot. RoboSoft 2018, 2018, 270–275.
- M. Sarac, A. M. Okamura and M. Di Luca, 2019, arXiv preprint arXiv:1911.08528.
- G. Moy, U. Singh, E. Tan and R. S. Fearing, Haptics-e, 2000, 1, 1–20 Search PubMed.
- M. Pielot and R. De Oliveira, Proceed. 15th Int. Conf. on Human-computer interaction with mobile devices and services, 2013, pp. 1–10.
- S. H. Yun and S. J. J. Kwok, Nat. Biomed. Eng., 2017, 1, 1–16 CrossRef.
- G. H. Lee, H. Moon, H. Kim, G. H. Lee, W. Kwon, S. Yoo, D. Myung, S. H. Yun, Z. Bao and S. K. Hahn, Nat. Rev. Mater, 2020, 5, 149–165 CrossRef.
- M. Yasumoto, T. Shimoda, R. Tsubouchi and S. Kawana, Nishinihon J. Dermatology, 2009, 71, 201–205 CrossRef.
- X. Huang and M. A. El-Sayed, Alexandria J. Med., 2011, 47, 1–9 CrossRef.
- M. Hennessy and M. R. Hamblin, J. Opt., 2017, 19, 013003 CrossRef.
- S. Kellner and S. Berlin, Appl. Sci., 2020, 10, 805 CrossRef CAS.
- J. Hopkins, L. Travaglini, A. Lauto, T. Cramer, B. Fraboni, J. Seidel and D. Mawad, Adv. Mater. Technol., 2019, 4, 1–10 Search PubMed.
- V. K. Samineni, A. D. Mickle, J. Yoon, J. G. Grajales-Reyes, M. Y. Pullen, K. E. Crawford, K. N. Noh, G. B. Gereau, S. K. Vogt, H. H. Lai, J. A. Rogers and R. W. Gereau, Sci. Rep., 2017, 7, 1–14 CrossRef CAS.
- L. Via and S. Magno, Curr. Med. Chem., 2001, 8, 1405–1418 CrossRef.
- M. Wegener, M. J. Hansen, A. J. M. Driessen, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2017, 139, 17979–17986 CrossRef CAS.
- S. G. R. Bade, X. Shan, P. T. Hoang, J. Li, T. Geske, L. Cai, Q. Pei, C. Wang and Z. Yu, Adv. Mater., 2017, 29, 1–7 CrossRef.
- H. E. Lee, D. Lee, T. I. Lee, J. H. Shin, G. M. Choi, C. Kim, S. H. Lee, J. H. Lee, Y. H. Kim, S. M. Kang, S. H. Park, I. S. Kang, T. S. Kim, B. S. Bae and K. J. Lee, Nano Energy, 2019, 55, 454–462 CrossRef CAS.
- H. E. Lee, J. H. Shin, J. H. Park, S. H. Hong, S. K. Park, S. H. Lee, J. H. Lee, I.-S. Kang and K. J. Lee, Adv. Funct. Mater., 2019, 29, 1808075 CrossRef.
- J. Kim, G. A. Salvatore, H. Araki, A. M. Chiarelli, Z. Xie, A. Banks, X. Sheng, Y. Liu, J. W. Lee, K. I. Jang, S. Y. Heo, K. Cho, H. Luo, B. Zimmerman, J. Kim, L. Yan, X. Feng, S. Xu, M. Fabiani, G. Gratton, Y. Huang, U. Paik and J. A. Rogers, Sci. Adv., 2016, 2, e1600418 CrossRef.
- A. D. Mickle, S. M. Won, K. N. Noh, J. Yoon, K. W. Meacham, Y. Xue, L. A. McIlvried, B. A. Copits, V. K. Samineni, K. E. Crawford, D. H. Kim, P. Srivastava, B. H. Kim, S. Min, Y. Shiuan, Y. Yun, M. A. Payne, J. Zhang, H. Jang, Y. Li, H. H. Lai, Y. Huang, S. Il Park, R. W. Gereau and J. A. Rogers, Nature, 2019, 565, 361–365 CrossRef CAS.
- G. Shin, A. M. Gomez, R. Al-Hasani, Y. R. Jeong, J. Kim, Z. Xie, A. Banks, S. M. Lee, S. Y. Han, C. J. Yoo, J. L. Lee, S. H. Lee, J. Kurniawan, J. Tureb, Z. Guo, J. Yoon, S. Il Park, S. Y. Bang, Y. Nam, M. C. Walicki, V. K. Samineni, A. D. Mickle, K. Lee, S. Y. Heo, J. G. McCall, T. Pan, L. Wang, X. Feng, T. il Kim, J. K. Kim, Y. Li, Y. Huang, R. W. Gereau, J. S. Ha, M. R. Bruchas and J. A. Rogers, Neuron, 2017, 93, 509–521.e3 CrossRef CAS.
- K. Yamagishi, I. Kirino, I. Takahashi, H. Amano, S. Takeoka, Y. Morimoto and T. Fujie, Nat. Biomed. Eng., 2019, 3, 27–36 CrossRef CAS.
- S. Il Park, D. S. Brenner, G. Shin, C. D. Morgan, B. A. Copits, H. U. Chung, M. Y. Pullen, K. N. Noh, S. Davidson, S. J. Oh, J. Yoon, K. I. Jang, V. K. Samineni, M. Norman, J. G. Grajales-Reyes, S. K. Vogt, S. S. Sundaram, K. M. Wilson, J. S. Ha, R. Xu, T. Pan, T. Il Kim, Y. Huang, M. C. Montana, J. P. Golden, M. R. Bruchas, R. W. Gereau and J. A. Rogers, Nat. Biotechnol., 2015, 33, 1280–1286 CrossRef.
- Y. Jeon, H. R. Choi, K. C. Park and K. C. Choi, J. Soc. Inf. Disp., 2020, 28, 324–332 CrossRef CAS.
- D. Han, Y. Khan, J. Ting, S. M. King, N. Yaacobi-Gross, M. J. Humphries, C. J. Newsome and A. C. Arias, Adv. Mater., 2017, 29, 1606206 CrossRef.
- H. E. Lee, S. H. Lee, M. Jeong, J. H. Shin, Y. Ahn, D. Kim, S. H. Oh, S. H. Yun and K. J. Lee, ACS Nano, 2018, 12, 9587–9595 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |