Open Access Article
Open Access ArticlePolymeric waste valorization at a crossroads: ten ways to bridge the research on model and complex/real feedstock
Idoia
Hita
 a,
S. Mani
Sarathy
a,
S. Mani
Sarathy
 b and
Pedro
Castaño
b and
Pedro
Castaño
 *a
*a
aMultiscale Reaction Engineering, KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900, Saudi Arabia. E-mail: pedro.castano@kaust.edu.sa
bClean Combustion Research Center (CCRC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900, Saudi Arabia
First published on 20th May 2021
Abstract
The valorization of polymeric wastes, such as biomass, tires, and plastics, via thermal depolymerization (i.e., pyrolysis or liquefaction) and simultaneous or subsequent catalytic treatment has gained enormous momentum. The inherent hurdles when using complex polymeric wastes or their products as feedstock have led researchers to conclude that obtaining a fundamental kinetic understanding of the catalytic stage is unfeasible. To overcome the issues related to feedstock complexity, the majority of researchers have decided to use representative model compounds or probe molecules (i.e., surrogates). Two separate mainstreams have emerged in this field: one focusing on the fundamental kinetic understanding of model molecules and the other focused on studying real feedstock. We aimed to merge these approaches to utilize and acknowledge their potential and drawbacks. Therefore, herein, we provide ten recommendations for exploiting the existing synergies between the two approaches. This manuscript first contextualizes our proposed recommendations with a short overview on the thermocatalytic valorization field for polymeric waste, the complex compositions of reactants and products, the progress made in the individual fields of model and real feedstock, comparisons of both feedstock types, and some previous history on hydrocarbon conversion. Subsequently, we present guidelines for a truly cooperative and synergetic research effort.
1. Introduction
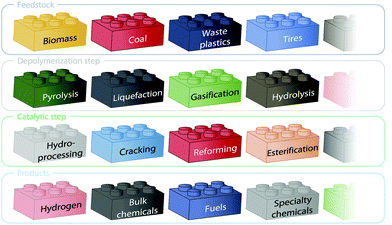
The valorization of polymeric wastes, such as biomass residues, tires, and polyolefins, is an overwhelming global challenge that targets circular economies and cradle-to-cradle objectives whilst simultaneously complying with the Green Chemistry principles;1,2 and it is aggravated by rapid population growth and the local scarcity of energy and chemical resources. Among the possibilities of recycling, chemical valorization (ternary recycling) through combined depolymerization and catalytic treatment (the so-called thermocatalytic valorization) has attracted particular research attention.3,4 The initial depolymerization stage can be accomplished using several methods, such as gasification, pyrolysis, liquefaction, hydrolysis, or torrefaction.5,6 The fractions produced from these processes generally lack the required stability, energy density, and quality for direct application or market placement. Thus, a sequential or simultaneous catalytic stage is typically required for upgrading these mixtures through various processes, such as reforming; hydroprocessing (i.e., hydrodesulfurization (HDS) and hydrodeoxygenation (HDO)); cracking (i.e., C–C bond cleavage, decarbonylation, and decarboxylation); Fischer–Tropsch synthesis; or esterification.7 The most valuable products obtained from these conversions are hydrogen, platform chemicals (i.e., methanol, light olefins, and aromatics), and fuels. For all the aforementioned processes, the resulting combinations of feeds, depolymerization strategies, catalytic processes, and products are overwhelming.8 This multiplicity can be envisioned as LEGO building blocks for feeds, depolymerization, catalytic stages, and products. The high number of process strategies can be easily inferred from the scheme shown in Fig. 1.Pyrolysis and hydrolysis are the most prolific waste depolymerization strategies based on publications (comprising ca. 78% of the publications in the field, according to Scopus™, 2020). Other well-established processes involve gasification, liquefaction, and torrefaction. Pyrolysis and hydrolysis depend on temperature and enzymes, respectively, when conducting depolymerization, and have been deemed successful in recent decades based on ample experiences and reviews associated with the valorization of plastics9,10 and biomass.11–13 This has led to a number of industrial initiatives, like for instance the biomass pyrolysis initiatives promoted within the framework of the International Energy Agency (IEA).14 A prominent example of such initiatives is the Empyro plant in Hengelo (the Netherlands), which became the first commercial-scale 24/7 biomass fast pyrolysis plant in the world, producing 20 million liters of bio-oil per year.15
Virtually, all scalable depolymerization pathways are intrinsically unselective owing to the highly heterogeneous feedstock and difficulty in controlling the polymerization at the chemical level. Thus, depolymerization products have heterogeneous and highly complex compositions.16 The logic is clear: elucidating the fundamental understanding of the catalytic steps by directly using depolymerization products is nearly impossible. Accordingly, simplifying the problem using a model compound (often diluted), which can be considered representative of depolymerized mixtures, is the best approach. Over time, this simplification resulted in the emergence of two research trends that have evolved in a relatively independent manner, achieving significant progress and pushing the frontiers of knowledge in their corresponding subfields. The model feedstock research community has progressed in terms of understanding the reaction mechanisms and developing new catalytic materials while attaining a fundamental understanding of the problem. Simultaneously, the real feedstock research community has progressed in the discovery of new process strategies and more stable catalyst materials while attaining a practical understanding of the problem and tackling the complex operational issues arising from handling such troublesome feedstock.
The thermal conversion of hydrocarbons via gasification, pyrolysis, and combustion has been extensively researched. With the advent of powerful computers, the chemical kinetics governing gas-phase oxidation and the pyrolysis of hydrocarbons have also been widely studied numerically.17 Consequently, extremely large chemical kinetic models comprising thousands of chemical species and tens of thousands of reactions have emerged.18 Traditionally, hydrocarbon combustion studies have explored representative fuel molecules (i.e., model compounds) both as pure components and in mixtures. Then, a synthetic mixture of these representative molecules was formulated to reproduce various characteristics of target real fuels.19,20 The key to developing appropriate surrogate mixtures is to have an in-depth understanding of the physical and chemical properties of the target real hydrocarbon mixtures. This has been enabled through improvements in the chemical analysis techniques available for relatively light hydrocarbon mixtures (e.g., gasoline and diesel) and for more complex heavy hydrocarbon mixtures (e.g., heavy fuel oil and vacuum residual oil).21,22
This work hypothesizes that a more fruitful and interactive collaboration between the two research subfields (real feedstocks and model molecules) is currently required to find unified and realistic solutions for polymeric waste valorization. Notably, these solutions need to comply with cradle-to-cradle objectives, circular economies, and Green Chemistry principles. However, they must also resolve a pressing societal and environmental problem. This work examines the compositions of depolymerization products, explores the benefits and limitations of each research trend, and highlights previous relevant works comparing the model and real feedstock. The past experiences in hydrocarbon conversion are used to build up a set of ideas and recommendations to attain a balance and take advantage of the knowledge already compiled through both research branches.
2. What is a “complex” composition?
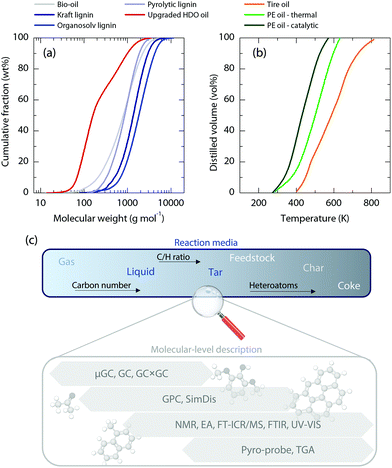
Resolving the composition of a single polymeric waste material (i.e., cellulose23 or lignin24–26) is a considerable analytical challenge. The problem is further complicated owing to the variable compositions of processed waste materials and the presence of additives and/or other impurities in them. In addition, concerning the products of initial depolymerization, two complexity levels must be assessed, namely, (i) the quantitative continuum of the reaction media and (ii) the molecular description of all fractions in such reaction media.• Quantitative continuum. The thermochemical depolymerization of a single polymeric waste material always results in a continuum distribution of species. This broad distribution of products comprises several fractions, such as gases, liquids (tar), and solids (char, soot, or coke). In some cases, liquids containing immiscible phases (aqueous and organic) can also be obtained. Closing the mass balance, which is often an intrinsically difficult task, can be further hindered by a fraction of the heaviest tar and char molecules that are virtually impossible to quantify, remaining deposited within reaction setups and pipes. Under a given set of conditions, this coexistence of liquid phases can cause some problems, such as phase separation, appearance of mists, loss of product materials, and additional clogging problems. The first crucial step is to quantify each of the obtained fractions. Ideally, this would be done simply by weighing products and quantifying gases through GC analysis using an internal standard (the use of which is recommended whenever possible). However, in thermo-chemical processes for polymeric waste valorization often a solvent-based protocol is required for product retrieval. Hence, when eliminating (evaporating) the solvent, we must also assume certain inevitable product mass losses, which will negatively affect our mass balances. The second step is to quantitatively classify the entire species distribution in each fraction based on the species’ physicochemical properties: elemental composition, molecular weight, and boiling point. This task is rather simple in gas products but is significantly more complicated in liquid and solid products. Some of the most applied analytical procedures for the latter fractions are elemental analysis, gravimetric–calorimetric analysis, rheometry, viscometry, and (simulated) distillation. Gel permeation chromatography (GPC) has been a recurrent tool for estimating the average molecular weights in polymeric feedstock.27–29Fig. 2 provides an overview of the ample ranges of molecular weights and boiling points that some of the most common biomass-derived (Fig. 2a) and plastic-derived (Fig. 2b) fractions present, and it showcases the need for advanced characterization techniques that can elucidate even the heaviest polymeric fractions. Thermogravimetric techniques are also well-established for understanding the thermal behavior of various samples (i.e., proximate analysis).30,31
• Molecular-level description. Because the current state-of-the-art analytical techniques can only detect the specific features or fractions of polymeric waste materials and depolymerization products, a multi-technique approach is unavoidable when aiming to obtain a complete description of a given feedstock, as depicted in Fig. 2c. Gas chromatography combined with mass spectrometry (GC/MS) can provide valuable qualitative and quantitative information on the composition of, for instance, pyrolysis liquids32,33 and sewage sludge,34 with an improved resolution by bi-dimensional GC (GC × GC/MS).35–37 Similarly, the boundaries of liquid chromatography (LC) have also been pushed for an improved resolving power of polymeric mixtures.38,39 Another approach that can be useful for better detection/quantification is to use pyroprobes (flash pyrolizers) connected online to a GC or GC × GC/MS system.40,41 Regardless, the analytical capacity of both the GC and LC techniques for polymeric samples is heavily limited by the volatility (hence, molecular weight) of the analyzed samples and the setup restrictions (i.e., maximum GC oven/column temperatures). More advanced techniques, such as mono- or bi-dimensional nuclear magnetic resonance (NMR), are also known to be powerful in the detection and quantification of certain functional groups.42,43 High-resolution Orbitrap and Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR/MS) have substantially progressed in recent years as they have provided insights into the compositions of very heavy feedstocks, such as crude oil derivatives and/or blends,44,45 oxygenated mixtures,37,46 and coke.47 To date, FT-ICR/MS offers the highest available mass resolution and accuracy not only for detecting specific molecules but also for identifying the compound classes present in mixtures.48
Another important aspect of polymeric waste processing is designing conversion systems that can stably and reliably operate over time. Two aspects are mainly responsible for such systems’ unstable behavior: (i) the challenges associated with feeding solids in a reaction system and (ii) the substantial amounts of char/coke formed in many cases during this process, thereby irreversibly blocking the reactor and/or equipment pipes. For instance, waste plastics present low thermal conductivity, which complicates their fast heating in the reaction environment. In addition, fused plastics cause rubber formation, resulting in severe operational difficulties in (semi)-continuous conversion systems.49 With such complex feedstock, the formation of undesired by-products including tar, char, and/or coke is also fast and barely controllable, causing a significant drop in catalyst activity. For instance, when processing bio-oils, the rapid repolymerization of highly unstable and reactive oxygenates quickly forms the so-called thermal lignin, which clogs the entrance of continuous reaction systems and rapidly evolves into more condensed coke.50
3. Benefits of each approach
Abundant data are reported in the literature on the catalytic treatment of either model or real feedstocks. Even though the correlation between the model and real data is not straightforward, we have inferred the interplay between both knowledge fields and schematized it in Fig. 3.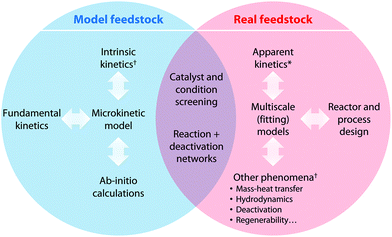 | ||
| Fig. 3 Interplay between the most significant knowledge that can be obtained from the studies dealing with model and real polymeric waste feedstock. | ||
• Model compounds allow the collection of valuable fundamental information and are most suitable for conducting operando studies, ab initio calculations, and microkinetic modeling or for establishing kinetic-deactivation mechanisms. Model feedstock can considerably aid in understanding the fundamental reaction pathways of individual compounds at the elemental reaction level and the synergies occurring among molecules with different reactivities while simultaneously avoiding the very pronounced catalyst deactivation occurring with real feedstock. The latter facilitates well-controlled continuous operation and hence allows for the easy and fast collection of kinetic data, which helps validate microkinetic models and thereby establish optimal reaction conditions. Numerous reviews exist on model compounds resembling mostly oxygenated feedstock, such as lignin51,52 and bio-oil.53,54 Furthermore, the ability to use detailed kinetic modeling data to develop lumped kinetic models for mixtures has been shown to be promising for hydrocarbon feedstocks,55 and the same could be applied to complex polymeric mixtures.56 This model approach also allows the detection of the potential inhibitory effects of specific reactants,57 which can be incorporated into models and simulations and are key for redesigning the reaction strategy.
• Real feedstock is indispensable for gathering realistic and industrially applicable results, addressing crucial operational issues, and tackling necessary reactor and catalyst design improvements. Embracing real feedstock is critical for understanding the true thermodynamics, hydrodynamics, kinetics, and mass and/or heat transport issues of catalysts as well as for addressing their deactivation and regeneration processes. This knowledge can later on be included in lump-based kinetic models, which can also reliably predict process behaviors in a relatively wide range of process conditions.58 The red line between the intrinsic kinetics and the use of real feeds is not that thick and a number of attempts have tried to bridge the two areas, e.g., in the field of biomass pyrolysis several groups including the one of Van Geem indicate that with suitable reaction (with negligible, predictable or measurable behavior of all the phenomena except the kinetics) and analytical systems, one can obtain the intrinsic kinetics of real feedstock pyrolysis.59–61 Operating with actual polymeric waste also yields a richer assortment of final products though less selectively than with model compounds. A valid strategy for facilitating operation with real feedstock is to use solvents for dilution, hence partially mitigating catalyst deactivation and facilitating reactor feeding, particularly in the case of very viscous and/or solid feeds.62 Furthermore, there are great reviews focusing mostly on real feedstock.63,64
The objective of this section is to convey that each approach is complementary; neither of them should fully stand alone as they rely on each other for attaining a useful understanding of the process. Although the data collected from both sides is abundant and well-interpreted, some of the behaviors observed with model compounds are difficult or even impossible to replicate with real feeds, and an in-depth physicochemical understanding of real feedstock processing remains extremely challenging. In addition, for practical reasons and commercial availability, the selected model compounds generally only represent the lightest fractions of the majority of waste materials, creating a big gap in the information available on the thermochemical conversion of heavier fractions, which can only be obtained by processing real feedstock; this also determines the overall feasibility and profitability of the process.
4. Comparison model vs. real feedstock
Fortunately, some works have compared the performance of model vs. real feedstock in industrially applicable thermocatalytic conversions and conditions. The most straightforward observation in the majority of these works is the massive drop in the catalytic activity that occurs in such chemical conversions when real feedstock is used, even when comparing individual reaction rates.36,65 The most extended approach is to start with model compounds and then use real feedstock.66 However, this feed switch forces researchers to use different conditions to obtain comparable kinetics or product distributions. Thus, the fundamental understanding obtained with the model molecules is neglected as it is not applicable (or replicable) when the conditions are changed. To avoid this, a more rational approach should be used to investigate real feedstock to establish a set of benchmark conditions. Then, intrinsic kinetic data should be obtained using model compounds under those predefined conditions.One of the main differences observed in the studies comparing model and real feedstock is the deactivation rate for a given reaction. For instance, during the steam reforming of bio-oil, accelerated catalyst deactivation is observed over time on the stream when comparing synthetic bio-oil/glycerol mixtures and real bio-oils; such deactivation translates into rapidly declining hydrogen yields over time on stream.67 Another relevant fact is that studies on model compounds are typically conducted under milder reaction conditions (e.g., temperature and pressure) owing to the higher reactivity of model mixtures, and often some potential operational difficulties remain overlooked. For instance, the conversion of phenolic compounds over a Ru/TiO2 catalyst yields cyclic hydrocarbons with a selectivity close to 100% at 280 °C. However, the hydrogenation capacity is considerably limited when converting real bio-oils, requiring an increase in the reaction temperature for increased hydrocarbon production.68 Another relevant aspect is that, because of their easy handling and reactivity, model compounds often lend themselves to research with little industrial applicability. This, for instance, is the case with regard to the studies in which in situ hydrogenation using hydrogen donors in batch reactors was reported.69,70 Despite obtaining valuable information, its industrial relevance is not meaningful considering the fact that industrial hydroprocessing occurs in continuous fixed bed systems and under hydrogen excess conditions using H2 streams. Another example of this can be the studies reporting the use of homogeneous catalysts for the mild hydrogenation or stabilization of bio-oils and synthetic mixtures.71 Although proven to be effective, the solubility of homogeneous catalysts in reaction products considerably limits further product applications and increases the operating costs for catalyst recovery and separation. A similar case is that of acid co-catalysts, like those studied on the hydrothermal liquefaction of model compounds for algal feedstock (polysaccharides and proteins).72
An interesting approach for understanding the individual compound reactivities in different environments is to isotopically label model compounds (i.e., phenol-d6, glucose-13C6) on both pyrolysis oil and water substrates.73 In this manner, in significantly complex reaction media, such as pyrolysis oil, the reactivity of phenol is limited in contrast to its reactivity in a water substrate when complete phenol conversion toward hydrogenated products is attained. However, glucose was found to be more reactive even on the basis of only thermal effects. The deuterium exchange reactions between labelled compounds and reaction media were also analyzed, occuring regardless of the presence of catalysts. A general tendency observed in all the reported works is that the lighter compounds in bio-oil (i.e., acids, aldehydes, and ketones) or tire oil (i.e., olefins, 1-ring aromatics) are significantly more reactive than the heteroatom-containing polyaromatics and unsaturated oxygenates contained within the most troublesome fractions in waste polymeric feedstock.
5. Recommendations
The problem we face can seem colossal, but the principle of “divide and conquer” can be the key to success. Fractionating an unfathomable task into a set of well-defined challenges based on everything learned from previous experiences and available literature is essential. In particular, the knowledge gathered on hydrocarbon engineering, processing, and chemical reactivity can be very valuable for valorizing polymeric waste more pragmatically. The most important principles to consider and progress toward the fusion of both research branches are as follows.1. Repurposing existing technology: We have built a worldwide system for converting, enhancing, and distributing hydrocarbons. From a sustainable and circular economy perspective, we should focus on “repurposing” the current infrastructure and also on its application to polymeric waste valorization. The downstream refining industry has a longstanding experience of treating all types of hydrocarbons from the lightest fractions down to bottom-of-the-barrel fractions. The developed technologies are ideal for the current case in most cases. From this perspective, assuming an allowed margin for design modifications, we should get inspiration from the previous publications and patents so as to create new process strategies.74 This message is probably far from new, but it is as important as ever in a scenario of a renewed interest in translating academic research into industry solutions. In terms of the use of refinery infrastructure to valorize waste, the new expression waste refinery has been coined75 and an important number of industrial advancements have been developed in this direction, e.g., Neste Oil has made a great effort in plastic and animal waste valorization repurposing technology.76,77
2. Wasteomics: Hydrocarbon chemistry has progressively evolved from a rough characterization strategy (API density or boiling point distribution) toward a much more detailed molecular classification. In fact, the groundbreaking advances of analytical techniques have led to coining the term petroleomics, which is used to refer to the field that explores the analytical aspects involved in petroleum chemistry.78 Inspired by this, we should take advantage of the momentum and create a new set of techniques for the chemical elucidation of polymeric waste materials: wasteomics. This new term would reflect the collective characterization and quantification of the pools of molecules that translate into the structure, function, and dynamics involved in polymeric waste valorization. The main deliverable of this stage would be an analytical workflow for characterizing polymeric waste materials and their derived streams, which would progressively become more standardized. This workflow requires a multi-technique approach, as discussed in section 2.
3. To fractionate or not to fractionate: The refining process always starts with distillation. This allows the division of very complex mixtures, such as crude oil, into fractions with similar boiling points (in this case). From this point downstream, all the postprocessing benefits can be imagined. Any pyrolysis oil, regardless of its source, can have a wide distribution of high molecular weight molecules (Fig. 2a and b) resembling crude oil, so proposing an initial fractionation seems coherent. Several in-depth studies have been conducted in this field.79 However, standardized approaches would help scientists narrow down more problems and challenges. Nevertheless, the refining industry is evolving from an expansive number of conversion steps toward a single one that aims at obtaining the most valued products in a single operation. These initiatives can be regarded as process intensification, and they have been investigated by the big players in the refining arena (Exxon Mobil, Aramco, or UOP, to mention a few). A common interest is processing crude oil and transforming it into highly valuable products, such as hydrogen, olefins, and aromatics (crude-oil to chemicals).80 Polymeric waste valorization already has a privileged position, and several initiatives have been developed to convert polymeric waste streams into chemicals.81,82 However, if our ambition is to treat feedstock as a whole, we will probably need to sacrifice a certain fundamental understanding of the process due to intrinsic limitations. To obtain the utmost benefits from the studies on model compounds, efforts must be directed toward establishing an experimental framework in which model compound studies are conducted. Also, the key “problems” that need to be solved should accurately be defined.
4. Benchmarking conditions: A preliminary investigation using real feedstock is required in the first place. The objective is to detect where the bottlenecks of the process are, what specific fundamental knowledge we lack, which set of conditions are realistic (i.e., temperature, pressure, phases), what kinds of materials or design can make sense, or how the performance is going to be evaluated. By following these steps, benchmarking conditions for future analyses can be established. This approach does not undermine the importance of model compound analysis. In contrast, it targets the narrowing down of the framework of these kinds of studies so that they can have a maximum impact on future applications. As explained later, the main aim of this work is to pass from a linear research strategy to a circular one.
5. Traceable model molecules with real feeds: A great research impact can be made by including traceable model molecules within real feedstocks to understand fundamental chemistry and kinetics. For instance, through 13C doping, it is possible to “chase down” the reactivity pathway of a given molecule immersed in a highly complex reaction medium, which is subjected to important reaction synergies. This method, even if conducted with a small number of compounds in a mixture, can provide greatly valuable information that can be complemented with extended studies with model compounds. This approach can also be done using specific detectors or analytical techiques, with molecular resolution. The model feedstock community must in turn collect a set of the main challenges established by the real feedstock community and push their own boundaries by incorporating more complex compounds/mixtures with a reactivity that realistically resembles that of actual polymeric waste. By investigating more realistic mixtures, various synergistic and/or antagonistic chemical phenomena resulting from cross-reactions can be more clearly understood. Unavoidably, this would imply taking a step forward in reactor design and always targeting continuous operation without losing sight of industrial applications. To exemplify the impact of this strategy let us consider the work of Fogassy et al.83 who used a traceable radiocarbon signal to analyze the fundamental mechanisms of bio-oil transformations when catalytically co-cracked with gasoil.
6. Use surrogates and be careful with the word “model”: By definition, a model molecule/compound should resemble the reactivity of a given chemical or mixture. One reiterative error is to assume that a representative or majoritarian species within a distribution of molecules is actually a (or “the”) model molecule. Much in contrast, the overall kinetics is dominated by the transformation of the most refractory and less reactive molecules (i.e., the rate-controlling compounds). For instance, the hydrodesulfurization kinetics of diesel is dominated by the transformation of the less reactive sulfur species, which happened to be 2,4-dimethyl dibenzothiophene-type structures. As a rule of thumb, these species typically correspond to the heaviest molecules present in reaction media. The concept is not far from the rate determining step (RDS) in chemical kinetics, taking the slowest reaction as representative of the whole reaction network. There are precise ways for calculating the molecular representations (surrogates) out of a mixture.84 These methodologies can lead to surrogate molecules, which may or may not be easily synthesized or purchased. In addition, they can put experimental work in jeopardy while ensuring fruitful theoretical or computational works. Valuable and irreplaceable information can be obtained from experimentation with single molecules, such as a fundamental understanding of the underlying kinetic networks, constants, and side reactions.85 However, simplifying a complex mixture into a “model molecule” is a statement that should not be lightly taken. Going beyond the use of surrogate or model compounds, researchers should also critically study the needed features for such compounds or their mixtures to reproduce the behavior of real feedstock. In the case of hydrocarbons, it has been shown that emulating the chemical functionalities (i.e., functional groups, molecular weight, etc.) of a real feedstock is critical for determining the mixture reactivity.86,87 This approach was recently extended to demonstrate that the combustion of hydrocarbon mixtures can be kinetically modeled only based on the functional group information derived from chemical analyses (e.g., GC/MS, NMR) and elegant reaction lumping strategies.88,89 Such an approach can be translated for a pyrolysis study of polymeric feedstock comprising various, albeit finite, chemical functionalities.
7. Analyze multiple molecules (and their interactions) simultaneously: Studying the kinetics of a single molecule first and then adding complexity to the system by increasing the number of species and/or their concentration is tremendously interesting. Using this approach, we can gain knowledge on the kinetic interrelations of these molecules (sometimes with the catalyst or catalyst surface) and their possible synergetic, neutral, or uncooperative interactions. Another interesting approach can be studying many single molecules under the same conditions and catalysts to extract tendencies and correlations using deep learning algorithms. Out of these algorithms, reinforced kinetic models can be expected to be applicable to realistic (industrial) applications. One interesting example of this powerful methodology is given by the work of Zhang et al.90 who developed the hydrogen-to-carbon effective ratio criteria upon reactivity analysis of several model components and real feedstock.
Irrespective of whether real or model feedstocks are being used, all the conducted research targeting a prospective industrial implementation of processes for waste valorization must comply with a series of precise guidelines. This set of rules ensures that maximum benefit is obtained by establishing the most crucial points for the synergy and interactions between both fields.
8. Rational catalyst design: The objective of catalytic research will always be determining the most active catalyst for a given application. Let us be mindful that according to the Green Chemistry principles the main factor driving catalysis design is selectivity, and above all, the best possible situation is not using a catalyst in the first place.91 However, as we investigate these materials, we must focus on the big picture. A number of parameters interplay and must be balanced when we aim for an optimized catalyst design, both from a chemical and an economic perspective, as shown in Fig. 4. Given the worldwide scale of the problem and the principles of Green Chemistry, we should also explore cheap, accessible, sustainable, non-hazardous and degradable materials. As we increase the reaction rate by synthesizing more active catalysts, the dominant phenomena in the reaction may switch from kinetics to heat-mass transfer or hydrodynamics. In this case, the material or the process need not be further enhanced because the rate-determining step is not the use of a catalyst. In other words, a more active catalyst means more problems in terms of side effects and potential limitations. Moreover, several studies on the valorization of polymeric waste have revealed that the practical limitations of the aforementioned technology (considering only catalytic performance factors) are not related to activity or selectivity but to catalyst deactivation.92
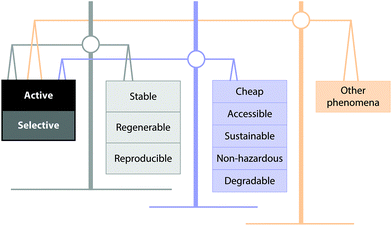 | ||
| Fig. 4 Parameters affecting the selection of an appropriate catalyst for the valorization of polymeric waste. | ||
Increased reaction rates cannot be attained at the expense of stability, regenerability, and reproducibility of a reaction. Take for example the main application of zeolite materials as catalysts at the industrial scale: in the fluid catalytic cracking (FCC) process, the zeolite is partially destroyed deliberately using steam to obtain a stable, regenerable, and reproducible catalyst. This steam treatment is known as equilibration of the catalyst. Thus, any new potential material-catalyst for FCC should survive the harsh conditions of equilibration which are designed not to have the most active catalyst but the most stable one.
9. Rational process design: Designing and developing robust continuous systems for real feedstock based on the knowledge acquired from model feedstock experimentation are important for overcoming the technical difficulties of dealing with polymeric waste. We must also be careful with the applications of batch reactors, which, on the one hand, can be very useful in the earliest stages of model research (i.e., for faster catalyst screening with easy operability), but on the other hand, lack industrial relevance. On the other side, batch reactors show important limitations to clarify the mechanisms of reactions with deactivation.93,94 Thus, a rational process design of these types of catalytic reactions may require a more careful selection of the reactor used.
10. Synergetic collaboration: Having the most complete overview can help in understanding to which extent the data of model compounds and/or model mixtures can be reliable and applicable to a given process. Such an overview is key for comparing the data obtained under similar conditions and for selecting model molecules or mixtures with a reactivity that is really representative of a given real feedstock. For a synergetic collaboration, the research conditions should be established by considering the actual industrial conditions and recreating them. In this context, the model feedstock research community must make the greatest effort in order to push their boundaries from both a reactor and catalyst design, and operational perspectives (bringing the more challenging representative feedstock into the picture). This way, more valuable and applicable information could be gathered on fundamental kinetics and chemistry. Then, the real feedstock research community would nurture from that knowledge and convert it into actionable models that simultaneously enable (i) the chemical and kinetic understanding of much more complex polymeric waste and (ii) progressing in the design of more viable and efficient conversion units for these kinds of wastes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was possible due to the financial support from the King Abdullah University of Science and Technology (KAUST).References
- W. R. Stahel, Nature, 2016, 531, 435–438 CrossRef CAS PubMed.
- A. Bosmans, I. Vanderreydt, D. Geysen and L. Helsen, J. Cleaner Prod., 2013, 55, 10–23 CrossRef.
- Y. Cao, S. S. Chen, S. Zhang, Y. S. Ok, B. M. Matsagar, K. C. W. Wu and D. C. W. Tsang, Bioresour. Technol., 2019, 291, 121878 CrossRef CAS.
- O. Dogu, M. Pelucchi, R. Van de Vijver, P. H. M. Van Steenberge, D. R. D'Hooge, A. Cuoci, M. Mehl, A. Frassoldati, T. Faravelli and K. M. Van Geem, Prog. Energy Combust. Sci., 2021, 84, 100901 CrossRef.
- H. C. Ong, W. H. Chen, A. Farooq, Y. Y. Gan, K. T. Lee and V. Ashokkumar, Renewable Sustainable Energy Rev., 2019, 113, 109266 CrossRef CAS.
- S. Pang, Biotechnol. Adv., 2019, 37, 589–597 CrossRef CAS PubMed.
- R. Trane, S. Dahl, M. S. Skjøth-Rasmussen and A. D. Jensen, Int. J. Hydrogen Energy, 2012, 37, 6447–6472 CrossRef CAS.
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493–1513 RSC.
- S. L. Wong, N. Ngadi, T. A. T. Abdullah and I. M. Inuwa, Renewable Sustainable Energy Rev., 2015, 50, 1167–1180 CrossRef CAS.
- B. Biswal, S. Kumar and R. K. Singh, J. Waste Manage., 2013, 2013, 1–7 CrossRef.
- H. B. Goyal, D. Seal and R. C. Saxena, Renewable Sustainable Energy Rev., 2008, 12, 504–517 CrossRef CAS.
- A. V. Bridgwater, Biomass Bioenergy, 2012, 38, 68–94 CrossRef CAS.
- T. J. Morgan and R. Kandiyoti, Chem. Rev., 2014, 114, 1547–1607 CrossRef CAS PubMed.
- D. Meier, B. Van De Beld, A. V. Bridgwater, D. C. Elliott, A. Oasmaa and F. Preto, Renewable Sustainable Energy Rev., 2013, 20, 619–641 CrossRef.
- BTG Bioliquids.
- M. S. Talmadge, R. M. Baldwin, M. J. Biddy, R. L. McCormick, G. T. Beckham, G. A. Ferguson, S. Czernik, K. A. Magrini-Bair, T. D. Foust, P. D. Metelski, C. Hetrick and M. R. Nimlos, Green Chem., 2014, 16, 407–453 RSC.
- C. K. Westbrook, Y. Mizobuchi, T. J. Poinsot, P. J. Smith and J. Warnatz, Proc. Combust. Inst., 2005, 30, 125–157 CrossRef.
- T. Lu and C. K. Law, Prog. Energy Combust. Sci., 2009, 35, 192–215 CrossRef CAS.
- W. J. Pitz and C. J. Mueller, Prog. Energy Combust. Sci., 2011, 37, 330–350 CrossRef CAS.
- S. M. Sarathy, A. Farooq and G. T. Kalghatgi, Prog. Energy Combust. Sci., 2018, 65, 67–108 CrossRef.
- J. C. Poveda and D. R. Molina, J. Pet. Sci. Eng., 2012, 84–85, 1–7 CrossRef CAS.
- S. Dooley, S. H. Won, J. Heyne, T. I. Farouk, Y. Ju, F. L. Dryer, K. Kumar, X. Hui, C.-J. Sung, H. Wang, M. A. Oehlschlaeger, V. Iyer, S. Iyer, T. A. Litzinger, R. J. Santoro, T. Malewicki and K. Brezinsky, Combust. Flame, 2012, 159, 1444–1466 CrossRef CAS.
- S. Rongpipi, D. Ye, E. D. Gomez and E. W. Gomez, Front. Plant Sci., 2019, 9, 1894 CrossRef PubMed.
- N. Giummarella, Y. Pu, A. J. Ragauskas and M. Lawoko, Green Chem., 2019, 21, 1573–1595 RSC.
- N. Baccile, C. Falco and M. M. Titirici, Green Chem., 2014, 16, 4839–4869 RSC.
- E. Terrell, L. D. Dellon, A. Dufour, E. Bartolomei, L. J. Broadbelt and M. Garcia-Perez, Ind. Eng. Chem. Res., 2020, 59, 526–555 CrossRef CAS.
- A. E. Harman-Ware and J. R. Ferrell, Energy Fuels, 2018, 32, 8905–8920 CrossRef CAS.
- N. Nciri and N. Cho, Mater. Today: Proc., 2018, 5, 23656–23663 CAS.
- J. C. Putman, S. Gutiérrez-Sama, C. Barrère-Mangote, R. P. Rodgers, R. Lobinski, A. G. Marshall, B. Bouyssière and P. Giusti, Energy Fuels, 2018, 32, 12198–12204 CrossRef CAS.
- N. Gao, A. T. Sipra and C. Quan, Fuel, 2020, 281, 118572 CrossRef CAS.
- Z. Kwoczynski and J. Čmelík, J. Cleaner Prod., 2021, 280, 124302 CrossRef CAS.
- Z. Echresh Zadeh, A. Abdulkhani and B. Saha, Energy, 2021, 214, 118930 CrossRef CAS.
- M. Arabiourrutia, G. Lopez, G. Elordi, M. Olazar, R. Aguado and J. Bilbao, Chem. Eng. Sci., 2007, 62, 5271–5275 CrossRef CAS.
- L. Martín-Pozo, B. de Alarcón-Gómez, R. Rodríguez-Gómez, M. T. García-Córcoles, M. Çipa and A. Zafra-Gómez, Talanta, 2019, 192, 508–533 CrossRef PubMed.
- J. H. Marsman, J. Wildschut, F. Mahfud and H. J. Heeres, J. Chromatogr. A, 2007, 1150, 21–27 CrossRef CAS PubMed.
- I. Hita, A. Gutiérrez, M. Olazar, J. Bilbao, J. M. Arandes and P. Castaño, Fuel, 2015, 145, 158–169 CrossRef CAS.
- I. Hita, T. Cordero-Lanzac, T. Kekäläinen, O. Okafor, J. Rodríguez-Mirasol, T. Cordero, J. Bilbao, J. Jänis and P. Castaño, ACS Sustainable Chem. Eng., 2020, 8, 18433–18445 CrossRef CAS.
- E. Lazzari, K. Arena, E. B. Caramão and M. Herrero, J. Chromatogr. A, 2019, 1602, 359–367 CrossRef CAS PubMed.
- C. Reymond, A. Le Masle, C. Colas and N. Charon, J. Chromatogr. A, 2021, 1636, 461716 CrossRef CAS PubMed.
- J. Dong, F. Li and K. Xie, J. Hazard. Mater., 2012, 243, 80–85 CrossRef CAS PubMed.
- J. Kong, R. Zhao, Y. Bai, G. Li, C. Zhang and F. Li, Fuel Process. Technol., 2014, 127, 41–46 CrossRef CAS.
- A. D. Ure, J. E. O'Brien and S. Dooley, Energy Fuels, 2019, 33, 11741–11756 CrossRef CAS.
- C. A. Mullen, G. D. Strahan and A. A. Boateng, Energy Fuels, 2009, 23, 2707–2718 CrossRef CAS.
- D. F. Smith, D. C. Podgorski, R. P. Rodgers, G. T. Blakney and C. L. Hendrickson, Anal. Chem., 2018, 90, 2041–2047 CrossRef CAS PubMed.
- R. Palos, T. Kekäläinen, F. Duodu, A. Gutiérrez, J. M. Arandes, J. Jänis and P. Castaño, Appl. Catal., B, 2019, 256, 117863 CrossRef CAS.
- R. L. Ware, S. M. Rowland, R. P. Rodgers and A. G. Marshall, Energy Fuels, 2017, 31, 8210–8216 CrossRef CAS.
- N. Wang, Y. Zhi, Y. Wei, W. Zhang, Z. Liu, J. Huang, T. Sun, S. Xu, S. Lin, Y. He, A. Zheng and Z. Liu, Nat. Commun., 2020, 11, 1079 CrossRef CAS PubMed.
- C. M. Michailof, K. G. Kalogiannis, T. Sfetsas, D. T. Patiaka and A. A. Lappas, Wiley Interdiscip. Rev.: Energy Environ., 2016, 5, 614–639 CAS.
- G. Lopez, M. Artetxe, M. Amutio, J. Bilbao and M. Olazar, Renewable Sustainable Energy Rev., 2017, 73, 346–368 CrossRef CAS.
- T. Cordero-Lanzac, R. Palos, I. Hita, J. M. Arandes, J. Rodríguez-Mirasol, T. Cordero, J. Bilbao and P. Castaño, Appl. Catal., B, 2018, 239, 513–524 CrossRef CAS.
- Z. Sun, B. Fridrich, A. de Santi, S. Elangovan and K. Barta, Chem. Rev., 2018, 118, 614–678 CrossRef CAS PubMed.
- W. Schutyser, T. Renders, S. Van Den Bosch, S. F. Koelewijn, G. T. Beckham and B. F. Sels, Chem. Soc. Rev., 2018, 47, 852–908 RSC.
- A. N. Kay Lup, F. Abnisa, W. M. A. Wan Daud and M. K. Aroua, Appl. Catal., A, 2017, 541, 87–106 CrossRef CAS.
- A. N. Kay Lup, F. Abnisa, W. M. A. Wan Daud and M. K. Aroua, J. Ind. Eng. Chem., 2017, 56, 1–34 CrossRef CAS.
- E. Ranzi, M. Dente, A. Goldaniga, G. Bozzano and T. Faravelli, Prog. Energy Combust. Sci., 2001, 27, 99–139 CrossRef CAS.
- A. Anca-Couce, Prog. Energy Combust. Sci., 2016, 53, 41–79 CrossRef.
- B. Puértolas, T. C. Keller, S. Mitchell and J. Pérez-Ramírez, Appl. Catal., B, 2016, 184, 77–86 CrossRef.
- T. Cordero-Lanzac, I. Hita, F. J. García-Mateos, P. Castaño, J. Rodríguez-Mirasol, T. Cordero and J. Bilbao, Chem. Eng. J., 2020, 124679 CrossRef CAS.
- G. SriBala, H.-H. Carstensen, K. M. Van Geem and G. B. Marin, Wiley Interdiscip. Rev.: Energy Environ., 2019, 8, e326 Search PubMed.
- A. Gonzalez-Quiroga, K. M. Van Geem and G. B. Marin, Biomass Convers. Biorefin., 2017, 7, 305–317 CrossRef CAS.
- P. N. Ciesielski, M. B. Pecha, A. M. Lattanzi, V. S. Bharadwaj, M. F. Crowley, L. Bu, J. V. Vermaas, K. X. Steirer and M. F. Crowley, ACS Sustainable Chem. Eng., 2020, 8, 3512–3531 CrossRef CAS.
- B. Valle, N. García-Gómez, A. Arandia, A. Remiro, J. Bilbao and A. G. Gayubo, Int. J. Hydrogen Energy, 2019, 44, 12593–12603 CrossRef CAS.
- J. Chen, J. Sun and Y. Wang, Ind. Eng. Chem. Res., 2017, 56, 4627–4637 CrossRef CAS.
- B. Valle, A. Remiro, N. García-Gómez, A. G. Gayubo and J. Bilbao, J. Chem. Technol. Biotechnol., 2019, 94, 670–689 CrossRef CAS.
- I. Hita, R. Palos, J. M. Arandes, J. M. Hill and P. Castaño, Fuel Process. Technol., 2016, 144, 239–247 CrossRef CAS.
- L. Yang, K. Seshan and Y. Li, Catal. Today, 2017, 298, 276–297 CrossRef CAS.
- K. Bizkarra, J. M. Bermudez, P. Arcelus-Arrillaga, V. L. Barrio, J. F. Cambra and M. Millan, Int. J. Hydrogen Energy, 2018, 43, 11706–11718 CrossRef CAS.
- R. Shu, B. Lin, J. Zhang, C. Wang, Z. Yang and Y. Chen, Fuel Process. Technol., 2019, 184, 12–18 CrossRef CAS.
- Y. Xu, J. Long, Q. Liu, Y. Li, C. Wang, Q. Zhang, W. Lv, X. Zhang, S. Qiu, T. Wang and L. Ma, Energy Convers. Manage., 2015, 89, 188–196 CrossRef CAS.
- Y. Xu, Y. Li, C. Wang, C. Wang, L. Ma, T. Wang, X. Zhang and Q. Zhang, Fuel Process. Technol., 2017, 161, 226–231 CrossRef CAS.
- L. Busetto, D. Fabbri, R. Mazzoni, M. Salmi, C. Torri and V. Zanotti, Fuel, 2011, 90, 1197–1207 CrossRef CAS.
- W. Yang, X. Li, D. Zhang and L. Feng, Energy Convers. Manage., 2017, 154, 336–343 CrossRef CAS.
- C. Boscagli, K. Raffelt and J.-D. Grunwaldt, Biomass Bioenergy, 2017, 106, 63–73 CrossRef CAS.
- M. A. Alabdullah, A. R. Gomez, J. Vittenet, A. Bendjeriou-Sedjerari, W. Xu, I. A. Abba and J. Gascon, ACS Catal., 2020, 10, 8131–8140 CrossRef CAS.
- R. Palos, A. Gutiérrez, F. J. Vela, M. Olazar, J. M. Arandes and J. Bilbao, Energy Fuels, 2021, 35, 3529–3557 CrossRef CAS.
- Focus Catal., 2021, 2021, 3, 4 Search PubMed.
- Focus Catal., 2020, 2020, 5, 5 Search PubMed.
- A. G. Marshall and R. P. Rodgers, Acc. Chem. Res., 2004, 37, 53–59 CrossRef CAS PubMed.
- D. E. Resasco and S. P. Crossley, Catal. Today, 2015, 257, 185–199 CrossRef CAS.
- A. Corma, E. Corresa, Y. Mathieu, L. Sauvanaud, S. Al-Bogami, M. S. Al-Ghrami and A. Bourane, Catal. Sci. Technol., 2017, 7, 12–46 RSC.
- D. C. Elliott, D. Meier, A. Oasmaa, B. van de Beld, A. V. Bridgwater and M. Marklund, Energy Fuels, 2017, 31, 5111–5119 CrossRef CAS.
- P. Bulsink, F. de Miguel Mercader, L. Sandström, B. van de Beld, F. Preto, A. Zacher, A. Oasmaa, N. Dahmen, A. Funke and B. Bronson, Energy Fuels, 2020, 34, 11123–11133 CrossRef CAS.
- G. Fogassy, N. Thegarid, Y. Schuurman and C. Mirodatos, Green Chem., 2012, 14, 1367 RSC.
- S. Cho, M. Kim, B. Lyu and I. Moon, Chem. Eng. J., 2021, 407, 126659 CrossRef CAS.
- X. Zhang and S. M. Sarathy, Fuel, 2021, 286, 119361 CrossRef CAS.
- S. Dooley, S. H. Won, M. Chaos, J. Heyne, Y. Ju, F. L. Dryer, K. Kumar, C.-J. Sung, H. Wang, M. A. Oehlschlaeger, R. J. Santoro and T. A. Litzinger, Combust. Flame, 2010, 157, 2333–2339 CrossRef CAS.
- A. G. Abdul Jameel, N. Naser, G. Issayev, J. Touitou, M. K. Ghosh, A.-H. Emwas, A. Farooq, S. Dooley and S. M. Sarathy, Combust. Flame, 2018, 192, 250–271 CrossRef CAS.
- H. Wang, R. Xu, K. Wang, C. T. Bowman, R. K. Hanson, D. F. Davidson, K. Brezinsky and F. N. Egolfopoulos, Combust. Flame, 2018, 193, 502–519 CrossRef.
- R. Xu, K. Wang, S. Banerjee, J. Shao, T. Parise, Y. Zhu, S. Wang, A. Movaghar, D. J. Lee, R. Zhao, X. Han, Y. Gao, T. Lu, K. Brezinsky, F. N. Egolfopoulos, D. F. Davidson, R. K. Hanson, C. T. Bowman and H. Wang, Combust. Flame, 2018, 193, 520–537 CrossRef CAS.
- H. Zhang, Y.-T. Cheng, T. P. Vispute, R. Xiao and G. W. Huber, Energy Environ. Sci., 2011, 4, 2297 RSC.
- R. A. Sheldon, Chem. Commun., 2008, 3352 RSC.
- A. Ochoa, J. Bilbao, A. G. Gayubo and P. Castaño, Renewable Sustainable Energy Rev., 2020, 119, 109600 CrossRef CAS.
- S. L. Scott, ACS Catal., 2018, 8, 8597–8599 CrossRef CAS.
- U. I. Kramm, R. Marschall and M. Rose, ChemCatChem, 2019, 11, 2563–2574 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |