Visible-light-promoted three-component cycloaddition reaction: synthesis of 4-functionalized 1,5-disubstituted 1,2,3-triazoles†
Abstract
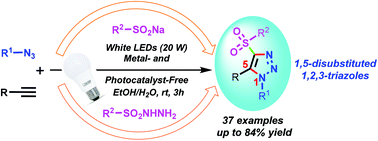
The first visible-light promoted and photocatalyst-free three-component reaction protocol has been developed to synthesize 4-functionalized 1,5-disubstituted 1,2,3-triazoles from terminal alkynes, aryl azides, and arylsulfinic acid sodium salts or arylsulfonyl hydrazides in an aqueous medium at room temperature. Notably, the reaction proceeds seamlessly without using any transition-metal catalysts, ligands, or photocatalysts, and also reduces chemical waste. It also features an easy operation procedure, high regioselectivity, scalability, a broad substrate scope, mild reaction conditions, and good functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...