Open Access Article
Open Access ArticleCombined ionic liquid and supercritical carbon dioxide based dynamic extraction of six cannabinoids from Cannabis sativa L.†
Christoph
Kornpointner‡
a,
Aitor
Sainz Martinez‡
 b,
Michael
Schnürch
b,
Michael
Schnürch
 b,
Heidi
Halbwirth
b,
Heidi
Halbwirth
 *a and
Katharina
Bica-Schröder
*a and
Katharina
Bica-Schröder
 *b
*b
aInstitute of Chemical, Environmental and Bioscience Engineering, TU Wien, Getreidemarkt 9/166, 1060, Vienna, Austria. E-mail: heidrun.halbwirth@tuwien.ac.at
bInstitute of Applied Synthetic Chemistry, TU Wien, Getreidemarkt 9/163, 1060, Vienna, Austria. E-mail: katharina.schroeder@tuwien.ac.at
First published on 18th November 2021
Abstract
The potential of supercritical CO2 and ionic liquids (ILs) as alternatives to traditional extraction of natural compounds from plant material is of increasing importance. Both techniques offer several advantages over conventional extraction methods. These two alternatives have been separately employed on numerous ocassions, however, until now, they have never been combined for the extraction of secondary metabolites from natural sources, despite properties that complement each other perfectly. Herein, we present the first application of an IL-based dynamic supercritical CO2 extraction of six cannabinoids (CBD, CBDA, Δ9-THC, THCA, CBG and CBGA) from industrial hemp (Cannabis sativa L.). Various process parameters were optimized, i.e., IL-based pre-treatment time and pre-treatment temperature, as well as pressure and temperature during supercritical fluid extraction. In addition, the impact of different ILs on cannabinoid extraction yield was evaluated, namely, 1-ethyl-3-methylimidazolium acetate, choline acetate and 1-ethyl-3-methylimidazolium dimethylphosphate. This novel technique exhibits a synergistic effect that allows the solvent-free acquisition of cannabinoids from industrial hemp, avoiding further processing steps and the additional use of resources. The newly developed IL-based supercritical CO2 extraction results in high yields of the investigated cannabinoids, thus, demonstrating an effective and reliable alternative to established extraction methods. Ultimately, the ILs can be recycled to reduce costs and to improve the sustainability of the developed extraction process.
Introduction
Cannabis sativa L. is an annual herbaceous blossoming plant that has been used throughout history in the textile industry, for recreational purposes and in medical applications. It is regarded as one of the oldest cultivated plants, and one of the most essential crops for the progress of humankind. Although native to Eastern Asia, its extensive applications led to its global spread.1The medicinal properties of Cannabis sativa L. can be attributed to the many bioactive compounds present in the plant, such as terpenes, polyphenols, phytosterols, tocopherols, fatty acids, and, specifically, cannabinoids, which are terpenophenolic secondary metabolites.2,3 It is important to mention that cannabinoids are not equally distributed in the plant. They are mainly found in the trichomes and in smaller to negligible amounts in the seeds, while roots contain none.4
Presently, over 100 cannabinoids have been identified.5 They are primarily encountered in their carboxylated form in the plant which constitutes a structure of 22 carbon atoms. So far, cannabinoids have been categorized into 11 subclasses: (1) (−)-Δ9-tetrahydrocannabinol (Δ9-THC), (2) (−)-Δ8-tetrahydrocannabinol (Δ8-THC), (3) cannabidiol (CBD), (4) cannabigerol (CBG), (5) cannabichromene (CBC), (6) cannabinol (CBN), (7) cannabinodiol (CBND), (8) cannabicyclol (CBL), (9) cannabielsoin (CBE), (10) cannabitriol (CBT) and (11) miscellaneous. The structures of cannabinoids from hemp investigated in this study are depicted in Fig. 1.6
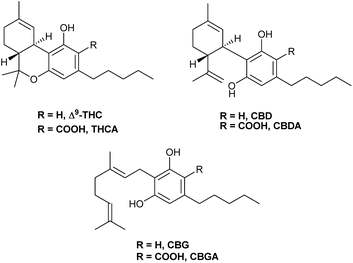 | ||
| Fig. 1 Structures of the investigated cannabinoids in this study: Δ9-THC, THCA, CBD, CBDA, CBG and CBGA. | ||
In terms of the biosynthesis of cannabinoids, CBGA is the main precursor for THCA and CBDA.7 However, under high temperatures, both acids are prone to degrade into their respective decarboxylated analogues, Δ9-THC and CBD.8
Δ9-THC and CBD are the most abundant cannabinoids present in cannabis plants. Δ9-THC is well-known as a psychoactive compound, which influences the central nervous and cardiovascular systems. Contrarily, CBD is non-psychoactive, but is regarded as a compound of enormous medical interest, as it has demonstrated numerous health benefits. It has been reported to have anti-inflammatory, antiepileptic and anticonvulsive properties, among many others.9–11 Excellent medicinal potential have been attributed to cannabinoids; thus, significant effort has been made in the past decades towards the research of the functions and mechanisms of cannabis-derived secondary metabolites in the human body.
Due to the growing medicinal interest in cannabinoids over the years, scientists have undertaken efforts in the development of extraction methods for these valuable bioactive compounds. Traditionally, Δ9-THC and other cannabinoids have been isolated by solvent-based extractions, with hydrocarbons and alcohols delivering the highest yields.12,13 Soxhlet extraction (SE) is also a commonly used technique,14,15 which is characterized by shortcomings, namely, long extraction times and high temperature that may promote thermal degradation of the target compounds.16
Other advanced extraction techniques, such as microwave-assisted extraction (MAE) allow higher yields, shorter extraction times, less solvent and reduced energy consumption.14,17 Nevertheless, uneven heating and/or overheating may cause thermal degradation, and thus negatively impact the extraction efficiency.18 Alternatively, the use of ultrasound-assisted extraction (UAE) achieves high yields in short times;19 however, the distribution of ultrasound energy lacks uniformity and over time the power decreases, which can lead to inefficient use of the ultrasound-generated energy.20
Supercritical fluid extraction (SFE) is an innovative separation technique, which has thus far been employed for extractions of valuable constituents from over 300 plant species.21 Carbon dioxide is a widespread choice for SFEs due to its several advantageous properties, such as low reactivity, non-toxicity, non-flammability, affordability, availability, and recyclability. Additionally, its selectivity can be adjusted by modification of pressure and temperature, while product fractionation and recovery with high purity is feasible. Nevertheless, due to its low polarity, addition of small quantities of organic solvents (co-solvents or modifiers) is necessary to access more polar compounds, thereby expanding its extraction range.22 The selection of an appropriate co-solvent is key for achieving optimum solubility of the bioactive compounds present in the plant.23 Supercritical carbon dioxide has previously been used to assess the solubility of individual cannabinoids, for example, Δ9-THC,24 CBD25 and CBG.25 Moreover, several extractions of cannabinoids from different parts of the cannabis plant, for instance, leaves, trimmings, buds, flowers and threshing residues, have been performed using ethanol as a co-solvent.26–29
Within the past years, ionic liquids have also emerged as alternative reaction media for the extraction of biomass that is regarded as a source of natural medicinally relevant complex compounds. Many different properties are attributed to ionic liquids, such as exceptional dissolution properties, high thermal stability and broad liquid range, to name a few. Furthermore, ILs display high tuneability, as the combination of different cations and anions leads to hydrophilicity or hydrophobicity and different polarity.30
The dissolution and processing of lignocellulosic biomass is a particularly interesting application of ionic liquids (ILs), as they can directly dissolve and fractionate (ligno-)cellulose in an overall less energy intensive process.31,32 The biomass dissolution capability of ILs is impacted by both their cation and anion, however, current publications suggest that anions have a more significant impact, since they play a role in breaking the many intermolecular hydrogen bonds.30 Regarding the cation, imidazolium-based ILs were the most successful for the direct dissolution of cellulose, followed by pyridinium- and ammonium-based ones.33 In addition, increasing the chain length of the cation had a negative influence on the dissolving capabilities of the ILs, as the viscosity increased, and the H-bond acidity decreased. As far as the anion is concerned, dissolving efficiency seems to be determined by the H-acceptor properties of the anion. In general, anions with weak H-bond basicity, for instance, [BF4]− and [PF6]−, could not successfully dissolve cellulose, while ionic liquids based on halide or acetate anions are typically the candidates of choice.30,34 The growing research on ILs as solvents for lignocellulosic biorefinery also prompted innovations for the extraction of valuable ingredients from plant materials.35 There are several aspects of ILs that are potentially advantageous for the extraction of high-value compounds: apart from their unique solvent properties and potential environmental benefits, the ability of ILs to dissolve biomass can lead to a better, and higher, yielding access to valuable ingredients embedded in the biopolymers and contribute to a value-added biorefinery.36,37 However, the recovery of natural products from ionic liquids is often more demanding than the mere extraction: many studies require extensive back-extraction with volatile solvents to actually isolate the valuable ingredients from ILs, thereby rendering the original solvent reduction less significant or even negating it altogether.
The combination of non-volatile polar ILs with volatile non-polar scCO2 has several advantages for extractions, as well as for catalysis. Since scCO2 is highly soluble in ILs, but ILs cannot dissolve in scCO2, it can easily penetrate the IL-phase. This allows the extraction of compounds from the IL-phase into the scCO2 phase, taken into account that the organic compound of interest is soluble in scCO2. Ultimately they are transported into an extraction vessel in a pure, solvent-free and solid form.38
Furthermore, ILs in the presence of CO2 expand their applicability, as their melting point and viscosity decrease, thus, promoting mass transportation.39 Consequently, the combination of ionic liquids with scCO2 has found application in several catalytic processes, such as hydroformylations, hydrogenations or carboxylations of alkenes in IL-scCO2 biphasic reaction media.40–43 In the IL-scCO2 reaction systems, the reactants and products are carried by the scCO2 and IL is used as a reaction media.44,45 Additionally, it is demonstrated that IL-scCO2 biphasic systems avoid cross-contamination of the extracted solute.38,46
Until now, IL-based pre-treatment and subsequent SFE (IL-SFE) for natural products has not been described, although ideal conditions arise from the unique properties of both media. Hence, by comparing IL-scCO2 extraction with the utilization of both applications individually or to traditional solvent-extraction, the IL-scCO2 approach is preferable. To begin with, less additional preparation, e.g., filtration of the raw material and consequent evaporation of solvents or separation of IL from the organic solvent is required to obtain a solvent-free and solid extract (Fig. 2). Consequently, there is a lower chance of loss of product or impurities, due to less post processing steps. On the other hand, IL-SFE is performed without additional co-solvents, therefore it reduces further solvent consumption and leads to lower expenses. Ultimately, if chosen appropriately, the ionic liquid can be recovered and re-used to improve the sustainability of the extraction process.
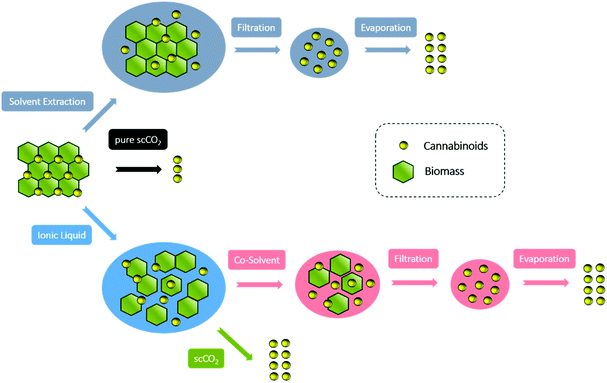 | ||
| Fig. 2 Conceptualization for the comparison of work up steps and yields of cannabinoids extraction techniques. | ||
Recently, an investigation of the extraction of cannabidiol with the aid of ILs has been published; however, isolation of cannabidiol required tedious back-extraction with organic solvents or with an aqueous AgNO3 solution.47 To the best of our knowledge, no data has been reported thus far regarding a combined extraction process that takes advantage of the complementing properties.
Herein, we present the first application of IL-SFE from industrial hemp of six cannabinoids (Δ9-THC, THCA, CBD, CBDA, CBG and CBGA). Several parameters during the IL-assisted pre-treatment, such as time, temperature and dilution with H2O, were investigated. In addition, pressure and temperature during SFE were evaluated. Ultimately, the optimized process for 1-ethyl-3-methylimidazolium acetate ([C2mim][OAc]) was additionally performed with choline acetate ([Ch][OAc]) and 1-ethyl-3-methylimidazolium dimethyl phosphate ([C2mim][DMP]) to compare the extraction efficiency of the investigated cannabinoids. In addition, the developed extraction process is complemented by a simple ionic liquid recovering process without the usage of additional organic solvents.
Results and discussion
The focus of this research was the investigation and optimization of various parameters for the extraction of CBD, CBDA, Δ9-THC, THCA, CBG and CBGA from partially pre-dissolved hemp in various room-temperature ILs with supercritical CO2. The optimization was divided into three successive stages (Scheme 1).In the first stage, the pre-treatment conditions to digest and partially dissolve hemp using [C2mim][OAc] before SFE were investigated. The lignocellulosic composition of hemp hurds is reported to contain 43.0% cellulose, 24.4% lignin and 29.0% hemicellulose.48 ILs are known to dissolve a variety of carbohydrates, e.g., cellulose, by combining strongly basic anions (e.g., Cl− or OAc−) with various cations.49–51 In particular, [C2mim][OAc] was selected in this study as it was used to pre-treat various lignocellulosic biomasses52 and it is known to effectively dissolve, hemicellulose53 and lignin.54 Furthermore, [C2mim][OAc] is liquid at room temperature, non-halogenated and miscible with H2O.
Subsequently, the best extraction conditions of stage 1 were employed in determining the most effective ratio of [C2mim][OAc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O during SFE. In the third stage, the previously optimized conditions from the first and second stage were utilized to investigate several combinations of pressure and temperature during SFE. Ultimately, the optimum parameters were employed with two additional ILs, namely [Ch][OAc] and [C2mim][DMP]. Both ILs are liquid at room temperature, non-halogenated and hydrophilic. Moreover, both ILs have been reported for pre-treatment of biomass.55,56 In addition, the positive rating of choline-based ILs in terms of toxicity and biodegradation renders them ideally suited for natural product extractions.57,58
H2O during SFE. In the third stage, the previously optimized conditions from the first and second stage were utilized to investigate several combinations of pressure and temperature during SFE. Ultimately, the optimum parameters were employed with two additional ILs, namely [Ch][OAc] and [C2mim][DMP]. Both ILs are liquid at room temperature, non-halogenated and hydrophilic. Moreover, both ILs have been reported for pre-treatment of biomass.55,56 In addition, the positive rating of choline-based ILs in terms of toxicity and biodegradation renders them ideally suited for natural product extractions.57,58
Pre-treatment with ionic liquid (Stage 1)
Herein, the influence of temperature and time for the partial dissolution of Cannabis sativa L. in [C2mim][OAc] before the scCO2 extraction is evaluated.Initially, the conditions to partially dissolve industrial hemp in [C2mim][OAc] were investigated in experiments 1–4 (Table 1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1
H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Stage 1)
2 (Stage 1)
| Exp. | t Pre/min | T Pre/°C | ∑(CBD) (mg g−1) | ∑(THC) (mg g−1) | ∑(CBG) (mg g−1) |
|---|---|---|---|---|---|
| Mean values with different letters (a, b, c, etc.) within the same column are statistically different (p < 0.05). | |||||
| 1 | 60 | 25 | 13.1 ± 0.8a | 0.464 ± 0.008b | 0.229 ± 0.011c |
| 2 | 60 | 70 | 12.9 ± 0.3a | 0.471 ± 0.019b | 0.244 ± 0.014c |
| 3 | 15 | 25 | 13.0 ± 0.8a | 0.48 ± 0.03b | 0.221 ± 0.019c |
| 4 | 15 | 70 | 13.6 ± 0.6a | 0.513 ± 0.017b | 0.247 ± 0.017c |
Therefore, the pre-treatments were carried out at 25 and 70 °C, each 15 and 60 min, afterwards diluted with H2O to a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and subsequently subjected to SFE at 20 MPa and 70 °C. To evaluate the quality of the performed experiments during the development of IL-SFE for hemp the yields of cannabinoids are expressed as the sum of cannabinoid types e.g. CBD and CBDA are referred to as ∑(CBD). Analogously ∑(THC) and ∑(CBG) are calculated. All experimental conditions and results for individual cannabinoid yields are shown in the ESI (Tables S1 and S2†).
2 and subsequently subjected to SFE at 20 MPa and 70 °C. To evaluate the quality of the performed experiments during the development of IL-SFE for hemp the yields of cannabinoids are expressed as the sum of cannabinoid types e.g. CBD and CBDA are referred to as ∑(CBD). Analogously ∑(THC) and ∑(CBG) are calculated. All experimental conditions and results for individual cannabinoid yields are shown in the ESI (Tables S1 and S2†).
The cannabinoids CBD and CBDA are predominantly accumulated in industrial hemp compared to THC, THCA, CBG and CBGA, which are considered minor compounds.
The pre-treatment with [C2mim][OAc] of industrial hemp at 25 °C and 70 °C indicated comparable cannabinoid yields. Increasing the time from 15 to 60 min at 70 °C in exp. 2 led to a small decrease of roughly 5% ∑(CBD) and 8% ∑(THC). However, similar ∑(CBD), but significantly more CBD (6.58 mg g−1) and less CBDA (6.3 mg g−1) at 60 min, was yielded in exp. 2 compared with exp. 4 (15 min), which led to 5.29 mg g−1 CBD and 8.8 mg g−1 CBDA, respectively (p < 0.05, Fig. 3, Table S2†). It was reported that an extraction process including [C6mim][NTf2] at 60 °C and 50 min leads to high amounts of CBD and that the IL preserves CBD,47 which correlates with the observations herein. In addition, the decarboxylation of cannabinoids at higher temperatures for longer times has been described before.8 The IL [C6mim][NTf2] was not utilized in this study, as the anion [NTf2]− renders it is less suitable to dissolve cellulose compared to the basic [OAc]− or [DMP]− and similarly, the longer alkyl side chain of the cation would be disadvantageous for this purpose.59 Ultimately, [NTf2]− was not considered for the extraction process, as it is hydrophobic and not mixable with H2O and thus, not suitable for the IL recovering process shown in here.
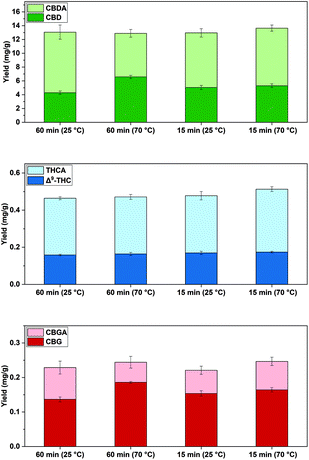 | ||
Fig. 3 Comparison of cannabinoid yields (mg g−1) at different pre-treatment temperatures and pre-treatment times with [C2mim][OAc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and subsequent SFE at 20 MPa and 70 °C, (n = 3 ± SD). Experiments refer to Table 1 for Stage 1. 2 and subsequent SFE at 20 MPa and 70 °C, (n = 3 ± SD). Experiments refer to Table 1 for Stage 1. | ||
A total time of 15 min instead of 60 min seems to be sufficient to release the investigated cannabinoids from the plant tissue with [C2mim][OAc] and hence, allows a significantly shorter pre-treatment time The highest cannabinoids yields were obtained at 70 °C for 15 min in exp. 4, namely 13.6 mg g−1∑(CBD), 0.513 mg g−1∑(THC)and 0.247 mg g−1∑(CBG) (Table 1).
Ratio of ionic liquid to water (Stage 2)
Optimization of temperature and time during the pre-treatment was performed with a constant ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [C2mim][OAc]
2 [C2mim][OAc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O. Here, the influence of several IL
H2O. Here, the influence of several IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios was investigated and compared with the sole use of IL as well as pure H2O in the extraction vessel (Table 2 and Fig. 4).
H2O ratios was investigated and compared with the sole use of IL as well as pure H2O in the extraction vessel (Table 2 and Fig. 4).
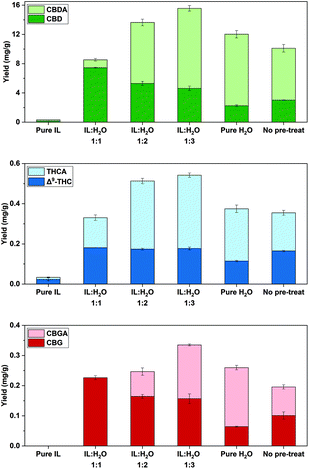 | ||
Fig. 4 Cannabinoid yields (mg g−1) for IL-SFE with pure IL and different IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios, using 15 min of pre-treatment time at 70 °C and for SFE with H2O (pure H2O) as well as for scCO2 (no pre-treat). All extractions were performed at 70 °C and 20 MPa; IL = [C2mim][OAc], (n = 3 ± SD). Experiments refer to Table 2 for Stage 2. H2O ratios, using 15 min of pre-treatment time at 70 °C and for SFE with H2O (pure H2O) as well as for scCO2 (no pre-treat). All extractions were performed at 70 °C and 20 MPa; IL = [C2mim][OAc], (n = 3 ± SD). Experiments refer to Table 2 for Stage 2. | ||
| Exp. | m IL/g | m H2O/g | ∑(CBD) (mg g−1) | ∑(THC) (mg g−1) | ∑(CBG) (mg g−1) |
|---|---|---|---|---|---|
| Mean values with different letters (a, b, c, etc.) within the same column are statistically different (p < 0.05). | |||||
| 4 | 3 | 6 | 13.6 ± 0.6b | 0.513 ± 0.017a | 0.247 ± 0.017b |
| 5 | 3 | 3 | 8.53 ± 0.19e | 0.330 ± 0.014c | 0.226 ± 0.007bc |
| 6 | 3 | 9 | 15.6 ± 0.7a | 0.542 ± 0.016a | 0.335 ± 0.016a |
| 7 | 3 | — | 0.322 ± 0.022f | 0.033 ± 0.006d | n.d. |
| 8 | — | 9 | 12.0 ± 0.6c | 0.375 ± 0.022b | 0.260 ± 0.008b |
| 9 | — | — | 10.1 ± 0.5d | 0.355 ± 0.009bc | 0.196 ± 0.019c |
A decrease of water in the IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio from 1
H2O ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in exp. 4 to 1
2 in exp. 4 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in exp. 5 led to a significant reduction of ∑(CBD) as well as ∑(THC) yield (Table 2) at 20 MPa and 70 °C. However, the significantly highest yield of CBD (7.45 mg g−1) of all performed IL-SFE was obtained under these conditions in exp. 5 (p < 0.05) and additionally, low yields of CBDA (1.09 mg g−1) and no CBGA were extracted (Fig. 4, Table S2†). Therefore, a ratio of 1
1 in exp. 5 led to a significant reduction of ∑(CBD) as well as ∑(THC) yield (Table 2) at 20 MPa and 70 °C. However, the significantly highest yield of CBD (7.45 mg g−1) of all performed IL-SFE was obtained under these conditions in exp. 5 (p < 0.05) and additionally, low yields of CBDA (1.09 mg g−1) and no CBGA were extracted (Fig. 4, Table S2†). Therefore, a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [C2mim][OAc]
1 [C2mim][OAc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O during SFE seems to favour the extraction of neutral CBD and CBG. Recently, it has been discovered that high yields of CBD are extracted by pre-heating hemp and subsequent extraction with supercritical CO2 combined with EtOH as a modifier.29 Similar behaviour can be observed under the previously mentioned IL-SFE conditions, without addition of co-solvents.
H2O during SFE seems to favour the extraction of neutral CBD and CBG. Recently, it has been discovered that high yields of CBD are extracted by pre-heating hemp and subsequent extraction with supercritical CO2 combined with EtOH as a modifier.29 Similar behaviour can be observed under the previously mentioned IL-SFE conditions, without addition of co-solvents.
On the other hand, significantly more ∑(CBD) and ∑(CBG) (p < 0.05) were obtained in exp. 6 by addition of more H2O to increase the ratio of [C2mim][OAc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O from 1
H2O from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The ∑(CBD) yield increased by 15% to 15.6 mg g−1, ∑(THC) by 6% to 0.542 mg g−1 and ∑(CBG) by 36% to (0.335 mg g−1) (Table 2). Adding more than 15 wt% H2O during [C2mim][OAc] pre-treatment does not allow complete cellulose dissolution, as reported by Le et al. in 2012.60 Therefore, H2O was added to the IL after the initial pre-treatment. The addition of H2O resulted in a reduction of the mixture's viscosity, and thus improved mass transport.60 It is reported that the viscosity of [C2mim][OAc] is reduced by 50% when mixed with 10 wt% H2O and that the IL is less viscous at higher temperatures.61 Lower viscosity of the IL
3. The ∑(CBD) yield increased by 15% to 15.6 mg g−1, ∑(THC) by 6% to 0.542 mg g−1 and ∑(CBG) by 36% to (0.335 mg g−1) (Table 2). Adding more than 15 wt% H2O during [C2mim][OAc] pre-treatment does not allow complete cellulose dissolution, as reported by Le et al. in 2012.60 Therefore, H2O was added to the IL after the initial pre-treatment. The addition of H2O resulted in a reduction of the mixture's viscosity, and thus improved mass transport.60 It is reported that the viscosity of [C2mim][OAc] is reduced by 50% when mixed with 10 wt% H2O and that the IL is less viscous at higher temperatures.61 Lower viscosity of the IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture led to higher yields, possibly due to the higher mobility of dissolved cannabinoids and better penetration of scCO2. An increase in carboxylated cannabinoids was observed by adding more water (Fig. 4). Furthermore, water is the only solvent without any negative impacts on the environment. Additionally, it is reported to have low solubility in scCO2
H2O mixture led to higher yields, possibly due to the higher mobility of dissolved cannabinoids and better penetration of scCO2. An increase in carboxylated cannabinoids was observed by adding more water (Fig. 4). Furthermore, water is the only solvent without any negative impacts on the environment. Additionally, it is reported to have low solubility in scCO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 and therefore less potential contamination of the extract.
62 and therefore less potential contamination of the extract.
The absence of H2O during the extraction with scCO2 and [C2mim][OAc] (pure IL) led to the lowest yields of all SFE in exp. 7 (Table 2 and Fig. 4). Low yields can be a result of the high viscosity of the IL, which leads to less permeability of scCO2 and subsequently lower mass transfer in the extraction. Therefore, dilution with H2O is essential during the extraction process.
However, the sole extraction with H2O (pure H2O) in the absence of [C2mim][OAc] in exp. 8 compared to exp. 6, leads to a significant reduction of ∑(CBD) by 23% to 12.0 mg g−1, ∑(THC) by 31% to 0.375 mg g−1 and ∑(CBG) by 22% to 0.260 mg g−1 (p < 0.05, Table 2). In particular, the use of H2O alone tends to yield fewer neutral cannabinoids (Fig. 4), which verifies what has previously been reported; ILs preserve neutral CBD.47 When comparing exp. 8 with exp. 4, even though the same total quantity of liquid was added in the high-pressure vessel, significantly less yields of ∑(CBD) by 12% and ∑(THC) by 27% are observed (p < 0.05, Table 2, Fig. 4) in the sole water-based SFE extraction. Therefore, a pre-treatment with IL to liberate the cannabinoids from the plant tissue and subsequent dilution with H2O positively affects the yield.
Ultimately, a reference scCO2 extraction in the absence of both IL and H2O in exp. 9 (no pre-treatment) yielded 10.1 mg g−1∑(CBD), 0.355 mg g−1∑(THC), 0.196 mg g−1∑(CBG) at 70 °C and 20 MPa (Table 2). Thus, IL-SFE with [C2mim][OAc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1
H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in exp. 6 and 1
3 in exp. 6 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in exp. 4, led to significantly higher yields of ∑(CBD, THC, CBG) than sole SFE (p < 0.05). It has been reported that the cannabinoid yields during SFE can be enhanced by adding EtOH as a modifier.26,28 In preliminary studies SFE with EtOH as a modifier at different temperatures and vol% EtOH as well as various conventional ethanolic extractions were carried out with another batch of industrial hemp. High yields of the targeted cannabinoids were obtained at 35 °C, 10 MPa and 120 min dynamic extraction with 10 and 20 vol% EtOH. In comparison to the performed conventional extraction, similar ∑(THC) yields, but less ∑(CBD) and ∑(CBG) were yielded (p < 0.05, Table S3†). All data is presented in the ESI.†
2 in exp. 4, led to significantly higher yields of ∑(CBD, THC, CBG) than sole SFE (p < 0.05). It has been reported that the cannabinoid yields during SFE can be enhanced by adding EtOH as a modifier.26,28 In preliminary studies SFE with EtOH as a modifier at different temperatures and vol% EtOH as well as various conventional ethanolic extractions were carried out with another batch of industrial hemp. High yields of the targeted cannabinoids were obtained at 35 °C, 10 MPa and 120 min dynamic extraction with 10 and 20 vol% EtOH. In comparison to the performed conventional extraction, similar ∑(THC) yields, but less ∑(CBD) and ∑(CBG) were yielded (p < 0.05, Table S3†). All data is presented in the ESI.†
The addition of EtOH as a co-solvent to IL-SFE would lead to the extraction of both IL and cannabinoids, thus, leading to impurities in the extract. In particular, IL-SFE does not require the use of a co-solvent to obtain cannabinoids in high yields, avoiding further solvent consumption.
Hence, the highest extraction yields were obtained with a IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 1
H2O ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in exp. 6, which achieved 15.6 mg g−1∑(CBD), 0.542 mg g−1∑(THC) and 0.335 mg g−1∑(CBG) (Table 2).
3 in exp. 6, which achieved 15.6 mg g−1∑(CBD), 0.542 mg g−1∑(THC) and 0.335 mg g−1∑(CBG) (Table 2).
SFE extraction parameters – pressure and temperature (Stage 3)
Apart from the optimization of pre-treatment conditions and the ratio of [C2mim][OAc] to H2O, temperature and pressure during SFE were investigated (Table 3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 1
H2O ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Stage 3)
3 (Stage 3)
| Exp. | P SFE/MPa | T SFE/°C | ∑(CBD) (mg g−1) | ∑(THC) (mg g−1) | ∑(CBG) (mg g−1) |
|---|---|---|---|---|---|
| Mean values with different letters (a, b, c, etc.) within the same column are statistically different (p < 0.05). | |||||
| 6 | 20 | 70 | 15.6 ± 0.7a | 0.542 ± 0.016a | 0.335 ± 0.016a |
| 10 | 10 | 70 | 3.66 ± 0.06d | 0.0885 ± 0.0026d | 0.045 ± 0.008c |
| 11 | 15 | 70 | 13.00 ± 0.19c | 0.457 ± 0.005c | 0.257 ± 0.009b |
| 12 | 30 | 70 | 14.7 ± 0.7ab | 0.500 ± 0.024ab | 0.36 ± 0.04a |
| 13 | 20 | 35 | 14.9 ± 0.7ab | 0.493 ± 0.014bc | 0.323 ± 0.009a |
| 14 | 10 | 35 | 13.7 ± 0.8bc | 0.468 ± 0.019bc | 0.248 ± 0.010b |
Initially, the pressure was reduced from 20 MPa in exp. 6 to 15 MPa in exp. 11 at 70 °C and led to a significant reduction by 17% ∑(CBD) to 13.00 mg g−1, 16% ∑(THC) to 0.457 mg g−1 and 23% ∑(CBG) to 0.245 mg g−1 (p < 0.05, Table 3). After further decreasing the pressure to 10 MPa in exp. 10, a significantly diminished yield of 3.66 mg g−1∑(CBD), 0.0885 mg g−1∑(THC), and 0.045 mg g−1∑(CBG) was observed (Table 3). Even though lower cannabinoid yields were obtained at 10 MPa and 70 °C in exp. 10, the extraction of neutral cannabinoids was favoured (Fig. 5). In literature, sole scCO2 extractions yield neither CBD nor CBDA at 10 MPa at 70 °C for 120 min,63 but SFE can be improved upon by adding EtOH26 or by the pre-treatment with IL, as herein reported. In addition, the pressure was increased to 30 MPa at 70 °C in exp. 12, which resulted in comparable yields of ∑(CBD, THC, CBG) as IL-SFE at 20 MPa in exp. 6 (Table 3). It can be assumed that 20 MPa at 70 °C are sufficient to extract cannabinoids during IL-SFE.
