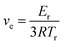Malleable and thermally recyclable polyurethane foam†
Xiang-Zhao
Wang
a,
Meng-Shi
Lu
a,
Jian-Bing
Zeng
 *a,
Yunxuan
Weng
b and
Yi-Dong
Li
*a
*a,
Yunxuan
Weng
b and
Yi-Dong
Li
*a
aChongqing Key Laboratory of Soft-Matter Material Chemistry and Function Manufacturing, School of Chemistry and Chemical Engineering, Southwest University, 2# Tiansheng Road, Chongqing 400715, China. E-mail: jbzeng@swu.edu.cn; liyidong@swu.edu.cn
bBeijing Key Laboratory of Quality Evaluation Technology for Hygiene and Safety of Plastics, Beijing Technology and Business University, 11# Fucheng Road, Beijing, 100048, China
First published on 11th December 2020
Abstract
Thermosetting polyurethane (PU) foams, which cannot be recycled economically and efficiently due to their permanently crosslinked structures, have caused significant environmental concerns after service. To improve the sustainable development of the PU foam industry, herein, we report malleable PU foams that contain a dynamic disulfide bond. The disulfide exchange reaction under heat enables the rearrangement of the network topology of the PU foam, imparting malleability and thermal processability. The disulfide containing PU foams (PUSFs) have similar appearance and physical properties to common PU foams and were prepared using conventional foaming technology without any modification. The PUSFs can be easily recycled into PU films through thermal compression molding. The recycled PU films show excellent and tunable mechanical properties depending on the compositions of the original malleable PU foams. Furthermore, the PU film recycled from PU foam with a well-designed composition can be further reprocessed several times without obvious loss in mechanical proprieties and change in chemical structures. This investigation provides a novel methodology to recycle and reuse PU foams and is expected to promote the sustainable development of the PU foam industry.
Versatile thermosetting polyurethanes (PUs), which account for 31% of the thermoset market, are widely used as adhesives, coatings, elastomers, and foams in various fields such as furniture, household appliances, automobile, sound and thermal insulations, construction materials, etc.1,2 Although durable products are preferred in most of these applications, thermosetting polyurethane wastes are growing drastically due to the inevitable wear and upgrading of polyurethane containing goods. Therefore, strategies to recycle PUs especially PU foams have attracted increasing attention. However, PU foams are featured by permanently crosslinked structures, which makes it impossible to recycle them by melt reprocessing and seriously restricts practical recycling.3 Like other thermosetting polymers, mechanical recycling and chemical degradation represent the most widely used approaches to recycle PU foams.4 Both approaches are types of downcycling in which the PU foams are recycled into lower value products.5 PU foams are either crushed and reused as fillers in mechanical recycling or recycled into polyols or other small molecules through chemical degradation with the aid of a catalyst.6,7 Therefore, it is highly desirable to develop alternative strategies to recycle PU waste into similar or even higher value products, which is of great significance for the sustainability of PU foams.
The incorporation of dynamic covalent bonds represents a promising strategy for preparing malleable thermosets, also known as covalent adaptable networks (CANs), which can be reconfigured, reprocessed, or reshaped via exchange reaction induced network topology rearrangement under external stimuli such as heat, pH, or UV-light.8–12 Based on the mechanism of the exchange reaction, CANs with different dynamic covalent bonds are normally categorized into dissociative and associative CANs.13 Dissociative CANs such as those containing Diels–Alder linkages undergo reversible reactions, resulting in network destruction and thus poor solvent resistance and dimensional stability.14,15 Associative CANs, usually known as vitrimers, maintain their network integrity as well as solvent resistance and dimensional stability due to the mechanism of the associative exchange reaction.16,17 Since Leibler et al. pioneered the first epoxy vitrimer based on Zn2+ catalyzed transesterification of a carboxylic acid cured epoxy network in 2011,18 a large number of vitrimers have been developed based on various dynamic covalent bonds including disulfides,19–21 Schiff base,22,23 transesterification,24 vinylogous urethane,25 thiol-Michael reaction,26 transthioesterification,27 boronic esters and boronates,28,29 and Si–O exchange in siloxanes and silyl ethers.30,31 The vitrimers with crosslinked structures are malleable and can be reprocessed with conventional processing techniques such as compression molding,32 injection molding,15 and extrusion.33
Some PU CANs based on hindered urea bonds,34,35 thiourethane bonds,36 hydroxyl-carbamate exchange reactions,37 and disulfide bonds38,39 have been reported in the recent literature. These PU CANs showed various advantages including malleability, shape memory, recyclability, self-healing and degradability.34–39 Despite the progress made, PU CANs reported in most studies were rigid plastics or elastomers rather than PU foams; however, more than 2/3rd of commercial PU products are produced as foams.4 In a recent study, Sheppard and coworkers have reported a new strategy to reprocess PU foams by introducing a carbamate exchange catalyst (dibutyltin dilaurate) and subsequent melt reprocessing at elevated temperatures.40 Despite successful reprocessing, this approach involves the use and removal of a large amount of organic solvents, which are used to swell PU foams for the incorporation of the catalyst, making the reprocessing costly and environmentally harmful. Therefore, it is urgent and highly desirable to develop more eco-friendly strategies for efficient recycling or reprocessing of PU foams.
Here, we report the design and fabrication of malleable PU foams bearing dynamic disulfide bonds. The malleable PU foams can be recycled directly into PU films under heat through compression molding. The recycled PU films have shown good mechanical properties and can be further reprocessed or recycled several times by the same method without obvious loss in mechanical properties. We have studied the effect of disulfide bonds on the structure and properties of malleable PU foams as well as the structure and properties of the recycled PU films.
A polysulfide oligomer (PSO) was used as the disulfide bond component. The active thiol groups enable the incorporation of PSO into the network structure of PU foam. Malleable PU foams are synthesized using polypropylene glycol, polysulfide oligomer, toluene diisocyanate, and various additives (Fig. 1). We prepared five PU foams with PPG/PSO weight ratios from 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 90
0 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and 60
30 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
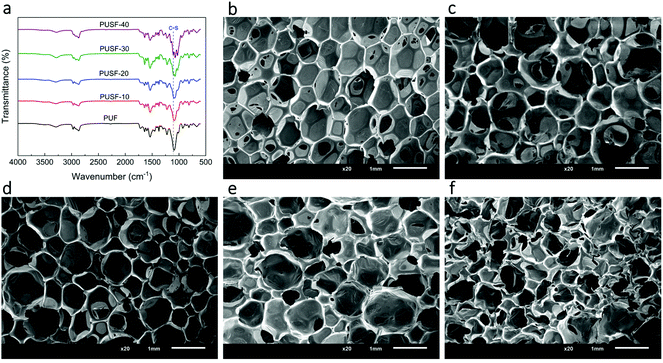
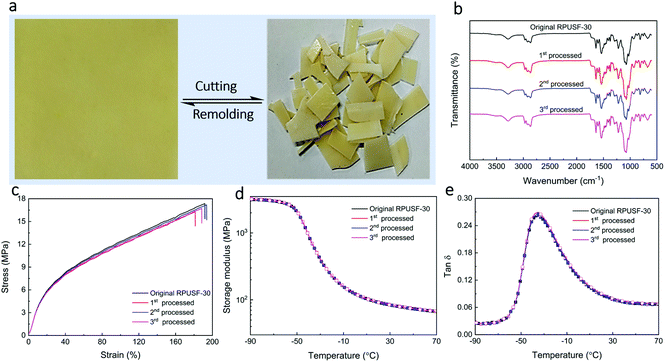
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (Table S1†). For brevity, the PU foam without a disulfide bond was designated as PUF and the four PU foams with different contents of PSO were designated as PUSF-10, PUSF-20, PUSF-30, and PUSF-40, respectively. The chemical structures of the PU foams were characterized by FT-IR spectroscopy (Fig. 2a). The stretching vibration of C–S, which was not observed for PUF, was observed at ∼1115 cm−1 for PUSFs and became more prominent with the increasing PSO content. In addition, the stretching vibration of S–H at ∼2556 cm−1 in the FT-IR spectra of PSO (Fig. S1†) disappeared in the spectra of PUSFs, indicating that thiol groups were completely consumed by reacting with the isocyanate group of TDI during the foaming process. These phenomena indicate that PSO, namely, the dynamic disulfide bond component was successfully incorporated into PU foams.
40 (Table S1†). For brevity, the PU foam without a disulfide bond was designated as PUF and the four PU foams with different contents of PSO were designated as PUSF-10, PUSF-20, PUSF-30, and PUSF-40, respectively. The chemical structures of the PU foams were characterized by FT-IR spectroscopy (Fig. 2a). The stretching vibration of C–S, which was not observed for PUF, was observed at ∼1115 cm−1 for PUSFs and became more prominent with the increasing PSO content. In addition, the stretching vibration of S–H at ∼2556 cm−1 in the FT-IR spectra of PSO (Fig. S1†) disappeared in the spectra of PUSFs, indicating that thiol groups were completely consumed by reacting with the isocyanate group of TDI during the foaming process. These phenomena indicate that PSO, namely, the dynamic disulfide bond component was successfully incorporated into PU foams.
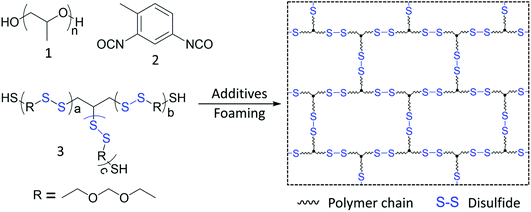 | ||
| Fig. 1 Synthesis of malleable PU foam containing a disulfide bond using 1 polypropylene glycol (PPG), 2 toluene diisocyanate (TDI), and 3 polysulfide oligomer (PSO) as the main starting materials. | ||
The cross sections of the PU foams were evaluated by SEM (Fig. 2b–f). A small amount of PSO (PPG/PSO ≥ 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) didn't obviously influence the cell morphology of the PU foams. When the PPG/PSO weight ratio decreased to 60
30) didn't obviously influence the cell morphology of the PU foams. When the PPG/PSO weight ratio decreased to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, the cell size decreased and the number of open cells obviously increased for PUSF-40 (Fig. 2f), which suggests that a high amount of PSO could affect the foaming process substantially due to the different structures and reactivities of PSO and PPG. The incorporation of PSO and the increase in its content increased the density of the PU foams slightly (Fig. 3a) because of the slightly increased content of the rigid TDI moiety (Table S1†) and the increased size of the strut joint of the PU foams with the increase in the PSO content (Fig. S2†). The incorporation of PSO almost did not influence the excellent resilience of the PU foams, all of which showed a resilience rate higher than 97% (Fig. 3b). These findings indicate that the polysulfide oligomer can be easily incorporated into PU foams without modifying foaming technology and without obviously changing the foam density and mechanical resilience, which is preferred for large scale production and practical application.
40, the cell size decreased and the number of open cells obviously increased for PUSF-40 (Fig. 2f), which suggests that a high amount of PSO could affect the foaming process substantially due to the different structures and reactivities of PSO and PPG. The incorporation of PSO and the increase in its content increased the density of the PU foams slightly (Fig. 3a) because of the slightly increased content of the rigid TDI moiety (Table S1†) and the increased size of the strut joint of the PU foams with the increase in the PSO content (Fig. S2†). The incorporation of PSO almost did not influence the excellent resilience of the PU foams, all of which showed a resilience rate higher than 97% (Fig. 3b). These findings indicate that the polysulfide oligomer can be easily incorporated into PU foams without modifying foaming technology and without obviously changing the foam density and mechanical resilience, which is preferred for large scale production and practical application.
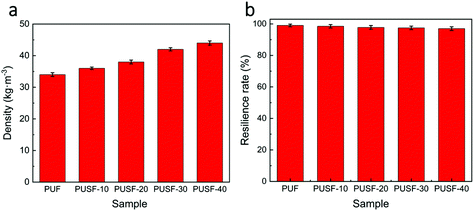 | ||
| Fig. 3 Physical properties of PUF and PUSFs. (a) Density of PUF and PUSFs with different compositions. (b) Resilience rate of PUF and PUSFs with different compositions. | ||
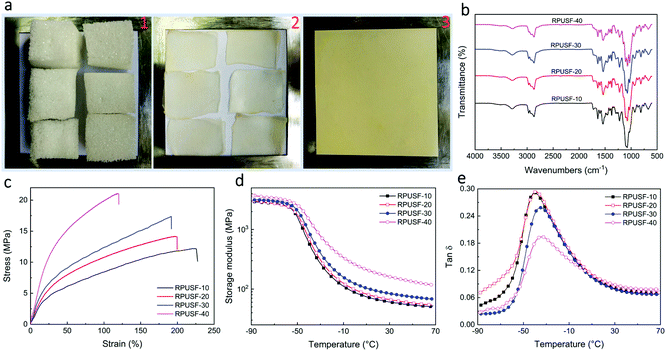
PU foams were cut into small pieces (Fig. 4a1). The small pieces of PU foams were recycled by compression molding at 180 °C under 20 MPa for 30 min. After compression molding, the pieces of PUF foams became compacted (Fig. 4a2) but didn't combine together to form a well-shaped film, indicating no malleability of the PUF foam due to the permanently crosslinked structure. In contrast, a well-developed PU film was obtained by compression molding of PUSF foams with PUSF-30 as a typical example (Fig. 4a3), attributing to the presence of a disulfide bond which could confer malleability to the PU foam via network topology rearrangement as a result of the disulfide exchange reaction under heat. Other PUSF foams can also be recycled into PU films (Fig. S3†). For brevity, the recycled PU films from PUSF-10, PUSF-20, PUSF-30, and PUSF-40 were designated as RPUSF-10, RPUSF-20, RPUSF-30, and RPUSF-40, respectively. The FT-IR spectra of recycled RPUSF films (Fig. 4b) were almost the same as those of the original PUSF foam counterparts (Fig. 2a), which indicates that the recycling process didn't change the chemical structure of the samples.
The mechanical properties of the recycled PU films were measured by tensile testing (Fig. 4c and Table S2†). It is interesting that the PU films show excellent mechanical properties with elongation at break in the range of 110–230%, tensile strength in the range of 12–21 MPa, and Young's modulus in the range of 22–61 MPa, depending on the content of PSO. Specifically, the elongation at break decreased while the tensile strength and Young's modulus increased gradually with the increasing content of PSO, which is reasonable because PSO with three thiol groups also acted as a crosslinking agent to increase the crosslink density and network stiffness of the recycled PU films. In order to study the effect of PSO on the crosslink density and network stiffness of the recycled PU films, the thermal and mechanical properties of the RPUSFs were characterized by DMA. The storage modulus plots (Fig. 4d) show that the storage modulus at a given temperature increased with increasing PSO, indicating increased network backbone stiffness. The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ plots (Fig. 4e) show that the glass transition temperature (Tg, peak temperature) slightly shifted to the higher temperature range with increasing PSO due to the increased backbone stiffness and crosslink density. The Tg values for RPUSF-10, RPUSF-20, RPUSF-30 and RPUSF-40 are −41.2, −41.0, −38.8 and −38.6 °C, respectively. The Tg values measured by DSC (Fig. S6†) show a similar variation tendency as the results obtained by DMA and the values are −49.0, −48.7, −47.5 and −47.0 °C for RPUSF-10, RPUSF-20, RPUSF-30, and RPUSF-40, respectively. The crosslinked density (ve) was determined by the rubber elasticity equation:
δ plots (Fig. 4e) show that the glass transition temperature (Tg, peak temperature) slightly shifted to the higher temperature range with increasing PSO due to the increased backbone stiffness and crosslink density. The Tg values for RPUSF-10, RPUSF-20, RPUSF-30 and RPUSF-40 are −41.2, −41.0, −38.8 and −38.6 °C, respectively. The Tg values measured by DSC (Fig. S6†) show a similar variation tendency as the results obtained by DMA and the values are −49.0, −48.7, −47.5 and −47.0 °C for RPUSF-10, RPUSF-20, RPUSF-30, and RPUSF-40, respectively. The crosslinked density (ve) was determined by the rubber elasticity equation:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 938 mol m−3, which increases to 19
938 mol m−3, which increases to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515, 23
515, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 311, and 48
311, and 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 678 mol m−3 for RPUSF-20, RPUSF-30, and RPUSF-40, respectively. The results of the swelling experiment also demonstrate that the crosslink density of the recycled PU films increased with the increasing PSO content since the swelling ratio decreased, while the gel fraction kept remained with the increasing content of PSO (Fig. S4†). Thermal stability was evaluated by the onset decomposition temperature T5 (5% weight loss temperature) measured by thermogravimetric analysis (Fig. S7†). RPUF and RPUSF-10 show similar T5 with a value of 269 °C. The T5 value decreases gradually with the increasing PSO content of RPUSF. The values are 252.0 °C, 243.3 °C and 219.4 °C for RPUSF-20, RPUSF-30 and RPUSF-40, respectively. The decreased thermal stability was attributed to the increased number of disulfide bonds, which should possess relatively lower thermal stability, attributing to the weak bond energy of the dynamic disulfide bonds.
678 mol m−3 for RPUSF-20, RPUSF-30, and RPUSF-40, respectively. The results of the swelling experiment also demonstrate that the crosslink density of the recycled PU films increased with the increasing PSO content since the swelling ratio decreased, while the gel fraction kept remained with the increasing content of PSO (Fig. S4†). Thermal stability was evaluated by the onset decomposition temperature T5 (5% weight loss temperature) measured by thermogravimetric analysis (Fig. S7†). RPUF and RPUSF-10 show similar T5 with a value of 269 °C. The T5 value decreases gradually with the increasing PSO content of RPUSF. The values are 252.0 °C, 243.3 °C and 219.4 °C for RPUSF-20, RPUSF-30 and RPUSF-40, respectively. The decreased thermal stability was attributed to the increased number of disulfide bonds, which should possess relatively lower thermal stability, attributing to the weak bond energy of the dynamic disulfide bonds.
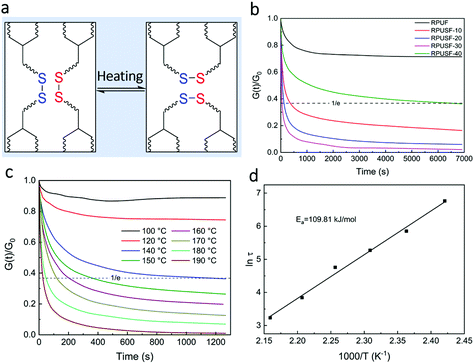
Malleability is of great importance for the processability of vitrimers. The recycled PU films are disulfide-based vitrimers, which can undergo network topology rearrangement as a result of the heat induced disulfide exchange reaction (Fig. 5a), endowing the recycled PU films with malleability and processability. The malleability of the recycled PU films was investigated by thermal stress relaxation (Fig. 5b), which shows the stress relaxation curves of RPUF and RPUSFs at 180 °C. The RPUF with a permanent crosslinking structure showed limited relaxation. The content of PSO plays a significant role in the stress relaxation of RPUSFs. The relaxation first accelerated and then decelerated on increasing the content of PSO and the fastest relaxation occurred for RPUSF-30 with a PPG/PSO weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. According to the Maxwell model, the time required to relax the initial modulus to 1/e is designated as the relaxation time (τ*) of a vitrimer.42 The relaxation times of RPUSF-10, RPUSF-20, RPUSF-30, and RPUSF-40 are 367, 130, 48, and 6509 s, respectively. Increasing content of PSO has two opposite impacts on the stress relaxation of the recycled PU films. On the one hand, the increasing content of PSO increases the number of dynamic disulfide bonds, which is favorable for accelerating stress relaxation. On the other hand, the increasing content of PSO increases the crosslink density of the recycled PU films, which decelerates stress relaxation. The crosslink density increases slightly from RPUSF-10 (17
30. According to the Maxwell model, the time required to relax the initial modulus to 1/e is designated as the relaxation time (τ*) of a vitrimer.42 The relaxation times of RPUSF-10, RPUSF-20, RPUSF-30, and RPUSF-40 are 367, 130, 48, and 6509 s, respectively. Increasing content of PSO has two opposite impacts on the stress relaxation of the recycled PU films. On the one hand, the increasing content of PSO increases the number of dynamic disulfide bonds, which is favorable for accelerating stress relaxation. On the other hand, the increasing content of PSO increases the crosslink density of the recycled PU films, which decelerates stress relaxation. The crosslink density increases slightly from RPUSF-10 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932 mol m−3) to RPUSF-20 (19
932 mol m−3) to RPUSF-20 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517 mol m−3) and RPUSF-30 (23
517 mol m−3) and RPUSF-30 (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 mol m−3), and the number of disulfide bonds dominates the stress relaxation of RPUSFs when the PPG/PSO weight ratio is ≥70
313 mol m−3), and the number of disulfide bonds dominates the stress relaxation of RPUSFs when the PPG/PSO weight ratio is ≥70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. With the decrease in the PPG/PSO weight ratio to 60
30. With the decrease in the PPG/PSO weight ratio to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, the crosslink density increased drastically to 46
40, the crosslink density increased drastically to 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 879 mol m−3 for RPUSF-40, which is about more than 2 times those of other RPUSFs. Thus, the stress relaxation of RPUSF-40 is retarded drastically in comparison with those of other RPUSFs.
879 mol m−3 for RPUSF-40, which is about more than 2 times those of other RPUSFs. Thus, the stress relaxation of RPUSF-40 is retarded drastically in comparison with those of other RPUSFs.
As a typical example, the stress relaxation of RPUSF-30 at different temperatures was investigated (Fig. 5c). At low temperature such as the temperature ≤120 °C, the stress relaxation is limited due to the low activity of the disulfide exchange reaction at low temperature. Although 120 °C is too low for the disulfide exchange reaction, it is much higher than the usual application temperature of PU foams in many areas, which may suggest that PU foams with disulfide bonds are thermally stable for general applications. When the temperature increases to 140 °C or higher, stress relaxation occurs substantially and accelerates with increasing temperature due to the enhanced network topology rearrangement as a result of activated disulfide exchange reaction at elevated temperatures. For vitrimers, the Arrhenius equation is usually used to describe the relationship between relaxation time (τ*) and temperature (T) by:
τ*(T) = τ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(Ea/RT) exp(Ea/RT) |
The relaxation time of the recycled PU film indeed follows the Arrhenius equation (Fig. 5d). According to the slope of the fitting straight line, an activation energy (Ea) for the disulfide exchange reaction in RPUSF-30 was calculated as 109.8 kJ mol−1, which is slightly higher than that of the aromatic disulfide exchange reaction with Ea in the range of 82.8–94.6 kJ mol−1 depending on the structures of different vitrimers.38,43,44
The sustainability of the recycled PU films can be substantially improved if they can be reprocessed after their lifetime. Therefore, we investigated the reprocessability of the recycled PU films. After cutting into small pieces, they were reprocessed into PU films again through compression molding at 180 °C for 30 min, taking RPUSF-30 as a typical example (Fig. 6a). We cut and remolded the sample for several cycles and investigated the structure and mechanical properties of the remolded PU films after each cycle to evaluate reprocessability. The FT-IR spectra (Fig. 6b) of the remolded RPUSF-30 films are the same as that of the original RPUSF-30 film and the gel fraction (Table S5†) almost remained unchanged after each reprocessing cycle, which indicates that the crosslinking structure of the PU film remained unchanged after reprocessing. The stress–strain curves (Fig. 6c), storage modulus plots (Fig. 6d), and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ plots (Fig. 6e) of remolded RPUSF-30 films almost coincide with those of original RPUSF-30 films, which demonstrates that the mechanical properties of RPUSF-30 recovered completely after reprocessing. It is noted that in the other three RPUSFs, the loss of some mechanical properties after each reprocessing cycle is observed (Fig. S5 and Tables S3, S4 and S6†) possibly due to either the insufficient number of disulfide bonds (for RPUSF-10 and RPUSF-20) or the high crosslink density (for RPUSF-40). These two aspects would reduce the stress relaxation rate and thus reduce the reprocessability of the recycled PU films.
δ plots (Fig. 6e) of remolded RPUSF-30 films almost coincide with those of original RPUSF-30 films, which demonstrates that the mechanical properties of RPUSF-30 recovered completely after reprocessing. It is noted that in the other three RPUSFs, the loss of some mechanical properties after each reprocessing cycle is observed (Fig. S5 and Tables S3, S4 and S6†) possibly due to either the insufficient number of disulfide bonds (for RPUSF-10 and RPUSF-20) or the high crosslink density (for RPUSF-40). These two aspects would reduce the stress relaxation rate and thus reduce the reprocessability of the recycled PU films.
In summary, we have demonstrated that thermosetting PU foams can be made thermally processable by incorporating dynamic disulfide bonds into the backbone of the PU network. The exchange reaction of the disulfide bond under heat enables the rearrangement of the network topology of the PU foam, imparting malleability to crosslinked PU foam, conventionally thought of as a thermoset. The malleable PU foams were facilely prepared by adding a polysulfide oligomer as a dynamic crosslinker without changing the foam processing conditions. The incorporation of the dynamic crosslinker does not obviously change the physical properties of the PU foam. The disulfide containing PU foams can be easily recycled through compression molding into PU films with tunable mechanical properties depending on the feed ratio of the dynamic crosslinker. Furthermore, we have shown that the recycled PU films can be further reprocessed several times without an obvious change in the structure and loss in the mechanical properties when a suitable content of a dynamic crosslinker was introduced. We believe that these findings point to a promising strategy for the sustainable development of the PU foam industry and a new methodology for the recycle and reuse of crosslinked PU foams.
Conflicts of interest
The authors declared no competing interest.Acknowledgements
We acknowledge financial support from the Basic and Frontier Research project of Chongqing (grant no. cstc2019jcyjjqX0023), Beijing Key Laboratory of Quality Evaluation Technology for Hygiene and Safety of Plastics (Beijing Technology and Business University) (grant no. QETHSP2020002) and the College Student Innovation and Entrepreneurship Training Program (Southwest University) (grant no. X202010635219).References
- M. F. Sonnenschein, Polyurethanes: Science, Technology, Markets, and Trends, Wiley: Hoboken, NJ, 2015, pp. 1–10 Search PubMed.
- H. W. Engels, H. G. Pirkl, R. Albers, R. W. Albach, J. Krause, A. Hoffmann, H. Casselman and J. Dormish, Angew. Chem., Int. Ed., 2013, 52, 9422–9441 CrossRef CAS.
- M. Cregut, M. Bedas, M. J. Durand and G. Thouand, Biotechnol. Adv., 2013, 31, 1634–1647 CrossRef CAS.
- N. Gamaa, B. Godinho, G. Marques, R. Silva, A. Barros-Timmons and A. Ferreira, Chem. Eng. J., 2020, 395, 125102 CrossRef.
- S. Thomas, A. V. Rane, K. Kanny and M. G. Thomas, Recycling of polyurethane foams, Elsevier Science, 2018, pp. 1–18 Search PubMed.
- S. M. Al-Salem, P. Lettieri and J. Baeyens, Waste Manage., 2009, 29, 2625–2643 CrossRef CAS.
- S. M. Al-Salem, P. Lettieri and J. Baeyens, Prog. Energ. Combust. Sci., 2010, 36, 103–129 CrossRef CAS.
- C. J. Kloxin and C. N. Bowman, Chem. Soc. Rev., 2013, 42, 7161–7173 RSC.
- W. Zou, J. Dong, Y. Luo, Q. Zhao and T. Xie, Adv. Mater., 2017, 29, 1606100 CrossRef.
- M. Delahaye, J. M. Winne and F. E. Du Prez, J. Am. Chem. Soc., 2019, 141, 15277–15287 CrossRef CAS.
- H. V. Niels, M. Diederick and U. Kami, Angew. Chem., 2020, 59, 3609–3617 CrossRef.
- J. Antoine, A. Rawnaq and O. Anaya, Macromolecules, 2020, 53, 1884–1900 CrossRef.
- M. Guerre, C. Taplan, J. M. Winne and F. E. Du Prez, Chem. Sci., 2020, 11, 4855–4870 RSC.
- B. J. Adzima, H. A. Aguirre, C. J. Kloxin, T. F. Scott and C. N. Bowman, Macromolecules, 2008, 41, 9112–9117 CrossRef CAS.
- X.-X. Chen, M. A. Dam, K. Ono, A. Mal, H.-B. Shen, S. R. Nutt, K. Sheran and F. A. Wudl, Science, 2002, 295, 1698–1702 CrossRef CAS.
- P. Maciej, B. D. Fairbanks and B. E. Kirkpatrick, Adv. Mater., 2020, 32, 1906876 CrossRef.
- J. L. Self, C. S. Sample, A. E. Levi, K.-X. Li, R.-X. Xie, J. R. de Alaniz and C. M. Bates, J. Am. Chem. Soc., 2020, 142, 7567–7573 CrossRef CAS.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS.
- Y.-L. Li, T. Liu and S. Zhang, Green Chem., 2020, 22, 870–881 RSC.
- Y. Liu, Y. Jia, Q. Wu and J. S. Moore, J. Am. Chem. Soc., 2019, 141, 17075–17080 CrossRef CAS.
- P. Mocny and H. A. Klok, Macromolecules, 2020, 53, 731–740 CrossRef CAS.
- H. Memon, H.-Y. Liu, M. A. Rashid, L. Chen, Q.-R. Jiang, L.-R. Zhang, Y. Wei, W.-S. Liu and Y.-P. Qiu, Macromolecules, 2020, 53, 621–630 CrossRef CAS.
- H.-W. Geng, Y.-L. Wang, Q.-Q. Yu, S.-J. Gu, Y.-S. Zhou, W.-L. Xu, X. Zhang and D.-Z. Ye, ACS Sustainable Chem. Eng., 2018, 6, 15463–15470 CrossRef CAS.
- J.-H. Chen, X.-P. An, Y.-D. Li, M. Wang and J.-B. Zeng, Chin. J. Polym. Sci., 2018, 36, 614–648 Search PubMed.
- W. Denissen, G. Rivero, R. Nicolay, L. Leibler, J. M. Winne and F. Z. Du Prez, Adv. Funct. Mater., 2015, 25, 2451–2457 CrossRef CAS.
- B. Shi and M. F. Greaney, Chem. Commun., 2005, 7, 886–888 RSC.
- M. D. Konieczynska, J. C. Villa-Camacho, C. Ghobril, M. Perez-Viloria, K. M. Tevis, W. A. Blessing, A. Nazarian, E. K. Rodriguez and M. W. Grinstaff, Angew. Chem., Int. Ed., 2016, 55, 9984–9987 CrossRef CAS.
- S. Delpierre, B. Willocq, G. Manini, V. Lemaur, J. Goole, P. Gerbaux, J. Cornil, P. Dubois and J. M. Raquez, Chem. Mater., 2019, 31, 3736–3744 CrossRef CAS.
- O. R. Cromwell, J. Chung and Z. Guan, J. Am. Chem. Soc., 2015, 137, 6492–6495 CrossRef CAS.
- X. Wu, X. Yang, R. Yu, X.-J. Zhao, Y. Zhang and W. Huang, J. Mater. Chem. A, 2018, 6, 10184–10188 RSC.
- Y. Nishimura, J. Chung, H. Muradyan and Z. Guan, J. Am. Chem. Soc., 2017, 139, 14881–14884 CrossRef CAS.
- M. Delahaye, J. M. Winne and F. E. Du Prez, J. Am. Chem. Soc., 2019, 141, 15277–15287 CrossRef CAS.
- C. Taplan, M. Guerre, J. M. Winne and F. E. Du Prez, Mater. Horiz., 2020, 7, 104–110 RSC.
- Y.-F. Zhang, H.-Z. Ying, K.-R. Hart, Y.-X. Wu, A. J. Hsu, A. M. Coppola, T. A. Kim, K. Yang, N. R. Sottos and S. R. White, Adv. Mater., 2016, 28, 7646–7651 CrossRef CAS.
- H.-Z. Ying, Y.-F. Zhang and J.-J. Cheng, Nat. Commun., 2014, 5, 4218 CrossRef.
- C.-J. Fan, Z.-B. Wen, Z.-Y. Xu, Y. Xiao, D. Wu, K. K. Yang and Y. Z. Wang, Macromolecules, 2020, 53, 4284–4293 CrossRef CAS.
- D. J. Fortman, J. P. Brutman, C. J. Cramer, M. A. Hillmyer and W. R. Dichtel, J. Am. Chem. Soc., 2015, 137, 14019–14022 CrossRef CAS.
- J.-H. Chen, W.-Q. Yuan, Y.-D. Li, Y.-X. Weng and J. B. Zeng, ACS Sustainable Chem. Eng., 2019, 7, 15147–15153 CrossRef CAS.
- A. Rekondo, R. Martin, A. R. de Luzuriaga, G. Cabanero, H. J. Grande and I. Odriozola, Mater. Horiz., 2014, 1, 237–240 RSC.
- D. T. Sheppard, K.-L. Jin, L. S. Hamachi, W. Dean, D. J. Fortman, C. J. Ellison and W. R. Dichtel, ACS Cent. Sci., 2020, 6, 921–927 CrossRef CAS.
- P. Cordier, F. Tournilhac, C. Soulie-Ziakovic and L. Leibler, Nature, 2008, 451, 977–980 CrossRef CAS.
- Y. Chen, Z.-H. Tang, X.-H. Zhang, Y.-J. Liu, S.-W. Wu and B.-C. Guo, ACS Appl. Mater. Interfaces, 2018, 10, 4224–2423 Search PubMed.
- J.-H. Chen, D.-D. Hu, Y.-D. Li, F.-L. Meng, J. Zhu and J.-B. Zeng, Polymer, 2018, 143, 79–86 CrossRef CAS.
- Y.-Y. Liu, J. He, Y.-D. Li, X.-L. Zhao and J.-B. Zeng, Ind. Crops Prod., 2020, 153, 112576 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc03471a |
| This journal is © The Royal Society of Chemistry 2021 |