Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Life cycle thinking case study for catalytic wet air oxidation of lignin in bamboo biomass for vanillin production†
Christopher S.
McCallum
 *ab,
Wanling
Wang
a,
W. John
Doran
*ab,
Wanling
Wang
a,
W. John
Doran
 b,
W. Graham
Forsythe
a,
Mark D.
Garrett
a,
Christopher
Hardacre
b,
W. Graham
Forsythe
a,
Mark D.
Garrett
a,
Christopher
Hardacre
 c,
J. J.
Leahy
d,
Kevin
Morgan
c,
J. J.
Leahy
d,
Kevin
Morgan
 a,
Dong-Soo
Shin
e and
Gary N.
Sheldrake
*a
a,
Dong-Soo
Shin
e and
Gary N.
Sheldrake
*a
aSchool of Chemistry and Chemical Engineering, David Keir Building, Queen's University Belfast, BT9 5AG, Northern Ireland, UK. E-mail: g.sheldrake@qub.ac.uk; cmccallum01@qub.ac.uk
bBryden Centre, School of Engineering, Letterkenny Institute of Technology, Donegal, F92 FC93, Ireland
cSchool of Chemical Engineering and Analytical Science, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK
dDepartment of Chemical Sciences, University of Limerick, Limerick, Ireland
eDepartment of Chemistry, Changwon National University, Changwon, South Korea
First published on 9th February 2021
Abstract
Life cycle thinking analysis (LCT) was conducted on the production of vanillin via bamboo wet air oxidation compared to vanillin production from crude oil or Kraft lignin. Synthetic vanillin has been produced from crude oil and paper pulping process “waste” for nearly a century. Bamboo is a quick growing, lignin rich biomass with the potential for the generation of aromatic products including vanillin. Wet air oxidation experiments were performed, using CuSO4 and Fe2O3 catalysts in alkaine environments, on raw bamboo with the purpose of determining the aromatic compound yields in order to investigate bamboo as a potential source of fine chemical products. From the LCT approach, production of vanillin from Kraft Lignin compared to crude oil has a lower environmental impact across all categories per kg produced of vanillin. Kraft lignin production of vanillin is shown to reduce smog formation and CO2 emissions from non energy sources to negligible levels while decreasing acidification potential by 6.8 times, toxic inhalation by 1.3 times and toxic ingestion potential by 0.3 times compared to crude oil. The proposed bamboo oxidation route has the potential to have the least environmental impact with negligible impact on smog formation potential and ozone depletion potential if a green polar extractive solvent can be utilised in future while also decreasing toxicity by inhalation by a further 115 fold and ingestion by 9458 fold. This work has the potential to contribute to the United Nation Sustainable Development Goal for Responsible Consumption and Production, Goal 12.
Introduction
An increasing effort is devoted to find processes for the conversion of biomass and biomass-derived products into fuels and organic chemicals,1 which is in part now driven by the United Nations Sustainable Development Goal 12 (SDG 12) for Responsible Consumption and Production.2 A scientific and rational approach to the utilization of biomass as a resource for both energy and chemical production can help society to achieve carbon neutrality. With diminishing global resources, biomass shows a great potential and its enhanced use could solve several societal needs. Advances in biotechnology, genetics, process chemistry, and engineering are leading to new manufacturing concepts for converting renewable biomass into valuable fuels and platform chemicals. The integration of agroenergy crops and bio-refinery manufacturing technologies offers the potential for the development of sustainable bio-power and biomaterials that will lead to a new manufacturing paradigm.3Conducting an LCA as part of a new process analysis has become more prevalent with the availability of LCA software and environmental databases.4,5 The benefits of LCA are the identification and quantification of impacts of particular processes or compounds to the environment allowing for adjustment of the process as to be more environmentally by design. This is a change from previous modes whereby the processes were shown to work, scaled and then an effort was made to mitigate against environmental impact. This has been adopted widely, as reviewed by Kralisch et al.,6 a number of case studies examining how LCA has, and is continuing, to generate more environmentally friendly processes for the chemical industry.
Life cycle thinking (LCT) is an approach, similar to life cycle assessments (LCA), that is utilised to assess the green credentials of a process by considering various synthesis routes.7,8 The aim of a LCT approach is to take into consideration the process from raw materials to processing and disposal. This requires a synthesis tree to be devised for each chemical within the process to determine points where a chemical could have an environmental impact.
In the current work we apply the LCT approach to the wet air oxidation of lignin using bamboo as substrate.
Bamboo is an important ecological, industrial and cultural resource, which has potential use in the bio-refinery industry.9–12 Some of the main transformations of biomass involve the use of rice husk, wood, bagasse and miscanthus, for example, as chemical feedstocks but there are limited equivalent studies for bamboo. Bamboos are perennial evergreen plants and are a viable lignocellulosic substrate for bio-ethanol production due the high cellulose percentage >48% and high production yields.10 For instance, China is home to one of the world's largest bamboo growing areas with a total bamboo forest of around 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010
010![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hectares.13 The conversion of bamboo for bio-ethanol, bio-methane, flavanoids, and functional xylo-oligosaccharides production is under development.14,15 Vanillin is one of the most widely used flavours, produced naturally via the vanilla pod (Vanilla planifoila) but this cannot meet current world demand and has been the focus of research to improve synthetic methods. Quantification of more environmentally friendly vanillin production processes have been reported previously through LCA. Isola et al.4 reported a quantified method to lower environmental impact of alkali lignin depolymerisation with vanillin using MoO, Lao and CoO catalysts. Wongtanyawat et al.5 reported a quantified environmental impact of process design, including solvent selection, for the production of vanillin using LCSoft.
000 hectares.13 The conversion of bamboo for bio-ethanol, bio-methane, flavanoids, and functional xylo-oligosaccharides production is under development.14,15 Vanillin is one of the most widely used flavours, produced naturally via the vanilla pod (Vanilla planifoila) but this cannot meet current world demand and has been the focus of research to improve synthetic methods. Quantification of more environmentally friendly vanillin production processes have been reported previously through LCA. Isola et al.4 reported a quantified method to lower environmental impact of alkali lignin depolymerisation with vanillin using MoO, Lao and CoO catalysts. Wongtanyawat et al.5 reported a quantified environmental impact of process design, including solvent selection, for the production of vanillin using LCSoft.
The total mass fraction of cellulose, hemicellulose and lignin in bamboo is between 89–93%.16 The cellulose and hemicellulose content are in the range of 63% to 85%, and since these are both more easily degraded than lignin, therefore, bamboo is a potentially valuable substrate for further conversion to valuable chemicals. Lignin is an amorphous, three-dimensional network polymer comprising of variously linked phenylpropane units.17,18 Previously, it has been shown that biomass rich in lignin can be used for the production of valuable chemicals via depolymerisation processes.19,20,47 Wet air oxidation (WAO) is an effective and extensively used industrial oxidation process21 for organic and inorganic aqueous solutions or suspensions. Typical conditions for wet air oxidation to produce phenolic compounds, depending on the source, ranges from 150–250 °C and 1–50 bar.22,45,46
In this study we have applied the life cycle thinking approach to the production of vanillin via bamboo wet air oxidation in comparison to the more traditional vanillin production routes from crude oil or kraft lignin. More details and background of this process are available in the ESI.†
Experimental
Life cycle thinking approach
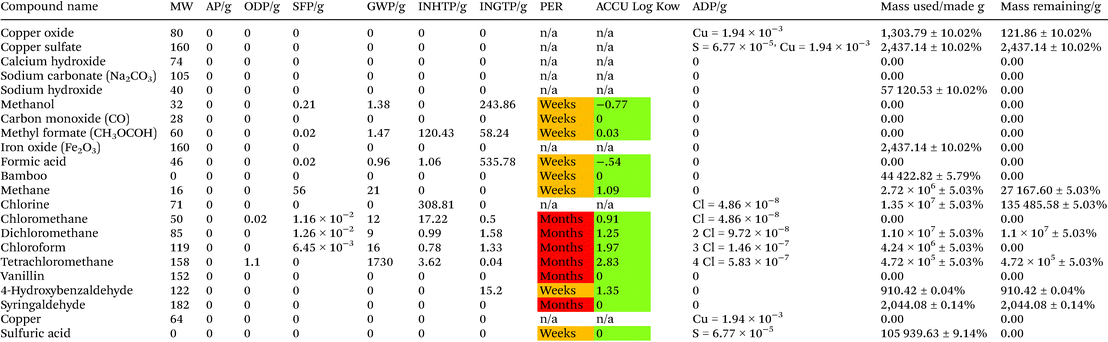
Life cycle thinking analysis was conducted via the method proposed by Jessop et al.23,24 The following factors were chosen for a comparison of synthesis pathways; acidification potential (AP), ozone depletion potential (ODP), Smog formation potential (SFP), global warming potential (GWP), human toxicity by inhalation potential (INHTP) and human toxicity by ingestion potential (INGTP), persistence (PER), biological accumulation (ACCU) and abiotic depletion potential (ADP). The following assumptions are made:• H2O, N2, O2 and H2 calculated but does not carry an environmental impact as it naturally occurs in large volumes.
• All unreacted starting material and by-products are released to the environment.
• Catalysts were assumed to be removed via filtration and not be recycled, as a worst case scenario for all reactions.
The following synthesis methods for vanillin were chosen for comparison with the bamboo oxidation presented
(1) Crude oil to vanillin production (crude oil → benzene → phenol → catechol → guaiacol → vanillin); more details in the ESI.†
(2) Paper pulping residue to vanillin.25
All information unless otherwise stated was obtained from either PubChem, National Institute for Standards and Technology or European Chemicals Agency (ECHA). Full details are reported in the ESI.†
Materials
Bamboo powder was obtained locally to Changwon and dried to 10% wetness prior to transport. Prior to use the bamboo was pulverised and sieved to <0.2 mm. The lignin content of this bamboo was determined with the Klason method26 and was found to be 28.8 wt%. Details of the Klason method are reported in the ESI.† Recycled lignin was obtained as a by-product of the acid pyrolysis of cellulosics in biomass from Miscanthus grass.27 This process produces lignin in formic acid (6.48 g L−1). The formic acid lignin solution needed to be neutralized with NaOH before applying the standard oxidation process (see ESI† for further details). CuSO4, Fe2O3, all solvents and other reagents used were obtained from Sigma Aldrich, unless otherwise stated.WAO of bamboo lignin
5 g of untreated bamboo wood powder was mixed with 120 mL water, catalyst and sodium hydroxide in a Parr 4560 Mini Bench Top batch reactor. The reactor was flushed three times with O2 to expel air and then the reactor was pressurised with O2 (6 bar) and heated to 195 °C. The reaction was then maintained at this temperature for 90 minutes.To determine the effect of base on the WAO, the CuSO4 catalyst different concentrations of sodium hydroxide were investigated (Table 1).
| NaOH (mol) | Bio-oil yield (lignin wt%) | Bio-oil yield ( bamboo wt%) |
|---|---|---|
| — | 19.6 | 5.6 |
| 0.075 | 28.0 | 8.1 |
| 0.15 | 51.6 | 14.9 |
| 0.3 | 31.2 | 9.0 |
For the comparison of the catalysts, different quantities of either CuSO4 or Fe2O3 were used singly (Table 2) or in combination (Table 3) with a constant quantity of NaOH (0.15 mole).
| Catalyst (mmol) | Bio-oil yield (wt% lignin) | Bio-oil yield (wt% bamboo) | |
|---|---|---|---|
| CuSO4 | Fe2O3 | ||
| 1.6 | 51.6 | 14.9 | |
| 3.2 | 40.2 | 11.6 | |
| 0.8 | 34.3 | 9.9 | |
| 1.6 | 32.4 | 9.3 | |
| Catalyst (mmol) | Bio-oil yield (wt% lignin) | Bio-oil yield (wt% bamboo) | |
|---|---|---|---|
| CuSO4 | Fe2O3 | ||
| 0.8 | 0.8 | 28.1 | 8.1 |
| 1.6 | 1.6 | 40.3 | 11.6 |
| 1.1 | 2.1 | 29.5 | 8.5 |
| 2.1 | 1.1 | 33.5 | 9.6 |
Analysis and characterisation
Analysis of the phenolic compounds from bamboo oxidation were performed via HPLC and GC analysis.HPLC analysis was conducted on an Agilent 1100 series HPLC system, fitted with a C18 ULTRASPHERE 5ODS column (Apex Scientific). Details of the HPLC method are reported in the ESI.†
Analysis of the lignin phenols were carried out on a Clarus 500 Gas Chromatograph. Details of the GC method are reported in the ESI.†
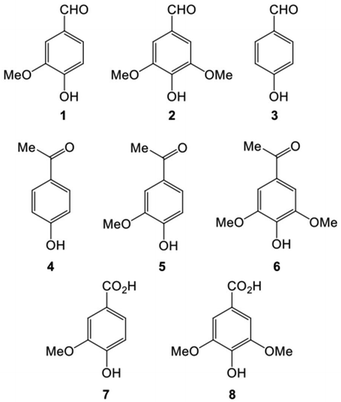
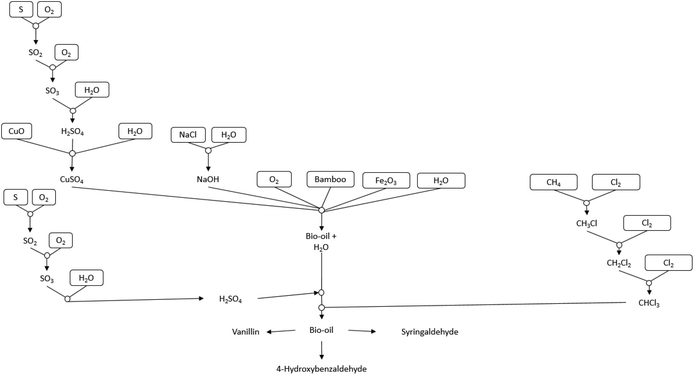
Under WAO of bamboo 8 main aromatic products were observed as shown in Fig. 1.
The aromatic aldehydes vanillin (1), syringaldehyde (2) and 4-hydroxybenzaldehyde (3) are the three main target products in the reaction (Fig. 1) and as such only the yields of these products are discussed. The ketones 4-hydroxyacetophenone (4), acetovanillone (5), and acetosyringone (6) as well as the acids vanillic acid (7) and syringic acid (8) are secondary products. Other, minor organic products include higher-molecular weight carboxylic and phenolic compounds, as well as volatile organic acids such as acetic acid. Substantial amounts of several inorganic compounds including sodium sulfate, sodium carbonate, and copper oxides (from catalysts) was also produced.
Results and discussion
Effect of addition of base on CuSO4 catalysed WAO of lignin
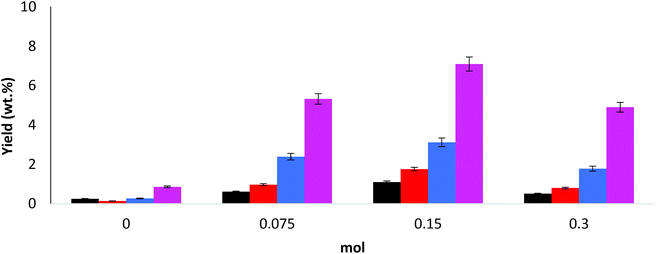
A number of variations of NaOH concentration were studied and were compared to a control experiment where no base was included. Generally, the bio-oil yields (as a percentage of starting material) are much higher with alkali present than without (see Table 1).Increasing the NaOH to 0.075 mol increased the yield from 5.6% to 8.1%. At 0.15 mol NaOH (as reported previously19) the yield of bio-oil increased further (up to 14.9%). Further increasing NaOH to 0.3 mol results in a reduction of the bio-oil yield (9.0%). The yield increases with concentration of NaOH, with the maximum yield occurring at 0.15 mol NaOH, but at higher concentrations than this (i.e. 0.3 mol) there is a reduction in the yield. A possible explanation for this is that at the highest alkaline levels, complete mineralization of the intermediates is taking place with the aldehydes being converted into CO2 and H2O when the available, more reactive lignin substrate has been exhausted and further oxidation becomes the dominant process. A distribution of the aldehyde products is reported in Fig. 2.
The desirable products in this process are the aromatic aldehydes and to a lesser extent the ketones and acids. Predominantly, the largest yields were observed for aldehydes (see Fig. 1), specifically 4-hydroxybenzaldehyde (4HB), vanillin (V) and syringaldehyde (S). The addition of NaOH has increased the yield of all the aldehydes, with the highest combined aldehyde yield (5.98 wt%) at 0.15 mol NaOH, which also corresponds to highest observed yield of bio-oil, as reported in Table 1. In all experiments with base added the product yields were in the order 4HB < V < S, a similar trend is reported by Zhou et al.28 reported a quantity of products in order of V < 4HB < S but only under catalytic conditions.
Fig. S1 in ESI† report the yields of acids and ketones, respectively. A 0.15 mol concentration of NaOH gives the highest acid yields (0.29 wt%), but the highest ketone yields (total of 1.34 wt%) were observed at 0.3 mol. This might indicate that peak production of both acids and aldehydes will occur in the concentration range greater than 0.075 mol but lower than 0.3 mol.
The product distribution within the acid and ketone groups also varied dependent on pH. From Fig. S1,† in most cases when the base was added, vanillic acid (VA) yield was higher than syringic acid (SA). At NaOH concentration of 0.3 mol, however, this trend is reversed and the yield of SA was approximately twice that of VA. For the ketones (Fig. S1†), acetosyringone (AS) was always the major product, while similar yields of acetovanillone (AV) and acetophenone (AP) were observed at 0.15 mol NaOH. At 0.075 mol NaOH, however, AV was the second most abundant ketone, while at 0.3 mol NaOH it was found to be AP.
Clearly, alkali is an important reagent in lignin oxidation.19,29,30 It helps to swell the wood, makes degradation and depolymerisation of the lignin polymer process more efficient and helps to stabilize the phenoxy aldehyde products (vanillin and syringaldehyde) towards further oxidative degradation.31 The most common degradation reactions that occurred during the lignin oxidation involving NaOH include the cleavage of α-aryl ether bonds.32 However, the reactivity of such linkages is sensitive to the type of moiety (i.e., free or etherified phenolic group) present at the para position relative to the phenylpropanoid side chain. The α-aryl ether linkage is readily cleaved, the reaction involving an alkali-assisted rearrangement of the phenolate structure to the corresponding quinone methide structure with elimination of the α-aryloxy substituent.
Another benefit of the high pH is to give aldehydes stability, at first the lignin oxidizes to aldehydes, then the aldehydes oxidize to carboxylic acids.33 From the literature on the Borregaard process it is known that the pH must be kept high to avoid further oxidation of vanillin to vanillic acid.34
Predominately it has been observed that 0.15 mol NaOH (pH 12.6) provides the best conditions, given that it resulted in the highest bio-oil yield, as well as the highest yield of aldehydes. For this reason, 0.15 mol NaOH (pH 12.6) was selected as the conditions for the comparison of the different catalysts (vide infra).
Effect of different catalysts for WAO of lignin
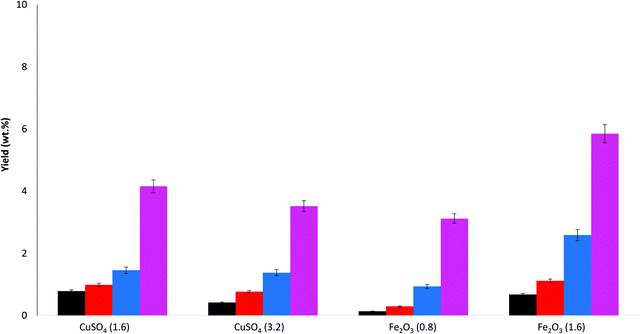
In order to optimise further the catalytic wet oxidation of lignin a number of other catalysts have been investigated. The catalysts studied are reported in Table 2, along with the bio-oil yields.Two different masses of catalyst were studied for both catalysts, and direct comparisons are made on the basis of equal metal content; i.e. 0.8 mmol of Fe2O3 contains the same amount of metal (Fe) as 1.6 mmol of CuSO4 (Cu). From the results it is clear that CuSO4 gives higher yields of bio-oil. It also interesting to note that increasing the amount of CuSO4 from 1.6 mmol to 3.2 mmol has a detrimental effect on the bio-oil yield (decreases from 14.9% to 11.6%). Increasing the amount of Fe2O3 from 0.8 to 1.6 mmol also had a slight detrimental effect on the bio-oil yield.
The yields of the main aldehyde products are reported in Fig. 3 (Fig. S2 in ESI† reports acids and ketones).
From Fig. 3, the trend in aldehyde product yields was S > V > 4HB for all the catalyst compositions studied. Again, overall yields of acids and ketones are relatively low compared to the aldehydes, Fig. S2.† For acids, increasing the amount of CuSO4 increased the yield of syringic acid, but decreased the yield of vanillic acid. Increasing the amount of Fe2O3 decreased the yield of syringic acid while increasing the yield of vanillic acid. For the ketones, increasing the amount of CuSO4 increased the acetosyringone yield, while increasing the amount of Fe2O3 decreased the acetosyringone yield.
In summary, CuSO4 and Fe2O3 are effective catalysts for reasonable overall bio-oil yields and also report good selectivity to aldehydes, particularly syringaldehyde. Consequently, a mixed catalyst of CuSO4 and Fe2O3 has been further studied for the WAO of lignin.
Mixed CuSO4 and Fe2O3 catalyst for WAO of lignin
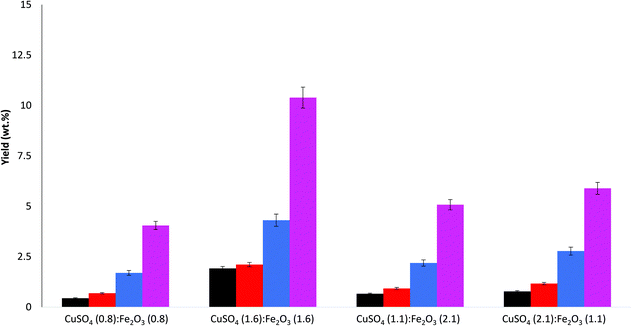
The catalyst mixture compositions are reported in Table 3.When the mixed catalyst was comprised of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of CuSO4
1 of CuSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 (0.8 mmol each) the bio-oil yield was 8.1%. When the total amount of each catalyst was doubled and the 1
Fe2O3 (0.8 mmol each) the bio-oil yield was 8.1%. When the total amount of each catalyst was doubled and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio maintained (i.e. to 1.6 mmol of both CuSO4 and Fe2O3) the bio-oil yield was increased to 11.7%. Two further compositions of ∼2
1 ratio maintained (i.e. to 1.6 mmol of both CuSO4 and Fe2O3) the bio-oil yield was increased to 11.7%. Two further compositions of ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios, with both components being the major constituent were also investigated. Interestingly, the compositions of 2
1 ratios, with both components being the major constituent were also investigated. Interestingly, the compositions of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios (total 3.2 mmol) both had lower yields than the 1
1 ratios (total 3.2 mmol) both had lower yields than the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio mixed catalyst (total 3.2 mmol).
1 ratio mixed catalyst (total 3.2 mmol).
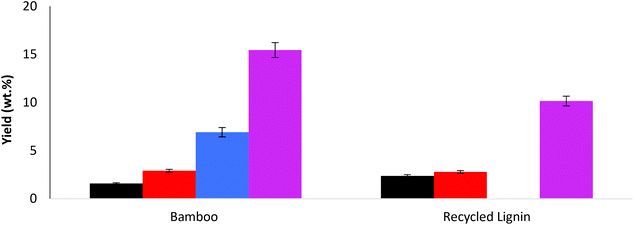
The aldehyde product yields for the mixed catalyst experiments are reported in Fig. 4. The trend is predominantly the same as before (S > V > 4HB) for all the catalyst compositions studied. The highest aldehyde yield was observed for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of CuSO4
1 ratio of CuSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 (total 3.2 mmol), which corresponds to the highest bio-oil yield. In fact, the aldehyde yield reflects the bio-oil yield in all cases.
Fe2O3 (total 3.2 mmol), which corresponds to the highest bio-oil yield. In fact, the aldehyde yield reflects the bio-oil yield in all cases.
The yields of acids and ketones (Fig. S3†) were substantially lower than the aldehyde yields. For acids, the highest yields were also observed for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of CuSO4
1 ratio of CuSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 (total 3.2 mmol). Vanillic acid was the main acid product in all cases, except for the 1
Fe2O3 (total 3.2 mmol). Vanillic acid was the main acid product in all cases, except for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CuSO4
1 CuSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 (total 1.6 mmol) catalyst. Interestingly, no syringic acid was produced for either catalyst with the 2
Fe2O3 (total 1.6 mmol) catalyst. Interestingly, no syringic acid was produced for either catalyst with the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. For the ketones, the yields (and trends) reflect those of the corresponding aldehydes.
1 ratio. For the ketones, the yields (and trends) reflect those of the corresponding aldehydes.
These results indicate that both copper and iron are working independently on different mechanistic pathways playing very different, but still very important roles during the oxidation process, as was indicated previously.22,48–50 It is clear that an equimolar catalyst concentration is best but that the reaction is more sensitive to a decrease in Cu2+ content than Fe3+ content.
Life cycle thinking analysis of bamboo oxidation for vanillin production
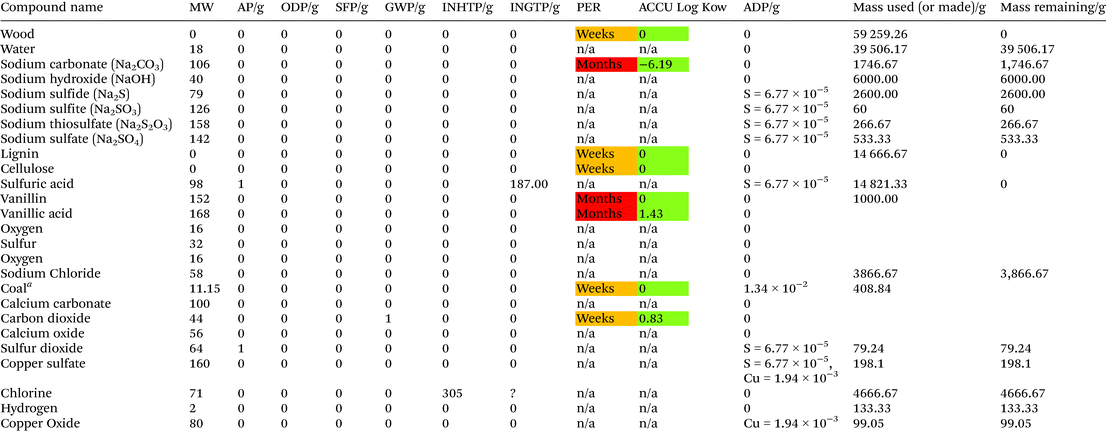
Table 4 and Fig. 5 show the LCT Inventory and synthesis tree of WAO of bamboo for vanillin production. The method analysed via LCT was for catalyst mixture CuSO4 (1.6 mmol): Fe2O3 (1.6 mmol), as shown in Fig. 4. Scaled to produce 1 kg of vanillin, scaling factor of 9494 ± 5.03%, this method produces an average of 910 g and 2044 g of 4-hydroxybenzaldehyde and syringaldehyde respectively. The reaction mixture is composed of 2437 g ± 10.02% of both CuSO4 and Fe2O3 with 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423 g ± 5.79% of bamboo and 57
423 g ± 5.79% of bamboo and 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121 g ± 10.02% NaOH. The chloroform required for separation is 4
121 g ± 10.02% NaOH. The chloroform required for separation is 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244
244![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 114 g ±5.03%.
114 g ±5.03%.
The production of 1 kg vanillin has an AP = 6.34 × 103, ODP = 5.19 × 105, SFP = 1.81 × 106, GWP = 9.16 × 108, INHTP = 5.98 × 107, INGTP = 1.75 × 107 and ADP = 3.33. The largest contribution to each category is attributed to the use of chloroform as an extractive solvent.
Mixed CuSO4/Fe2O3 catalyst for oxidation of recycled lignin
Treatment of bamboo with WAO there is a significant amount of residual material between 90–95% of starting material. Therefore, if wet oxidation is found to be viable to further process this “recycled” lignin, it opens the possibility of two complementary processes generating different added-value product streams from different parts of biomass. This recycled lignin oxidation reaction was conducted to investigate if the lignin by-product could also be a source of the lignin oxidation products that have already been observed from the oxidation of raw bamboo herein. The intention being the investigation of the complimentary of the wet air oxidation of lignin (from bamboo) and the acid pyrolysis of cellulosic material (from miscanthus); i.e. can the by-products of each process become feedstocks for the other.Miscanthus grass is a well-studied energy crop with a number of reviews detailing benefit to the bio-economy.35–37 The standard conditions described in the Experimental section (0.15 mol NaOH, 6 bar O2, 195 °C) were used with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CuSO4
1 CuSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 (total 3.2 mmol) catalyst for a 15 min reaction.
Fe2O3 (total 3.2 mmol) catalyst for a 15 min reaction.
The bamboo and recycled lignin feedstocks have been compared under these conditions, and the bio-oil yields are reported in Table 5. The actual yield from the bamboo (467.1 mg per 32.4 wt%) was higher than that obtained from the recycled lignin (117.8 mg per 36.4 wt%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe2O3 (1.6))
Fe2O3 (1.6))
The actual lignin content for bamboo was 1.44 g (based on 28.8 wt%) and 0.324 g for the recycled lignin (see ESI† for determination); i.e. ∼4.5 times more lignin was available in bamboo. The actual bio-oil yield from bamboo is ∼4 times that from the recycled lignin, and as such there is a reasonable pro rata comparison of the bio-oil yields. The aldehyde product yields for both feedstocks are reported in Fig. 6, while the acid and ketone yields can be found in the ESI in Fig. S4.†
The trends for bamboo are the same as those reported for the 90 min test reported in the previous section, although the total yields are lower due to shorter reaction time. It is evident that the recycled lignin can be converted to useful value-added products. As would be expected from the Miscanthus grass lignin, only the vanillin series of products is generated. Vanillin (2.7 wt%) occupies the largest yield of the products, followed by 4-hydroxybenzaldehyde (2.4 wt%), acetovanillone (2.1 wt%), acetophenone (1.9 wt%) and vanillic acid (1.0 wt%).
NaOH is playing an important role in the oxidation of raw bamboo powder, it can accelerate the reaction by helping swell the wood, makes degradation and depolymerisation of the lignin polymer process more efficient and also helps to stabilize the phenoxy aldehyde products (4-hydroxybenzaldehyde, vanillin and syringaldehyde) against further oxidative degradation.
Experiments with different catalysts addressed the effects of Cu2+ and Fe3+ on the yields. A combination of Cu2+ and Fe3+, however, was far more effective in improving the yields and selectivity toward the oxidation reactions. The best results show that the use of both Cu2+ and Fe3+ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio improves the yields of aldehydes.
1 ratio improves the yields of aldehydes.
Life cycle thinking comparison
In order to assess the environmental impact of the new production method for vanillin compared to established methods a life cycle thinking analysis was conducted. A more detailed analysis is reported in the ESI.†Crude oil synthesis route
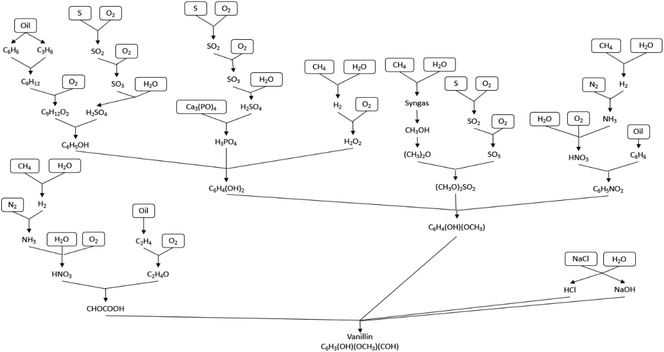
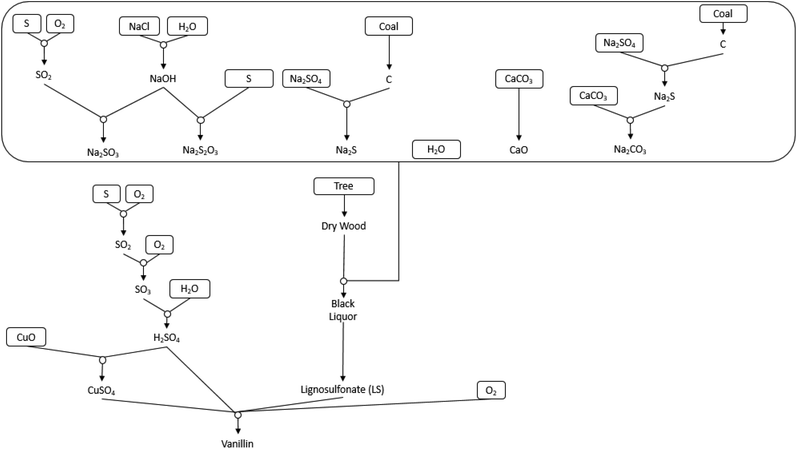
A synthesis tree used for the production of vanillin from crude oil is shown in Fig. 7, excluding side products. A life cycle thinking analysis of the side product processes is shown in the ESI.†Fig. 7 shows the synthesis of vanillin from oil via:
1. Production of phenol from benzene and propene
2. Hydroxylation of phenol to catechol
3. Methylation of catechol to guaiacol
4. Synthesis of vanillin from guaiacol via Vilsmeier formylation.
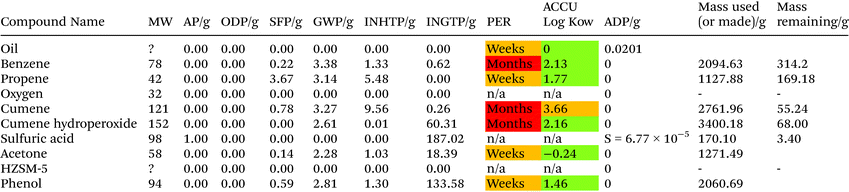
Phenol is assumed to be produced from benzene and propene, via a reaction with oxygen then sulfuric acid, cumene-hydroperoxide process38
In the production of 1 kg of vanillin the reaction process as shown in eqn 1 and 2 was used:
| Guaiacol + glyoxylic acid + NaOH → vanillylmandelic acid + HCl | (1) |
| Vanillylmandelic acid + HCl → vanillin + NaCl | (2) |
For the production of guaiacol methylation of catechol was used via a dimethyl sulfate in nitrobenzene, as shown in eqn 3:
| Catechol + dimethyl sulfate + NaOH → guaiacol + methyl sulfate | (3) |
Catechol is assumed to be produced from phenol, as shown in eqn 4:
 | (4) |
Phenol is assumed to be produced from benzene and propene, as shown in (eqn 5, 6 and 7):
| Benzene + propene → cumene | (5) |
| Cumene + O2 → cumene hydroperoxide | (6) |
| Cumene hydroperoxide + H2SO4 (500 ppm) → phenol + acetone | (7) |
Tables 6–9 report the synthesis of vanillin from oil starting with benzene. Due the mature nature of the process has developed to overcome any handling issues and increase selectivity/yield.
Crude oil to vanillin life cycle thinking
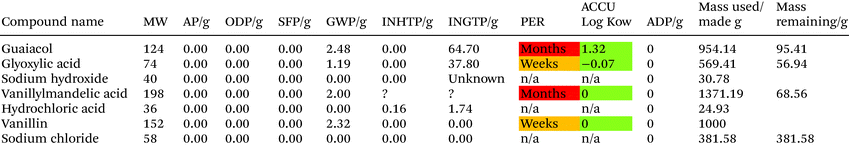
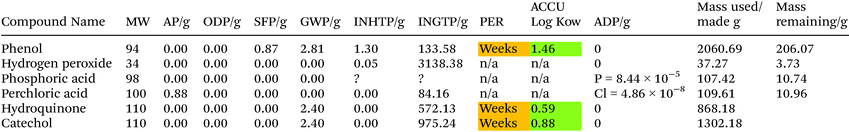
To produce 1 kg of vanillin 954 g of guaiacol is required with an assumed 90% yield for step 1 and a 95% yield for step 2.39 The areas of concern within this process are accumulation of material with the environment if released, PER, and the high ingestion toxicity to humans, INGTP, of compounds used within this mixture. The process limits the negative effects of this process due to the mature nature of the reaction with selectivity and yields to limit an oversupply of potentially unwanted environmentally negative compounds. Glyoxylic acid, while itself not a major concern within the LCT, has several compounds of concern within its synthesis process which would raise concern and it would be environmentally beneficial to explore alternatives, see ESI.†To produce 954.14 g of guaiacol 1302 g of catechol is required at an assumed 65% yield.40 The production of guaiacol presents an increase number of issues with ADP from sulfur a concern along with the high INGTP of catechol. The production of both dimethyl sulfate and nitrobenzene also require the use of sulfuric acid which has the potential to highly environmentally damaging to the presence of a number of compounds, see ESI.† If an alternative to both dimethyl sulfate and nitrobenzene can be found, which don't have sulfuric acid in their synthesis routes, this process could become more environmentally benign. The production of 1302.18 g of catechol requires 2060.69 g of phenol at an assumed 90% yield. There are four values over 100 for INGTP per g, 0.87 SFP per g, 0.88 AP per g and depletion of sulfur. The production of H2O2, phosphoric acid and perchloric acid each have environmental concerns associated with them, see ESI.†
The production of 2060.69 g of phenol requires 1016.47 g and 547.33 g of benzene and propene respectively. This process is the one of most optimised and industrially utilised processes in the production of vanillin from crude oil. It was assumed the eqn (5) has a 85% yield while the other reactions are 98% respectively.38 The AD value for this process is assumed to be greater than 1000 due to the use of crude oil as a starting material. The generation of benzene alone as a starting material could generate a value of over 1000 as benzene is between 3.9–7% of crude oil.41 The main areas of environmental concern are SFP, AD and INGTP.
Kraft lignin to vanillin life cycle thinking
A synthesis tree for vanillin synthesis for Kraft Lignin is shown in Fig. 8, excluding side products. A life cycle thinking analysis of the process is shown in ESI.† The method published by Pacek et al.25 for vanillin production from Kraft lignin was chosen for comparison with other vanillin production methods.The method was scaled to produce 1 kg of vanillin by a scaling factor of 66.6 from the published details. Assuming 15 g L−1 of vanillin is produced per 220 g L−1 of lignin entering the reaction with a lignin content in raw biomass of 27.5%.
For the production of 1 kg of vanillin it is found the majority of the reactants do not have an environmental impact across the majority of categories measured except copper sulfate, chlorine, sulfuric acid and the compounds associated with the production sulfuric acid, see ESI.†Table 10 shows the LCT inventory for the production of vanillin from Kraft Lignin. Sulfuric acid is associated with 1.88% of the acidification potential of the reaction while the production of it is 98.12%. All of the INGTP of the reaction is associated with sulfuric acid while the INHTP is associated with chlorine in the production of NaOH from seawater.
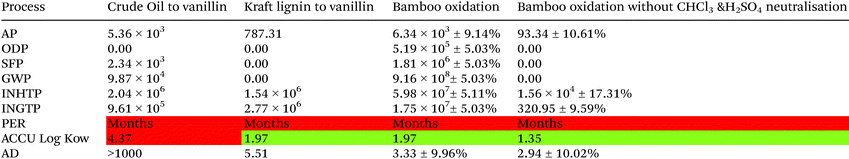
A comparison of the synthesis route from crude oil versus the synthesis route from Kraft lignin and the bamboo oxidation explored in this paper is shown in Table 11.
Vanillin synthesis from crude oil, as discussed previously (Tables 6–9), shows significant environmental impacts in every area, except for ozone depletion potential, as there are no CFCs used in the production route analysed. The major areas of concern are human toxicity by inhalation potential (INHTP) and human toxicity by ingestion potential (INGTP). The large value for accumulation, 4.37, could potentially be reduced as this is associated with 2-ethylanthraquinone. This compound is reused extensively in the production of hydrogen peroxide and therefore the mass of the compound could be reduced.
The production of vanillin from kraft lignin, as discussed in Table 10, has a minimal environmental impact with any environmental impact associated with the use of sulfuric acid (H2SO4). The process is scaled by a factor of 66.6 times to achieve 1 kg of vanillin compared to the method by Pacek et al.25
The synthesis of vanillin from catalytic wet oxidation of bamboo has a significantly higher environmental impact when compared to the crude oil and Kraft lignin methods of production. The impact is, in part, as a scaling factor of 9494 ± 5.03% is required to produce 1 kg of vanillin from the bamboo wet oxidation. With this increase, there is a requirement for 4244 kg ± 5.03% of chloroform per kg of vanillin. If an alternative solvent and alternative acid for neutralisation is utilised in the process, the oxidation of bamboo for vanillin improves significantly. Therefore, a greener extraction solvent is key to this process being sustainable. This is certainly possible and other solvent systems are already showing some promise in this area.19,43,44
Other areas of improvement are lowering acidification potential from 5362 or 787 using crude oil and kraft lignin process respectively to 93.34 using the bamboo oxidation. Human toxicity by inhalation potential is also lowered significantly from 2.4 million and 1.54 million respectively to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631 ± 17.31%. Both values remain elevated due the use of sulfuric acid, and associated values, in the production of copper sulfate.
631 ± 17.31%. Both values remain elevated due the use of sulfuric acid, and associated values, in the production of copper sulfate.
Conclusions
The transition from production of synthetic vanillin from oil to kraft lignin would be environmentally beneficial across all assessed impact categories, particularly in ODP, SFP and GWP were it is calculated there would be minimal impact to the environment from processing. Production of vanillin from bamboo via wet air oxidation still requires optimisation but a change of solvent utilised to extract the bio-oil from chloroform to a green solvent could significantly improve the environmental impact of the process.It has also been demonstrated that this oxidative depolymerisation method could be applied successfully to a lignin waste stream from another process, the acid pyrolysis of cellulosics in biomass. This opens the possibility of two complementary processes generating different added-value product streams from different parts of biomass, a key goal in the bio-refinery principle.
Consequently, the life cycle thinking approach can facilitate the success of the United Nation Sustainable Development Goal 12 for Responsible Consumption and Production.
Disclaimer
The views and opinions expressed in this paper do not necessarily reflect those of the European Commission or the Special EU Programmes Body (SEUPB).Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work is funded as part of the ReNEW Network project under INTERREG IVB NWE Subsidy Contract 317J and The Bryden Centre project which is supported by the European Union's INTERREG VA Programme, managed by the Special EU Programmes Body (SEUPB). Funding under the CASTech Grant (EP/G012156/1) from the EPSRC is also gratefully acknowledged.References
- A. Corma, G. Huber, L. Sauvanaud and P. O'Connor, Biomass to chemicals: Catalytic conversion of glycerol/water mixtures into acrolein, reaction network, J. Catal., 2008, 257(1), 163–171 CrossRef CAS.
- United Nations. United Nations Sustainable Development Goals. [Online]. [cited 2020 July 7. Available from: https://sustainabledevelopment.un.org/?menu=1300.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney and C. A. Eckert, et al. The path forward for biofuels and biomaterials, Science, 2006, 311(5760), 484–489 CrossRef CAS.
- C. Isola, H. L. Sieverding, A. M. Numan-Al-Mobin, R. Rajappagowda, E. A. Boakye and D. E. Raynie, et al., Vanillin derived from lignin liquefaction: a sustainability evaluation, LCA for Agricultural Practices and Biobased Industrial Products, 2018, vol. 23, pp. 1761–1772 Search PubMed.
- N. Wongtanyawat, P. Lusanandana, N. Khwanjaisakun, P. Kongpanna, J. Phromprasit and L. Simasatitkul, et al. Comparison of different kraft lignin-based vanillin production processes, Comput. Chem. Eng., 2018, 117, 159–170 CrossRef CAS.
- D. Kralisch, D. Ott and D. Gericke, Rules and benefits of Life Cycle Assessment in green chemical process and synthesis design: a tutorial review, Green Chem., 2015, 17, 123–145 RSC.
- P. Jessop, Editorial: Evidence of a significant advance in green chemistry, Green Chem., 2020, 22, 13–15 RSC.
- P. Jessop, Searching for green solvents, Green Chem., 2011, 13, 1391–1398 RSC.
- J. Vogtländer, P. Van Der Lugt and H. Brezet, The sustainability of bamboo products for local and Western European applications. LCAs and land-use, J. Cleaner Prod., 2010, 18(13), 1260–1269 CrossRef.
- M. F. Silva, M. E. Menis-Henrique, M. H. Felisberto, R. Goldbeck and M. T. Clerici, Bamboo as an eco-friendly material for food and biotechnology industries, Curr. Opin. Food Sci., 2020, 33, 124–130 CrossRef.
- F. C. Chang, K. S. Chen, P. Y. Yang and C. H. Ko, Environmental benefit of utilizing bamboo material based on life cycle assessment, J. Cleaner Prod., 2018, 204, 60–69 CrossRef.
- Q. L. Zhu, B. Wu, F. R. Tan, K. Pan, Q. C. Hu and L. C. Dai, et al. Bamboo: A new source of carbohydrate for biorefinery, Carbohydr. Polym., 2014, 111, 645–654 CrossRef.
- W. Liu, C. Hui, F. Wang, M. Wang and G. Liu, Review of the Resources and Utilization of Bamboo in China. In., 2018.
- M. Kuttiraja, R. Sindhu, P. E. Varghese, S. V. Sandhya, P. Binod and S. Vani, et al. Bioethanol production from bamboo (Dendrocalamus sp.) process waste, Biomass Bioenergy, 2013, 59, 142–150 CrossRef CAS.
- S. Kumar, L. K. S. Gujjala and R. Banerjee, Simultaneous pretreatment and saccharification of bamboo for biobutanol production, Ind. Crops Prod., 2017, 2017, 21–28 CrossRef.
- L. Nayak and S. P. Mishra, Prospect of bamboo as a renewable textile fiber, historical overview, labeling, controversies and regulation, Fashion and Textiles, 2016, 3(1), 1–23 CrossRef.
- R. Vanholme, B. Demedts, K. Morreel, J. Ralph and W. Boerjan, Lignin biosynthesis and structure, Plant Physiol., 2010, 153(3), 895–905 CrossRef CAS.
- A. Sakakibara, A structural model of softwood lignin, Wood Sci. Technol., 1980, 14(2), 89–100 CrossRef CAS.
- C. S. McCallum, N. Strachan, S. C. Bennett, W. G. Forsythe, M. D. Garrett and C. Hardacre, et al. Catalytic depolymerisation of suberin rich biomass with precious metal catalysts, Green Chem., 2018, 20, 2702–2705 RSC.
- M. D. Garrett, S. C. Bennett, C. Hardacre, R. Patrick and G. N. Sheldrake, New methods in biomass depolymerisation: catalytic hydrogenolysis of barks, RSC Adv., 2013, 3(44), 21552 RSC.
- M. Florea and V. I. Parvulescu, Selective Oxidation of Biomass Constitutive Polymers to Valuable Platform Molecules and Chemicals, in Chemicals and Fuels from Bio-Based Building Blocks, 2016, pp. 379–402 Search PubMed.
- L. Zhou, H. Cao, C. Descorme and Y. Xie, Phenolic compounds removal by wet air oxidation based processes, Front. Environ. Sci. Eng., 2018, 12, 1 CrossRef CAS.
- P. Jessop, Green Chemistry Editorial: Evidence of a significant advance in green chemistry, Green Chem., 2020, 22, 13 RSC.
- P. Jessop, Searching for Green Solvents, Green Chem., 2011, 13, 1391–1398 RSC.
- A. W. Pacek, P. Ding, M. Garrett, G. Sheldrake and A. W. Nienow, Catalytic conversion of sodium lignosulfonate to vanillin: Engineering aspects. part 1. effects of processing conditions on vanillin yield and selectivity, Ind. Eng. Chem. Res., 2013, 52(25), 8361–8372 CrossRef CAS.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton, Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (Revised July 2011)., 2008.
- K. Dussan, B. Girisuta, D. Haverty, J. J. Leahy and M. H. B. Hayes, The effect of hydrogen peroxide concentration and solid loading on the fractionation of biomass in formic acid, Carbohydr. Polym., 2014, 111, 374–384 CrossRef CAS.
- S. Zhou, H. Y. Zhan and S. Fu, Study on the degradation of bamboo alkaline lignin by catalytic wet air oxidation to obtain aromatic aldehydes, Trans. China Pulp Pap., 2010, 25(3), 47–51 CAS.
- M. Talawar, T. Jyothi, P. Sawant, T. Raja and B. Rao, Calcined Mg–Al hydrotalcite as an efficient catalyst for the synthesis of guaiacol, Green Chem., 2000, 2, 266–268 RSC.
- R. E. O'Neill, L. Vanoye, C. de Bellefon and F. Aiouache, Aldol-condensation of furfural by activated dolomite catalyst, Appl. Catal., B, 2014, 144, 46–56 CrossRef.
- V. E. Tarabanko, D. V. Petukhov and G. E. Selyutin, New mechanism for the catalytic oxidation of lignin to vanillin, Kinet. Catal., 2004, 45(4), 569–577 CrossRef CAS.
- R. B. Santos, P. W. Hart, H. Jameel and H. M. Chang, Wood based lignin reactions important to the biorefinery and pulp and paper industries, BioResources, 2013, 8(1), 1456–1477 CrossRef.
- V. E. Tarabanko and N. Tarabanko, Catalytic oxidation of lignins into the aromatic aldehydes: General process trends and development prospects, Int. J. Mol. Sci., 2017, 18(11), 2421 CrossRef.
- D. S. Argyropoulos and F. Asgari, Fundamentals of oxygen delignification. Part II. Functional group formation/elimination in residual kraft lignin, Can. J. Chem., 1998, 76, 606–161 Search PubMed.
- C. P. Witzel and R. Finger, Economic evaluation of Miscanthus production - A review, Renewable Sustainable Energy Rev., 2016, 53, 681–696 CrossRef.
- A. I. Osman, A. Abdelkader, C. R. Johnston, K. Morgan and D. W. Rooney, Thermal Investigation and Kinetic Modeling of Lignocellulosic Biomass Combustion for Energy Production and Other Applications, Ind. Eng. Chem. Res., 2017, 56(42), 12119–12130 CrossRef CAS.
- N. Ben Fradj, S. Rozakis, M. Borzęcka and M. Matyka, Miscanthus in the European bio-economy: A network analysis, Ind. Crops Prod., 2020, 148, 112281 CrossRef.
- Phenol – The Essential Chemical Industry - online. [Online]. 2017 [cited 2020 July 1. Available from: https://www.essentialchemicalindustry.org/chemicals/phenol.html.
- J. Kamlet, inventor; Manufacture of Vanillin and its homlogues, United States patent, US 264083, 1953 Search PubMed.
- H. Fiege, H. Voges, T. Hamamoto, S. Umemura, T. Iwata and H. Miki, et al., Ullmann's Encyclopedia of Industrial Chemistry, Phenol Derivatives, 2000 Search PubMed.
- International Agency for Research on Cancer, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 1989 Search PubMed.
- L. Krotz and G. Guido, Elemental Analysis: CHNS/O Determination in Carbon, 2017 Search PubMed.
- C. J. Clarke, W. C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Green and Sustainable Solvents in Chemical Processes, Chem. Rev., 2018, 118(2), 747–800 CrossRef CAS.
- J. Sherwood, M. De bruyn, A. Constantinou, L. Moity, C. R. McElroy and T. J. Farmer, et al. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents, Chem. Commun., 2014, 50(68), 9650–9652 RSC.
- H. Debellefontaine, M. Chakchouk, J. N. Foussard, D. Tissot and P. Striolo, Treatment of organic aqueous wastes: Wet air oxidation and wet peroxide oxidation, Environ. Pollut., 1996, 92(2), 155–164 CrossRef CAS.
- K. R. Alunga, Y. Y. Ye, S. R. Li, D. Wang and Y. Q. Liu, Catalytic oxidation of lignin-acetoderivatives: A potential new recovery route for value-added aromatic aldehydes from acetoderivatives, Catal. Sci. Technol., 2015, 5(7), 3746–3753 RSC.
- P. C. R. Pinto, C. E. Costa and A. E. Rodrigues, Oxidation of lignin from eucalyptus globulus pulping liquors to produce syringaldehyde and vanillin, Ind. Eng. Chem. Res., 2013, 52(12), 4421–4428 CrossRef CAS.
- K. Morgan, K. J. Cole, A. Goguet, C. Hardacre, G. J. Hutchings and N. Maguire, et al. TAP studies of CO oxidation over CuMnOX and Au/CuMnOX catalysts, J. Catal., 2010, 276(1), 38–48 CrossRef CAS.
- K. Morgan, R. Burch, M. Daous, J. J. Delgado, A. Goguet and C. Hardacre, et al. Re-dispersion of gold supported on a ‘mixed’ oxide support, Catal., Struct. React., 2015, 1(3), 120–124 CrossRef.
- G. Wu and M. Heitz, Catalytic mechanism of Cu2 + and Fe3 + in alkaline O2 oxidation of lignin, J. Wood Chem. Technol., 1995, 15(2), 189–202 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc03685d |
| This journal is © The Royal Society of Chemistry 2021 |