Rapid boron isotope and concentration measurements of silicate geological reference materials dissolved through sodium peroxide sintering†
Abstract
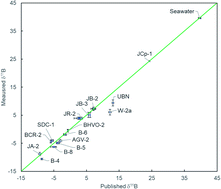
Understanding the movement of fluids in the solid Earth system is crucial for answering a wide range of important questions in Earth science. Boron (B) is a perfect tracer for geofluids because of its high solubility and large isotopic fractionation that depends on both temperature and alkalinity. However, the high volatility of boron in acidic solutions at moderate temperatures presents a significant challenge for accurate measurements of the boron concentration and boron isotopic ratios for silicate rock samples. To circumvent this problem, most laboratories use low-temperature dissolution methods that involve concentrated hydrofluoric acid with or without mannitol. However, hydrofluoric acid is highly hazardous and the controlled temperature condition may be difficult to monitor. As a result, relatively few silicate samples have been analyzed for high precision B concentration and isotopic composition measurements, which hinders our understanding of the behavior of B in the solid earth system and the utility of this powerful tracer. Here we report B concentrations and isotopic compositions of the most commonly used geological reference standards dissolved through sodium peroxide sintering and purified using a rapid single-column exchange chromatographic procedure. This streamlined method effectively removes Na and Si from the sample matrix and generates accurate B concentration and isotopic data in as little as a day without the need for expensive lab equipment and reagents. Sintering is already routinely used to dissolve zircon-bearing silicate samples as it ensures complete dissolution. Besides the analysis of boron, other elemental and isotopic analyses can be performed using aliquots of the same dissolution, which greatly speeds up the chemical processing time and reduces uncertainties associated with sample heterogeneity. Using this method, large amounts of material can be processed for ion-exchange chromatography without the need of splitting each sample into separate beakers for dissolution as is often required for the HF + mannitol dissolution method. This new method can rapidly expand the available dataset of the boron concentration and boron isotopes of silicate materials which will certainly advance our understanding of many geologic problems involving fluids.


 Please wait while we load your content...
Please wait while we load your content...