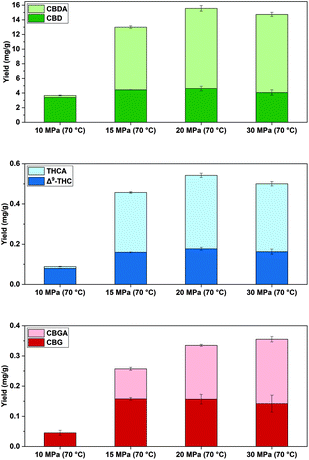 | ||
Fig. 5 Cannabinoid yields (mg g−1) at 10, 15, 20 and 30 MPa at 70 °C by scCO2 extraction combined with [C2mim][OAc]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and a pre-treatments at 70 °C for 15 min, (n = 3 ± SD). Experiments refer to Table 3 for Stage 3. 3 and a pre-treatments at 70 °C for 15 min, (n = 3 ± SD). Experiments refer to Table 3 for Stage 3. | ||
Furthermore, the temperature was lowered to 35 °C at 20 MPa during SFE in exp. 13. This led to comparable yields of ∑(CBD) and ∑(CBG), but significantly lower ∑(THC) yields (0.493 mg g−1) compared to 70 °C in exp. 6 (p < 0.05, Table 3). This corresponds to literature data, where similar yields of ∑(CBD) were extracted during SFE at 35 °C and 70 °C at 50 MPa.63 Lower temperatures are known to reduce the viscosity of H2O and additionally, have been reported to decrease the viscosity of [C2mim][OAc].61 Hence, the mixture is less penetrable for scCO2 to extract the target cannabinoids. In comparison of exp. 13 and exp. 6, the yields of decarboxylated cannabinoids decreased significantly (CBD by 28%; Δ9-THC by 16%; CBG by 33%) and similar yields of THCA and CBDA, but significantly more CBGA by 23% was obtained in exp. 13 (p < 0.05, Table S3†).
A further decrease from 20 MPa at 35 °C in exp. 13 to 10 MPa in exp. 14 led to a slight reduction in ∑(CBD) by 8% and ∑(THC) by 5%, and significant reduction in ∑(CBG) by 23% (p < 0.05, Table 3). Therefore, a combination of 35 °C and 10 MPa seems to affect the total cannabinoid yield negatively, but increasing the temperature to 70 °C at the same pressure further reduces the yields. Lower CBD and CBDA yields at 10 MPa at 70 °C compared with 35 °C during SFE have been described in literature.63 Thus, 10 MPa at 70 °C during SFE seem to be unfeasible to extract cannabinoids from industrial hemp. At 20 MPa, the temperature seems to have a minor effect on the total yields of cannabinoids.
Consequently, the optimum cannabinoid yields were obtained at 20 MPa and 70 °C in exp. 6 during supercritical CO2 extraction.
Type of ionic liquid
Two additional ILs, namely, [Ch][OAc] and [C2mim][DMP], were selected for evaluation alongside [C2mim][OAc]. The optimized extraction conditions, with a pre-treatment at 70 °C for 15 min and subsequent SFE at 70 °C and 20 MPa with a IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 1
H2O ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, were additionally applied to these two ILs to observe differences in cannabinoid yields (Table 4 and Fig. 6).
3, were additionally applied to these two ILs to observe differences in cannabinoid yields (Table 4 and Fig. 6).
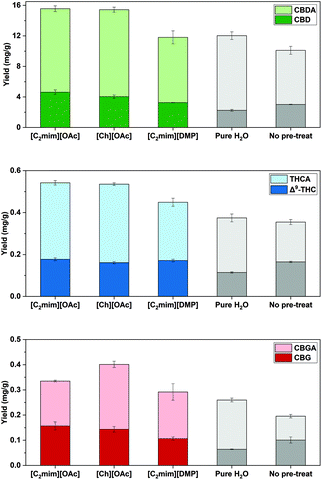 | ||
Fig. 6 Cannabinoids yields (mg g−1) for IL-SFE with [C2mim][OAc], [Ch][OAc] as well as [C2mim][DMP] (IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), for SFE with H2O (pure H2O) and for scCO2 (no pre-treat). All extractions were performed at 70 °C and 20 MPa, (n = 3 ± SD). Pure H2O (exp. 8) and scCO2 (no pre-treat) (exp. 9) refer to Table 2. [C2mim][OAc] (exp. 6), [Ch][OAc] (exp. 15) and [C2mim][DMP] (exp. 16) refer to Table 4. 3), for SFE with H2O (pure H2O) and for scCO2 (no pre-treat). All extractions were performed at 70 °C and 20 MPa, (n = 3 ± SD). Pure H2O (exp. 8) and scCO2 (no pre-treat) (exp. 9) refer to Table 2. [C2mim][OAc] (exp. 6), [Ch][OAc] (exp. 15) and [C2mim][DMP] (exp. 16) refer to Table 4. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 IL
3 IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O at 70 °C and 20 MPa during SFE
H2O at 70 °C and 20 MPa during SFE
| Exp. | IL or solvent | ∑(CBD) (mg g−1) | ∑(THC) (mg g−1) | ∑(CBG) (mg g−1) |
|---|---|---|---|---|
| Mean values with different letters (a, b, c, etc.) within the same column are statistically different (p < 0.05).a At 70 °C. | ||||
| 6 | [C2mim][OAc] | 15.6 ± 0.7a | 0.542 ± 0.016a | 0.335 ± 0.016c |
| 15 | [Ch][OAc] | 15.4 ± 0.5a | 0.535 ± 0.010ab | 0.401 ± 0.024b |
| 16 | [C2mim][DMP] | 11.8 ± 0.9b | 0.449 ± 0.025c | 0.292 ± 0.028c |
| 17a | EtOH for 2 h | 15.4 ± 0.4a | 0.498 ± 0.018b | 0.452 ± 0.019a |
| 18a | EtOH for 24 h | 14.84 ± 0.15a | 0.447 ± 0.005c | 0.440 ± 0.004ab |
| 19a | H2O for 2 h | 1.6 ± 0.3c | 0.057 ± 0.012d | 0.031 ± 0.007d |
Ionic liquid assisted SFE with [Ch][OAc] in exp. 15 yielded comparable yields of ∑(CBD) (15.4 mg g−1) and ∑(THC) (0.535 mg g−1), but significantly more ∑(CBG) (0.401 mg g−1) than IL-SFE with [C2mim][OAc] in exp. 6 (p < 0.05, Table 4). The change of cation does affect the yields of cannabinoids, however, the role of the cation during the dissolution of lignocellulose structure is not yet fully understood.64 On the other hand, anions, such as [OAc]−, are described to effectively support the dissolution of cellulose by forming hydrogen bonds.34
To investigate the influence of the anion in IL-SFE of cannabinoids from industrial hemp, the imidazolium-based IL [C2mim][DMP] was used in exp. 16. This resulted in a significant reduction of ∑(CBD) to 11.8 mg g−1 and total THC to 0.449 mg g−1 compared with the acetate-based ILs in exp. 6 and exp. 15 (p < 0.05, Table 4). [C2mim][DMP] is described as effectively dissolving biomass, but has a high viscosity,56,65 which could affect the extraction at supercritical conditions, due to the weaker penetration of scCO2. Nonetheless, phosphate based and acetate based IL-SFE yielded higher amounts of ∑(CBD, THC, CBG) compared with sole supercritical CO2 extraction without IL pre-treatment (Fig. 6).
The following mechanism can be proposed for IL-SFE. Firstly, the biomass is partially dissolved by breaking down the lignocellulose structure of the industrial hemp powder. This depends on the anion and cation of the ILs.34,64 The cannabinoids are released from the plant tissues and the IL possibly stabilizes them.47 Secondly, the water is added, which reduces the viscosity of the mixture61 and lowers the solubility of the target cannabinoids. Due to the lower surface tension and higher mobility of cannabinoids, a higher mass transfer between the scCO2 phase and the IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O phase is generated. As reported the scCO2 dissolves in ILs, however, neither the IL nor the H2O does dissolve in scCO2.38,62 Finally, these synergic effects allow the scCO2 to extract the targeted cannabinoids, due to better solubility in the supercritical phase without contaminating it with IL or H2O. Thus, no further organic solvents are necessary to purify the compounds from the IL phase and consequently, no additional work up is needed to obtain a solid and solvent free product (Fig. 2).
