Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A simple and reversible glass–glass bonding method to construct a microfluidic device and its application for cell recovery†
Shun-ichi
Funano
,
Nobutoshi
Ota
 and
Yo
Tanaka
and
Yo
Tanaka
 *
*
Laboratory for Integrated biodevice, Center for Biosystems Dynamics Research, RIKEN, 1-3 Yamadaoka, Suita, Osaka 565-0871, Japan. E-mail: yo.tanaka@riken.jp
First published on 27th April 2021
Abstract
Compared with polymer microfluidic devices, glass microfluidic devices have advantages for diverse lab-on-a-chip applications due to their rigidity, optical transparency, thermal stability, and chemical/biological inertness. However, the bonding process to construct glass microfluidic devices usually involves treatment(s) like high temperature over 400 °C, oxygen plasma or piranha solution. Such processes require special skill, apparatus or harsh chemicals, and destroy molecules or cells in microchannels. Here, we present a simple method for glass–glass bonding to easily form microchannels. This method consists of two steps: placing water droplets on a glass substrate cleaned by neutral detergent, followed by fixing a cover glass plate on the glass substrate by binding clips for a few hours at room temperature. Surface analyses showed that the glass surface cleaned by neutral detergent had a higher ratio of SiOH over SiO than glass surfaces prepared by other cleaning steps. Thus, the suggested method could achieve stronger glass–glass bonding via dehydration condensation due to the higher density of SiOH. The pressure endurance reached over 600 kPa within 6 h of bonding, which is sufficient for practical microfluidic applications. Moreover, by exploiting the reversibility of this bonding method, cell recoveries after cultivating cells in a microchannel were demonstrated. This new bonding method can significantly improve both the productivity and the usability of glass microfluidic devices and extend the possibility of glass microfluidic applications in future.
Introduction
Lab-on-a-chip technology has generated substantial interest due to the reduced reagent consumption, space requirements and analysis times.1,2 Major substrate materials of microfluidic devices include silicon, glass, polydimethylsiloxane (PDMS), polymethylmethacrylate (PMMA), paper or 3D printed materials.3–7 Currently, PDMS is the most common material used in microfluidics because it is low cost and easy to fabricate.8 On the other hand, glass is a major substrate material for nanofluidic devices. Glass has excellent properties including suppression of deformation of channel walls by its hardness, superior optical transparency, thermal stability, chemical/biological inertness, and capability of diverse surface modification that allows smooth introduction of liquid.4,9 Furthermore, by exploiting the flexibility of ultra-thin glass to compensate the hardness of glass, glass-based valves10 and pumps11 have also been developed.Based on these glass properties and fundamental technologies, many unique applications such as detection by Raman microscopy,12 thermal lens microscopy,13 organic synthesis,14,15 gas analysis,16,17 cell patterning and analysis,18,19 electrophoretic separations,20 nanostructure-based molecule separations21,22 and cell analysis by ultra-fast flow imaging23 have been developed. These applications are difficult to realize with polymer-based microfluidic devices because polymers show lower performance of light transmission, chemical stability against organic solvents, retaining gas in microchannels, have lower values of Young's modulus which is a fundamental factor in cell scaffolding, and are less suitable for precise fabrication at microscale than glass does.
However, glass microfluidic devices have a major challenge in their glass bonding process. To bond glass components of a glass microfluidic device, thermal bonding method is classically employed which applies high temperature over 400 °C to the glasses overnight.24–26 This process typically ruins chemical or biochemical components on glass surface. Also, dangerous chemicals,27–29 including piranha solution for thorough cleaning, and special handling skill are necessary for glass bonding. Various low-temperature bonding methods have been developed to solve these issues in microfluidic fabrication.30–34 Another challenge of a glass microfluidic device is its repeated use because the bonding process is usually irreversible and inside of a glass device is difficult to maintain functional. For example, it is difficult to recover a clogged microchannel in a glass microfluidic device with permanent glass bonding. Compared with microfluidic devices made of other materials, repeated use with maintained functions of glass devices is highly desired because glass microfluidic devices are expensive and labour-intensive to produce. These challenges have prevented wide use of glass microfluidic devices.
Previously, we reported low temperature bonding method for glass microfluidic devices by using a machine to control pressure and temperature applied to glass components for bonding.35 Practical pressure resistance was obtained by the bonding below 100 °C at 450 N bonding force. However, it still required a dedicated apparatus and special chemicals including oxygen plasma, piranha solution, or hydrochloric acid. Also, the bonding operation is complicated and needs special skills. Such issues can be barriers to entry in this field and should be addressed to promote wide use of glass microfluidic devices. Other glass bonding techniques performed at low temperature have also been reported including the methods using two-step plasma treatment (O2 etching plasma irradiation followed by a nitrogen microwave radical activation),36 O2/CF4 plasma irradiation,37 vacuum ultraviolet (VUV) light irradiation,38 irradiation only by O2 plasma,39 treatment with hydrogen fluoride steam40 or hydrofluoric acid,41 pulse laser irradiation,42 or an intermediate gluing layer.43 Recently, glass bonding techniques for reuse have also been reported the methods with O2 plasma irradiation and heating at 110 °C (ref. 44) or O2 plasma irradiation and annealing for reinforcement at 150 °C.45 However, these methods require glass surface treatments immediately before glass bonding. These surface treatments cannot keep molecules or cells intact on glass surface. Overall, these methods are not simple and limit applications of glass microfluidics.
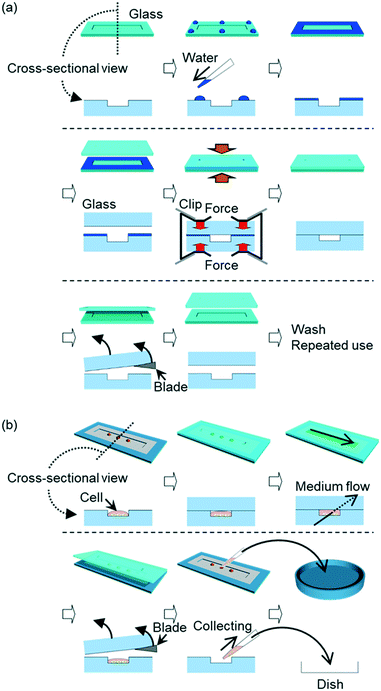
Here, we propose a new method of reversible glass–glass bonding, which is achieved by settling water droplets on a cleaned glass surface at room temperature, as shown in Fig. 1a. This bonding method increases utility of glass microfluidics and allows wider biological applications; the method enables (1) maintaining biological materials intact, (2) positioning these materials before forming microfluidic channels, (3) recovering materials from the channels, and (4) using the same glass components repeatedly (Fig. 1b). When two glass surfaces are clean and face to each other, the surfaces can be chemically bonded only by applying pressure for sufficient time. The chemical bond is produced by water dehydration between glass surfaces. By using neutral detergent for cleaning glass surfaces, this bonding method overcomes the difficulties derived from dangerous chemicals. Also, this method stabilizes glass–glass bonding achieved by binding clips instead of using special facilities like pressing apparatus. Moreover, the present method addresses skill requirement for glass bonding. This method employs wet glass bonding which uses water as a bonding agent. Although our wet bonding method requires longer bonding time than bonding methods for dry glass surfaces, this wet bonding method can be performed without special skills of manual handling for temporal glass bonding and fine alignment of glass components. In these perspectives, this method is beneficial for both fabrication and use of glass microfluidic devices.
The aim of this report is to establish a simple fabrication method of glass microfluidic devices with reversible glass bonding. Firstly, bonding chemical groups on the glass surfaces were investigated to find the influence of different steps for glass surface cleaning. Secondly, the pressure resistance was evaluated to determine whether this bonding method is appropriate for practical uses. Finally, cultivating cells of multiple types in single microchannel, followed by recovery of cells from the microchannel, was demonstrated as an application to show reversible glass bonding which is a unique characteristic of this method.
Experimental
Glass bonding
Borosilicate glass (TEMPAX Float, 30 × 70 × 0.7 mm, Matsunami Glass Industry, Osaka, Japan), soda-lime glass (S1215, Matsunami Glass Industry), and fused silica (VIOSIL-SQ, Shin-Etsu Chemical, Tokyo, Japan) were purchased. Flow channels and patterns of immobilized hydrophilic and hydrophobic areas were formed on glass plates by reported procedures (ESI†).35,46 Each glass plate, subjected to channel fabrication and surface treatment, was polished with melamine resin to remove dirt from the surface. After rinsing with water, the glass components were cleaned by 60 min sonication in 5% neutral detergent (Scat 20X-N, DKS, Kyoto, Japan) solution, followed by rinse by running water.Two bonding procedures were investigated (Fig. S1 (ESI†)). In one procedure, two wet glass components were attached, and fixed by binding clips (Kokuyo, Osaka, Japan) (hereinafter referred to whole-surface wet bonding). In the other procedure, two glass components were dried with blowing nitrogen gas, and 6 droplets of 2 μL water were placed on periphery of one glass component. Then, the two glass components were fixed by binding clips (hereinafter referred to water-droplet bonding). These fixed glass components were left at room temperature with humidity of approximately 40%.
Surface condition measurement after surface treatment
Five borosilicate glass plates were prepared for different surface conditions. One glass plate was prepared as an untreated glass surface. Four glass plates were polished by melamine resin, followed by sonication in water for 60 min. One of the four plates was kept for investigating glass surface rinsed by water only. The remaining glass plates were treated by either oxygen plasma (30 W, 1 min at oxygen flow rate of 8 mL min−1) in a compact etcher (FA-1, Samco, Kyoto, Japan), sonication in aqueous solution containing 5% neutral detergent for 60 min followed by water rinse, or piranha solution followed by water rinse. The surface conditions of the glass plates were investigated by sputtered neutral mass spectrometry (SNMS)47–49 in the following conditions. Primary ion beams of 0.3 μs pulse and 1 μm diameter accelerated by 30 kV were irradiated onto the surface of a glass sample at a repetition rate of 500 Hz to generate sample particles. The sample particles sputtered by the primary ion beam were ionized by femtosecond laser. The femtosecond laser with 100 μm focusing diameter produced 3.5 mJ per pulse of 800 nm, 50 fs width, and 1 kHz frequency. Ions produced by the femtosecond laser were accelerated by 5 kV to enter the mass analyser.Evaluation of bonding performance
The bonding area was evaluated by the following procedure. After detergent treatment or by water-only treatment, each pair of glass components was prepared by whole-surface wet bonding through pressing by binding clips or a press machine for glass–glass bonding. After a day, the bonded glass components were photographed by a camera. Each photograph of the bonded glasses was adjusted to a rectangular shape by perspective tool of GIMP 2 software (developed by The GIMP Team, https://www.gimp.org/) for evaluating the bonded area. The bonded area was measured by threshold function of ImageJ software (developed by Wayne Rasband, https://imagej.nih.gov/ij/).Bonding strength of glass components was evaluated by applying pressure to check the resistance of a glass microfluidic device against leakage described in a previous report.35 The glass components were treated by whole-surface wet bonding to have main channels and detour channels. The main channel with ports at both ends was filled with water. One port was blocked, and pressure was applied from the other port. The pressure was gradually increased with 10 kPa increment at every 30 s. When the pressure was high enough to break the glass bonding, leakage of water was observed in the detour channels, adjacent to the main channel, indicated by change in appearance of the detour channels from opaque to transparent. The pressure value immediately before leakage was defined as the value of pressure resistance. The upper limit of measurement was 600 kPa due to the performance of the employed air-compressor (PC4-10H, Yaezaki Kūatsu, Tokyo, Japan).
Detachment of bonded glass components and reversible bonding
The bonded glass components were detached in a simple manner. A razor blade was made wet by water and was applied to one corner of the bonded glass components to insert between the components. For investigating reversible bonding, the same bonding procedure, which was initially selected according to the channel design and application of a glass device, was employed repeatedly to bond the same set of detached glass components.Cell cultivation in a channel
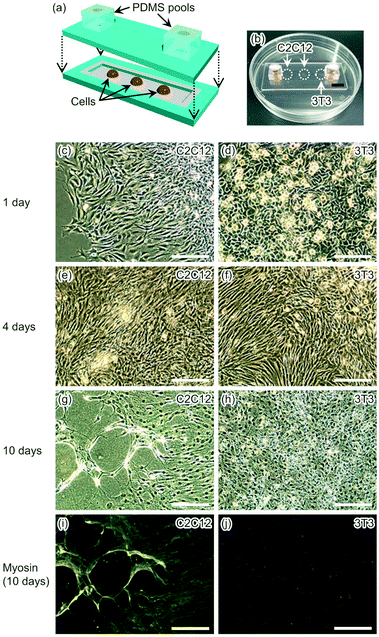
Glass components were modified to provide patterns of hydrophilic and hydrophobic areas (ESI†)35,46 prior to introduction of cells. For a glass component with a channel, periphery and inside of the channel except the cell adhesion areas were hydrophobic and the other areas were hydrophilic. For a glass component with two ports, periphery of the glass component was hydrophilic for glass–glass bonding. The other area was hydrophobic and aligned to be above the channel of the other glass component when the two components were bonded.The glass component with a channel was placed on a heater set at 37 °C. Pools made of PDMS (SILPOT 184, DuPont Toray Specialty Materials, Tokyo, Japan) were placed on the hydrophilic areas for cell adhesion on the glass channel. Ten μg mL−1 fibronectin (F1141, Sigma-Aldrich, MO, USA) dissolved in Dulbecco's phosphate buffer saline (PBS) (048-29805, Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) solution were introduced into the pools to coat the areas of cell adhesion. After 10 min of coating, the solution was sucked up with a pipette, and the coated areas were dried by air for 5 min. Cell suspensions of myoblast cell line C2C12 cells, fibroblast-like cell line 3T3 cells, or human cervix adenocarcinoma cell line HeLa cells were prepared to contain 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL in Dulbecco's modified Eagle's medium (DMEM) (D6429, Sigma-Aldrich, MO, USA) with 10% (v/v) fetal bovine serum (35-010-CV, Corning, NY, USA) and 1% (v/v) penicillin and streptomycin antibiotics (168-23191, Fujifilm Wako Pure Chemical Corporation). Each type of the suspensions was introduced into a PDMS pool independently. After 3 h of introducing cell suspensions, most of medium was sucked up with a pipette and small amount of medium was left on the cells. Then the PDMS pools were removed from the glass component.
000 cells per mL in Dulbecco's modified Eagle's medium (DMEM) (D6429, Sigma-Aldrich, MO, USA) with 10% (v/v) fetal bovine serum (35-010-CV, Corning, NY, USA) and 1% (v/v) penicillin and streptomycin antibiotics (168-23191, Fujifilm Wako Pure Chemical Corporation). Each type of the suspensions was introduced into a PDMS pool independently. After 3 h of introducing cell suspensions, most of medium was sucked up with a pipette and small amount of medium was left on the cells. Then the PDMS pools were removed from the glass component.
The glass component with the introduced cells was covered by a glass component with ports, via water-droplet bonding. After bonding, the binding clips were removed, and the PDMS pools were placed on the ports to supply culture medium. Medium was introduced into a channel, and the cultivation was conducted at 37 °C under humidified conditions with 5% CO2. The supernatant was changed with the fresh culture medium every day during the subsequent cultivation. For observing differentiation, medium was changed to DMEM with 2% (v/v) horse serum (H1138, Sigma-Aldrich, MO, USA) and 1% (v/v) penicillin and streptomycin antibiotics when one day passed from the beginning of cultivation.
Collecting cells from a channel and re-cultivation on culture dish
After cell cultivation, PDMS pools were removed from the ports, and the glass components were detached. Medium in a channel was sucked up with a pipette, and PBS droplets were placed on each cell adhesion area and sucked up to wash. Droplets of 0.05% (w/v) trypsin (208-17251, Fujifilm Wako Pure Chemical Corporation) in PBS were then placed on each cell adhesion area for cell removal. After 3 min, each cell suspension was transferred to centrifuge tubes containing 3 mL of the culture medium to spin down cells at 800 rpm for 5 min. Then, the supernatants were aspirated, and 3 mL of the medium were added to each centrifuge tube. After pipetting the medium in each centrifuge tube to re-suspend the collected cells, the whole amount of the new cell suspension was transferred to a culture dish. The cultivation was conducted at 37 °C under humidified conditions with 5% CO2. The supernatant was changed with the fresh culture medium every day during the subsequent cultivation.Optical detection and data processing
Optical and fluorescence images of cells were observed with an inverted microscope (IX71, Olympus, Tokyo, Japan) equipped with a charge-coupled device (CCD) camera (24-bit RGB colour) using cellSens software. Fluorescent images were collected by using the following optical filters: excitation/emission filter sets of 360–370 nm bandpass/420–460 nm bandpass, 460–495 nm bandpass/510 nm longpass, and 530–550 nm bandpass/575 nm longpass. Fluorescent images were merged by merge channels function of ImageJ software.Results and discussion
Bonding chemical groups on glass surface after cleaning
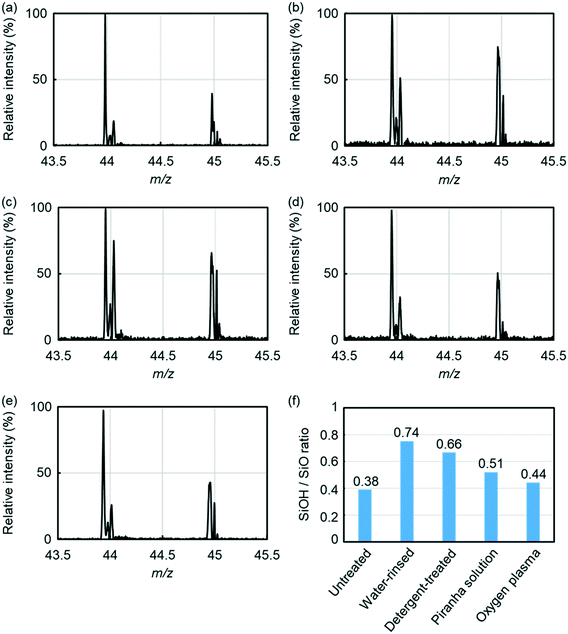
Conditions of borosilicate glass surfaces are varied by applied chemicals for surface cleaning. Based on the reported glass–glass bonding mechanism,50,51 two SiOH form an Si–O–Si covalent bond between two glass plates via dehydration while SiO does not form the covalent bond between glass plates. It means that larger density of SiOH, comparing with that of SiO, on a glass surface provides more bonding opportunities between two glass plates. Hence, larger ratio of SiOH/SiO is considered to form stronger glass–glass bonding.For evaluating chemical groups on a glass surface, various glass surfaces were investigated by SNMS.47–49 The measuring surfaces were prepared through glass cleaning by water only, neutral detergent solution followed by water rinse, oxygen plasma, or piranha solution followed by water rinse. Fig. 2 shows the results of SNMS measurements on SiOH and SiO groups in relative intensities to show typical mass spectra for each type of the prepared glass surfaces. These typical spectra were obtained after measurement conditions were determined by using the surface samples of the corresponding type. Also, mass spectral results expressed in absolute intensities are shown in Fig. S2 (ESI†). Mass spectral peaks at m/z of 44 are of SiO group and the peaks at m/z of 45 are of SiOH group. Based on the intensities of these peaks, SiOH/SiO values of glass surfaces were calculated. Among the measurements, the glass surface rinsed by water only showed the highest SiOH/SiO value (0.74) while untreated glass showed the lowest value (0.38). These values indicated hydration was important to form SiOH groups on a glass surface.
Other treatments provided SiOH/SiO values between the value of the untreated glass surface and the value of the glass surface rinsed by water only. These results indicated that chemicals in cleaning agents, other than hydrating chemicals like water, prohibited formation of SiOH group. Among these measurements, detergent-treated glass showed the SiOH/SiO value of 0.66, which was closest to the SiOH/SiO value of water-rinsed glass (0.74). This could occur because surfactant molecules were easily removed by water rinse prior to bonding.
Glass cleaned by piranha solution showed a relatively low value (0.51). This could be caused by sulfate ions, which adsorbed on glass surface through piranha treatment. Glass treated by oxygen plasma showed a lower value (0.44). This could be induced by oxidation of a glass surface in vacuum; a part of SiOH groups reacted with oxygen plasma, and some neighbouring SiOH groups spontaneously formed a Si–O–Si bonding to release a water molecule into dry atmosphere. In short, hydration with limited adsorbents was required to glass cleaning agents for glass–glass bonding.
To determine which treatment is most suitable for glass–glass wet bonding, however, investigating in surface chemical groups is insufficient. Indeed, glass–glass bonding of a microfluidic device is strongly influenced by presence of debris on surfaces of glass components.
Area of glass–glass bonding
Area of successful bonding between two glass components worked as an indicator for surface cleanness, or degree of removing debris, on glass surface. For this evaluation, glasses were bonded through various steps.The bonding area formed by whole-surface wet bonding was investigated. The area, where Newton rings were observed, was defined as not bonded. The bonded area was estimated by GIMP 2 software and ImageJ software and was compared with the total area of a glass microfluidic device. Area of glass–glass bonding through detergent cleaning showed similar degrees of successful bonding either by applying 96 N from 6 clips (16 N per clip, measured by a spring balance) to glasses (Fig. 3a, 98.1%) or by applying 450 N to glasses via a press machine (98.9%). In contrast, glasses rinsed by water only and clip-pressing showed smaller bonding area (Fig. 3b, 90.8%) than those prepared by detergent cleaning did. These results indicated that detergent cleaning could remove adsorbed chemicals and particles on a glass surface more than water rinse could. Thus, larger area of glass–glass bonding was achieved by the combination of detergent cleaning, which removed debris, and clip-pressing than the combination of rinse by water only and clip-pressing.
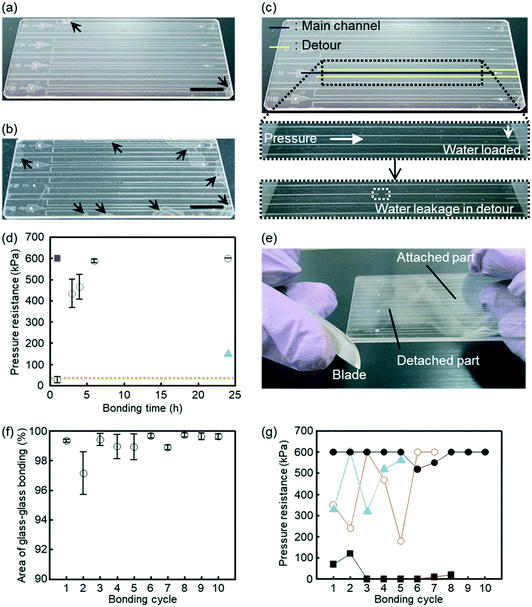 | ||
| Fig. 3 Glass microfluidic devices fabricated by the described wet bonding method. (a and b) Area of glass–glass bonding. Arrowheads indicate area that was not bonded. The black bars indicate 1 cm. (a) A photograph of a glass microfluidic device made by neutral detergent cleaning followed by water rinse. (b) A photograph of a glass microfluidic device made via glass rinse by water only. (c and d) Investigation of pressure resistance of glass–glass bonding. (c) A glass microfluidic device used for this investigation. The glass microfluidic device had main channels and detours. Water was loaded by applying pressure to the main channel. The channels appeared white if dry and transparent if wet. The pressure continued to be applied to the main channel, and the value of the pressure just before the water leaked into the detour was defined as the pressure resistance. (d) Pressure resistance of each bonding procedure. A glass microfluidic device made via (open circles) cleaning with neutral detergent, (red rectangle) fusion bonding, or (blue triangle) water rinse. The orange dashed line shows the pressure resistance of glass microfluidic devices made through the method reported by Yamashita et al.57 error bars indicate standard errors. The number of experiments were as follows. Cleaning with neutral detergent and bonding for 1 h: n = 8, 3 h: n = 7, 4 h: n = 5, 6 h: n = 10, and 24 h: n = 3. Fusion bonding: n = 1. Water rinse: n = 1. (e) Detachment of bonded glass components by a razor blade. (f) Bonding area of glass–glass components employed for 10 cycles of bonding/detaching procedures. Three sets of the same glass components were repeatedly used through the series of measurements. Each data point with a standard error is n = 3. (g) Pressure resistance of glass–glass bonding at various bonding periods and cycles. Prior to bonding, glass components were cleaned by neutral detergent. Periods of clip-pressed bonding were (black rectangles) 1 h, (open red circles) 3 h, (blue triangles) 4 h, and (black circles) 6 h. Each data point is n = 1. | ||
Pressure resistance of glass–glass bonding
The pressure resistance of glass–glass bonding was investigated. Fig. 3c and d show leaking resistance of glass microfluidic devices through different cleaning and/or bonding steps. Glass microfluidic devices produced by detergent wash with clip-pressing showed water leakage below 600 kPa when clip-pressing were performed for 4 h or less. When clip-pressing was performed for 6 h or more, the devices were stable against almost maximum pressure of our measuring system (600 kPa). This value was also attained by fusion bonding (630 °C for 1 h). Even though these values of bonding strength could not tell exact leaking pressure, 600 kPa were high enough to cover practical uses of microfluidic devices.52–55 In addition, 6 h of clip-pressing had advantages in low risk of damaging glasses by thermal expansion/contraction and total length of bonding period. Meanwhile, fusion bonding was overnight process, typically requiring more than 8 h for glass heating, bonding, and cooling in a furnace with risk of thermally damaging glasses.By comparing the glass devices produced by 24 h of clip-pressing, the devices produced by water rinse showed water leakage at 150 kPa, significantly weaker than that produced by detergent cleaning showed. Considering these outcomes with SiOH/SiO values and bonding area of corresponding conditions, rinsing by water only was concluded to provide highest SiOH density with insufficient removal of chemicals or particles adsorbed on glass surfaces. Since SNMS was performed before microfabrication processes, these processes appeared to provide adsorbents to lower bonding area and leaking resistance between two glass components. Hence, detergent cleaning was found to play an important role for glass–glass bonding by high SiOH generation as well as for removal of adsorbents. For further investigations, detergent cleaning and clip-pressing were employed.
Repeated use and bonding of the same glass microfluidic device
Capability of repeated use and reversible glass–glass bonding was validated. The same pair was washed by detergent solution, bonded by clip-pressing, and then detached by a wet razor blade (Fig. 3e). After each bonding, bonding area and pressure resistance were investigated.Bonding/detaching procedures were repeated for 10 times using 3 pairs of glass components, and the area of glass–glass bonding was evaluated at each bonding cycle (Fig. 3f). It was shown that the bonding area did not decrease significantly by repeating bonding/detaching cycles. Bonding/detaching procedures were also repeated for various clip-pressing periods (1, 3, 4, and 6 h) to measure pressure resistance to find the influence of multiple times of bonding/detaching operations on the same pair of glass components (Fig. 3g). In the case of 6 h clip-pressing, the measured values of pressure resistance were close to or above 600 kPa even after repeating the bonding/detaching cycles. In contrast, clip-pressing periods of 1, 3, and 4 h resulted in pressure resistance with large variation or significantly lower than 600 kPa.
It worth to note that, for the experiments of Fig. 3d and g except for fusion bonding, the same pair of glass components was used. For example, tenth cycle of the 6 h bonding was corresponded to thirty-fourth cycle of all bonding cycles, and this pair showed 600 kPa of pressure resistance. These results suggested that bonding/detaching cycles could be repeated for more than 34 times without major defects. Thus, for repeated use and reversible bonding, the present wet bonding method generated negligible changes or deficient on glass surfaces by detachment. It is also worth to note that this method saves time and cost of glass microfabrication.
Formation of a glass channel after placing liquid droplets
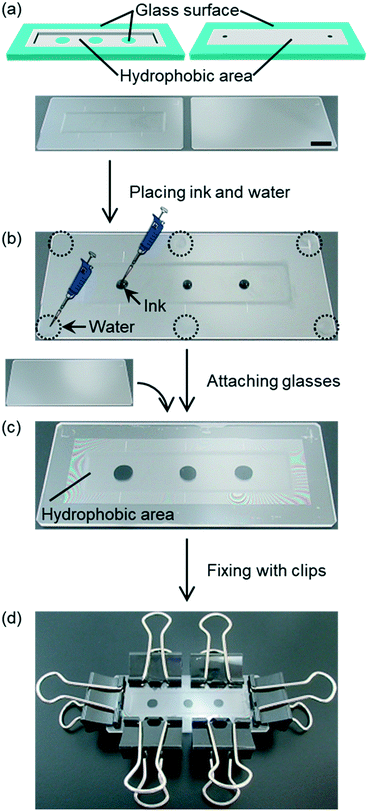
Low/room temperature bonding methods, including wet bonding methods, can be applicable for liquid-based samples due to their mild bonding conditions. However, when liquid-based samples such as cell suspensions are placed in a microchannel, these samples are easily spread by mixing with other liquids and/or are difficult to set at specific positions in a channel. Thus, liquid-based samples are not compatible with wet bonding procedures that apply liquid on whole surfaces of glass components. For example, glass bonding in water flow50 cannot keep molecules or cells at certain positions. This reported method needs water flow during the bonding process to maintain a clean glass surface. This water flow makes it difficult to maintain molecules or cells that are physically adsorbed or settled, not covalently bonded, on the surface of a microfluidic device. To avoid losing these molecules or cells significantly from a glass surface, it is necessary to use a glass bonding method without water flow.Contrarily, water-droplet bonding procedure does not need water flow for glass–glass bonding. Thus, this procedure is possible to maintain molecules or cells settled on the surface of a microfluidic device. With the hydrophobic patterning method that separates liquid-based samples from water droplets for bonding, this bonding procedure can keep dry area to place samples at designated positions without mixed to the droplets. In addition, glass–glass bonding via clip-pressing at room temperature allows placing samples before formation of channels in a glass microfluidic device. These features are advantageous because liquid-based samples are easily placed at specific positions on an open glass channel. These samples can be enclosed in a glass channel later through glass–glass bonding. Fig. 4a–d show the procedure of enclosing liquid droplets via glass bonding after detergent cleaning. Although the same procedure could be applied to different types of glasses, borosilicate glass was selected for further experiments because this type of glass is frequently employed for glass microfluidics. The post-bonding photographs of other glass types, soda-lime glass and fused silica treated by detergent cleaning, are shown in Fig. S3 (ESI†).
A pair of borosilicate glass components washed with detergent and rinsed with water was prepared (Fig. 4a), and black ink droplets were placed on a glass channel as samples (Fig. 4b). Then, 6 droplets of 2 μL water were placed on edges of a glass plate (Fig. 4b). Subsequently, the etched glass plate was attached with a plain glass plate (Fig. 4c). The ink was sandwiched between hydrophobic areas of two glass plates. The hydrophobic area was formed by a silane coupling agent containing fluoro-functional groups prior to placing the ink droplets. When the ink droplets were pressed by two glass plates, the droplets changed their shapes without spreading to hydrophobic area. Simultaneously, the water droplets spread within the hydrophilic area, and did not overflow into the hydrophobic area. These results indicate that this procedure is applicable even for enclosing liquid in a channel. At the end of the bonding procedure, glass components were fixed by 6 binding clips (Fig. 4d). Through this demonstration, multiple ink droplets were easily enclosed in a glass microchannel via water-droplet bonding and showed no mixing with water droplets for glass–glass bonding.
Cultivating multiple cell types in a glass channel
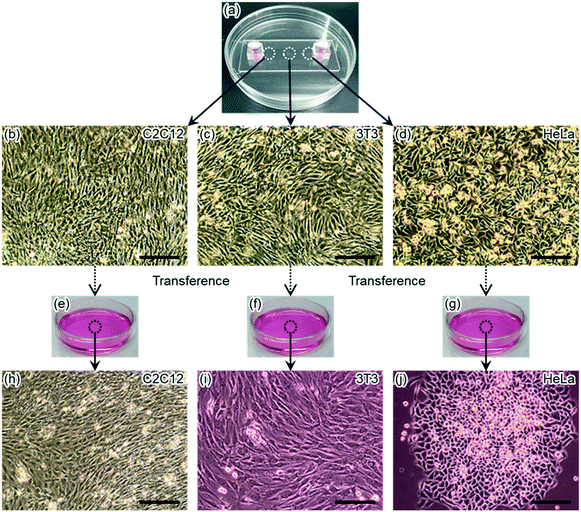
To show the usefulness of water-droplet bonding procedure performed at room temperature without harsh conditions or special equipment, cell packaging and cultivation in a glass microfluidic device were demonstrated. In addition, this procedure does not wash molecules or cells away from glass surfaces. Hence, water-droplet bonding is compatible to wider biological applications.For cell cultivation, aliquots of suspended cells were placed on an open glass microchannel. Then, the microchannel was confined by a glass plate when the glass microfluidic device was bonded by water placed on the edges of the glass device. With surface modification around the microchannel, water for bonding the glass components did not disturb cell suspension placed in the microchannel. Hence, the same cleaning and bonding steps were applied for cultivating multiple types of cells in a glass microfluidic device (Fig. 5 and S4 (ESI†)). In water-droplet bonding, cells were placed on an open glass microchannel, and then the channel was covered by another glass plate. All types of cells were observed to grow in the glass microfluidic device for 10 days. During 10 days of cell cultivation, myoblast showed myosin formation through differentiation from myoblast cells (C2C12 cells) into myotubes.56 Thus, it was shown that cells could be successfully cultivated in the microchannel for at least 10 days using this procedure. Four mL of culture medium were used in this period. Therefore, water-droplet bonding procedure took an advantage of microchannels, reduced consumption of precious cells and reagents. In addition, unlike a conventional microfluidic device, this procedure allowed cell manipulations at an open channel, like a conventional condition for cell manipulations on a culture dish.
Recovery of cells from a detached glass microfluidic device
In most of the cell cultivating applications in a microfluidic device, cells are not recovered. However, cell recovery is important in some cases. For example, cell recovery of vascular systems constructed in a microfluidic device is desired to analyse the cells furthermore for regenerative medicine.57 For such applications, microfluidic devices with reversible bonding would be useful. Therefore, we demonstrated the recovery of cells after cultivation.After 4 days of cell cultivation, the cells were collected from the glass microfluidic device, the same device used in the last section, by detaching the glass components. Upon detaching, the cultivating microchannel was opened to the atmosphere. Then, the cultured cells were collected from the open channel and were transferred to culture dishes. The transferred cells were found to keep growing for 10 days after transfer (Fig. 6). A procedure of collecting live cells from a microchannel has been reported using a separable microfluidic device.57 The upper and the bottom glass components of this device used a thick metal jig to fix the glass components only by physically sandwiching them. However, this bonding method has low leaking resistance and is difficult to culture multiple types of cells. On the other hand, water-droplet bonding procedure can be widely applied for collecting cells, particles and substances that are difficult to collect separately or tend to remain in microchannels by resisting liquid flow. This operation cannot be realized by devices with components bonded permanently, for example, a typical PDMS microfluidic device with its components bonded by plasma treatment.
The same pair of glass components was employed for the experiments that used cells in this and the last sections. Therefore, the same pair of the glass components were repeatedly used for two independent experiments. For repeated use of the glass components, the cleaning and bonding procedures were unchanged. In this experiment the cells did not show significantly different behaviour, including cell viability, from the cells cultured in the last experiment. These results indicated that repeated use of glass components had no negative effect on the cells clearly.
Conclusions
In this report, we have demonstrated a glass–glass bonding method using only neutral detergent for cleaning the surface before glass–glass bonding. Chemical analysis of the glass surface shows that the ratio of silanol group remarkably increased in this cleaning step, compared with other cleaning steps employing different cleaning chemicals. These results meant that the glass–glass bonding formed after cleaning by neutral detergent was stronger than the bonding formed after other cleaning steps. The pressure resistance could reach over 600 kPa within 6 h of bonding, sufficient pressure endurance for practical uses of microfluidic devices. This endurance was achieved by fixing glass components only with binding clips. Furthermore, reversible glass–glass bonding of the microfluidic devices was demonstrated by recovering cultured cells. These features are attractive in terms of preparation and use of glass microfluidic devices.This glass–glass bonding method does not use any dangerous chemicals, special equipment, or technically difficult operations. This method significantly improves both the productivity and the usability of glass microfluidic devices and extend the possibility of glass microfluidic applications in addition to the cell recovery system demonstrated in this study. For instance, with a miniaturized pump,58 an on-chip valve59 and a cell separator,60,61 the present microfluidic system can be utilized as a portable analysis system which can be constructed to confine analytes on-site. Such systems should be user-friendly and useful for high throughput on-site biomedical or environmental analysis.
Author contributions
S. F. and Y. T. conceived this study and provided materials and reagents. S. F. and N. O. performed the experiments. S. F. designed the experiments, organized, and visualized the data, and wrote the original draft. Y. T. acquired funding and supervised this study. All authors analysed the data, discussed the results, and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the JSPS Grant-in-Aid for Scientific Research (B) (No. 20H02596) and the RIKEN engineering network project, Japan. We also thank Asst. Prof. Yosuke Kawai and Dr. Yumi Miyake at Osaka University, Japan, for their technical advices and supports on sputtered neutral mass spectrometry.References
- N. Convery and N. Gadegaard, Micro and Nano Eng., 2019, 2, 76–91 CrossRef.
- D. T. Chiu, A. J. deMello, D. Di Carlo, P. S. Doyle, C. Hansen, R. M. Maceiczyk and R. C. R. Wootton, Chem, 2017, 2, 201–223 CAS.
- D. Mark, S. Haeberle, G. Roth, F. Von Stetten and R. Zengerle, Chem. Soc. Rev., 2010, 39, 1153–1182 RSC.
- P. N. Nge, C. I. Rogers and A. T. Woolley, Chem. Rev., 2013, 113, 2550–2583 CrossRef CAS PubMed.
- J. M. K. Ng, A. D. Stroock and G. M. Whitesides, Electrophoresis, 2002, 23, 3461–3473 CrossRef CAS PubMed.
- T. Ozer, C. McMahon and C. S. Henry, Annu. Rev. Anal. Chem., 2020, 13, 85–109 CrossRef PubMed.
- A. V. Nielsen, M. J. Beauchamp, G. P. Nordin and A. T. Woolley, Annu. Rev. Anal. Chem., 2020, 13, 45–65 CrossRef PubMed.
- J. B. Nielsen, R. L. Hanson, H. M. Almughamsi, C. Pang, T. R. Fish and A. T. Woolley, Anal. Chem., 2020, 92, 150–168 CrossRef CAS PubMed.
- K. Ren, J. Zhou and H. Wu, Acc. Chem. Res., 2013, 46, 2396–2406 CrossRef CAS PubMed.
- Y. Tanaka, RSC Adv., 2013, 3, 10213–10220 RSC.
- Y. Yalikun and Y. Tanaka, Micromachines, 2016, 7, 83 CrossRef PubMed.
- N. Ota, Y. Yonamine, T. Asai, Y. Yalikun, T. Ito, Y. Ozeki, Y. Hoshino and Y. Tanaka, Anal. Chem., 2019, 91, 9631–9639 CrossRef CAS PubMed.
- T. Kitamori, M. Tokeshi, A. Hibara and K. Sato, Anal. Chem., 2004, 76, 52A–60A CrossRef CAS.
- H. Hisamoto, Y. Shimizu, K. Uchiyama, M. Tokeshi, Y. Kikutani, A. Hibara and T. Kitamori, Anal. Chem., 2003, 75, 350–354 CrossRef CAS PubMed.
- H. Hisamoto, T. Saito, M. Tokeshi, A. Hibara and T. Kitamori, Chem. Commun., 2001, 2662–2663 RSC.
- S. Hiki, K. Mawatari, A. Aota, M. Saito and T. Kitamori, Anal. Chem., 2011, 83, 5017–5022 CrossRef CAS PubMed.
- M. Goto, K. Sato, A. Murakami, M. Tokeshi and T. Kitamori, Anal. Chem., 2005, 77, 2125–2131 CrossRef CAS PubMed.
- K. Jang, K. Sato, Y. Tanaka, Y. Xu, M. Sato, T. Nakajima, K. Mawatari, T. Konno, K. Ishihara and T. Kitamori, Lab Chip, 2010, 10, 1937–1945 RSC.
- K. Sugioka and Y. Cheng, Lab Chip, 2012, 12, 3576–3589 RSC.
- F. Shang, E. Guihen and J. D. Glennon, Electrophoresis, 2012, 33, 105–116 CrossRef CAS PubMed.
- T. Yasui, N. Kaji, M. R. Mohamadi, Y. Okamoto, M. Tokeshi, Y. Horiike and Y. Baba, ACS Nano, 2011, 5, 7775–7780 CrossRef CAS PubMed.
- N. Kaji, Y. Okamoto, M. Tokeshi and Y. Baba, Chem. Soc. Rev., 2010, 39, 948–956 RSC.
- Y. Yalikun, N. Ota, B. Guo, T. Tang, Y. Zhou, C. Lei, H. Kobayashi, Y. Hosokawa, M. Li, H. E. Muñoz, D. Di Carlo, K. Goda and Y. Tanaka, Cytometry, Part A, 2020, 97, 909–920 CrossRef PubMed.
- C.-H. Lin, G.-B. Lee, Y.-H. Lin and G.-L. Chang, J. Micromech. Microeng., 2001, 11, 726–732 CrossRef CAS.
- A. Daridon, V. Fascio, J. Lichtenberg, R. Wütrich, H. Langen, E. Verpoorte and N. F. de Rooij, Fresenius' J. Anal. Chem., 2001, 371, 261–269 CrossRef CAS PubMed.
- Y. Akiyama, K. Morishima, A. Kogi, Y. Kikutani, M. Tokeshi and T. Kitamori, Electrophoresis, 2007, 28, 994–1001 CrossRef CAS PubMed.
- T. Ito, K. Sobue and S. Ohya, Sens. Actuators, B, 2002, 81, 187–195 CrossRef CAS.
- T. Mayer, A. N. Marianov and D. W. Inglis, Mater. Res. Express, 2018, 5, 085201 CrossRef.
- I. K. Shaik, L. Zhang, S. Pradhan, A. K. Kalkan, C. P. Aichele and P. K. Bikkina, J. Pet. Sci. Eng., 2021, 198, 108231 CrossRef CAS.
- D. R. Reyes, D. Iossifidis, P.-A. Auroux and A. Manz, Anal. Chem., 2002, 74, 2623–2636 CrossRef CAS PubMed.
- T. Vilkner, D. Janasek and A. Manz, Anal. Chem., 2004, 76, 3373–3386 CrossRef CAS PubMed.
- P. S. Dittrich, K. Tachikawa and A. Manz, Anal. Chem., 2006, 78, 3887–3907 CrossRef CAS PubMed.
- J. West, M. Becker, S. Tombrink and A. Manz, Anal. Chem., 2008, 80, 4403–4419 CrossRef CAS PubMed.
- A. Arora, G. Simone, G. B. Salieb-Beugelaar, J. T. Kim and A. Manz, Anal. Chem., 2010, 82, 4830–4847 CrossRef CAS PubMed.
- S.-i. Funano, N. Ota, A. Sato and Y. Tanaka, Chem. Commun., 2017, 53, 11193–11196 RSC.
- Y. Xu, C. Wang, Y. Dong, L. Li, K. Jang, K. Mawatari, T. Suga and T. Kitamori, Anal. Bioanal. Chem., 2012, 402, 1011–1018 CrossRef CAS PubMed.
- Y. Xu, C. Wang, L. Li, N. Matsumoto, K. Jang, Y. Dong, K. Mawatari, T. Suga and T. Kitamori, Lab Chip, 2013, 13, 1048–1052 RSC.
- K. Shirai, K. Mawatari and T. Kitamori, Small, 2014, 10, 1514–1522 CrossRef CAS PubMed.
- K. Shoda, M. Tanaka, K. Mino and Y. Kazoe, Micromachines, 2020, 11, 804 CrossRef PubMed.
- L. Chen, G. Luo, K. Liu, J. Ma, B. Yao, Y. Yan and Y. Wang, Sens. Actuators, B, 2006, 119, 335–344 CrossRef CAS.
- L. Chen, H. Nishimura, K. Fukumi, J. Nishii and K. Hirao, Appl. Surf. Sci., 2007, 253, 4906–4910 CrossRef CAS.
- K. Cvecek, S. Dehmel, I. Miyamoto and M. Schmidt, Int. J. Extreme Manuf., 2019, 1, 042001 CrossRef CAS.
- J. Bart, R. Tiggelaar, M. Yang, S. Schlautmann, H. Zuilhof and H. Gardeniers, Lab Chip, 2009, 9, 3481–3488 RSC.
- R. Ohta, K. Mawatari, T. Takeuchi, K. Morikawa and T. Kitamori, Biomicrofluidics, 2019, 13, 024104 CrossRef PubMed.
- C. Wang, H. Fang, S. Zhou, X. Qi, F. Niu, W. Zhang, Y. Tian and T. Suga, J. Mater. Sci. Technol., 2020, 46, 156–167 CrossRef.
- S.-i. Funano, N. Tanaka and Y. Tanaka, RSC Adv., 2016, 6, 96306–96313 RSC.
- Y. Higashi, Spectrochim. Acta, Part B, 1999, 54, 109–122 CrossRef.
- Y. Kawai, T. Hondo, J.-L. Lehmann, K. Terada and M. Toyoda, Nucl. Instrum. Methods Phys. Res., Sect. A, 2019, 942, 162427 CrossRef CAS.
- K. Ninomiya, T. Kudo, P. Strasser, K. Terada, Y. Kawai, M. Tampo, Y. Miyake, A. Shinohara and K. M. Kubo, J. Radioanal. Nucl. Chem., 2019, 320, 801–805 CrossRef CAS.
- Z.-J. Jia, Q. Fang and Z.-L. Fang, Anal. Chem., 2004, 76, 5597–5602 CrossRef CAS PubMed.
- H. Y. Wang, R. S. Foote, S. C. Jacobson, J. H. Schneibel and J. M. Ramsey, Sens. Actuators, B, 1997, 45, 199–207 CrossRef CAS.
- F. H. J. van der Heyden, D. J. Bonthuis, D. Stein, C. Meyer and C. Dekker, Nano Lett., 2007, 7, 1022–1025 CrossRef CAS PubMed.
- K. Morikawa, K. Mawatari, M. Kato, T. Tsukahara and T. Kitamori, Lab Chip, 2010, 10, 871–875 RSC.
- K. Yamamoto, K. Morikawa, H. Imanaka, K. Koreyoshi and T. Kitamori, Analyst, 2020, 145, 5801–5807 RSC.
- Y. Tsuyama, K. Morikawa and K. Mawatari, J. Chromatogr. A, 2020, 1624, 461265 CrossRef CAS PubMed.
- Datasheet, https://www.phe-culturecollections.org.uk/products/celllines/generalcell/detail.jsp?refId=91031101&collection=ecacc_gc Search PubMed.
- T. Yamashita, Y. Tanaka, N. Idota, K. Sato, K. Mawatari and T. Kitamori, Biomaterials, 2011, 32, 2459–2465 CrossRef CAS PubMed.
- N. Sasaki, M. Shinjo, S. Hirakawa, M. Nishinaka, Y. Tanaka, K. Mawatari, T. Kitamori and K. Sato, Electrophoresis, 2012, 33, 1729–1735 CrossRef CAS PubMed.
- Y. Tanaka, T. Fujikawa, Y. Kazoe and T. Kitamori, Sens. Actuators, B, 2013, 184, 163–169 CrossRef CAS.
- Y. Zhang and X. Chen, J. Braz. Soc. Mech. Sci. Eng., 2020, 42, 89 CrossRef CAS.
- Y. Zhang and X. Chen, J. Braz. Soc. Mech. Sci. Eng., 2020, 42, 206 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1lc00058f |
| This journal is © The Royal Society of Chemistry 2021 |