Instantaneous hydrolysis of PET bottles: an efficient pathway for the chemical recycling of condensation polymers†
Abstract
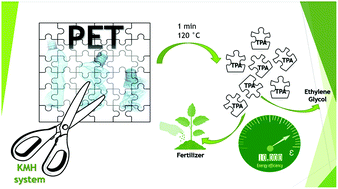
Finding ways to restore the environmental balance that has been disturbed by the rise in the use of plastic commodities is urgent for researchers and the wider community. It is imperative that the increasing amount of plastic waste and the high amount of petrochemical resources consumed during the constant replacement of single-use plastics be reduced. Poly(ethylene terephthalate) (PET) is one of the most commonly produced single-use polymers in the world, and its mechanical recycling is challenging due to the loss of properties during reprocessing. Chemical recycling is a feasible alternative to reclaim the monomers, however, its viability relies on establishing a straightforward, fast, and inexpensive procedure to turn the end-of-use polymer into new pure monomers. This work reports on the fastest known procedure for PET chemical recycling to produce terephthalic acid and ethylene glycol in an efficient and straightforward manner, thanks to microwave-assisted heating that permitted 100% PET conversion into TPA in just 1 minute at 120 °C. The depolymerization kinetics of this new procedure were studied and its improved efficiency over other reported hydrolysis procedures was attributed to a thicker shrinking layer. This new procedure may form a major breakthrough in chemical depolymerization. Evidence pointing to the higher reactivity of free OH species enabled us also to obtain dimethyl terephthalate (DMT) through the use of an anhydrous depolymerization system that is able to convert PET into DMT in 4 min at 80 °C, but requiring careful humidity control. The activation energy for the proposed depolymerization system was estimated as 120 kJ mol−1. An additional advantage of the proposed process is the production of potassium sulfate as a side-product, a valuable fertilizer with high market value. This product compensates for the lack of recoverability of the catalyst. Our KOH-in-methanol hydrolysis (KMH) process has the potential to become a widely used depolymerization solution for a wide range of (heterogeneous) condensation polymers, which is currently under study by our research group.


 Please wait while we load your content...
Please wait while we load your content...