Implications of laser shot dosage on image quality in LA-ICP-QMS imaging
Martin
Šala
 *a,
Vid Simon
Šelih
*a,
Vid Simon
Šelih
 *a,
Ciprian C.
Stremtan
b,
Tudor
Tămaş
*a,
Ciprian C.
Stremtan
b,
Tudor
Tămaş
 c and
Johannes T.
van Elteren
c and
Johannes T.
van Elteren
 a
a
aDepartment of Analytical Chemistry, National Institute of Chemistry, Hajdrihova 19, SI-1000 Ljubljana, Slovenia. E-mail: martin.sala@ki.si; vid.selih@ki.si
bTeledyne Photon Machines, 384 Gallatin Park Drive, Bozeman, MT 59715, USA
cDepartment of Geology, Babeş-Bolyai University, M. Kogălniceanu 1, 400084 Cluj-Napoca, Romania
First published on 20th November 2020
Abstract
In the last few years elemental imaging by laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) has advanced rapidly, both due to hardware development associated with fast aerosol transport technologies and a deeper understanding of the influence of operational parameters on the image quality. Herein we describe the effect of dosage, i.e., the number of laser pulses per pixel, on the image quality attainable by LA-ICP-QMS as illustrated by mapping of a biological (murine brain tissue) and a mineralogical (asbestos fibers) sample. The usage of higher dosages results in better S/N ratios and is crucial when elements are present at lower concentration levels, or if mapping of more than one element is required. While this potentially increases the mapping time, elemental images with higher dosages will generally be of better quality.
1. Introduction
The last years have been momentous in advancing laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS), especially with regard to hardware development.1–6 Because of this progress, as well as the ever-growing interest in high resolution chemical imaging techniques, the number of publications utilizing elemental and isotopic ratio mapping has increased exponentially.7–15 Fast aerosol transfer devices coupled to newly designed ablation chambers, fast repetition rate lasers and the availability of data reduction software (Weiskirchen et al., 2019 and references therein),16 have paved the way to transform ultrafast imaging into a routine analysis technique.17Different approaches are being used for elemental imaging by LA-ICP-MS such as single pulse analysis (each pixel is generated by a single laser shot, i.e., dosage D = 1) and continuous scanning (each pixel is generated by multiple laser shots, i.e., dosage D > 1). Even though the latter approach samples material beyond the pixel size, potentially leading to extra blur, less noise is generated than in the former approach as more counts are accumulated per pixel. The dosage D is not only an important factor in the attainable image quality, but also defines the number of elements that can be measured per pixel with LA-ICP-QMS instruments.18,19 In combination with the latest generation of fast aerosol transfer devices, such instruments can only measure one element in single pulse mode (dosage D = 1); however, dosages of D ∼ 10 allow for measurement of more elements although the mapping time may be extended. Especially the development of new LA cells and transfer devices have led to much shorter particle washout times and therefore much faster responding systems. This has led to mapping times which are 1–2 orders of magnitude faster for the same beam size or increased spatial resolution by generating 10–100 times more pixels which are 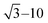 smaller in the same mapping time. However, smaller pixels generated by smaller beam sizes imply that the amount of material ablated is reduced, leading to a lower sensitivity. A higher dosage is therefore crucial for mapping of elements present at low concentration levels to keep S/N ratios as high as possible, even though higher dosages slightly increase the amount of blur. Image quality is thus a delicate balance between image noise and image blur, which are a direct function of the dosage.
smaller in the same mapping time. However, smaller pixels generated by smaller beam sizes imply that the amount of material ablated is reduced, leading to a lower sensitivity. A higher dosage is therefore crucial for mapping of elements present at low concentration levels to keep S/N ratios as high as possible, even though higher dosages slightly increase the amount of blur. Image quality is thus a delicate balance between image noise and image blur, which are a direct function of the dosage.
The image quality obtainable by single pulse analysis (D = 1) and continuous scanning (D = 10) was compared by mapping of single or several elements in complex mineralogical (fibrous asbestos compounds) and biological (murine brain tissue sections) samples. Because of the heterogeneous nature of fibrous asbestos compounds, it is difficult to ablate them using single pulse analysis, especially if the laser fluence is not set above the ablation threshold of all the mineral components comprising the mixture. This is also valid for biological samples that contain tissues with different density. However, if the dosage is set correctly, in conjunction with the correct fluence, these types of samples can yield high quality elemental or isotopic images. This has been shown theoretically through modeling,18 but the underlying work shows the practical implications of different dosages used for mapping of a mineralogical and a biological sample.
While modelling allows us to fundamentally study LA-ICP-MS optimization of parameters such as beam size, repetition rate, scanning speed, dwell time and acquisition time in the quest for best image quality, practical application also requires a deeper understanding of instrumental intricacies such as differences in aerosol transport and communication problems between laser and ICP-MS (e.g., triggering, acquisition start, synchronization, etc.). For these reasons, this work focuses on practical assessment of dosage related to image quality for a mineralogical and a biological sample.
2. Experimental
2.1 Samples
Asbestos is a general term used to define a non-homogeneous mixture of six naturally occurring silicate minerals pertaining to two mineralogical groups. The asbestos fibres used, (i.e., actinolite, generally Ca2(MgFe2+)Si8O22(OH)2 with various ion substitutions) were inspected under a stereomicroscope to remove any contaminants; they were embedded in epoxy resin and polished to ensure that the fibres were sufficiently exposed. Prior to LA-ICP-MS imaging the embedded asbestos fibres were sonicated in DI water to remove any particulates generated by the polishing materials; and dried overnight at low temperature (50 °C) in a vacuum desiccator.The murine brain tissue sample was prepared by freezing of tissue in LN2; sections of 16 μm thickness were then cut on a cryotome, placed on a glass slide and dried in air. No fixing or staining was performed (similar to the procedure described in Opačić et al.).20
2.2 LA-ICP-MS imaging
The instrumental setup used in this work comprised a laser ablation system (193 nm ArF* excimer; Analyte G2 Teledyne Photon Machines Inc., Bozeman, MT). The LA-system was equipped with a standard active two-volume ablation cell (HelEx II), including the Aerosol Rapid Introduction System (ARIS, Teledyne CETAC Technologies) for fast aerosol washout (ca. 20 ms for imaging of the samples in this work). The LA unit was coupled to a quadrupole ICP-MS instrument (Agilent 7900x, Agilent Technologies, Santa Clara, CA); Ar makeup gas was added before the ICP torch to the ARIS torch adaptor.Both types of samples were measured in single pulse (D = 1) and multiple pulse (D = 10) mode for imaging of one or several elements. Mapping was carried out on adjacent locations to clearly see the continuation of sample features and allow direct comparison of different mapping conditions. Table 1 summarizes the operational LA-ICP-MS settings; nuclides in bold are the ones related to images indicated in the text. The selection of elements was based on prior knowledge of the concentrations in the samples. In all experiments a beam size of 20 μm (square mask) was used, and other settings were based on model predictions for fastest possible mapping times, avoidance of aliasing, minimal blur and maximal S/N ratios.18 Data processing and image analysis were performed using the software packages HDIP (Teledyne Photon Machines Inc., Bozeman, MT) and ImageJ.21
| Brain | Asbestos | |||||||
|---|---|---|---|---|---|---|---|---|
| LA (Analyte G2, ARIS) | ||||||||
| Washout time, ms | ca. 20 | |||||||
| Beam size (square), μm | 20 | |||||||
| Fluence, J cm−2 | 0.5 | 4 | ||||||
| Dosage | 1 | 10 | 1 | 10 | ||||
| Repetition rate, Hz | 40 | 294 | 40 | 294 | ||||
| Scanning speed, μm s−1 | 800 | 588 | 800 | 588 | ||||
| He carrier flow rate, L min−1 cup|cell | 0.3|0.3 | |||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| ICP-MS (Agilent 7900x) | ||||||||
| R f power, W | 1500 | |||||||
| Sampling depth, mm | 6.5 | |||||||
| Ar makeup flow rate, L min−1 | 0.8 | |||||||
| Isotopes measured, dwell time, ms | 56 Fe, 25 | 56 Fe, 10.5 | 55Mn, 7 | 56 Fe, 34 | 59 Co, 25 | 139 La, 25 | 52 Cr, 6 | 59 Co, 34 |
| ( Fig. 1a ) | 66Zn, 10.5 | 56 Fe, 7 | ( Fig. 1d ) | ( Fig. 3b ) | ( Fig. 3c ) | 59Co, 6 | ( Fig. 3a ) | |
| ( Fig. 1b ) | 63 Cu, 7 | 139 La, 6 | ||||||
| 66 Zn, 7 | 146 Nd, 6 | |||||||
| ( Fig. 1c ) | ( Fig. 3d ) | |||||||
| ( Fig. 2 ) | ( Fig. 4 ) | |||||||
| Duty cycle time, ms | 25 | 34 | 25 | 34 | ||||
| Mapping rate, kpx h−1 | 144 | 105.9 | 144 | 105.9 | ||||
3. Results and discussion
Asbestos fibres and murine brain tissue were measured in this work to show the implications of laser shot dosage D on image quality. Differences, advantages and drawbacks of two approaches, viz., single pulse analysis (D = 1) and continuous scanning (D = 10), will be highlighted. Similar findings have already been seen in time-of-flight (TOF) instruments,22,23 but the consequences are even more crucial when using QMS instruments.Commonly, biological samples are “thin” tissue slices with a thickness ranging from less than one to several tens of micrometres, which of course determines the useable dosage as too high a dosage will lead to complete consumption of the sample. Unless one chooses to do this intentionally to increase the spatial resolution in the scan direction, by removing only a small fraction of a pixel using a single laser shot with a high fluence, this may cause sensitivity issues. In geological samples usually this problem does not arise as samples are thick enough so that even at higher dosage, the sample is not fully ablated through.
Although quantification-related issues are not the topic of this paper, some discussion about this important issue is in place. The ability of high-speed, high-dosage LA-ICP-MS for quantitative mapping still has its restrictions as only several milliseconds are available for measurement of each and every pixel in the map. Consequently, only a handful of elements can be measured, and a calibration approach based on e.g. sum normalisation for elemental mapping (citations), requiring the measurement of tens of elements, is out of the question for sequential scanning QMS instruments but feasible for simultaneous TOFMS systems. However, samples with a homogeneously distributed element that can be used for internal standardisation can be easily quantified for a limited number of elements using LA-ICP-QMS mapping.
3.1 Murine brain tissue
Fig. 1 presents the elemental distribution of 56Fe acquired at different LA-ICP-MS settings summarized in Table 1. Very poor image quality can be noticed in Fig. 1b due to the fact that in QMS instruments the elements are measured sequentially, in contrast to TOFMS instruments which measure elements simultaneously. As such the synchronisation of LA and ICP-MS becomes crucial, especially for high repetition rates and short dwell times. Only a few milliseconds of de-synchronisation can result in measuring the single pulse signal at the beginning or the end of the washout profile, leading to elevated noise levels in the resulting images. Reasons for de-synchronisation may be associated with irreproducible laser ablation, aerosol transport delay, erratic washout of aerosol, non-triggered ICP-MS acquisition, etc. To exclude the effects of poor pulse-to-pulse stability, the energy output of the laser was monitored using the on-board energy meter which a high level of repeatability (RSD < 1.9%). The gas flows for the ablation chamber were tuned to avoid any aerosol transport-induced artefacts.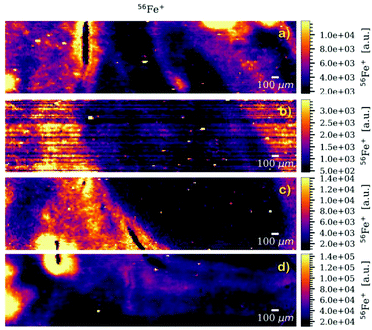 | ||
| Fig. 1 LA-ICPMS images of murine brain tissue showing the distribution of 56Fei for measurement of (a) one nuclide (D = 1), (b) two nuclides (D = 1), (c) four nuclides (D = 10) and (d) one nuclide (D = 10). See Table 1 for details. | ||
Although Fig. 1a (measurement at D = 1 of one nuclide) shows a relatively high-detail image, it has to be compared to Fig. 1c and d (measurement at D = 10 of four and one nuclides, respectively). As expected, Fig. 1d has a ca. ten times higher count rate than Fig. 1a due to a dosage difference of ten, but still a (subjective) similar visual image quality. Fig. 1c has roughly the same count rate, and also a similar (subjective) visual image quality than Fig. 1a, in spite of the fact that in Fig. 1c three more nuclides are measured next to 56Fe. However, to accommodate the measurement of four elements the mapping rate decreases from 144 to 105.9 kpx h−1. This is due to the fact that we run into the repetition rate ceiling, i.e., 300 Hz, for multiple pulse analysis at a dosage D = 10 in the LA instrument used. A laser head with a repetition rate of 400 Hz would facilitate the measurement at 144 kpx h−1 as well, but then the four elements would need to be measured in 25 ms. According to Table 1 this would be possible as hopping and settling takes only 6 ms (hopping and settling times are instrument-specific), leaving 19 ms to be distributed among measurement of these elements. The use of high repetition rate laser heads for imaging applications have already been documented.17
A significant advantage of the multiple dosage analysis technique compared to the single dosage approach is its multielement capabilities as shown in Fig. 2 where an RGB overlay of the maps of three elements is presented. This map clearly shows different distributions and associations of elements in the sample that can (in general) help researchers understand physiological and metallomic implications connected to the samples analysed.
 | ||
| Fig. 2 Multielement RGB map of the murine brain tissue, overlaying the maps of three elements (56Fe, 63Cu and 66Zn). | ||
3.2 Asbestos fibres
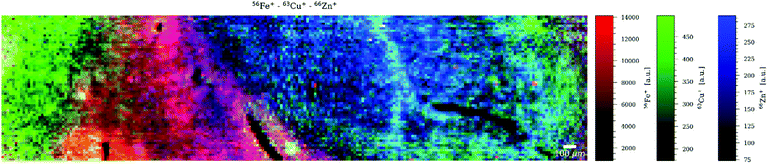
Although the sample chosen is not the most obvious choice to represent a mineralogical sample, it was selected because of its complex physical properties and chemical composition, as well as the growing interest in the study of asbestos in mesothelioma-related clinical studies.24 Co and La were selected for mapping as representatives of elements in asbestos fibres with high and low concentration, respectively. Similar to the murine brain tissue, there are very obvious differences when comparing the images generated with different dosages. Fig. 3 shows the mapping of Co as single element at D = 10 (a) and D = 1 (b), La as single element at D = 1 (c) and La as multielement in a sequence of four at D = 10 (d). It can be seen that the image quality of the (high-concentration) Co maps is visually very similar although a dosage D = 10 (Fig. 3a) results in significantly higher counts rates than a dosage D = 1 (Fig. 3b). Also the (low-concentration) La maps look visually quite similar although in Fig. 3d three more elements are measured than in Fig. 3c, and even less than ca. 10 counts per pixel are recorded. The higher dosage still allows high-resolution mapping of low concentrations in a multielement setting. The multielemental character is clearly shown in Fig. 4 which overlays the maps of three elements. | ||
| Fig. 3 LA-ICPMS images of asbestos fibres showing the distribution of 59Co upon measurement of (a) one nuclide (D = 10) and (b) one nuclide (D = 1), and 139La upon measurement of (c) one nuclide (D = 1) and (d) four nuclides (D = 10). See Table 1 for details. | ||
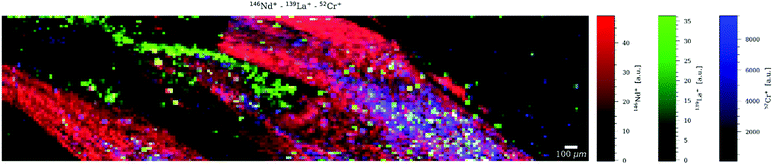 | ||
| Fig. 4 Multielement RGB map of the asbestos fibres, overlaying the maps of three elements (52Cr, 139La and 146Nd). | ||
Although not visually obvious from the maps shown, higher dosages result in less image noise and higher S/N ratios as illustrated in Table 2. A NIST SRM612 glass reference standard was mapped with LA-ICP-QMS parameters similar to those used for mapping of the asbestos samples. We selected aluminium and lanthanum as elements representative for high (0.537 ± 0.011% m/m) and low (36 ± 7 μg g−1) concentrations, respectively. Al and La were mapped individually and simultaneously for dosages of 1, 2, 5 and 10. From Table 2 it is clear that higher dosages result in better S/N ratios, more pronounced for the lower concentration element La. Measurement of two elements simultaneously yields an overall lower sensitivity and thus an even higher influence of dosage on the S/N ratio.
| Dosage D | Al | La | Al (+La) | La (+Al) |
|---|---|---|---|---|
| 1 | 19.6 | 12.7 | 2.6 | 1.7 |
| 2 | 19.6 | 18.2 | 7.0 | 6.7 |
| 5 | 21.3 | 20.8 | 20.8 | 14.9 |
| 10 | 24.4 | 23.8 | 23.8 | 16.1 |
4. Conclusions
We have shown that higher dosages in LA-ICP-QMS imaging may be beneficial, not only to overall image quality but also as a means of multielement analysis. Increased image quality results from better counting statistics, especially noticeable for low elemental concentrations, and generally dominating the slight loss in spatial resolution. We believe that this approach is also useful in the field of LA-ICP-TOFMS imaging as evidenced by some recent publications.Conflicts of interest
The authors declare the following competing financial interest(s): Ciprian C. Stremtan is employed by Teledyne CETAC Technologies.Acknowledgements
The authors acknowledge financial support from the Slovenian Research Agency (ARRS), contract numbers P1-0034 and N1-0060.References
- M. Burger, L. Hendriks, J. Kaeslin, A. Gundlach-Graham, B. Hattendorf and D. Gunther, J. Anal. At. Spectrom., 2019, 34, 135–146 RSC.
- M. Burger, G. Schwarz, A. Gundlach-Graham, D. Kaser, B. Hattendorf and D. Gunther, J. Anal. At. Spectrom., 2017, 32, 1946–1959 RSC.
- Y. Bussweiler, O. Borovinskaya and M. Tanner, Spectroscopy, 2017, 32, 14–20 CAS.
- D. N. Douglas, A. J. Managh, H. J. Reid and B. L. Sharp, Anal. Chem., 2015, 87, 11285–11294 CrossRef CAS.
- S. J. M. Van Malderen, A. J. Managh, B. L. Sharp and F. Vanhaecke, J. Anal. At. Spectrom., 2016, 31, 423–439 RSC.
- S. J. M. Van Malderen, T. Van Acker and F. Vanhaecke, Anal. Chem., 2020, 92, 5756–5764 CrossRef CAS.
- Y. Bussweiler, F. Gervasoni, M. Rittner, J. Berndt and S. Klemme, Lithos, 2020, 352, 10 Search PubMed.
- D. Chew, K. Drost and J. A. Petrus, Geostand. Geoanal. Res., 2019, 43, 39–60 CrossRef CAS.
- K. Drost, D. Chew, J. A. Petrus, F. Scholze, J. D. Woodhead, J. W. Schneider and D. A. T. Harper, Geochem., Geophys., Geosyst., 2018, 19, 4631–4648 CrossRef CAS.
- R. Dubosq, C. J. M. Lawley, A. Rogowitz, D. A. Schneider and S. Jackson, Lithos, 2018, 310, 86–104 CrossRef.
- A. Gundlach-Graham, M. Burger, S. Allner, G. Schwarz, H. A. O. Wang, L. Gyr, D. Grolimund, B. Hattendorf and D. Gunther, Anal. Chem., 2015, 87, 8250–8258 CrossRef CAS.
- P. M. Kopittke, E. Lombi, A. van der Ent, P. Wang, J. S. Laird, K. L. Moore, D. P. Persson and S. Husted, Plant Physiol., 2020, 182, 1869–1882 CrossRef CAS.
- T. J. Stewart, Metallomics, 2019, 11, 29–49 RSC.
- T. Ubide, J. Caulfield, C. Brandt, Y. Bussweiler, S. Mollo, F. Di Stefano, M. Nazzari and P. Scarlato, Front. Earth Sci., 2019, 7, 23 CrossRef.
- M. Burger, A. Gundlach-Graham, S. Allner, G. Schwarz, H. A. O. Wang, L. Gyr, S. Burgener, B. Hattendorf, D. Grolimund and D. Gunther, Anal. Chem., 2015, 87, 8259–8267 CrossRef CAS.
- R. Weiskirchen, S. Weiskirchen, P. Kim and R. Winkler, J. Cheminf., 2019, 11, 21 Search PubMed.
- M. Šala, V. S. Šelih, C. C. Stremtan and J. Teun van Elteren, J. Anal. At. Spectrom., 2020, 35, 1827–1831 RSC.
- J. T. van Elteren, V. S. Šelih and M. Šala, J. Anal. At. Spectrom., 2019, 34, 1919–1931 RSC.
- J. T. van Elteren, D. Metarapi, M. Šala, V. S. Šelih and C. C. Stremtan, J. Anal. At. Spectrom., 2020, 35, 2494–2497 RSC.
- M. Opačić, A. J. Ristić, D. Savić, V. S. Šelih, M. Živin, D. Sokić, S. Raičević, V. Baščarević and I. Spasojević, Metallomics, 2017, 9, 141–148 RSC.
- C. A. Schneider, W. S. Rasband and K. W. Eliceiri, Nat. Methods, 2012, 9, 671–675 CrossRef CAS.
- C. Neff, P. Keresztes Schmidt, P. S. Garofalo, G. Schwarz and D. Günther, J. Anal. At. Spectrom., 2020, 35, 2255–2266 RSC.
- D. Rubatto, M. Burger, P. Lanari, B. Hattendorf, G. Schwarz, C. Neff, P. K. Schmidt, J. Hermann, A. Vho and D. Gunther, Contrib. Mineral. Petrol., 2020, 175, 19 CrossRef.
- C. J. Greenhalgh, O. M. Voloaca, P. Shaw, A. Donard, L. M. Cole, M. R. Clench, A. J. Managh and S. L. Haywood-Small, J. Anal. At. Spectrom., 2020, 35, 2231–2238 RSC; M. Burger, L. Hendriks, J. Kaeslin, A. Gundlach-Graham, B. Hattendorf and D. Gunther, J. Anal. At. Spectrom., 2019, 34, 135–146 RSC.
| This journal is © The Royal Society of Chemistry 2021 |
