Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of dense porous layered double hydroxides from struvite†
W. L. Joyce
Kwok
 ,
Hongri
Suo
,
Chunping
Chen
,
D. W. Justin
Leung
,
Jean-Charles
Buffet
,
Hongri
Suo
,
Chunping
Chen
,
D. W. Justin
Leung
,
Jean-Charles
Buffet
 and
Dermot
O'Hare
and
Dermot
O'Hare
 *
*
Chemistry Research Laboratory, Department of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: dermot.ohare@chem.ox.ac.uk
First published on 15th February 2021
Abstract
We report an atom economic synthesis of a new type of dense porous layered double hydroxides (SLDHs) using struvite which is a commonly produced undesirable waste in wastewater treatment plants. The obtained SLDHs exhibit significantly higher specific surface areas and larger pore volumes than conventional coprecipitated LDHs but retain a comparable particle density.
Layered double hydroxides (LDHs) are a class of 2D layered materials with the general formula [(M1−xz+M′xy+(OH)2)]w+(An−)w/n·mH2O], where M and M′ are typically di- or trivalent cations, respectively; and A is an n-valent anionic moiety, either organic or inorganic. The overall metal hydroxide layer charge (w) is controlled by w = z(1 − x) + xy − 2 which is compensated by w/n An− interlayer anions.1 Owing to their distinguished properties such as compositional flexibility, biocompatibility, acid/base tunability and anion exchangeability, LDHs have garnered increasing attention across a wide range of fields (e.g.: catalysis, biology, environment and energy) for both fundamental research and commercial applications.2–6 LDHs are commonly synthesised at scale using metal salts, such as nitrates and chlorides,7 or oxides.8,9 There continues to be scope for both increasing the atom efficiency of LDH syntheses and utilising resources in a circular economic way. For example, at an commercial-scale LDH plant in Dalian (China), magnesium aluminium LDHs were prepared using magnesium chloride (MgCl2) from the bitterns remaining after the crystallisation of sodium and potassium chloride from seawater.7
Struvite (MgNH4PO4·6H2O) is a naturally occurring orthophosphate mineral that precipitates in aqueous systems containing high concentrations of ammonium and phosphate. It was first identified in wastewater treatment plants (WWTPs) in the late 1930s.10 It can precipitate spontaneously in some areas of WWTPs where the pH, temperature, and magnesium/phosphorus/ammonium ion concentrations are suitable.11 Struvite is sparingly soluble in water (169.2 mg cm−3 at 25 °C)12 and so creates significant problems for WWTPs, it easily forms hard crystalline deposits that lead to pipeline blockages, resulting in costly and time consuming cleaning and maintenance.10 The intentional and controlled precipitation of struvite in a dedicated vessel can be deployed by regulating the concentrations of Mg2+, PO43− and NH4+ (eqn (1)).13
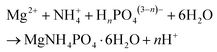 | (1) |
This approach can effectively alleviate pipeline blockages, but it results in the annual production of large quantities of struvite solid discharge from WWTPs. It is of significant importance to develop new efficient circular economic approaches to reuse this waste mineral resource. The low solubility and high nutrient content (magnesium/phosphorus/nitrogen) of struvite make it a potential slow-release fertiliser for agricultural use.14 However, there are some concerns that the struvite sourced from WWTPs present a potential health risk and as a result is not certified as end-of-waste in many countries.15,16 Its application as a fertiliser is currently limited by local policy. Therefore, the development of alternative uses of this material is desirable in order to reduce the cost of waste disposal and to increase the efficiency of our chemical processes.
Herein, we explore struvite as a low-cost starting material to synthesise novel morphology magnesium aluminium LDHs using a bespoke in situ solid–solid growth method. This method has the advantage over traditional route of LDH synthesis of reducing the amount of waste from water treatment plant, the use of expensive magnesium salts and the production of salt-rich effluent streams that are harmful to the environment.17
Although struvite can be used directly to make LDHs we have determined that a pre-treatment step significantly improves the efficiency of the process. Struvite is initially suspended in aqueous sodium hydroxide (1 M) at 85 °C for 1 hour. Ammonia is liberated that can be collected using a HCl trap to yield NH4Cl, an additional valuable by-product. To obtain phase pure struvite-derived [Mg0.75Al0.25(OH)2](CO3)0.175 (Mg3Al-CO3 SLDH) sodium carbonate and aluminium nitrate are then introduced and this suspension is maintained at pH 11.5, 85 °C for 2 hours (by addition of NaOH). We do not observe dissolution of the mineral precursor; imaging the particles before and after synthesis (vide infra) suggest that it may be a solid–solid transformation. The struvite starting material is highly crystalline with an orthorhombic unit cell containing hydrogen-bonded PO43− tetrahedra, NH4+ tetrahedra and distorted Mg(H2O)62+ octahedra (Fig. 1a).11 After the synthesis reaction, filtration and drying the XRD pattern of the isolated solid is shown in Fig. 1b. The Bragg reflections observed in the XRD can indexed to 00l, 01l, 11l, corresponding to unit cell dimensions of Mg3Al-CO3 SLDH, no other crystalline phases are observed.
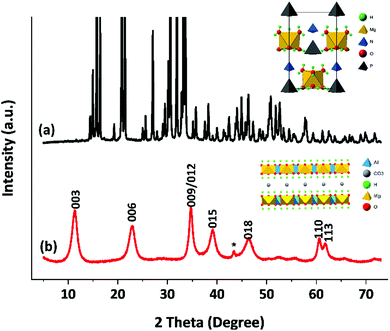 | ||
| Fig. 1 Powder XRD diffractograms of (a) struvite and (b) Mg3Al-CO3 SLDH. *Bragg reflection due to the sample holder. | ||
Thermogravimetric analysis (TGA) of struvite (Fig. S1†) exhibits one main weight loss (approximately 45% of its mass) before 150 °C, which is attributed to the liberation of ammonia and surface water. Further heating causes the loss of structural water and the formation of magnesium phosphates.18 At 800 °C, the material has lost around 55% of its original mass. In comparison, the Mg3Al-CO3 SLDH presents the archetypical thermal decomposition profile of an LDH:19 loss of surface and interlayer water (17.5 wt%) below 200 °C, followed by dehydroxylation and loss of interlayer carbonate as CO2 above 200 °C, leading a total loss of 44.3 wt% at 800 °C.
A series of further spectroscopic studies were carried out in order to fully understand the conversion process and the nature of the Mg3Al-CO3 SLDH product. Fourier-transform infrared (FTIR) spectra are shown in Fig. S2,† four main band regions are observed for struvite (Fig. S2a†). The broad bands in the range 2550–3300 cm−1 are assigned to the antisymmetric stretching vibration of NH4 and the OH stretching mode of H2O. The weak band between 2200–2500 cm−1 is due to hydrogen bonding between H2O and PO4. The bands between 1300–1800 cm−1 are ascribed to the deformations of H–O–H and H–N–H. Strong PO43− vibration bands can be observed around 1000 cm−1.20 After the synthesis reaction, vibrational bands characteristic of LDH can be clearly observed (Fig. S2b†): 3200–3700 cm−1 (OH− and H2O hydrogen bonding), 1364 cm−1 (ν4/ν2 vibrations of CO32−) and 600–1100 cm−1 (O–M–O bending/stretching/deformation), indicating the successful formation of an LDH.21 In addition, all vibrations due to NH4+ were absent, confirming that ammonia had been liberated during the synthesis. Vibrations due to phosphate ions were much weaker in the final LDH product, indicating phosphate is not the main interlayer anion within the interlayer galleries. However, a small amount of PO43− remains in the Mg3Al-CO3 SLDH, which can be detected by solid state 31P NMR spectroscopy (Fig. S3†) and ICP-MS (Table S1†). The total phosphorus content in the sample was typically 1.4 wt%. The ICP-MS data indicates that the Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio in the SLDH is 2.99, which is close to the theoretical value (3), indicating the magnesium has been fully incorporated in Mg3Al-CO3 SLDH. Powder XRD diffractograms of carbonate and phosphate SLDHs for two different Mg/Al ratios (Fig. S4†) and their corresponding d-spacing (Table S2†) show the expected 00l and interlayer distances.
Al ratio in the SLDH is 2.99, which is close to the theoretical value (3), indicating the magnesium has been fully incorporated in Mg3Al-CO3 SLDH. Powder XRD diffractograms of carbonate and phosphate SLDHs for two different Mg/Al ratios (Fig. S4†) and their corresponding d-spacing (Table S2†) show the expected 00l and interlayer distances.
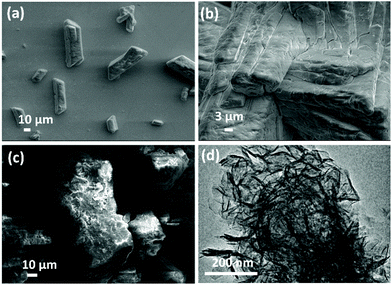
The morphologies of the materials before and after the synthesis reaction have been investigated using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As shown in Fig. 2a, struvite presents as coffin-shaped prismatic crystals with dimensional ranges 20–80 μm × 10–20 μm. The SEM images reveal the surface of the struvite crystals are stone-like and smooth (Fig. 2b). After the reaction, the isolated material retains a large particle morphology, but the surface of the particles is now much rougher with small particles observed on the surface (Fig. 2c). TEM imaging after sonication of these particles in ethanol reveals a classical rosette shape clusters that are composed of primary LDH nanosheets (Fig. 2d).
To better understand the formation process of Mg3Al-CO3 SLDH from struvite, we first isolated the material following pre-treatment of struvite in sodium hydroxide at 85 °C for 1 hour. The high resolution (HR) SEM and energy dispersive X-ray (EDX) reveal that the original coffin-lid crystals become cracked and fragmented (Fig. S6†). Sodium, magnesium and phosphorus are uniformly distributed over these particles, which suggests the formation of a metastable intermediate in which the ammonium ions within the original struvite structure are replaced by smaller sodium cations (eqn (2)).
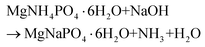 | (2) |
ICP-MS data of this material has a Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na
Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 2
P ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 indicating partial exchange of the ammonium cations (Table S3†).
2 indicating partial exchange of the ammonium cations (Table S3†).
Hence, we postulate that Mg3Al-CO3 SLDH formation proceeds via a reactive intermediate that is unique to the synthesis route. The struvite-like structure of proposed “MgNax(NH4)yPO4·6H2O” (MgNa0.5(NH4)0.5PO4·6H2O according to the ICP-MS data in this case) material provides a framework to grow Mg3Al-CO3 SLDH nanosheets. Nucleation and growth of the Mg3Al-CO3 SLDH proceeds directly on the surface of this intermediate via a dissolution-crystallisation process, giving rise to a struvite-like porous framework composed of hierarchical LDH rosettes. This is confirmed by characterisation by powder XRD, solid state NMR spectroscopy and specific surface area and pore volume measurements (Fig. S7–S11†).
We ascribe this dissolution-crystallisation process on the surface of the intermediate magnesium phosphate as the origin of the unique porosity of Mg3Al-CO3 SLDH. The specific surface area and pore volume of Mg3Al-CO3 SLDH as measured by N2 BET measurements are 184 m2 g−1 and 0.75 cm3 g−1, respectively (Fig. 3 and S5†). These values are significantly higher than conventional water-washed Mg3Al-CO3 LDH (43 m2 g−1 and 0.11 cm3 g−1) synthesised by traditional co-precipitation methods.22 In fact, these porosity figures resemble those obtained in the aqueous miscible organic solvent treatment method (AMOST) to yield AMO-Mg3Al-CO3 LDH (212 m2 g−1 and 0.75 cm3 g−1).22–24 Fig. S5† also shows the different shapes of N2 adsorption/desorption isotherms of SLDH and AMO-SLDH above P/P0 = 0.7 which suggests different origins of the porosity. It is likely inter-particle porosity for AMO-SLDH with straight and parallel adsorption and desorption branches until higher adsorbed N2 volume while the hysteresis loop suggests intra-particle porosity SLDH. AMO-LDHs with high specific surface area typically possess very low powder densities (tap density ca. 0.2 g cm−3),22 this feature can create some powder handling challenges especially during large scale production. Interestingly, the Mg3Al-CO3 SLDH developed in this study exhibits not only high surface area and pore volume, but also relatively high particle density (tap density = 0.64 g cm−3) which is three times higher than that of a typical AMO-LDH. We concluded that the high surface area and pore volume created in Mg3Al-CO3 SLDH is derived by the internal void structure of the aggregated SLDH particles that is a direct consequence of using a struvite-derived precursor during synthesis.
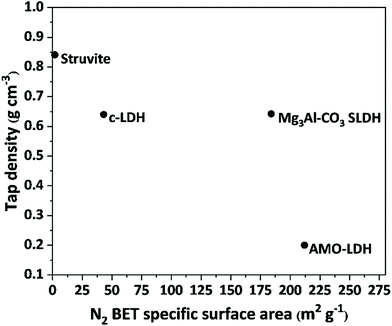 | ||
| Fig. 3 N2 BET specific surface areas and powder tap densities of different materials. The c-LDH is a conventional water-washed Mg3Al-CO3 LDH. The AMO-LDH is an Mg3Al-CO3 LDH post synthesis dispersed in acetone.22 | ||
We have also prepared porous Mg2Al-CO3 SLDH by adjusting the ratio of struvite to aluminium nitrate (Fig. S4†). By omitting the Na2CO3 in the synthesis and operating under a nitrogen atmosphere we can utilise the phosphate anions within the struvite to make MgnAl-PO4 SLDH (n = 2, 3) (Fig. S4†). We observe a slight increase in the interlayer separation from 7.78 Å to 7.93 Å between Mg3Al-CO3 SLDH and Mg3Al-PO4 SLDH (Table S2†). We have also carried out SLDH synthesis using two commercial waste water treatment plant (WWTP) struvite samples (Table S4, Fig. S13†). Although technologies used to precipitate these commercial struvites differ and as a result the composition and morphology (Fig. S14–S17†) of the struvite samples differ from that observed of the pure struvite purchased from Alfa Aesar both produced SLDHs with excellent yield and purity. Some differences in pore volume were observed (Fig. S18†).
We believe that this new class of dense and porous SLDH will be an attractive material in many applications such as heterogeneous catalysis, adsorption, and energy storage. Therefore we have performed some preliminary studies on carbon dioxide (CO2) capture to demonstrate that an LDH derived from struvite waste could be a promising candidate as a low-cost, efficient carbon capture material. It is well known that LDHs can be transformed into uniquely structured mixed metal oxides (LDOs) by calcination at temperatures in the range 300–550 °C.25,26 Calcination of Mg3Al-CO3 SLDH above 400 °C produces an SLDO which shows a complex aluminium/magnesium phase that possess both basicity and acidity in the medium-high strength range (Fig. S19†).27–29 In addition, the specific surface area (Fig. S20†) and pore volume (Fig. S21†) further increases up to 203 m2 g−1 and 0.84 cm3 g−1, respectively, when it is calcined at 400 °C for 2 hours. All these materials features are particularly appropriate for the adsorption of CO2.
Fig. 4 shows the CO2 adsorption performances of struvite and SLDO. Struvite exhibits poor CO2 adsorption capacity (0.18 mmol g−1 at 40 °C and 100 kPa), which includes 0.16 mmol g−1 of physisorption and 0.02 mmol g−1 of chemisorption. This is mainly due to its low specific surface area and almost no active sites for CO2 adsorption. In contrast, the CO2 adsorption capability of Mg3Al-CO3 SLDH is 0.9 mmol g−1, four times higher than that of struvite, 74% of this capacity is due to physisorption, which is ascribed to high specific surface area and large pore volume, whilst 26% is chemisorption that is ascribed to the presence of moderate basic sites in the SLDO.28,30
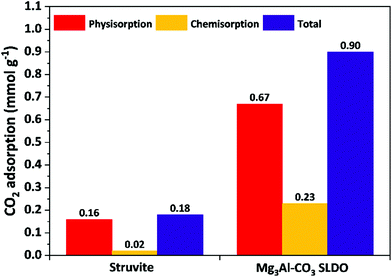 | ||
| Fig. 4 CO2 adsorption of struvite and Mg3Al-CO3 SLDO at 40 °C and 100 kPa. The samples were initially calcined at 400 °C for 2 hours. | ||
In summary, we have shown that struvite (including waste water treatment plant samples) can be both an efficient, green source of magnesium and phosphate and create a structural scaffold for the preparation of a new class of dense porous MgnAl-A SLDHs (n = 2, 3; A = CO3, PO4). Our preliminary studies suggest the transformation proceeds via a dissolution-crystallisation mechanism; possesses high surface area, large pore volume and high tap densities, a combination not found in conventional LDHs and AMO-LDHs.
Additionally, the SLDO derived from Mg3Al-CO3 SLDH exhibits four times higher CO2 adsorption than that of struvite. These findings demonstrate that the synthesis in this study can upgrade the struvite mineral into valuable LDH-based materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank SCG Chemicals Co., Ltd, Thailand for funding and Dr Nicholas Rees (University of Oxford) for solid state NMR spectroscopy.References
- Layered Double Hydroxides, ed. X. Duan and D. G. Evans, Springer-Verlag Berlin Heidelberg, 2005 Search PubMed.
- S. Miyata, Clays Clay Miner., 1983, 31, 305–311 CrossRef CAS.
- A. I. Khan, A. Ragavan, B. Fong, C. Markland, M. O'Brien, T. G. Dunbar, G. R. Williams and D. O'Hare, Ind. Eng. Chem. Res., 2009, 48, 10196–10205 CrossRef CAS.
- G. Fan, F. Li, D. G. Evans and X. Duan, Chem. Soc. Rev., 2014, 43, 7040–7066 RSC.
- S. He, Z. An, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2013, 49, 5912–5920 RSC.
- Z. Cao, B. Li, L. Sun, L. Li, Z. P. Xu and Z. Gu, Small Methods, 2020, 4, 1900343 CrossRef CAS.
- D. G. Evans and X. Duan, Chem. Commun., 2006, 485–496 RSC.
- S. P. Newman, W. Jones, P. O'Connor and D. N. Stamires, J. Mater. Chem., 2002, 12, 153–155 RSC.
- Z. P. Xu and G. Q. Lu, Chem. Mater., 2005, 17, 1055–1062 CrossRef CAS.
- Y. Jaffer, T. A. Clark, P. Pearce and S. A. Parsons, Water Res., 2002, 36, 1834–1842 CrossRef CAS.
- K. S. Le Corre, E. Valsami-Jones, P. Hobbs and S. A. Parsons, Crit. Rev. Environ. Sci. Technol., 2009, 39, 433–477 CrossRef CAS.
- M. I. H. Bhuiyan, D. S. Mavinic and R. D. Beckie, Environ. Technol., 2007, 28, 1015–1026 CrossRef CAS.
- S. Kataki, H. West, M. Clarke and D. C. Baruah, Resour., Conserv. Recycl., 2016, 107, 142–156 CrossRef.
- H. D. Ryu, C. S. Lim, M. K. Kang and S. I. Lee, J. Hazard. Mater., 2012, 221, 248–255 CrossRef.
- Q.-L. Chen, X.-L. An, Y.-G. Zhu, J.-Q. Su, M. R. Gillings, Z.-L. Ye and L. Cui, Environ. Sci. Technol., 2017, 51, 8149–8157 CrossRef CAS.
- M. Otoo and P. Drechsel, Resource recovery from waste: business models for energy, nutrient and water reuse in low- and middle-income countries, Routledge - Earthscan, Oxon, UK, 2018, pp. 316–546 Search PubMed.
- F. J. W. J. Labuschagné, A. Wiid, H. P. Venter, B. R. Gevers and A. Leuteritz, Green Chem. Lett. Rev., 2018, 11, 18–28 CrossRef.
- M. I. H. Bhuiyan, D. Mavinic and F. Koch, Chemosphere, 2008, 70, 1347–1356 CrossRef CAS.
- W. Yang, Y. Kim, P. K. T. Liu, M. Sahimi and T. T. Tsotsis, Chem. Eng. Sci., 2002, 57, 2945–2953 CrossRef CAS.
- G. Kurtulus and A. Tas, Mater. Lett., 2011, 65, 2883–2886 CrossRef CAS.
- C. Chen, K. Ruengkajorn, J.-C. Buffet and D. O'Hare, RSC Adv., 2018, 8, 34650–34655 RSC.
- C. Chen, M. Yang, Q. Wang, J.-C. Buffet and D. O'Hare, J. Mater. Chem. A, 2014, 2, 15102–15110 RSC.
- Q. Wang and D. O'Hare, Chem. Commun., 2013, 49, 6301–6303 RSC.
- C. Chen, A. Wangriya, J.-C. Buffet and D. O'Hare, Dalton Trans., 2015, 44, 16392–16398 RSC.
- M. A. Aramendía, Y. Avilés, V. Borau, J. M. Luque, J. M. Marinas, J. R. Ruiz and F. J. Urbano, J. Mater. Chem., 1999, 9, 1603–1607 RSC.
- F. Rey, V. Fornés and J. M. Rojo, J. Chem. Soc., Faraday Trans., 1992, 88, 2233–2238 RSC.
- Y. Gao, Z. Zhang, J. Wu, X. Yi, A. Zheng, A. Umar, D. O'Hare and Q. Wang, J. Mater. Chem. A, 2013, 1, 12782–12790 RSC.
- D. W. J. Leung, C. Chen, J.-C. Buffet and D. O'Hares, Dalton Trans., 2020, 49, 9306–9311 RSC.
- F. Prinetto, G. Ghiotti, R. Durand and D. Tichit, J. Phys. Chem. B, 2000, 104, 11117–11126 CrossRef CAS.
- X. Zhu, C. Chen, H. Suo, Q. Wang, Y. Shi, D. O'Hare and N. Cai, Energy, 2019, 167, 960–969 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Powder XRD, ICP-MS, TGA, FTIR, solid state NMR spectroscopy, BET, BJH and HR-SEM. See DOI: 10.1039/d0gc04410e |
| This journal is © The Royal Society of Chemistry 2021 |