Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enzyme-catalyzed synthesis of malonate polyesters and their use as metal chelating materials†
Fergal P.
Byrne
 *a,
Jamie M. Z.
Assemat
a,
Amy E.
Stanford
a,
Thomas J.
Farmer
*a,
Jamie M. Z.
Assemat
a,
Amy E.
Stanford
a,
Thomas J.
Farmer
 a,
James W.
Comerford
a,
James W.
Comerford
 ab and
Alessandro
Pellis
ab and
Alessandro
Pellis
 *ac
*ac
aGreen Chemistry Centre of Excellence, Department of Chemistry, University of York, Heslington, York YO10 5DD, UK. E-mail: alessandro.pellis@boku.ac.at; fergal.byrne@york.ac.uk
bSINTEF, Forskningsveien 1A, 0373, Oslo, Norway
cUniversity of Natural Resources and Life Sciences, Vienna, Department of Agrobiotechnology, Institute of Environmental Biotechnology, Konrad Lorenz Strasse 20, 3430, Tulln an der Donau, Austria
First published on 29th June 2021
Abstract
Following the environmental problems caused by non-degradable plastics there is a need to synthesise greener and more sustainable polymers. In this work we describe, for the first time, the facile enzyme-catalysed synthesis of linear polyesters using dimethyl malonate as the diester. These polymers, containing a different aliphatic diol component (C4, C6 or C8), were synthesised in solventless conditions using immobilized Candida antarctica lipase B as the biocatalyst. The potential of enzymes for catalysing this reaction is compared with the unsuccessful antimony- and titanium-catalysed synthesis (T > 150 °C). The application of the synthesized polymers as effective metal chelators in biphasic, green solvent systems was also described, together with the characterisation of the synthesised materials.
Introduction
Due to the functionality limitations and the pollution caused by fossil-derived polymers,1 the chemical industry urges the development of greener routes to produce novel bio-based, degradable (or compostable) materials. Several steps in this direction were recently made, especially in the polyesters field, with the development of poly(ethylene 2,5-furandicarboxylate) (PEF) as a poly(ethylene terephthalate) (PET) substitute due to its similar mechanical and barrier properties2,3 and better biodegradability.4,5 Other furan- and pyridine-based polymers potentially useful for packaging and film applications were developed, but their synthesis remains limited to laboratory-scale.6Recently, the potential of enzymes as green and selective biocatalysts has been demonstrated on several aromatic and aliphatic monomers, which is of great interest when the polycondensation of monomers carrying lateral functionalities is desired, as highlighted in several review articles.7,8 Such functionalities – the vinyl group of itaconic acid, the secondary hydroxy group of glycerol, sorbitol or mucic acid, etc. – are prone to a wide array of side reactions (such as Ordelt saturation, radical crosslinking, etc.) when traditional metal- or acid-catalyzed polymerization reactions are carried out.9,10 In fact, previously synthesized metal-chelating polymers based on a diethylenetriaminepentaacetic acid pendant group required a Michael addition of a thiol to be carried out in a second reaction step, and the double bond of the itaconate moiety was preserved only thanks to the use of very toxic chemicals such as 2-furanmethanethiol and a phosphazene base as the initiator.11
In this work, attention was focused on malonate-derived aliphatic polyesters. Malonic acid is a source of bio-based 1,3-diketone functionality, and it is produced commercially by Lygos using engineered yeast strains.12 To the best of our knowledge, malonate-derived polyesters were reported before in the literature only in the form of short oligomers having a maximum DP of around 5.13 In fact, the reports dealing with malonate polymers describe mainly the synthesis of aliphatic hyperbranched polyesters (HBPE) from various monomers derived in one step from commercial diethyl malonate.14 The acid- and metal-catalyzed polycondensation of malonate derivatives bearing aliphatic residues15 was also reported together with the sequential anionic polymerization of ethylene oxide and methylidene malonate to obtain poly(ethylene oxide)-block-poly(methylidene malonate 2.1.2) block copolymers bearing a primary amino group at the PEO chain end.16 The only work where the malonate unit was changed from a malonic acid or dialkyl ester to being part of the main chain, is the work of Doğan and Küsefoğlu, published in 2008, that reported the 1,4-diazabicyclo[2.2.2]octane-catalyzed synthesis of a biodegradable polymeric foam from epoxidized soybean oil and malonic acid.17
There has been increased interest in elemental sustainability in recent years. Commonly used metals such as cobalt, nickel, copper and zinc have reserves expected to last only 50–100 years. Recovery of these metals from waste streams is vital to maintain supplies of these dwindling resources.18 One method of recovering metals from aqueous waste streams is solvent-based hydrometallurgy.19,20 This involved contacting an organic solution containing metal chelators with an aqueous metal solution. A biphasic system emerges, in which metal ions can pass from the aqueous phase to the metal chelators in the organic phase. The metals can be recovered from the organic phase by re-extraction by an acidic solution, allowing the free chelators in the organic phase to be reused.20 However, issues of toxicity, bioaccumulation and persistence in the environment of chelators are common.21 In addition, most currently available chelators are petroleum-derived with few examples of bio-based products (Nouryon Dissolvine range being a rare example).22 As such, bio-based, safe, water-insoluble metal chelators are sought after.
Many functional groups can be used as chelators, such as oximes, carboxylates, phosphorous acids, and 1,3-diketones. 1,3-Diketones such as LIX54 (Fig. 1) are commercially available for this purpose,19,23 but naturally occurring other 1,3-diketones such as 14,16-hentriacontanedione are present in plant waxes.24,25 Indeed, biphasic extraction systems have been proposed in the past using bio-derived lipophilic chelators sourced from wheat straw wax,25 as well as modified wax products to produce super-chelators.24
 | ||
| Fig. 1 Structures of the previously described 1,3-diketone chelators LIX54, 14,16-hentriacontanedione and the malonate-based polymers described in this work. | ||
Herein we present the facile enzyme-catalyzed synthesis of dimethyl malonate-based linear polyesters having a different aliphatic diol component (C4, C6 or C8). The reaction was conducted in solventless conditions using immobilized Candida antarctica lipase B as the biocatalyst. The potential of enzymes for catalysing this reaction is compared with the largely unsuccessful chemo-catalytic metal-catalyzed synthesis. The application of the synthesized polymers as effective metal chelators in biphasic, solvent-based hydrometallurgy is also described together with a detail characterization of the synthesized materials. Moreover, these aliphatic polyesters are known to be easily degraded to their constituent monomers (diacids and polyols) using a variety of hydrolytic enzymes (e.g. lipases, cutinases),26,27 therefore allowing the recovery of such building blocks and the re-synthesis of the polymer in a closed-loop circular economy concept.
Synthesis of malonate-based aliphatic polymers
Quite surprisingly, very few reports describing the use of malonic acid (or its esters) as the diacid component of polyesters were found in the literature28,29 and none of them focuses on the chelating properties of these polymers. We therefore initially attempted to synthesize malonate polyesters using the most commonly known metal catalysts for polycondensation reactions: antimony oxide and titanium butoxide, catalysts widely known for the synthesis of PET,30 PEF31 and a wide range of other aliphatic and aromatic polyesters.32 Unfortunately, when using dimethyl malonate (DMM) as the diester in combination with various aliphatic polyols, the metal-catalyzed synthesis was unsuccessful, with obtained Mns between 1000 and 2600 Da (DP of 6 and 16 respectively, Table 1).| Diol | Catalyst |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Da]
[Da] |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Da]
[Da] |
Đ |
|---|---|---|---|---|
| a Calculated via GPC. | ||||
| 1,4-BDO | Sb2O3 | 2100 | 4000 | 1.90 |
| Ti(OtBu)4 | 2600 | 4600 | 1.79 | |
| Sb2O3 | 1000 | 2400 | 2.28 | |
| 1,8-ODO | 1600 | 2800 | 1.68 | |
The rather low molecular weights obtained in this work using metal catalysts can be explained with the fact that β-diketones, such as malonates, are known chelating agents and can therefore competitively chelate the catalyst metal ions, reducing their capacity to promote the transesterification reaction. This idea is supported by the fact that titanium forms complexes with dimethyl malonate33 creating a useful catalyst for the polymerization of polypropylene.34 One of the few available reports on malonate polyesters is the P2O5-catalyzed synthesis of poly(1,3-propyl malonate), but also in this case the obtained molecular weights were really limited since the maximum achieved DP was approximately 5.13
Taking inspiration from recent papers on environmentally friendly synthesis of polymers, and due to the impossibility to obtain polymers using traditionally-used methods, an enzymatic approach was used in order to synthesize a series of malonate-containing aliphatic polyesters using diols having a chain length from 4 to 8 carbon atoms. For the synthesis performed in this work, an immobilized preparation of Candida antarctica lipase B (iCaLB) was used as the biocatalyst since this enzyme was reported to be an excellent candidate for such synthesis reactions.7,35 The polycondensation reaction progressed at very mild (85 °C, 1000/20 mbar, 6 + 18 h) solventless conditions. The application of such environmentally-friendly synthesis protocol was possible since DMM is a liquid at the used operational temperature while the corresponding diacid, malonic acid, has a melting temperature reported to be between 135 °C and 137 °C.
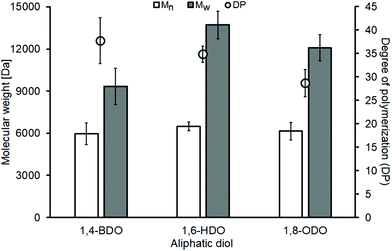
The enzymatic synthesis experiments show similar Mn values of around 6000 Da for all the used diols while the Mw values increase from 9000 Da to 12–14 K Da with the increase of the diol's chain length (Fig. 1). The reported values are in line with previous reports of solvent-free enzymatic polycondensations where the used diester was dimethyl adipate that also showed similar Mn (∼7000 Da) and increasing Mw (from 11 to 14 K Da) when the same three aliphatic diols having increasing carbon chain length were used.35 The decrease of the DP as the diol increases, as seen for both the malonate and the adipate polyesters, is also a common trait (Fig. 2).7,35
All synthesized malonate-based polyesters have 29 < DP < 38, therefore presenting molecular masses significantly higher in comparison of the short oligomers previously synthesized using P2O5 and our own chemocatalytic synthesis approach (Table 1). The polymers were recovered using a simple vacuum filtration that allowed the removal of the immobilized enzyme and the work-up solvent was then removed via rotary evaporation. All isolated polyesters were colourless viscous liquids and subsequently used for the chelation experiments without further purification.
Polymer structures and relative monomer conversions were elucidated via1H-NMR spectroscopy (Fig. S1–S5 in ESI†). Upon reaction, the –CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–OH signal from the diol has a characteristic change of chemical shift from 3.65 ppm to 4.14 ppm, proving the formation of an ester bond. Additionally, the –OC
2–OH signal from the diol has a characteristic change of chemical shift from 3.65 ppm to 4.14 ppm, proving the formation of an ester bond. Additionally, the –OC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3 signal from the malonate, observable at 3.75 ppm disappears due to the release of MeOH with the progression of the reaction. The signals at 3.38 ppm (C–C
3 signal from the malonate, observable at 3.75 ppm disappears due to the release of MeOH with the progression of the reaction. The signals at 3.38 ppm (C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–C of the malonate) and in the 1.3–1.8 ppm range (–C
2–C of the malonate) and in the 1.3–1.8 ppm range (–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH2–OH of the diols) do not noticeably change chemical shift upon elongation of the polymer chain. 13C-NMR spectroscopy of the polymer reveals the typical signals for these aliphatic polyesters (Fig. S6 in ESI†).
2–CH2–OH of the diols) do not noticeably change chemical shift upon elongation of the polymer chain. 13C-NMR spectroscopy of the polymer reveals the typical signals for these aliphatic polyesters (Fig. S6 in ESI†).
Metal chelation in biphasic systems based on green solvents
The malonate polyesters were assessed for their ability to extract metal ions from aqueous streams in a biphasic system. The biphasic system involved dissolving the polyester in an organic solvent and mixing it with a metal-containing aqueous solution. Upon contact between the two phases, metal transfer occurs from the aqueous phase to the diketone chelating points on the polyester in the organic phase, purifying the aqueous phase. Separation of the two phases and re-extraction of the organic phase with an acidic stripping solution can recover the metal from the polyester for reuse, and also regenerate the diketone chelating point on the polyester.Copper was chosen as the target metal for chelation using the polyesters due to it being a common pollutant in metallurgy waste streams.36 Cl− was selected as the counter ion as it has previously been shown to be effective in the chelation of copper using diketone species due to it being a strong inner-sphere ligand.24,25 The chelation tests were carried out across as pH range of 8.4–12.3.
Several requirements exist for the choice of organic solvent for this purpose. It must partition well with water; the chelating agent must favour solubility in the organic solvent over water; it must facilitate enol formation; and it must not be reactive in the extraction conditions. As the solvent does not need to be evaporated at any stage of the extraction process, a low boiling point is not necessary. In fact, a higher boiling point will reduce losses to the atmosphere, improving the economics of such an extraction process. In addition, it will add to the green credentials of the process, as emissions, exposure to workers and solvent demand are reduced. The CHEM21 solvent selection guide recommends a boiling point of between 70–139 °C.37
Three candidate solvents were ultimately selected using the CHEM21 solvent guide:37para-cymene, ethyl levulinate and anisole. para-Cymene and anisole have boiling points within the ideal range (70–139 °C), while that of ethyl levulinate is 206 °C. However, as the solvent/polymer mixture can be used repeatedly, this higher than preferred boiling point is not a significant issue. All three candidates are aprotic, meaning the enol form is more likely to be favoured25 which is a prerequisite for chelation ability (keto form is stabilised by intermolecular hydrogen-bonding in protic solvents).38 Polymer insolubility in BDO MAL polymer prevented para-cymene from being tested, while ethyl levulinate likely chelated with metal ions during extraction, indicated by a green complex being formed when mixed with copper solutions. Anisole could dissolve all polymers, and also scores well in the CHEM21 solvent selection guide, being classified as “recommended”.37 As such, anisole was chosen as the most appropriate solvent for this process.
The 1,3-diketones functionality can exist in keto and enol forms (Fig. 3). In basic conditions, deprotonation of the acidic proton in the 2-position forms an anionic bidentate ligand which are weak chelators. A square planar complex is suggested, similar to that of bis(acetylacetonato)copper(II), as previously determined by single crystal X-ray diffraction.39 As the polymers existed as a viscous liquid, possibly due to residual solvent that could not be removed in vacuo, powder XRD could not be carried out to confirm the square planar complex in the malonate polymers of this work.
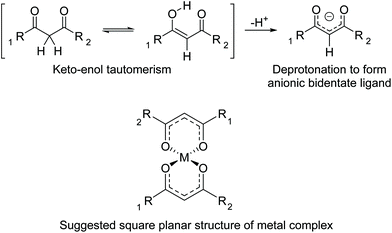 | ||
| Fig. 3 Keto–enol tautomerism of 1,3-diketones followed by deprotonation to form negatively charged bidentate ligand. | ||
The polymers with the highest (ODO MAL) and lowest (BDO MAL) M0 were chosen as model to perform chelation tests with CuCl2. A pH range of 8.4–12.3 was used for the tests. Chelation tests were carried out in a biphasic system consisting of a CuCl2-rich aqueous phase (0.05 M CuCl2, 0.25 M NH3) and an organic solution of the chelating polymer (0.05 M). Interestingly, the density of the aqueous metal solutions at higher pH (10.4–12.3) changed such that the layers switched in the biphasic system when the BDO MAL was used. This is due to the proximity of the density of pure anisole (0.995 g mL−1) to that of water (1.000 g mL−1), which must be taken into consideration in an industrial process.
Fig. 4 shows that optimal pH for extraction was similar in both cases, with ∼pH 10 being optimal for ODO MAL and BDO MAL polymers (specific pH's and absorbances for chelation tests are shown in Tables S2 and S3 in ESI†). This is consistent with previous observations of 1,3-diketones being more effective in basic conditions.17 Superior extraction was obtained with the ODO MAL, with mean Cu extraction of 22.7% compared to 15.7% for the BDO MAL polymer. Extraction with the malonate polymers is comparable with the commercial LIX54 (18% in a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mix of LIX54/kerosene, 40% using pure LIX54),23 demonstrating the potential of such a chelating polymer for use in an industrial setting. Further work is required on optimising the conditions for extraction (polymer loading, further solvent investigation, more robust pH control), their stability over multiple uses and their affinities for other metals in mixed aqueous streams which will be the focus of a subsequent full article.
50 mix of LIX54/kerosene, 40% using pure LIX54),23 demonstrating the potential of such a chelating polymer for use in an industrial setting. Further work is required on optimising the conditions for extraction (polymer loading, further solvent investigation, more robust pH control), their stability over multiple uses and their affinities for other metals in mixed aqueous streams which will be the focus of a subsequent full article.
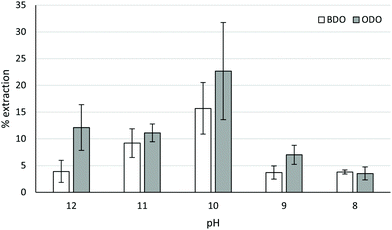 | ||
Fig. 4 The % extraction of Cu(II) from ammoniacal solutions using “BDO” MAL and “ODO” MAL at different pHs and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight loading of chelator 1 by weight loading of chelator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(II). The pH shown in the graph has been rounded to the nearest whole number for visual clarity. Exact pH values are shown in the ESI.† Cu(II). The pH shown in the graph has been rounded to the nearest whole number for visual clarity. Exact pH values are shown in the ESI.† | ||
Conclusions
A series of malonate polymers were successfully synthesized using environmentally-friendly conditions (enzymatic catalyst, T < 90 °C, solvent for the workup: MeTHF) and used to achieve the efficient chelation of copper, a common pollutant in metallurgy waste streams. A superior extraction efficiency of 23% was obtained with the ODO MAL polymer that is comparable with commercially-available LIX54 (18% in a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mix of LIX54/kerosene, 40% using pure LIX54), demonstrating the potential of this new class of polyesters for use in an industrial setting. Enzymatic catalysis, showing high selectivity, low operational temperatures and benign reaction conditions, is emerging as a useful tool to complement chemo-catalytic routes for the synthesis of multifunctional polymers having structures that are otherwise not possible to obtain using traditional metal- and acid based-methods.
50 mix of LIX54/kerosene, 40% using pure LIX54), demonstrating the potential of this new class of polyesters for use in an industrial setting. Enzymatic catalysis, showing high selectivity, low operational temperatures and benign reaction conditions, is emerging as a useful tool to complement chemo-catalytic routes for the synthesis of multifunctional polymers having structures that are otherwise not possible to obtain using traditional metal- and acid based-methods.
Author contributions
A. P., J. M. Z. A. and A. E. S. performed the enzymatic polymer synthesis and material's characterization. F. P. B., J. M. Z. A. and A. E. S. performed the chelation experiments. A. P. and J. W. C. performed the chemo-catalytic synthesis. A. P. and F. P. B. planned the experiments and wrote the manuscript. F. P. B., A. P. and T. J. F. supervised the work. All authors corrected the manuscript and discussed the data prior to submission.Conflicts of interest
The authors declare no conflict of interests.Acknowledgements
A. P. thanks the FWF Erwin Schrödinger fellowship (grant agreement J4014-N34) for financial support. A. E. S. thanks the Department of Chemistry of the University of York for funding her summer vacation placement to undertake this research.References
- A. Lechner, H. Keckeis, F. Lumesberger-Loisl, B. Zens, R. Krusch, M. Tritthart, M. Glas and E. Schludermann, The Danube so Colourful: A Potpourri of Plastic Litter Outnumbers Fish Larvae in Europe's Second Largest River, Environ. Pollut., 2014, 188, 177–181, DOI:10.1016/j.envpol.2014.02.006.
- G. Z. Papageorgiou, V. Tsanaktsis and D. N. Bikiaris, Synthesis of Poly(Ethylene Furandicarboxylate) Polyester Using Monomers Derived from Renewable Resources: Thermal Behavior Comparison with PET and PEN, Phys. Chem. Chem. Phys., 2014, 16(17), 7946–7958, 10.1039/c4cp00518j.
- G. Z. Papageorgiou, D. G. Papageorgiou, Z. Terzopoulou and D. N. Bikiaris, Production of Bio-Based 2,5-Furan Dicarboxylate Polyesters: Recent Progress and Critical Aspects in Their Synthesis and Thermal Properties, Eur. Polym. J., 2016, 83, 202–229, DOI:10.1016/j.eurpolymj.2016.08.004.
- S. Weinberger, K. Haernvall, D. Scaini, G. Ghazaryan, M. T. Zumstein, M. Sander, A. Pellis and G. M. Guebitz, Enzymatic Surface Hydrolysis of Poly(Ethylene Furanoate) Thin Films of Various Crystallinities, Green Chem., 2017, 19(22), 5381–5384, 10.1039/c7gc02905e.
- S. Weinberger, J. Canadell, F. Quartinello, B. Yeniad, A. Arias, A. Pellis and G. Guebitz, Enzymatic Degradation of Poly(Ethylene 2,5-Furanoate) Powders and Amorphous Films, Catalysts, 2017, 7(11), 318, DOI:10.3390/catal7110318.
- A. Pellis, J. W. Comerford, S. Weinberger, G. M. Guebitz, J. H. Clark and T. J. Farmer, Enzymatic Synthesis of Lignin Derivable Pyridine Based Polyesters for the Substitution of Petroleum Derived Plastics, Nat. Commun., 2019, 10(1), 1–9, DOI:10.1038/s41467-019-09817-3.
- S. Kobayashi, Lipase-catalyzed polyester synthesis–a green polymer chemistry, Proc. Jpn. Acad. Ser. B Phys. Biol. Sci., 2010, 86(4), 338–365, DOI:10.2183/pjab.86.338.
- R. A. Gross, A. Kumar and B. Kalra, Polymer Synthesis by In Vitro Enzyme Catalysis, Chem. Rev., 2001, 101, 2097–2124 CrossRef CAS PubMed.
- A. Pellis, P. A. Hanson, J. W. Comerford, J. H. Clark and T. J. Farmer, Enzymatic Synthesis of Unsaturated Polyesters: Functionalization and Reversibility of the Aza-Michael Addition of Pendants, Polym. Chem., 2019, 10(7), 843–851, 10.1039/c8py01655k.
- T. Farmer, R. Castle, J. Clark and D. Macquarrie, Synthesis of Unsaturated Polyester Resins from Various Bio-Derived Platform Molecules, Int. J. Mol. Sci., 2015, 16(12), 14912–14932, DOI:10.3390/ijms160714912.
- N. Illy, D. Majonis, I. Herrera, O. Ornatsky and M. A. Winnik, Metal-Chelating Polymers by Anionic Ring-Opening Polymerization and Their Use in Quantitative Mass Cytometry, Biomacromolecules, 2012, 13, 2359–2369, DOI:10.1021/bm300613x.
- Malonic acid https://lygos.com/products/(accessed June 15, 2021).
- A. Ravve, G. Pasternack, K. H. Brown and S. B. Radlove, Synthesis of Photocrosslinkable Polyesters by the Knoevenagel Reaction, J. Polym. Sci., Polym. Chem. Ed., 1973, 11(7), 1733–1752, DOI:10.1002/pol.1973.170110720.
- S. Santra and A. Kumar, Facile Synthesis of Aliphatic Hyperbranched Polyesters Based on Diethyl Malonate and Their Irreversible Molecular Encapsulation, Chem. Commun., 2004,(No. (18), 2126–2127, 10.1039/b404447a.
- N. Kolb and M. A. R. Meier, Monomers and Their Polymers Derived from Saturated Fatty Acid Methyl Esters and Dimethyl Carbonate, Green Chem., 2012, 14(9), 2429–2435, 10.1039/c2gc35793c.
- P. Studer, V. Larras and G. Riess, Amino End-Functionalized Poly(Ethylene Oxide)-Block-Poly(Methylidene Malonate 2.1.2) Block Copolymers: Synthesis, Characterization, and Chemical Modification for Targeting Purposes, Eur. Polym. J., 2008, 44(6), 1714–1721, DOI:10.1016/j.eurpolymj.2008.04.003.
- E. Doǧan and S. Küsefoǧlu, Synthesis and in Situ Foaming of Biodegradable Malonic Acid ESO Polymers, J. Appl. Polym. Sci., 2008, 110(2), 1129–1135, DOI:10.1002/app.28708.
- J. R. Dodson, A. J. Hunt, H. L. Parker, Y. Yang and J. H. Clark, Elemental Sustainability: Towards the Total Recovery of Scarce Metals, Chem. Eng. Process., 2012, 51, 69–78, DOI:10.1016/j.cep.2011.09.008.
- F. J. Alguacil and M. Alonso, Effect of Ammonium Sulphate and Ammonia on the Liquid-Liquid Extraction of Zinc Using LIX 54, Hydrometallurgy, 1999, 53(2), 203–209, DOI:10.1016/S0304-386X(99)00045-6.
- A. M. Wilson, P. J. Bailey, P. A. Tasker, J. R. Turkington, R. A. Grant and J. B. Love, Solvent Extraction: The Coordination Chemistry behind Extractive Metallurgy, Chem. Soc. Rev., 2014, 43, 123–134, 10.1039/c3cs60275c.
- B. Nowack and J. M. VanBriesen, Chelating Agents in the Environment; UTC, 2005; vol. 14, pp. 1–18. DOI:10.1021/bk-2005-0910.ch001.
- Discover our Dissolvine® product range https://www.nouryon.com/products/chelates/dissolvine/(accessed Apr 7, 2021).
- G. Kyuchoukov, M. B. Bogacki and J. Szymanowski, Copper Extraction from Ammoniacal Solutions with LIX 84 and LIX 54, Ind. Eng. Chem. Res., 1998, 37(10), 4084–4089, DOI:10.1021/ie980192v.
- K. Asemave, F. P. Byrne, J. H. Clark, T. J. Farmer and A. J. Hunt, Modification of Bio-Based β-Diketone from Wheat Straw Wax: Synthesis of Polydentate Lipophilic Super-Chelators for Enhanced Metal Recovery, RSC Adv., 2019, 9(7), 3542–3549, 10.1039/c8ra09426h.
- K. Asemave, F. Byrne, T. J. Farmer, J. H. Clark and A. J. Hunt, Rapid and Efficient Biphasic Liquid Extraction of Metals with Bio-Derived Lipophilic β-Diketone, RSC Adv., 2016, 6(98), 95789–95792, 10.1039/c6ra24104b.
- C. Gamerith, M. Vastano, S. M. Ghorbanpour and S. Zitzenbacher, et al., Enzymatic Degradation of Aromatic and Aliphatic Polyesters by P. pastoris Expressed Cutinase 1 from Thermobifida cellulosilytica, Front. Microbiol., 2017, 8, 938, DOI:10.3389/fmicb.2017.00938.
- K. Shi, J. Jing, L. Song, T. Su and Z. Wang, Enzymatic hydrolysis of polyester: Degradation of poly(ε-caprolactone) by Candida antarctica lipase and Fusarium solani cutinase, Int. J. Biol. Macromol., 2020, 144, 183–189, DOI:10.1016/j.ijbiomac.2019.12.105.
- P. D. Tomke, X. Zhao, P. P. Chiplunkar, B. Xu, H. Wang, C. Silva, V. K. Rathod and A. Cavaco-Paulo, Lipase-Ultrasound Assisted Synthesis of Polyesters, Ultrason. Sonochem., 2017, 38, 496–502, DOI:10.1016/j.ultsonch.2017.03.051.
- Diester-Based Polymer Electrolytes for High Voltage Lithium Ion Batteries; 2017.
- L. Finelli, C. Lorenzetti, M. Messori, L. Sisti and M. Vannini, Comparison between Titanium Tetrabutoxide and a New Commercial Titanium Dioxide Based Catalyst Used for the Synthesis of Poly(Ethylene Terephthalate), J. Appl. Polym. Sci., 2004, 92(3), 1887–1892, DOI:10.1002/app.20171.
- K. Loos, R. Zhang, I. Pereira, B. Agostinho, H. Hu, D. Maniar, N. Sbirrazzuoli, A. J. D. Silvestre, N. Guigo and A. F. Sousa, A Perspective on PEF Synthesis, Properties, and End-Life, Front. Chem., 2020, 585, DOI:10.3389/fchem.2020.00585.
- G. Guidotti, M. Gigli, M. Soccio, N. Lotti, M. Gazzano, V. Siracusa and A. Munari, Poly(Butylene 2,5-Thiophenedicarboxylate): An Added Value to the Class of High Gas Barrier Biopolyesters, Polymers, 2018, 10(2), 167, DOI:10.3390/polym10020167.
- A. Marrone, A. Renzetti, P. De Maria, S. Gérard, J. Sapi, A. Fontana and N. Re, Condensation of β-Diester Titanium Enolates with Carbonyl Substrates: A Combined DFT and Experimental Investigation, Chem. – Eur. J., 2009, 15(43), 11537–11550, DOI:10.1002/chem.200901595.
- T. Gueta-Neyroud, B. Tumanskii, M. Botoshansky and M. S. Eisen, Synthesis, Characterization and Catalytic Activity of the Complex Titanium Bis(Dimethylmalonate)-Bis(Diethylamido) in the Polymerization of Propylene, J. Organomet. Chem., 2007, 692(5), 927–939, DOI:10.1016/j.jorganchem.2006.10.031.
- A. Pellis, J. W. Comerford, A. J. Maneffa, M. H. Sipponen, J. H. Clark and T. J. Farmer, Elucidating Enzymatic Polymerisations: Chain-Length Selectivity of Candida Antarctica Lipase B towards Various Aliphatic Diols and Dicarboxylic Acid Diesters, Eur. Polym. J., 2018, 106, 79–84, DOI:10.1016/j.eurpolymj.2018.07.009.
- E. Matinde, G. S. Simate and S. Ndlovu, Mining and Metallurgical Wastes: A Review of Recycling and Re-Use Practices, J. South. Afr. Inst. Min. Metall., 2018, 118, 825–844, DOI:10.17159/2411-9717/2018/v118n8a5.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, CHEM21 Selection Guide of Classical- and Less Classical-Solvents, Green Chem., 2015, 18(1), 288–296, 10.1039/c5gc01008j.
- P. O. Sandusky, Expansion of the Classic Acetylacetone Physical Chemistry Laboratory NMR Experiment: Correlation of the Enol-Keto Equilibrium Position with the Solvent Dipole Moment, J. Chem. Educ., 2014, 91, 739–742, DOI:10.1021/ed400583q.
- P. C. Lebrun, W. D. Lyon and H. A. Kuska, Crystal Structure of Bis(2,4-Pentanedionato)Copper(II), J. Crystallogr. Spectrosc. Res., 1986, 16(6), 889–893, DOI:10.1007/BF01188194.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc01783g |
| This journal is © The Royal Society of Chemistry 2021 |