 Open Access Article
Open Access ArticleA biobased, bioactive, low CO2 impact coating for soil improvers†
Renate
Weiß
 *a,
Sebastian
Gritsch
*a,
Sebastian
Gritsch
 a,
Günter
Brader
a,
Günter
Brader
 b,
Branislav
Nikolic
b,
Marc
Spiller
b,
Branislav
Nikolic
b,
Marc
Spiller
 c,
Julia
Santolin
c,
Julia
Santolin
 c,
Hedda K.
Weber
d,
Nikolaus
Schwaiger
c,
Hedda K.
Weber
d,
Nikolaus
Schwaiger
 d,
Sylvain
Pluchon
e,
Kristin
Dietel
f,
Georg
Gübitz
ag and
Gibson
Nyanhongo
ag
d,
Sylvain
Pluchon
e,
Kristin
Dietel
f,
Georg
Gübitz
ag and
Gibson
Nyanhongo
ag
aUniversity of Natural Resources and Life Sciences, Vienna, Institute of Environmental Biotechnology, Konrad Lorenz Straße 20, 3430, Tulln an der Donau, Austria. E-mail: renate.weiss@boku.ac.at
bAIT, Austrian Institute of Technology, Konrad-Lorenz-Straße 24, 3430 Tulln, Austria
cUniversity of Antwerp, Research Group of Sustainable Energy, Air and Water Technology, Department of Bioscience Engineering, Groenenborgerlaan 171, 2020 Antwerpen, Belgium
dSAPPI Papier Holding GmbH, Brucker Strasse 21, 8101 Gratkorn, Austria
eCentre Mondial de l'Innovation Roullier – Laboratoire de Nutrition Végétale, 18 avenue Franklin Roosevelt, 35400 Saint-Malo, France
fABiTEP GmbH, Glienicker Weg 185, 12489 Berlin, Germany
gAustrian Centre for Industrial Biotechnology (ACIB), Konrad Lorenz Strasse 20, 3430, Tulln an der Donau, Austria
First published on 9th August 2021
Abstract
Lignosulfonate-based bioactive coatings as soil improvers for lawns were developed using laccase as a biocatalyst. Incorporation of glycerol, xylitol and sorbitol as plasticizers considerably reduced the brittleness of the synthesized coatings of marine carbonate granules while thermal enzyme inactivation at 100 °C enabled the production of stable coatings. Heat inactivation produced stable coatings with a molecular weight of 2000 kDa and a viscosity of 4.5 × 10−3 Pas. The desired plasticity for the spray coating of soil improver granules was achieved by the addition of 2.7% of xylitol. Agriculture beneficial microorganisms (four different Bacillus species) were integrated into the coatings. The stable coatings protected the marine calcium carbonate granules, maintained the viability of the microorganisms and showed no toxic effects on the germination and growth of model plants including corn, wheat, salad, and tomato despite a slight delay in germination. Moreover, the coatings reduced the dust formation of soil improvers by 70%. CO2 emission analysis showed potential for the reduction of up to 3.4 kg CO2-eq. kg−1 product, making it a viable alternative to fossil-based coatings.
Introduction
Lawns have become a key part of landscape designs in urban centres occupying parks; golf courses; botanical, zoological and home gardens; institutional green spaces; playground/school grounds; street spaces, etc. Lawn management requires frequent mowing, irrigation and application of fertilizers for optimal performance and aesthetic quality, which are attributed to many negative environmental impacts.1 European homeowners spend billions of euros and typically apply 10 times the amount of pesticides and fertilizers per hectare on their lawns than farmers do on crops.2,3 Most of these agrochemicals end up polluting the environment, for example, fertilizers emit greenhouse gases and also cause eutrophication of local streams, lakes, and coastal zones.1The negative effects caused by the release of fertilizers into the environment are putting pressure on agrochemical producers to improve the efficiency of their products. Ideal fertilisers should have a higher nutrient use efficiency, as a consequence of which detrimental effects on soil, water, and atmospheric environments are reduced while at the same time enabling a single fertiliser application during a growing season.4 However, currently, the nutrient use efficiency (NUE), defined as the percentage of a nutrient that contributes to biomass production, for nitrogen based fertilizers ranges from 30 to 50%.5 To compensate for these losses, agrochemicals are applied in excess amounts,5 meaning at least 70% of the fertilizer is lost either through leaching thereby causing eutrophication and also increasing the release of nitrous oxide,6 a powerful greenhouse gas emitted into the atmosphere that contributes to climate change. Intensely managed lawns have 70% higher global warming potential from direct emissions compared to lawns that are not fertilized or irrigated.7
To reduce the loss and negative effects of agrochemicals on the environment, coating materials for fertilisers have been developed. However, many coatings are fossil based and/or non-biodegradable such as those based on polyethylene, polysulfone, polyvinyl chloride, polystyrene, polyacrylic acid latex, polyvinyl alcohol, polyethylene, polydopamine, cellulose acetate, polyacrylonitrile, copolymers of butylene-succinate-co-dilinoleate and polyethylene-succinate-co-terephthalate copolymers of polyvinyl alcohol (PVA) and polyvinylpyrrolidone.4,5,8,9 Some of these polymer-coatings decompose extremely slowly or not at all in soil, leading to undesirable accumulation of microplastics potentially posing a threat to terrestrial wildlife and food security.10,11 Apart from accumulating in the soil, microplastics can be transported to water bodies, contributing to marine plastic pollution and putting marine organisms at risk.12 Furthermore, several conventional fertilizer coatings can even be toxic to plants or change the soil's pH, which is undesirable.4 The European Commission, in the frame of the Circular Economy Action Plan, has put forward a proposal to restrict some intentionally added microplastics in products, including polymers used in fertilizers, by 2021.13 By 2026 only polymers that meet defined biodegradability standards will be allowed on the market.14
Therefore, there is growing interest in developing bio-based coating materials obtained from renewable sources. Among the bio-based polymers, lignosulfonate is emerging as a highly promising versatile material. Unlike many other bio-based materials, lignosulfonates have an inherent structure containing abundant reactive functional groups (aliphatic and phenolic hydroxyl groups and carbonyl groups) and unshared electron pairs on the oxygen atoms in these groups, making them natural chelators of many important agricultural metal ions such as potassium, calcium, magnesium, manganese, etc.15 Furthermore, the abundant phenolic functionalities of lignosulfonates stabilize the soil's organic nitrogen and thereby inhibit two detrimental events occurring in soils: nitrification and the unwanted volatilization due to soil urease activity.15 Currently only 2% of the 100 million tonnes of lignin side streams produced yearly by the global pulp and paper industry is utilised,16 and the remaining 98% is burned in the processes releasing 1.63 kg CO2 kg−1 lignin.17 Contrary to this, applying lignosulfonates to the soil can enable the capture of CO2 and improve the soil properties. Lignosulfonates biodegrade in the soil and transform it into humic acids which improve the soil structure, nutrients and water retention and are hence beneficial for crop growth.18 Lignosulfonates are approved for use in organic crop production in the USA as published in the identification of petitioned substances report of the U.S. Department of Agriculture.19 Therefore their broad application in agriculture as fertilizer components20 and even attempts to develop gels formed by chemical synthesis using formaldehyde as an agent21 have been reported. Although the suitability of lingnosulfonates for agriculture is well-known, as fertilizer carriers, binders, and dispersants are in commercial use, to date no suitable technologies have been developed to exploit their remarkable properties for fertilizer coatings.22,23 This would both allow the replacement of currently used petrol-based coatings and exploit the beneficial effect of lignins on the soil. Several strategies including the use of simple physical coatings without modification of technical lignins (kraft, soda and lignosulfonates), chemically modified coatings through ammoxidation, acetylation,24 Mannich reaction, esterification, and hydroxymethylation combined with blending and even attempts to use glycerol as a plasticizer resulted in defective coatings.18,25 Furthermore, chemical processes such as acetylation,26 hydroxymethylation, and esterification of lignin use toxic chemicals like formaldehyde.18,27,28 Likewise other applications such as the application as dispersing agents or stabilizers have even been patented, but they often involve the necessity of harsh reaction conditions and toxic or harmful chemicals.29,30 Often lignosulfonates are used as is without any modification merely as fillers or in rather low amounts.31,32
Unlike these previous studies, this study investigates a whole green chemistry process, that employs laccase enzymes to polymerize and tailor the properties of lignosulfonate coatings. Laccases are oxidoreductase enzymes that are widely distributed in nature, able to react with a variety of aromatic substrates including lignosulfonates, and generate reactive species with the concomitant reduction of molecular oxygen to water. The most attractive feature of laccase lies in its ability to generate reactive species that are able to cross-react among themselves or with other (functional) molecules. This characteristic enables the tailoring of lignosulfonate properties to synthesize materials with certain property profiles. Despite earlier promising studies on lignin modification using laccases, the need for electron mediators, the high enzyme costs, and the lack of mechanistic understanding had considerably limited the viable use of laccases to process lignin. Recently we developed a versatile laccase-based technology that allows tailoring the lignosulfonate polymerization process and enables us to transform low molecular weight water-soluble lignosulfonates into high molecular weight water-insoluble polymers with potential applications for making many different functional materials33,34 including agrochemical delivery systems35 without the need for mediators. Based on this success, this study involved the enzyme-based synthesis of novel multifunctional biobased, bioactive non-toxic lignosulfonate coatings that reduce fertilizer leaching and hence its negative environmental impact. This involved specifically controlled enzymatic polymerization combined with enzyme activation and the addition of precise amounts of plasticizer to achieve certain properties suitable for coating synthesis (such as the use in coating machines and/or for spray coating) as well as improved coating performance (stability, caking, etc.). This study also, for the first time, shows the possibility of incorporating plant growth promoting microorganisms i.e. Bacillus species into the formulation. The enzymatically polymerized lignin coatings incorporating plant growth promoting Bacillus species are used for promoting the growth of turf grass. It is expected that incorporating Bacillus spp, widely known as plant growth promoters, which under stress conditions solubilize nutrients needed by the host plant,36 will help promote plant growth. Their ability to support the nutrient uptake of the plant in soils by interacting with the root system has been described37,38 and a number of products have been commercialized such as Kodiak (B. subtilis GB03), Quantum-400 (B. subtilis GB03), Rhizovital42 (B. amyloliquefaciens FZB42)39 and Rhizovital C5 (B. atrophaeus, included in this study).
Materials and methods
Laccase activity assay
The activity of laccase from Myceliopthera thermophila was determined by photometrically monitoring the oxidation of ABTS (azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) at 420 nm as previously described by Weiss et al. 2020.35 ABTS is oxidised by laccases and the concentration of the resulting green-blue coloured cation radical can be correlated with the enzyme activity.40 For the enzyme activity assay, 50 mM sodium phosphate buffer pH 7 and 10 mM ABTS solution were prepared. The reaction was performed in a 96-microwell plate. For the blank, 170 μL of phosphate buffer were added to 50 μl of the ABTS solution. For the enzymatic reaction, 170 μL of the enzyme solution diluted at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in sodium phosphate buffer were prepared in triplicate and added to 50 μl of ABTS solution. The laccase activity was determined as follows:
000 in sodium phosphate buffer were prepared in triplicate and added to 50 μl of ABTS solution. The laccase activity was determined as follows:The enzyme activity refers to the amount of enzyme necessary to catalyse the conversion of 1 μmol of substrate per minute under specific assay conditions.
Enzymatic synthesis of lignosulfonate coatings
Liquid magnesium lignosulfonates supplied by SAPPI were used as a raw material for all polymerisation experiments (Specifications on the composition are provided in the ESI.†). The liquid lignosulfonates were dried in large ceramic pans for 3–7 days in a 70 °C oven until it was bone dry (i.e. 100% dry matter). The dried lignosulfonates were then weighed and dissolved in MilliQ water to obtain the desired lignosulfonate concentration (7% w/v, 8% w/v, 9% w/v) and pH adjusted to 7 using 5 M sodium hydroxide solution.The level of oxygen saturation in the solution was monitored by using oxygen sensors positioned on optical spots. The reaction was started by introducing 10 Units per mL of laccase and continuously aerating at 30 cm3 min−1 at room temperature. A laccase with an average activity of 1273 U mL−1 from Myceliophthora thermophila (MtL) purchased from Novozymes (Novozym 51003) was used for lignosulfonate cross-linking.
To reduce the brittleness of pure lignosulfonate polymers, various plasticizers (sorbitol, xylitol and glycerol of reagent grade and purchased from Sigma-Aldrich GmbH) at different concentrations (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratios of plasticizer: dry matter content of lignosulfonates) were separately added to the lignosulfonates during polymerization.
4 ratios of plasticizer: dry matter content of lignosulfonates) were separately added to the lignosulfonates during polymerization.
Size exclusion chromatography
The changes in the molecular weight of lignosulfonates before and during polymerization as well as during the inactivation process were measured using a high performance liquid chromatography (HPLC) system equipped with a quaternary/binary pump, a 1260 series autosampler from Agilent Technologies (Palo Alto, CA), a DAD (diode array detector) and a refractive index (RI) detector system (Agilent Technologies 1260 Infinity), as well as a MALS HELEOS DAWN II detector from Wyatt Technologies (Dernbach, Germany) was used. The column system consisted of a PL aquagel-OH MIXED Guard precolumn (PL1149-1840, 8 μm, 7.5 × 50 mm2, Agilent, Palo Alto, CA) and a PL aquagel-OH MIXED H separation column (PL1549-5800, 4.6 × 250 mm2, 8 μm, Agilent, Palo Alto, CA) with a mass range of 6–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kDa. Lignin samples were diluted with the eluent to a concentration of 1 mg mL−1. The injection volume was 100 μL. The mobile phase consisted of 50 mM NaNO3/3 mM NaN3 and had a total runtime of 92 min. The Agilent Software Openlab Chemstation CDS and ASTRA 7 software from Wyatt Technologies were used for data acquisition and data analysis.
000 kDa. Lignin samples were diluted with the eluent to a concentration of 1 mg mL−1. The injection volume was 100 μL. The mobile phase consisted of 50 mM NaNO3/3 mM NaN3 and had a total runtime of 92 min. The Agilent Software Openlab Chemstation CDS and ASTRA 7 software from Wyatt Technologies were used for data acquisition and data analysis.
BSA was used for the normalization, band broadening, and peak alignment of the MALS detector.
Viscosity measurement
The rheological properties of the samples were measured using a CVO 50 rotational rheometer (Bohlin Instruments, U.K.). This device permits the study of viscosity and elasticity of materials under different conditions. For the viscosity measurements, 1 mL of the defrosted sample was placed on a fixed plate. The shear force was applied with a 4° conical plate with a diameter of 40 mm. The measurement was carried out at a constant temperature of 20 °C and a shear rate of 200 s−1.Inactivation of laccase
The laccase was heat inactivated after polymerization to the desired size in order to produce stable coatings. To determine the optimal conditions for inactivation, 10 mL of the respective 7%, 8% and 9% coatings were treated at different temperatures (80 °C, 90 °C, 100 °C) for 5, 10, and 15 minutes in a water bath. Falcon tubes with a max volume of 15 ml were used. The stability of the polymers was then followed by monitoring the changes in viscosity during storage.Synthesis of bioactive soil improver coatings
Marine calcium carbonate granules (4 mm diameter) provided by Roullier were first coated with different concentrations of enzymatically polymerised lignosulfonates. Finally, the 8% coating was determined as the optimum incorporating 2.7% xylitol as a plasticizer. Six types of granules were produced, namely, (i) uncoated marine calcium carbonate granules which served as a blank, (ii) lignosulfonate coated granules which represented the negative control and (iii) granules that contained different Bacillus species in the lignosulfonate coating at a concentration of 2 × 106 cfu g−1 (Table 1).| Calcium carbonate | Coated | Bacillus species | Abbreviation | Strain collection |
|---|---|---|---|---|
| Yes | — | None | — | — |
| Yes | Yes | None | — | — |
| Yes | Yes | B. subtilis | A0 44 | AIT_EKA044BA16 |
| Yes | Yes | B. licheniformis | Abi 53 | ABITEP Abi 53 |
| Yes | Yes | B. velezensis | Abi 25 | ABITEP Abi 25 |
| Yes | Yes | B. atrophaeus | Abi 05 | ABITEP Abi 05 |
| Rhizovital C5 |
Spore viability in coated granules
Bacillus species were provided by ABITEB and AIT and spore formulations were produced by ABITEP. One gram of differently coated and uncoated granules was transferred under sterile conditions into 15 ml tubes filled with 10 ml of sterile 0.9% NaCl solution and 1 g of sterile glass beads (1 mm diameter). Tubes were vigorously vortexed for a couple of minutes to homogenize and suspend spores. Serial dilutions of spore suspensions were prepared by combining 100 μl of suspension with 900 μl of sterile 0.9% NaCl up to a dilution of 10−5. From each spore dilution, 25 μl were inoculated onto each of the three Trypsin-Soy (100%) agar plates (6 cm diameter) and spread using well sterilized glass rods. Agar plates were inoculated overnight at 37% and the number of colony forming units (CFUs) were counted. In calculations, the most outliers for CFUs per plate were removed and the average of CFUs (germinated spores) per plate and dilution was calculated.Attrition of granules
Attrition (the gradual destruction of dried granules in subsequent handling after production and during storage is generally a disastrous situation to be avoided, also called roll off)41 of uncoated and coated granules was measured. Twenty grams of the respective granules were transferred in 50 ml Falcon tubes and stored at room temperature. Due to mechanical attrition, the generated dust was gravimetrically analysed after 1 month and 1 year.Application of coatings on plants
The effects of modified lignosulfonates and/or coatings on plants were tested in plant pot trials. Lignosulfonates (3.6 w/v%) were put into 1000 ml plant pot soil volume. As plant models, corn, wheat, salad, and tomato were chosen and 16 seeds per pot were planted. Sterilized, fertile soil was used for all tests. Experiments were performed in triplicate. Germination and growth were monitored. After germination, seedlings were manually removed and only 3 plants per pot for corn, salad, and tomato, and 4 plants per pot for wheat left to grow.Application of soil improvers on the golf lawn
The effect of the soil improver, when coated with different variants of the bioactive coating, was tested in greenhouse trials on the golf lawn. The grass was regularly cut and the fresh weight of the cut was gravimetrically analysed to show the biomass production over 84 days.CO2 emission equivalent of coating production
The system boundaries applied in the CO2-eq. assessment are cradle-to-gate, assessing the upstream and foreground processes leading to the production of a market ready coating material. The impact category assessed is the Global Warming Potential for a 100-year time horizon (GWP100a). The modelled processes are shown in Fig. 1. Information about the process parameters was obtained from SAPPI for their sulphite paper pulp factory in Austria (Gratkorn). In this installation, lignosulfonates (LS) are currently a side product of the pulping process. LS are presently used as a fuel to generate heat and power in the installation (Fig. 1: process B′). In the innovative processes investigated, LS are processed into a coating material (process train B). The processing comprises the purification of the lignosulfonate containing wastewater. The purified LS are further refined by polymerisation and addition of a plasticizer before being market ready (i.e. at gate). Inputs to these processes are electrical energy and heat energy, respectively (see the ESI† for assumptions). Diversion of LS from incineration implies that another energy source must be substituted for the marginal decrease in energy generation (process A). At the paper pulp plant it is envisioned to use wood residues for this purpose. Wood residues and LS are further assumed to have the same CO2-eq. emission per unit energy (i.e. MJ) released. Therefore, the substitution between the two materials is assumed to exhibit a net zero emission (zero burden assumption). Finally, the novel LS coating material is assumed to substitute the state-of-the-art coating products of the current industrial use (process D). Therefore, scenario analysis has been applied in this research, in which the impact of different state-of-the-art coatings (polyurethane, polyethylene) is compared to LS coatings that used different plasticizers (xylitol, glycerol).42 Plasticizers were varied as they were found to cause the major share of CO2-eq. emissions.Results and discussion
Production of lignosulfonate coatings
Multiple parameters were monitored during the enzymatic synthesis of lignosulfonate soil improver coatings to determine optimum conditions and concentrations of lignosulfonates required. As shown in Fig. 2, aeration followed by the addition of enzyme leads to an immediate sharp decrease of the oxygen concentration to zero. The oxygen saturation is one of the most crucial drivers of enzymatic polymerization and the most influential tool to change the polymerization rate. This was utilized in previous studies for the production of oxygen scavenging films.43 The online measurement of dissolved oxygen results in saturation curves giving insight into the evolution of the reaction. Previous studies44 demonstrated the remarkable activity of laccase in oxidizing lignin while simultaneously reducing molecular oxygen to water. Similar to previous findings on sodium lignosulfonates the molecular weight of the coatings is highly dependent on the used enzyme as well as the substrate concentration and the initial molecular weight of the substrate.45 The results show that at constant oxygen supply significant differences can be observed depending on the lignosulfonate dry matter content. After enzyme application at 7% DM saturation levels are attained earlier compared to higher dry matter percentages (Fig. 2). The 8% DM curve remains on a lower oxygen saturation level, suggesting a higher demand for oxygen due to the better mobility of the radicals in the solution as well as the better steric availability of the substrate.46–48 | ||
| Fig. 2 Oxygen saturation during laccase catalysed polymerisation of lignosulfonates at different concentrations. | ||
Laccase polymerization of 7, 8 and 9% lignosulfonates resulted in an increase in the molecular weight from between 30 and 80 kDa to over 1500, 2000 and 2500 kDa, respectively (Fig. 4). This clearly shows that the initial concentration of lignosulfonates has a direct effect on the size of the formed polymers. As also shown in Fig. 4, the size of the formed lignosulfonate polymer has also a direct effect on the viscosity of the produced coatings. These observations enable the tailoring of the properties of the produced coatings depending on the initial lignosulfonate concentration.
Stabilization of lignosulfonate coatings
A stable and consistent coating is required for product reproducibility. Hence, the reaction must be stopped when reaching the desired degree of polymerisation to avoid ongoing laccase action during storage. To address this challenge, thermal inactivation of the laccase was investigated at 80 °C, 90 °C and 100 °C for 5, 10 and 15 minutes, respectively. Fig. 3 shows the enzyme activity in the coatings after each treatment. MtL was very stable at 80 °C retaining 40–90% of its activity in lignin samples as shown in Fig. 3. The enzyme could only be completely inactivated when incubated at 100 °C for 10 minutes. Since heat inactivation at 100 °C is a relatively harsh tool in respect of the polymer structure, the effect of the thermal inactivation on the molecular weight and viscosity of the produced polymers was investigated (Fig. 4). The structure of the lignosulfonate polymer produced with 9% dry substance was significantly stronger affected by higher temperatures and residence time compared to the other polymeric coatings. The 7% polymer was in general less viscous and had a lower average molecular weight, but was much less affected by the inactivation. Contrary to that the 8% coating showed an intermediate decrease of molecular weight and viscosity due to temperature and residence time, having similar viscosity (5 mPa s) and molecular weight (2000 kDa) values at the minimal required parameters for heat inactivation. Heat inactivation of the enzyme generally led to a decrease in both the final molecular weight and viscosity of the coatings. However, these studies show that, considering all these factors, well defined conditions can be achieved to produce a consistent coating product.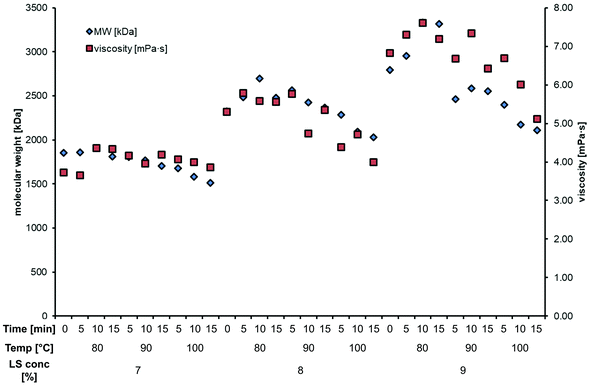 | ||
| Fig. 4 Change of molecular weight and viscosity after thermal inactivation of laccase of polymerized lignosulfonates. | ||
Screening for plasticizers and anti-dust properties
Preliminary trials with polymerized lignosulfonates produced unstable randomly disintegrating coatings that completely detached from the soil improver granules. This is in line with previous observations which demonstrated that using lignosulfonates and other industrial lignins without plasticizers or binder materials leads to brittle and shrinking materials.49,50 To reduce the brittleness of the coatings, the effects of glycerol, sorbitol and xylitol as plasticizers at different concentrations were studied. The addition of these plasticizers showed no significant effect on the molecular weight of the coatings. However, viscosity was significantly affected by the addition of plasticizers during polymerization (Fig. 5). In general, the application of plasticizers led to an increase of viscosity as previously reported by Jimenez Bartolome et al.34 An increase in viscosity was directly proportional to the amount of added plasticizer showing the highest gain when they were added in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 proportion.
2 proportion.
 | ||
| Fig. 5 Effect of different plasticisers on the viscosity of enzymatically polymerised lignosulfonates. | ||
The performance and properties of plasticized lignosulfonate coatings were investigated on marine calcium carbonate granules. These were coated with all variants of the coatings. All the used plasticizers applied were able to reduce the brittleness of the polymerized lignosulfonates even at the lowest applied concentration. Plasticizers can be defined as low molecular weight, non-volatile compounds used as additives in order to enhance the flexibility and processability of polymers. In general, plasticizers manage to achieve this role by occupying intermolecular spaces between polymer chains, reducing secondary forces among them, changing the three-dimensional molecular organization of polymers and at the same time thereby lowering the second order transition temperature, the glass transition temperature (Tg), hardness, density, viscosity and electrostatic charge of the polymers.34 For hydrophilic biopolymers like pullulan plasticizing effects by glycerol, sorbitol and xylitol have been reported due to the enhancement of molecular mobility by disrupting the hydrogen bonding interaction between polymer chains amongst other phenomena. The effect on various properties (mechanical, moisture content, etc.) depended on both the plasticizer concentration and size.51 Therefore, it was imperative to investigate in this study their overall effect for this particular application in LS based coatings.
The most promising coatings in terms of sprayability, adhesion to the granules and their anti-dust properties were selected. In Fig. 6, the effect of the coating on roll-off production can be seen. In polymerized lignosulfonates, without the use of plasticisers, a reduction of 28–56% of dust production could be achieved. The addition of plasticisers, however, reduced the attrition even further up to 70%, revealing xylitol to be the most suitable in the coating process.
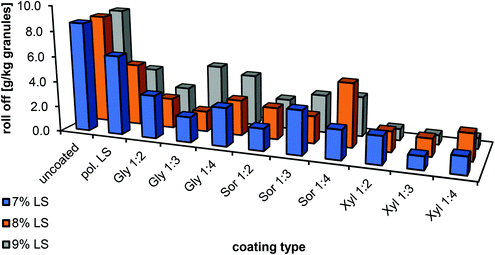 | ||
| Fig. 6 Effect of different plasticisers on the roll-off (attrition) of enzymatically polymerised lignosulfonates fertiliser coatings. | ||
By combining all information from these experiments, the most suitable coating was determined to be an 8% lignosulfonate containing coating with 2.7% xylitol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dry substance ratio) being added as a plasticizer, polymerized for 6 hours at room temperature and inactivated for 10 minutes at 100 °C.
3 dry substance ratio) being added as a plasticizer, polymerized for 6 hours at room temperature and inactivated for 10 minutes at 100 °C.
Toxicity and safety of polymerized lignosulfonates
New materials intended for field application require extensive in vitro testing to ensure that they are not toxic to plants and that they support plant growth. The importance of the safety of new fertilizer products is also stated in the EU Regulation 2019/1009 of the European Parliament and of the Council.52 Consequently, the effects of the synthesized coatings on the germination and growth of plants were investigated by applying 3.6% v/v of the coatings to the soil before seeding with wheat, corn, tomato, or iceberg salad under greenhouse conditions. Germination and growth were monitored for up to 31 days. Fig. 7 shows the performance of salad. No significant differences in germination or plant growth between control plants and polymerized lignosulfonate treated plants were observed.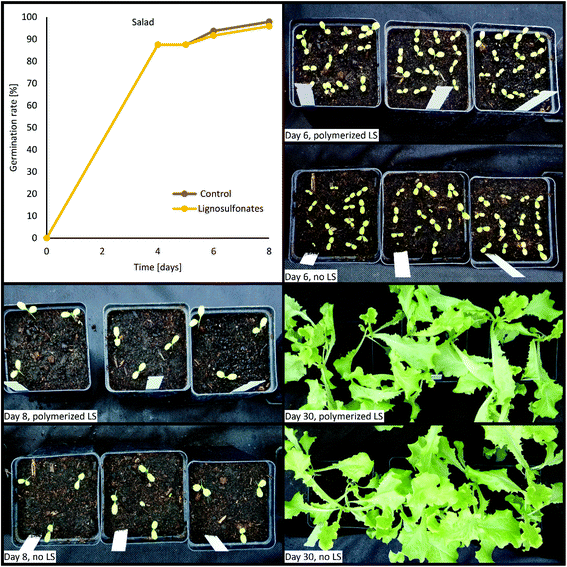 | ||
| Fig. 7 Comparison of the germination rate and growth development of ice berg salad on fertile soil and fertile soil with 3.6% v/v polymerized lignosulfonates being added. | ||
Fig. 11–13 in the ESI† show the performances of wheat, corn, and tomato. In wheat germination rates reached their maximum after 14 days amounting to 90% in the control, and 88% in experiments containing polymerized lignosulfonates. The overall trend of the graph suggests a delay in germination between day 5 and day 14 which can be appreciated in Fig. 12 in the ESI† due to the reduced size of the seedlings. The delayed germination led to a slower growth rate at first in lignosulfonate treated plants. After decollation plant growth in control and trial pots was equivalent as little difference in the development stages were observed. In corn the germination rates reached their maximum after 8 days amounting to 98% in both the control and polymerized lignosulfonate containing experiments. The overall trend of the graph suggests a slight delay in germination between day 5 and day 8 in samples containing lignosulfonates. The delayed germination had no effect on further plant development. No significant difference in plant growth was observed. Similar to corn, tomato plants also showed a slight delay in germination between day 5 and day 11 with plants reaching their maximum germination rate of 94% in the controls and 92% in the trial plants after 11 days. No difference in plant growth and development was observed after this time point.
Overall a safe application of polymerized lignosulfonate as coatings can be deducted from the above experiments. Even if applied in excessive amounts (324 g m−2versus 1 g m−2) no or only minor negative effects on the germination and growth rate were observed depending on plant species which was similarly reported by Stapanian et al. and others.53–55
In addition, to plant toxicity, the safety of coatings during transport and storage must be ensured. A detailed investigation of this was beyond the scope of this research but should be pursued in the future. Based on literature evidence it is not expected that LS coatings pose a safety hazard. The exception to this may be the coating of fertilizers containing a high concentration of ammonium nitrate. As ammonium nitrate is a strong oxidizing agent the coating with combustible organic carbon (acting as a fuel/electron donor) such as LS will likely pose a fire or explosion hazard.56 According to the UN transport classification this is the case when straight ammonium nitrate with a concentration of >90% and a combustible organic carbon content of >2% are combined (classification: UN 0222 Class 1).57 The coating applied in this study was 2–4% w/w of the fertilizer and does hence exceed the specified level. However, research has also shown that LS purified by dialysis can act as fire retardants in wood flour-based particleboards by increasing char formation.58 Whether LS can also improve fire safety for fertilizers should be subject to further research.
Viability of beneficial Bacillus strains in lignosulfonate coatings
The viability of B. subitilis, B. licheniformis, B. velezensis and B. atrophaeus incorporated into the coatings was investigated. Bacillus spp. are known to aid plant roots in uptake of essential nutrients, such as P and N39 and reduce pathogen pressure, either directly or indirectly by stimulating plant defence.59 Four strains of these well-known plant growth promoters were added in the form of spore solutions to the finished coating solution before spray application. In Fig. 8 the spore viability on the surface of the granules is shown. The applied number of spores on 1 g of granules was 2 × 106. Despite vigorous mixing and very careful preparation of dilutions, the variation of the number of CFUs per plate of three replicates was rather high. Differences are not to be taken as of technical nature, rather due to the nature of granule coating. Differences in the number of spores per 1 g of granules between different time points might be due to the unequal coating of granules with spores. For three species we observed a slight increase of the CFU from day 1 to day 28 potentially indicating bacterial growth. However, after 173 days of storage the CFUs were equal to or slightly lower than those at day 0. This shows that the polymerized lignosulfonate coating is a potent carrier for plant growth promoting Bacillus spores, which will not affect their viability. After 173 days of storage under non-sterile conditions low amounts of growth of most likely airborne Bacilli were observed in untreated and negative controls. | ||
| Fig. 8 Number of germinated Bacillus spores after 0, 1, 7, 14, 28 and 173 days of storage at room temperature. | ||
Effect of coated soil improvers on lawn cut weight
The application of the differently coated soil improvers showed significant positive effects on the biomass production of the lawns. Non-treated lawns produced significantly fewer lawn cuttings whereas samples treated with bioactive coatings had increased biomass production (Fig. 9). In addition, leave colour and growth density were also improved. Therefore, not only the viability of the Bacilli but also their positive effects as plant growth promoters could be shown.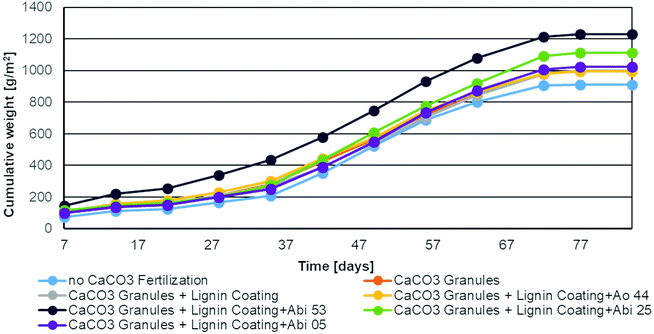 | ||
| Fig. 9 Cumulative fresh weight of lawn cut when different coated soil improvers were added after germination. | ||
CO2 emission equivalent of coating production
The results show that the impact of LS coating production with xylitol as a plasticizer exceeds the impact of coating with polyurethane by 1.6–3.9 kg CO2-eq kg−1 product (Fig. 10a). However, the use of glycerol as a plasticizer leads to avoided impacts between 1.1 and 3.4 kg CO2-eq. kg−1 product. The major impact of the choice of the plasticizer is evident in the fact that it accounts for about 85% of the impact in the xylitol min scenario, but for only between 19 and 43% in the glycerol scenario. | ||
| Fig. 10 Comparison of the CO2-eq. emission of LS coatings with polyurethane (a) and polyethylene (b) based coatings. Different plasticizers were modelled for each LS coating. | ||
When comparing polyethylene-based coatings, the net impact of the LS coating with xylitol is further emphasized due to the lower avoided emissions of the substitution of polyethylene coatings (yellow bar – Fig. 10b). This is evident in the higher overall emissions of 4.8–7 kg CO2-eq. kg−1 product in the xylitol scenario. When glycerol is used as a plasticizer, the importance of maximizing the energy efficiency is highlighted. At maximal heat energy efficiency (i.e. min scenario), a net CO2-eq. reduction of 0.2 kg CO2-eq. kg−1 product can be realized. However, in a process with a higher heat demand (max scenario) this may lead to a net impact of 2.1 kg CO2-eq. kg−1 product (Fig. 10b).
