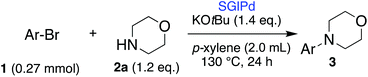Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Product selective reaction controlled by the combination of palladium nanoparticles, continuous microwave irradiation, and a co-existing solid; ligand-free Buchwald–Hartwig amination vs. aryne amination†
Makito
Yamada
 a,
Ryousuke
Ohta
a,
Kazuo
Harada
a,
Ryousuke
Ohta
a,
Kazuo
Harada
 a,
Tsunayoshi
Takehara
a,
Tsunayoshi
Takehara
 b,
Hitoshi
Haneoka
b,
Yosuke
Murakami
b,
Takeyuki
Suzuki
b,
Hitoshi
Haneoka
b,
Yosuke
Murakami
b,
Takeyuki
Suzuki
 b,
Yuuta
Ohki
c,
Naoyuki
Takahashi
c,
Toshiki
Akiyama
b,
Yuuta
Ohki
c,
Naoyuki
Takahashi
c,
Toshiki
Akiyama
 a,
Natchanun
Sirimangkalakitti
a,
Natchanun
Sirimangkalakitti
 a,
Makoto
Sako
a,
Makoto
Sako
 a,
Kenichi
Murai
a,
Kenichi
Murai
 a,
Masayoshi
Arai
a,
Masayoshi
Arai
 a and
Mitsuhiro
Arisawa
a and
Mitsuhiro
Arisawa
 *a
*a
aGraduate School of Pharmaceutical Sciences, Osaka University, Yamada-oka 1-6, Suita, Osaka 565-0871, Japan. E-mail: arisaw@phs.osaka-u.ac.jp
bComprehensive Analysis Centre, SANKEN (The Institute of Scientific and Industrial Research), Osaka University, Mihogaoka 8-1, Ibaraki, Osaka 567-0047, Japan
cTokyo Rikakikai Co. Ltd (Brand: EYELA), TN Koishikawa Bldg. 1-15-17 Koishikawa Bunkyo-ku, Tokyo 112-0002, Japan
First published on 31st August 2021
Abstract
We have developed a continuous microwave irradiation-assisted Buchwald–Hartwig amination using our original Pd nanoparticle catalyst with a copper plate as a co-existing metal solid. In this methodology, a microwave-controlled product selectivity was achieved between Buchwald–Hartwig amination and aryne amination performed under strongly basic conditions and at a high reaction temperature, because a polar chemical species such as Ar–Pd–halogen might be activated selectively by microwave radiation. Moreover, our catalyst could be used repeatedly over 10 times, and the amount of Pd leaching could be suppressed to a low level.
Introduction
Arylamines are widely utilized as functional organic materials in fields such as pharmaceuticals, agrochemicals, dyes, and polymers. Among methodologies for convenient synthesis of arylamines, carbon–nitrogen bond forming reactions are very important.1The Pd-catalyzed cross-coupling of tin amides and aryl halides to generate arylamines in homogeneous systems was first studied by Migita and colleagues in 1983.2 Followed by this report, the Buchwald and Hartwig groups developed a Pd-catalyzed amination under relatively mild reaction conditions independently.3,4 However, sophisticated dialkylaryl phosphine ligands are often used to promote these reactions.1
To develop a ligand-free and environmentally benign metal-catalyzed coupling reaction, metal nanoparticle (NP) catalysts have been widely used to catalyze the coupling reaction.5 We have also developed Pd NP catalysts immobilized on gold or glass and applied them to organic reactions.6
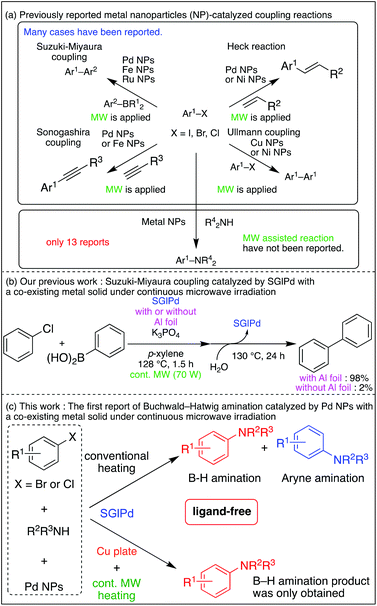
Although many examples of Suzuki–Miyaura coupling, Heck reaction, and Sonogashira coupling have been reported,7 Buchwald–Hartwig amination catalyzed by a metal NP catalyst was reported in only 13 examples (Scheme 1a).8 Moreover, the catalysts used in 13 examples have four drawbacks: (1) ligand addition, (2) inconvenient removal of NPs, (3) insufficient recyclability of NP catalysts (up to six times), and (4) a few reports about leaching analysis of NPs.
On the other hand, external energy-assisted (visible light, microwave) organic synthesis has been enthusiastically researched to perform reactions that are difficult or impossible and to access eco-friendly methodologies.9 Among them, microwave radiation has been applied for the metal NP-catalyzed coupling reaction (Suzuki–Miyaura coupling, Ullmann coupling, and Heck reaction).10 A conventional microwave machine which was used in a previous report turns off within a very short period when the temperature limit was reached and cannot use microwave efficiently. Therefore, we developed a continuous irradiation type microwave machine, and then we have reported continuous microwave irradiation-assisted ligand-free Suzuki–Miyaura coupling of inert aryl chlorides using a Ru NP catalyst on sulfur-modified gold (henceforth referred to as SARu) or a Pd NP catalyst on sulfur-modified glass (henceforth referred to as SGlPd).11 In the latter case, it is unique that the addition of a solid metal promotes the reaction due to increased microwave absorption of the reaction system (Scheme 1b).12 We wondered whether this methodology could be applicable to Buchwald–Hartwig amination.
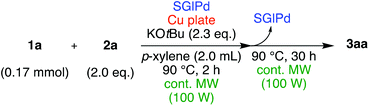
Herein, we report the first example of continuous microwave irradiation-assisted ligand-free Buchwald–Hartwig amination using a Pd NP catalyst and a co-existing solid metal (copper plate, Scheme 1c), and we also found that this combination can work together to control the product selectivity to give only a Buchwald–Hartwig amination product.
Results and discussion
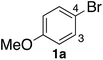
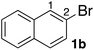
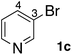
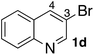
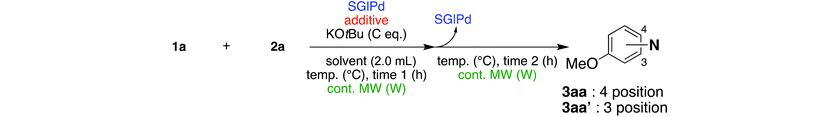
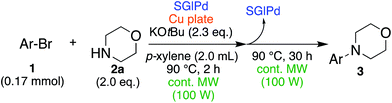
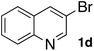
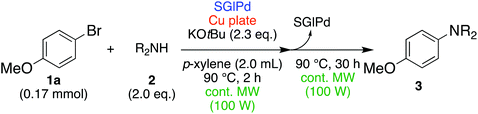
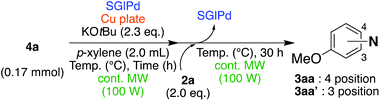
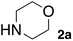
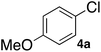
In the beginning, we attempted the ligand-free Buchwald–Hartwig amination between aryl bromides and morpholine (2a) using SGlPd,13 without microwave irradiation under the conditions quoted (Table 1). Regioisomers were obtained from 1a, 1b, 1c, and 1d respectively because an aryne was generated by strongly basic conditions and high temperatures14 (entries 1–4).Therefore, we investigated the optimization of reaction conditions to prevent the generation of regioisomers (Table 2). When the reaction was carried out at lower temperatures such as 80 or 90 °C, the corresponding coupling product was barely formed (entries 1 and 2). Moreover, the regioisomer was formed by heating at 100 °C (entry 3); hence the reaction using continuous microwave irradiation was carried out at temperatures lower than 90 °C. The substrates were heated to 80 or 90 °C under microwave irradiation for 24 h (entries 4 and 5). Even though the yield was improved, it hit the ceiling of 36%. We considered that this might be because a large amount of Pd was leached and aggregated, so it lost its catalytic activity. Then, we conducted the reaction in two steps and removed the SGlPd between the first step and second step, as shown in the equation in Table 3. The reaction mixture was heated for a short time to elute the Pd NPs into the reaction system (1st step) and then heated for a long time to effect the reaction. The yield was slightly increased with longer reaction time, and higher microwave power and temperature (entries 1–4). When the reaction was carried out with an aluminum foil which is effective for Suzuki–Miyaura coupling of aryl chlorides based on our previous work,11b the corresponding coupling product was obtained in just only 11% yield (entry 5). It is because aluminum foil decayed under the strongly basic conditions, and the microwave absorption in the reaction system was not increased. Therefore, we used copper plates instead of aluminum foil as the co-existing metal, and we found that the yield of the product improved to 64% (entry 6). Encouraged by these results, we continued our experiments to optimize the equivalence of reagents and the reaction time of the first and second steps (entries 7 and 8); and finally, we found that the coupling product was obtained in 90% yield under the conditions indicated in entry 9.15 When the reaction was performed under the conditions of entry 9 without microwave irradiation, the product was not obtained (entry 10). In addition, the reaction did not proceed with only a Cu plate as the metal source (entry 11). According to this result, Pd is the active species for the reaction, but Cu is not (example of Cu-catalyzed C–N coupling, see ref. 4). Subsequently, we investigated the reusability of SGlPd for the Buchwald–Hartwig amination. When the SGlPd was used repeatedly 10 times in the reaction under the optimal conditions, the coupling product in each case was obtained in high yield (Table 4). In addition, the amount of Pd leaching into each reaction solution was measured by inductively coupled plasma mass spectrometry (ICP-MS; Table 4). As a result, it was found that up to 0.33 μg (1.84 mmol%) of Pd leached into the reaction solution. Under the optimized reaction conditions (entry 9, Table 4), a tiny quantity of Pd NPs on SGlPd is leached into the reaction solution because it is firmly immobilized on glass. Therefore, we considered that SGlPd could be used repeatedly for the reaction and low leaching of Pd NPs succeeded in giving the product in good yield in each reaction.
| Entry | 1a (mmol) | 2a (eq.) | Additive | KOtBu (eq.) | Temp. (°C) | Cont. MW (W) | 1st step time 1 (h) | 2nd step time 2 (h) | Yield (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 3aa | 3aa′ | |||||||||
| a SGlPd was not used for the reaction, ND = not detected. N = 4-morpholinyl. | ||||||||||
| 1 | 0.27 | 1.2 | — | 1.4 | 80 | 50 | 1.5 | 12 | 4 | ND |
| 2 | 0.27 | 1.2 | — | 1.4 | 90 | 50 | 2 | 12 | 5 | ND |
| 3 | 0.27 | 1.2 | — | 1.4 | 90 | 100 | 2 | 12 | 7 | ND |
| 4 | 0.27 | 1.2 | — | 1.4 | 90 | 100 | 2 | 24 | 9 | ND |
| 5 | 0.27 | 1.2 | Al foil | 1.4 | 90 | 100 | 2 | 24 | 11 | Trace |
| 6 | 0.27 | 1.2 | Cu plate | 1.4 | 90 | 100 | 2 | 24 | 64 | Trace |
| 7 | 0.17 | 2.0 | Cu plate | 2.3 | 90 | 100 | 2 | 24 | 84 | Trace |
| 8 | 0.17 | 2.0 | Cu plate | 2.3 | 90 | 100 | 1 | 24 | 45 | Trace |
| 9 | 0.17 | 2.0 | Cu plate | 2.3 | 90 | 100 | 2 | 30 | 90 | Trace |
| 10 | 0.17 | 2.0 | Cu plate | 2.3 | 90 | — | 2 | 30 | 8 | ND |
| 11a | 0.17 | 2.0 | Cu plate | 2.3 | 90 | 100 | 2 | 30 | Trace | ND |
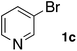
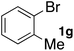
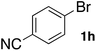
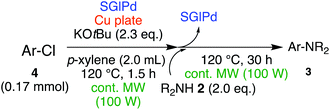
Next, SGlPd was used repeatedly for the Buchwald–Hartwig amination between several aryl bromides 1 and morpholine (2a) to give eight different types of products (sequential re-use of SGlPd; Table 5).16 The reaction proceeded, with bromobenzene (1e) and aryl bromides 1f, and 1h bearing electron-donating and electron-withdrawing groups (runs 1, 2 and 7). Also, ortho-substituted aryl bromide 1g was converted to the corresponding compound 3ga in 90% yield (run 3). Also, the desired coupled products 3ba, 3ca, and 3da were obtained in good yields from 2-bromonaphthalene (1b), 3-bromopyridine (1c) and 3-bromoquinoline (1d), respectively (runs 5, 6 and 8).
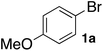
This was also the case when the amines were changed. SGlPd was also repeatedly used for the Buchwald–Hartwig amination of 4-bromoanisole (1a) with several amines 2 (Table 6). Every corresponding coupled product was obtained in around 90% yield.
Ligand-free Buchwald–Hartwig amination between aryl chlorides and amines using SGlPd
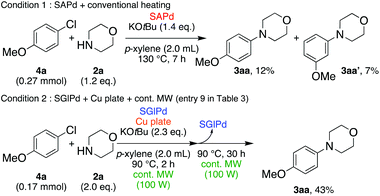
Next, we investigated the Buchwald–Hartwig amination of aryl chloride as a more inert compound than aryl bromides. At first, the SGlPd was utilized for the reaction under the conditions as described in Table 1. In addition to the desired product, regioisomers were also obtained in low yield (Scheme 2, Condition 1). Next, the optimal reaction conditions for aryl bromides (entry 9, Table 3) were utilized for the Buchwald–Hartwig amination of 4-chloroanisole (4a) and morpholine (2a; Scheme 2). However, the desired coupled product 3aa was obtained in only 43% yield and the 4-chloroanisole remained (Scheme 2, Condition 2).Therefore, we continued our experiments to determine appropriate reaction conditions for the Buchwald–Hartwig amination of aryl chlorides and amines. We changed each condition in both step 1 and step 2; finally, we found the experimental conditions as outlined in the equation in Table 7, in which amine 2a was added in the second step. When the reaction temperature was higher, the yield of 3aa was higher (100 °C: 59% 120 °C: 75%, 130 °C: 78%, entries 1–3). However, when the reaction was carried out at 130 °C, the regioisomer 3aa′via the aryne intermediate was obtained in 5% yield (entry 3). Next, we investigated the reaction time for the first step (entries 4–8). When the reaction was performed for 3 h, the yield of the desired product was decreased to 61% (entry 4). It was considered that a large number of Pd NPs leached into the reaction solution were agglomerated and deactivated. Moreover, a shorter reaction time, such as 60–80 min, also decreased the yield of the product between 73 and 78% respectively, because the amount of Pd NPs leached into the reaction system was not enough for this reaction to proceed (entries 6–8). When the reaction was performed for 1.5 hours in the 1st step, the product was obtained in 81% yield (entry 5).
From the above results, we investigated the substrate scope of aryl chlorides and amines under the optimum conditions (entry 8, Table 7). When chlorobenzene (4b) or 4-chloroanisole (4a) was subjected to the reaction with amines such as morpholine (2a), N,N-dibutylamine (2i), benzylamine (2j) or N,N-dicyclohexylamine (2f), the corresponding coupled products were obtained in good yields, respectively (entries 1–7, Table 8).
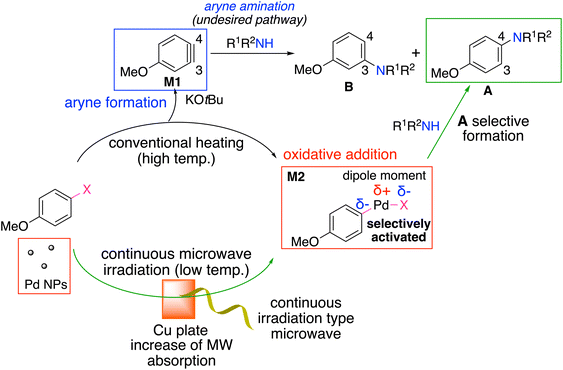
Based on the generally known effect of microwave irradiation on compounds with a dipole moment and the experimental results of the product selective formation of the desired coupled product under continuous microwave irradiation, we proposed the plausible reaction mechanism (Fig. 1). In conventional heating at high temperature, not only the typical Buchwald–Hartwig amination but also the carbon–nitrogen bond forming reaction via an aryne intermediate (M1) proceeded.
That is to say, the aryne was formed under the strongly basic conditions at a high temperature, and the amines then attacked the aryne intermediate. In contrast, under continuous microwave irradiation conditions at a lower temperature, Buchwald–Hartwig amination might have been selectively activated due to the specific activation of Pd NPs and/or the polar chemical species, Ar–Pd–Br (M2).
Conclusion
In summary, we have developed a product selective Buchwald–Hartwig amination controlled by the combination of palladium nanoparticles, continuous microwave irradiation, and a co-existing metal solid. In conventional heating, the aryne formation occurred and generated the undesired regioisomer due to the high temperature and strongly basic conditions. On the other hand, the Buchwald–Hartwig amination could proceed selectively to obtain the corresponding coupled product by this methodology because the microwave-assisted heating system suppressed the reaction temperature and might have selectively activated the species with a dipole moment. The Buchwald–Hartwig amination of aryl chlorides, which are inert compounds, was also promoted by the microwave-assisted system. Moreover, we found that the SGlPd can be used repeatedly over 10 times for the reaction, and the amount of Pd leached into the reaction mixture was detected at a quite low level (ppb level) by ICP-MS.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The XAFS measurements were performed at beamlines BL14B2 of SPring-8, with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2019B1907). This work was partially supported by a Grant-in-Aid from the JSPS KAKENHI for Precisely Designed Catalysts with Customized Scaffolding (Grant No. JP 15KT0063), by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from the AMED under Grant Number JP21am0101084, by Yazaki Memorial Foundation for Science and Technology and Sasagawa Science Research Foundation of The Japan Science Society.Notes and references
- R. Dorel, C. P. Grugel and A. M. Haydl, Angew. Chem., Int. Ed., 2019, 58, 17118–17129 ( Angew. Chem. , 2019 , 131 , 17276–17287 ) CrossRef CAS PubMed.
- M. Kosugi, M. Kameyama and T. Migita, Chem. Lett., 1983, 12, 927–928 CrossRef.
- (a) A. S. Guram, R. A. Rennels and S. L. Buchwald, Angew. Chem., Int. Ed. Engl., 1995, 34, 1348–1350 ( Angew. Chem. , 1995 , 107 , 1456–1459 ) CrossRef CAS; (b) J. Louise and J. F. Hartwig, Tetrahedron Lett., 1995, 36, 3609–3612 CrossRef.
- Cu-Catalysed C–N couplings were also reported. For examples; (a) F. Ullmann and J. Bielecki, Chem. Ber., 1901, 34, 2174–2185 CrossRef CAS; (b) D. M. T. Chan, K. L. Monaco, R.-P. Wang and M. P. Winters, Tetrahedron Lett., 1998, 39, 2933–2936 CrossRef CAS; (c) D. A. Evans, J. L. Katz and T. R. West, Tetrahedron Lett., 1998, 39, 2937–2940 CrossRef CAS; (d) W. Zhou, M. Fan, J. Yin, Y. Jiang and D. Ma, J. Am. Chem. Soc., 2015, 137, 11942–11945 CrossRef CAS PubMed; (e) M. M. Islam, M. Halder, A. S. Roy, S. Chatterjee, A. Bhaumik and S. M. Islam, RSC Adv., 2016, 6, 109692–109701 RSC.
- (a) T. Akiyama, Y. Wada, M. Yamada, Y. Shio, T. Honma, S. Shimoda, K. Tsuruta, Y. Tamenori, H. Haneoka, T. Suzuki, K. Harada, H. Tsurugi, K. Mashima, J. Hasegawa, Y. Sato and M. Arisawa, Org. Lett., 2020, 22, 7244–7249 CrossRef CAS PubMed; (b) H. Alinezhad, M. Cheraghian and S. Ghasemi, J. Organomet. Chem., 2020, 907, 121069–121077 CrossRef CAS; (c) H. Alamgholiloo, S. Rostamnia and N. N. Pesyan, Appl. Organomet. Chem., 2020, 34, 5452–5462 CrossRef; (d) I. Yavari, A. Mobaraki, Z. Hosseinzadeh and N. Sakhaee, J. Organomet. Chem., 2019, 897, 236–246 CrossRef CAS.
- (a) N. Hoshiya, M. Shimoda, H. Yoshikawa, Y. Yamashi, S. Shuto and M. Arisawa, J. Am. Chem. Soc., 2010, 132, 7270–7272 CrossRef CAS PubMed; (b) M. Xiao, N. Hoshiya, K. Fujiki, T. Honma, Y. Tamenori, S. Shuto, H. Fujioka and M. Arisawa, Chem. Pharm. Bull., 2016, 64, 1154–1160 CrossRef CAS PubMed; (c) M. Al-Amin, T. Honma, N. Hoshiya, S. Shuto and M. Arisawa, Adv. Synth. Catal., 2012, 354, 1061–1068 CrossRef CAS; (d) K. Takagi, M. Al-Amin, N. Hoshiya, J. Wouters, H. Sugimoto, Y. Shiro, H. Fukuda, S. Shuto and M. Arisawa, J. Org. Chem., 2014, 79, 6366–6371 CrossRef CAS PubMed; (e) M. Al-Amin, M. Arisawa, S. Shuto, Y. Ano, M. Tobisu and N. Chatani, Adv. Synth. Catal., 2014, 356, 1631–1637 CrossRef CAS; (f) M. Al-Amin, S. Arai, N. Hoshiya, T. Honma, Y. Tamenori, T. Sato, M. Yokoyama, A. Ishii, M. Takeuchi, T. Maruko, S. Shuto and M. Arisawa, J. Org. Chem., 2013, 78, 7575–7581 CrossRef CAS PubMed; (g) M. Al-Amin, M. Akimoto, T. Tameno, Y. Ohki, N. Takahashi, N. Hoshiya, S. Shuto and M. Arisawa, Green Chem., 2013, 15, 1142–1145 RSC; (h) M. Arisawa, T. Sato, N. Hoshiya, M. Al-Amin, Y. Kogami and S. Shuto, ACS Comb. Sci., 2014, 16, 215–220 CrossRef CAS PubMed; (i) K. Takagi, H. Fukuda, S. Shuto, A. Otaka and M. Arisawa, Adv. Synth. Catal., 2015, 357, 2119–2124 CrossRef CAS; (j) K. Urakawa, M. Sumimoto, M. Arisawa, M. Matsuda and H. Ishikawa, Angew. Chem., Int. Ed., 2016, 55, 7432–7436 ( Angew. Chem. , 2016 , 128 , 7558–7562 ) CrossRef CAS PubMed.
- (a) B. F. dos Santos, B. A. L. da Silva, A. R. de Oliveira, M. H. Sarragiotto and N. L. C. Dominiques, Synthesis, 2021, 53, 933–942 CrossRef CAS; (b) I.-R. Oliva, N.-L. Garcia, A. S. Santos, M. M. B. Marques and J. M. Palomo, Asian J. Org. Chem., 2021, 10, 872–878 CrossRef; (c) K. Hong, M. Sajjadi, J. M. Suh, K. Zhang, M. Nasrollahzadeh, H. W. Jang, R. S. Varma and M. Shokouhimehr, ACS Appl. Nano Mater., 2020, 3, 2070–2103 CrossRef CAS; (d) T. Tamoradi, M. Daraie and M. M. Heravi, Appl. Organomet. Chem., 2020, 34, 5538–5547 Search PubMed; (e) B. Lakshminarayana, T. Vinodkumar, G. Satyanarayara and Ch. Sabrahanyam, RSC Adv., 2020, 10, 4568–4578 RSC.
- (a) D. Petkova, N. Borlinghaus, S. Sharma, J. Kaschel, T. Lindner, J. Klee, A. Jolit, V. Haller, S. Heitz, K. Britze, J. Dietrich, W. M. Braje and S. Handa, ACS Sustainable Chem. Eng., 2020, 8, 12612–12617 CrossRef CAS; (b) P. Sun, J. Yang, C. Chen, K. Xie and J. Peng, Catal. Lett., 2020, 150, 2900–2910 CrossRef CAS; (c) T. N. Ansari, A. Taussat, A. H. Clark, M. Nachtegaal, S. Plummer, F. Gallou and S. Handa, ACS Catal., 2019, 9, 10389–10397 CrossRef CAS; (d) I. C. Watson, A. Schumann, H. Yu, E. C. Davy, R. McDonald, M. J. Ferguson, C.-H. Junghans and E. Rivard, Chem. – Eur. J., 2019, 25, 9678–9690 CrossRef CAS PubMed; (e) M. Ghotbinejad, A. R. Khosropour, I.-M. Baltork, M. Moghadam, V. Mirkhani and S. Tangestaninejad, Nanochem. Res., 2017, 2, 230–236 CAS; (f) J. Cho, J. Cho, H. Kim, M. Lim, H. Jo, H. Kim, S.-J. Min, H. Rhee and J. W. Kim, Green Chem., 2018, 20, 2840–2844 RSC; (g) M. Sarvestani and R. Azadi, Appl. Organomet. Chem., 2018, 32, 3906–3914 CrossRef; (h) S. Pradhan, A. Bhattacharyya and R. P. John, Tetrahedron Lett., 2016, 57, 1532–1536 CrossRef CAS; (i) R. S. Shelkar, S. S. Shendage and J. M. Nagarkar, Tetrahedron Lett., 2015, 56, 4463–4467 CrossRef CAS; (j) F. Heshmatpour and R. Abazari, RSC Adv., 2014, 4, 55815–55826 RSC; (k) G. Yun, Z. Hassan, J. Lee, J. Kim, N.-S. Lee, N. H. Kim, K. Baek, I. Hwang, C. G. Park and K. Kim, Angew. Chem., Int. Ed., 2014, 53, 6414–6418 ( Angew. Chem. , 2014 , 126 , 6532–6536 ) CrossRef CAS PubMed; (l) A. H. Ghasemi and H. Naeimi, New J. Chem., 2020, 44, 5056–5063 RSC.
- (a) R. S. J. Proctor, P. Chuentragool, A. C. Colgan and R. J. Phipps, J. Am. Chem. Soc., 2021, 143, 4928–4934 CrossRef CAS PubMed; (b) M. Gecht, G. Kantin, D. Dar'in and M. Krasavin, Tetrahedron Lett., 2019, 60, 151120–151123 CrossRef CAS.
- (a) M. K. Das, J. A. Bobb, A. A. Ibrahim, A. Lin, K. M. AbouZeid and M. S.-E. Shall, ACS Appl. Mater. Interfaces, 2020, 12, 23844–23852 CrossRef CAS PubMed; (b) F. Benyettou, L. Motte, H. Traboulsi, J. Mazher, R. Pasricha, J.-C. Olsen, A. Trabolsi and E. Guenin, Chem. – Eur. J., 2018, 24, 2349–2353 CrossRef CAS PubMed; (c) Q. Zhang, Z. Mao, K. Wang, N. T. S. Phan and F. Zhang, Green Chem., 2020, 22, 3239–3247 RSC; (d) M. Sako and M. Arisawa, Synthesis, 2021, 53, 3513–3521 CrossRef CAS.
- (a) T. Akiyama, T. Taniguchi, N. Saito, R. Doi, T. Honma, Y. Tamenori, Y. Ohki, N. Takahashi, H. Fujioka, S. Shuto, Y. Sato and M. Arisawa, Green Chem., 2017, 19, 3357–3369 RSC; (b) M. Yamada, Y. Shio, T. Akiyama, T. Honma, Y. Ohki, N. Takahashi, K. Murai and M. Arisawa, Green Chem., 2019, 21, 4541–4549 RSC.
- In our previous work, it was considered that a minute local hot spot is generated between the aluminum foil and Pd NPs. Therefore, the Suzuki–Miyaura coupling of aryl chlorides is promoted.
- We performed XPS, Powder XRD and HR TEM experiments for SGlPd, see ESI.† We also analysed the SGlPd by XAFS and it showed that Pd NPs on SGlPd is 0 valences. See ref. 6b.
- W. Kleista, S. S. Pröckla, M. Dreesa, K. Köhlera and L. Djakovitch, J. Mol. Catal. A: Chem., 2009, 303, 15–22 CrossRef.
- When the reaction was performed under optimized reaction conditions (entry 9) without continuous microwave irradiation, the desired coupled product was obtained in only 8% yield (entry 10), and we confirmed that the continuous microwave irradiating is necessary for the reaction.
- SGlPd was removed from the reaction solution with a pair of tweezers after completing of the first step of the reaction. Then, the surface was washed with ethanol, dried under reduced pressure, and used repeatedly.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc01782a |
| This journal is © The Royal Society of Chemistry 2021 |