H2O phase is generated. As reported the scCO2 dissolves in ILs, however, neither the IL nor the H2O does dissolve in scCO2.38,62 Finally, these synergic effects allow the scCO2 to extract the targeted cannabinoids, due to better solubility in the supercritical phase without contaminating it with IL or H2O. Thus, no further organic solvents are necessary to purify the compounds from the IL phase and consequently, no additional work up is needed to obtain a solid and solvent free product (Fig. 2).
Ultimately, IL-SFE was compared with reference solvent extraction (exp. 17–19). Ethanol is one of the most commonly used solvents to extract cannabinoids.66 Herein, a conventional extraction for 2 h, at 70 °C, with EtOH in exp. 17, sufficiently extracted the investigated cannabinoids; however, employing H2O in exp. 19 alone under the same conditions, low yields of cannabinoids were obtained (Table 4). A control extraction in EtOH for 24 h was carried out in exp. 18 to investigate the influence of longer extraction times. Longer times at high temperatures seem to degrade carboxylated cannabinoids significantly, reducing CBDA by 52%, THCA by 65% and CBGA by 53% (p < 0.05, Table S2†). The decarboxylation of cannabinoic acids at high temperatures for longer times is described in literature.8 However, it can be reported that the degradation over time does not affect the overall cannabinoid yields.
By comparing the two-hour ethanolic extraction (exp. 17) with acetate based ionic liquid-SFE, several differences can be observed. Firstly, the yields of ∑(CBD) by SFE with [C2mim][OAc] in exp. 6 and [Ch][OAc] in exp. 15 are slightly higher but comparable to the 2 h ethanolic extraction (Table 4). Secondly, significantly more ∑(THC) with [C2mim][OAc] compared with the two-hour reference extraction with EtOH is obtained. Ultimately, the reference extraction yielded more ∑(CBG) than IL-SFE using [Ch][OAc] or [C2mim][OAc] (p < 0.05, Table 4). Hence, the results underline the importance of appropriately selecting the IL cation and anion, as well as the optimal extraction parameters for IL-SFE to extract cannabinoids from industrial hemp. Ultimately, [Ch][OAc] based SFE yields high amounts of the investigated cannabinoids and also provides environmental and economic benefits. Not only is [Ch][OAc] biodegradable, but it is also considered relatively cheap (88 € for 25 g), easy to synthesize, as well as less toxic compared to other ionic liquids.57,58,67,68 Furthermore, no co-solvents are applied during IL-SFE, which avoids additional solvent consumption and consequently leads to a purer, solid extract (Fig. S2†). Ultimately, all three ILs were purified without any additional use of organic solvents. Neither water nor significant impurities were detected by NMR spectroscopic analysis for the purified ILs (Table S4 and Fig. S5–S7†) and thus, can be re-used for IL-SFE.
Conclusions
Herein, we report a novel IL-based dynamic supercritical CO2 extraction process for the isolation of cannabinoids from Cannabis sativa L. The investigation showed that 15 min at 70 °C pre-treatment of hemp with [C2mim][OAc] and [Ch][OAc], dilution of IL with H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and ultimately, scCO2 extraction at 20 MPa and 70 °C for 2 h, led to high yields of the investigated cannabinoids. Acetate-based ILs resulted in higher yields of cannabinoids compared to phosphate-based ILs. In addition, IL-SFE with [C2mim][OAc] yielded significantly more ∑(THC) than conventional extraction with EtOH. Hence, the type of IL is of great importance and affects the cannabinoid yield significantly. However, not only the type of IL needs to be selected carefully, also the SFE parameters. In dependence of various parameters, e.g. IL pre-treatment temperature or the ratio of IL
3) and ultimately, scCO2 extraction at 20 MPa and 70 °C for 2 h, led to high yields of the investigated cannabinoids. Acetate-based ILs resulted in higher yields of cannabinoids compared to phosphate-based ILs. In addition, IL-SFE with [C2mim][OAc] yielded significantly more ∑(THC) than conventional extraction with EtOH. Hence, the type of IL is of great importance and affects the cannabinoid yield significantly. However, not only the type of IL needs to be selected carefully, also the SFE parameters. In dependence of various parameters, e.g. IL pre-treatment temperature or the ratio of IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O during SFE, it is possible to adjust the proportion of carboxylated and decarboxylated cannabinoids in the extracts. In addition, IL-SFE allows extracting cannabinoids in highest yields and, therefore, it can be reported as a novel competitive alternative to traditional extraction techniques or supercritical fluid extraction with co-solvents. Ultimately, the ILs can be recycled without additional usage of further organic solvents to reduce costs and improve the sustainability of the process. IL-SFE offers the opportunity to extract secondary metabolites from different natural sources without volatile organic solvents and the presented process has great potential for future industrial applications.
H2O during SFE, it is possible to adjust the proportion of carboxylated and decarboxylated cannabinoids in the extracts. In addition, IL-SFE allows extracting cannabinoids in highest yields and, therefore, it can be reported as a novel competitive alternative to traditional extraction techniques or supercritical fluid extraction with co-solvents. Ultimately, the ILs can be recycled without additional usage of further organic solvents to reduce costs and improve the sustainability of the process. IL-SFE offers the opportunity to extract secondary metabolites from different natural sources without volatile organic solvents and the presented process has great potential for future industrial applications.
Experimental
Plant material
The type III chemovar Futura 75 was cultivated in Austria, in the fields of Biobloom (Apetlon, Austria, 7°41′23.4′′N 16°56′26.7′′E), in September 2020. After the harvest, the plants (flowers, leaves and stems) were stored under mild conditions at 40 °C for 14 h. The samples were milled with a Fritsch Universal Pulverisette 19 mill through a 2 mm sieve (Fritsch, Oberstein, Germany). The dry matter was 94.73 ± 0.05 wt% (n = 3). A second batch of the same industrial hemp harvested in 2019 was used for the preliminary experiments, mentioned in section Results and discussion. The dry matter was 93.68 ± 0.03 wt% (n = 3). The hemp raw material was stored in the dark, at −20 °C, between experiments.Ionic liquid-supercritical fluid extraction
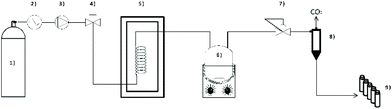
For pre-treatment, a high-pressure vessel of approximately 50 mL (EV-3), produced by Jasco (Jasco Corporation, Tokyo, Japan), containing one input and one output connections on the lid, was used. The batch reactor was charged with 0.20 g milled hemp and 3 g of IL. [C2mim][OAc] (≥90%) was purchased from BASF (Ludwigshafen am Rhein, Germany), [Ch][OAc] (98%) from IoLiTec (Heilbronn, Germany) and [C2mim][DMP] (98%) from ABCR (Karlsruhe, Germany). Pre-treatment optimization was performed for 15 min and 60 min, at 25 °C and 70 °C, respectively, with [C2mim][OAc]. Furthermore, [C2mim][OAc] was diluted with different amounts of H2O (filtered through a Milli-Q ion exchange system) after the pre-treatment to evaluate the effect on extraction efficiency. Therefore, 3 g of IL were mixed with 3 g, 6 g and 9 g of H2O and stirred for 10 min before SFE. In addition, extraction purely with [C2mim][OAc], without the addition of water, was tested.The SFE setup is presented in Fig. 7. All extractions were performed with a scCO2 device manufactured by Jasco (Jasco Corporation, Tokyo, Japan). Liquid CO2 (>99.995% purity; with ascension pipe; Messer GmbH, Vienna, Austria) was pressurized by two CO2-pumps (PU-2086, Jasco Corporation, Tokyo, Japan) with cooled heads (CF40, JULABO GmbH, Seelbach, Germany). An oven (CO-2060, Jasco Corporation, Tokyo, Japan) with a heating coil was used and was thermostated to the desired temperature. The vessel containing the IL pre-treated hemp was placed on a heating mantle set to a certain temperature and a stirring rate of 500 rpm and, subsequently, connected to the supercritical carbon dioxide (scCO2) device. A back-pressure regulator (BP-2080, Jasco Corporation, Tokyo, Japan), a gas/liquid separator (HC-2086-01, Jasco Corporation, Tokyo, Japan), and a product collector (SCF-Vch-Bp, Jasco Corporation, Tokyo, Japan) were used to obtain the extracts.
The conditions employed for the SFE of cannabinoids were based on literature data63,69 and adapted for our purposes. The CO2 flow rate, the static extraction and the dynamic extraction were set to 5.0 mL min−1, 30 min and 120 min, respectively. Different variables were evaluated during SFE, e.g., oven temperature, heating mantle temperature (35 °C and 70 °C, respectively) and pressure (10 MPa, 15 MPa, 20 MPa and 30 MPa), using [C2mim][OAc]. Ultimately, the optimized conditions were applied to [Ch][OAc] and [C2mim][DMP]. After each extraction, the extracts were collected and diluted to a defined volume with ethanol and prepared for analysis by HPLC. EtOH was purchased from Chem-Lab (Zedelgem, Belgium, abs.).
Solvent-based extraction
For comparison, conventional solvent extractions were performed in 30 mL Teflon screw cap vials. The hemp quantity used in each extraction was 0.2 g. Two extractions were performed in triplicate using 2 mL solvent, more precisely, H2O and EtOH, for 2 h at 70 °C and a third one, also in triplicate, using 10 mL EtOH for 24 h and 70 °C.70Ionic liquid recovering
After extraction, the scCO2 device was depressurized, the metallic extraction reactor was disconnected and brought to room temperature. The IL–water–hemp mixture (Fig. S3†) was filtered to remove hemp particles, the water was evaporated in vacuo and the remaining ionic liquid was dried under vacuum (0.65 mbar) for 24 h. Afterwards 20 mg of purified IL (Fig. S4†) were dissolved in chloroform-d3 (Sigma Aldrich, St Louis, USA) and a 1H-NMR was recorded with a 400 MHz Bruker Advanced Ultra Shield 400 spectrometer (Bruker, Billerica, USA). Spectroscopic data and NMR spectra are given in the ESI (Table S4 and Fig. S5–7†)Cannabinoid quantification
The determination of CBDA, CBD, CBGA, CBG, THCA, Δ9-THC, was carried out on a High-Performance Liquid Chromatography (HPLC) in a Dionex UltiMate© RSLC System, with DAD-3000RS Photodiode Array Detector (Thermo Scientific, Germering, Germany), on a Dionex Acclaim™ RSLC 120 C18 (2.2 μm, 120 Å, 2.1 × 150 mm, Bonded Silica Products: no. 01425071, Thermo Scientific, Germering, Germany). A mobile phase flow rate of 0.2 mL min−1 was employed and the oven temperature was set to 25 °C. As a mobile phase, H2O with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B) were used. The following gradient was carried out: 2 min of pre-equilibration at 70% B, 6 min hold at 70% B, 6 min from 70% B to 77% B, 18 min hold at 77% B, 0.5 min from 77% B to 95% B, 1.5 min at 95% B, 0.5 min from 95% B to 70% B, and 5 min at 70% B.71 Acetonitrile was purchased from VWR Chemicals (Radnor, PA, USA) and formic acid from Merck (Darmstadt, Germany). All solvents for HPLC were of analytical grade.The cannabinoid standards CBD, CBDA, THCA, Δ9-THC, CBG and CBGA were provided by Medical Cannabinoids Research and Analysis GmbH (Brunn am Gebirge, Austria) in the course of previous joint research. A mixed cannabinoid stock solution (1 mg mL−1) in MeOH of the investigated cannabinoids diluted for calibration.
Statistical analysis
Statistical data analysis was performed with Origin 2021. One-way ANOVA for multiple groups, followed by Tukey honestly significant difference (HSD) post hoc test at the 0.05 significance level, was carried out.Addendum
The authors would like to point out that the focus of this study was the extraction of cannabinoids as a class, not THC specifically. Any THC extraction is purely incidental, and bound to be negligible, given that industrial hemp was used, which in the EU must have a THC content not in excess of 0.2%.The relevant EU law can be perused under: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32013R1307&from=de.
In particular, we refer to Article 32, paragraph 6.
Additionally, the authors do hold a licence to for the purposes of research, in accordance with Austrian law, available under:
https://www.ris.bka.gv.at/GeltendeFassung.wxe?Abfrage=Bundesnormen&Gesetzesnummer=10011053.
Author contributions
C.K. & A.S.M.: conceived the research, designed and performed the experiments, analysed the data, wrote the original draft, edited and reviewed the manuscript. M.S.: supervised the research, edited and reviewed the manuscript. K.S. & H.H.: conceived and supervised the research, designed the experiments, edited and reviewed the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.Acknowledgements
The authors acknowledge TU Wien for the Open Access Funding Programme of TU Wien Bibliothek for financial support and for the funding of the Doctoral College “Bioactive” (https://bioactive.tuwien.ac.at/home/), Christian Löfke (Biobloom, Apetlon, Austria) for kindly providing the plant material, Renate Paltram for the technical assistance during cannabinoid quantification and Kristof Stagel for the support of recovering ionic liquids. This project has also received funding from the European Research Council (ERC) under the Horizon 2020 research and innovation programme (Grant agreement No. 864991)References
- J. F. Hancock, Plant evolution and the origin of crop species, CABI, 2012 Search PubMed.
- E. Small, Cannabis: a complete guide, CRC Press, 2016 Search PubMed.
- C. Kornpointner, A. Sainz Martinez, S. Marinovic, C. Haselmair-Gosch, P. Jamnik, K. Schröder, C. Löfke and H. Halbwirth, Ind. Crops Prod., 2021, 165, 113422 CrossRef CAS.
- D. J. Potter, in Handbook of Cannabis, ed. R. Pertwee, Oxford Scholarship Online, 2014, pp. 82–83 Search PubMed.
- M. ElSohly and W. Gul, in Handbook of Cannabis, ed. R. Pertwee, Oxford Scholarship Online, 2014, p. 1093 Search PubMed.
- R. Brenneisen, in Marijuana and the Cannabinoids, ed. M. A. ElSohly, Springer, 2007, pp. 17–49 Search PubMed.
- I. J. Flores-Sanchez and R. Verpoorte, Phytochem. Rev., 2008, 7, 615–639 CrossRef CAS.
- M. Wang, Y.-H. Wang, B. Avula, M. M. Radwan, A. S. Wanas, J. van Antwerp, J. F. Parcher, M. A. ElSohly and I. A. Khan, Cannabis Cannabinoid Res., 2016, 1, 262–271 CrossRef CAS PubMed.
- N. M. Kogan and R. Mechoulam, Dialogues Clin. Neurosci., 2007, 9, 413–430 Search PubMed.
- R. Mechoulam, L. A. Parker and R. Gallily, J. Clin. Pharmacol., 2002, 42, 11–19 CrossRef PubMed.
- S. P. H. Alexander, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2016, 64, 157–166 CrossRef CAS PubMed.
- A. Hazekamp, R. Simons, A. Peltenburg-Looman, M. Sengers, R. van Zweden and R. Verpoorte, J. Liq. Chromatogr. Relat. Technol., 2004, 27, 2421–2439 CrossRef CAS.
- L. L. Romano and A. Hazekamp, Cannabinoids, 2013, 1, 1–11 Search PubMed.
- C.-W. Chang, C.-C. Yen, M.-T. Wu, M.-C. Hsu and Y.-T. Wu, Molecules, 2017, 22, 1894 CrossRef PubMed.
- A. C. Gallo-Molina, H. I. Castro-Vargas, W. F. Garzón-Méndez, J. A. M. Ramírez, Z. J. R. Monroy, J. W. King and F. Parada-Alfonso, J. Supercrit. Fluids, 2019, 146, 208–216 CrossRef CAS.
- Q. W. Zhang, L. G. Lin and W. C. Ye, Chin. Med., 2018, 13, 1–26 CrossRef CAS PubMed.
- Z. Drinić, J. Vladić, A. Koren, T. Zeremski, N. Stojanov, B. Kiprovski and S. Vidović, J. Chem. Technol. Biotechnol., 2020, 95, 831–839 CrossRef.
- S. Al Jitan, S. A. Alkhoori and L. F. Yousef, in Studies in Natural Products Chemistry, ed. A. Rahman, Elsevier, 2018, vol. 58, pp. 389–417 Search PubMed.
- C. Agarwal, K. Máthé, T. Hofmann and L. Csóka, J. Food Sci., 2018, 83, 700–710 CrossRef CAS PubMed.
- C. Ajila, S. Brar, M. Verma, R. Tyagi, S. Godbout and J. Valero, Crit. Rev. Biotechnol., 2011, 31, 227–249 CrossRef CAS PubMed.
- M. De Melo, A. Silvestre and C. Silva, J. Supercrit. Fluids, 2014, 92, 115–176 CrossRef CAS.
- B. A. S. Machado, C. G. Pereira, S. B. Nunes, F. F. Padilha and M. A. Umsza-Guez, Sep. Sci. Technol., 2013, 48, 2741–2760 CrossRef CAS.
- A. Sainz Martinez, C. Kornpointner, C. Haselmair-Gosch, M. Mikulic-Petkovsek, K. Schröder and H. Halbwirth, LWT, 2021, 138, 110633 CrossRef CAS.
- H. Perrotin-Brunel, P. C. Perez, M. J. van Roosmalen, J. van Spronsen, G.-J. Witkamp and C. J. Peters, J. Supercrit. Fluids, 2010, 52, 6–10 CrossRef CAS.
- H. Perrotin-Brunel, M. C. Kroon, M. J. Van Roosmalen, J. Van Spronsen, C. J. Peters and G.-J. Witkamp, J. Supercrit. Fluids, 2010, 55, 603–608 CrossRef CAS.
- L. J. Rovetto and N. V. Aieta, J. Supercrit. Fluids, 2017, 129, 16–27 CrossRef CAS.
- E. Vági, M. Balázs, A. Komoczi, M. Mihalovits and E. Székely, J. Supercrit. Fluids, 2020, 164, 104898 CrossRef.
- J. Omar, M. Olivares, M. Alzaga and N. Etxebarria, J. Sep. Sci., 2013, 36, 1397–1404 CrossRef CAS PubMed.
- D. R. Grijó, I. A. V. Osorio and L. Cardozo-Filho, J. CO2 Util., 2018, 28, 174–180 CrossRef.
- H. Wang, G. Gurau and R. D. Rogers, in Structures and Interactions of Ionic Liquids. Structure and Bonding, ed. S. Zhang, J. Wang, X. Lu and Q. Zhou, Springer, 2014, vol. 151, pp. 79–105 Search PubMed.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC.
- Y. Li, J. Wang, X. Liu and S. Zhang, Chem, 2018, 9, 4027–4043 CAS.
- S. P. Ventura, F. A. e Silva, M. V. Quental, D. Mondal, M. G. Freire and J. A. Coutinho, Chem. Rev., 2017, 117, 6984–7052 CrossRef CAS PubMed.
- A. K. Ressmann, K. Strassl, P. Gaertner, B. Zhao, L. Greiner and K. Bica, Green Chem., 2012, 14, 940–944 RSC.
- E. G. García, A. K. Ressmann, P. Gaertner, R. Zirbs, R. L. Mach, R. Krska, K. Bica and K. Brunner, Anal. Bioanal. Chem., 2014, 406, 7773–7784 CrossRef PubMed.
- L. A. Blanchard, Z. Gu and J. F. Brennecke, J. Phys. Chem. B, 2001, 105, 2437–2444 CrossRef CAS.
- R. Liu, P. Zhang, S. Zhang, T. Yan, J. Xin and X. Zhang, Rev. Chem. Eng., 2016, 32, 587–609 CAS.
- M. F. Sellin, P. B. Webb and D. J. Cole-Hamilton, Chem. Commun., 2001, 781–782 RSC.
- P. B. Webb, M. F. Sellin, T. E. Kunene, S. Williamson, A. M. Slawin and D. J. Cole-Hamilton, J. Am. Chem. Soc., 2003, 125, 15577–15588 CrossRef CAS PubMed.
- F. Liu, M. B. Abrams, R. T. Baker and W. Tumas, Chem. Commun., 2001, 433–434 RSC.
- A. Sainz Martinez, C. Hauzenberger, A. R. Sahoo, Z. Csendes, H. Hoffmann and K. Bica, ACS Sustainable Chem. Eng., 2018, 6, 13131–13139 CrossRef CAS.
- U. Hintermair, G. Zhao, C. C. Santini, M. J. Muldoon and D. J. Cole-Hamilton, Chem. Commun., 2007, 1462–1464 RSC.
- R. A. Brown, P. Pollet, E. McKoon, C. A. Eckert, C. L. Liotta and P. G. Jessop, J. Am. Chem. Soc., 2001, 123, 1254–1255 CrossRef CAS PubMed.
- L. A. Blanchard, D. Hancu, E. J. Beckman and J. F. Brennecke, Nature, 1999, 399, 28–29 CrossRef.
- C. Cai, Y. Wang, Y. Yi, F. Li and Z. Tan, Ind. Crops Prod., 2020, 155, 112796 CrossRef CAS.
- K. S. Salem, V. Naithani, H. Jameel, L. Lucia and L. Pal, Global Challenges, 2021, 5, 2000065 CrossRef PubMed.
- Y. Li, X. Liu, S. Zhang, Y. Yao, X. Yao, J. Xu and X. Lu, Phys. Chem. Chem. Phys., 2015, 17, 17894–17905 RSC.
- H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peters, Green Chem., 2008, 10, 696–705 RSC.
- Q. Zhang, J. Hu and D.-J. Lee, Renew. Energy, 2017, 111, 77–84 CrossRef CAS.
- E. Bahcegul, S. Apaydin, N. I. Haykir, E. Tatli and U. Bakir, Green Chem., 2012, 14, 1896–1903 RSC.
- L. Hu, H. Peng, Q. Xia, Y. Zhang, R. Ruan and W. Zhou, J. Mol. Struct., 2020, 1210, 128067 CrossRef CAS.
- S. H. Lee, T. V. Doherty, R. J. Linhardt and J. S. Dordick, Biotechnol. Bioeng., 2009, 102, 1368–1376 CrossRef CAS PubMed.
- F. Cheng, H. Wang, G. Chatel, G. Gurau and R. D. Rogers, Bioresour. Technol., 2014, 164, 394–401 CrossRef CAS PubMed.
- Q. Li, X. Jiang, Y. He, L. Li, M. Xian and J. Yang, Appl. Microbiol. Biotechnol., 2010, 87, 117–126 CrossRef CAS PubMed.
- T. P. T. Pham, C.-W. Cho and Y.-S. Yun, Water Res., 2010, 44, 352–372 CrossRef CAS PubMed.
- A. Jordan and N. Gathergood, Chem. Soc. Rev., 2015, 44, 8200–8237 RSC.
- K. C. Badgujar and B. M. Bhanage, Bioresour. Technol., 2015, 178, 2–18 CrossRef CAS PubMed.
- K. A. Le, R. Sescousse and T. Budtova, Cellulose, 2012, 19, 45–54 CrossRef CAS.
- S. Fendt, S. Padmanabhan, H. W. Blanch and J. M. Prausnitz, J. Chem. Eng. Data, 2011, 56, 31–34 CrossRef CAS.
- Z. Wang, Q. Zhou, H. Guo, P. Yang and W. Lu, Fluid Ph. Equilibria, 2018, 476, 170–178 CrossRef CAS.
- V. Kitryte, D. Bagdonaite and P. R. Venskutonis, Food Chem., 2018, 267, 420–429 CrossRef CAS PubMed.
- B. D. Rabideau, A. Agarwal and A. E. Ismail, J. Phys. Chem. B, 2013, 117, 3469–3479 CrossRef CAS PubMed.
- B. Zheng, C. Harris, S. R. Bhatia and M. F. Thomas, Polym. Adv. Technol., 2019, 30, 1751–1758 CrossRef CAS.
- F. Fathordoobady, A. Singh, D. D. Kitts and A. Pratap Singh, Food Rev. Int., 2019, 35, 664–684 CrossRef CAS.
- D. Rengstl, V. Fischer and W. Kunz, Phys. Chem. Chem. Phys., 2014, 16, 22815–22822 RSC.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- V. Brighenti, F. Pellati, M. Steinbach, D. Maran and S. Benvenuti, J. Pharm. Biomed. Anal., 2017, 143, 228–236 CrossRef CAS PubMed.
- M. Szalata and K. Wielgus, New Biotechnol., 2016, S162 CrossRef.
- S. Serna-Loaiza, J. Adamcyk, S. Beisl, C. Kornpointner, H. Halbwirth and A. Friedl, Processes, 2020, 8, 1334 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc03516a |
| ‡ These two authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |