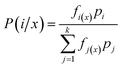Open Access Article
Open Access ArticleClassification of minerals and the assessment of lithium and beryllium content in granitoid rocks by laser-induced breakdown spectroscopy
Patrick
Janovszky
ab,
Krisztián
Jancsek
c,
Dávid J.
Palásti
ab,
Judit
Kopniczky
d,
Béla
Hopp
 bd,
Tivadar
M. Tóth
bd,
Tivadar
M. Tóth
 c and
Gábor
Galbács
c and
Gábor
Galbács
 *ab
*ab
aDepartment of Inorganic and Analytical Chemistry, University of Szeged, Dóm Square 7, 6720 Szeged, Hungary. E-mail: galbx@chem.u-szeged.hu
bDepartment of Materials Science, Interdisciplinary Excellence Centre, University of Szeged, Dugonics Square 13, 6720 Szeged, Hungary
cDepartment of Mineralogy, Geochemistry and Petrology, University of Szeged, Egyetem Street 2, 6720 Szeged, Hungary
dDepartment of Optics and Quantum Electronics, University of Szeged, Dóm Square 9, 6720 Szeged, Hungary
First published on 19th March 2021
Abstract
This study demonstrates that LIBS mapping and spatially resolved local analysis is an efficient and practical approach for the classification of mineral grains (quartz, feldspar, biotite, amphibole) and for prospecting of technologically relevant, low-Z elements (e.g. Be and Li) in granitoid rock samples. We tested three statistical approaches (classification tree (CT) based on indicator elements, linear discriminant analysis (LDA) and random forest (RF)) for the classification of the mineral grains and found that each of the three methods provides fairly similar, very good classification accuracies. RF and LDA provided better than 92% accuracy for all minerals, whereas CT showed a somewhat poorer (around 80%) accuracy for quartz in particular. Our results also demonstrate that using multiple analytical locations within each grain and resting the classification on the majority vote of these individual analysis gives more reliable discrimination (grain-based accuracy is better than location-based accuracy). We also demonstrated that LIBS elemental mapping can provide valuable information about the distribution of chemical elements among the minerals, especially if it is combined with matrix-matched calibration of emission intensity data. We illustrated this by the successful assessment of ng to μg amounts of Be and Li in the studied mineral grains. Our results suggest that mining for Be and Li in granitoid rocks should be aiming for biotite and amphibole grains.
1. Introduction
As it is known, LIBS is a versatile, laser ablation-based atomic emission spectroscopy technique, which allows the fast and direct analysis of solid and liquid (even gaseous) samples with minimum sample preparation, in a non-contact, marginally destructive way.1–3 Its routine configuration already makes trace (ppm level) elemental analysis possible for all elements of the periodic table, but more sophisticated laboratory setups (e.g. in double- or multi-pulse configurations) can even provide ppb-range detection limits.1,4 Time-resolved analysis of the plasma emission also permits isotope-selectivity.5 There is ample demonstration in the literature that the feature-rich LIBS spectra can be successfully used for the accurate identification or discrimination of a variety of samples (chemical fingerprinting).6–11 Micrometer-resolution local analysis or elemental mapping can also be done on solid samples, allowing for material science, medical, environmental or industrial applications,1–3,9,12–14 even in the field.One of the most appealing characteristics of LIBS is the possibility of direct solid sample analysis, which makes it of interest also to geologists and mineralogists. In this context, the analytical performance and package of attributes of LIBS have often been compared to those of other spatially resolving solid sampling atomic spectroscopy techniques. Although it is not a perfect analytical technique, but LIBS does offer a uniquely advantageous combination of features. For example, laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS), which has several decades of success in geology, has a similar overall analytical potential, but is not portable, cannot be used in a stand-off configuration, struggles with the detection of some lighter elements and has challenges in the quantitative analysis due to issues related to the need for the transportation of ablated sample matter. Micro X-ray fluorescence spectrometry (μ-XRF) is a popular desktop instrument in geochemical elemental analysis, but in contrast to LIBS it cannot sensitively detect low-Z elements (below Na), it has a narrower dynamic range, its spectra contain far less chemical information and it cannot be used remotely. Electron-probe micro analyzers (EPMA, similar to SEM-EDS or SEM-WDS) can provide very high spatial resolution and an elemental map within a very short time, but require tedious sample preparation and vacuum-ready samples and are very bulky and costly instruments. In addition they are not capable for measuring light elements or in the field or stand-off, and have quite limited accuracy and dynamic range.1,15–17
Based on the above-discussed aspects, it is no wonder that LIBS is being increasingly explored by geologists and the mining and mineral processing industry in the last 10–15 years and is now more and more used for the analysis of geological material (GEOLIBS).15,16,18 A further, highly related, although more specialized application is planetary exploration using the LIBS instrumentation in the ChemCam system of the Curiosity Mars rover.9,19,20
LIBS geochemical analysis is generally directed towards one or the other of two primary and related goals: quantitative analysis of the elemental contents of rocks/minerals (e.g. ore prospecting) and identification of the minerals (e.g. mapping of geochemical and mineralogical footprints, provenance analysis). For example, LIBS measurements with field-applicable bespoke or laboratory-based instrumentation were successfully demonstrated for the compositional analysis of silicate and carbonate minerals,21,22 iron and phosphate ores,23–25 speleothems,26,27 monazite sand,28 volcanic rocks,29,30 soils,31 fluid inclusions32 and other materials. In these studies, the concentration of several elements including Au, Fe, Cu, Zn, Pb, Ca, Mg, Sr, Mn, Si, Cr, Al, K, REEs, etc. were assessed by using principal components analysis (PCA), multivariate regression (PCR), artificial neural network (ANN) analysis or partial least-squares regression (PLSR) for the construction of calibration models. Either drill-core or ground rock samples were tested. In both cases, a large number of measurements are carried out to provide stratification or average concentration data.15,16,18 Another important field, where the on-site quantitative analysis of natural solid resources is needed and LIBS has already proven itself useful is the energy industry, more precisely coal analysis. For instance, the LIBS determination of carbon content, volatile content and the calorific value was shown to be practical and accurate enough33,34 so that this information to be used for fuel type discrimination or control of coal-fueled power plants.
Mineral and rock type identification also necessitates the use of statistical data analysis. Broad discrimination of ore mineralogy by PCA was demonstrated in selected wavelength windows in Australian iron ores.23 Pořízka et al. also used PCA to classify 27 igneous rock samples.35 In another study, PCA and soft independent modelling of class analogy (SIMCA) methods were used to generate a model and predict the type of samples.36 Harmon et al. used a spectral library approach to rapidly identify and classify samples based on their dominant elements with a high degree of confidence. It was observed that a maximum variance weighted – maximum correlation approach performs best. Minerals of different classes were correctly identified at a success rate of >95% for carbonates and >85% for feldspars and pyroxenes.37 PCA and partial least squares discriminant analysis (PLS-DA) were used by Gottfried et al.38 to identify the distinguishing characteristics of geological samples and to classify them based on their minor impurities. The PLS-DA approach was later successfully extended to the provenance analysis of garnets,39 obsidian glasses,40 igneous and sedimentary rocks9 as well as conflict minerals (e.g. columbite and tantalite).41 Most recently, the application of the advanced spectral angle mapper algorithm (SAM) for the identification of variations in the chemical composition in a complex chromite ore sample was also successfully demonstrated by Meima and Rammlmair.42 Nardecchia et al. introduced a new, LIBS-based spectral analysis strategy and named it embedded k-means clustering, for the simultaneous detection of major and minor compounds and the generation of associated localization maps for the characterization of complex and heterogeneous rock samples at the micro-scale level.43
The above examples only illustrate the unique analytical potential of LIBS in geology-related or raw material exploration applications. This potential is expected to unfold in the coming years and more and more industrial, green-field or brown-field (mine), stand-off LIBS applications will be developed. This development is also propelled by the increased demand and declining reserves for raw materials needed by advanced technologies. Two of the metals that are in high demand in recent years are Li and Be. Beryllium is widely used, e.g. in telecommunications infrastructure, advanced medical diagnostics instrumentation, automobile components and aeroplane equipment. Lithium is also a greatly sought-after metal, as it is used in large amounts in batteries, ceramics and glass, lubricating greases and polymer production. The uneven distribution and limited availability made critical raw materials to be a subject of geopolitics and made the governments and companies44,45 realize that the mineral industry has to adopt new, cost-effective methodologies and technologies. LIBS is one of the promising and flexible novel exploration tools, considering its sensitivity towards all elements, speed, information-rich spectra, as well as field- and stand-off applicability.15
In the present study, we assess the potential of LIBS for the identification of minerals (biotite, feldspar, quartz and, partially, amphibole) and the distribution and quantitative amount of lithium and beryllium in granitoid rock samples. The pros and cons of several analytical and data evaluation approaches are discussed and tested.
2. Experimental
2.1. Instrumentation
LIBS experiments were performed on a J-200 Tandem LA/LIBS instrument (Applied Spectra, USA), in the LIBS mode. This instrument is equipped with a 266 nm, 6 ns Nd:YAG laser source and a six-channel CCD spectrometer with a resolution of 0.07 nm. For every laser shot, the full LIBS spectra over the wavelength range of 190 to 1040 nm were recorded in the Axiom data acquisition software, using a 0.5 μs gate delay and 1 ms gate width. During the experiments, a 40 μm laser spot size was maintained, as it allows for the sampling of sub-millimetre grains (small pieces of minerals making up a rock) at several locations, but is large enough to provide ample LIBS signal for trace element detection. The pulse energy was generally set at 17.5 mJ and the laser repetition frequency was 10 Hz. The number of repeated measurements in one sampling location (without translation) was ten. The first shots were clean-up shots, so the spectra originating from them were discarded. Measurements were performed at 4–5 sampling points in each mineral grain (sampling was done in a total of 128 locations for biotite, 155 for feldspar, 83 for quartz and 4 for amphibole). LIBS experiments were carried out under argon, continuously rinsing the ablation cell with a gas flow rate of 1 L min−1. Argon gas increases the signal intensities and the continuous flow decreases the fallout of ablation debris around at the crater.Contact profilometry measurements performed on a Veco Dektak 8 Advanced Development Profiler. The tip had a radius of curvature of 2.5 μm and the force applied to the surface during scanning was 30 μN. The horizontal resolution was set to 0.267 μm and 3.175 μm in the x and y scan directions, respectively. The vertical resolution was 40 Å.
Optical images of the rock samples were taken with an Olympus BX-43 microscope equipped with an Olympus DP-73 camera, under polarised and transmitted light.
2.2. Materials
Our studied samples are of the monzogranite type, i.e. a granite variant with 35–65% feldspar. The fact that two different feldspars appear in the rock (orthoclase (K-rich) and plagioclase (Na-rich) feldspars) suggests that it had crystallized from magma saturated with water. Besides the two feldspars, the Mórágy Granite contains the most common rock-forming minerals, quartz, biotite and, to a lesser extent, amphibole.
2.3. Methods
The cutting was done using a diamond cutter (Struers DiscoPlan) to form 35 × 20 × 10 mm rectangular bodies. These then underwent vacuum impregnation using ARALDITE AY103 and REN HY956 epoxy resins in a Struers CitoVac equipment. After a full day of setting, a fresh surface was created on the impregnated rock body using the diamond cutter. The surface was ground using a Struers LaboPol-35 machine equipped with 80, 220, 500 and 1200 grit Struers MD-Piano diamond grinding wheels. As a final step, the aqueous suspension of SiC abrasive powder (Buehler) was applied to smoothen the sample surface. 30 μm thin sections of these rock bodies were cut using a Buehler PetroThin cutting and grinding machine. The thin sections were mounted on microscope slides using EpoFix epoxy resin. After 24 hours the samples were ready for optical microscopy. The mineral grains within the thin sections were identified and categorized under polarized light using optical microscopy, according to the standardized methods of petrology.48 The remaining part of the prepared rock body (bulk) was used for the LIBS measurements.
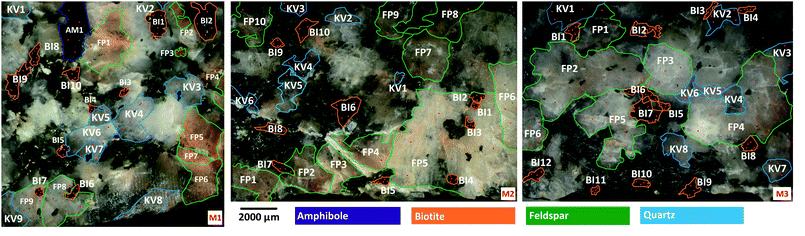
A reasonable number of the four most common mineral grains in each sample were identified, labelled and numbered in the samples (Fig. 1). The total set of mineral grains in the three samples consisted of 33 biotite, 27 feldspar, 22 quartz grains and a single amphibole grain. Plagioclase and potassium feldspar grains were not distinguished.
2.4. Data evaluation
Spectral line identification was carried out using version 18.0 of the built-in Clarity Software (Applied Spectra, USA) of the LIBS instrument. Data processing was done mainly in the open-source RStudio Desktop software package (v1.3), via developing custom codes using the chemometrics, MASS, ALS, RPart and random forest modules of RStudio. The Nelder–Mead simplex optimization algorithm was programmed and applied in MS Quick Basic programming language. The Image Lab software (Epina, Austria) was used to visualize LIBS elemental maps, whereas the open access ImageJ software was used to extract the surface area of mineral grains in microscopy images of the rock samples. The overall LIBS dataset submitted to RF and LDA contained as many as 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288 × 370 = 4
288 × 370 = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 546
546![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 data points.
560 data points.
3. Results and discussion
3.1. Compositional heterogeneities of the mineral grains
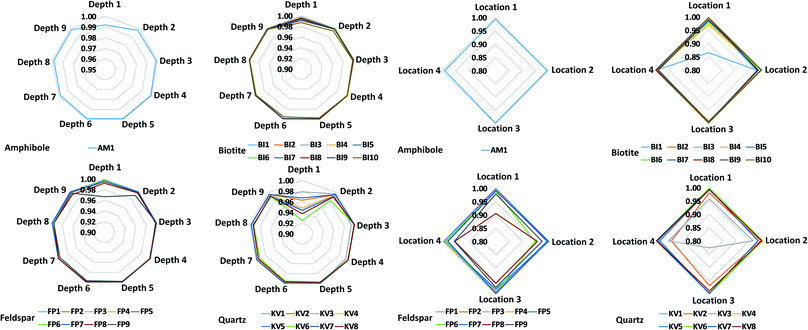
Most mineral grains in igneous rocks grow during a longer time under various physical (first of all pressure and temperature) circumstances and changing chemical conditions resulting in internal chemical zoning patterns. This is, from a chemical point of view, the manifestation of spatial changes of the composition inside a grain. The two most common types of zoning patterns are concentric and sector zoning, but other types, such as patchy, oscillatory, step and others also occur.53,54 These changes in chemical composition usually can be detected by different optical methods (e.g. Nomarski Differential Interference Contrast (NDIC) microscopy, cathodoluminescence (CL), etc.), if the compositional changes also induce changes in the optical properties, or by scanning elemental mapping techniques (e.g. electron microprobe (EMP), secondary ion mass spectrometry (SIMS), proton microprobe (PIXE), etc.).53 Minerals of magmatic rocks, such as the granitoid rocks studied here, are usually zoned. Although the lateral resolution (40 μm) used in the present LIBS experiments is not capable to fully resolve zoning features of the smaller mineral grains investigated, the effect can still influence the LIBS spectra collected at various locations and depths. Therefore, the extent of heterogeneity of the mineral grains in the samples was first investigated by repeated measurements.Individual LIBS spectra were collected from 10 shots delivered at 4–5 locations within each mineral grain. LIBS data from the first shot (“cleaning shot”) were discarded and data from 9 depth levels were retained. Spectra within each mineral across locations or depths (intra-mineral variations) were then compared to each other using the linear correlation function, which indicates full similarity with a Pearson correlation coefficient value of 1, and full dissimilarity with a value of 0 (assuming positive intensities).55,56 Ray plots in Fig. 2 show the observed intra-mineral variations of each mineral grain in sample M1.
It was generally found that there is a reasonable similarity of spectra, indicated by correlation factors of at least 0.85 in most cases. It can also be seen that the inter-location (lateral) variations, or heterogeneities, are significantly larger than the inter-depth variations. This can be attributed to the larger spatial distance between locations (ca. 100–500 μm, cf.Fig. 1) than depth levels, which makes location-related changes from the same zone generally more observable. It is also apparent that the LIBS spectra from depth level 1 are quite dissimilar from the rest the depth-resolved data, hence the data not only the first (already discarded) but also from the second laser shot should be considered as a cleaning shot. Based on these observed variations, we decided that in the mineral classification part of our study, we use the depth-averaged LIBS spectra from depths 2–9 of each location in each mineral grain as a statistical data element.
Another observation made in data in Fig. 2 is that zoning in the present samples is most pronounced in quartz and feldspar grains, whereas variations between different grains of the same mineral are also clearly identifiable. These observations should be considered when the accuracy of mineral grain classifications is evaluated in Section 3.3.
3.2. Laser ablation characteristics of the mineral grains
The studied minerals are all silicates, but their generalized composition is disparate.53 Besides, their colours are also different, hence it can be expected that their laser ablation behaviours are different as well (because of the different light absorption characteristics). To assess this, we investigated the laser ablation craters in the mineral grains by using contact profilometry after delivering ten repeated laser shots under the same conditions as described in the Experimental section. The cross-sectional profilometry curves (Fig. 3) reveal that the crater depths and volumes are indeed highly different, which indicates that quantitative analysis (or certain discriminative analysis) can only be attempted with reasonable accuracy if matrix-matched calibration or at least crater volume normalization (with a general silicate standard, such as the NIST 6XX glass series) is performed. The ablation depth per a laser shot was approximately 1.4 μm for quartz, 4 μm for biotite and 11 μm for feldspar.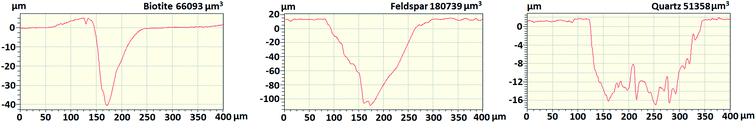 | ||
| Fig. 3 Ablation crater cross-sections of the mineral grains from ten repeated laser shots, as obtained by contact profilometry. The crater volume is indicated in the upper right corner of each graph. | ||
3.3. Qualitative discrimination of mineral grains
Considering the classification character of the analytical problem addressed here, we tested the performance of mainly multivariate chemometric methods (RF and LDA), which can also be called machine learning (ML) methods. In these methods, we used uncompressed data sets, as in our experience, data compression often leads to a distortion, which in turn may decrease the discrimination power and reliability (robustness) of the classification. This requires chemometric (or ML) methods that can cope with uncompressed data, which is the case with RF and LDA. In addition, we also assessed the performance of a more conventional approach in which the presence of spectral lines of indicator elements (characteristic of the mineral composition) were used for discrimination.For model construction (training), we used minerals in sample M1. The model then was validated by using it on sample M2 and M3. The accuracy of the methods was established by comparing the predicted and actual mineral types; the accuracy was expressed in terms of correct classifications. Moreover, we give calculated accuracy results according to two approaches: a location- and a grain-based metric. The grain-based accuracy was obtained in the way that separate laser ablation locations (4–5 within each grains) were evaluated individually and the majority “vote” for the mineral type was associated with that grain. The location-based accuracy was calculated as the overall accuracy obtained when spectra from ablation locations in each grain were evaluated individually.
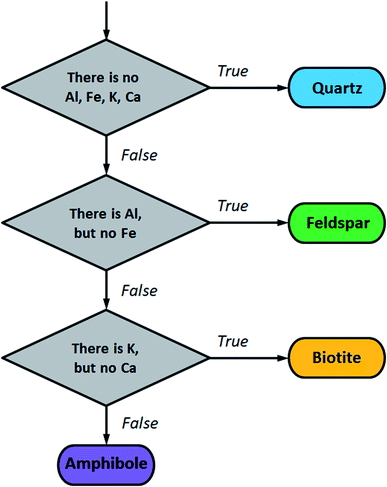 | ||
| Fig. 4 Flow chart for mineral grain classification based on the LIBS detection of major components Al, Fe, K and Ca. | ||
For the testing of this protocol, the spectral lines of Al I 308.21 nm, Fe I 371.99 nm, Ca II 393.37 nm and K I 769.93 nm were selected from the NIST Atomic Spectra Database. Each of these spectral lines is free from interference, at the resolution of our LIBS instrument, from the other three elements, as well as from Si, O, Na and Mg, the other commonly occurring components of these minerals. It is essential for the functioning of the protocol that a given element is only considered as present if the intensity of its spectral line exceeds a threshold intensity corresponding to a concentration level that classifies as a major component (e.g. 0.5 m/m%). Corresponding intensity thresholds for the above four spectral lines were taken from calibration plots obtained using the NIST glass (silicate) standards. These four intensity thresholds were then used as initial estimates for the Nelder–Mead simplex optimization algorithm52 which was performed to maximize the accuracy of the classification of all mineral grains in sample M1. As described earlier, depth-averaged LIBS spectra taken at each location in each grain were used as statistical elements in this classification. When the simplex algorithm terminated, the optimized intensity thresholds were used to evaluate LIBS data from the mineral grains in samples M2 and M3. A grain was only considered to be accurately identified if the majority of the locations within the grain gave correct identification. As can be seen, the classification is very accurate for biotite and feldspar but is significantly poorer for quartz, for which it is around 80% only. This result is in line with the former finding, namely that quartz grains are rather impure in the samples. The results also strongly suggest that sampling at several locations within each grain makes the identification more robust. Table 1 gives an overview of the accuracies obtained.
| Indicator lines | Random forest (RF) | Linear discriminant analysis (LDA) | ||||
|---|---|---|---|---|---|---|
| As per sampling locations | As per grains | As per sampling locations | As per grains | As per sampling locations | As per grains | |
| Biotite | 97.60% | 100.00% | 95.30% | 97.00% | 95.30% | 97.00% |
| Feldspar | 95.50% | 100.00% | 88.40% | 92.60% | 89.00% | 92.60% |
| Quartz | 80.70% | 77.30% | 84.30% | 95.40% | 87.90% | 95.40% |
In the present application, we trained the RF with datasets on sample M1 and optimized the number of trees as well as the number of nodes. Up to 500 trees with up to 20 nodes were evaluated by monitoring the out-of-bag error. It was found that the OOB initially steeply decreases with the increase in the number of trees and asymptotically reaches its minimum at around 50. Simultaneously, the increase of the number of nodes clearly deteriorated the OOB; the minimum was found with as little as two nodes. All RF classification results were therefore obtained by using 100 trees with two nodes.
As can be seen in Table 1, RF gave good, well-balanced results. The accuracy for grain-based classification was at least 92.6% for all three minerals. Similarly to the indicator line approach, the grain-based accuracy (majority vote of sampling locations within the grain) was better than when classification by each sampling locations was considered. By scrutinizing the nodes, it is also possible to estimate the most important (most frequent) classifiers. This analysis interestingly revealed that 274.29 nm, 400.84 nm, 345.65 nm and 326.08 nm were these variables, which may be associated with Fe, Th and V, instead of major components of the rock-forming minerals.
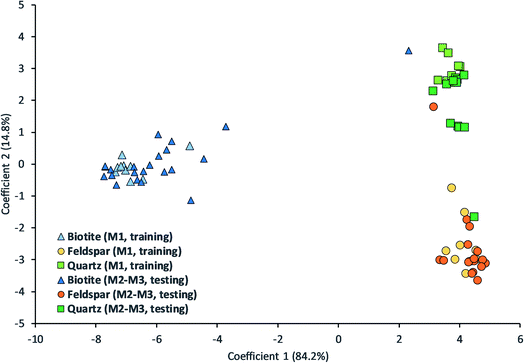
We tested Bayesian LDA on our uncompressed LIBS data. The results can be seen in Table 1 and Fig. 5. The overall classification accuracy was good, over 87% based on separate sampling locations and over 92% for grains (based on the majority vote within a grain). False classifications can be mostly associated with quartz and feldspar.
3.4. Quantitative assessment of the distribution of selected trace elements
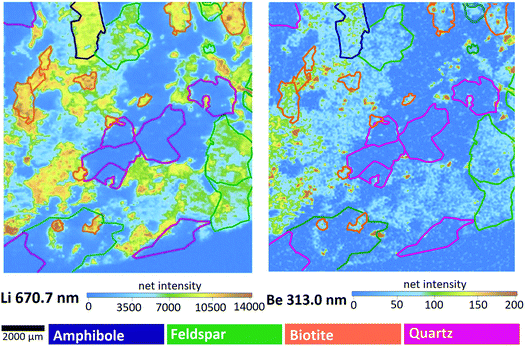 | ||
| Fig. 6 Intensity-based step-scan LIBS elemental maps of the M1 sample. Colors of the contours are indicating the mineral types, as seen in Fig. 1. | ||
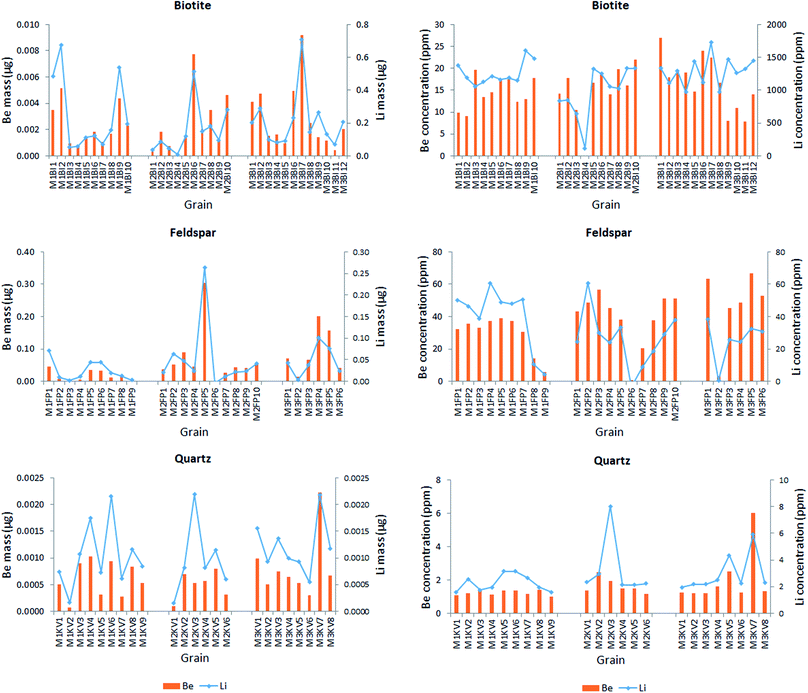 | ||
| Fig. 7 The Li and Be content of each analyzed mineral grain, expressed in terms of mass (on the left) and ppm concentration (on the right). | ||
Data in Fig. 7 justify the assumption suggested by the intensity-based elemental maps namely that the Li and Be content varies concertedly in all three minerals – the pattern show that generally (with very rare exceptions) wherever the Li content is high, so is the Be content. The mass of the metals present in the grains naturally changes with the size of the grain, thus the concentration distribution does not follow the same pattern, but the relative contents of Be and Li follow the same trend. Be concentrations (amounts) are in the same magnitude as Li concentrations (amounts) in quartz and feldspar, whereas they are significantly different in biotite, in which Li concentrations are almost one hundred times higher than those of Be. Not surprisingly, quartz contains the smallest amounts of both metals. It is also apparent that feldspar is the best source of Be, whereas biotite is for Li. According to the relevant report published by the Geological Institute of Hungary, the mineral composition of Mórágy Granite samples is 10% biotite, 70% feldspar and 15% quartz, with the remaining 5% being mostly amphibole.65 Using this average mineral composition data, an estimate can be given for the overall Be and Li content of these rocks: 1 kg of these rocks contain ca. 28 mg of Be and 144 mg of Li.
4. Conclusions
We have shown that LIBS mapping and spatially resolved local analysis is an efficient and practical approach for the classification of mineral grains (quartz, feldspar, biotite, amphibole) and for prospecting of technologically relevant elements in granitoid rock samples. We have tested three statistical approaches for the classification and it was demonstrated that better than 92% classification accuracy is achievable by using random forests and linear discriminant analysis. Direct classification by assessing the presence of the characteristic elements (decision tree based on indicative spectral lines) is a powerful method but can lead to great failure rates in case of relatively pure minerals, such as quartz. Our results also revealed that using multiple analytical locations within each grain and resting the classification on the majority vote of these individual analysis gives more reliable discrimination. We also demonstrated that LIBS elemental mapping can provide valuable information about the distribution of chemical elements in the minerals, especially if it is combined with the matrix-matched calibration of emission intensity data.It is also worth emphasizing that the appeals of LIBS in this and similar geochemical and mining industry-oriented applications include that it is a highly versatile analytical technique which is portable, robust, can be used in a stand-off situation and is equally sensitive to light and heavy elements. This is a unique set of features among atomic spectroscopy techniques. In our opinion, the sensitivity of the technique is well demonstrated by the fact that we successfully assessed ng to μg amounts of Be and Li in the studied mineral grains. We also point out that the described LIBS analytical and data evaluation approaches can be potentially fairly easily generalized and automated. For example, once grain boundaries in a rock sample are automatically identified by modern computer vision and machine learning methods (e.g. ref. 42), spatially resolved LIBS analysis can identify the mineral types and use this information to select the proper calibration standards (calibration curves) that can be used to convert spectral intensity data to concentration (mass) data of relevant elements.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support received from various sources including the Ministry of Innovation and Technology (through project no. TUDFO/47138-1/2019-ITM FIKP and Tématerületi Kiválósági Program TKP 2020) and the National Research, Development and Innovation Office (through projects no. K_129063, EFOP-3.6.2-16-2017-00005 and GINOP-2.3.3-15-2016-00040) of Hungary is kindly acknowledged.References
- G. Galbács, Anal. Bioanal. Chem., 2015, 407, 7537–7562 CrossRef PubMed.
- F. J. Fortes, J. Moros, P. Lucena, L. M. Cabalin and J. J. Laserna, Anal. Chem., 2013, 85, 640–669 CrossRef PubMed.
- D. W. Hahn and N. Omenetto, Appl. Spectrosc., 2012, 66, 347–419 CrossRef CAS PubMed.
- N. Jedlinszki and G. Galbács, Microchem. J., 2011, 97, 255–263 CrossRef CAS.
- A. Bol'shakov, X. Mao, J. J. González and R. E. Russo, J. Anal. At. Spectrom., 2016, 31, 119–134 RSC.
- A. Metzinger, R. Rajkó and G. Galbács, Spectrochim. Acta, Part B, 2014, 94, 48–57 CrossRef.
- J. Moros, J. Serrano, C. Sanchez, J. Macias and J. J. Laserna, J. Anal. At. Spectrom., 2012, 27, 2111–2122 RSC.
- S. Manzoor, S. Moncayo, F. Navarro-Villoslada, J. A. Ayala, R. Izquierdo-Hornillos, F. J. Manuel de Villena and J. O. Caceres, Talanta, 2013, 121, 65–70 CrossRef.
- A. M. Ollila, J. Lasue, H. E. Newsom, R. A. Multari, R. C. Wiens and S. M. Clegg, Appl. Opt., 2012, 51, B130–B142 CrossRef CAS.
- D. J. Palásti, A. Metzinger, T. Ajtai, Z. Bozóki, B. Hopp, É. Kovács-Széles and G. Galbács, Spectrochim. Acta, Part B, 2019, 153, 34–41 CrossRef.
- G. Vitkova, K. Novotny, L. Prokes, A. Hrdlicka, J. Kaiser, J. Novotny, R. Malina and D. Prochazka, Spectrochim. Acta, Part B, 2012, 73, 1–6 CrossRef CAS.
- J. Klus, P. Mikysek, D. Prochazka, P. Pořízka, P. Prochazková, J. Novotný, T. Trojek, K. Novotný, M. Slobodník and J. Kaiser, Spectrochim. Acta, Part B, 2016, 123, 143–149 CrossRef CAS.
- V. K. Singh, J. Sharma, A. K. Pathak, C. T. Ghany and M. A. Gondal, Biophys. Rev., 2018, 10, 1221–1239 CrossRef CAS.
- L. Wanting, L. Xiangyou, L. Xin, H. Zhongqi, L. Yongfeng and Z. Xiaoyan, Appl. Spectrosc. Rev., 2020, 55, 1–25 CrossRef.
- R. S. Harmon, C. J. Lawley, J. Watts, C. L. Harraden, A. M. Somers and R. R. Hark, Minerals, 2019, 9, 718 CrossRef CAS.
- R. S. Harmon, R. E. Russo and R. R. Hark, Spectrochim. Acta, Part B, 2013, 87, 11–26 CrossRef CAS.
- J. D. Winefordner, I. B. Gornushkin, T. L. Correll, E. Gibb, B. W. Smith and N. Omenetto, J. Anal. At. Spectrom., 2004, 19, 1061–1083 RSC.
- S. Qiao, Y. Ding, D. Tian, L. Yao and G. Yang, Appl. Spectrosc. Rev., 2015, 50, 1–26 CrossRef.
- T. F. Boucher, M. V. Ozanne, M. L. Carmosino, M. D. Dyar, S. Mahadevan, E. A. Breves, K. H. Lepore and S. M. Clegg, Spectrochim. Acta, Part B, 2015, 107, 1–10 CrossRef CAS.
- M. D. Dyar, M. L. Carmosino, E. A. Breves, M. V. Ozanne, S. M. Clegg and R. C. Wiens, Spectrochim. Acta, Part B, 2012, 70, 51–67 CrossRef CAS.
- N. J. McMillan, C. E. McManus, R. S. Harmon, F. C. Lucia Jr and A. W. Miziolek, Anal. Bioanal. Chem., 2006, 385, 263–271 CrossRef CAS.
- N. J. McMillan, R. S. Harmon, F. C. De Lucia and A. M. Miziolek, Spectrochim. Acta, Part B, 2007, 62, 1528–1536 CrossRef.
- D. L. Death, A. P. Cunningham and L. J. Pollard, Spectrochim. Acta, Part B, 2009, 64, 1048–1058 CrossRef.
- Q. Sun, M. Tran, B. W. Smith and J. D. Winefordner, Anal. Chim. Acta, 2000, 413, 187–195 CrossRef CAS.
- M. Gaft, I. Sapir-Sofer, H. Modiano and R. Stana, Spectrochim. Acta, Part B, 2007, 62, 1496–1503 CrossRef.
- J. Cunat, S. Palanco, F. Carrasco, M. D. Simon and J. J. Laserna, J. Anal. At. Spectrom., 2005, 20, 295–300 RSC.
- G. Galbács, I. Kevei-Bárány, E. Szőke, N. Jedlinszki, I. B. Gornushkin and M. Z. Galbács, Microchem. J., 2011, 99, 406–414 CrossRef.
- K. M. Abedin, A. F. M. Y. Haider, M. A. Rony and Z. H. Khan, Opt. Laser Technol., 2011, 43, 45–49 CrossRef CAS.
- Y. Hu, Z. Li and T. Lü, J. Anal. At. Spectrom., 2017, 32, 2263–2270 RSC.
- M. A. Gondal, M. M. Nasr, Z. Ahmed and Z. H. Yamani, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2009, 44, 528–535 CrossRef CAS.
- P. K. Srungaram, K. K. Ayyalasomayajula, F. Yu-Yueh and J. P. Singh, Spectrochim. Acta, Part B, 2013, 87, 108–113 CrossRef CAS.
- C. Fabre, M.-C. Boron, J. Dubessy, M. Cathlineau and D. Banks, Chem. Geol., 2002, 182, 249–264 CrossRef CAS.
- L. Zhang, Z. Hu, W. Yin, D. Huang, W. G. Ma, D. Lei, H. P. Wu, Z. X. Li, L. T. Xiao and S. T. Jia, Front. Phys., 2012, 7, 690–700 CrossRef.
- A. Metzinger, D. J. Palásti, É. Kovács-Széles, T. Ajtai, Z. Bozóki, Z. Kónya and G. Galbács, Energy Fuels, 2016, 30, 10306–10313 CrossRef CAS.
- P. Pořízka, A. Demidov, J. Kaiser, J. Keivanian, I. Gornushkin, U. Panne and J. Riedel, Spectrochim. Acta, Part B, 2014, 101, 155–163 CrossRef.
- S. M. Clegg, E. Sklute, M. D. Dyar, J. E. Barefield and R. C. Wiens, Spectrochim. Acta, Part B, 2009, 64, 79–88 CrossRef.
- R. S. Harmon, J. J. Remus, C. McManus, F. C. DeLucia, J. Gottfried and A. W. Miziolek, Appl. Geochem., 2009, 24, 1125–1141 CrossRef CAS.
- J. L. Gottfried, R. S. Harmon, F. C. De Lucia and A. W. Miziolek, Spectrochim. Acta, Part B, 2009, 64, 1009–1019 CrossRef.
- D. C. Alvey, K. Morton, R. S. Harmon, J. L. Gottfried, J. J. Remus, L. M. Collins and M. A. Wise, Appl. Opt., 2010, 49, C168–C180 CrossRef CAS.
- J. J. Remus, R. S. Harmon, R. R. Hark, G. Haverstock, D. Baron, I. K. Potter, S. K. Bristol and L. J. East, Appl. Opt., 2012, 51, B65–B73 CrossRef.
- R. S. Harmon, K. M. Shughrue, J. J. Remus, M. A. Wise, L. J. East and R. R. Hark, Anal. Bioanal. Chem., 2011, 400, 3377–3382 CrossRef CAS PubMed.
- J. A. Meima and D. Rammlmair, Chem. Geol., 2020, 532, 119376 CrossRef CAS.
- A. Nardecchia, C. Fabre, J. Cauzid, F. Pelascini, V. Motto-Ros and L. Duponchel, Anal. Chim. Acta, 2020, 1114, 66–73 CrossRef CAS.
- S. Kalantzakos, China and the Geopolitics of Rare Earths, Oxford University Press, New York, 1st edn, 2017 Search PubMed.
- Raw materials scoreboard: European innovation partnership on raw materials, 2018, https://op.europa.eu/en/publication-detail/-/publication/117c8d9b-e3d3-11e8-b690-01aa75ed71a1, accessed 24 September 2020 Search PubMed.
- A Magyar Állami Földtani Intézet Évi Jelentése, 2004, http://epa.oszk.hu/02900/02934/00031/pdf/EPA02934_mafi_evi_jelentes_2004_041-064.pdf, accessed 24 September 2020 Search PubMed.
- Z. Kercsmár, T. Budai, G. Csillag, I. Selmeczi and O. Sztanó, Magyarország felszíni képződményeinek földtana, Magyar Földtani és Geofizikai Intézet, Budapest, 1st edn, 2015 Search PubMed.
- J. D. Winter, Principles of igneous and metamorphic petrology, Pearson Education Limited, London, 2nd edn, 2014 Search PubMed.
- L. Breiman, Mach. Learn., 2001, 45, 5–32 CrossRef.
- L. Breiman and A. Cutler, Random forest, 2020, https://www.stat.berkeley.edu/%7Ebreiman/RandomForests/cc_home.htm, accessed 24 September 2020 Search PubMed.
- K. Varmuza and P. Filzmoser, Introduction to Multivariate Statistical Analysis in Chemometrics, CRC Press, Taylor & Francis Group, New York, 1st edn, 2009 Search PubMed.
- J. A. Nelder and R. Mead, Comput. J., 1965, 7, 308–313 CrossRef.
- W. D. Nesse, Introduction to Mineralogy, Oxford University Press, New York, 1st edn, 2000 Search PubMed.
- M. J. Streck, Rev. Mineral. Geochem., 2008, 69, 595–622 CrossRef CAS.
- G. Galbács, I. B. Gornushkin, B. W. Smith and J. D. Winefordner, Spectrochim. Acta, Part B, 2001, 56, 1159–1173 CrossRef.
- G. Galbács, I. B. Gornushkin and J. D. Winefordner, Talanta, 2004, 63, 351–357 CrossRef.
- L. Brunnbauer, S. Larisegger, H. Lohninger, M. Nelhiebel and A. Limbeck, Talanta, 2020, 209, 120572 CrossRef CAS.
- J. Qi, T. Zhang, H. Tang and H. Li, Spectrochim. Acta, Part B, 2018, 149, 288–293 CrossRef CAS.
- T. Zhang, D. Xia, H. Tang, X. Yang and H. Li, Chemom. Intell. Lab. Syst., 2016, 157, 196–201 CrossRef CAS.
- L. Sheng, T. Zhang, G. Niu, K. Wang, H. Tang, Y. Duand and H. Li, J. Anal. At. Spectrom., 2015, 30, 453–458 RSC.
- G. Vítková, L. Prokeš, K. Novotný, P. Pořízka, J. Novotný, D. Všianský, L. Čelko and J. Kaiser, Spectrochim. Acta, Part B, 2014, 101, 191–199 CrossRef.
- W. T. Li, Y. N. Zhu, X. Li, Z. Q. Hao, L. B. Guo, X. Y. Li, X. Y. Zenga and Y. F. Lu, J. Anal. At. Spectrom., 2020, 35, 1486 RSC.
- F. Rivera-Hernández, D. Y. Sumner, N. Mangold, K. M. Stack, O. Fornie, H. Newsomg, A. Williams, M. Nachon, J. L'Haridon, O. Gasnault, R. Wiens and S. Maurice, Icarus, 2019, 321, 82–98 CrossRef.
- W. P. Geddes, Energy Explor. Exploit., 1983, 3, 197–218 CrossRef.
- V. A. Utenkov, Z. Balla and E. Sallay, Annual Report of the Geological Institute of Hungary 2000–2001, 2003, vol. 1, pp. 153–188 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |