 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Modification of cellulose through physisorption of cationic bio-based nanolatexes – comparing emulsion polymerization and RAFT-mediated polymerization-induced self-assembly†
Alexandros E.
Alexakis
 ab,
Joakim
Engström
ab,
Joakim
Engström
 ab,
Arne
Stamm
ab,
Arne
Stamm
 a,
Anastasia V.
Riazanova
b,
Calvin J.
Brett
a,
Anastasia V.
Riazanova
b,
Calvin J.
Brett
 abc,
Stephan V.
Roth
ac,
Per-Olof
Syrén
abc,
Stephan V.
Roth
ac,
Per-Olof
Syrén
 ab,
Linda
Fogelström
ab,
Michael S.
Reid
ab,
Linda
Fogelström
ab,
Michael S.
Reid
 d and
Eva
Malmström
d and
Eva
Malmström
 *ab
*ab
aKTH Royal Institute of Technology, School of Engineering Sciences in Chemistry, Biotechnology and Health, Department of Fibre and Polymer Technology, Division of Coating Technology, Teknikringen 56-58, SE-100 44 Stockholm, Sweden. E-mail: mavem@kth.se
bWallenberg Wood Science Centre (WWSC), Teknikringen 56-58, SE-100 44 Stockholm, Sweden
cDeutsches Elektronen-Synchrotron (DESY), Notkestrasse 85, 22603 Hamburg, Germany
dKTH Royal Institute of Technology, School of Engineering Sciences, Department of Engineering Mechanics, Osquarsbacke 18, SE-100 44 Stockholm, Sweden
First published on 25th February 2021
Abstract
The polymerization of a bio-based terpene-derived monomer, sobrerol methacrylate (SobMA), was evaluated in the design of polymeric nanoparticles (nanolatexes). Their synthesis was accomplished by using emulsion polymerization, either by free-radical polymerization in the presence of a cationic surfactant or a cationic macroRAFT agent by employing RAFT-mediated polymerization-induced self-assembly (PISA). By tuning the length of the hydrophobic polymer, it was possible to control the nanoparticle size between 70 and 110 nm. The average size of the latexes in both wet and dry state were investigated by microscopy imaging and dynamic light scattering (DLS). Additionally, SobMA was successfully copolymerized with butyl methacrylate (BMA) targeting soft-core nanolatexes. The comparison of the kinetic profile of the cationically stabilized nanolatexes highlighted the differences of both processes. The SobMA-based nanolatexes yielded high Tg ∼ 120 °C, while the copolymer sample exhibited a lower Tg ∼ 50 °C, as assessed by Differential Scanning Calorimetry (DSC). Thereafter, the nanolatexes were adsorbed onto cellulose (filter paper), where they were annealed at elevated temperatures to result in polymeric coatings. Their morphologies were analysed by Field Emission Scanning Electron Microscopy (FE-SEM) and compared to a commercial sulfate polystyrene latex (PS latex). By microscopic investigation the film formation mechanism could be unravelled. Water contact angle (CA) measurements verified the transition from a hydrophilic to a hydrophobic surface after film formation had occured. The obtained results are promising for the toolbox of bio–based building blocks, focused on sobrerol-based monomers, to be used in emulsion polymerizations either for tailored PISA–latexes or facile conventional latex formation, in order to replace methyl methacrylate or other high Tg-monomers.
Introduction
(Meth)acrylate monomers are widely used, particularly in coating and adhesive applications.1 Their respective macromolecules are prepared mainly by free radical polymerization techniques from fossil-based building blocks.2,3 However, there are bio-based alternatives that can replace fossil-based monomers, but there are problems concerning upscaling their production.4 Therefore, it is of significant interest to increase the availability of bio-based monomers, especially in waterborne systems. A special interest has been devoted to wood-based sources, due to its large abundance.5 In this context, turpentine, a pine-tree resin, is comprised primarily from α-pinene.6 The high annual production of turpentine, which exceeds 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons, makes it a promising bio-based substrate.7 Furthermore, through the transformation of α-pinene, a new diol is derived, sobrerol.8 Sobrerol is a cyclic, high temperature profile monomer, which possesses a secondary hydroxyl group that is more reactive than the tertiary and its six-member ring carries a double bond. Its multifunctionality equips sobrerol with interesting properties and possibilities for orthogonal chemistry. Sobrerol can be readily methacrylated to form SobMA (Fig. 1)8,9 and has been shown that it can be polymerized by controlled radical polymerization (CRP) techniques such as both reversible addition–fragmentation chain-transfer (RAFT) and atom transfer radical polymerization (ATRP).9
000 tons, makes it a promising bio-based substrate.7 Furthermore, through the transformation of α-pinene, a new diol is derived, sobrerol.8 Sobrerol is a cyclic, high temperature profile monomer, which possesses a secondary hydroxyl group that is more reactive than the tertiary and its six-member ring carries a double bond. Its multifunctionality equips sobrerol with interesting properties and possibilities for orthogonal chemistry. Sobrerol can be readily methacrylated to form SobMA (Fig. 1)8,9 and has been shown that it can be polymerized by controlled radical polymerization (CRP) techniques such as both reversible addition–fragmentation chain-transfer (RAFT) and atom transfer radical polymerization (ATRP).9
The production of colloidal polymer particles in aqueous media, latexes, has primarily relied on oil-based (meth)acrylates and styrenics using low molecular weight stabilizers. However, this approach faces challenges, such as the scarcity of bio-based alternatives and the tailoring of particle dimensions and charge on-demand.3,10–17 The use of CRP techniques, such as RAFT polymerization, provides a versatile possibility on tailoring the synthesis technique in order to bypass the use of surfactants.13,18,19 A prerequisite is the use of chain-extension of a macromolecule called macroRAFT agent by hydrophobic monomers in water, leading to the formation of a stable latex. This process is called polymerization-induced self-assembly (PISA) and it has been demonstrated that the applied macroRAFT agent could possess different functionalities and thus tailor the surface properties of the formed latexes.20–23 For instance, it could be hydrophilic by using a carbohydrate such as xyloglucan24 or zwitterionic by using an amino acid such as cysteine.25 The choice of the macroRAFT is based on the final application of the produced latexes. For example, when targeting anionic cellulose surfaces, the use of cationic polymers 2-(dimethylamino)ethyl methacrylate (PDMAEMA) and N-[3-(dimethylamino)propyl]methacrylamide (PDMAPMA) has shown interesting properties. Specifically, they not only have been shown to preserve the control of the polymerization, but also to allow for efficient tailoring of particle size and charge density.26–28 The necessity to modify cellulose is primarily derived from its incompatibility in composite applications due to its hydrophilic nature. Hence, the modification of its surface properties is of utmost importance. Within this context, filter papers, being a facile cellulose substrate, have been coated with the aforementioned nanolatexes in order to alter their surface hydrophilicity. In detail, latexes of which the corona is comprised of PDMAEMA27 or xyloglucan24 were successfully adsorbed on filter paper in order to convert the substrate to a hydrophobic surface.
Notwithstanding the previous work on SobMA polymerizations, this study aims to utilize SobMA in emulsion polymerization using only water and no organic solvents, which according to the best of the authors’ knowledge has never been performed. For this reason, SobMA is homo- and copolymerized with butyl methacrylate (BMA), resulting in high and low glass transition temperature nanolatexes, respectively, the adhesion of which is investigated on cellulose surfaces. To widely explore the use of SobMA, conventional latexes using low molecular weight cationic surfactant (cetyltrimethylammonium chloride (CTAC)) and P(DMAEMA-co-MAA) macroRAFT agent through RAFT-mediated surfactant-free emulsion polymerization with PISA are evaluated in parallel. The latter will also be reported for the first time, which expands the scope of the monomer and the use of the previously investigated macroRAFT agent, as well as its ability to successfully stabilize bio-based latexes. Additionally, PISA-based nanolatexes differ from the surfactant-based counterparts in terms of the length of the stabilizing agent, which is expected to affect their film formation properties. The use of SobMA is motivated by its renewability, as it is derived from α-pinene; at the same time, it also brings functionality into the hydrophobic core of the latex, due to the tertiary hydroxyl group and the alkene present in every repeating unit.
The SobMA-containing latexes were characterized with respect to chemical composition (1H-nuclear magnetic resonance (1H-NMR)), molecular weight (size exclusion chromatography (SEC)), thermal properties (differential scanning calorimetry (DSC)), and their particle size/size distribution (dynamic light scattering (DLS), atomic force microscopy (AFM) imaging and field emission scanning electron microscopy (FE-SEM)). Based on the already published findings that P(DMAEMA-co-MAA)-stabilized PISA latexes are readily adsorbed to cellulose,27,28 the latexes were also adsorbed onto cellulose (filter papers) and the resulting surfaces were analysed in terms of morphology and wetting (FE-SEM and contact angle (CA)).
Experimental
Materials
n-Butyl methacrylate (BMA, 99%), 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AIBA, 97%), cetyltrimethylammonium chloride (CTAC, 25 wt% water solution) and (2-dimethylaminoethyl) methacrylate (DMAEMA, 98%) were all purchased from Sigma Aldrich and used as received. Dimethyl sulfoxide-d6 (DMSO-d6, 99.9%), chloroform-d (CDCl3, 99.8%) and deuterium oxide (D2O, 99.9%) were all purchased from VWR and used as received. Sulfate polystyrene latex (PS latex, 8% w/v, 0.2 μm) were purchased from Thermo Fischer Scientific. The hydrophobic monomer, sobrerol methacrylate (SobMA), was synthesized in analogy to previously published protocols either with the enzymatic or the typical methacrylation approach.8,9 The water used was either deionized or Milli-Q water. RAFT agent 4-cyano-4-thiothiopropylsulfanyl pentanoic acid (CTPPA) was synthesized according to literature procedure.29,30 Munktell cellulose filter paper grade 3 (Ahlstrom, Munktell) was used for adsorption of latexes.Characterization techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) from PSS GmbH, using DMF as solvent with 0.01 M LiBr as the mobile phase at 50 °C with a flow rate of 0.2 mL min−1. A calibration method with PMMA standards was used ranging from 700 to 2000
000 g mol−1) from PSS GmbH, using DMF as solvent with 0.01 M LiBr as the mobile phase at 50 °C with a flow rate of 0.2 mL min−1. A calibration method with PMMA standards was used ranging from 700 to 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. All samples were freeze dried prior to their characterization. The results of this analysis can be found in Table S2.†
000 g mol−1. All samples were freeze dried prior to their characterization. The results of this analysis can be found in Table S2.†
Synthetic procedures
| Materials | p (%) | D AFM (nm) | D SEM (nm) | D H (nm) | PdI | Z (mV) | N P 1014h(mL−1) | T g (°C) |
|---|---|---|---|---|---|---|---|---|
| a Synthesized using CTAC as surfactant and AIBA as initiator targeting 10 wt% dry content. b Synthesized using macroRAFT as stabilizer and AIBA as initiator targeting 10 wt% dry content. The target DP is shown in subscript. c Commercial sulfate polystyrene latex. d Total monomer conversion (p) calculated from 1H-NMR in DMSO-d6 and CDCl3 (eqn (S2)). e Measured by AFM on spin coated silica wafers with 0.1 wt% of latex dispersion. f Measured by FE-SEM on the same samples used for AFM. g Measured from DLS with 10 vol% dispersion in MilliQ water. The DLS values (DH, PdI and ζ) are the average of three samples (Table S3†). h Obtained from eqn (S6) by using DH. i Obtained from the second heating run of DSC measurement and averaged over three samples. j Not applicable. | ||||||||
| PSobMACTACa | 91 | 76 ± 24 | 78 ± 23 | 109 ± 1 | 0.09 ± 0.01 | 51 ± 4 | 1.21 ± 0.02 | 123 ± 2 |
| P(SobMA-co-BMA)CTACa | 98 | 81 ± 20 | 93 ± 28 | 110 ± 3 | 0.03 ± 0.01 | 49 ± 4 | 1.31 ± 0.10 | 50 ± 2 |
| PSobMA370b | 83 | 38 ± 17 | 52 ± 16 | 73 ± 1 | 0.08 ± 0.01 | 53 ± 2 | 3.94 ± 0.55 | 119 ± 7 |
| PSobMA1000b | 83 | 58 ± 28 | 73 ± 23 | 112 ± 17 | 0.09 ± 0.03 | 54 ± 10 | 1.13 ± 0.55 | 127 ± 11 |
| PS latexc | —j | —j | —j | 215 ± 1 | 0.03 ± 0.02 | −40 ± 1 | 0.150 ± 0.002 | 104 ± 1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.25 molar ratio to the macroRAFT). Deionized water was added (10.3 mL) to reach a final dry content of 10 wt% and the flask was immersed in an ice/water bath and degassed with argon for 30 min. Finally, it was immersed into an oil bath which was pre-heated to 70 °C, which is the time zero for the polymerization. All reactions were performed for 120 min. Aliquots were taken during polymerization and analysed with DLS, AFM and FE-SEM and thereafter freeze dried and characterized using 1H-NMR, SEC and DSC. The amounts of the reagents are listed in Table S1.†
8.25 molar ratio to the macroRAFT). Deionized water was added (10.3 mL) to reach a final dry content of 10 wt% and the flask was immersed in an ice/water bath and degassed with argon for 30 min. Finally, it was immersed into an oil bath which was pre-heated to 70 °C, which is the time zero for the polymerization. All reactions were performed for 120 min. Aliquots were taken during polymerization and analysed with DLS, AFM and FE-SEM and thereafter freeze dried and characterized using 1H-NMR, SEC and DSC. The amounts of the reagents are listed in Table S1.†
The latexes synthesized with CTAC were abbreviated PSobMACTAC (homopolymerization of SobMA) and P(SobMA-co-BMA)CTAC (copolymerization of SobMA with BMA), whereas PISA-latexes were abbreviated with the core polymer and target DP in subscript, i.e. PSobMA370 and PSobMA1000.
Results and discussion
Kinetic results of cationic nanolatexes
Striving towards renewable monomers for radical polymerization, the methacrylation of sobrerol, to produce sobrerol methacrylate (SobMA) was accomplished in accordance to previously published protocols.8,9 In this work, SobMA is being investigated in waterborne systems and specifically in emulsion polymerizations with and without surfactant in order to expand its versatility.The results are divided into three parts. In the first part, the kinetic profiles of the surfactant-based and surfactant-free emulsion homo- and copolymerizations of SobMA and butyl methacrylate (BMA) are discussed in detail. Also, in this part, the two different approaches are compared. In the second part, the final morphological and thermal properties of latexes originating from both approaches are investigated. In both parts, analyses of the polymeric constituents and their particle properties in the wet state, being nanosized colloids, are discussed. The third part focuses on the film formation ability of latexes as well as their dry state particle structures when adsorbed onto cellulose (filter paper) surfaces.
Initially, SobMA was either successfully homo- or copolymerized with BMA in water using a commercial cationic surfactant, cetyltrimethylammonium chloride (CTAC) (Fig. 1, upper and middle arrows, respectively). Additionally, a cationic macroRAFT agent was chain extended with SobMA in water with RAFT-mediated surfactant-free emulsion polymerization during polymerization-induced self-assembly (PISA) (Fig. 1, lower arrow). In both approaches, the polymerization was initiated by the cationic azo-initiator 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AIBA) and the resulting latex properties are summarized in Table 1. Throughout, sample names are abbreviated with respect to their nanoparticle's core monomer and in subscript either the stabilizer CTAC (e.g. PSobMACTAC) for surfactant-based, or the target degree of polymerization (DP) (e.g. PSobMA370) for the surfactant-free latexes, respectively.
The synthesis and the colloidal stability of the polymerization can primarily be evaluated from three aspects: the kinetics of the polymerization, the size and control of the resulting polymer stabilized latexes and the resulting block copolymer formation in the case of PISA-latexes (RAFT-mediated synthesis to be the cause of self-assembly). The reproducibility of all polymerizations was evaluated by producing triplicate latex batches (except for PSobMACTAC where only two batches were used) (Table S3†).
Usually, kinetics is monitored by the gravimetry, which rely on the fact that the monomer/s used is/are volatile and thus evaporate/s. However, that is not the case for SobMA. It is reported that SobMA has a high boiling point and is therefore difficult to evaporate without causing autopolymerization.9 Hence, a more accurate way to follow the polymerization kinetics is by 1H-NMR. The conversion was calculated by comparing the methine proton next to the methacrylate peak while in the monomer (5.2 ppm) and polymer configuration (broad peak around 5.0 ppm) (Fig. S3†). Additionally, the double bond of the six-membered-ring of SobMA remained unaffected by the polymerization as suggested by 1H-NMR (Fig. S5†). The kinetic data suggest that surfactant-based latexes polymerize more rapidly compared with PISA-latexes. Furthermore, only the PISA-systems exhibit inhibition periods of approximately 15 min (Fig. 2). Inhibition periods are frequently observed in PISA-systems when the kinetics is monitored by 1H-NMR31,32 or gravimetry.27 Additionally, in Fig. 2 it is shown that the surfactant-based systems reach higher conversions at shorter reaction times compared to the PISA-based. Specifically, the plateau is reached after approximately 45 min, corresponding to 90% of conversion and 75 min corresponding to 83% of conversion for surfactant and PISA-based systems, respectively (Table 1). One of the reasons why neither of the systems reach full conversion may be the fact that air could have been introduced in the reaction flask when aliquots were taken for analysis during the polymerization. Additionally, SEC results showed high deviations between the theoretical and experimental molecular weight obtained (Table S2†). This can be attributed to different reasons. Firstly, SEC is a relative technique where the investigated diblock copolymer samples are compared to conventional homopolymer (PMMA calibration standard). Secondly, similarly charged nanolatexes have shown inaccurate estimation of the molecular weight in the literature.27 Finally, although the discrepancy is high, increasing the target DP of the core resulted in increasing molecular weight.
The aforementioned observations based on the kinetic profile of the samples are further strengthened by monitoring the evolution of particles size by DLS (Fig. 3). In the beginning, the emulsion polymerization is characterized by the presence of monomer droplets and surfactant. For all samples, the formation of latexes is characterized by a sharp decrease in the monitored size. For PISA synthesized latexes, this decrease signals that the critical chain length of the hydrophobic block has been achieved, meaning that the block copolymer starts to self-assemble into a latex (Fig. 3c and d).20 Afterwards, the formed latex nanoparticles start to grow by diffusion of monomer to their core, which is translated into an increase of the particle size. It is observed that this diffusion takes longer time for PISA-systems than surfactant-based systems, which can be correlated to the faster monomer consumptions (Fig. 2). Finally, the latexes obtain their final size which is indicated by the plateau observed in both particles’ size (Fig. 3) and number of particles (Np) (Fig. S6†). As mentioned previously, the final size of the latex nanoparticle is achieved earlier for surfactant-based latexes (Fig. 2a). However, it is worth noting that P(SobMA-co-BMA)CTAC exhibits a slight increase in size at higher reaction times, where the final diameter recoded is similar to that of PSobMACTAC, being 110 and 109 nm, respectively (Table 1). Furthermore, the polydispersity index (PdI) obtained from DLS (Fig. S4†) verified the equilibrium which is reached at higher reaction times, where all samples have reached a constant value listed in Table 1.
For PISA-latexes, the final size of the nanoparticles is sensitive to the initial ratio of monomer-to-macroRAFT which can be affected if monomers are evaporated during degassing (especially for small scale). Also, initiator efficiency plays a key role in both the conversion and particle size. For the emulsion polymerization, discrepancies can take place when the surfactant concentration is very close to or below the critical micelle concentration (CMC). Although in this work, the concentration of the surfactant was chosen to be almost two times higher than its CMC in order to avoid the aforementioned discrepancies.33 Larger standard deviations are observed in stage C for the PSobMA1000 which indicates batch-to-batch size variations. The difficulty to reach consistent final average size as the DP in the core grows too large, comparing the two PISA-latexes, seems specific to the SobMA monomer as it has not been shown in the same degree for MMA and BMA previously.27 One can speculate that it is due to the stiffer character of the growing PSobMA chain in the core that might not allow for monomer diffusion as homogenously as for MMA/BMA systems, which is needed for the higher target DP to reach monodisperse results. Regardless of that, the diameter of PSobMA1000 was found to be similar to that of the surfactant-based latex, i.e., 112 nm (Table 1).
An advantage of the RAFT-PISA process is the control of the final size of the latex by tuning the ratio between the hydrophobic and hydrophilic block, while using the same macroRAFT agent. Hence, the size and in extension the properties of the formed latexes can both be tailored. This was indeed the result of using the cationic PDMAEMA-based macroRAFT for the homopolymerization of SobMA in a surfactant-free PISA system. By comparing PSobMA370 and PSobMA1000 the particle diameter was increased by increasing the DP of the core, i.e. 73 and 112 nm, respectively (Table 1). In order to correlate the DLS data with the data obtained from 1H-NMR, the kinetic plot (natural logarithm) is compared with the particle diameter (Fig. 3). The colloidal stability, which is governed by the cationic nature of the corona, as shown by ζ potential measurements, is maintained cationic at >+49 mV for all latexes (Table 1). Furthermore, the colloidal stability can be investigated by visual observations. None of the latexes exhibited phase separation or sedimentation during the experiments as well as after storage at 4 °C for approximately 8 months.
Morphological and thermal properties of nanolatexes
It is important to understand the differences between the wet and the dry state of a latex sample and, in extension, what effect that has on the final coating applications. Hence, in this part, the morphological properties in both states as well as their thermal properties will be discussed. The morphological characterization of dry samples was achieved by using atomic force microscopy (AFM) and field emission scanning electron microscopy (FE-SEM). The results obtained are further compared with the DLS results.The latex samples were diluted (0.1 wt%) and spin coated on clean silica wafers and characterized by AFM (Fig. 4) and FE-SEM (Fig. S8†) without prior dialysis. Both microscopy techniques verified the spherical geometry of nanoparticles (Table 1). The size values obtained differ from those obtained by DLS, but they exhibit the same pattern. The differences in size we observed by comparing FE-SEM, AFM with DLS are due to two reasons: In DLS, the nanolatexes are in the wet state whereas in AFM and FE-SEM, in the dry state. When a latex sample is dried all water present in the core will evaporate, thus the size will be decreased. Furthermore, in DLS larger particles influence the final diameter more, due to the relation between the incident light and the particle size.34–36 Additionally, the large standard deviations in the FE-SEM and AFM diameter (Table 1) are the result of using the whole area of the micrographs.
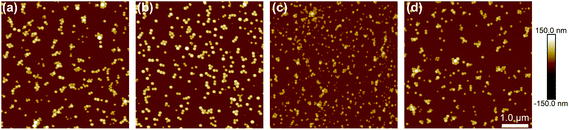 | ||
| Fig. 4 AFM Imaging results of the investigated nanolatexes. (a) PSobMACTAC, (b) P(SobMA-co-BMA)CTAC, (c) PSobMA370 and (d) PSobMA1000. The scale bar is 1 μm for all images. | ||
For the coating applications of latexes and in order to create a film of the nanolatexes onto cellulose, the knowledge of the film formation behaviour and the polymeric components glass transition temperature (Tg) is of utmost importance.37 Therefore, their thermal properties were investigated by DSC (Table 1, Fig. S7†). It has been reported that PSobMA exhibit a rather high Tg, ranging from 116 to 155 °C, depending on both the polymerization technique and molecular weight used.9 In this work, that observation is verified. All SobMA-based latexes reach high Tg values. Additionally, by increasing the molecular weight of SobMA, the Tg is also slightly increasing, i.e. 119 and 127 °C for PSobMA370 and PSobMA1000, respectively. However, considering the standard deviations, the Tg values are not significantly different. According to the Flory-Fox equation and assuming full miscibility of monomers, it is expected that the Tg will be decreased when SobMA is copolymerized with BMA.38 The final Tg of P(SobMA-co-BMA)CTAC was found to be 50 °C, which is slightly higher than the theoretical value obtained from the Flory-Fox equation, i.e. 38 °C (eqn (S7)†). Similar discrepancies were also observed in a previous study.9
Adsorption of nanolatexes onto cellulose
The film formation properties of the nanolatexes are investigated and discussed in this part. Specifically, the ability of the nanolatexes to adsorb onto cellulose was investigated thoroughly with FE-SEM, where the morphological characteristics are studied. Additionally, with the formation of a film, the wetting is expected to change, thus, the contact angle (CA) against water was measured. Furthermore, the adsorption is compared with commercial sulfate polystyrene latex (PS latex), which are larger, anionically stabilized particles and exhibit a lower Tg (104 °C) than the latexes synthesized in this work (Table 1).To investigate the film formation that the nanolatexes undergo on cellulose, pieces of cellulose (filter paper) were immersed in latex dispersion (1 wt%) for 24 h and either annealed above their Tg (150 °C) for 1 and 8 h or left to dry without annealing. In order to verify the successful adsorption, the filter papers were studied by FT-IR (Fig. S9†). It can be confirmed that the carbonyl peak at approximately 1730 cm−1 is present for all samples, which originates from the ester groups present in all investigated latex polymers.
In order to verify that the substrate remains unaffected by the heat treatment, the surface morphology of pristine cellulose was studied by FE-SEM (Fig. S10†). The unmodified cellulose samples were subjected to the same annealing protocol as the latex-modified samples, which showed no macroscopic differences. Additionally, low magnification images of modified cellulose samples were also taken (Fig. S11†). The cellulose samples modified with the surfactant-based latexes were the only ones to show clear features of film formation after 8 h.
In order to highlight the morphological differences originating from the two different synthetic procedures, cellulose modified with PSobMACTAC and PSobMA370 were compared with samples modified with PS latex (Fig. 5). It can be observed that spherical particles occupy most of the untreated substrate, which indicates successful adsorption. For PSobMA1000 (Fig. S12†) a range of differently sized nanolatexes is observed which can be directly correlated to the high standard deviation in the size values obtained by DLS (Table 1). When the latex samples are annealed a film is created, which is characterized by two stages; polymer interdiffusion and the final film formation.39,40 In our case, after 1 h of annealing, the latexes start to coalesce and fuse together with their neighbouring nanoparticles through polymer interdiffusion (Fig. 5b, e and h). The coalescence is most efficient for the P(SobMA-co-BMA)CTAC (Fig. S12†) and PS latex samples, which exhibit clear features of film formation. The reason for that lies in the low Tg value of these samples compared with the high Tg latexes. When the samples were annealed for 8 h, all nanoparticles have coalesced and coherent films are formed. The copolymer modified cellulose sample did not exhibit any morphological differences after 8 h, which suggest that a thermally stable film is formed. Interestingly, for the PISA latexes it can be observed that even after 8 h of annealing there are locations where aggregates of uncoalesced nanoparticles are apparent. The observed thermal stability seems to be an effect of the structural stability imposed by the charged corona, corroborating previous studies of PISA-latexes in which very high colloidal stability was observed in the wet state but also to some degree in dried state where tougher annealing was needed to disrupt and fuse the nanoparticle boundaries, despite a low Tg core polymer.28,40
An alternative and indirect way to study the film formation on cellulose is by monitoring the difference in wetting before and after annealing. For the case of non-modified cellulose, the CA could not be assessed due to the instant absorption of the water droplet, confirming the hydrophilic nature of the substrate. Consequently, the formation of a polymer coating with hydrophobic units is expected to result in increased hydrophobicity. In order to study this, the CAs against water were measured (Table 2). The CA for all modified samples increases after 1 h and 8 h of annealing, respectively, where the highest value recorded belongs to P(SobMA-co-BMA)CTAC (106°). In Table 2 it can be observed that P(SobMA-co-BMA)CTAC-modified cellulose sample exhibits a high CA value even before annealing, which can be explained by the fact that a partial polymeric film was created even before the annealing, due to the low Tg of the nanolatex (Fig. S13†). However, all of the PISA-modified samples exhibited lower CA values when compared with similar systems where PMMA and PBMA were used as the hydrophobic core.27
| Samples | CA (°) | ||
|---|---|---|---|
| 0 h | 1 h | 8 h | |
| PSobMACTAC | 30 ± 9 | 78 ± 20 | 85 ± 13 |
| P(SobMA-co-BMA)CTAC | 90 ± 11 | 104 ± 4 | 106 ± 13 |
| PSobMA370 | 29 ± 9 | 67 ± 20 | 63 ± 13 |
| PSobMA1000 | 33 ± 8 | 101 ± 23 | 70 ± 15 |
| PS latex | 16 ± 2 | 63 ± 10 | 86 ± 9 |
Conclusions
In this work, bio-based cationic nanolatexes, comprised of SobMA, a derivative of the terpene α-pinene, were successfully synthesized with and without surfactant. In the former case, nanolatexes stabilized by the cationic surfactant CTAC were produced by employing emulsion polymerization. In the latter case, RAFT-mediated emulsion polymerization with PISA was employed and the nanolatexes were stabilized by the cationic macroRAFT agent comprised of P(DMAEMA-co-MAA). Both cases yielded spherical nanosized latexes with diameters ranging between 70 and 110 nm, according to DLS, the geometry of which was further verified with AFM and FE-SEM. The kinetic profile of these nanolatexes highlighted the differences between the two polymerization techniques. Thereafter, they were adsorbed on cellulose (filter paper) and annealed at 150 °C for different amounts of time. The annealing resulted in the creation of a polymer coating on top of the cellulose substrate which exhibited a hydrophobic character according to CA. Finally, the film formation mechanism was studied by FE-SEM and gave a better understanding on how the investigated nanolatexes were fused together to yield the polymeric film, also indicating that PISA-nanolatexes are more thermally stable and resist coalescence to larger degree than the conventional CTAC-based latexes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the Knut and Alice Wallenberg foundation through the Wallenberg Wood Science Centre (WWSC) for financial support. C. J. Brett and S. V.Roth acknowledge the kind financial support from the DESY strategic fund (DSF) “Investigation of processes for spraying and spray-coating of hybrid cellulose-based nanostructures”. DESY is a member of the Helmholtz Association (HGF). Per Olof Syrén and Arne Stamm would like to acknowledge funding from FORMAS young-research leader grant (#942-2016-66).Notes and references
- R. Lambourne and T. A. Strivens, Paint and surface coatings: theory and practice, 1999 Search PubMed.
- B. Yamada and P. B. Zetterlund, Handbook of Radical polymerization, 2002, pp. 117–186 Search PubMed.
- G. Odian, Emulsion Polymerization, 2004, pp. 350–371 Search PubMed.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6(25), 4497–4559 RSC.
- F. L. Hatton, Polym. Chem., 2020, 11(2), 220–229 RSC.
- Y. Zhu, C. Romain and C. K. Williams, Sustainable polymers from renewable resources, Nature, 2016, 540, 354–362 CrossRef CAS.
- A. Gandini and T. M. Lacerda, Prog. Polym. Sci., 2015, 48, 1–39 CrossRef CAS.
- M. S. Lima, C. S. M. F. Costa, J. F. J. Coelho, A. C. Fonseca and A. C. Serra, Green Chem., 2018, 20(21), 4880–4890 RSC.
- A. Stamm, M. Tengdelius, B. Schmidt, J. Engström, P. O. Syrén, L. Fogelström and E. Malmström, Green Chem., 2019, 21(10), 2720–2731 RSC.
- J. T. P. Derksen, F. P. Cuperus and P. Kolster, Ind. Crops Prod., 1995, 3(4), 225–236 CrossRef.
- M. Moreno, M. Goikoetxea, J. C. de la Cal and M. J. Barandiaran, Polym. Chem., 2014, 52(24), 3543–3549 CAS.
- G. Lligadas, J. C. Ronda, M. Galia and V. Cadiz, Mater. Today, 2013, 16(9), 337–343 CrossRef CAS.
- J. Qui, B. Charleux and K. Matyajaszewski, Prog. Polym. Sci., 2001, 26(10), 2083–2134 CrossRef.
- F. Ozer, M. O. Beskardes and E. Piskin, J. Appl. Polym. Sci., 2000, 78(3), 569–575 CrossRef CAS.
- S. A. F. Bon, M. Bosveld, B. Klumperman and A. L. German, Macromolecules, 1997, 30(2), 324–326 CrossRef CAS.
- H. A. S. Schoonbrood and J. M. Asua, Macromolecules, 1997, 30(20), 6034–6041 CrossRef CAS.
- S. C. Thickett and R. G. Gilbert, Polymer, 2007, 48(24), 6965–6991 CrossRef CAS.
- P. B. Zetterlund, S. C. Thickett, S. Perrier, E. Bourgeat-Lami and M. Lansalot, Chem. Rev., 2015, 115(18), 9745–9800 CrossRef CAS.
- M. F. Cunningham, Prog. Polym. Sci., 2002, 27(6), 1039–1067 CrossRef CAS.
- C. J. Ferguson, Macromolecules, 2005, 38(6), 2191–2204 CrossRef CAS.
- A. M. dos Santos, J. Pohn, M. Lansalot and F. D'Agosto, Macromol. Rapid Commun., 2007, 28(12), 1325–1332 CrossRef CAS.
- I. Chaduc, W. J. Zhang, J. Rieger, M. Lansalot, F. D'Agosto and B. Charleux, Macromol. Rapid Commun., 2011, 32(16), 1270–1276 CrossRef CAS.
- M. Lansalot, J. Rieger and F. D'Agosto, Macromolecular Self-Assembly, 2016, pp. 33–82 Search PubMed.
- F. L. Hatton, M. Ruda, M. Lansalot, F. D'Agosto, E. Malmström and A. Carlmark, Biomacromolecules, 2016, 17(4), 1414–1424 CrossRef CAS.
- V. Ladmiral, A. Charlot, M. Semsarilar and S. P. Armes, Polym. Chem., 2015, 6(10), 1805–1816 RSC.
- L. Carlsson, A. Fall, I. Chaduc, L. Wågberg, B. Charleux, E. Malmström, F. D'Agosto, M. Lansalot and A. Carlmark, Polym. Chem., 2014, 5(20), 6076–6086 RSC.
- J. Engström, F. L. Hatton, L. Wågberg, F. D'Agosto, M. Lansalot, E. Malmström and A. Carlmark, Polym. Chem., 2017, 8(6), 1061–1073 RSC.
- J. Engström, T. Benselfelt, L. Wågberg, F. D'Agosto, M. Lansalot, A. Carlmark and E. Malmström, Nanoscale, 2019, 11, 4287–4302 RSC.
- S. H. Thang, Y. K. Chong, R. T. A. Mayadunne, G. Moad and E. Rizzardo, Tetrahedron Lett., 1999, 40(12), 2435–2438 CrossRef CAS.
- G. Bouhadir, N. Legrand, B. Quiclet-Sire and S. Z. Zard, Tetrahedron Lett., 1999, 40(2), 277–280 CrossRef CAS.
- W. J. Zhang, F. D'Agosto, O. Boyron, J. Rieger and B. Charleux, Macromolecules, 2011, 44(19), 7584–7593 CrossRef CAS.
- I. Chaduc, E. Reynaud, L. Dumas, L. Albertin, F. D'Agosto and M. Lansalot, Polymer, 2016, 106, 218–228 CrossRef CAS.
- C. H. Tan, Z. J. Huang and X. G. Huang, Anal. Biochem., 2010, 401(1), 144–147 CrossRef CAS.
- C. M. Hoo, N. Starostin, P. West and M. L. Mecartney, J. Nanopart. Res., 2008, 10, 89–96 CrossRef CAS.
- P. Rademeyer, D. Carugo, J. Y. Lee and E. Stride, Lab Chip, 2105, 15(2), 417–428 RSC.
- S. Bhattacharjee, J. Controlled Release, 2016, 235, 337–351 CrossRef CAS.
- J. Engström, C. J. Brett, V. Körstgens, P. Müller-Buschbaum, W. Ohm, E. Malmström and S. V. Roth, Adv. Funct. Mater., 2020, 30, 1907720 CrossRef.
- T. G. Fox Jr. and P. J. Flory, J. Appl. Phys., 1950, 21(6), 581–591 CrossRef.
- H. Kast, Die Makromol. Chem., 1985, 10(S19851), 447–461 CrossRef.
- J. L. Keddie and F. Routh, Fundamentals of latex film formation, 2010 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc04266h |
| This journal is © The Royal Society of Chemistry 2021 |




