 Open Access Article
Open Access ArticleComparative life cycle assessment of NAD(P)H regeneration technologies†
Joseph W. H.
Burnett
ab,
Ziying
Sun
c,
Jianwei
Li
a,
Xiaonan
Wang
 *c and
Xiaodong
Wang
*c and
Xiaodong
Wang
 *a
*a
aChemical Engineering, Department of Engineering, Lancaster University, Lancaster LA1 4YW, UK. E-mail: xiaodong.wang@lancaster.ac.uk
bChemical and Materials Engineering, School of Engineering, University of Aberdeen, Aberdeen AB24 3UE, UK
cDepartment of Chemical and Biomolecular Engineering, National University of Singapore, Singapore 117585, Singapore. E-mail: chewxia@nus.edu.sg
First published on 17th August 2021
Abstract
NAD(P)H is a key cofactor widely used in biocatalytic reductive transformations, facilitating a wide range of industrially significant reactions which ultimately result in the consumption of the costly cofactor. To make NAD(P)H dependent biotransformations sustainable and economically feasible, different catalytic routes have been investigated to regenerate NAD(P)H. Here we report a comprehensive life cycle assessment (LCA) of these catalytic regeneration methods. Midpoint characterisation and normalisation show that the synthesis of the catalyst, specifically the use of noble metals and energy consumption, dominate the environmental impacts and have the greatest contribution to all considered impact categories. This comparative LCA highlights the need for future investigation into noble metal based catalyst alternatives, to provide cleaner and more sustainable methods of regenerating the cofactor NAD(P)H.
1. Introduction
The importance of biocatalytic processes, specifically using enzymes, for the synthesis of chemicals is becoming increasingly recognised in both academic and industrial fields.1 As well as satisfying many of the principles of a green chemical process (e.g., ambient reaction conditions, no dependence on organic solvents, etc.) enzymes also exhibit far superior chemo, regio, and enantioselectivities compared to conventional synthetic methods.2,3 Oxidoreducatases are the largest class of commercial enzymes and catalyse a wide range of industrially significant transformations, including enzymatic hydrogenation reactions such as the synthesis of chiral intermediates for the production of pharmaceuticals.4–6 To perform these reactions, 90% of oxidoreductases are dependent on the biocofactor NAD(P)H which provides a source of hydride ions.7 The application of these enzymes on a commercial scale is hindered by their dependence on expensive cofactors ($2600 mol−1 NADH and $70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mol−1 NADPH) that are stoichiometrically consumed during the reaction. Hence, there has been significant investigation to find an efficient method for the in situ regeneration of NAD(P)H under biologically favourable conditions.8
000 mol−1 NADPH) that are stoichiometrically consumed during the reaction. Hence, there has been significant investigation to find an efficient method for the in situ regeneration of NAD(P)H under biologically favourable conditions.8
The conversion of NAD(P)+ to NAD(P)H has been extensively studied with attempts to increase the rate of cofactor regeneration whilst maintaining the regioselectivity to the enzymatically active isomer, 1,4-NAD(P)H.9–11 The in situ regeneration of NAD(P)H has thus far focused on five different catalytic methods; enzymatic, electrochemical, photochemical, homogeneous and heterogeneous regeneration (Fig. 1). Since NAD(P)H is a biomolecule that participates in cellular reactions, applying enzymes for the regeneration seems like the most obvious route and so has been the only method applied in industry. Enzymatic regeneration utilises a second enzyme and/or a second substrate to selectively produce 1,4-NAD(P)H.12 Although enzymes offer high selectivities, their dependence on a second enzyme and/or a second substrate results in downstream separation issues and along with their inherent instability, other means of regeneration have been investigated. Electrochemical regeneration has focused on both direct (electrodes) and indirect (mediated) regeneration, utilising a range of electrodes such as Ni, Cu and Au.13–16 Homogeneous regeneration has been dominated by the use of Rh and Ir complexes,17–20 with photochemical regeneration using various TiO2-doped and 2-dimensional photocatalysts.21–25 Heterogeneous regeneration has thus far applied supported Pt catalysts.26 Whilst almost each regeneration method claims to be sustainable, green or clean (e.g., using solar energy, clean electricity or renewable H2), there has not been any direct evidence or comparisons, regardless of the qualitative or quantitative nature of the analysis.
 | ||
| Fig. 1 Schematic representation of the current methods of catalytic NAD(P)H regeneration coupled to an enzymatic reduction. | ||
It is widely agreed that there is a significant need to move towards a more sustainable future and so it is important to determine the sustainability of new and upcoming processes.27 In 2015, the UN developed a blueprint consisting of 17 sustainable development goals (SDGs) to address the wide range of global challenges we currently face. The utilisation and application of enzymes on an industrial scale offers numerous tangible solutions to many of the SDGs including Goal 6 via enzymatic conversion of water contaminants, Goal 12 via the environmentally benign reaction conditions under which these transformations readily occur and Goal 13 via CO2 fixation and subsequent conversion etc. There is, however, a current knowledge gap in the sustainability performance of NAD(P)H regeneration. We recently utilised E-Factor (i.e., mass ratio of waste to target product) as a green chemistry metric to assess the sustainability of NAD(P)H regeneration methods,28 and now we extend that to perform, for the first time, a thorough and comparative life cycle assessment (LCA). Although E-Factor is the simplest and most common metric, it does not consider the types of waste produced nor the energy requirements of processes.29 Applying LCA provides a means of quantitative comparison of the five NAD(P)H regeneration methods of biocatalytic regeneration (Bio), homogeneous regeneration (Homo), electrochemical regeneration (Electro), photochemical regeneration (Photo) and heterogeneous regeneration (Hetero). Here, we examine the environmental ramifications of chemical synthesis with different NAD(P)H regeneration systems and then focus on the catalyst synthesis aspect of each process, with the aim of directing research, particularly catalyst choice, in this newly emerging field.
2. Methodology
Life cycle assessment is a supportive approach for evaluating environmental impacts, energy consumption, resource depletion and other impact categories for an entire product system. Although LCA is typically applied to existing industrial scale processes, prospective LCAs may be performed on processes still on the laboratory scale in an early stage, and can highlight areas that require improvements, providing invaluable guidance for the development of catalyst composition and synthesis.30,31 In this study, involved LCA methodology was done according to the recommendation in the ‘ISO 14040: Principles and Framework’ and ‘ISO 14044: Requirements and Guidelines’ international environmental standards.32,332.1. Goal and scope definition
2.1.2.1. System boundary. The scope of the LCA study was set based on cradle-to-gate processes, and the related system boundaries for the whole process and the synthesis of the catalysts can be found in Fig. 2a and b, respectively. The overall cradle-to-gate processes encompass the extraction of raw materials, the production of the catalyst precursors, the preparation of the catalysts and the in situ regeneration of NAD(P)H coupled to the enzymatic reduction reaction (Fig. 2). Use of the enzymatic reduction product, downstream separation and transportation were excluded from the system boundary as they are not encompassed in the scope of this study to assess the environmental performance of the different processes. For the fulfilment of the aforementioned LCA goal, two functional units (FU) acting as the reference for the included processes were employed respectively for conducting the corresponding LCA for the systems illustrated in Fig. 2a and b. For the system shown in Fig. 2a, the FU was defined as 1 kg of the synthesised active catalyst. For the system shown in Fig. 2b, 1 kg of the desired product from the enzymatic reduction reaction was used as the FU.
2.1.2.2. Impact categories. LCA was performed on the basis of the ReCiPe characterisation methods. ReCiPe is one of the most advanced LCA methodologies, encompassing the broadest set of midpoint indicators.34,35 Midpoint indicators focus on single environmental problems (such as global warming) and endpoint indictors show the impact of the midpoint indicators on the three aggregation levels (damage to human health, damage to ecosystems and damage to resource availability).36 The number of points derived from the characterisation results express the environmental load. Since the units of the points associated with each midpoint and endpoint indicator vary, comparison between the indicators is difficult. Hence, normalisation of the midpoint and endpoint characterisation results by dividing each indictor value with a set reference removes the unit incompatibility issue and allows for comparison.37
Ten ReCiPe midpoint indicators were selected: agricultural land occupation (ALOP), climate change (GWP100), fossil depletion (FDP), freshwater ecotoxicity (FETPinf), human toxicity (HTPinf), ionising radiation (IRP_HE), marine ecotoxicity (METPinf), metal depletion (MDP), terrestrial acidification (TAP100) and urban land occupation (ULOP). The other indicators were discarded as they contributed to less than 1% of the normalised points for each of the five regeneration methods. The eight excluded indicators were freshwater eutrophication (FEP), marine eutrophication (MEP), natural land transformation (NLTP), ozone depletion (ODPinf), particulate matter formation (PMFP), photochemical oxidant formation (POFP), terrestrial ecotoxicity (TETPinf) and water depletion (WDP). For the endpoint characterisation method, included impact indicators are damage to human health, damage to ecosystems and damage to resource availability.
2.2. Life cycle inventory
The inventory data for enzymatic, electrochemical, photochemical, homogeneous and heterogeneous regeneration methods were collected from literature and are shown in detail in the ESI.†13,38–40 One example was taken from each regeneration method whereby the example was chosen based on the minimal amount of waste produced determined by the E-Factor metric, the relative simplicity of the system (e.g., independence of mediators in the case of Electro and Photo) and was a representative example of each method of regeneration (specific details of each process and the associated assumptions are shown in the ESI: Fig. S1–5 and Tables S1–5†). For the catalyst preparation, the data collected covered the entire synthesis process, including all raw materials, energies and wastes. Information of the commercial catalyst preparation in Hetero was unavailable and so the most common and traditional lab scale preparation (wet impregnation) was assumed and applied. Where data was not available for specific molecules, such as the niche ligands used in the preparation of the homogeneous catalyst, the synthesis of these ligands, even though not directly involved in the preparation procedures, were taken into account. Any unreacted reactants and by-products formed were treated as waste. Due to insufficient information, the lifetime and deactivation of the catalysts used in each method was not considered, as a result of which the system in each case was treated as a batch process. When data were unavailable and had to be extrapolated, both scale-up and scale-down were considered as linear.Emission factors for conducting further impact assessment were collected from Ecoinvent database. In order to reflect the environmental performance of studied regeneration processes in the future. For the consumption of electricity, corresponding emission factors were calculated based on the electricity generation scenario in 2030 and 2050 in EU.41
3. Results and discussion
In this section, the life cycle impact assessment results based on ReCiPe midpoint levels and endpoint levels are presented to capture the full picture of the environmental performance of studied regeneration processes. Normalisation for the assessment results based on midpoint levels was done to illustrate the most environmentally-friendly pathway of regeneration in a more straightforward way. Meanwhile, scenario analysis was conducted to assess the environmental loads of the types of employed metal.3.1. Reaction impact assessment
Normalisation of the midpoint results are shown in Fig. 4. Evidently, Electro has the largest environmental impact to all impact categories, especially freshwater ecotoxicity, human toxicity and marine ecotoxicity from the use of Au and Hg in the catalyst. Bio and Hetero have the second and third largest impact in all impact categories, respectively, except metal depletion and urban land occupation in which Homo scores higher than both Bio and Hetero, due to the Rh in the catalyst. Photo has the smallest environmental impact in all considered impact categories.
3.2. Catalyst synthesis impact assessment
 | ||
| Fig. 5 Characterisation results of the five regeneration methods for catalyst synthesis corresponding to (a) Bio (b) Homo (c) Electro (d) Photo and (e) Hetero. | ||
While the characterisation results for Hetero showing energy consumption being the major contributor to agricultural land occupation, climate change, fossil fuel depletion and ionising radiation, Pt is the major contributor to freshwater ecotoxicity, human toxicity, marine ecotoxicity, metal depletion, terrestrial acidification and urban land occupation. This results from the use of a supported Pt catalyst in the regeneration. The catalyst was prepared via a traditional wet impregnation method whereby a Pt salt precursor, in this case H2PtCl6, was first deposited onto the Al2O3 support. After this step, the catalyst undergoes calcination at 773 K for 4 h in air and then a reduction at 573 K for 2 h in 5% H2/N2, forming metallic Pt supported on Al2O3 (the active form of the catalyst). Unlike Bio and Photo, which depend on carbon based materials, it is clear that the use of a noble metal, in this case Pt, has negative environmental ramifications. This is most apparent when considering the characterisation results for Homo and Electro, also shown in Fig. 5. Electro utilises a cholesterol-modified Au amalgam electrode for the regeneration. During the synthesis, Au foil was immersed in Hg to form the amalgam, then a solution of cholesteryl oleate dissolved in hexane was added to the surface. Since the synthesis is very straightforward and does not require any thermal treatments, it is not surprising that electricity consumption has a minimal contribution and the chemicals required for the synthesis, especially the metals, are the highest contributors to the impact categories. Due to the high mass of Au required for the synthesis of the electrode, it is the dominant contributor to all impact categories and contributes to at least 97% in all categories. The exception to this is human toxicity, whereby the use of Hg contributes to 12% of the impact category. The catalyst used in Homo is an organometallic Rh complex, [(η5-C5Me5)Rh(1,10-phenanthroline)Cl]+, whereby the use of Rh is the major contributor to 7 out of the 10 impact categories and dichloromethane (the solvent used in the preparation of the active catalyst) being the major contributing to climate change, fossil depletion and ionising radiation.
Normalisation of the ReCiPe midpoint results allows for direct comparison between the regeneration methods. The results were evaluated using normalisation values from Europe and expressed in Fig. 6. Electro constitutes the highest amount of normalised points to every impact category. This is due to high mass of noble metal required for the preparation of that catalyst, for example Hetero requires 0.01 kg of Pt per kg of active catalyst, Homo requires 0.006 kg of Rh, whereas Electro requires 0.34 kg of Au per kg of active catalyst. Although Homo uses less mass of noble metal than Hetero, it has the second largest environmental impact to all impact categories apart from metal depletion, showing not only the importance of the amount of noble metal, but also the type of noble metal used. Bio and Photo have the lowest and second lowest environmental impacts, respectively, showing that although energy is a significant contributor to the environmental impacts, it is not as significant as the use of noble metals.
 | ||
| Fig. 6 Normalisation results for the synthesis of the catalysts used in the five regeneration methods. | ||
Although Electro and Photo have both been topical new technologies that are supposed to be “green”, Photo appears to be better than Electro across the board. This is not surprising since such a relatively large mass of metal is required for the preparation of the electrodes when compared with Photo, and indeed the other metal based regeneration methods. To understand how to reduce the environmental impacts of Electro, a scenario analysis was performed and explained later (section Scenario Analysis).
3.3. Scenario analysis
The different methods of NAD(P)H cofactor regeneration clearly have a significant influence on the degree of environmental impact. Due to the benign reaction conditions in which these transformations readily occur, in most cases the synthesis of catalyst is the largest contributor to the impact categories, specifically the use of noble metals in the catalysts and the energy required for catalyst synthesis.To assess the influence of the type of metal used in the two methods where the metal had the largest contribution to the environmental impact (Electro and Hetero), the substitution of noble metals was considered and its effect on the 3 endpoint impact categories (ecosystem quality, human health and resources) were studied. For Electro the alternate metals, Ag and Cu, were selected due to the similar electronic structure and potentially similar chemical behaviour. The use of Ag and Cu electrodes for NADH regeneration have also been reported, showing the feasibility of the metal substitution.16,42 Pd and Ni were chosen as substitutions for Pt in Hetero, with the same justification as Electro, where Pd and Ni have also been applied to NADH regeneration.8
Fig. 7 shows the effect on the impact to the 3 endpoint categories when the same mass of Au required for the catalyst is substituted for Ag or Cu (for Electro) or when Pt is substituted for Pd or Ni (for Hetero). Substitution of Pt with Pd shows a reduction of 1702 to 287 points (83%) of the contribution to ecosystem quality, a reduction from 9920 to 2750 (72%) to human health and reduction from 4574 to 1081 (76%) reduction to resources. Nickel substitution reduces the contribution to ecosystem quality to 0.43 (99.9%), human health to 2.7 (99.9%) and resources to 6.3 (99.8%). For Electro, substitution of Au with Ag reduces the contribution to ecosystem quality, human health, and resources from 1167 to 11.6 (99%), 5699 to 50.2 (99.1%) and 7455 to 127.6 (98.3%), respectively, and with Cu to 0.5 (99.9%), 1.6 (99.9%) and 3.6 (99.9%), respectively. This demonstrates the considerable impact of noble metal substitution on the environmental impacts of the regeneration methods.
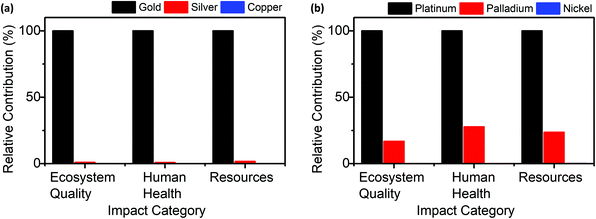 | ||
| Fig. 7 Effect of metal substitution on the ReCiPe endpoint impact categories in (a) Electro and (b) Hetero. | ||
4. Conclusions
The environmental impact of the five NAD(P)H regeneration technologies were evaluated using an early stage cradle-to-gate life cycle assessment. ReCiPe midpoint characterisation showed that for Bio, Hetero, Homo and Electro the production of the catalyst was the largest contributor to all impact categories, whereas energy consumption was the largest contributor for Photo. Normalisation of the characterisation results revealed that Photo had the lowest environmental impact in all impact categories and the trend of increasing environmental impacts was Photo, Homo, Hetero, Bio, and Electro, respectively. A scenario analysis was performed to show the effect of noble metal substitution, resulting in a potential 98% reduction in total environmental impacts when Au is substituted for Ag and a potential 75% reduction when Pt is substituted for Pd.This comparative LCA highlights the importance of catalyst composition and synthesis and can inspire investigation into transition metal based catalysts as an alternative to noble metals, leading to more environmentally friendly and sustainable methods of regenerating NAD(P)H and thus the associated biosynthesis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by The Royal Society (IES\R3\170162).Notes and references
- G. Hughes and J. C. Lewis, Chem. Rev., 2018, 118, 1–3 CrossRef CAS PubMed.
- R. Wichmann and D. Vasic-Racki, Technology Transfer in Biotechnology: from Lab to Industry to Production, 2005, vol. 92, pp. 225–260 Search PubMed.
- D. Monti, G. Ottolina, G. Carrea and S. Riva, Chem. Rev., 2011, 111, 4111–4140 CrossRef CAS PubMed.
- M. Hambourger, G. F. Moore, D. M. Kramer, D. Gust, A. L. Moore and T. A. Moore, Chem. Soc. Rev., 2009, 38, 25–35 RSC.
- U. T. Bornscheuer, G. W. Huisman, R. J. Kazlauskas, S. Lutz, J. C. Moore and K. Robins, Nature, 2012, 485, 185–194 CrossRef CAS PubMed.
- J. S. Rowbotham, M. A. Ramirez, O. Lenz, H. A. Reeve and K. A. Vincent, Nat. Commun., 2020, 11, 1454 CrossRef CAS PubMed.
- L. S. Vidal, C. L. Kelly, P. M. Mordaka and J. T. Heap, Biochim. Biophys. Acta, Proteins Proteomics, 2018, 1866, 327–347 CrossRef.
- X. Wang, T. Saba, H. H. P. Yiu, R. F. Howe, J. A. Anderson and J. F. Shi, Chem, 2017, 2, 621–654 CAS.
- S. Fukuzumi, Y. M. Lee and W. Nam, Chemphotochem, 2018, 2, 121–135 CrossRef CAS.
- S. Fukuzumi, Y. M. Lee and W. Nam, J. Inorg. Biochem., 2019, 199, 110777 CrossRef CAS PubMed.
- H. Wu, C. Y. Tian, X. K. Song, C. Liu, D. Yang and Z. Y. Jiang, Green Chem., 2013, 15, 1773–1789 RSC.
- L. Han and B. Liang, World J. Microbiol. Biotechnol., 2018, 34, 141 CrossRef PubMed.
- S. H. Baik, C. Kang, J. C. Jeon and S. E. Yun, Biotechnol. Tech., 1999, 13, 1–5 CrossRef CAS.
- T. Theodore, K. Cheikhou, R. Jerome, G. S. Karine and R. Olivier, Electrochim. Acta, 2010, 55, 2286–2294 CrossRef.
- N. Ullah, I. Ali and S. Omanovic, Mater. Chem. Phys., 2015, 149, 413–417 CrossRef.
- R. Barin, S. Rashid-Nadimi, D. Biria and M. A. Asadollahi, Electrochim. Acta, 2017, 247, 1095–1102 CrossRef CAS.
- A. Bucci, S. Dunn, G. Bellachioma, G. M. Rodriguez, C. Zuccaccia, C. Nervi and A. Macchioni, ACS Catal., 2017, 7, 7788–7796 CrossRef CAS.
- V. Ganesan, D. Sivanesan and S. Yoon, Inorg. Chem., 2017, 56, 1366–1374 CrossRef CAS PubMed.
- F. Hollmann, B. Witholt and A. Schmid, J. Mol. Catal. B: Enzym., 2002, 19, 167–176 CrossRef.
- L. Tensi and A. Macchioni, ACS Catal., 2020, 10, 7945–7949 CrossRef CAS.
- Q. Y. Pan, H. Liu, Y. J. Zhao, S. Q. Chen, B. Xue, X. N. Kan, X. W. Huang, J. Liu and Z. B. Li, ACS Appl. Mater. Interfaces, 2019, 11, 2740–2744 CrossRef CAS.
- J. Liu and M. Antonietti, Energy Environ. Sci., 2013, 6, 1486–1493 RSC.
- Q. Shi, D. Yang, Z. Y. Jiang and J. Li, J. Mol. Catal. B: Enzym., 2006, 43, 44–48 CrossRef CAS.
- S. Roy, V. Jain, R. K. Kashyap, A. Rao and P. P. Pillai, ACS Catal., 2020, 10, 5522–5528 CrossRef CAS.
- B. B. Ma, S. Y. Sun, H. C. He, R. Lv, J. J. Deng, T. T. Huo, Y. L. Zhao, H. L. Yu and L. Zhou, Ind. Eng. Chem. Res., 2019, 58, 23567–23573 CrossRef CAS.
- X. Wang and H. H. P. Yiu, ACS Catal., 2016, 6, 1880–1886 CrossRef CAS.
- X. Z. Zhu, C. H. Ho and X. N. Wang, ACS Sustainable Chem. Eng., 2020, 8, 11141–11151 CrossRef.
- T. Saba, J. W. H. Burnett, J. Li, X. N. Wang, J. A. Anderson, P. N. Kechagiopoulos and X. Wang, Catal. Today, 2020, 339, 281–288 CrossRef CAS.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- D. Kralisch, D. Ott and D. Gericke, Green Chem., 2015, 17, 123–145 RSC.
- B. Agarski, V. Nikolic, Z. Kamberovic, Z. Angic, B. Kosec and I. Budak, J. Cleaner Prod., 2017, 162, 7–15 CrossRef CAS.
- ISO 14040:2006a – Environmental management – Life cycle assessment – Principles and framework International Organisation for Standardisation, 2nd edn, 2006 Search PubMed.
- ISO 14040:2006 – Life cycle assessment – Requirements and guidelines, The International Journal of Life Cycle Assessment, 1st edn, 2006 Search PubMed.
- Pre, SimaPro Database Manual, Methods Library. 2014.
- G. Mark, H. Reinout, H. Mark, S. A. De and S. Jaap, ReCiPe 2008 Report I: characterisation (v 1.08). 2013.
- M. A. J. Huijbregts, Z. J. N. Steinmann, P. M. F. Elshout, G. Stam, F. Verones, M. Vieira, M. Zijp, A. Hollander and R. van Zelm, Int. J. Life Cycle Assess., 2017, 22, 138–147 CrossRef.
- A. W. Sleeswijk, L. van Oers, J. B. Guinee, J. Struijs and M. A. J. Huijbregts, Sci. Total Environ., 2008, 390, 227–240 CrossRef CAS PubMed.
- D. Yang, H. J. Zou, Y. Z. Wu, J. F. Shi, S. H. Zhang, X. Wang, P. P. Han, Z. W. Tong and Z. Y. Jiang, Ind. Eng. Chem. Res., 2017, 56, 6247–6255 CrossRef CAS.
- C. Zor, H. A. Reeve, J. Quinson, L. A. Thompson, T. H. Lonsdale, F. Dillon, N. Grobert and K. A. Vincent, Chem. Commun., 2017, 53, 9839–9841 RSC.
- J. Canivet, G. Suss-Fink and P. Stepnicka, Eur. J. Inorg. Chem., 2007, 30, 4736–4742 CrossRef.
- P. Capros, EU Reference Scenario 2016 – Energy, transport and GHG emissions, Trends to 2050, European Commission, 2016 Search PubMed.
- Y. T. Long and H. Y. Chen, J. Electroanal. Chem., 1997, 440, 239–242 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc02349g |
| This journal is © The Royal Society of Chemistry 2021 |



