Open Access Article
Open Access ArticleA catalytic approach via retro-aldol condensation of glucose to furanic compounds†
Rui
Zhang
 a,
Aleksi
Eronen
a,
Xiangze
Du
b,
Enlu
Ma
a,
Ming
Guo
a,
Aleksi
Eronen
a,
Xiangze
Du
b,
Enlu
Ma
a,
Ming
Guo
 a,
Karina
Moslova
a and
Timo
Repo
a,
Karina
Moslova
a and
Timo
Repo
 *a
*a
aDepartment of Chemistry, University of Helsinki, A. I. Virtasen aukio 1, P.O. Box 55, 00014, Finland. E-mail: timo.repo@helsinki.fi
bKey Laboratory of Green Chemistry and Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064, P. R. China
First published on 5th July 2021
Abstract
The synthesis of new types of furan-based compounds other than 5-hydroxymethylfurfural from glucose is a very attractive yet underexploited strategy. We report here a catalytic conversion of glucose with acetylacetone (acac) to furan-centered chemicals, 2-methyl-3-acetylfuran (MAF) and 1-(5-(1,2-dihydroxyethyl)-2-methylfuran-3-yl)ethan-1-one (DMAF), which are potential building blocks for the synthesis of fine chemicals. The experimentally supported reaction mechanism is cascade-type, including glycolaldehyde (GA) formation by H2MoO4-catalysed retro-aldol condensation (C2 + C4) of glucose and immediate capture of transient C2 and C4 intermediates by acac to yield MAF and DMAF. To the best of our knowledge, this is the first report on the straightforward synthesis of MAF and DMAF from glucose, providing a new but generic synthesis strategy for GA-based C2 and erythrose-based C4 chemistry in biorefining.
Introduction
The high dependence of modern society on fossil fuel-based resources, together with the associated adverse environmental impacts, motivates research to identify renewable raw materials and develop new production methods for fuels and chemicals.1 Glucose has attracted global attention as a representative monosaccharide of non-edible cellulose. Numerous studies have been devoted to converting glucose into valuable platform chemicals, such as levulinic acid, 5-hydroxymethylfurfural (5-HMF), lactic acid, sugar alcohols and ethylene glycol (Fig. 1), through isomerization, dehydration, hydrogenation and retro-aldol condensation (RAC).2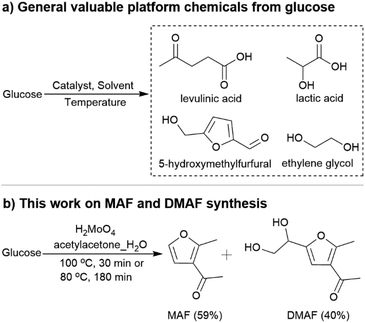 | ||
| Fig. 1 (a) General platform chemicals derived from glucose and (b) summary of this work: furanic compounds via RAC from glucose. | ||
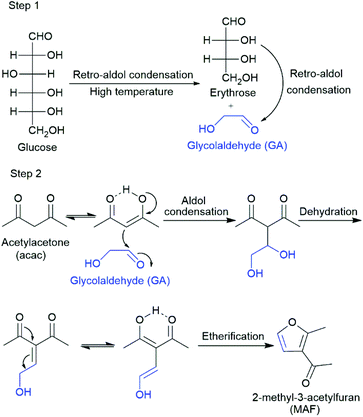
RAC of glucose through a C2 + C4 pathway generates glycolaldehyde (GA) and erythrose (Scheme 1, step 1). GA is a remarkable small molecule with both aldehyde and alcohol functionalities and has high potential to be a renewable alternative for petroleum-based ethylene oxide.3 GA is prone to many side reactions due to its highly reactive nature; thus, it is often sequentially stabilized after formation, such as by hydrogenation to ethylene glycol.4 Other synthesis methods have also been developed for the transformation of GA, mainly including oxidation, aldol reaction, amination, etc., for the production of glycolic acid, α-hydroxy acid esters and amines, as summarized recently by Faveere et al.3–5 Nevertheless, new transformations that create platform chemicals or building blocks for fine chemicals are greatly needed to boost contemporary biorefinery concepts toward a sustainable world.
In this study, acetylacetone (acac), a typical β-dicarbonyl compound, was employed to capture the in situ formed, reactive GA. The rapid interconversion between keto and enol tautomers of acac makes it a good nucleophilic reagent to attack aldehyde groups.6 As shown here, high-temperature treatment (220 °C) of aqueous glucose solution in the presence of acac gives a highly intriguing furan-derived product 2-methyl-3-acetylfuran (MAF) (Table 1, entry 1). Addition of H2MoO4 as a catalyst improves the efficiency of the reaction and enables glucose transformation to MAF under significantly milder conditions. In addition, the catalytic process opens simultaneously a unique possibility to synthesize 1-(5-(1,2-dihydroxyethyl)-2-methylfuran-3-yl)ethan-1-one (DMAF), which is derived from the reaction between erythrose (C4 fragment) and acac (Fig. 1). It is noteworthy that MAF and DMAF were previously only accessible by multi-step chemical synthesis7 or using isolated and expensive GA and erythrose as starting materials in a ZrCl4-catalysed reaction with acac.8 From the furan-derived products, MAF is considered a useful intermediate for the synthesis of photochromic molecules,9 pharmaceuticals,10 secoprostacyclins and food additives,11 while DMAF is seen as an underexploited chemical with potential for application in the pharmaceutical and fine chemical industries.8,12
| Entry | Solvent | Substrate | MAF yielda (mol %) |
|---|---|---|---|
| Reaction conditions: 300 mg of substrate, total solvent volume = 13 mL, H2O/solvent = 1/1, 220 °C, 30 min, 2.5 MPa N2, 600 rpm.a MAF yield = mol of MAF per mol of glucose × 100%; the amount of MAF is determined by GC using acetophenone as an internal standard.b 80 °C. | |||
| 1 | H2O/acac | Glucose | 46 |
| 2 | H2O/acetone | Glucose | — |
| 3 | H2O/ethyl acetate | Glucose | — |
| 4 | acac | Glucose | 18 |
| 5 | EtOH/acac | Glucose | 16 |
| 6 | H2O/acac | GA | 83 |
| 7b | H2O/acac | GA | 82 |
| 8 | H2O/acac | Mannose | 46 |
| 9 | H2O/acac | Xylose | 12 |
| 10 | H2O/acac | Fructose | 9 |
| 11 | acac | GA | 66 |
Results and discussion
Investigation on MAF formation
To open the RAC pathway for glucose conversion in the presence of acac, we chose high-temperature reaction conditions (220 °C). It was confirmed to be an essential reaction parameter for the high yield of MAF (Table S1 and Fig. S1–4†). The indispensable role of acac as a nucleophile in this transformation was further confirmed by a series of experiments. H2O/acetone and H2O/ethyl acetate reaction media were unsuccessful in this transformation and gave only insoluble humins, while all reactions involving acac gave MAF. From the studied combinations, H2O with acac offered a satisfactory yield of 46% for this cascade-type reaction (Table 1, entries 1–5).We also studied the role of GA as the key intermediate. Indeed, the use of pure GA as a starting material instead of glucose increases the MAF yield significantly to 83% under the same reaction conditions (Table 1, entry 6). The central role of GA in the MAF formation reaction is also consistent with the results obtained from a series of carbohydrate substrates. Mannose, as a C-2 epimer of glucose, gave a comparable yield of MAF to glucose, while xylose and fructose gave much lower yields (Table 1, entries 8–10). Xylose as an aldopentose only gave around 1/3 of MAF compared with the amount derived from glucose, in accordance with the C2 + C3 RAC of xylose. Fructose is prone to undergo C3 + C3 RAC (which produces glyceraldehyde and dihydroxyacetone) rather than the desired C2 + C4 pathway and thus gave only a small amount of MAF.13 We confirmed this observation by a direct reaction between acac and glyceraldehyde at 220 °C, yielding only 4% of MAF and other unidentifiable products. Further studies revealed that acetic acid as a general carbohydrate decomposition product along with typical dehydration products with furan structures (such as furfural, 2-methylfuran and 5-HMF) is not involved in the MAF formation reaction (Table S2†). These results confirm the pivotal role of GA in the condensation reaction with acac and further the formation of the furan structure of MAF under the applied conditions.
The reaction pathway involves the cleavage of glucose through C2 + C4 RAC, followed by the aldol condensation of GA with acac (Scheme 1, steps 1 and 2). In this reaction, the presence of water is necessary for high yields in both steps (Table 1, entries 1 vs. 4, 6 vs. 11). It is known that water, besides being an efficient proton carrier, undergoes autoprotolysis. Therefore, the concentration of hydronium (H3O+) and hydroxide (OH−) ions increases with increasing temperature and, e.g., at 200 °C, the pKw is 11.31.14 Water is a prominent proton or hydroxide ion source at high temperatures. However, in these MAF formation reactions, the measured pH of the aqueous phase ranges from 2.7 to 3.0 pH units depending on the applied reaction conditions (Table S3†). This phenomenon is likely due to the dissociation of acac in water,15 as the pH value for the H2O/acac reaction medium at room temperature is measured as 3.1. In addition to the above, water is beneficial as it dissolves glucose well and generates a homogeneous reaction medium for the reaction.
As we searched for ways to improve the efficiency of the reaction, it soon became clear that the RAC, which forms GA, is a high-temperature step in the concerted reaction. The high yield reaction between pure GA and acac can occur even at 80 °C (Table 1, entry 7). An additional 1H NMR study revealed that GA reacts fast with acac and the GA signal disappears in 1 min at 100 °C (Fig. S5 and 6†). Previous publications support the reasoning; high GA yields are normally obtained under supercritical water (>373 °C, >22 MPa) and in a flow reactor where the formed GA can be rapidly separated.13e,16 In general, the elevated reaction temperature is related to the high activation energy of the RAC of glucose.17 Following this idea and increasing the reaction temperature, we achieved a yield of 49% for MAF at 240 °C, but at 260 °C, the yield decreased slightly (Table S1†). Therefore, increasing the temperature quickly hit the limit, and we had to look for alternative solutions.
H2MoO4 catalysed reaction
The ideal catalyst to enhance the RAC towards GA should be able to reduce the required activation energy while minimizing the isomerization of glucose to fructose. In this respect, Mo(VI) compounds (i.e., molybdic acid, molybdenum oxide, and polyoxometalates) are attractive, as they are known to catalyse the epimerization of glucose to mannose through a 1,2-intramolecular carbon shift (1,2-CS), known as the Bilik reaction.18 Mo-containing catalysts also show reactivity in the production of formic acid and glycolic acid from cellulose via the RAC pathway combined with oxidation.19 Therefore, we introduced commercially available H2MoO4 to our studies. When we performed the H2MoO4-catalysed reaction at 220 °C, a significant increase in MAF yield to 66% was observed (Table 2, entry 9). To our surprise, reducing the reaction temperature as low as 100 °C gave a MAF yield of 59% (Table 2, entry 7), while in contrast no transformation occurs without an H2MoO4 catalyst. This is a marked enhancement when compared to an uncatalyzed reaction with a record yield of 46% at 220 °C (Table 1, entry 1).| Entry | T (°C) | Time (min) | Conversiona (mol %) | Mannose yielda (mol %) | MAF yield (mol %)/C(%)c | DMAF yieldb (mol %)/C(%)c |
|---|---|---|---|---|---|---|
| Reaction conditions: 300 mg (46 g L−1) of glucose, 100 mg of H2MoO4, H2O/acac = 1/1 (6.5 mL/6.5 mL), 2.5 MPa N2, 600 rpm.a Measured by HPLC using authentic glucose and mannose as standards.b DMAF yield = mol of DMAF per mol of glucose × 100%; the amount of DMAF is determined by 1H NMR in MeOD using 2-methylfuran as an internal standard.c Carbon yield is calculated based on carbon atoms in glucose. Carbon yield of MAF = 2 × mol of MAF/6 × mol of glucose × 100%; carbon yield of DMAF = 4 × mol of DMAF/6 × mol of glucose × 100%.d In the absence of acac.e GA as a starting material.f 975 mg (150 g L−1) of glucose and 321 mg of H2MoO4. | ||||||
| 1 | 60 | 30 | 54 | 8 | 16/5 | 19/13 |
| 2 | 80 | 30 | 84 | 6 | 45/15 | 36/23 |
| 3 | 80 | 120 | 93 | 4 | 56/19 | 36/24 |
| 4 | 80 | 180 | 98 | 2 | 59/20 | 41/28 |
| 5 | 80 | 240 | 99 | 1 | 59/20 | 42/28 |
| 6 | 100 | 15 | 98 | 2 | 55/18 | 40/27 |
| 7 | 100 | 30 | 100 | 1 | 59/20 | 39/26 |
| 8 | 120 | 30 | 100 | — | 59/20 | 37/25 |
| 9 | 220 | 30 | 100 | — | 66/22 | — |
| 10d | 100 | 30 | 64 | 44 | — | — |
| 11e | 80 | 30 | — | — | 87 | — |
| 12f | 100 | 120 | 100 | — | 52/17 | 37/25 |
We studied the reaction parameters to optimize the yield and gain further insight into the H2MoO4-catalysed transformations at low temperatures. At a fixed reaction time (30 min), the yield of MAF improved markedly from 16% to 59% as the temperature increased from 60 °C to 100 °C (Table 2, runs 1, 2 and 7). Similarly, there was a positive correlation with the MAF yield when the catalyst loading amount ranged from 10 to 33 wt%; above this, the generation of MAF remained consistent at 59% even when a nearly stoichiometric amount of H2MoO4 was used (Table S5†). The extension of reaction time from 30 to 180 min at 80 °C resulted in the same MAF yield as that at 100 °C for 30 min (Table 2, entries 2–4 vs. 7).
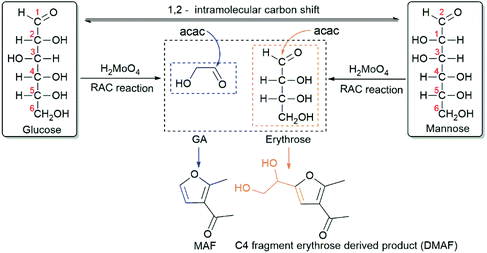
In the catalysed, low-temperature reactions, our attention was drawn to the formation of a new product. Detailed 1H, 13C, and 2D NMR and HRESI-MS (high-resolution electrospray-ionization mass spectra) analyses confirmed that the isolated product is 1-(5-(1,2-dihydroxyethyl)-2-methylfuran-3-yl)ethan-1-one (DMAF), an aldol condensation product of acac and the C4 fragment, erythrose (Table 2; Figs. S7–10†). DMAF can be obtained with a yield of up to 42%, thus offering an efficient approach for underexplored erythrose-based C4 chemistry in biorefining.
Under the applied reaction conditions, H2MoO4 can epimerize glucose to mannose. Our control experiment in the absence of acac showed that 64% of glucose was converted and 44% of mannose was formed (Table 2, entry 10). This result is in line with previous glucose epimerization studies18b,d and with the low activation barrier reported for Mo-catalysed 1,2-CS (21.1 kcal mol−1).20 Mechanistically, for successful glucose/mannose epimerization, a carbon skeleton rearrangement should occur through the cleavage of the C2–C3 bond with the subsequent formation of a new C1–C3 bond.18b,d,21 However, if the main catalytic process is only the formation of mannose, the uncatalysed retro-aldol reaction would still remain the rate-limiting, high energy step in the MAF formation, requiring high reaction temperature. H2MoO4 is able to catalyse the reaction at 80 °C with high yield. Therefore, the substantial reduction of reaction temperature indicates a catalysed reaction pathway for RAC, employing H2MoO4-catalysed breaking of the C2–C3 bond. As acac is prone to react with GA under the applied conditions (Figs. S5 and 11†), a mechanistic scheme is proposed (Table 2). The H2MoO4 catalyst lowers the high activation energy of RAC and enables the efficient formation of GA at mild temperature for MAF synthesis. Accordingly, the same mechanistic process applies for DMAF, where the released C4 fragment (erythrose) directly reacts with acac. DMAF seems to be unstable at high temperature (Table 2, entry 9). Thus, the integration of RAC by H2MoO4 and high reactivity between acac and GA or erythrose are needed for the high yields of MAF and DMAF at mild reaction temperature. Simultaneously with the RAC reaction, H2MoO4 also catalyses the epimerization of glucose to mannose by 1,2-CS. Both glucose and mannose can undergo C2 + C4 RAC. There is an open discussion in the literature as to whether there is a mechanistic relationship between RAC and 1,2-CS.4a,13b,13c,22 However, comprehensive theoretical publications or direct experimental evidence are lacking.
It is noteworthy that the yield of MAF remained satisfactory (52%) when the glucose concentration was up to 150 g L−1, which is of great significance for reaction upscaling (Table 2, entry 12; Table S6†). In addition, the system is very promising as it enables the straightforward conversion of microcrystalline cellulose and wood (pine), as an example of lignocellulosic biomass, to MAF (45% and 59%, respectively; the MAF yield is calculated based on the mol of product per the mol of sugar units in cellulose and wood) with the aid of NaCl (Tables S7 and 8,† see also the explanation and detailed calculation methods in the ESI). Based on the reaction scheme, two carbon atoms of MAF are from GA, whereas in erythrose-derived DMAF, four carbon atoms originate from glucose. When looking at the amount of renewable carbon in the products and the carbon yield of the syntheses, attention is drawn not only to glucose but also to acac; the 5/7 and 5/9 carbon atoms of MAF and DMAF are derived from acac. From this point of view, acac is an almost perfect reagent. It is here a component of very high atom economy and can be prepared directly from glucose via the biosynthetic pathway23 or via the bio-based triacetic acid lactone pathway in almost quantitative yield (Fig. S12†).24 Carbon yield towards glucose, as a sum of MAF and DMAF formation in the catalysed reaction under optimized conditions, is 48% (Table 2, entry 4). Although this is a rather good number for a RAC-derived cascade-type reaction, there is scope for the development of a further catalysis or synthesis strategy to improve the carbon efficiency.
Conclusions
In summary, we have developed a new strategy to convert glucose directly to MAF and DMAF via RAC with subsequent aldol condensation. The reaction benefits from several elementary steps. The non-catalytic approach requires high temperature for C2 + C4 RAC, and it was shown that GA is a key intermediate in the MAF formation reaction. Acac, which is a good nucleophile and weak acid in water, is essential to capture GA in situ through aldol condensation. The catalytic approach for MAF was performed with the use of H2MoO4. Its capability for the 1,2-CS transformation of glucose is known, but here, it demonstrates a pivotal role in C2 + C4-type RAC and enables GA and erythrose formation under significantly mild reaction conditions (80 °C). As a result, a novel route to MAF and DMAF synthesis is established. Both of them can be seen as chemicals with potential for application in the pharmaceutical and fine chemical industries. Notably, natural carbohydrates including cellulose and raw wood materials can be converted to MAF using the presented approaches. Further studies are focused on catalyst design to improve the carbon efficiency and the synthesis of other value-added chemicals with this strategy.Author contributions
Rui Zhang: investigation, methodology, data curation, formal analysis, software, writing – original draft, and writing – review and editing; Aleksi Eronen: data curation, formal analysis, and software; Xiangze Du: methodology and data curation; Enlu Ma: methodology and formal analysis; Ming Guo: methodology; Karina Moslova: resources; Prof. Timo Repo*: conceptualization, funding acquisition, supervision, resources, project administration, and writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the University of Helsinki. R. Z. is grateful for financial support from CSC (China Scholarship Council); A. E. received support from Magnus Ehrnrooth foundation; and E. M. and M. G. received support from CSC.References
- (a) Y. Liao, S.-F. Koelewijn, G. Van den Bossche, J. Van Aelst, S. Van den Bosch, T. Renders, K. Navare, T. Nicolaï, K. Van Aelst, M. Maesen, H. Matsushima, J. M. Thevelein, K. Van Acker, B. Lagrain, D. Verboekend and B. F. Sels, Science, 2020, 367, 1385–1390 CrossRef CAS PubMed; (b) Y. Liu, Y. Nie, X. Lu, X. Zhang, H. He, F. Pan, L. Zhou, X. Liu, X. Ji and S. Zhang, Green Chem., 2019, 21, 3499–3535 RSC; (c) P. Sudarsanam, E. Peeters, E. V. Makshina, V. I. Parvulescu and B. F. Sels, Chem. Soc. Rev., 2019, 48, 2366–2421 RSC; (d) Z. Sun, G. Bottari, A. Afanasenko, M. C. A. Stuart, P. J. Deuss, B. Fridrich and K. Barta, Nat. Catal., 2018, 1, 82–92 CrossRef CAS; (e) P. Ferrini and R. Rinaldi, Angew. Chem., Int. Ed., 2014, 53, 8634–8639 CrossRef CAS PubMed; (f) R. Gérardy, D. P. Debecker, J. Estager, P. Luis and J.-C. M. Monbaliu, Chem. Rev., 2020, 120, 7219–7347 CrossRef PubMed; (g) X. Luo, Y. Li, N. K. Gupta, B. Sels, J. Ralph and L. Shuai, Angew. Chem., Int. Ed., 2020, 59, 11704–11716 CrossRef CAS PubMed; (h) Y. Liu, C. Luo and H. Liu, Angew. Chem., Int. Ed., 2012, 51, 3249–3253 CrossRef CAS PubMed; (i) P. N. R. Vennestrøm, C. M. Osmundsen, C. H. Christensen and E. Taarning, Angew. Chem., Int. Ed., 2011, 50, 10502–10509 CrossRef PubMed.
- (a) J. Song, H. Fan, J. Ma and B. Han, Green Chem., 2013, 15, 2619–2635 RSC; (b) S. Kang, J. Fu and G. Zhang, Renew. Sustain. Energy Rev., 2018, 94, 340–362 CrossRef CAS; (c) Y. Yang, C.-w. Hu and M. M. Abu-Omar, Green Chem., 2012, 14, 509–513 RSC; (d) X. Zhang, P. Murria, Y. Jiang, W. Xiao, H. I. Kenttämaa, M. M. Abu-Omar and N. S. Mosier, Green Chem., 2016, 18, 5219–5229 RSC; (e) M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS PubMed; (f) L. T. Mika, E. Cséfalvay and Á. Németh, Chem. Rev., 2018, 118, 505–613 CrossRef CAS PubMed.
- W. Faveere, S. Van Praet, B. Vermeeren, K. N. R. Dumoleijn, K. Moonen, E. Taarning and B. F. Sels, Angew. Chem., Int. Ed., 2021, 60, 12204–12223 CrossRef CAS PubMed.
- (a) M. Zheng, J. Pang, R. Sun, A. Wang and T. Zhang, ACS Catal., 2017, 7, 1939–1954 CrossRef CAS; (b) J. Pang, M. Zheng, R. Sun, A. Wang, X. Wang and T. Zhang, Green Chem., 2016, 18, 342–359 RSC.
- (a) W. Faveere, T. Mihaylov, M. Pelckmans, K. Moonen, F. Gillis-D'Hamers, R. Bosschaerts, K. Pierloot and B. F. Sels, ACS Catal., 2020, 10, 391–404 CrossRef CAS; (b) G. Liang, A. Wang, L. Li, G. Xu, N. Yan and T. Zhang, Angew. Chem., Int. Ed., 2017, 56, 3050–3054 CrossRef CAS PubMed.
- (a) W. Caminati and J.-U. Grabow, J. Am. Chem. Soc., 2006, 128, 854–857 CrossRef CAS PubMed; (b) Y. Jing, Y. Zhang, Q. Lv, Y. Guo, X. Liu and Y. Wang, Green Chem., 2019, 21, 6236–6240 RSC.
- (a) E. Baciocchi and R. Ruzziconi, Synth. Commun., 1988, 18, 1841–1846 CrossRef CAS; (b) Y. Ji, J. Pan, P. Dauenhauer and R. J. Gorte, Appl. Catal., A, 2019, 577, 107–112 CrossRef CAS.
- N. Ronaghi, D. M. Fialho, C. W. Jones and S. France, J. Org. Chem., 2020, 85, 15337–15346 CrossRef CAS PubMed.
- (a) H. G. Heller and G. A. Jenkins, J. Chem. Soc., Perkin Trans. 1, 1984, 2877–2880 RSC; (b) A. P. Glaze, H. G. Heller and J. Whittall, J. Chem. Soc., Perkin Trans. 2, 1992, 591–594 RSC.
- (a) Y. O. Remizov, L. M. Pevzner and M. L. Petrov, Russ. J. Gen. Chem., 2018, 88, 1402–1410 CrossRef CAS; (b) L. M. Pevzner, Y. O. Remizov and M. L. Petrov, Russ. J. Gen. Chem., 2015, 85, 61–70 CrossRef CAS.
- J. Saunders, D. C. Tipney and P. Robins, Tetrahedron Lett., 1982, 23, 4147–4150 CrossRef CAS.
- The possible reaction routes for DMAF like polyhydroxyalkyl furans as platform molecules for other final products has been reported previously; see the reference: V. Escande, T. K. Olszewski, E. Petit and C. Grison, ChemSusChem, 2014, 7, 1915–1923 CrossRef CAS PubMed.
- (a) S. Yamaguchi and T. Baba, Molecules, 2016, 21, 937 CrossRef PubMed; (b) M. Orazov and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 11777 CrossRef CAS PubMed; (c) Y. Yan, L. Feng, G. Li, S. Lin, Z. Sun, Y. Zhang and Y. Tang, ACS Catal., 2017, 7, 4473–4478 CrossRef CAS; (d) A. Bayu, A. Abudula and G. Guan, Fuel Process. Technol., 2019, 196, 106162 CrossRef CAS; (e) C. B. Schandel, M. Høj, C. M. Osmundsen, A. D. Jensen and E. Taarning, ChemSusChem, 2020, 13, 688–692 CrossRef CAS PubMed.
- (a) C. Luo, S. Wang and H. Liu, Angew. Chem., Int. Ed., 2007, 46, 7636–7639 CrossRef CAS PubMed; (b) A. Kruse and E. Dinjus, J. Supercrit. Fluids, 2007, 39, 362–380 CrossRef CAS.
- J. Stary and J. O. Liljenzin, Pure Appl. Chem., 1982, 54, 2557–2592 CAS.
- M. Sasaki, K. Goto, K. Tajima, T. Adschiri and K. Arai, Green Chem., 2002, 4, 285–287 RSC.
- J. Zhang, B. Hou, A. Wang, Z. Li, H. Wang and T. Zhang, AIChE J., 2014, 60, 3804–3813 CrossRef CAS.
- (a) V. Bílik, L. Petruš and V. Farkaš, Chem. Zvesti, 1975, 5, 690–693 Search PubMed; (b) L. Petruš, M. Petrušová and Z. Hricovíniová, in Glycoscience: Epimerisation, Isomerisation and Rearrangement Reactions of Carbohydrates, ed. A. E. Stütz, Springer Berlin Heidelberg, Berlin, Heidelberg, 2001, pp. 15–41, DOI:10.1007/3-540-44422-x_2; (c) A. Takagaki, S. Furusato, R. Kikuchi and S. T. Oyama, ChemSusChem, 2015, 8, 3769–3772 CrossRef CAS PubMed; (d) F. Ju, D. VanderVelde and E. Nikolla, ACS Catal., 2014, 4, 1358–1364 CrossRef CAS; (e) I. Delidovich and R. Palkovits, ChemSusChem, 2016, 9, 547–561 CrossRef CAS PubMed; (f) M. Rellán-Piñeiro, M. Garcia-Ratés and N. López, Green Chem., 2017, 19, 5932–5939 RSC.
- (a) J. Albert, R. Wölfel, A. Bösmann and P. Wasserscheid, Energy Environ. Sci., 2012, 5, 7956–7962 RSC; (b) J. Zhang, X. Liu, M. Sun, X. Ma and Y. Han, ACS Catal., 2012, 2, 1698–1702 CrossRef CAS; (c) T. Lu, M. Niu, Y. Hou, W. Wu, S. Ren and F. Yang, Green Chem., 2016, 18, 4725–4732 RSC.
- B. K. Chethana, D. Lee and S. H. Mushrif, J. Mol. Catal. A: Chem., 2015, 410, 66–73 CrossRef CAS.
- M. L. Hayes, N. J. Pennings, A. S. Serianni and R. Barker, J. Am. Chem. Soc., 1982, 104, 6764–6769 CrossRef CAS.
- J. Iglesias, I. Martínez-Salazar, P. Maireles-Torres, D. Martin Alonso, R. Mariscal and M. López Granados, Chem. Soc. Rev., 2020, 49, 5704–5771 RSC.
- Y. Zhou, Y. Ding, W. Gao, J. Wang, X. Liu, M. Xian, X. Feng and G. Zhao, Biotechnol. Biofuels, 2020, 13, 88 CrossRef CAS PubMed.
- (a) B. H. Shanks and P. L. Keeling, Green Chem., 2017, 19, 3177–3185 RSC; (b) M. Chia, T. J. Schwartz, B. H. Shanks and J. A. Dumesic, Green Chem., 2012, 14, 1850–1853 RSC; (c) L. P. Saunders, M. J. Bowman, J. A. Mertens, N. A. Da Silva and R. E. Hector, J. Ind. Microbiol. Biotechnol., 2015, 42, 711–721 CrossRef CAS PubMed; (d) D. Xie, Z. Shao, J. Achkar, W. Zha, J. W. Frost and H. Zhao, Biotechnol. Bioeng., 2006, 93, 727–736 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc01429c |
| This journal is © The Royal Society of Chemistry 2021 |