Despite the potentially higher CO2-eq. emissions, LS coatings are likely to reduce the pressure on the environment as they avoid the pollution with non-biodegradable coatings, including the pollution of the soils and oceans for example with micro-plastics. Instead, LS based coatings may increase the soil organic matter and soil organic carbon content, due to their relatively slow biodegradability and potential transformation to humic compounds.18 As a result, they are likely to improve the nutrient and water retention of the soil, leading to better plant yields and a higher nutrient use efficiency.
Conclusion
In this work a bio-based, biocompatible, anti-dust soil improver coating was investigated. The environmental non-hazardous application of polymerized lignosulfonates was demonstrated in plant trials. The model plants corn, wheat, tomato and salad showed no significant negative response to the application of the coatings. The CO2 emission analysis of the coatings produced with xylitol and glycerol compared to polyurethane and polyethylene coatings showed potential CO2 savings of up to 3.4 kg CO2-eq. kg−1 product. The required plasticity of the coatings was best achieved by the addition of xylitol, a natural plasticizer, and the optimization of the polymerization conditions. The potential of the coating to not interfere with the combined application of the plant growth promoting microorganisms and fertilizer was further demonstrated. The coated marine calcium carbonate granules significantly improve golf lawn/sport lawn fertilization, as they provide the chance of long-term fertilization as compared to the regular liquid application of single nutrients. Future studies should investigate the safety of an LS coating in combination with other fertilizers, in particular the fire safety/explosion of combinations with ammonium nitrate.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Writing – original draft: Renate Weiß, Gibson Nyanhongo, Marc Spiller, Julia Santolin, and Günter Brader. Writing – review & editing: all authors. Visualization: Renate Weiß, Sebastian Gritsch, Marc Spiller, and Julia Santolin. Supervision: Gibson Nyanhongo and Georg Gübitz. Resources: Georg Gübitz, Sylvain Pluchon, Kristin Dietel, Günter Brader, Hedda Weber, and Nikolaus Schwaiger. Funding acquisition: Georg Gübitz, Gibson Nyanhongo, and Günter Brader. Conceptualization: Renate Weiß. Data curation: Renate Weiß, Gibson Nyanhongo, Branislav Nikolic, and Günter Brader. Formal Analysis: Renate Weiß, Sebastian Gritsch, and Branislav Nikolic. Investigation: Renate Weiß, Sebastian Gritsch, and Branislav Nikolic. Methodology: Renate Weiß, Sebastian Gritsch, Branislav Nikolic, and Marc Spiller. Software: Renate Weiß and Marc Spiller.Conflicts of interest
No conflicts of interest are to be reported.Acknowledgements
This work was supported by the NÖ Forschungs- und Bildungsges.m.b.H. [grant number SC17-007].The SUFERT project has received funding from the Bio-based Industries Joint Undertaking (BBI-JU) under grant agreement No. 792021.
The BBI-JU receives support from the European Union's Horizon 2020 research and innovation programme and the Bio-Based Industries Consortium.
References
- A. R. Carrico, U. S. Raja, J. Fraser and M. P. Vandenbergh, Household and block level influences on residential fertilizer use, Landsc. Urban Plan, 2018, 178, 60–68 CrossRef.
- L. Polycarpou, The Poblem of Lawns, State of the Planet Columbia University, 2010. Available from: https://blogs.ei.columbia.edu/2010/06/04/the-problem-of-lawns/ Search PubMed.
- R. Jurin, Principles of Sustainablility: Lawns-Water use and lawn chemicals, Mariana Cove, 2020. Available from: http://www.marianacove.org/principles-of-sustainability-lawns-water-use-and-lawn-chemicals/ Search PubMed.
- J. Behin and N. Sadeghi, Utilization of waste lignin to prepare controlled-slow release urea, Int. J. Recycl. Org. Waste Agric., 2016, 5(4), 289–299 CrossRef.
- J. Chen, F. Cao, H. Xiong, M. Huang, Y. Zou and Y. Xiong, Effects of single basal application of coated compound fertilizer on yield and nitrogen use efficiency in double-cropped rice, Crop J., 2017, 5(3), 265–270 CrossRef.
- P. Tidåker, T. Wesström and T. Kätterer, Energy use and greenhouse gas emissions from turf management of two Swedish golf courses, Urban For. Urban Green., 2017, 21, 80–87 CrossRef.
- C. Gu, J. Crane, G. Hornberger and A. Carrico, The effects of household management practices on the global warming potential of urban lawns, J. Environ. Manage., 2015, 151, 233–242 CrossRef.
- C. O. Dimkpa, J. Fugice, U. Singh and T. D. Lewis, Development of fertilizers for enhanced nitrogen use efficiency – Trends and perspectives, Sci. Total Environ., 2020, 731, 139113 CrossRef CAS PubMed.
- C. Feng, S. Lü, C. Gao, X. Wang, X. Xu and X. Bai, et al., “Smart” Fertilizer with Temperature- and pH-Responsive Behavior via Surface-Initiated Polymerization for Controlled Release of Nutrients, ACS Sustainable Chem. Eng., 2015, 3(12), 3157–3166 CrossRef CAS.
- M.E. Trenkel, Slow- and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Efficiency in Agriculture, IFA, Paris, France, 2nd edn, 2010. Available from: http://www.fertilizer.org/imis20/images/Library_Downloads/2010_Trenkel_slowreleasebook.pdf?WebsiteKey=411e9724-4bda-422f-abfc-8152ed74f306&=404%3Bhttp%3A%2F%2Fwww.fertilizer.org%3A80%2Fen%2Fimages%2FLibrary_Downloads%2F2010_Trenkel_slow+release+book.pdf Search PubMed.
- N. Katsumi, T. Kusube, S. Nagao and H. Okochi, Accumulation of microcapsules derived from coated fertilizer in paddy fields, Chemosphere, 2021, 267, 129185 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0045653520333828.
- N. Katsumi, T. Kusube, S. Nagao and H. Okochi, The role of coated fertilizer used in paddy fields as a source of microplastics in the marine environment, Mar. Pollut. Bull., 2020, 161, 111727 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0025326X20308456.
- ECHA, Microplastics, 2019. Available from: https://echa.europa.eu/de/hot-topics/microplastics Search PubMed.
- F. Europe, Microplastics, 2020. Available from: https://www.fertilizerseurope.com/circular-economy/micro-plastics/ Search PubMed.
- M. H. Sipponen, O. J. Rojas, V. Pihlajaniemi, K. Lintinen and M. Österberg, Calcium Chelation of Lignin from Pulping Spent Liquor for Water-Resistant Slow-Release Urea Fertilizer Systems, ACS Sustainable Chem. Eng., 2017, 5(1), 1054–1061 CrossRef CAS.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, A concise review of current lignin production, applications, products and their environment impact, Ind. Crops Prod., 2019, 139, 111526, DOI:10.1016/j.indcrop.2019.111526.
- M. Secchi, V. Castellani, M. Orlandi and E. Collina, Use of Lignin Side-Streams from Biorefineries as Fuel or Co-product? Life Cycle Analysis of Bio-ethanol and Pulp Production Processes, BioResources, 2019, 14(2), 4832–4865 CAS.
- J. Chen, X. Fan, L. Zhang, X. Chen, S. Sun and R.-C. Sun, Research Progress in Lignin-Based Slow/Controlled Release Fertilizer, ChemSusChem, 2020, 13(17), 4356–4366 CrossRef CAS PubMed.
- United States D of A, Lignin Sulfonate Technical Evaluation Report, 2011, vol. 705707. Available from: https://www.ams.usda.gov/sites/default/files/media/LigninSulfonateTR2011.pdf Search PubMed.
- Wordpress, Lignosulfonate used in agriculture, 2021. Available from: https://www.greenagrochem.com/industry/lignosulfonate-used-in-agriculture/ Search PubMed.
- J. Steinar and I.B Gunn, Product for soil improvement comprises lignin sulphonate gel, Germany, DE19828483A1, 1998. Available from: https://patents.google.com/patent/DE19828483A1/en Search PubMed.
- S. Fertahi, M. Ilsouk, Y. Zeroual, A. Oukarroum and A. Barakat, Recent trends in organic coating based on biopolymers and biomass for controlled and slow release fertilizers, J. Controlled Release, 2021, 330, 341–361 CrossRef CAS PubMed.
- J. Li, M. Wang, D. She and Y. Zhao, Structural functionalization of industrial softwood kraft lignin for simple dip-coating of urea as highly efficient nitrogen fertilizer, Ind. Crops Prod., 2017, 109, 255–265 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0926669017305228.
- A. C. S. dos Santos, H. M. Henrique, V. L. Cardoso and M. H. M. Reis, Slow release fertilizer prepared with lignin and poly(vinyl acetate) bioblend, Int. J. Biol. Macromol., 2021, 185, 543–550 CrossRef CAS PubMed.
- R. Gil-Ortiz, M.Á Naranjo, A. Ruiz-Navarro, M. Caballero-Molada, S. Atares and C. García, et al., New Eco-Friendly Polymeric-Coated Urea Fertilizers Enhanced Crop Yield in Wheat, Agronomy, 2020, 10(3), 438 CrossRef CAS . Available from: https://www.mdpi.com/2073-4395/10/3/438.
- N. Sadeghi, K. Shayesteh and S. Lotfiman, Effect of Modified Lignin Sulfonate on Controlled-Release Urea in Soil, J. Polym. Environ., 2017, 25(3), 792–799 CrossRef CAS.
- F. Rotondo, R. Coniglio, L. Cantera, I. Di Pascua, L. Clavijo and A. Dieste, Lignin-based coatings for controlled P-release fertilizer consisting of granulated simple superphosphate, Holzforschung, 2018, 72(8), 637–643 CAS.
- S. Fertahi, I. Bertrand, M. Amjoud, A. Oukarroum, M. Arji and A. Barakat, Properties of Coated Slow-Release Triple Superphosphate (TSP) Fertilizers Based on Lignin and Carrageenan Formulations, ACS Sustainable Chem. Eng., 2019, 7(12), 10371–10382 CrossRef CAS.
- E. Windeisen and G. Wegener, Lignin as Building Unit for Polymers, in Reference Module in Materials Science and Materials Engineering, Elsevier, 2016. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128035818092407 Search PubMed.
- R. Al-Hellani, C.-E. Cimpeanu, J. Schmidt-Thuemmes, D. Lawrenz, K. Georgieva and A. Song, Aqueous Polymer Dispersion Obtainable By Free-Radically Initiated Emulsion Polymerization In The Presence Of Lignosulfonate, US Pat., 14375937, 2013, Available from: https://patents.justia.com/patent/20140342171 Search PubMed.
- L. Munk, M. L. Andersen and A. S. Meyer, Influence of mediators on laccase catalyzed radical formation in lignin, Enzyme Microb. Technol., 2018, 116, 48–56 CrossRef CAS.
- G. G. Allan, J. A. Dalan and N. C. Foster, Modification of Lignins for Use in Phenolic Resins, ACS Symp. Ser., 1989,(385), 55–67 CrossRef CAS . Available from: http://pubs.acs.org/doi/abs/10.1021/bk-1989-0385.ch005.
- B. Beer, M. J. Bartolome, L. Berndorfer, G. Bochmann, G. M. Guebitz and G. S. Nyanhongo, Controlled enzymatic hydrolysis and synthesis of lignin cross-linked chitosan functional hydrogels, Int. J. Biol. Macromol., 2020, 161, 1440–1446 CrossRef CAS PubMed.
- M. Jimenez Bartolome, S. Bischof, A. Pellis, J. Konnerth, R. Wimmer and H. Weber, et al., Enzymatic synthesis and tailoring lignin properties: A systematic study on the effects of plasticizers, Polymer, 2020, 202, 122725 CrossRef CAS.
- R. Weiss, E. Ghitti, M. Sumetzberger-Hasinger, G. M. Guebitz and G. S. Nyanhongo, Lignin-Based Pesticide Delivery System, ACS Omega, 2020, 5(8), 4322–4329, DOI:10.1021/acsomega.9b04275 , Available from: https://pubs.acs.org/doi/10.1021/acsomega.9b04275.
- E. Oleńska, W. Małek, M. Wójcik, I. Swiecicka, S. Thijs and J. Vangronsveld, Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review, Sci. Total Environ., 2020, 743, 140682 CrossRef PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0048969720342042.
- D. D. Figueiredo, R. A. Batista, P. J. Roszak, L. Hennig and C. Köhler, Auxin production in the endosperm drives seed coat development in Arabidopsis, eLife, 2016, 5, 20542, DOI:10.7554/eLife.20542.
- A. Hashem, B. Tabassum and E. Fathi Abd_Allah, Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress, Saudi J. Biol. Sci., 2019, 26(6), 1291–1297 CrossRef CAS PubMed.
- R. Radhakrishnan, A. Hashem and E. F. Abd_Allah, Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments, Front. Physiol., 2017, 8, 00667 CrossRef PubMed , Available from: http://journal.frontiersin.org/article/10.3389/fphys.2017.00667/full.
- L. P. Christopher, B. Yao and Y. Ji, Lignin biodegradation with laccase-mediator systems, Front. Energy Res., 2014, 2(MAR), 1–13 Search PubMed.
- R. Utsumi, T. Hirano, H. Mori, J. Tsubaki and T. Maeda, An attrition test with a sieve shaker for evaluating granule strength, Powder Technol., 2002, 122(2–3), 199–204 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0032591001004168.
- B. Azeem, K. KuShaari, Z. B. Man, A. Basit and T. H. Thanh, Review on materials & methods to produce controlled release coated urea fertilizer, J. Controlled Release, 2014, 181, 11–21 CrossRef CAS . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168365914001205.
- K. Johansson, S. Winestrand, C. Johansson, L. Järnström and L. J. Jönsson, Oxygen-scavenging coatings and films based on lignosulfonates and laccase, J. Biotechnol., 2012, 161(1), 14–18 CrossRef CAS PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0168165612002970.
- D. Huber, A. Ortner, A. Daxbacher, G. S. Nyanhongo, W. Bauer and G. M. Guebitz, Influence of Oxygen and Mediators on Laccase-Catalyzed Polymerization of Lignosulfonate, ACS Sustainable Chem. Eng., 2016, 4(10), 5303–5310 CrossRef CAS.
- N. Madad, L. Chebil, C. Charbonnel, I. Ioannou and M. Ghoul, Enzymatic polymerization of sodium lignosulfonates: Effect of catalysts, initial molecular weight, and mediators, Can. J. Chem., 2013, 91(3), 220–225 CrossRef CAS.
- A. Ortner, D. Huber, O. Haske-Cornelius, H. K. Weber, K. Hofer and W. Bauer, et al., Laccase mediated oxidation of industrial lignins: Is oxygen limiting?, Process Biochem., 2015, 50(8), 1277–1283, DOI:10.1016/j.procbio.2015.05.003.
- D. Huber, A. Pellis, A. Daxbacher, G. Nyanhongo and G. Guebitz, Polymerization of Various Lignins via Immobilized Myceliophthora thermophila Laccase (MtL), Polymer, 2016, 8(8), 280 Search PubMed . Available from: http://www.mdpi.com/2073-4360/8/8/280.
- P. K. Robinson, Enzymes: principles and biotechnological applications, Essays Biochem., 2015, 59, 1–41 CrossRef PubMed.
- T. Hatakeyama, Y. Asano and H. Hatakeyama, Mechanical and thermal properties of rigid polyurethane foams derived from sodium lignosulfonate mixed with diethylene-, triethylene- and polyethylene glycols, Macromol. Symp., 2003, 197(1), 171–180 CrossRef CAS.
- H. Hatakeyama and T. Hatakeyama, Lignin Structure, Properties, and Applications, in Biopolymers, 2009, pp. 1–63 Search PubMed.
- Q. Tong, Q. Xiao and L.-T. Lim, Effects of glycerol, sorbitol, xylitol and fructose plasticisers on mechanical and moisture barrier properties of pullulan-alginate-carboxymethylcellulose blend films, Int. J. Food Sci. Technol., 2013, 48(4), 870–878 CrossRef CAS . Available from: https://onlinelibrary.wiley.com/doi/10.1111/ijfs.12039.
- E. Commission, Regul. Eur. Parliament Counc., 2011, 1(2011), 1–119 Search PubMed . Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1009.
- M. A. Stapanian and D. W. Shea, Lignosulfonates: effects on plant growth and survival and migration through the soil profile, Int. J. Environ. Stud., 1986, 27(1–2), 45–56 CrossRef CAS . Available from: http://www.tandfonline.com/doi/abs/10.1080/00207238608710276.
- W. M. A. N. Wan Abdullah, L.-Y. Low, S. B. Mumaiyizah, Q.-Y. Chai, J.-Y. Loh and J. Ong-Abdullah, et al., Effect of lignosulphonates on Vanilla planifolia shoot multiplication, regeneration and metabolism, Acta Physiol. Plant., 2020, 42(7), 107 CrossRef CAS . Available from: http://link.springer.com/10.1007/s11738-020-03099-9.
- A. Ertani, O. Francioso, V. Tugnoli, V. Righi and S. Nardi, Effect of Commercial Lignosulfonate-Humate on Zea mays L. Metabolism, J. Agric. Food Chem., 2011, 59(22), 11940–11948 CrossRef CAS PubMed . Available from: https://pubs.acs.org/doi/10.1021/jf202473e.
- V. Babrauskas, Explosions of ammonium nitrate fertilizer in storage or transportation are preventable accidents, J. Hazard. Mater., 2016, 304, 134–149 CrossRef CAS PubMed . Available from: https://linkinghub.elsevier.com/retrieve/pii/S0304389415301680.
- F. Europe, Guidance For Un Transport Classification Of Ammonium Nitrate Based Substances, 2011. Available from: https://www.fertilizerseurope.com/wp-content/uploads/2019/08/Guidance_of_UN_class_of_ammonium_ntirate_based_substances.pdf Search PubMed.
- S. Angelini, A. Barrio, P. Cerruti, G. Scarinzi, J. Garcia-Jaca and D. Savy, et al., Lignosulfonates as Fire Retardants in Wood Flour-Based Particleboards, Int. J. Polym. Sci., 2019, 2019, 1–10 CrossRef . Available from: https://www.hindawi.com/journals/ijps/2019/6178163/.
- S. Nigris, E. Baldan, A. Tondello, F. Zanella, N. Vitulo and G. Favaro, et al., Biocontrol traits of Bacillus licheniformis GL174, a culturable endophyte of Vitis vinifera cv. Glera, BMC Microbiol., 2018, 18(1), 133 CrossRef CAS PubMed . Available from: https://bmcmicrobiol.biomedcentral.com/articles/10.1186/s12866-018-1306-5.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc02221k |
| This journal is © The Royal Society of Chemistry 2021 |



