Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improving the flame retardancy of wood using an eco-friendly mineralisation process†
Andreja
Pondelak
 *,
Andrijana Sever
Škapin
*,
Andrijana Sever
Škapin
 ,
Nataša
Knez
,
Friderik
Knez
and
Tomaž
Pazlar
,
Nataša
Knez
,
Friderik
Knez
and
Tomaž
Pazlar
Slovenian National Building and Civil Engineering Institute, Dimičeva ulica 12, 1000, Ljubljana. E-mail: andreja.pondelak@zag.si
First published on 26th January 2021
Abstract
A novel environmentally friendly method for in situ formation of CaCO3 deep inside a wood's structure is presented. The method is based on vacuum-pressure impregnation using a one-component treatment medium – a water solution of calcium acetoacetate - and a single stage process to significantly improve the fire retardancy of the treated material.
Wood has become a widely used building material in recent years due to its unique combination of properties: easy processing, excellent mechanical and physical properties, aesthetic appeal, environmental friendliness and high sustainability.1,2 Wood also presents a number of significant drawbacks, however, including high ignitability and susceptibility to fire spread.1,3 It has a gross calorific value of approximately 20 MJ kg−1, meaning wood easily burns and essentially contributes to the fire.1,4 Numerous methods1,3,5–7 have already been developed to improve the flame retardancy of wood, the most environmental friendly of which is mineralisation with inorganic fire retardant materials such as carbonates.1,3,8,9 Calcium carbonate is the most common raw material, which is known to be environmentally friendly and highly efficient as a gas-phase flame retardant with no reported environmental hazards, and no adverse effect on the biggest causes of death and injury in fire – namely, smoke and toxicity.3,10–12 Upon endothermic decomposition carbonates absorb some of the combustion heat and lower the wood temperature near the flame by releasing CO2, which dilutes the gaseous reactants in the flame.13 Carbonates may, however, require unachievably high loading of the treatment to have sufficient fire-proofing properties, and as such are rarely used for the protection of timber products.3 On the other hand, the solubility of CaCO3 in water is 0.013 g L−1,14 meaning the leaching of carbonates from wood is expected to be very low.
The insertion of CaCO3 into the structure of wood has been reported in several studies, using various processing routes: CaCl2 and NaOH with supercritical carbon dioxide;15 aqueous Na2CO3 solution followed by a CaCl2 solution with added dodecanoic acid;16 calcium di(methylcarbonate) in methanol, which hydrolyses in the presence of residual moisture from the wood;17 CaCl2 and NaHCO3;13,18,19 CaCl2 and (NH4)2CO3;20 CaCl2 and Na2CO3;21 and finally the alkaline hydrolysis of dimethyl carbonate (creating a source of liquid CO2) in the presence of calcium ions.8 These technologies often require either an expensive technical set-up for the use of supercritical gases,15 or harmful precursors (e.g., methanol16,17), reactants with limited solubility,13,18–21 or undesirable side effects (such as NaCl, NH4Cl) are formed.13,18–21 Another drawback of the majority of technologies reported in the literature is that precipitation of CaCO3 usually takes place on the wood's surface, which can impede the wood treatment. In a recent study by Merk et al.,13 mineralised wood with an improved reaction to fire was obtained by the in situ formation of CaCO3 inside the lumina of the wood, by vacuum impregnation of aqueous NaHCO3 and alcoholic CaCl2 (1–4 reaction cycles). Using this process, a substantial mass of inorganic compounds were incorporated into the wood. The mass gain of CaCO3 obtained in the study by Merk et al.,13 however, contains also the mass of by-products such as NaCl or unreacted salts. Based on our knowledge, only the group led by Merk8,13 succeeded in depositing CaCO3 deep into the wood's structure, but unfortunately this procedure is quite complicated and time-consuming. Moreover, the by-products formed, such as NaCl, may have a negative effect on the wood's appearance, and can also cause corrosion of the metal fasteners which are commonly fixed to the wood. Authors used different testing methods8,13,15,18,19,21 to evaluate fire resistance, so between studies it is difficult to compare the improvement in fire resistance as a result of mineralization, if not even impossible. In the studies using cone calorimetry13,21 however, no significant improvements in fire retardancy were found following the mineralization of wood, for example with respect to ignition time.
In order to address the issues mentioned above, we here propose a novel approach to improving flame retardancy: an eco-friendly method for the mineralisation of wood using a water solution of calcium acetoacetate (Ca(OAcAc)2) which has been patented in our laboratory.22 In the presence of water, Ca(OAcAc)2 transforms to CaCO3, acetone and carbon dioxide.23–25 Under standard laboratory conditions (RT and 50% RH), this reaction is very slow, but the transformation is accelerated as temperature and relative humidity are increased.23 The impregnation process of the mineralization procedure was therefore conducted at room temperature, then following treatment the temperature and relative humidity were increased to ensure that the desired reaction took place only once the impregnation solution had penetrated inside the wood. This way the protective compounds become deeply incorporated into the structure of the wood. The efficiency of this novel method of mineralisation of wood, and the effect on its essential property i.e. improved flame retardancy, is discussed in this paper. Beech, was selected as a representative of hardwoods and used as the wood species to be investigated, as it is probably the most important commercial hardwood in Europe, with many different uses. Beech forest grows indigenously in central Europe because it adapts more easily than spruce forest to the changing climate. The difference between beech and other lignocellulosic biomasses, such as softwood (i.e. spruce), is their cellular structures. Beech comprises several different cell types - vessels, responsible for conducting water, and fibres, which offer structural support. Spruce, on the other hand, contains only fibrous cells called tracheides.26
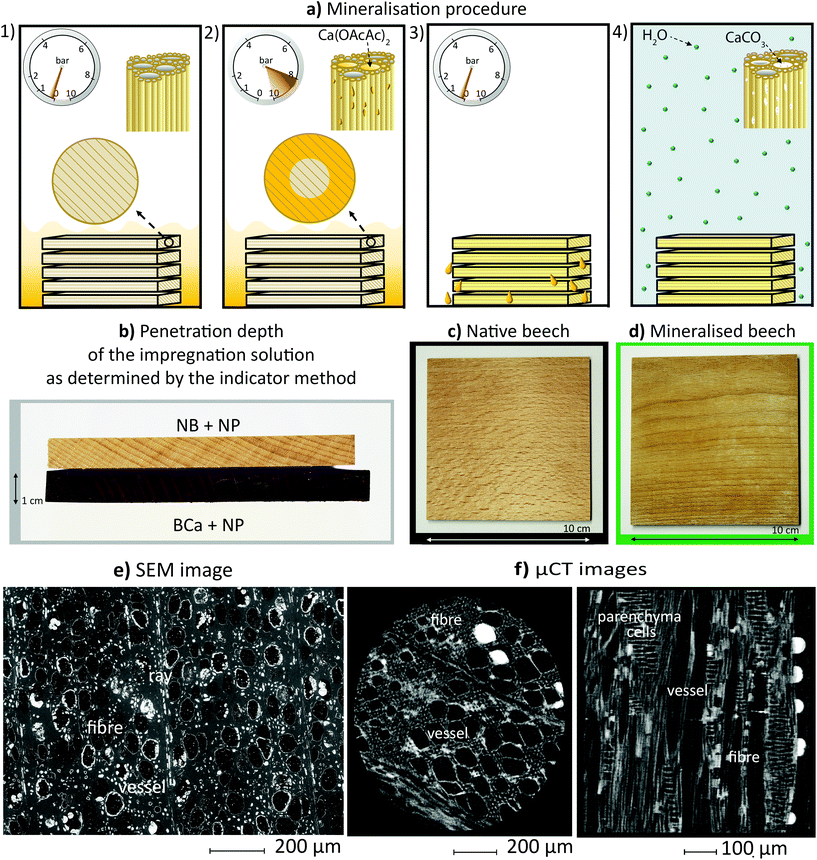
To accomplish the formation of CaCO3 particles deep in the wood's structure, a vacuum–pressure process (Fig. 1a – 1st–3rd step) was used to impregnate the samples with a water solution of Ca(OAcAc)2. This process generally provides the best impregnation properties (i.e. a more homogeneous distribution, deeper penetration depth, and higher adsorption of the impregnating agent).27 We thereby assume that this is the best solution to incorporate the novel fire retardant treatment into the structure of the wood, and that upscaling the newly proposed method should not be a problem. Once the wood samples are fully impregnated they are then further dried at an elevated temperature (for example 80 °C) and relative humidity (for example cycling between 40% and 90% RH – see the 4th step in Fig. 1a). Ca(OAcAc)2 is thermally unstable and spontaneously decarboxylates to CaCO3, acetone and CO2.23 For this reason, an increased temperature accelerates its decomposition. Additionally, an increase in relative humidity accelerates the transformation of amorphous CaCO3 into vaterite crystal modification, since water is quickly physisorbed on the particle surface and dehydration, partial dissolution and then re-precipitation promptly occur, resulting in the rapid formation of vaterite particles.23 One key advantage of this new method is fully-effective mineralisation deep into the wood's structure, as a result of impregnating the wood with the Ca(OAcAc)2 solution. Fig. 1b demonstrates the penetration depth of the Ca(OAcAc)2, as determined by the indicator method. The purple colour obtained on the cross-section of the impregnated beech sample – indicating the reaction of acetoacetate with nitroprusside28 – is evident throughout the whole depth of the impregnated sample (1 cm). As shown in Fig. 1c and d, the mineralised wood retains its natural appearance.
In order to maximize the flame retardancy of the mineralised wood, the aim was to input a high amount of CaCO3 into the structure of the wood; a 20 wt% solution of Ca(OAcAc)2 was therefore impregnated (theoretically meaning that 8.3% CaCO3 would be formed). A single impregnation with the Ca(OAcAc)2 solution resulted in 7.4 ± 1.3% of CaCO3 formed (Table 1). Please note that the difference in mass between the reference and mineralized samples (+18% in Table 1) does not equate to differences between the true amount of CaCO3 inserted, since this value also includes the weight of water content within the wood (Table S1†), which is greater in the case of the mineralized wood sample. To avoid this error, the amount of CaCO3 was calculated from the difference in total weight loss (Δm25–1000 °C) between the native (i.e. reference, non-treated) and mineralised beech samples (see Table S1†), as determined by thermogravimetric analyses (TG). A similar mass gain was reported by Merk et al.,13 when CaCO3 was added using a different mineralization method.
| Native beech | CaCO3 beech | Difference (%) | |
|---|---|---|---|
| a Determined by means of TG. b Determined by cone calorimetry by exposing specimens to a constant irradiation of 50 kW m−2. The results are given as average values and standard deviations of five specimens. PHRR – Peaks of heat release rate; TRH600s – total heat release for the first 600s of the test; FIGRA – fire growth rate index. | |||
m
CaCO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (%)
(%) |
— | 7.4 ± 1.3 | — |
Initial mass0 s![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (g) b (g) |
69.1 ± 2.5 | 81.8 ± 1.5 | +18 |
| Total mass loss (%)b | 85.0 ± 1.1 | 73.3 ± 1.9 | +14 |
t
ignition![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /s
/s |
29.8 ± 3.8 | 46.8 ± 6.2 | +57 |
t
1. PHRR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /°C
/°C |
36.4 ± 5.9 | 52.0 ± 7.6 | +43 |
1. PHRR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/kWm−2 b/kWm−2 |
219.2 ± 9.1 | 179.4 ± 11.6 | −18 |
t
2. PHRR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /°C
/°C |
277.0 ± 19.6 | 392.0 ± 4.5 | +42 |
2. PHRR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/kWm−2 b/kWm−2 |
509.6 ± 53.4 | 374.8 ± 33.0 | −26 |
THR600 s![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/MJ b/MJ |
43.5 ± 2.7 | 31.8 ± 2.1 | −27 |
FIGRA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/W s−1 b/W s−1 |
530.3 ± 51.0 | 217.2 ± 9.4 | −59 |
As shown in the SEM image in Fig. 1e, the CaCO3 particles are deposited in fibres cells (Fig. S1b, c, d, g†), rays (Fig. 1e), and fully inside or around the beech vessels (Fig. S1a, b, c, e, f†). Additionally, the images in Fig. S1a, e, f, g† reveal that CaCO3 is deposited throughout the beech vessels. This was also confirmed by μCT images (see the CaCO3 particles to the far right in Fig. 1f). Detailed SEM and EDS analyses revealed that CaCO3 is also present in the cell walls of the parenchyma (Fig. S2b†), in middle lamellae (Fig. S1c†) and in pits (Fig. S1f†). Since the fibres contain more CaCO3 than the vessels, the capillarity effect is most probably controlling the CaCO3 deposition as reported by Merk et al.13
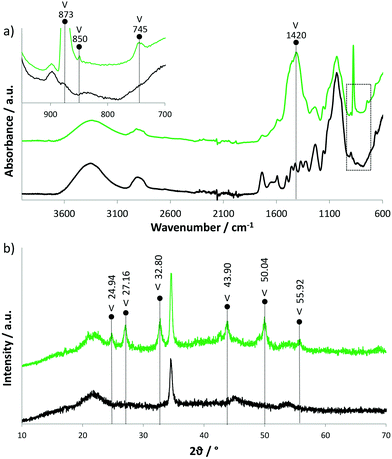
The CaCO3 particles formed in the vessels are either distributed around the cell walls or deposited in the form of spheres and hemispheres with diameters up to 100 μm (see Fig. S1f† and Fig. 1f). In the case of fibres the CaCO3 either completely or partly fills the vessels (Fig. S2b,c,d and Fig. S2a†). CaCO3 aggregate particles are composed of nanoparticles between 13 nm and 30 nm (Fig. S1h†), which is in agreement with our previous research.23 The formation of a particular CaCO3 phase (amorphous, vaterite, aragonite or calcite), as well as the morphology and size of the particles formed, can all be controlled by synthesis parameters, such as pH value, initial supersaturation and temperature, and the presence of various additives or impurities.29–31Fig. 2a shows the FT-IR spectra of the native and mineralised beech. The presence of a band at 1420 cm−1, representing the ν3 mode, along with the ν2 mode split at 873–850 cm−1, indicate vaterite crystal modification.32 This was further confirmed by the XRD analysis presented in Fig. 2b, where XRD patterns of native and mineralised beech are shown. The characteristic reflections at 21.7° and 34.6° correspond to cellulose,33 while other visible reflections in the patterns for mineralised beech belong to vaterite crystal modification. In a previous study23 we proposed that the presence of an organic component, such as Ca(OAcAc)2, could stabilise or promote the stabilisation of the vaterite phase. Moreover, as an organic material, wood may also act as a template for vaterite heterogeneous nucleation34 – the formation of vaterite modification was therefore expected.
Cone calorimetry was performed in order to simulate combustion behaviour in a developing fire. Bimodal heat release rate (HRR) profiles of the average of five measurements of untreated (black curve) and mineralised beech (green curve) are presented in Fig. 3 (raw data for the reaction to fire for all wood specimens can be found in Fig. S3†). Peaks of heat release rate (PHRR) represent the flaming combustion caused by a rapid spread of fire.
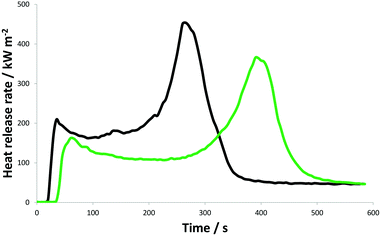 | ||
| Fig. 3 Heat release profiles (average of the measurements of five specimens) of the native beech (black curve) and beech mineralised by CaCO3 (green curve). | ||
The first peak occurs at the beginning of the test, when volatile pyrolysis gases are present in a sufficient amount to allow ignition.3,13 It was demonstrated that the time of ignition of mineralised beech was significantly delayed from approximately 30 s for native beech to 47 s for mineralised samples (Table 1). For purposes of comparison, the mineralisation strategy performed by Merk et al.13 caused hardly any change in ignition time (from 28 ± 4 s for native to 30 ± 5 s for CaCO3 mineralised beech). As the heat generated by combustion sustains the pyrolysis, more volatiles are released. The dip in the curve corresponds to the formation of an insulating char layer, in which transformation of heat is more difficult, meaning that the process of pyrolysis is slowed. Once the protective char structure disappears, more volatiles evaporate from the wood and a second peak appears in the HRR curves.3,13 This peak corresponds to an extended burning surface, as the lower surface of the specimen also starts to burn, meaning the time of the second peak strongly depends on the thickness of the specimen tested, as well as on the level of progression of the front burning through the wood.35 When there are no more volatiles to exhaust, the flaming ends and smouldering combustion with gradual glowing begins, causing the baseline of the HRR curves to return to steady-state. As shown in Table 1 and Fig. 3, both HRR peaks (1st and 2nd PHRR) significantly decreased and shifted to higher temperatures in the case of mineralised beech, indicating enhanced char stabilization. Furthermore, the total heat release in the first 600 s of the test (THR600s) and fire growth rate index (FIGRA) are significantly lower than those for native wood. THR600s decreased by approximately a quarter (27%), while the FIGRA reduced by more than half (59%) (Table 1). Incorporated CaCO3 decreases the wood combustion process, primarily through the release of non-flammable gas (i.e. CO2), which would result from the endothermic decomposition of CaCO3. This process may cool and dilute the flammable pyrolysis gases.3,13 Moreover, it was shown that CaCO3 mineralised beech contains about 2% more water than native beech (see Table S1†), which can additionally improve the fire characteristics of the wood. The higher content of water in the mineralised samples can be explained by the hydrophilic properties of CaCO3, which lead to greater amounts of moisture being absorbed from the atmosphere.36
We have managed to successfully reduced the total heat release (THR600s) by inserting only ∼7 wt% of CaCO3 using a single one-step impregnation of Ca(OAcAc)2 solution. We believe that the method presented is less time-consuming and far more environmentally friendly than the two-, three- or even four-step process of vacuum pressure immersion using either CaCl2 and Na2CO321 or NaHCO313 solutions. With only a one-stage process we have succeeded to significantly improve the fire retardancy of the wood, and the ignition time of beech has been prolonged using the novel mineralised method far more than has been seen by any other method. The originality of this novel method lies in two parts: firstly, its simple single-stage impregnation process, and secondly, by providing a source of CaCO3 in the form of a Ca(OAcAc)2 solution, which transforms into the end product in situ without creating any solid by-products. During the first step the CaCO3 particles formed can become trapped on the surface of the wood and block pore accessibility, which then hampers subsequent steps of the impregnation process and reduces the generation of CaCO3 inside the wood's structure.21 Since in our case the impregnation agent is a solution, it can penetrate deep inside the structure of the wood, where it then transforms into CaCO3.
Conclusions
A new treatment method for the in situ formation of CaCO3 in wood is presented, with the purpose of improving its properties of fire resistance. A calcium acetoacetate (Ca(OAcAc)2) water solution is impregnated under vacuum in order to form CaCO3 deep inside the wood. In the presence of water and at elevated temperature the calcium acetoacetate transforms to CaCO3. This process occurs once the CaCO3 has penetrated into the wood. For purposes of demonstration beech wood was selected. SEM and μCT analysis showed that CaCO3 particles are primarily incorporated into the beech fibres and vessels in the form of spheres. Using FT-IR spectroscopy and XRD analysis it was found that the CaCO3 formed as vaterite crystal modification.Cone calorimetry tests indicated a significant improvement in the fire retardant properties of wood following the mineralisation process. This improvement was evident following the addition of a relatively low amount of CaCO3 (∼7 wt%). Significant reductions were seen in the total heat release over the first 600 s of the test (THR600s) as well as in the fire growth rate index (FIGRA), both of which define the behaviour of the treated wood during the initial stages of fire. The time to ignition of the treated wood was delayed in comparison to that of the native wood, leading to a reduction in fire propagation and the combustion process. All these parameters, together with the prolonged time between peaks of heat release rate (PHRR), indicate an obvious improvement in the fire behaviour of the mineralised wood.
The new method is recognized to have great potential as an eco-friendly fire retardant, because non-flammable CO2 is released upon endothermic decomposition. Due to low solubility of CaCO3 in water,14 low leaching of CaCO3 from wood is expected. For this reason, wood modified in such a way may be suitable for outdoor applications. The method proposed involves the formation of CaCO3 deep in the wood structure, as a result of the vacuum-pressure process. Moreover, impregnation involves the use of only a single component – a water solution of calcium acetoacetate – and a one-stage process, in order to significantly improve the fire retardancy of the material. The factors stated above clearly show the huge potential of this eco-friendly method for wood mineralisation, and we firmly believe that it is possible to easily up-scale such a process to an industrial level.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The research leading to these results received funding from the Slovenian Research Agency (No. Z4-9298, P2-0273). We thank Srečo D. Škapin from the Department of Advanced Materials, Jožef Stefan Institute, for the XRD analysis and FE-SEM EDS analysis. We would also like to thank Miha Humar from the Department of Wood Science and Technology, Biotechnical Faculty, University of Ljubljana for wood impregnation via the vacuum-pressure process. We also acknowledge Rožle Repič from the Slovenian National Building and Civil Engineering Institute for his help in preparing the SEM samples. We are very much obliged to Catherine Earles for proof-reading the English manuscript.Notes and references
- C. M. Popescu and A. Pfriem, Fire Mater., 2020, 44, 100–111 CrossRef CAS.
- Y. Lu, M. Feng and H. B. Zhan, Cellulose, 2014, 21, 4393–4403 CrossRef CAS.
- L. A. Lowden and T. R. Hull, Fire Sci. Rev., 2013, 2, 1–19 CrossRef.
- V. Babrauskas and S. J. Grayson, Heat release in fires, Interscience Communications Ltd, London, 2013 Search PubMed.
- Q. F. Sun, Y. Lu, Y. Z. Xia, D. J. Yang, J. Li and Y. X. Liu, Surf. Eng., 2012, 28, 555–559 CrossRef CAS.
- H. Q. Qu, W. H. Wu, L. Liu and J. Z. Xu, Adv. Compos., 2011, 150–151, 475–479 CAS.
- S. Lv, X. G. Kong, L. R. Wang, F. Z. Zhang and X. D. Lei, New J. Chem., 2019, 43, 16359–16366 RSC.
- V. Merk, M. Chanana, T. Keplinger, S. Gaan and I. Burgert, Green Chem., 2015, 17, 1423–1428 RSC.
- L. A. Berglund and I. Burgert, Adv. Mater., 2018, 30, e1704285 CrossRef.
- A. B. Morgan and J. W. Gilman, Fire Mater., 2013, 37, 259–278 CrossRef CAS.
- A. Witkowski, L. Hollingbery and T. R. Hull, Fire and Polymers VI: New Advances in Flame Retardant Chemistry and Science, American Chemical Society, Washington, 2012 Search PubMed.
- O. A. Jimoh, K. S. Ariffin, H. Bin Hussin and A. E. Temitope, Carbonates and Evaporites, 2018, 33, 331 CrossRef CAS.
- V. Merk, M. Chanana, S. Gaan and I. Burgert, Holzforschung, 2016, 70, 867–876 CAS.
- W. Tagethoff, Calcium Carbonate: From the Cretaceous Period Into the 21st Century, Springer, Boston, Berlin, Birkhäuser, 2001 Search PubMed.
- C. Tsioptsias and C. Panayiotou, J. Mater. Sci., 2011, 46, 5406–5411 CrossRef CAS.
- C. Wang, C. Liu and J. Li, Adv. Mater. Res., 2010, 113–116, 1712–1715 CAS.
- S. Klaithong, D. Van Opdenbosch, C. Zollfrank and J. Plank, ZNB, 2013, 68, 533–538 CAS.
- R. Moya, J. Gaitan-Alvarez, A. Berrocal and F. Araya, BioResouces, 2020, 15, 4802–4822 CAS.
- T. Yang, G. Yuan, M. Xia and S. Chen, Eur. J. Wood Prod., 2020 DOI:10.1007/s00107-020-01606-w.
- Z. Jia, G. Li, J. Li, C. Wang and Z. Zhang, Appl. Mech. Mater., 2012, 184–185, 1129–1133 CAS.
- L. Huang, X. Yao, Y. Huang and Q. Wang, BioResources, 2018, 13, 6694–6706 CAS.
- A. Pondelak, A. Sever Škapin, N. Knez, F. Knez, T. Pazlar and A. Legat, SI Pat, P-201900260, 2019 Search PubMed.
- A. Pondelak, F. Rosi, C. Maurich, C. Miliani, S. Škapin and A. Sever Škapin, Appl. Surf. Sci., 2020, 506, 144768 CrossRef CAS.
- A. Pondelak, S. Kramar, M. L. Kikelj and A. Sever Škapin, J. Cult. Herit., 2017, 28, 1–8 CrossRef.
- A. Pondelak, S. Kramar, J. Ranogajec, L. Škrlep, S. Vucetic and A. Sever Škapin, Materials, 2019, 12, 521 CrossRef CAS.
- J. Walker, Primary wood processing: principle and practice, Springer, Dordrecht, Netherlands, 2006 Search PubMed.
- H. Yorur and K. Kayahan, BioResources, 2018, 13, 1829–1842 CrossRef CAS.
- P. Ropret, L. Legan, K. Retko, T. Špec, A. Pondelak, L. Škrlep and A. Sever Škapin, J. Cult. Herit., 2016, 23, 158–156 Search PubMed.
- D. Konopacka-Lyskawa, B. Koscielska and J. Karczewski, J. Cryst. Growth, 2015, 418, 25–31 CrossRef CAS.
- Y. Han, G. Hadiko, M. Fuji and M. Takahashi, J. Cryst. Growth, 2006, 289, 269–274 CrossRef CAS.
- B. Feng, A. K. Yong and H. An, Mater. Sci. Eng., A, 2007, 445–446, 170–179 CrossRef.
- F. A. Andersen and L. Brecevic, Acta Chem. Scand., 1991, 45, 1018–1024 CrossRef CAS.
- D. M. Chu, J. Mu, L. Zhang and Y. S. Li, Holzforschung, 2017, 71, 217–223 CAS.
- C. Rodriguez-Navarro, C. Jimenez-Lopez, A. Rodriguez-Navarro, M. T. Gonzalez-Munoz and M. Rodriguez-Gallego, Geochim. Cosmochim. Acta, 2007, 71, 1197–1213 CrossRef CAS.
- B. Schartel and T. R. Hull, Fire Mater., 2007, 31, 327–354 CrossRef CAS.
- R. J. Gustafsson, A. Orlov, C. L. Badger, P. T. Griffiths, R. A. Cox and R. M. Lambert, Atmos. Chem. Phys., 2005, 5, 3415–3421 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, additional SEM, FE-SEM and EDS analysis, raw cone calorimetry data, TG analysis. See DOI: 10.1039/d0gc03852k |
| This journal is © The Royal Society of Chemistry 2021 |