Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aqueous one-pot synthesis of well-defined zwitterionic diblock copolymers by RAFT polymerization: an efficient and environmentally-friendly route to a useful dispersant for aqueous pigments†
Shannon M.
North
 and
Steven P.
Armes
and
Steven P.
Armes
 *
*
Department of Chemistry, University of Sheffield, Dainton Building, Brook Hill, Sheffield, South Yorkshire S3 7HF, UK. E-mail: s.p.armes@shef.ac.uk
First published on 25th January 2021
Abstract
Various examples of well-defined zwitterionic diblock copolymers have been reported in the literature. However, synthetic routes to such copolymers have almost invariably involved protecting group chemistry and/or multi-step syntheses. Herein we use reversible addition–fragmentation chain transfer (RAFT) polymerization to develop an atom-efficient, wholly aqueous one-pot synthesis of zwitterionic diblock copolymers comprising anionic methacrylic acid (MAA) and cationic 2-(dimethylamino)ethyl methacrylate (DMA) repeat units. Empirically, we find that polymerizing DMA first leads to a more well-defined block architecture and a narrower molecular weight distribution as judged by 1H NMR spectroscopy and gel permeation chromatography, respectively. Aqueous electrophoresis studies indicate that the isoelectric point (IEP) exhibited by such zwitterionic diblock copolymers in aqueous solution can be tuned by varying the relative proportions of the anionic and cationic comonomers. The convenient removal of trithiocarbonate-based RAFT end-groups can be achieved using aqueous hydrazine, with subsequent macroscopic precipitation of the crude zwitterionic diblock copolymer at its IEP facilitating a highly convenient wholly aqueous work-up. This augurs well for potential applications of these fascinating materials. In this context, we show that such zwitterionic diblock copolymers serve as highly effective dispersants for nano-sized transparent yellow iron oxide nanoparticles, a notoriously problematic aqueous pigment.
Introduction
Zwitterionic polymers, also known as polyampholytes, contain both cationic and anionic monomer repeat units.1–7 Thus they differ from polybetaines, which possess anionic and cationic groups within the same repeat unit.8–11 Unlike polybetaines – and indeed zwitterionic statistical copolymers12 – many zwitterionic diblock copolymers, sometimes known as ‘block polyampholytes’, exhibit an isoelectric point (IEP) in aqueous solution owing to charge compensation. Such copolymers are soluble in their cationic form below this IEP, become insoluble in their neutral form at around the IEP and redissolve (or redisperse) in their anionic form above the IEP. In principle, zwitterionic diblock copolymers offer potential applications in protein purification,13 ion exchange,14 trace metal chelation,15 and sewage treatment.16 Interestingly, zwitterionic diblock copolymers comprising poly(methacrylic acid) (PMAA) and poly(2-(dimethylamino)ethyl methacrylate) (PDMA) have been shown to act as so-called ‘universal’ pigment dispersants for aqueous pigment dispersions owing to their ability to confer colloidal stability via electrosteric stabilization.6,17,18 More specifically, Creutz and co-workers prepared zwitterionic PMAA-PDMA diblock, random and tapered copolymers via anionic polymerization at −78 °C. These three copolymers were subsequently evaluated as putative dispersants for an iron oxide red pigment, a diketopyrrolopyrrole red pigment, and a copper phthalocyanine blue pigment.18 The well-defined diblock copolymer architecture proved to be the most effective, while the random copolymer exhibited the poorest dispersant performance. Thus ‘blockiness’ appears to be a prerequisite for efficient pigment dispersion, presumably because this results in stronger anchoring of the copolymer chains at the surface of the pigment particles. In a related study, Creutz and Jérôme evaluated PMAA-PDMA diblock copolymers as dispersants for alumina-coated titanium dioxide particles.17 In this case, efficient stabilization was achieved at just 0.3% dispersant relative to the mass of pigment. Again, ‘blockiness’ was demonstrated to be an important criterion. Moreover, longer PDMA anchoring blocks produced better-quality dispersions offering higher color strength and considerably lower viscosity compared to a reference commercial formulation.17Unfortunately, the traditional synthesis of well-defined zwitterionic diblock copolymers is synthetically demanding and typically requires protecting group chemistry for the anionic block.19 Indeed, Creutz and co-workers employed protecting group chemistry to prepare their PMAA-PDMA diblock copolymers: t-butyl methacrylate was used to prepare the polyacid block, with the t-butyl group being subsequently removed via acid hydrolysis. Similarly, Kamachi et al. copolymerized 2-vinylpyridine with either trimethylsilyl methacrylate or tert-butyl acrylate via sequential monomer addition using living anionic polymerization to produce zwitterionic diblock copolymers after appropriate deprotection.20,21 In related work, first Patrickios et al.4 and later Lowe and co-workers22 prepared PDMA-PMAA diblock copolymers via group transfer polymerization, with 2-tetrahydropyranyl methacrylate being used as a protecting group for the polyacid block. Subsequently, Liu et al. prepared poly(4-vinylbenzoic acid)-poly(2-(diethylamino)ethyl methacrylate) diblock copolymers via ATRP using protecting group chemistry for the polyacid block.5 Such weak polyacid/weak polybase copolymers exhibit so-called ‘schizophrenic’ behavior: they form either cationic or anionic micelles in aqueous solution via micelle inversion on switching the solution pH from 2 to 10. Apart from the prohibitive cost of such multi-step syntheses, removal of protecting ester groups to generate the acidic block can lead to broader molecular weight distributions via formation of intermolecular cross-links, yielding ill-defined branched architectures.22 A more atom-efficient approach was reported by Bories-Azeau and co-workers,23 who synthesized a series of poly(tertiary amine methacrylate)-poly(2-hydroxyethyl methacrylate) diblock copolymers using atom transfer radical polymerization (ATRP). The hydroxyl groups on these precursors were subsequently reacted with succinic anhydride under mild conditions to introduce the desired acid functionality. Nevertheless, this two-step route involved organic solvents, excess reagents and a relatively long reaction time (48 h) for the second step.
In principle, controlled radical polymerization techniques such as ATRP,24,25 nitroxide-mediated polymerization (NMP)26,27 and reversible addition–fragmentation chain transfer (RAFT) polymerization28,29 should enable the direct synthesis of zwitterionic diblock copolymers without requiring any protecting group chemistry. An early example of such an approach was reported by Gabaston et al.,30 who utilized NMP to prepare block copolymers of poly(sodium 4-styrenesulfonate) and poly(4-(dimethylamino)methylstyrene) in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol-water mixture at 120 °C. The use of RAFT polymerization to directly prepare PDMA-PMAA diblock copolymers has also been reported,1,31,32 albeit using one or more organic solvents. For example, Xin et al. used a two-step synthetic protocol, with the polybase precursor being prepared in anisole in 84–86% yield and the final zwitterionic diblock copolymers being obtained directly in a 4
1 ethylene glycol-water mixture at 120 °C. The use of RAFT polymerization to directly prepare PDMA-PMAA diblock copolymers has also been reported,1,31,32 albeit using one or more organic solvents. For example, Xin et al. used a two-step synthetic protocol, with the polybase precursor being prepared in anisole in 84–86% yield and the final zwitterionic diblock copolymers being obtained directly in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v methanol–water mixture.1 As expected, such copolymers exhibited rich aqueous solution behavior, including macroscopic precipitation close to their IEP.
3 v/v methanol–water mixture.1 As expected, such copolymers exhibited rich aqueous solution behavior, including macroscopic precipitation close to their IEP.
Over the past decade or so, polymerization-induced self-assembly (PISA) has become widely recognized as a powerful technique for the synthesis of a wide range of block copolymer nano-objects.33–35 Indeed, Canning et al. recently reported the PISA synthesis of zwitterionic diblock copolymers in the form of sterically-stabilized nanoparticles using an aqueous formulation.3 In this case, rhodamine and fluorescein labels were incorporated into the polybase and polyacid blocks respectively to produce self-reporting pH-responsive nanoparticles. However, in this prior study the poly(2-(diethylamino)ethyl methacrylate precursor was prepared separately in toluene and extensively purified prior to its chain extension. In contrast, we report herein the first wholly aqueous one-pot synthesis of zwitterionic PDMA-PMAA diblock copolymers using RAFT solution polymerization (see Scheme 1). The resulting copolymers are characterized by 1H NMR spectroscopy, GPC analysis and their aqueous solution properties are studied using DLS and aqueous electrophoresis. Moreover, we devise a highly convenient wholly aqueous protocol for the efficient removal of the organosulfur RAFT end-groups from such copolymers which takes advantage of the macroscopic precipitation that occurs at their isoelectric point. Finally, the effectiveness of such copolymers as dispersants for a somewhat problematic nano-sized transparent yellow iron oxide pigment is briefly evaluated.
 | ||
| Scheme 1 Wholly aqueous one-pot synthetic route to zwitterionic diblock copolymers via RAFT solution polymerization, where the first block comprises (protonated) PDMA and the second block is PMAA. Given its pKa of 8.44, most of the DMA monomer units are assumed to be protonated when the solution pH is adjusted to pH 7.0 using HCl.38 | ||
Experimental
Materials
4-Cyano-4-(2-phenylethanesulfanylthiocarbonyl)sulfanylpentanoic acid (PETTC) was synthesized as previously reported.36,37 2-(Dimethylamino)ethyl methacrylate (DMA), trimethylsilyldiazomethane (supplied as a 2.0 M solution in diethyl ether) and hydrazine hydrate (reagent grade, 50–60% in water) were purchased from Sigma-Aldrich (Dorset, UK) and were used as received. Methacrylic acid (MAA) was purchased from Merck (Germany) and was used as received. 2,2′-Azobis(2-(2-imidazolin-2-yl)propane) dihydrochloride (VA-044) was purchased from Wako Pure Chemical Industries (Japan). 4,4′-Azobis(4-cyanovaleric acid) (ACVA; 98%) and 2,2,3,3-d(4)-3-(trimethylsilyl)propionic acid sodium salt was purchased from Alfa Aesar (Heysham, UK) and was used as received. CD3OD and CD2Cl2 were purchased from Goss Scientific Instruments Ltd (Cheshire, UK). CDCl3, D2O, sodium deuteroxide (NaOD) and deuterium chloride (DCl) were purchased from Sigma-Aldrich (Dorset, UK). All other solvents were purchased from Fisher Scientific (Loughborough, UK) and were used as received. Deionized water was used for all experiments and the solution pH was adjusted using either HCl or NaOH. Finally, transparent yellow iron oxide (Lanox 8916) pigment and eChem DF1519 defoamer were kindly provided by The Lubrizol Corporation (Manchester, UK).One-pot synthesis of poly(2-(dimethylamino)ethyl methacrylate)-poly(methacrylic acid) (PDMA-PMAA) diblock copolymer
A typical protocol for the synthesis of a PDMA50-PMAA50 zwitterionic diblock copolymer was conducted as follows: DMA (4.0 g, 25.4 mmol), PETTC (0.17 g, 0.51 mmol), VA-044 (32.9 mg, 0.10 mmol), and deionized water (9.81 g) were added to a 100 ml two-necked round-bottomed flask. 36% HCl (2.18 g, 25.4 mmol) was added to protonate the DMA monomer. This aqueous reaction mixture was then purged for 30 min with nitrogen and heated to 44 °C. In a separate vial, MAA (2.19 g, 25 mmol), VA-044 initiator (55 mg, 0.51 mmol) and water (15.9 g) were purged with nitrogen for 30 min. The DMA polymerization had reached approximately full conversion after 3 h, as determined by 1H NMR spectroscopy. Then the degassed aqueous solution containing monomer and initiator was added under a nitrogen atmosphere. The MAA polymerization was allowed to proceed for 5 h at 44 °C. A final MAA conversion of more than 99% was achieved as determined by 1H NMR, yielding a low-viscosity yellow solution at pH 2. The amounts of DMA and/or MAA, and the PETTC concentration were adjusted accordingly when targeting other copolymer compositions. Depending on the target diblock composition, relatively viscous transparent yellow solutions can be obtained.One-pot synthesis of poly(methacrylic acid)-poly(2-(dimethylamino)ethyl methacrylate) (PMAA-PDMA) diblock copolymer
A typical protocol for the synthesis of a PMAA50-PDMA50 (target DP) zwitterionic diblock copolymer was conducted as follows: MAA (1.0 g, 11.6 mmol), PETTC (78.9 mg, 0.23 mmol), ACVA (13 mg, 46.4 μmol) and deionized water (6.19 g) were added to a 50 mL two-necked round-bottomed flask. This aqueous reaction mixture was then purged for 30 min with nitrogen and heated to 70 °C. In a separate vial, DMA (1.83 g, 11.6 mmol), ACVA (25 mg, 77.4 μmol), NaOH (0.46 g, 11.6 mmol) and water (5.53 g) were purged with nitrogen gas for 30 min. The MAA polymerization had reached approximately full conversion after 3 h, as determined by 1H NMR spectroscopy. Then the degassed aqueous solution containing DMA monomer and ACVA initiator was added under a nitrogen atmosphere. The DMA polymerization was allowed to proceed for 9 h at 70 °C (final solution pH = 8.5). A final DMA conversion of 95% was reached as determined by 1H NMR analysis, yielding a viscous yellow dispersion. The amounts of DMA and/or MAA, and the PETTC or CPDB concentration were adjusted accordingly when targeting other copolymer compositions.Aqueous gel permeation chromatography (GPC)
Molecular weight distributions of diblock copolymers or homopolymers were analysed in a basic aqueous buffer (pH 10) containing 1 M NaNO3 solution (adjusted to pH 10 with concentrated NaOH) at a flow rate of 1.0 mL min−1. The GPC set-up comprised an Agilent 1260 Infinity series degasser and pump, an Agilent PL Aquagel-OH 30 8 μm column and an Agilent PL Aquagel-OH 40 8 μm column. Calibration was conducted using a series of near-monodisperse PEO standards ranging from 600 g mol−1 to 969![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. Chromatograms were analyzed using Agilent GPC/SEC software.
000 g mol−1. Chromatograms were analyzed using Agilent GPC/SEC software.
THF gel permeation chromatography (GPC)
The THF GPC set-up comprised two 5 μm (30 cm) Mixed C columns and a WellChrom K-2301 refractive index detector operating at a wavelength of 950 ± 30 nm. The mobile phase contained 2.0% v/v triethylamine and 0.05% w/v butylhydroxytoluene and the flow rate was 1.0 mL min−1. A series of ten near-monodisperse poly(methyl methacrylate) standards (Mp values ranging from 645 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480
480![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) were used for calibration. Chromatograms were analyzed using Agilent GPC/SEC software.
000 g mol−1) were used for calibration. Chromatograms were analyzed using Agilent GPC/SEC software.
Dynamic light scattering
Dilute (0.10% w/w) aqueous copolymer dispersions were analyzed at 25 °C using a Malvern NanoZS instrument. Scattered light was detected at 173° and hydrodynamic diameters were calculated using the Stokes–Einstein equation, which assumes dilute non-interacting spheres. Data were averaged over three consecutive measurements comprising eleven runs per measurement.Aqueous electrophoresis
Zeta potentials were calculated from electrophoretic mobilities using a Malvern NanoZS instrument. Measurements were recorded on 0.05–0.10% w/w copolymer solutions as a function of pH in the presence of 1 mM KCl background salt and averaged over 20 runs. In each case the solution pH was gradually lowered by adding 0.1 M HCl.1H NMR spectroscopy
All 1H NMR spectra were recorded using a 400 MHz Bruker Avance-400 spectrometer. The NMR solvent was CD3OD or D2O and typically 64 scans were averaged per spectrum.For in situ NMR studies during the synthesis of PDMA50 homopolymer at 40% w/w, the reaction mixture was prepared as described above (albeit with D2O being used as a solvent rather than H2O) and a 0.75 mL aliquot was placed into an NMR tube equipped with a J-Young tap. The D2O in the reaction mixture was used as the lock solvent. This NMR tube assembly was inserted in a Bruker AVANCE III HD spectrometer operating at 500.13 MHz (1H frequency) and a reference spectrum was recorded at 25 °C (no polymerization) prior to heating up to 44 °C. Spectra were recorded every 5 min for 4 h. All spectra were phase-adjusted and baseline-corrected using Bruker TopSpin 3.1 software. DMA conversions were determined by comparing integrated monomer and polymer signals relative to the aromatic signals from the PETTC RAFT agent. 2,2,3,3-d(4)-3-(trimethylsilyl)propionic acid sodium salt (15 mg) was used as a reference signal at 0.0 ppm.
UV spectroscopy
Absorption spectra were recorded between 200 and 800 nm at 25 °C using a PC-controlled Shimadzu UV-1800 spectrophotometer equipped with a 1.0 cm path length quartz cell. End-group removal was recorded at a copolymer concentration of 0.20% w/w, recording spectra at regular intervals over 3.5 h.Removal of trithiocarbonate end-groups
Hydrazine hydrate (1.02 mL of a 50% w/w aqueous solution; 0.0159 mmol) was added to a 0.50% w/w aqueous solution of PDMA50-PMAA50 copolymer (40.0 mg, 0.0159 mmol; hydrazine/trithiocarbonate molar ratio = 1.0) in deionized water at pH 9 and 20 °C. The solution pH was then adjusted to the IEP (approximately pH 6.0) to induce precipitation. The aqueous supernatant was carefully decanted and the crude precipitate was then washed three times with deionized water (pH 6) to remove small molecule contaminants generated during removal of the organosulfur-based chain-ends.Helium pycnometry
The solid-state density of transparent yellow iron oxide pigment particles was determined using a calibrated helium pycnometer (Micromeritics AccuPyc 1330 instrument) at 20 °C.Surface area analysis
BET surface area measurements were performed using a Quantachrome Nova 1000e instrument with dinitrogen gas (mean area per molecule = 16.2 Å2) as an adsorbate at 77 K. Transparent yellow iron oxide pigment was degassed under vacuum at 100 °C for at least 16 h prior to analysis. The particle diameter, d, was calculated using the equation d = 6/(ρ × As), where As is the BET specific surface area in m2 g−1 and ρ is the pigment density in g m−3 obtained from helium pycnometry.Milling of transparent yellow iron oxide to produce aqueous pigment dispersions
Lanox 8916 pigment (1.00 g) was added in turn to fifty-four 14 mL Trident vials. Varying amounts of copolymer were added, the dispersion pH was adjusted using 0.5 M HCl or 0.5 M NaOH, and the final volume was made up to 10 mL with deionized water. Defoamer (eChem DF1519, 0.10 mL) and 17 g of 3 mm glass beads were then added before sealing each vial. These vials were then placed on a high energy shaker for 16 h prior to analysis.Dispersion viscosity measurements
An AR-G2 rheometer equipped with a variable temperature Peltier plate and a 40 mm 2° aluminium cone was used for all experiments. The strain was fixed at 1.0%, and a rotational mode was used to measure the dispersion viscosity in Pa s as a function of shear rate. Viscosities were recorded at shear rates between 0.1 and 1000 s−1 with each measurement equilibrated at 25 °C. Viscosity measurements were compared at a constant shear rate of 34 s−1.Transmission electron microscopy (TEM)
A 10 μL droplet of diluted copolymer-stabilised pigment dispersion (0.1% solids) was placed onto a glow discharge-treated carbon-coated copper/palladium TEM grid (Agar Scientific, UK) for 30 seconds. Excess solution was then removed carefully using filter paper. To ensure sufficient contrast, a 10 μL droplet of 0.75% w/w aqueous uranyl formate staining solution was then placed onto the dried grid for 30 seconds prior to careful drying using a vacuum hose. TEM images were recorded using a Philips CM100 instrument operating at 100 kV and equipped with a Gatan 1k CCCD camera.Results and discussion
In principle, the one-pot synthesis of zwitterionic diblock copolymers in aqueous solution should be feasible. In this context, an important question is whether the order of monomer addition makes any difference to the outcome.A one-pot protocol utilizing PDMA as the first block was optimized as follows: firstly, a PDMA50 precursor was prepared at 40% w/w solids by RAFT aqueous solution polymerization of DMA at 44 °C using PETTC and VA-044 initiator, see Scheme 1.
The initial solution pH was lowered from 9.5 to 7.0 prior to polymerization by adding 35% HCl; this adjustment is required to suppress in situ hydrolysis of the DMA monomer, which would otherwise afford 2-(dimethylamino)ethanol and MAA.391H NMR studies indicated that this side reaction produced approximately 1.5% MAA residues within the PDMA chains using the above conditions. However, this is significantly lower than that reported by Carlsson et al.39 who observed more than 7% hydrolysis within 2 h when conducting the RAFT aqueous solution polymerization of DMA at 70 °C. Presumably, hydrolysis is minimized by the lower reaction temperature selected in the present study. After 3 h at 44 °C, the DMA polymerization had reached approximately full conversion. Empirically, we found that the subsequent MAA polymerization is best performed at pH 1.5–2.0 (see Scheme 1). This suppresses ionization of the acidic PMAA block, which possesses essentially neutral character under such conditions. Moreover, at this low pH the PDMA precursor also remains stable towards further hydrolysis during the MAA polymerization.38 This is because the polybasic PDMA precursor block is fully protonated under such conditions, which also ensures its aqueous solubility as a cationic polyelectrolyte.
Then MAA was added to the reaction mixture and the ensuing polymerization proceeded to approximately 99% conversion to produce a well-defined PDMA50-PMAA50 diblock copolymer with a relatively narrow molecular weight distribution (Mn = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Mw/Mn = 1.23). The conversion vs. time curves obtained from in situ NMR studies (see Fig. 1 and S2†) confirm that high conversions can be obtained for each block within 8 h at 44 °C. Similarly, in situ NMR studies during the synthesis of PDMA100 and PDMA200 precursor blocks at 40% w/w solids indicate that essentially full monomer conversion is achieved within 3.5 h and 4 h, respectively (Fig. S1, S2 and S3†).
000 g mol−1, Mw/Mn = 1.23). The conversion vs. time curves obtained from in situ NMR studies (see Fig. 1 and S2†) confirm that high conversions can be obtained for each block within 8 h at 44 °C. Similarly, in situ NMR studies during the synthesis of PDMA100 and PDMA200 precursor blocks at 40% w/w solids indicate that essentially full monomer conversion is achieved within 3.5 h and 4 h, respectively (Fig. S1, S2 and S3†).
 | ||
| Fig. 1 Selected 1H NMR spectra recorded in D2O at the start (blue) and end (green) of the RAFT aqueous solution polymerization of DMA and the start (red) and end (pink) of the subsequent MAA polymerization (see Scheme 1); 2,2,3,3-d(4)-3-(trimethylsilyl)propionic acid sodium salt (labelled as ‘TMS’) is used to provide a reference at 0 ppm. | ||
A summary of the overall comonomer conversions, molecular weights and isoelectric points (IEP) is provided in Table 1. In each case, high conversions (typically at least 99%) and relatively low dispersities (Mw/Mn < 1.32) are obtained. As expected for such zwitterionic diblock copolymers,4,22 the IEP can be adjusted simply by varying the relative proportions of the DMA and MAA comonomers.
| Entry | Diblock copolymer composition | PDMA 1H NMR conversion (%) | PMAA 1H NMR conversion (%) | THF GPC (PDMA homopolymer) | Aqueous GPC (PDMA-PMAA diblock) | IEP | ||
|---|---|---|---|---|---|---|---|---|
| M n (g mol−1) | M w/Mn | M n (g mol−1) | M w/Mn | |||||
| 1 | PDMA49-PMAA100 | >99 | >99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.21 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.27 | 5.4 |
| 2 | PDMA200-PMAA200 | >99 | >99 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.29 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.25 | 6.6 |
| 3 | PDMA50-PMAA50 | 99 | >99 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.29 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.23 | 6.7 |
| 4 | PDMA100-PMAA50 | 99 | >99 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.12 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.32 | 8.6 |
In principle, these zwitterionic diblock copolymers can also be prepared by polymerizing the MAA monomer first. Accordingly, a series of such RAFT aqueous solution polymerizations were conducted under the conditions outlined in Scheme S1† (see Table S1†). However, MAA conversions remained incomplete (89–97%), despite performing these syntheses at 70 °C for up to 6 h. In this case, a solution pH of 8.5 was required to prevent phase separation during the polymerization. Furthermore, these reaction conditions led to significant hydrolysis of DMA (up to 7.3%). Moreover, discoloration of the final polymer solution (from yellow to brown) was observed during the second-stage polymerization, which suggests premature loss of the RAFT chain-ends. GPC studies indicated relatively high blocking efficiencies and unimodal, reasonably narrow molecular weight distributions (see Fig. S4†). Nevertheless, bearing in mind the above disadvantages it is clear that the preferred order of monomer addition for the synthesis of well-defined zwitterionic diblock copolymers under the stated reaction conditions is DMA first, rather than MAA first. Thus, all of the copolymers characterized in the rest of this study were prepared using this optimized protocol.
1H NMR spectroscopy can be used to assess changes in the degree of solvation of each block for a representative PDMA50-PMAA50 zwitterionic diblock copolymer, see Fig. 2. At pH 6, almost no NMR signals can be observed for this copolymer owing to macroscopic precipitation at its IEP. However, addition of sufficient background salt at the same pH screens the electrostatic attractions between the cationic and anionic blocks, which prevents macroscopic precipitation. Under such conditions, NMR signals assigned to the protonated PDMA block (see a, b and c) can be observed. In contrast, the ionized PMAA block exhibits barely exhibits any unique NMR signals because its methacrylic backbone signals overlap with those of the PDMA block. However, there is some evidence for a weak broad signal e at around 1.7 ppm; this is assigned to the two methylene backbone protons associated with the anionic carboxylate form of the MAA repeat units.
At pH 2 (DCl/D2O), the PDMA block is fully protonated whereas ionization of the PMAA block is suppressed. Hence signals a, b and c observed at 2.90, 3.51 and 4.32 ppm, respectively are assigned to the former block while the methacrylic backbone signals f and g (at 1.0 and 1.9 ppm, respectively) are also prominent. However, the methacrylic backbone signals e and d for the neutral PMAA block at 0–2.5 ppm are suppressed. At pH 9 (NaOD/D2O), the PMAA block becomes ionized and acquires highly anionic character, whereas the PDMA block becomes deprotonated (while remaining partially solvated). As expected, some of the NMR signals assigned to the PDMA block are shifted to lower δ values: in particular, a, b, and c now appear at 2.23, 2.63 and 4.08 ppm. The methacrylic backbone protons d and e assigned to the anionic PMAA block are now much more solvated and appear at around 0.9 and 1.6 ppm, respectively. The PDMA signals f and g remain visible at δ 1.0 and 1.9 ppm. As expected, the NMR shifts for these methacrylic backbone signals are less sensitive to the degree of protonation of the PDMA block than the oxymethylene, azamethylene and methyl proton signals associated with the pendent 2-(dimethylamino)ethyl groups.
At the IEP, the mean number of cationic and anionic charges per chain are equal. Thus, the zwitterionic copolymer exhibits no overall charge and is typically water-insoluble under such conditions.4,22 The IEP can be determined from aqueous electrophoresis studies while DLS can be used to assess the colloid instability window, as illustrated in Fig. 3 for three PDMA-PMAA zwitterionic diblock copolymers. Moreover, the IEPs exhibited by such copolymers can be tuned from pH 5.4 to 8.6 by systematic variation of the relative proportions of the DMA and MAA comonomers. More specifically, increasing the DMA mol% of such copolymers leads to higher IEPs. These findings are in good agreement with those reported by Lowe et al.,22 who observed an IEP of 6.74 for a PDMA-PMAA zwitterionic diblock copolymer comprising 50 mol% DMA.
RAFT end-group removal
Various methods for removing RAFT end-groups have been reported in the literature.40–42 One of the most common approaches involves addition of excess free radical initiator.40,43 However, this is a highly atom-inefficient process that introduces various impurities and by-products.43 In the present study, removal of the trithiocarbonate end-groups from a PDMA50-PMAA50 diblock copolymer was achieved within 3.5 h at 20 °C using a weakly basic aqueous solution of hydrazine hydrate (pH 9) at a hydrazine/trithiocarbonate molar ratio of 1.0.44 UV spectroscopy was used to monitor the disappearance of the trithiocarbonate band at 314 nm. The progressive reduction in absorbance at this wavelength over time is shown in Fig. 4a. However, the decaying signal never reaches the baseline. At first sight, this suggests that end-group removal remains incomplete under such conditions. However, this is simply an artefact caused by the appearance of a new band at approximately 304 nm, which is assigned to UV-active small-molecule by-products generated during chain-end removal. Similar observations were reported by Jesson et al. when using UV spectroscopy to monitor the removal of dithiobenzoate end-groups using excess H2O2.45 Fortunately, this technical problem can be circumvented by using UV GPC (THF eluent; λ = 314 nm; after exhaustive methylation of the PMAA block using excess trimethylsilyldiazomethane) to monitor the extent of end-group removal, because this technique leads to fractionation of the copolymer species from the small-molecule by-products prior to detection. Comparison of the UV signals for the diblock copolymer chains before and after end-group removal using hydrazine indicates substantial loss (approximately 98%) of the original trithiocarbonate end-groups. Moreover, the insolubility of such zwitterionic diblock copolymers at around their IEP enables their purification after hydrazine derivatization using a wholly aqueous work-up. Thus, after hydrazine treatment of an initially yellow PDMA50-PMAA50 copolymer at pH 9 to remove its end-groups, the solution pH was lowered to the isoelectric point of the zwitterionic diblock copolymer using 0.25 M HCl to induce macroscopic precipitation. The insoluble crude copolymer was then washed four times using deionized water (pH 6) to remove the small-molecule by-products. The resulting purified white copolymer was then analyzed by 1H NMR spectroscopy in CD3OD to confirm disappearance of the aromatic signals at 7–8 ppm (data not shown). According to the literature,46 the end-group on the PMAA chains after hydrazine treatment is likely to be a thiol. However, further studies are required to confirm this hypothesis. In principle, this organic solvent-free end-group removal protocol should be well-suited for industrial scale-up.Dispersion of transparent yellow iron oxide pigment
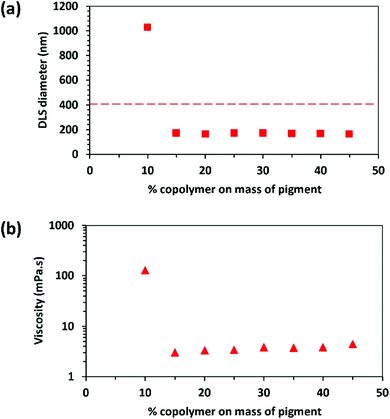
The effectiveness of PDMA50-PMAA50 as a dispersant for transparent yellow iron oxide was investigated. This pigment was deliberately selected because it is known to be technically challenging to achieve a high degree of dispersion in aqueous media: the primary particles are completely unstable at pH 5–7 and form large flocs. In the absence of any suitable dispersant, the apparent DLS diameter is approximately 0.9 μm at pH 9.5 and 2.1 μm at pH 3.5. However, according to the pigment manufacturer, the BET specific surface area of this pigment is 108–120 m2 g−1 and our helium pycnometry measurements indicate a solid-state density of 4.21 g cm−3. Assuming that the pigment particles are non-porous, this indicates a primary grain diameter of around 12–13 nm. TEM studies confirm that this pigment has a distinctive ‘rice grain’ morphology. This should be borne in mind when interpreting DLS data because this particle sizing technique reports a sphere-equivalent hydrodynamic diameter. Aqueous dispersions containing the pigment and a zwitterionic diblock copolymer dispersant were subjected to high energy mixing for 16 h; this is a common industrial protocol to assess the performance of a new dispersant. PDMA50-PMAA50 was used as a dispersant at pH 8.5 and pH 4.0, which correspond to either side of its IEP at pH 6.7.At pH 8.5, the PDMA block is deprotonated and hence has neutral character, while the PMAA block is ionized and so acquires anionic character. In principle, the latter block should act as a steric stabilizer, while the neutral PDMA chains adsorb onto the surface of the pigment particles. For this particular pigment, if the apparent DLS diameter for the final aqueous dispersion is below 400 nm then the copolymer is considered to be an effective dispersant. This particle size represents a safety margin on the standard minimum size of 500 nm required for inkjet applications to ensure that such formulations pass through a printer nozzle head without blocking it. DLS studies of the aqueous copolymer/pigment dispersions suggest that this copolymer acts as a good dispersant at pH 8.5, with an optimum concentration of approximately 25% copolymer with respect to pigment being observed (Fig. 5).
Moreover, the sphere-equivalent diameter of 118 nm for the pigment particles is well below the minimum threshold of 400 nm, indicating a sufficiently high degree of dispersion. Using these data, we calculate an upper limit adsorbed amount of approximately 2.9 mg m−2 for the copolymer chains on the pigment particles, which is a physically realistic value.
Dispersant performance can also be assessed by viscosity measurements: a minimum in dispersion viscosity at a fixed pigment concentration is known as the ‘surfactant demand’ of the pigment.47 This is where the particle are assumed to be fully dispersed. Excess surfactant – or, in this case, copolymer dispersant – can cause depletion flocculation, which increases the dispersion viscosity.47 This is because some of the dispersant is located in the continuous phase, as well as adsorbed at the surface of the pigment particles.48 On the other hand, adding too little dispersant leads to bridging flocculation because the particles are not sufficiently coated (submonolayer coverage).
The sphere-equivalent DLS diameter is 206 nm when using 10% copolymer based on pigment, which is still well below the minimum acceptable diameter. However, this dispersion is relatively viscous, which suggests an unstable dispersion. This is confirmed by TEM studies, which indicate the presence of large flocs (see Fig. 6a). This suggests bridging flocculation owing to insufficient dispersant. In contrast, the pigment particles are well dispersed when the mass of copolymer relative to pigment is increased to 25% (see Fig. 6b), with both DLS and viscosity data suggesting an optimum degree of dispersion under such conditions. However, using 50% copolymer based on pigment leads to the formation of relatively large aggregates (see Fig. 6c). In this case, it is hypothesized that free, non-adsorbed copolymer chains leads to a depletion flocculation mechanism.49,50 This is consistent with the relatively large apparent particle diameter reported by DLS and a correspondingly high dispersion viscosity (45 mPa s).
Similar pigment dispersion experiments were also performed at pH 4.0, which is below the copolymer IEP. Under these conditions, the PMAA block is in its neutral form, whereas the PDMA block is protonated and hence acquires cationic character. In this case, the cationic block most likely acts as the steric stabiliser, while the PMAA block adsorbs at the surface of the pigment particles.
DLS and viscosity measurements suggest that this copolymer dispersant is effective over a wider range of concentrations at pH 4.0, with any copolymer concentration above 15% based on the mass of pigment resulting in a stable dispersion (Fig. 7). The minimum dispersion viscosity is 3.3 mPa s, which is comparable to the minimum value of 3.2 mPa s observed at pH 8.5. However, DLS studies indicate an apparent minimum diameter of 165 nm, which is somewhat larger than that achieved at pH 8.5.
TEM studies confirm the presence of flocs when using 10% copolymer at pH 4.0, suggesting a bridging flocculation mechanism (Fig. 8a). However, TEM images recorded when using either 15% or 25% copolymer relative to pigment mass (Fig. 8b and c) indicate that a high degree of dispersion is achieved under these conditions. In this case, there is no upturn in either the diameter or viscosity. This suggests that either there is no depletion flocculation or that this mechanism operates at a much higher copolymer concentration.
Aqueous electrophoresis studies were conducted on bare pigment particles, the PDMA50-PMAA50 copolymer alone, and copolymer-dispersed pigment particles prepared using the optimum copolymer concentration to minimize the presence of non-adsorbed copolymer chains (Fig. 9). The zeta potential curve obtained for the dispersed pigment particles is similar to that observed for the copolymer alone and differs significantly from that obtained for the pigment alone. This provides good evidence for adsorption of the copolymer chains at the surface of the pigment particles.
Two other zwitterionic diblock copolymers were also assessed as putative dispersants for the transparent yellow iron oxide pigment. Again, the dispersion pH was selected to be either above and below the IEP of the copolymer in question (see Table S2†). PDMA49-PMAA100 also acts as an effective copolymer dispersant, with a relatively low apparent pigment particle diameter of 201 nm being achieved below the copolymer IEP. This suggests that the neutral PMAA100 block acts as an effective anchor. However, the smallest particle size that could be reached above the copolymer IEP was 369 nm, which is only just below the minimum acceptable diameter. Similarly, using a PDMA100-PMAA50 copolymer produced a high degree of dispersion at pH 10.5 but only a very poor degree of dispersion at pH 5.0.
These results highlight the importance of optimizing both the zwitterionic diblock copolymer composition and also the dispersion pH to achieve the highest possible degree of pigment dispersion. In particular, the PDMA50-PMAA50 copolymer seems to be a particularly promising dispersant for transparent yellow iron oxide particles, since it led to the smallest apparent particle diameter and lowest dispersion viscosity. Moreover, a high pH is normally required for pigment dispersion when formulating aqueous inkjet inks, making this copolymer well-suited for potential industrial use.
Conclusions
In summary, we report the first one-pot synthesis of zwitterionic diblock copolymers via RAFT solution polymerization. Such syntheses are wholly aqueous, highly efficient and do not require any protecting group chemistry. Aqueous GPC analysis indicates relatively narrow molecular weight distributions for such zwitterionic diblock copolymers (Mw/Mn < 1.30) and systematic variation of the copolymer composition enables the isoelectric point to be readily tuned. Moreover, we have exploited the aqueous insolubility behavior exhibited by such zwitterionic diblock copolymers at their isoelectric point to devise a wholly aqueous protocol for the removal of trithiocarbonate-based RAFT end-groups using a stoichiometric reagent (hydrazine). Finally, it is demonstrated that PDMA50-PMAA50 can act as an effective dispersant for nano-sized transparent yellow iron oxide particles in aqueous formulations. In summary, this attractive new synthetic route to zwitterionic diblock copolymers is well-suited to industrial scale-up with promising results being obtained for their use as a commercial pigment dispersant.Conflicts of interest
There are no conflicts to declare for this work.Acknowledgements
EPSRC (EP/L016281) and The Lubrizol Corporation (Blackley, UK) are thanked for funding a CDT PhD studentship for S. M. N. EPSRC is also acknowledged for a four-year Established Career Particle Technology Fellowship for S. P. A. (EP/R003009). Dr E. Coulbeck is thanked for preparing the aqueous pigment dispersions at The Lubrizol Corporation (Blackley, UK).Notes and references
- X. Xin, Y. Wang and W. Liu, Eur. Polym. J., 2005, 41, 1539 CrossRef CAS.
- V. Bütün, S. Liu, J. V. M. Weaver, X. Bories-Azeau, Y. Cai and S. P. Armes, React. Funct. Polym., 2006, 66, 157 CrossRef.
- S. L. Canning, T. J. Neal and S. P. Armes, Macromolecules, 2017, 50, 6108 CrossRef CAS.
- C. S. Patrickios, W. R. Hertler, N. L. Abbott and T. A. Hatton, Macromolecules, 1994, 27, 930 CrossRef CAS.
- S. Liu and S. P. Armes, Angew. Chem., Int. Ed., 2002, 41, 1413 CrossRef CAS.
- A. B. Lowe and C. L. McCormick, Chem. Rev., 2002, 102, 4177 CrossRef CAS.
- T. L. Sun, T. Kurokawa, S. Kuroda, A. Bin Ihsan, T. Akasaki, K. Sato, M. A. Haque, T. Nakajima and J. P. Gong, Nat. Mater., 2013, 12, 932 CrossRef CAS.
- A. Laschewsky, Polymers, 2014, 6, 1544 CrossRef.
- K. E. B. Doncom, N. J. Warren and S. P. Armes, Polym. Chem., 2015, 6, 7264 RSC.
- C. L. McCormick and A. B. Lowe, Acc. Chem. Res., 2004, 37, 312 CrossRef CAS.
- L. D. Blackman, P. A. Gunatillake, P. Cass and K. E. S. Locock, Chem. Soc. Rev., 2019, 48, 757 RSC.
- G. Ehrlich and P. Doty, J. Am. Chem. Soc., 1954, 76, 3764 CrossRef CAS.
- T. Chakrabarty, M. Kumar and V. K. Shahi, Ind. Eng. Chem. Res., 2012, 51, 3015 CrossRef CAS.
- J. Liu, Y. Ma, T. Xu and G. Shao, J. Hazard. Mater., 2010, 178, 1021 CrossRef CAS.
- P. Liu, A. J. Boyle, Y. Lu, J. Adams, Y. Chi, R. M. Reilly and M. A. Winnik, Biomacromolecules, 2015, 16, 3613 CrossRef CAS.
- C. Liu, J. Lee, J. Ma and M. Elimelech, Environ. Sci. Technol., 2017, 51, 2161 CrossRef CAS.
- S. Creutz and R. Jérôme, Prog. Org. Coat., 2000, 40, 21 CrossRef CAS.
- S. Creutz, R. Jérôme, G. M. P. Kaptijn, A. W. Van Der Werf and J. Akkerman, J. Coat. Technol., 1998, 70, 41 CrossRef CAS.
- A. B. Lowe and C. L. McCormick, Prog. Polym. Sci., 2007, 32, 283 CrossRef CAS.
- M. Kamachi, M. Kurihara and J. K. Stille, Macromolecules, 1972, 5, 161 CrossRef CAS.
- V. Butun, N. C. Billingham and S. P. Armes, J. Am. Chem. Soc., 1998, 120, 11818 CrossRef.
- A. B. Lowe, N. C. Billingham and S. P. Armes, Macromolecules, 1998, 31, 5991 CrossRef CAS.
- X. Bories-Azeau, S. P. Armes and H. J. W. Van Den Haak, Macromolecules, 2004, 37, 2348 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS.
- X. Zhang, J. Ma, S. Yang and J. Xu, Soft Mater., 2013, 11, 394 CrossRef CAS.
- J. Nicolas, Y. Guillaneuf, C. Lefay, D. Bertin, D. Gigmes and B. Charleux, Prog. Polym. Sci., 2013, 38, 63 CrossRef CAS.
- L. Couvreur, C. Lefay, J. Belleney, B. Charleux, O. Guerret and S. Magnet, Macromolecules, 2003, 36, 8260 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379 CrossRef CAS.
- J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559 CrossRef CAS.
- L. I. Gabaston, S. A. Furlong, R. A. Jackson and S. P. Armes, Polymer, 1999, 40, 4505 CrossRef CAS.
- Y. Xia, X. Xu, H. Yu, C. Zhou, Z. Nie, J. Yang, J. Qian and H. Ni, Colloid Polym. Sci., 2020, 70 DOI:10.1007/s00396-020-04790-6.
- L. Upadhyaya, M. Semsarilar, R. Fernández-Pacheco, G. Martinez, R. Mallada, I. M. Coelhoso, C. A. M. Portugal, J. G. Crespo, A. Deratani and D. Quemener, Polym. Chem., 2017, 8, 605 RSC.
- S. L. Canning, G. N. Smith and S. P. Armes, Macromolecules, 2016, 49, 1985 CrossRef CAS.
- J. Tan, H. Sun, M. Yu, B. S. Sumerlin and L. Zhang, ACS Macro Lett., 2015, 4, 1249 CrossRef CAS.
- L. D. Blackman, K. E. B. Doncom, M. I. Gibson and R. K. O'Reilly, Polym. Chem., 2017, 8, 2860 RSC.
- M. Semsarilar, V. Ladmiral, A. Blanazs and S. P. Armes, Langmuir, 2012, 28, 914 CrossRef CAS.
- N. J. W. Penfold, J. R. Whatley and S. P. Armes, Macromolecules, 2019, 52, 1653 CrossRef CAS.
- P. van de Wetering, N. J. Zuidan, M. J. van Steenbergen, O. A. G. J. van der Houwen, W. J. M. Underberg and W. E. Hennink, Macromolecules, 1998, 31, 8063 CrossRef CAS.
- L. Carlsson, A. Fall, I. Chaduc, L. Wagberg, B. Charleux, E. Malmstrom, F. D'Agosto, M. Lansalot and A. Carlmark, Polym. Chem., 2014, 5, 6076 RSC.
- H. Willcock and R. K. O'Reilly, Polym. Chem., 2010, 1, 149 RSC.
- S. Perrier, Macromolecules, 2017, 50, 7433 CrossRef CAS.
- Y. K. Chong, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 2007, 40, 4446 CrossRef CAS.
- S. Perrier and P. Takolpuckdee, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5347 CrossRef CAS.
- W. Shen, Q. Qiu, Y. Wang, M. Miao, B. Li, T. Zhang, A. Cao and Z. An, Macromol. Rapid Commun., 2010, 31, 1444 CrossRef CAS.
- C. P. Jesson, C. M. Pearce, H. Simon, A. Werner, V. J. Cunningham, J. R. Lovett, M. J. Smallridge, N. J. Warren and S. P. Armes, Macromolecules, 2017, 50, 182 CrossRef CAS.
- J. Xu, J. He, D. Fan, X. Wang and Y. Yang, Macromolecules, 2006, 39, 8616 CrossRef CAS.
- E. Kissa, Dispersions: Characterization, Testing, and Measurement, Marcel Dekker Inc., New York, 1999 Search PubMed.
- E. Kostansek, J. Coat. Technol. Res., 2007, 4, 375 CrossRef CAS.
- M. J. Snowden, S. M. Clegg, P. A. Williams and I. D. Robb, J. Chem. Soc., Faraday Trans., 1991, 87, 2201 RSC.
- M. J. Snowden, P. A. Williams, M. J. Garvey and I. D. Robb, J. Colloid Interface Sci., 1994, 166, 160 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional kinetic data for in situ1H NMR of PDMA-first polymerizations, and GPC curves obtained by aqueous and THF GPC. Scheme for methacrylic acid-first polymerization, conversion and kinetic data by 1H NMR and aqueous GPC. Dispersion data including DLS, viscosity and TEM for dispersion of transparent yellow iron oxide with PDMA49-PMAA100 and PDMA100-PMAA50. See DOI: 10.1039/d0gc04271d |
| This journal is © The Royal Society of Chemistry 2021 |