 Open Access Article
Open Access ArticlePlanar hydrodynamic traps and buried channels for bead and cell trapping and releasing†
Clémentine
Lipp
 *,
Kevin
Uning
,
Jonathan
Cottet‡
*,
Kevin
Uning
,
Jonathan
Cottet‡
 ,
Daniel
Migliozzi
,
Daniel
Migliozzi
 ,
Arnaud
Bertsch
and
Philippe
Renaud
,
Arnaud
Bertsch
and
Philippe
Renaud
Laboratory of Microsystems LMIS4, Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland. E-mail: philippe.renaud@epfl.ch
First published on 14th September 2021
Abstract
We present a novel concept for the controlled trapping and releasing of beads and cells in a PDMS microfluidic channel without obstacles present around the particle or in the channel. The trapping principle relies on a two-level microfluidic configuration: a top main PDMS channel interconnected to a buried glass microchannel using round vias. As the fluidic resistances rule the way the liquid flows inside the channels, particles located in the streamlines passing inside the buried level are immobilized by the round via with a smaller diameter, leaving the object motionless in the upper PDMS channel. The particle is maintained by the difference of pressure established across its interface and acts as an infinite fluidic resistance, virtually cancelling the subsequent buried fluidic path. The pressure is controlled at the outlet of the buried path and three modes of operation of a trap are defined: idle, trapping and releasing. The pressure conditions for each mode are defined based on the hydraulic–electrical circuit equivalence. The trapping of polystyrene beads in a compact array of 522 parallel traps controlled by a single pressure was demonstrated with a trapping efficiency of 94%. Pressure conditions necessary to safely trap cells in holes of different diameters were determined and demonstrated in an array of 25 traps, establishing the design and operation rules for the use of planar hydrodynamic traps for biological assays.
Microfluidics is very popular for its ability to manipulate objects in the micron range at the single particle resolution.1 Beads and cells can be manipulated in microfluidic devices using different forces, such as dielectrophoresis,2,3 optics,4,5 acoustics,6,7 hydrodynamics8,9 or magnetics.10 Among these, hydrodynamic trapping is the most convenient way of handling a large number of particles with single object resolution.11 In particular, large arrays of parallel single cell hydrodynamic traps in microfluidic chips are popular as they reveal single cell behavior among a population when subjected to various stimuli or when brought into contact with another cell type.12–14 Similarly, manipulation of beads in microfluidic channels has applications in the biomedical field among others for performing ultrasensitive immunoassays on beads15 and capturing cells16 or extracellular vesicles.17 Hydrodynamic trapping consists in placing an obstacle smaller than the particle dimension in its flow path. The force maintaining the particle against the obstacle is derived from the shear stress coming from the leaks around the cell, and/or from the difference of pressure built at the interface of the trapped particle. One very popular type of hydrodynamic trap, here named the pachinko array, consists of dense arrays with obstacles placed so that the particles have a large probability of encountering one when flowed in the devices.8,18 Another popular hydrodynamic trapping principle, here named the serpentine array, relies on splitting a channel into two flow paths, with the lowest resistance path comprising a restriction. The first particle arriving in the channel will be dragged to the restriction, increasing its flow resistance. Once the first restriction path is blocked, the following particle follows the bypass channel and is trapped in the next restriction.19,20 Both types of hydrodynamic trapping principles are adapted to beads and cells and require the presence of the material around the trapped particle, and unless coupled with pneumatic soft valve actuation, they cannot be dynamically controlled.21–23 Massive immobilization of single cells can be achieved using single wells,24 micropore arrays sandwiched between two microfluidic PDMS channels,25 or micropore arrays combined with dielectrophoresis.26 However, these methods use non standard materials that are non compatible with further microfabrication processing and lack controllability in both design and operation.
We thus propose a hydrodynamic trapping principle that overcomes the problem of space congestion and its resulting low trap density intrinsic to the presented well-known hydrodynamic traps. This novel trapping principle relies on two superimposed levels of microchannels, connected by round traps smaller than the particle of interest. As the hydrodynamic flow resistance rules the way the liquid flows in the channels, particles lying in the streamlines passing inside the vias will be stopped due to their size and will generate an infinite fluidic resistance, virtually cancelling the lower hydrodynamic path. Once a particle is trapped, it is immobilized in the top channel without any material surrounding it, free for interactions with other particles or for biological assays.
These planar hydrodynamic traps can be dynamically switched between different modes of operation by simply tuning the pressure at the outlet of the microfluidic buried level, controlling the magnitude and direction of the flow inside the buried channel layer. We propose three modes of operations for the trapping and releasing of beads and cells with their respective design criteria. The process for the microfabrication of the buried level of microchannels and the micron-sized vias using standard materials and processes is presented, together with the bonding of a top channel made of a PDMS replica. The final chip is totally transparent and compatible with widely used inverted microscopes.
The trapping and releasing of beads and cells are then investigated theoretically and experimentally, and demonstrate good trapping efficiency in compact arrays composed of a large number of traps. To our knowledge, this is the first demonstration of the controlled planar trapping and releasing of beads and cells in microfluidic channels.
1 Experimental
1.1 Fabrication
The top layer channels are made by PDMS casting, a well documented method that is widely used for fabricating microfluidic devices. The microfluidic traps and lower (buried) fluidic channels are manufactured using another microfabrication process presented in 1994 by Kaplan et al.,27 in which channels are obtained by opening small access holes in a thin structural layer for the subsequent selective under-etching of the substrate. The access holes are then closed by the deposition of a layer, sealing the channel.The top layer fabrication, alignment and bonding were previously precisely described.28 Shortly, PDMS is molded on a DRIE-etched silicon substrate, cured and punched (steps 2). The PDMS is then aligned and permanently bonded to the bottom substrate using a mask aligner (MJB4, Süss) (step 3). The height of the PDMS channel was set to 30 μm. The inset of Fig. 1b shows an SEM picture of a trap fabricated using this process.
1.2 Materials
5 μm diameter polystyrene beads were purchased from Sigma-Aldrich and suspended in deionized water containing 0.1% TWEEN 20 (Sigma-Aldrich) at a concentration of 5 × 105 beads per ml.1.3 Cell culture and preparation
The semi-adherent cell line Colo205 (ATCC) was cultured in RPMI 1640 supplemented with 10% FBS and 1% penicillin–streptomycin at 37 °C in a 5% CO2 atmosphere. Cell staining was performed by incubating the adherent part of the cell population for 1 h in RPMI with 1 μM calcein AM (Calbiochem). The supernatant was removed and the cells were washed with PBS, detached using trypsin and centrifuged at 1200 rpm for 2 minutes. The cells were resuspended in PBS containing 1 mM EDTA and 1% BSA and passed through a 40 μm cell strainer for the experiment. All the reagents are from Gibco unless specified.The distribution in the cell diameter was measured using an image analysis method on 260 cells. The mean diameter was 16.9 μm with a standard deviation of 3.1 μm.
1.4 Experimental set-up
The PDMS chip was degassed in a desiccator for 30 minutes prior to the experiment to avoid formation of bubbles, and primed with Pierce™ protein-free (PBS) blocking buffer for 1 h to prevent proteins from adhering to the surfaces. The cells or beads were placed in a chromatography vial connected to the punched PDMS with 360 μm outer diameter tubing for tight sealing. Pressure was applied to the vial using Fluigent Flow-EZ pressure controllers. The chip was mounted on the stage of a Leica DMI3000 B inverted microscope and observed using a uEye (IDS) camera.2 Results
2.1 Principle of operation
Fig. 1b shows a brightfield view of a buried channel and trap connecting two PDMS channels. An orange filter is superimposed to the image and indicates the regions where the PDMS is bonded to the substrate. The control channel on the right is short and wide in order to minimize its fluidic resistance and is connected to the pressure control Pc, while beads or cells flow in the left PDMS channel from the top to the bottom under the effect of the inlet pressure Pin. The top inset shows an SEM picture of a trap, whose diameter is smaller than the dimension of the particle to be trapped. Pc is the control pressure, Pin is the inlet pressure, Qin is the flow upstream of the trap and Qbc is the flow inside the buried channel. The chip presented here can be operated in four different modes as presented in Fig. 2a.In order to model the behaviour of the chip, we assumed a non-compressible Newtonian fluid and used the Hagen–Poiseuille law ΔP = RhydQ,29 where ΔP is the difference of pressure across a channel with fluidic resistance Rhyd in which a flow Q is present, and used the electric–hydraulic circuit equivalence30 to model the behaviour of the chip. The equivalent circuit is shown in the bottom inset of Fig. 1b where Rin is the resistance of the PDMS channel upstream of the trap, Rout is the resistance of the PDMS channel downstream of the trap, Rh is the resistance of a trap, Rbc is the resistance of the buried channel, Pin is the inlet pressure and Pc is the control pressure. We used these values to determine the conditions to operate in each of the four modes that are illustrated in Fig. 2a:
 | (1) |
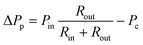 | (2) |
2.2 Characterization of single cell trapping
Although polystyrene beads are rigid and can withstand large pressures, special consideration must be taken when trapping cells with this novel kind of hydrodynamic traps. Indeed, cells are deformable bodies whose shapes are impacted by the difference of pressure built across them.31 When immobilizing cells in traps similar to the one presented in Fig. 1b, one must adapt the pressures and trap dimensions such that the cells stay intact.In this study, we consider the cell membrane integrity as a gauge of success, such that the cells can be used for a subsequent assay on the cell membrane on the chip. Cells were immobilized in traps of 3, 5, 7, and 9 μm in diameter using a chip with a single trap and control pressure channel, to find the critical ΔPp where the membrane fails. For the visualization of membrane failure, the cell cytosol was loaded with fluorescent calcein as it is a small molecule with no covalent binding to cell compounds that quickly diffuses in case of membrane rupture. To avoid fluorophore bleaching, the cells were imaged every minute up to 15 minutes. This time threshold was chosen by overestimating the maximum time the filling of an array could take.
ΔPp was controlled by setting Pc to a negative value and setting Pin to zero, thus stopping the flow in the main channel. The different outcomes that could be observed after 15 minutes are shown in Fig. 3b. Intact cells could either have a round appearance and no visible deformation, or have a protrusion visible in the buried channel. Membrane failure was identified as dye leaking in the buried channel, or as an engulfed cell that could be found inside the buried channel or in the control PDMS channel.
 | ||
| Fig. 3 (a) Graph representing the percentage of intact cells after 15 minutes for different trap diameters and different pressure gradients at the interface of the trapped cells ΔPp. The number of experiments is N ≥ 5 for each condition. (b) Fluorescence pictures of the different cell outcomes when trapped. (i) The cell is intact without any visible deformation. (ii) The cell is intact and a protrusion inside the buried channel is visible. (iii) The membrane is ruptured and the fluorescent dye diffuses in the buried channel. (iv) The cell passed through the trap and is found inside the buried channel or in the PDMS control channel. (c) The minimum pressure with less than 50% intact cells after 15 minutes of trapping as a function of the trap diameter is represented in red. The blue area corresponds to the fit of eqn (3) for a range of cell diameters of 13.8–20 μm. The inset shows the range of membrane tension at rupture extracted from the fit measured in this work compared to the range of vesicle membrane tension at rupture from the literature. | ||
The graph in Fig. 3a shows the percentage of intact cells after 15 minutes, as a function of the trap diameter and the pressure difference across the trapped cells ΔPp. A pressure threshold under which the cells did not suffer any membrane rupture or engulfment was found for each trap diameter. The minimum pressure with a percentage of intact cells lower than 50% is represented as a function of trap diameter in red in Fig. 3c, indicating that the cells resist higher pressures in smaller traps. Previous studies on vesicle and cell deformation in pipettes indicate that the tension τ of a spherical membrane of radius Rv can be determined via the difference of pressure ΔPp between the inside and outside of the pipette and the radius of curvature Rp in the pipette.32–34
 | (3) |
2.3 Hydrodynamic trapping of beads in large arrays
We demonstrate here the trapping of 5 μm polystyrene beads in an array composed of 522 parallel traps arranged in an equilateral triangle tiling pattern spaced by 9 μm. Fig. 4a shows a brightfield picture of an array of 522 traps used for this study, partially filled with beads and connected by a buried channel to a control PDMS channel on the right. The triangles visible on the membrane are the regions where no access holes or traps were designed to prevent under-etch and mechanically support the membrane. An orange filter is superimposed to the image where the PDMS is bonded to the substrate, and the inset shows an SEM picture of the 2 μm diameter traps. We made the choice of operating the chip at constant pressure in order to avoid the need to use complex feedback control systems. Supplementary file S1† shows a video of the filling of the arrays with 5 μm diameter beads at an inlet pressure of Pin = 40 mbar and a control pressure Pc = 0 mbar.
Fig. 4b shows the experimentally measured fraction of lost beads, defined as  , as a function of the percentage of filled traps for different pressures Pin and pressure ratios Pc/Pin, measured while filling the arrays. Using these measurements, the trapping efficiency, here defined as η = 1 − LF, at 98% filling was measured and was found to be constant for experiments with the same pressure ratio and different inlet pressures. The trapping efficiency at 98% filling percentage and its standard deviation for a pressure ratio Pc/Pin = 0.2 were respectively 70.4% and 1.1%, whereas for a pressure ratio of Pc/Pin = 0, the trapping efficiency and its standard deviation were 94.4% and 2.7%. By adapting the circuit in Fig. 2 and replacing the resistance of a single trap of resistance Rh by n traps in parallel of the total resistance Rh/n, the ratio of the inlet flow going in the buried channel is calculated as follows:
, as a function of the percentage of filled traps for different pressures Pin and pressure ratios Pc/Pin, measured while filling the arrays. Using these measurements, the trapping efficiency, here defined as η = 1 − LF, at 98% filling was measured and was found to be constant for experiments with the same pressure ratio and different inlet pressures. The trapping efficiency at 98% filling percentage and its standard deviation for a pressure ratio Pc/Pin = 0.2 were respectively 70.4% and 1.1%, whereas for a pressure ratio of Pc/Pin = 0, the trapping efficiency and its standard deviation were 94.4% and 2.7%. By adapting the circuit in Fig. 2 and replacing the resistance of a single trap of resistance Rh by n traps in parallel of the total resistance Rh/n, the ratio of the inlet flow going in the buried channel is calculated as follows:
 | (4) |
Furthermore, if the only criteria are fast and efficient filling, the resistances can be tailored for that application with a small inlet and buried channel resistances, Rin and Rbc, together with large outlet resistance Rout. This combination will increase the flow ratio Qbc/Qin, improve the trapping efficiency and reduce the filling time. In order to achieve 100% trapping efficiency, the downstream path should be of infinite resistance Rout = ∞. To that end, the downstream path could either not exist and be designed as a dead-end, which would remove the possibility of flowing subsequent analytes over the array and the releasing in a channel path different than the inlet, or be blocked with a valve that could be switched off once the array is totally filled.
98% of the beads could be released from the array and brought to the outlet channel by increasing the control pressure together with the inlet pressure.
2.4 Hydrodynamic trapping of cells in the arrays
The design used for the trapping of cells was modified to allow the passage of flowing cells around the immobilized ones, and the triangular tiling pattern forces the contact between the trapped and flowing cells. The distance between two traps was thus set to 50 μm. During the filling of an array of traps with cells, the already immobilized cells are subjected to a pressure gradient ΔPp that varies with 1/n, with n being the number of remaining free traps as follows: | (5) |
 . Once the array is totally filled, the control pressure Pc can be increased to minimize ΔPp while maintaining the trapped cells intact and flowing additional material on top of the trapped cells, as illustrated in supplementary file S3.†
. Once the array is totally filled, the control pressure Pc can be increased to minimize ΔPp while maintaining the trapped cells intact and flowing additional material on top of the trapped cells, as illustrated in supplementary file S3.†
Fig. 5 shows the fluorescence picture of the successful trapping of 24 Colo205 cells stained with calcein AM in a compact array of 25 traps. Supplementary file S2† shows the trapping of the Colo205 cells in the array using an inlet pressure of Pin = 40 mbar and a control pressure of Pc = 0 mbar. We chose traps with a diameter of 5 μm to allow working at reasonable pressures without compromising the successful filling of the array because of debris clogging the traps, which was the case with the 3 μm diameter traps. The inlet pressure was set to 40 mbar in a chip with Rin = Rout such that the maximum pressure the cells were subjected to was 20 mbar, below the threshold pressure corresponding to that of the trap diameter, while working at a pressure ratio of Pc/Pin = 0 for a maximum trapping efficiency. The trapping efficiency η was 88.4% at 92% filling percentage and η = 68.6% at 96% filling percentage. 88% of the cells could be released from the array and brought to the outlet channel by increasing the control pressure together with the inlet pressure.
3 Discussion
This novel type of hydrodynamic traps differs significantly from the typical traps found in the literature. It resolves the problem of space cluttering around the trapped object while allowing a dynamic control over the trapping state. We compare here different characteristics of the array of planar hydrodynamic traps with the two common types of hydrodynamic trapping arrays presented in the introduction in the case of particle trapping: the pachinko and the serpentine arrays.Scalability and density of traps
An interesting parameter to compare is the scalability of the array, or how many traps the system can handle. Each trap of the serpentine and pachinko trapping principles is independent of each other and relies on local flow lines. Once the geometry of one trap is optimized, they can theoretically be scaled indefinitely. Both types can be optimized for density,18,38 however the pachinko arrays are more dense and can accommodate more traps per unit surface because the serpentine arrays require the presence of a space-consuming bypass channel. In contrast, the planar hydrodynamic traps proposed here are interdependent when used in an array: eqn (4) applied to a single trap shows that the flow passing in each trap depends on the percentage of filled traps of an array. It was also shown that the traps close to the border opposite to the control pressure channel were more difficult to fill due to the additional resistance under the membrane. To scale up the planar traps and limit interdependency, the array should be divided into separate parts controlled by independent control channels and filled sequentially: while the first part of the array is being filled, the other parts can be operated in idle mode and so forth until all parts are filled. This comes to the cost of increasing the complexity of the control. However, the density of the traps can be higher than that for the other two mechanisms since there is no material around the trap and more particles can be observed in a defined field of view.Addressability and multiplexing
We suggested using independent control channels for different parts of an array to allow scalability, and this property can be exploited to multiplex and capture different species in the different parts of the channel. The possibility of running the chip in the idle mode and the possibility of multiplexing are also available using the serpentine array only when coupled with side pneumatic valve actuation.21Compliance with the particle size
A limitation of the serpentine and pachinko trapping methods comes from the fact that the single-particle trapping efficiency depends on the height of the channel. Although the height has to be larger than the size of the particle to avoid clogging for any type of trapping, a height larger than twice that of the particle causes multiple particles to stack in a single trap in the pachinko and serpentine arrays. In contrast, the height of the main channel is not limited in the case of coplanar traps, as proved by the trapping of beads of 5 μm diameter in a channel of 30 μm height.Trapping efficiency and filling percentage
Due to its deterministic trapping property, the serpentine trapping can reach 100% trapping efficiency and filling percentage. The pachinko array was shown to have a filling percentage up to >99%18 but no reported trapping efficiency was found for beads. In comparison, the planar hydrodynamic traps have both good trapping efficiency and filling percentage with respective values of 94% and 98%.Space availability and complexity of fabrication
The planar hydrodynamic traps have the advantage of having the space around the trapped particle completely available for the interaction with another particle, which can be manipulated with different forces, such as hydrodynamics, optical tweezers, dielectrophoresis, or others39 as the microfabrication process with standard materials and processes allows an additional layer to be easily added to fabricate microelectrodes aligned to the buried and PDMS channels. Supplementary file S3† shows the flow of subsequent cells around the ones trapped in the array, demonstrating the potential of this technology for studying in flow cell–cell interaction. This space could also be used to place electrodes for the impedance measurement of the particle for example. However, this possibility comes to the cost of an increased complexity of fabrication: while the serpentine and pachinko devices can be fabricated by a single PDMS casting step, the planar hydrodynamic traps require an extra photolithography step for the fabrication of the buried channels.4 Conclusions
We presented novel hydrodynamic traps that enable the trapping of beads and cells in flow with no material surrounding the trapped particles. The traps can be dynamically operated in different modes thanks to the presence of a pressure control channel and are able to trap and release particles, but also stand in an idle mode. We studied the resistance to pressure difference of cells trapped in holes of different diameters and fitted the result using a theoretical model from pipette assays to extract the tension at rupture of the cell membrane. We proposed a model to understand the properties of this type and showed that this technology could be used for the trapping and releasing of beads and cells in compact arrays, opening new possibilities in the field of biological assays. We believe that the presented fabrication method for multi-level microfluidic channels connected by vias compatible with electrode fabrication can open new possibilities in different fields of study such as microfluidic filtering, impedance measurements, on-chip patch clamping, flow focusing, microfluidic mixing and droplet microfluidics, among others.Author contributions
Conceptualization: C. L., J. C., A. B. and P. R.; Investigation: C. L. and K. U.; Data Curation: C. L., K. U. and D. M.; Writing original draft: C. L.; Editing: C. L., K. U., J. C., D. M., A. B. and P. R.; Supervision: A. B. and P. R.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
We would like to deeply thank Margaux Duchamp for her precious help in the laboratory and for the discussion. This work was possible thanks to the outstanding platform for micro and nanofabrication of EPFL: the Center for MicroNanoTechnology (CMi). This work was supported by the French Agence Nationale de la Recherche and the Swiss National Science Foundation through the CoDiCell project (contract “ANR-17-CE33-0009” and “No. 00021E_175592/1”, respectively).References
- P. Shinde, L. Mohan, A. Kumar, K. Dey, A. Maddi, A. Patananan, F.-G. Tseng, H.-Y. Chang, M. Nagai and T. Santra, Int. J. Mol. Sci., 2018, 19, 3143 CrossRef PubMed.
- N. Mittal, A. Rosenthal and J. Voldman, Lab Chip, 2007, 7, 1146 RSC.
- M. Kim, J. Lee, K. Nam, I. Park, M. Son, H. Ko, S. Lee, D. Yoon, W.-J. Chang and S. Lee, Sensors, 2017, 17, 2272 CrossRef PubMed.
- P. Polimeno, A. Magazzù, M. A. Iatì, F. Patti, R. Saija, C. D. Esposti Boschi, M. G. Donato, P. G. Gucciardi, P. H. Jones, G. Volpe and O. M. Maragò, J. Quant. Spectrosc. Radiat. Transfer, 2018, 218, 131–150 CrossRef CAS.
- X. Wang, S. Chen, M. Kong, Z. Wang, K. D. Costa, R. A. Li and D. Sun, Lab Chip, 2011, 11, 3656 RSC.
- D. J. Collins, B. Morahan, J. Garcia-Bustos, C. Doerig, M. Plebanski and A. Neild, Nat. Commun., 2015, 6, 8686 CrossRef CAS PubMed.
- X. Ding, S.-C. S. Lin, B. Kiraly, H. Yue, S. Li, I.-K. Chiang, J. Shi, S. J. Benkovic and T. J. Huang, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11105–11109 CrossRef CAS PubMed.
- D. Di Carlo, N. Aghdam and L. P. Lee, Anal. Chem., 2006, 78(14), 4925–4930 CrossRef CAS PubMed.
- M. Nagai, K. Oohara, K. Kato, T. Kawashima and T. Shibata, Biomed. Microdevices, 2015, 17, 41 CrossRef PubMed.
- N.-T. Huang, Y.-J. Hwong and R. L. Lai, Microfluid. Nanofluid., 2018, 22, 16 CrossRef.
- L. Pang, J. Ding, X.-X. Liu, H. Yuan, Y. Ge, J. Fan and S.-K. Fan, TrAC, Trends Anal. Chem., 2020, 129, 115940 CrossRef CAS.
- B. Dura, S. K. Dougan, M. Barisa, M. M. Hoehl, C. T. Lo, H. L. Ploegh and J. Voldman, Nat. Commun., 2015, 6, 5940 CrossRef CAS PubMed.
- B. Dura, M. M. Servos, R. M. Barry, H. L. Ploegh, S. K. Dougan and J. Voldman, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E3599–E3608 CrossRef CAS PubMed.
- M. Duchamp, T. Dahoun, C. Vaillier, M. Arnaud, S. Bobisse, G. Coukos, A. Harari and P. Renaud, RSC Adv., 2019, 9, 41066–41073 RSC.
- I. F. Pinto, R. R. G. Soares, M. E.-L. Mäkinen, V. Chotteau and A. Russom, ACS Sens., 2021, 6(3), 842–851 CrossRef CAS PubMed.
- A.-E. Saliba, L. Saias, E. Psychari, N. Minc, D. Simon, F.-C. Bidard, C. Mathiot, J.-Y. Pierga, V. Fraisier and J. E. A. Salamero, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14524–14529 CrossRef CAS PubMed.
- M. Tayebi, Y. Zhou, P. Tripathi, R. Chandramohanadas and Y. Ai, Anal. Chem., 2020, 92, 10733–10742 CrossRef CAS PubMed.
- X. Xu, P. Sarder, Z. Li and A. Nehorai, Biomicrofluidics, 2013, 7, 014112 CrossRef PubMed.
- W.-H. Tan and S. Takeuchi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 1146–1151 CrossRef CAS PubMed.
- J. Chung, Y.-J. Kim and E. Yoon, Appl. Phys. Lett., 2011, 98, 123701 CrossRef PubMed.
- H. Kim and J. Kim, Microfluid. Nanofluid., 2013, 16, 623–633 CrossRef.
- H. S. Kim, T. P. Devarenne and A. Han, Lab Chip, 2015, 15, 2467–2475 RSC.
- L. Pang, W. Liu, C. Tian, J. Xu, T. Li, S.-W. Chen and J. Wang, Lab Chip, 2016, 16, 4612–4620 RSC.
- C.-H. Lin, Y.-H. Hsiao, H.-C. Chang, C.-F. Yeh, C.-K. He, E. M. Salm, C. Chen, I.-M. Chiu and C.-H. Hsu, Lab Chip, 2015, 15, 2928–2938 RSC.
- Y. Tang, J. Shi, S. Li, L. Wang, Y. E. Cayre and Y. Chen, Sci. Rep., 2014, 4, 6052 CrossRef CAS PubMed.
- Y. Kimura, M. Gel, B. Techaumnat, H. Oana, H. Kotera and M. Washizu, Electrophoresis, 2011, 32, 2496–2501 CrossRef CAS PubMed.
- W. Kaplan, H. Elderstig and C. Veider, A novel fabrication method of capillary tubes on quartz for chemical analysis applications, Proceedings IEEE Micro Electro Mechanical Systems An Investigation of Micro Structures, Sensors, Actuators, Machines and Robotic Systems, 1994, pp. 63–68, DOI:10.1109/MEMSYS.1994.555599.
- J. Cottet, C. Vaillier, F. Buret, M. Frénéa-Robin and P. Renaud, Biomicrofluidics, 2017, 11, 064111 CrossRef CAS PubMed.
- H. Bruus, Theoretical microfluidics, Oxford Univ. Press, 2011 Search PubMed.
- K. W. Oh, K. Lee, B. Ahn and E. P. Furlani, Lab Chip, 2012, 12, 515–545 RSC.
- C. Lim, E. Zhou and S. Quek, J. Biomech., 2006, 39, 195–216 CrossRef CAS PubMed.
- R. Kwok and E. Evans, Biophys. J., 1981, 35, 637–652 CrossRef CAS PubMed.
- S. C. W. Tan, T. Yang, Y. Gong and K. Liao, J. Biomech., 2011, 44, 1361–1366 CrossRef PubMed.
- P. Sens and J. Plastino, J. Phys.: Condens. Matter, 2015, 27, 273103 CrossRef PubMed.
- S. U. Alam Shibly, C. Ghatak, M. A. Sayem Karal, M. Moniruzzaman and M. Yamazaki, Biophys. J., 2016, 111, 2190–2201 CrossRef CAS PubMed.
- A. Sohrabi Kashani and M. Packirisamy, Sci. Rep., 2019, 9, 5511 CrossRef PubMed.
- S. Kobel, A. Valero, J. Latt, P. Renaud and M. Lutolf, Lab Chip, 2010, 10, 857 RSC.
- D. Jin, B. Deng, J. X. Li, W. Cai, L. Tu, J. Chen, Q. Wu and W. H. Wang, Biomicrofluidics, 2015, 9, 014101 CrossRef CAS PubMed.
- M. Arnaud, M. Duchamp, S. Bobisse, P. Renaud, G. Coukos and A. Harari, Curr. Opin. Biotechnol., 2020, 65, 52–59 CrossRef CAS PubMed.
Footnotes |
| † Supplementary files are available externally at DOI: 10.5281/zenodo.5483561 |
| ‡ Present address: Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. |
| This journal is © The Royal Society of Chemistry 2021 |




