Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sesamin attenuates PM2.5-induced cardiovascular injury by inhibiting ferroptosis in rats
Jing-yi
Ren
a,
Bo-wen
Yin
a,
Xiang
Li
b,
Si-qi
Zhu
b,
Jin-liang
Deng
c,
Yi-ting
Sun
c,
Zhen-ao
Zhang
b,
Zi-hao
Guo
b,
Huan-ting
Pei
b,
Fan
Zhang
a,
Rui-qiang
Li
a,
Feng-ge
Chen
d and
Yu-xia
Ma
 *a
*a
aDepartment of Nutrition and Food Hygiene, School of Public Health, Hebei Medical University, Hebei Province Key Laboratory of Environment and Human Health, Shijiazhuang, 050017, China. E-mail: mayuxia@hebmu.edu.cn
bUndergraduate of College of Public Health, Hebei Medical University, Shijiazhuang, 050017, China
cUndergraduate of College of Basic Medicine, Hebei Medical University, Shijiazhuang, 050017, China
dShijiazhuang Center for Disease Control and Prevention, Shijiazhuang, 050017, China
First published on 9th November 2021
Abstract
Objective: This study aimed to elucidate the pharmacological effects of sesamin (Ses) and its mechanism of action towards PM2.5-induced cardiovascular injuries. Method: Forty Sprague Dawley (SD) rats were randomly divided into five groups: a saline control group; a PM2.5 exposure group; and low-, middle-, and high-dose Ses pretreatment groups. The SD rats were pretreated with different concentrations of Ses for 21 days. Afterward, the rats were exposed to ambient PM2.5 by intratracheal instillation every other day for a total of three times. The levels of inflammatory markers, including tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), and interleukin-6 (IL-6), and indicators related to oxidative responses, such as total superoxide dismutase (SOD), reduced glutathione (GSH), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA), were measured in the blood and heart. The expression of ferroptosis-related proteins in heart tissues was determined via western blot and immunohistochemistry. Results: Ses pretreatment substantially ameliorated cardiovascular injuries in rats as evidenced by the decrease in the pathological score and collagen area. The decreased levels of SOD, GSH, and GSH-Px in the heart and serum were inhibited by Ses. In addition, Ses not only notably increased the activity of antioxidant enzymes but also reduced the levels of MDA, CK, LDH, CK-MB, IL-6, TNF-α, IL-1β, and IL-6. Furthermore, Ses pretreatment upregulated the expression levels of GPX4, SLC7A11, TFRC, and FPN1 and inhibited the expression levels of FTH1 and FTL. Conclusion: Ses pretreatment could ameliorate PM2.5-induced cardiovascular injuries perhaps by inhibiting ferroptosis. Therefore, Ses pretreatment may be a novel strategy for the prevention and treatment of PM2.5-induced cardiovascular injury.
1 Introduction
Air pollution is a major environmental risk factor that affects both ecosystems and human health worldwide. According to the World Health Organization, 92% of individuals live in places where air quality levels exceed the recommended limits. Many epidemiological investigations have identified that both short- and long-term exposure to ambient particulate matter (PM), especially to fine particulate matter (PM2.5), is strongly related to the occurrence and progression of various cardiovascular diseases (CVDs).1 PM2.5 is defined as an atmospheric particle with an aerodynamic diameter of less than 2.5 μm. Substantial evidence indicates that the main sources of PM2.5 are coal combustion, automobile exhausts, and industrial production2 and that the surface of PM2.5 contains many kinds of toxic substances, such as organic carbon, heavy metals, bacteria, and viruses.3–5 Accumulating evidence indicates that PM2.5 is associated with the adverse effects of respiratory and cardiovascular diseases.6,7 Yang et al.8 reported that PM2.5, PM10, NO2, and O3 exposures are closely related to increased risks of CVD mortality. Compared with other pollutants, PM2.5 exposure poses a greater risk of stroke incidence and ischemic heart disease incidence. PM2.5 is strongly related to markers of early atherosclerosis.9 Therefore, novel effective therapeutic strategies for preventing cardiovascular injuries due to PM2.5 exposure must be developed.Although the precise molecular mechanisms of PM2.5-induced cardiovascular injuries have not been fully elucidated yet, inflammation and oxidative stress have been proved to be closely associated with cardiovascular dysfunctions linked to PM2.5.10,11 PM2.5 causes general inflammation by releasing inflammatory factors, including interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-a).11 Meanwhile, the role of oxidative stress in cardiovascular injury has also attracted increased attention. Oxidation imbalance and antioxidation play an important role in the development of CVDs.12,13 Antioxidation can attenuate the risk of CVDs.13–15 Ferroptosis, which is a recently discovered kind of cell death, is triggered under the typical conditions of iron accumulation and lipid peroxidation.16 Ferroptosis facilitates the development and progression of CVDs, and inhibition of ferroptosis improves human endothelial cell death and cardiac functions in both in vivo and in vitro models of CVDs.17,18 However, the protective effects of ferroptosis inhibition on PM2.5-induced cardiovascular damage require further study.
Hundreds of millions of people worldwide are exposed to high concentrations of PM2.5, especially in China and other developing countries. Therefore, efforts have been exerted to discover dietary improvement strategies that can prevent the adverse health effects of PM2.5 exposure. Dietary supplements can be considered an effective way of preventing cardiovascular injury.19,20 Several intervention studies have noted that fish oil and other dietary antioxidant supplements may alleviate PM2.5-induced oxidative stress and inflammation in humans.20,21 Sesamin (Ses), a fat-soluble lignin isolated from sesame seeds and sesame oil, has attracted considerable attention owing to its wide range of biological and pharmacological activities, including antioxidant, anti-inflammatory, and anticarcinogenic activities.22 However, the effects of Ses on PM2.5-induced cardiovascular toxicity remain unclear. Moreover, the underlying mechanism must be elucidated.
In this study, rats were pretreated with Ses and then exposed to PM2.5. The main goal of this study was to determine whether Ses can antagonize the cardiovascular toxicity of PM2.5 by inhibiting ferroptosis.
2 Materials and methods
2.1 PM2.5 collection and preparation
PM2.5 samples were collected using an air sampler from November 2019 to March 2020 in a nonindustrial district in Shijiazhuang, China. The collected PM2.5 quartz fiber filters were cut into small pieces, immerged in three-fold distilled water, and sonicated three times for 30 min each time with a sonicator. The PM2.5 suspensions were then dried at a constant temperature for 48 h to recover PM2.5 samples. The collected PM2.5 samples were then weighed and stored at −80 °C. The water-soluble components and metal elements in the PM2.5 samples were analyzed via ion chromatography (ICS1100, Deoxon, USA) and inductively coupled plasma mass spectrometry (PerkinElmer, USA). Polycyclic aromatic hydrocarbons (PAHs) were analyzed via high-performance liquid chromatography. The concentrations of the water-soluble components, metal elements, and PAHs in the PM2.5 samples are shown in Table 1.| Component | Concentration | Component | Concentration | |
|---|---|---|---|---|
| The unit of soluble anions is μg mg−1 and the unit of metal elements and PAHs is ng mg−1. | ||||
| Soluble anions | F− | 0.28 | NO3− | 210.13 |
| NH4+ | 104.66 | SO42− | 164.87 | |
| Cl− | 15.17 | |||
| Metal elements | Tl | 4.01 | Pb | 404.58 |
| Cd | 11.85 | Al | 544.93 | |
| Cr | 16.07 | Ni | 8.70 | |
| Se | 50.41 | Be | 0.63 | |
| Sb | 26.72 | Hg | 1.63 | |
| Mn | 252.45 | As | 36.20 | |
| PAHs | Naphthalene | <0.18 | Benzo[a] pyrene | 71.02 |
| Acenaphthene | <0.18 | Benzo[a] anthracene | 77.22 | |
| Phenanthrene | 50.67 | Benzo[k] fluoranthene | 66.18 | |
| Fluoranthene | 76.54 | Benzo[b] fluoranthene | 88.02 | |
| Pyrene | 73.63 | Dibenzo[a, h]anthracene | 60.35 | |
| Acenaphthylene | <0.18 | Benzo [g, h, i]perylene | 70.34 | |
| Fluorene | 72.45 | Indene[1,2,3-c,d]pyrene | 71.97 | |
| Anthracene | 47.06 | |||
2.2 Animals
Forty specific pathogen-free normal Sprague Dawley (SD) rats (7 weeks old and 251–275 g in weight) were supplied by Charles River Laboratories. The rats were adaptively acclimatized for 1 week. All rats were housed in an environmentally controlled room (22 ± 2 °C, 50%–70% humidity) with a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light
12 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark cycle and provided with a standard diet and water ad libitum. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Hebei Medical University and the experiments were approved by the Animal Ethics Committee of Hebei Medical University.
dark cycle and provided with a standard diet and water ad libitum. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Hebei Medical University and the experiments were approved by the Animal Ethics Committee of Hebei Medical University.
2.3 Preparation of Ses
Ses was obtained from Aladdin Company (Aladdin, Shanghai, China) and dissolved in 0.5% carboxymethylcellulose.2.4 PM2.5 exposure and Ses intervention
The SD rats were randomly allocated into five groups (n = 8). In the PM2.5 exposure group, the rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times. In the saline control group, the SD rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and intratracheally instilled with 0.9% saline (1 mL per kg b.w.) every other day for a total of three times. In the Ses pretreatment groups, the SD rats were gavaged with low (L-Ses, 40 mg per kg b.w), medium (M-Ses, 80 mg per kg b.w.), and high (H-Ses, 160 mg per kg b.w.) doses of Ses. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times.2.5 Determination of inflammatory cytokines
Twenty-four hours after the final intratracheal instillation, the SD rats were briefly anesthetized with pentobarbital sodium (50 mg kg−1, i.p.) and blood samples were collected from the abdominal aorta. Heart tissues from the sacrificed rats were then obtained. Levels of IL-6, TNF-a, and interleukin 1β (IL-1β) in serum and heart tissues were measured via enzyme-linked immunosorbent assay (Shanghai Enzyme-linked Biotechnology Co., Ltd) in strict accordance with the manufacturer's instructions.2.6 Measurement of oxidative stress
The heart of each animal was homogenized (10%, w/v) in ice-cold 0.9% normal saline by using a high-speed homogenizer. The homogenate was then centrifuged at 3000 rpm for 10 min at 4 °C and the supernatant was collected. The content of malondialdehyde (MDA) and the activities of superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GSH-Px) were measured using assay kits (Nanjing Jiancheng Bioengineering Institute, China).2.7 Measurement of tissue iron
Iron concentration in the heart was measured using an iron determination kit (Nanjing Jiancheng Bioengineering Institute, China) by following the manufacturer's instructions.2.8 Histological examination
The heart samples were routinely fixed and embedded in paraffin. The wax blocks were sectioned and stained with hematoxylin–eosin and sirius red staining according to the manufacturer's protocol. The severity of cardiac inflammation was evaluated using a 0 to 4 grading system according to a previous study.23 For immunohistochemistry, the sections were incubated overnight with primary antibodies against the ferritin light chain (#A11241, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, ABclonal, China) at 4 °C. Subsequently, the slides were incubated with corresponding secondary antibodies at room temperature for 50 min. Semiquantitative analyses of the area were performed using the Image-Pro plus 6.0. Data are expressed as the ratio of the integrated optical density (IOD = area × density) of the heart-positive area to the total IOD of corresponding heart tissues.
100, ABclonal, China) at 4 °C. Subsequently, the slides were incubated with corresponding secondary antibodies at room temperature for 50 min. Semiquantitative analyses of the area were performed using the Image-Pro plus 6.0. Data are expressed as the ratio of the integrated optical density (IOD = area × density) of the heart-positive area to the total IOD of corresponding heart tissues.
2.9 Heart functional parameters
Heart functions were investigated by measuring the serum and heart levels of lactate dehydrogenase (LDH), creatine kinase (CK), and creatine kinase isoenzyme MB (CK-MB). The assay kits of LDH and CK were purchased from Nanjing Jiancheng Bioengineering Institute (China), whereas the assay kits of CK-MB were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd (China).2.10 Western blot
Fresh heart tissues were sufficiently lysed with RIPA buffer containing protease inhibitors. The proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In brief, the resolved proteins were electrophoretically transferred onto polyvinylidene difluoride membranes. The blots were blocked with 5% defatted milk at room temperature for 2 h. Subsequently, the membranes were washed with Tris-buffered saline tween (TBST) and incubated overnight with primary antibodies at 4° C. The following antibodies were used: anti-rabbit FTH1 (#A19544, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ABclonal, China), anti-rabbit FTL (#A11241, 1
1000, ABclonal, China), anti-rabbit FTL (#A11241, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ABclonal, China), anti-rabbit TFRC (#A5865, 1
1000, ABclonal, China), anti-rabbit TFRC (#A5865, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ABclonal, China), anti-rabbit FPN1 (#A14884, 1
1000, ABclonal, China), anti-rabbit FPN1 (#A14884, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ABclonal, China), anti-rabbit SLC7A11 (#A2413, 1
1000, ABclonal, China), anti-rabbit SLC7A11 (#A2413, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ABclonal, China), anti-rabbit GPX4 (#A13309, 1
1000, ABclonal, China), anti-rabbit GPX4 (#A13309, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, ABclonal, China), and anti-rabbit GAPDH (#AC027, 1
1000, ABclonal, China), and anti-rabbit GAPDH (#AC027, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, ABclonal, China) and incubated overnight at 4 °C. The membranes were then washed four times by using TBST every 5 min and probed with an HRP-conjugated secondary antibody (#AB0101, 1
000, ABclonal, China) and incubated overnight at 4 °C. The membranes were then washed four times by using TBST every 5 min and probed with an HRP-conjugated secondary antibody (#AB0101, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Abways, USA) at room temperature for 1 h. Afterward, TBST was added for washing twice and Tris-buffered saline (TBS) for washing once. Finally, the membranes were washed again and detected using an enhanced chemiluminescence solution. GAPDH served as the internal loading control for normalization.
000, Abways, USA) at room temperature for 1 h. Afterward, TBST was added for washing twice and Tris-buffered saline (TBS) for washing once. Finally, the membranes were washed again and detected using an enhanced chemiluminescence solution. GAPDH served as the internal loading control for normalization.
2.11 Statistical analyses
Statistical analysis was performed using the SPSS 21.0 software and the GraphPad Prism software. Data are reported as means ± SEM. Comparison of the means among groups was performed using one-way ANOVA. Differences with a p-value of <0.05 were considered significant.3 Results
3.1 Effect of Ses on heart histology
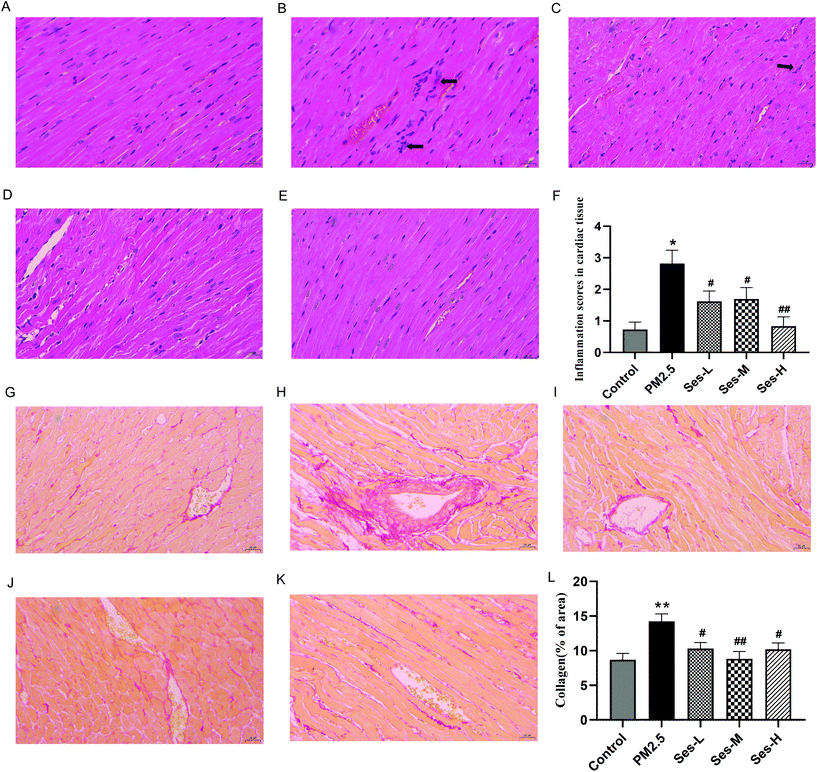
As shown in Fig. 1A–E, the heart tissues of the rats in the control group had a normal ultrastructure, whereas those of the rats in the PM2.5 exposure group had obvious cellular inflammatory infiltrates. Compared with those in the PM2.5 exposure group, edema, hemorrhage, and dense inflammatory cell infiltrations were substantially reduced in the Ses-treated groups. The inflammation score was evaluated histopathologically in the heart tissues. As shown in Fig. 1F, the inflammation score was considerably higher in the PM2.5 exposure group than that in the saline control group and the score was lower in the Ses pretreatment groups than that in the PM2.5 exposure group. Myocardial fibrosis was also assessed to further explore the effects of Ses on PM2.5-induced cardiovascular injury (Fig. 1F–K). The results showed that PM2.5 exposure led to irreversible cardiac interstitial fibrosis. Interstitial fibrosis remarkably decreased in the Ses-treated groups compared with that in the PM2.5 exposure group.3.2 Effects of Ses on the levels of LDH, CK, and CK-MB in heart tissues and serum
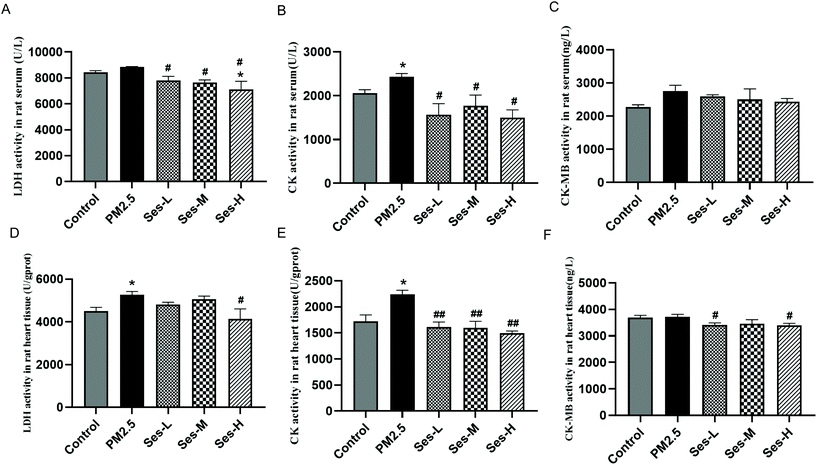
Exposure to PM2.5 notably elevated but Ses pretreatment markedly reduced the levels of LDH and CK in heart tissues and serum (Fig. 2).3.3 Effects of Ses on the levels of TNF-α, IL-1β, and IL-6 in heart tissues and serum
The levels of TNF-α, IL-1β, and IL-6 in the heart tissues and the serum of the SD rats were measured to explore whether Ses affects the inflammatory response. The levels of TNF-α, IL-1β, and IL-6 substantially increased in the PM2.5 exposure group compared with that in the saline control group (Fig. 3). However, this upregulation was alleviated by Ses. Thus, Ses pretreatment largely blocked the PM2.5-induced inflammatory response.3.4 Effects of Ses on the levels of SOD, MDA, GSH, and GSH-PX in heart tissues and serum
The activities of SOD, MDA, GSH, and GSH-PX in the heart tissues and serum of the SD rats were measured to investigate whether Ses affects the degree of oxidative stress (Fig. 4). Compared with those in the saline control group, the activities of SOD, GSH, and GSH-Px in the heart tissues and serum of the SD rats in the PM2.5 exposure group remarkably decreased. The contents of SOD, GSH, and GSH-Px increased in the Ses pretreatment groups compared with those in the PM2.5 exposure group. In addition, PM2.5 instillation substantially increased but Ses pretreatment inhibited the level of MDA in the heart tissues and serum of the SD rats.3.5 Effects of Ses on iron accumulation
Iron plays a vital role in the pathogenesis of cardiovascular disorders and the initiation of ferroptosis. Therefore, the effects of PM2.5 exposure on the level of iron in the heart tissues of the SD rats were measured. The concentration of iron in the heart tissues of the SD rats in the PM2.5 exposure group remarkably increased compared with that in the saline control group (Fig. 5A). However, Ses pretreatment led to a decrease in iron concentration in heart tissues of the SD rats. The expression of the ferroportin (FPN1) protein, the only known cellular iron exporter, was considerably decreased by PM2.5 exposure. In contrast, Ses pretreatment notably inhibited the downregulation of FPN1 protein expression. The expression of transferrin receptor 1 (TfR1), a transporter protein thought to be important for cellular iron uptake, was downregulated by PM2.5 exposure but Ses pretreatment restored its expression. The expression levels of ferritin heavy chain (FTH1) and ferritin light chain (FTL), which are iron-storage proteins, increased in the PM2.5 exposure group. Ses pretreatment remarkably decreased the expression levels of FTH1 and FTL.3.6 Effects of Ses on ferroptosis
The expression levels of the ferroptosis-related proteins GPX4 and SLC7A11 were analyzed to understand the potential mechanisms by which Ses attenuates PM2.5-induced cardiovascular injuries. The expression levels of SLC7A11 and GPX4 in the PM2.5 exposure group were substantially lower than those in the saline control group, and Ses pretreatment restored their expression (Fig. 6).4 Discussion
This study demonstrated that Ses pretreatment exerted beneficial effects on PM2.5-induced cardiovascular injuries by inhibiting ferroptosis. These findings not only enable us to understand a new mechanism involved in PM2.5-induced cardiovascular injuries but also provide a new approach for the prevention and treatment of PM2.5-induced injuries. PM2.5, as an environmental pollutant in China, has been demonstrated to increase the hospitalization and mortality rate of people with CVDs.24,25 However, few studies have investigated the molecular mechanisms involved in cardiovascular injuries as induced by PM2.5 inhalation. Ferroptosis plays a vital role in the occurrence and development of CVDs, such as in doxorubicin- and ischemia/reperfusion (I/R)-induced cardiomyopathy.18,26 The present study focused on the role of ferroptosis in PM2.5-induced cardiovascular injury. Our results indicated that PM2.5 exposure increased the iron load and substantially reduced the expression levels of ferroptosis-related proteins. However, Ses pretreatment attenuated these PM2.5-induced changes, suggesting that Ses may have an inhibitory effect on ferroptosis induced by PM2.5 exposure.PM2.5 exposure may damage the pericardium, myocardium, and vasculature of the heart. Li et al.27 found that PM2.5 exposure induces pathological changes and ultrastructural damage in the heart, such as mitochondrial swelling and cristae disorder. In the present study, the histological examination of cardiac tissues revealed obvious inflammation and injury in the PM2.5 exposure group. In comparison, less inflammation and injury were observed in the Ses pretreatment groups. This protective effect of Ses towards cardiovascular injury was verified by the decrease in myocardial inflammation and fibrosis. The activities of cardiac marker enzymes, including LDH, CK, and CK-MB, which are well-known diagnostic indicators of myocardial cellular injury, are related to myocardial infarction, coronary atherosclerosis, and heart failure.28–30 In the current study, the activity of CK in the heart and serum and the activity of LDH in the heart were remarkably higher in the saline control group than those in the PM2.5 exposure group. However, no notable differences in the serum levels of CK-MB were observed among the five groups. Owing to the tolerance of normal heart tissue, a small degree of myocardial damage may not be obvious.31,32 This fact may explain why only some abnormalities of myocardial damage indicators were detected herein.
Ses, a bioactive component extracted from sesame seeds and sesame oil,33 exhibits multiple biological functions, including immunomodulatory, antioxidant, and anti-inflammatory functions. Many studies have confirmed that Ses plays a vital role in various CVDs, such as hypertension, progression of atherosclerosis, thrombosis, and hypercholesterolemia.34 Kong35 demonstrated that Ses treatment for 8 weeks reduces systolic blood pressure and improves vasodilatation in renovascular hypertensive rats. Atherosclerosis is a chronic vascular inflammatory disease that is a risk factor for the development of CVDs. Sesame oil can reduce atherosclerotic lesions, triglycerides, and plasma cholesterol in mice.36 The rupture of atherosclerotic plaques is a common cause of arterial thrombosis.37 Noguchi38 reported that Ses administration for 5 weeks increases the number of laser pulses required to induce a thrombus. Although Ses has numerous potential health impacts, researchers are still unconvinced whether Ses is beneficial to PM2.5-induced cardiovascular injury. The results of the present study showed that Ses pretreatment substantially reduced both inflammation and oxidative stress following PM2.5 exposure, consistent with heart histological injury and the amelioration of cardiovascular dysfunction.
Proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β, play a vital role in cardiovascular damage caused by PM2.5 and they can initiate inflammatory cascade, reinforce macrophage migration, and aggravate cardiovascular injury.11,23 Our results showed that after PM2.5 stimulation, the infiltration of inflammatory cells in heart tissues substantially increased and the levels of IL-6, TNF-a, and IL-1β in heart tissues and serum markedly increased. These effects were ameliorated by Ses. PM2.5 exposure can also lead to a measurable oxidative stress response in the heart and serum.31 PM2.5 exposure can stimulate the extensive release of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Excessive ROS and RNS generation can impair the activity of antioxidant enzymes, such as SOD, GSH, and GSH-Px.39 As a marker of lipid peroxidation products, MDA can reflect the extent of lipid peroxidation and the degree of cellular damage attacked by free radicals.40 In the current study, we found that the activities of SOD, GSH, and GSH-Px in the heart tissues and serum of the SD rats exposed to PM2.5 remarkably decreased. In addition, PM2.5 exposure led to a notable increase in MDA content in the heart tissues and serum of the SD rats. However, Ses attenuated these PM2.5-induced changes.
In 2012, ferroptosis was first identified as an iron-dependent form of programmed cell death and it can be initiated by the production of ROS and iron overload.16 In fact, iron deposition and lipid peroxidation, the major features of ferroptosis, are strongly associated with inflammatory response and oxidative stress.41,42 Glutathione acts as an important antioxidant and a free radical scavenger in vivo and it can be categorized as either reduced (GSH) or oxidized (GSSG). GPx4 converts GSH into GSSG and GSH/GSSG constitutes an antioxidant system that provides reducing equivalents to eliminate oxidative species.41,43 Cellular iron overload can lead to mitochondrial dysfunction and increased ROS production that even exceeds the scavenging capacity of antioxidant systems (e.g., GSH and GPX4), thereby forming lipid peroxides, enhancing oxidative stress, and promoting the release of pro-inflammatory mediators.41,42 A recent review highlighted that ferroptosis may be the initiating factor for inflammation or at least has proinflammatory effects.42 These findings suggest that ferroptosis may play a major role in the occurrence and development of CVDs. Therefore, to further explore the mechanisms by which Ses attenuates cardiovascular injury due to PM2.5 exposure, we detected the expression of ferroptosis-related proteins. Iron is an indispensable trace element for various metabolic processes and biological functions in the human body. Iron homeostasis is mediated at the systemic, organelle, and cellular levels by iron acquisition, storage, utilization, and export and is tightly regulated by multiple proteins.44,45 Broadly speaking, iron is coupled with transferrin following dietary intake and then transferrin-bound iron is imported into various organelles by binding to its receptor TFR1 on the cell membrane, which is internalized by endocytosis.46,47 Excess cellular iron is stored and detoxified in ferritin, which is a spherical shell protein composed of FTH1 (heavy chain) and FTL (light chain). Cellular iron can be exported into body fluids through FPN1, which is currently the only known cellular iron exporter.48 A previous study reported that PM2.5 exposure can obviously cause iron intake and storage disorders.17 Consistent with this report, we also found that Ses pretreatment decreased iron accumulation in the heart tissues of the SD rats exposed to PM2.5. GPX4, a lipid repair enzyme, is a key player in ferroptosis. GPX4 can reduce toxic phospholipid hydroperoxides and oxidized lipoproteins generated in biological membranes.49 SLC7A11 is a cystine/glutamate xCT transporter used in GSH synthesis50 and it is a key protein involved in regulating “iron overload-ferroptosis”.51 Wang et al.17 found that PM2.5 exposure substantially reduces the expression of SLC7A11 and GPX4. Our results were consistent with previous reports that state PM2.5 exposure can remarkably decrease the expressions of SLC7A11 and GPX4. However, Ses pretreatment increases the expressions of SLC7A11 and GPX4. These results suggested that Ses effectively inhibits PM2.5-induced cardiovascular damage via its anti-ferroptosis activity.
In this study, we preliminarily elucidated the mechanisms by which Ses attenuates PM2.5-induced cardiovascular injuries in rats. The results indicated that this effect is attributed, at least in part, to the inhibition of the ferroptosis pathway. However, this study has several limitations. First, the SD rats were treated with Ses supplementation by gavage, which did not completely represent the food consumption by humans. Second, this study only focused on the ferroptosis pathway. Other signaling pathways, such as autophagy, apoptosis, and necrosis, should be explored in future studies. Finally, whether the anti-ferroptosis activity of Ses is related to its structure remains unknown. Thus, further investigation is warranted to clarify the potential mechanism and the structure–activity relationship of Ses.
5 Conclusions
The present study provided the first in vivo evidence that Ses pretreatment potentially represents a novel and pragmatic therapeutic strategy to protect the cardiovascular system from damage via its anti-ferroptosis activity. In addition, this study offered a new perspective on the therapeutic potential of Ses for the intervention of PM2.5-induced cardiovascular injury. Clinical trials are required to prove its safety and effectiveness against PM2.5-induced cardiovascular injury.Author contributions
Jing-yi Ren roles: data curation, conceptualization, formal analysis, project administration, and writing-original draft; Bo-wen Yin, Xiang Li, and Si-qi Zhu roles: formal analysis, validation, and software; Jin-liang Deng, Yi-ting Sun, Zhen-ao Zhang, Zi-hao Guo, Huan-ting Pei, Fan Zhang, and Rui-qiang Li roles: data curation, supervision, investigation, and project administration; Feng-ge Chen roles: methodology; and Yu-xia Ma roles: conceptualization, funding acquisition, project administration, resources, supervision, and writing-review & editing.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This study was supported by the National Natural Science Foundation of China (no. 81874264).References
- N. Martinelli, O. Olivieri and D. Girelli, Air particulate matter and cardiovascular disease: a narrative review, Eur. J. Intern. Med., 2013, 24, 295–302 CrossRef CAS PubMed.
- S. Archer-Nicholls, E. Carter, R. Kumar, Q. Xiao, Y. Liu, J. Frostad, M. H. Forouzanfar, A. Cohen, M. Brauer, J. Baumgartner and C. Wiedinmyer, The Regional Impacts of Cooking and Heating Emissions on Ambient Air Quality and Disease Burden in China, Environ. Sci. Technol., 2016, 50, 9416–9423 CrossRef CAS PubMed.
- Y. Liu, J. Hu, X. Wang, J. Jia, J. Li, L. Wang, L. Hao and P. Gao, Distribution, bioaccessibility, and health risk assessment of heavy metals in PM(2.5) and PM(10) during winter heating periods in five types of cities in Northeast China, Ecotoxicol. Environ. Saf., 2021, 214, 112071 CrossRef CAS PubMed.
- Z. Yin, X. Huang, L. He, S. Cao and J. J. Zhang, Trends in ambient air pollution levels and PM(2.5) chemical compositions in four Chinese cities from 1995 to 2017, J. Thorac. Dis., 2020, 12, 6396–6410 CrossRef PubMed.
- H. Zeng, L. Zhang, F. Sun, J. Liu, B. Fang, W. Yang, C. Meng, M. Wang, Q. Wang and Y. Hao, Inhalation bioaccessibility, health risk assessment, and source appointment of ambient PM(2.5)-bound polycyclic aromatic hydrocarbons (PAHs) in Caofeidian, China, Environ. Sci. Pollut. Res. Int., 2021, 28, 47574–47587 CrossRef CAS PubMed.
- M. Kajbafzadeh, M. Brauer, B. Karlen, C. Carlsten, S. van Eeden and R. W. Allen, The impacts of traffic-related and woodsmoke particulate matter on measures of cardiovascular health: a HEPA filter intervention study, Occup. Environ. Med., 2015, 72, 394–400 CrossRef PubMed.
- L. F. Guan, X. K. Geng, J. M. Shen, J. Yip, F. W. Li, H. S. Du, Z. L. Ji and Y. C. Ding, PM2.5 inhalation induces intracranial atherosclerosis which may be ameliorated by omega 3 fatty acids, Oncotarget, 2018, 9, 3765–3778 CrossRef PubMed.
- H. X. Yang, S. Li, L. Sun, X. Y. Zhang, Z. Cao, C. J. Xu, X. X. Cao, Y. Y. Cheng, T. Yan, T. Liu and Y. G. Wang, Smog and risk of overall and type-specific cardiovascular diseases: A pooled analysis of 53 cohort studies with 21.09 million participants, Environ. Res., 2019, 172, 375–383 CrossRef CAS PubMed.
- K. S. Woo, P. Chook, Y. J. Hu, X. Q. Lao, C. Q. Lin, P. Lee, C. Kwok, A. N. Wei, D. S. Guo, Y. H. Yin, K. Lau, K. S. Leung, Y. Leung and D. S. Celermajer, The impact of particulate matter air pollution (PM2.5) on atherosclerosis in modernizing China: a report from the CATHAY study, Int. J. Epidemiol., 2021, 50, 578–588 CrossRef CAS PubMed.
- W. Li, E. H. Wilker, K. S. Dorans, M. B. Rice, J. Schwartz, B. A. Coull, P. Koutrakis, D. R. Gold, J. F. Keaney Jr., H. Lin, R. S. Vasan, E. J. Benjamin and M. A. Mittleman, Short-Term Exposure to Air Pollution and Biomarkers of Oxidative Stress: The Framingham Heart Study, J. Am. Heart Assoc., 2016, 5, e002742 Search PubMed.
- X. J. Zeng, J. Liu, X. H. Du, J. Zhang, K. Pan, W. Shan, Y. Q. Xie, W. M. Song and J. Z. Zhao, The protective effects of selenium supplementation on ambient PM(2.5-)induced cardiovascular injury in rats, Environ. Sci. Pollut. Res., 2018, 25, 22153–22162 CrossRef CAS PubMed.
- M. H. Long, X. M. Zhu, Q. Wang, Y. Chen, X. D. Gan, F. Li, W. L. Fu, W. W. Xing, D. Q. Xu and D. G. Xu, PM2.5 exposure induces vascular dysfunction via NO generated by iNOS in lung of ApoE-/- mouse, Int. J. Biol. Sci., 2020, 16, 49–60 CrossRef CAS PubMed.
- S. A. Weichenthal, K. Godri-Pollitt and P. J. Villeneuve, PM2.5, oxidant defence and cardiorespiratory health: a review, Environ. Health, 2013, 12, 40 CrossRef PubMed.
- Y. Liu, M. Li, X. Du, Z. Huang and N. Quan, Sestrin 2, a potential star of antioxidant stress in cardiovascular diseases, Free Radicals Biol. Med., 2021, 163, 56–68 CrossRef CAS PubMed.
- H. Tian, Y. M. Kang, H. L. Gao, X. L. Shi, L. Y. Fu, Y. Li, X. Y. Jia, K. L. Liu, J. Qi, H. B. Li, Y. M. Chen, W. S. Chen, W. Cui, G. Q. Zhu and X. J. Yu, Chronic infusion of berberine into the hypothalamic paraventricular nucleus attenuates hypertension and sympathoexcitation via the ROS/Erk1/2/iNOS pathway, Phytomedicine, 2019, 52, 216–224 CrossRef CAS PubMed.
- S. J. Dixon, K. M. Lemberg, M. R. Lamprecht, R. Skouta, E. M. Zaitsev, C. E. Gleason, D. N. Patel, A. J. Bauer, A. M. Cantley, W. S. Yang, B. Morrison 3rd and B. R. Stockwell, Ferroptosis: an iron-dependent form of nonapoptotic cell death, Cell, 2012, 149, 1060–1072 CrossRef CAS PubMed.
- Y. Wang and M. Tang, PM2.5 induces ferroptosis in human endothelial cells through iron overload and redox imbalance, Environ. Pollut., 2019, 254, 112937 CrossRef CAS PubMed.
- X. Fang, H. Wang, D. Han, E. Xie, X. Yang, J. Wei, S. Gu, F. Gao, N. Zhu, X. Yin, Q. Cheng, P. Zhang, W. Dai, J. Chen, F. Yang, H. T. Yang, A. Linkermann, W. Gu, J. Min and F. Wang, Ferroptosis as a target for protection against cardiomyopathy, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 2672–2680 CrossRef CAS PubMed.
- I. Romieu, R. Garcia-Esteban, J. Sunyer, C. Rios, M. Alcaraz-Zubeldia, S. R. Velasco and F. Holguin, The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM(2.5), Environ. Health Perspect., 2008, 116, 1237–1242 CrossRef CAS PubMed.
- Z. Lin, R. Chen, Y. Jiang, Y. Xia, Y. Niu, C. Wang, C. Liu, C. Chen, Y. Ge, W. Wang, G. Yin, J. Cai, V. Clement, X. Xu, B. Chen, H. Chen and H. Kan, Cardiovascular Benefits of Fish-Oil Supplementation Against Fine Particulate Air Pollution in China, J. Am. Coll. Cardiol., 2019, 73, 2076–2085 CrossRef CAS PubMed.
- Y. Jiang, C. Wang, Z. Lin, Y. Niu, Y. Xia, C. Liu, C. Chen, Y. Ge, W. Wang, G. Yin, J. Cai, B. Chen, R. Chen and H. Kan, Alleviated systemic oxidative stress effects of combined atmospheric oxidant capacity by fish oil supplementation: A randomized, double-blinded, placebo-controlled trial, Ecotoxicol. Environ. Saf., 2019, 184, 109598 CrossRef CAS PubMed.
- A. F. Majdalawieh, S. M. Yousef, I. A. Abu-Yousef and G. K. Nasrallah, Immunomodulatory and anti-inflammatory effects of sesamin: mechanisms of action and future directions, Crit. Rev. Food Sci. Nutr., 2021, 5, 1–32 CrossRef PubMed.
- X. Du, S. Jiang, L. Bo, J. Liu, X. Zeng, Y. Xie, Q. He, X. Ye, W. Song and J. Zhao, Combined effects of vitamin E and omega-3 fatty acids on protecting ambient PM(2.5)-induced cardiovascular injury in rats, Chemosphere, 2017, 173, 14–21 CrossRef CAS PubMed.
- Y. Yao, L. Liu, G. Guo, Y. Zeng and J. S. Ji, Interaction of Sirtuin 1 (SIRT1) candidate longevity gene and particulate matter (PM2.5) on all-cause mortality: a longitudinal cohort study in China, Environ. Health, 2021, 20, 25 CrossRef CAS PubMed.
- W. Yue, L. Tong, X. Liu, X. Weng, X. Chen, D. Wang, S. C. Dudley, E. K. Weir, W. Ding, Z. Lu, Y. Xu and Y. Chen, Short term Pm2.5 exposure caused a robust lung inflammation, vascular remodeling, and exacerbated transition from left ventricular failure to right ventricular hypertrophy, Redox Biol., 2019, 22, 101161 CrossRef CAS PubMed.
- L. J. Tang, Y. J. Zhou, X. M. Xiong, N. S. Li, J. J. Zhang, X. J. Luo and J. Peng, Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion, Free Radicals Biol. Med., 2021, 162, 339–352 CrossRef CAS PubMed.
- R. Li, X. Kou, H. Geng, J. Xie, J. Tian, Z. Cai and C. Dong, Mitochondrial damage: an important mechanism of ambient PM2.5 exposure-induced acute heart injury in rats, J. Hazard. Mater., 2015, 287, 392–401 CrossRef CAS PubMed.
- Y. Chen, L. Peng, S. Q. Shi, G. Guo and H. L. Wen, Boeravinone B alleviates gut dysbiosis during myocardial infarction-induced cardiotoxicity in rats, J. Cell. Mol. Med., 2021, 25, 6403–6416 CrossRef CAS PubMed.
- F. Cao, M. L. Maguire, D. J. McAndrew, H. A. Lake, S. Neubauer, S. Zervou, J. E. Schneider and C. A. Lygate, Overexpression of mitochondrial creatine kinase preserves cardiac energetics without ameliorating murine chronic heart failure, Basic Res. Cardiol., 2020, 115 Search PubMed.
- A. Wojtkowska, T. Zapolski, J. Wysokinska-Miszczuk and A. P. Wysokinski, The inflammation link between periodontal disease and coronary atherosclerosis in patients with acute coronary syndromes: case-control study, BMC Oral Health, 2021, 21, 5 CrossRef CAS PubMed.
- Z. A.-O. Wang, W. Pang, C. He, Y. Li, Y. Jiang and C. Guo, Blueberry Anthocyanin-Enriched Extracts Attenuate Fine Particulate Matter (PM(2.5))-Induced Cardiovascular Dysfunction, J. Agric. Food Chem., 2017, 65, 87–94 CrossRef CAS PubMed.
- E. Golomb, A. Nyska and H. Schwalb, Occult Cardiotoxicity-Toxic Effects on Cardiac Ischemic Tolerance, Toxicol. Pathol., 2009, 37, 572–593 CrossRef CAS PubMed.
- M. Hemshekhar, R. M. Thushara, S. Jnaneshwari, S. Devaraja, K. Kemparaju and K. S. Girish, Attenuation of adjuvant-induced arthritis by dietary sesamol via modulation of inflammatory mediators, extracellular matrix degrading enzymes and antioxidant status, Eur. J. Nutr., 2013, 52, 1787–1799 CrossRef CAS PubMed.
- S. Dalibalta, A. F. Majdalawieh and H. Manjikian, Health benefits of sesamin on cardiovascular disease and its associated risk factors, Saudi Pharm. J., 2020, 28, 1276–1289 CrossRef CAS PubMed.
- X. Kong, J. R. Yang, L. Q. Guo, Y. Xiong, X. Q. Wu, K. Huang and Y. Zhou, Sesamin improves endothelial dysfunction in renovascular hypertensive rats fed with a high-fat, high-sucrose diet, Eur. J. Pharmacol., 2009, 620, 84–89 CrossRef CAS PubMed.
- C. A. Narasimhulu, K. Selvarajan, D. Litvinov and S. Parthasarathy, Anti-Atherosclerotic and Anti-Inflammatory Actions of Sesame Oil, J. Med. Food, 2015, 18, 11–20 CrossRef CAS PubMed.
- N. Mackman, Triggers, targets and treatments for thrombosis, Nature, 2008, 451, 914–918 CrossRef CAS PubMed.
- T. Noguchi, K. Ikeda, Y. Sasaki, J. Yamamoto, J. Seki, K. Yamagata, Y. Nara, H. Hara, H. Kakuta and Y. Yamori, Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats, Hypertens. Res., 2001, 24, 735–742 CrossRef CAS PubMed.
- S. Feng, D. Gao, F. Liao, F. Zhou and X. Wang, The health effects of ambient PM2.5 and potential mechanisms, Ecotoxicol. Environ. Saf., 2016, 128, 67–74 CrossRef CAS PubMed.
- K. Huang, C. Shi, J. Min, L. Li, T. Zhu, H. Yu and H. Deng, Study on the Mechanism of Curcumin Regulating Lung Injury Induced by Outdoor Fine Particulate Matter (PM2.5), Mediators Inflammation, 2019, 2019, 8613523 Search PubMed.
- J. Li, Y. Zhou, H. Wang, J. Lou, C. Lenahan, S. Gao, X. Wang, Y. Deng, H. Chen and A. Shao, Oxidative Stress-Induced Ferroptosis in Cardiovascular Diseases and Epigenetic Mechanisms, Front. Cell Dev. Biol., 2021, 9, 685775 CrossRef PubMed.
- Y. Sun, P. Chen, B. Zhai, M. Zhang, Y. Xiang, J. Fang, S. Xu, Y. Gao, X. Chen, X. Sui and G. Li, The emerging role of ferroptosis in inflammation, Biomed. Pharmacother., 2020, 127, 110108 CrossRef CAS PubMed.
- Y. Xie, W. Hou, X. Song, Y. Yu, J. Huang, X. Sun, R. Kang and D. Tang, Ferroptosis: process and function, Cell Death Differ., 2016, 23, 369–379 CrossRef CAS PubMed.
- A. K. Tripathi, S. Haldar, J. Qian, A. Beserra, S. Suda, A. Singh, U. Hopfer, S. G. Chen, M. D. Garrick, J. R. Turner, M. D. Knutson and N. Singh, Prion protein functions as a ferrireductase partner for ZIP14 and DMT1, Free Radicals Biol. Med., 2015, 84, 322–330 CrossRef CAS PubMed.
- J. S. Calla-Choque, E. E. Figueroa-Angulo, L. Avila-Gonzalez and R. Arroyo, alpha -Actinin TvACTN3 of Trichomonas vaginalis is an RNA-binding protein that could participate in its posttranscriptional iron regulatory mechanism, BioMed Res. Int., 2014, 2014, 424767 Search PubMed.
- N. Li, W. Jiang, W. Wang, R. Xiong, X. Wu and Q. Geng, Ferroptosis and its emerging roles in cardiovascular diseases, Pharmacol. Res., 2021, 166, 105466 CrossRef CAS PubMed.
- J. Ju, Y. N. Song and K. Wang, Mechanism of Ferroptosis: A Potential Target for Cardiovascular Diseases Treatment, Aging Dis., 2021, 12, 261–276 CrossRef PubMed.
- A. Tsatsanis, S. Dickens, J. C. F. Kwok, B. X. Wong and J. A.-O. Duce, Post Translational Modulation of β-Amyloid Precursor Protein Trafficking to the Cell Surface Alters Neuronal Iron Homeostasis, Neurochem. Res., 2019, 44, 1367–1374 CrossRef CAS PubMed.
- M. Matsushita, S. Freigang, C. Schneider, M. Conrad, G. W. Bornkamm and M. Kopf, T cell lipid peroxidation induces ferroptosis and prevents immunity to infection, J. Exp. Med., 2015, 212, 555–568 CrossRef CAS PubMed.
- Y. Chen, S. S. Hu, L. Mu, B. H. Zhao, M. M. Wang, N. S. Yang, G. L. Bao, C. G. Zhu and X. S. Wu, Slc7a11 Modulated by POU2F1 is Involved in Pigmentation in Rabbit, Int. J. Mol. Sci., 2019, 20, 2493 CrossRef CAS PubMed.
- Y. Xie, W. Hou, X. Song, Y. Yu, J. Huang, X. Sun, R. Kang and D. Tang, Ferroptosis: process and function, Cell Death Differ., 2016, 23, 369–379 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |