Open Access Article
Open Access ArticleDistinct fermentation of human milk oligosaccharides 3-FL and LNT2 and GOS/inulin by infant gut microbiota and impact on adhesion of Lactobacillus plantarum WCFS1 to gut epithelial cells†
Chunli
Kong
 *ab,
Renate
Akkerman
*ab,
Renate
Akkerman
 b,
Cynthia E.
Klostermann
c,
Martin
Beukema
b,
Cynthia E.
Klostermann
c,
Martin
Beukema
 b,
Marjolein M. P.
Oerlemans
b,
Henk A.
Schols
d and
Paul
de Vos
b
b,
Marjolein M. P.
Oerlemans
b,
Henk A.
Schols
d and
Paul
de Vos
b
aSchool of Food and Health, Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Engineering and Technology Research Center of Food Additives, Beijing Technology and Business University, Beijing 100048, China. E-mail: kongc0606@hotmail.com
bDepartment of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Hanzeplein 1, 9700 RB Groningen, The Netherlands
cBiobased Chemistry and Technology, Wageningen University & Research, Wageningen, The Netherlands
dLaboratory of Food Chemistry, Wageningen University & Research, Wageningen, The Netherlands
First published on 23rd November 2021
Abstract
Human milk oligosaccharides (hMOs) are unique bioactive components in human milk. 3-Fucosyllactose (3-FL) is an abundantly present hMO that can be produced in sufficient amounts to allow application in infant formula. Lacto-N-triaose II (LNT2) can be obtained by acid hydrolysis of lacto-N-neotetraose (LNnT). Both 3-FL and LNT2 have been shown to have health benefits, but their impact on infant microbiota composition and microbial metabolic products such as short-chain fatty acids (SCFAs) is unknown. To gain more insight in fermentability, we performed in vitro fermentation studies of 3-FL and LNT2 using pooled fecal microbiota from 12-week-old infants. The commonly investigated galacto-oligosaccharides (GOS)/inulin (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) served as control. Compared to GOS/inulin, we observed a delayed utilization of 3-FL, which was utilized at 60.3% after 36 h of fermentation, and induced the gradual production of acetic acid and lactic acid. 3-FL specifically enriched bacteria of Bacteroides and Enterococcus genus. LNT2 was fermented much faster. After 14 h of fermentation, 90.1% was already utilized, and production of acetic acid, succinic acid, lactic acid and butyric acid was observed. LNT2 specifically increased the abundance of Collinsella, as well as Bifidobacterium. The GOS present in the GOS/inulin mixture was completely fermented after 14 h, while for inulin, only low DP was rapidly utilized after 14 h. To determine whether the fermentation might lead to enhanced colonization of commensal bacteria to gut epithelial cells, we investigated adhesion of the commensal Lactobacillus plantarum WCFS1 to Caco-2 cells. The fermentation digesta of LNT2 collected after 14 h, 24 h, and 36 h, and GOS/inulin after 24 h of fermentation significantly increased the adhesion of L. plantarum WCFS1 to Caco-2 cells, while 3-FL had no such effect. Our findings illustrate that fermentation of hMOs is very structure-dependent and different from the commonly applied GOS/inulin, which might lead to differential potencies to stimulate adhesion of commensal cells to gut epithelium and consequent microbial colonization. This knowledge might contribute to the design of tailored infant formulas containing specific hMO molecules to meet the need of infants during the transition from breastfeeding to formula.
1) served as control. Compared to GOS/inulin, we observed a delayed utilization of 3-FL, which was utilized at 60.3% after 36 h of fermentation, and induced the gradual production of acetic acid and lactic acid. 3-FL specifically enriched bacteria of Bacteroides and Enterococcus genus. LNT2 was fermented much faster. After 14 h of fermentation, 90.1% was already utilized, and production of acetic acid, succinic acid, lactic acid and butyric acid was observed. LNT2 specifically increased the abundance of Collinsella, as well as Bifidobacterium. The GOS present in the GOS/inulin mixture was completely fermented after 14 h, while for inulin, only low DP was rapidly utilized after 14 h. To determine whether the fermentation might lead to enhanced colonization of commensal bacteria to gut epithelial cells, we investigated adhesion of the commensal Lactobacillus plantarum WCFS1 to Caco-2 cells. The fermentation digesta of LNT2 collected after 14 h, 24 h, and 36 h, and GOS/inulin after 24 h of fermentation significantly increased the adhesion of L. plantarum WCFS1 to Caco-2 cells, while 3-FL had no such effect. Our findings illustrate that fermentation of hMOs is very structure-dependent and different from the commonly applied GOS/inulin, which might lead to differential potencies to stimulate adhesion of commensal cells to gut epithelium and consequent microbial colonization. This knowledge might contribute to the design of tailored infant formulas containing specific hMO molecules to meet the need of infants during the transition from breastfeeding to formula.
1. Introduction
Human milk (HM) is the gold standard for early life nutrition and promotes life-long health.1,2 An important component in HM is the human milk oligosaccharides (hMOs). HMOs are the third most abundant component in HM and exert many health-promoting effects, such as modulating the gut microbiota composition,3 reducing pathogen infection,4 and regulating the gut immune barrier functions.5 The World Health Organization recommends exclusively breastfeeding for up to six months of age. However, about 50% of infants rely on formula feeding even in the first month of life.6 Most of the infant formulas available on the market are based on cow milk. Cow milk lacks the unique oligosaccharides that are present in HM, i.e., hMOs.7Improving the nutritional quality of infant formula is essential to prevent the development of health issues in later life, especially in allergy-prone children.8 HMO analogs are considered to be an important family of molecules for preventing these health issues. Until now, only 2′-fucosyllactose (2′-FL) and lacto-N-neotetraose (LNnT) are used in infant formula, while there are over 200 different hMO structures identified in HM.9,10 To mimic some functions of hMOs, other non-digestible carbohydrates (NDCs) such as galacto-oligosaccharides (GOS), fructo-oligosaccharides (FOS), and inulin have also been tested and added in infant formula.11,12 In recent years, hMOs have been produced microbiologically in a cost-effective way, such as 3-FL, a highly abundant hMO in HM, and lacto-N-triaose II (LNT2), which are of interest to be applied in infant formula as well.10,13
One important function of hMOs and NDCs in infant formula is to guide the colonization of the gut microbiota.3,14 HMOs and NDCs are resistant to human enzymes in the upper gastrointestinal tract, and cannot be directly utilized by the host. Therefore, they reach the colon intact where they can serve as substrates for bacterial fermentation.15,16 In this way, hMOs and NDCs can specifically enrich beneficial species such as Bifidobacterium and Lactobacillus,17,18 and lower the abundance of pathogenic species belonging to Clostridia, Campylobacter, and stable toxin of entero-toxigenic E. coli.19 These enriched bacterial species may ferment hMOs and NDCs, which results in the production of short chain fatty acids (SCFAs) and other organic acids.5,20 These breakdown products are not only a primary energy source for the gut epithelial cells, but are also important for tolerogenic immune responses21 and in controlling undesired inflammatory responses.22 Moreover, SCFAs and other organic acids produced during fermentation serve as nutrients for cross-feeding between gut bacteria, which might influence colonization and adherence of commensal bacteria to the host epithelium.23
Despite these proposed benefits, it still remains unknown how the currently developed hMO molecules for infant formula influence the gut microbiota composition as individual molecules. It is also not known whether they can be fermented by microbiota of babies, and whether they stimulate the production of SCFAs and other accessible organic acids. It is also still unknown how these hMOs influence, e.g., adhesion of commensal bacteria to gut epithelium. To investigate this, we performed in vitro fermentation of NDCs and hMOs using the pooled fecal inoculum of 12-week-old infants. GOS/inulin, which has been widely investigated for infant formula, was taken as a reference. We tested fermentation of 3-FL, which has a similar structure as 2′-FL, the most abundant hMOs in mother's milk, and LNT2. LNT2 can be obtained by acid hydrolysis of LNnT. LNnT has shown health benefits and can be produced in sufficient amounts for supplementation in infant formula.24–26 During the fermentation, we collected digesta at 0 (T0), 14 (T14), 24 (T24), and 36 (T36) hours to study glycosidic breakdown, microbiota changes and SCFA formation. In addition, we investigated the effect of these digesta on the adhesion of a commensal bacterium L. plantarum WCFS1 to the intestine epithelial Caco-2 cells.
2. Materials and methods
2.1 Carbohydrates
The human milk oligosaccharides (hMOs) 3-FL and LNT2 were provided by Elicityl, France, and Frutafit TEX! inulin with oligomers in the range of degree of polymerization. DP8-DP50 was provided by Sensus, Roosendaal, the Netherlands. An overview of the structures of the carbohydrates is listed in Table 1.2.2 Fermentation by infant fecal samples
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82 (v/v).20
82 (v/v).20
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were combined with the prepared infant fecal inoculum in a ratio of 1
1) were combined with the prepared infant fecal inoculum in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) with a final concentration of 10 mg mL−1 in sterile glass fermentation bottles. The infant fecal inoculum without the tested carbohydrates served as the control. The fermentation bottles were closed with a rubber stopper and sealed with a metal lid to guarantee an anaerobic condition. Fermentations were performed in duplicate in an incubator shaker at 37 °C, 100 rpm.
10 (v/v) with a final concentration of 10 mg mL−1 in sterile glass fermentation bottles. The infant fecal inoculum without the tested carbohydrates served as the control. The fermentation bottles were closed with a rubber stopper and sealed with a metal lid to guarantee an anaerobic condition. Fermentations were performed in duplicate in an incubator shaker at 37 °C, 100 rpm.
Fermentation samples were collected at the start (T0), and after 14 (T14), 24 (T24) and 36 h (T36). Three samples were collected from each group, one of which was centrifuged at 3000g for 10 min, snap frozen in liquid nitrogen and stored at −80 °C for microbial analysis. The other two samples were collected in a safe-lock Eppendorf tube to determine carbohydrate degradation, short chains fatty acids (SCFAs) and organic acids. These two samples were heated at 100 °C in a water bath for 10 min to inactivate the enzymes produced by the bacteria before storing at −80 °C.27 Safe-lock Eppendorf tubes were applied to avoid evaporation of the SCFAs during heating. The cap of the tube was only removed when the sample was at room temperature. The method of inactivating/heating to stop fermentation and enzyme action did not influence SCFA analysis as validated using standards, as well as fermentation samples (ESI Fig. 1–4†).
2.3 Carbohydrates degradation
High performance anion exchange chromatography (HPAEC) was used to study the degradation of the carbohydrates in the infant fecal sample fermentation, as an indicator of the utilization of the carbohydrates by the gut microbiome.20,30 Samples were diluted to 313 μg mL−1 (GOS/inulin) or 10 μg mL−1 (3-FL and LNT2) and centrifuged at 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. Ten μL of sample was injected to an ICS 5000 HPAEC system (Dionex, Sunnyvale, USA), in combination with a CarboPac PA-1 (250 mm × 2 mm ID) column and a CarboPac PA guard column (25 mm × 2 mm ID) (Dionex). The samples were eluted using solvent A (0.1 M NaOH solution) and solvent B (1 M NaOAc in 0.1 M NaOH) at a flow of 0.3 mL min−1 through an electrochemical pulsed amperometric detector (Dionex) to detect the elution of the oligosaccharides. Two different gradients were applied, i.e., for inulin, 0–50% B (0–50 min), 50–100% (50–55 min), 100% B (55–60 min), 0% B (60–75 min), and for GOS, 3-FL, and LNT2, 0–20% B (0–20 min), 100% B (20–26 min), 0% B (26–41 min).
000g for 10 min. Ten μL of sample was injected to an ICS 5000 HPAEC system (Dionex, Sunnyvale, USA), in combination with a CarboPac PA-1 (250 mm × 2 mm ID) column and a CarboPac PA guard column (25 mm × 2 mm ID) (Dionex). The samples were eluted using solvent A (0.1 M NaOH solution) and solvent B (1 M NaOAc in 0.1 M NaOH) at a flow of 0.3 mL min−1 through an electrochemical pulsed amperometric detector (Dionex) to detect the elution of the oligosaccharides. Two different gradients were applied, i.e., for inulin, 0–50% B (0–50 min), 50–100% (50–55 min), 100% B (55–60 min), 0% B (60–75 min), and for GOS, 3-FL, and LNT2, 0–20% B (0–20 min), 100% B (20–26 min), 0% B (26–41 min).
2.4 Microbiological analysis
2.5 Short chain fatty acids and other organic acids production
High performance liquid chromatography (HPLC) was used to measure the production of SCFAs and other organic acids during the fermentation, as an indicator of the activity of the gut microbiome.20,31 Samples were centrifuged at 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min after five-fold dilution with Milli-Q water (Arium mini essential UV Ultrapure water filter, Sartorius, Göttingen, Germany). Ten μL of the diluent was injected to an Ultimate 3000 HPLC system (Dionex), with an Aminex HPX-87H column (Bio-Rad laboratories Inc., Hercules, USA). The SCFAs acetic acid, propionic acid, butyric acid, and the organic acids lactic acid and succinic acid were detected with a refractive index detector (RI-101, Shodex, Yokohama, Japan) and an UV detector at 210 nm. The samples were eluted in 50 mM sulphuric acid at a flow rate of 0.5 mL min−1 at 50 °C. Standard curves in a range of 0.05–2 mg mL−1 of acetate (VWR chemicals), propionate (Sigma Aldrich), butyrate (Sigma Aldrich), lactate (Alfa Aeser) and succinate (VWR chemicals) were used for quantification. The production was calculated based on each corresponding standard curves and the dilution factors. Results were presented as μmol mg−1 of the fermentation substrate.
000g for 10 min after five-fold dilution with Milli-Q water (Arium mini essential UV Ultrapure water filter, Sartorius, Göttingen, Germany). Ten μL of the diluent was injected to an Ultimate 3000 HPLC system (Dionex), with an Aminex HPX-87H column (Bio-Rad laboratories Inc., Hercules, USA). The SCFAs acetic acid, propionic acid, butyric acid, and the organic acids lactic acid and succinic acid were detected with a refractive index detector (RI-101, Shodex, Yokohama, Japan) and an UV detector at 210 nm. The samples were eluted in 50 mM sulphuric acid at a flow rate of 0.5 mL min−1 at 50 °C. Standard curves in a range of 0.05–2 mg mL−1 of acetate (VWR chemicals), propionate (Sigma Aldrich), butyrate (Sigma Aldrich), lactate (Alfa Aeser) and succinate (VWR chemicals) were used for quantification. The production was calculated based on each corresponding standard curves and the dilution factors. Results were presented as μmol mg−1 of the fermentation substrate.
2.6 Bacteria adhesion to intestine epithelial Caco-2 cells with the fermentation samples
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were seeded in 24-well plates per well, and were cultured for 21 days before use.
000 cells were seeded in 24-well plates per well, and were cultured for 21 days before use.
2.7 Statistical analysis
The carbohydrate degradation, and the SCFAs and other organic acids production were analyzed with ChromeleonTM 7.2.6 (Thermo Fisher Scientific, Massachusetts, USA). For the gut microbiome composition analysis, the high-quality clean reads were obtained from the sequencing by merging raw reads with FLASH (v1.2.7), filtering with QIIME (v1.7.0), and removal of chimera sequences detected with UCHIME algorithm. The clean reads were then analyzed with Uparse software (v7.0.1001). Sequences with similarities of ≥97% were assigned to the same operational taxonomic units (OTUs). Principal coordinate analysis (PCoA) was performed with R software by the WGCNA package, stat packages and ggplot2 package (v2.15.3). Data analysis of the bacteria adhesion assay was performed with GraphPad Prism 8 (v8.4.2, GraphPad Software LLC.). Results were shown as the mean ± SD. Normal distribution of the data was confirmed with a Shapiro–Wilk test. Statistical differences were evaluated using a two-way ANOVA, followed by a Dunnet's multiple comparisons test. A p-value of <0.05 was considered as a significant difference (*, #p < 0.05; **, ##p < 0.01).3. Results
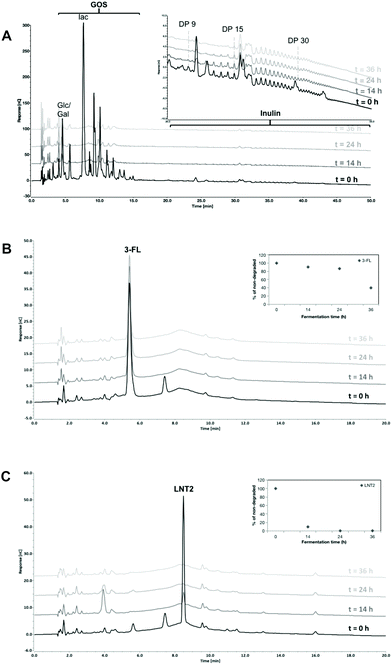
3.1 Delayed fermentation of 3-FL compared to GOS/inulin and LNT2
We first determined the fermentability of the hMOs 3-FL and LNT2 by microbiota of 12-week-old infants, and compared it with the fermentability of GOS/inulin (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a reference.12,32 We studied fermentability at 0 (T0), 14 (T14), 24 (T24) and 36 (T36) hours.
1) as a reference.12,32 We studied fermentability at 0 (T0), 14 (T14), 24 (T24) and 36 (T36) hours.
In the GOS/inulin fermentation, GOS was completely fermented at T14 by the fecal microbiome of the 12 weeks-old infants (Fig. 1A). This was different for inulin present in the GOS/inulin mixture, i.e., only the inulin oligomers eluting between retention time of 25–30 min (DP8–14) were fermented slowly over time until completely utilized at T36.
Fermentation of 3-FL was delayed when compared to GOS/inulin. At T14 and T24, only 9.5% and 13.4% was fermented but at T36, 60.3% of the 3-FL was utilized (Fig. 1B). LNT2 was more readily fermented and was already degraded for 90.1% at T14, and further fermented by 99.4% at T24 (Fig. 1C).
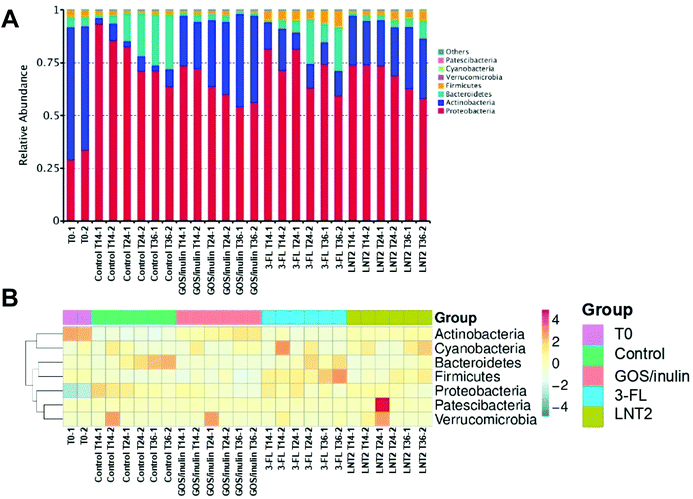
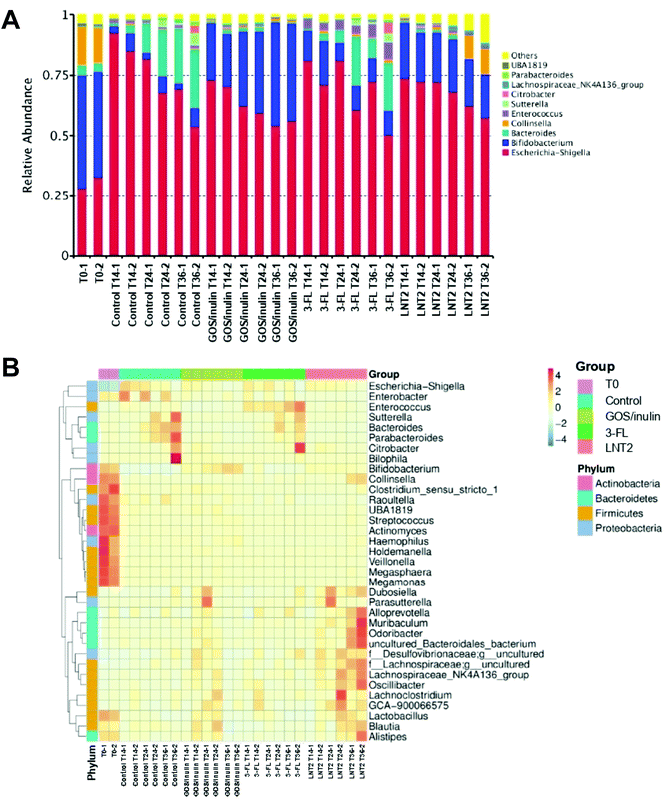
3.2 GOS/inulin and LNT2 increase abundance of Actinobacteria, and 3-FL specifically increases the relative abundance of Bacteroidetes at the phylum level
Next, we investigated which groups of gut bacteria were changed during the fermentation of SIEM medium, GOS/inulin, 3-FL, or LNT2, and contributed to the diversity differences. To this end, the relative abundance of different phyla during fermentation at T0, T14, T24, and T36 were first determined.At the start of the fermentation, the gut microbiome communities were dominated by 60.8% of Actinobacteria, 31.3% of Proteobacteria, 4.9% Bacteroidetes, and 3.1% of Firmicutes. This microbiome composition changed during fermentation (Fig. 2A). In the control fermentation of the SIEM medium, which only contained pectin, xylan, amylopectin, and starch as a carbohydrate source, but without GOS/inulin or hMO, Actinobacteria was decreased from 60.8% at T0 to 5% at T14, and the level remained about 5.3% until T36. Proteobacteria, however, was nearly triplicated to 89.5% at T14, and the relative abundance was 67.5% at T36 of fermentation. This reduction might be induced by the increase of Bacteroidetes, which was increased from 2.8% (T14) to 25.1% (T36).
The microbiota composition was clearly influenced by GOS/inulin and the two tested hMOs. For the GOS/inulin fermentation at T14, we observed already a high level of Actinobacteria of 23.0% (Fig. 2A), and this abundance increased to 32.9% (T24) and 42.5% (T36) during the fermentation. The relative abundance of Proteobacteria was doubled to 72.9% at T14 of fermentation, but lowered to 55.3% at T36. GOS/inulin fermentation resulted in a relative low level of both Bacteroidetes and Firmicutes of around 2% during the fermentation.
With 3-FL, we observed a relative abundance of Actinobacteria of 16.1% at T14 (Fig. 2A), and the level decreased to 9.6% (T24) and 11.7% (T36) during the fermentation. Despite its low fermentability, the relative abundance of Proteobacteria was doubled at T14 of fermentation compared to T0, and it decreased to 66.8% at T36 of fermentation. The relative abundance of Bacteroidetes was decreased from 4.9% at T0 to 2.7% at T14, but it kept increasing to 14.8% at T36.
Results were different with LNT2, which induced a high abundance of Actinobacteria of 22% at T14 (Fig. 2A), and 22% at T24 and 28.8% at T36. The relative abundance of Proteobacteria at T14 was 74.1%, with LNT2 fermentation, the level decreased to 60.4% at T36.
Fig. 2B shows a heat map directly illustrating the differences of each phylum in different groups.
3.3 GOS/inulin and LNT2 fermentation increase the relative abundance of Bifidobacterium, and LNT2 specifically increases the relative abundance of Collinsella at the genus level
Then, the relative abundance of different genera during the fermentation at T0, T14, T24, and T36 were determined. At the start of fermentation (T0), the microbiome compositions in the fecal samples were dominated by Bifidobacterium (45.2%), Escherichia-Shigella (30.4%), Collinsella (15.2%), and Bacteroides (3.9%).In the SIEM control without added GOS/inulin or hMO, the relative abundance of Bifidobacterium was dramatically decreased from 45.2% at T0 to 4.9% at T14, and remained around 5% at T24 and T36 (Fig. 3A). However, the relative abundance of Escherichia-Shigella belonging to Proteobacteria was nearly triplicated to 88.9% at T14, and kept decreasing to 61.5% at T36 of fermentation. The relative abundance of Bacteroides at T14 was 2.3%, but it kept increasing to 23.6% (T36). There were 16 genera belonging to Firmicutes phylum of the top 35 (Fig. 3B), but they were all of low abundance.
GOS/inulin induced a relative abundance of 11.5% of Bifidobacterium at T14 (Fig. 3A), which was increased to 32.3% at T24, and finally achieved a level of 41.5% at T36. Although GOS/inulin induced a high abundance of Escherichia-Shigella of 71.8% at T14, the relative abundance kept decreasing to 60.9% (T24) and 55.0% (T36). The change of Escherichia-Shigella can also be recognized from the heat map, which gradually changed from dark blue at T0 to shallow orange at T14–T36 (Fig. 3B).
Despite its low fermentability, 3-FL also induced a high relative abundance of Bifidobacterium of 15.41% at T14 (Fig. 3A), but it decreased during fermentation to 8.8% (T24) and 10.0% (T36). The lowering of Bifidobacterium might be induced by the increase in relative abundance of Bacteroides and Enterococcus during the fermentation, as Bacteroides increased from 1.9% at T14, to 12.8% at T24, and reached a level of 13.8% at T36. The relative abundance of Bacteroides was as low as 0.2% at T0, but it increased to 3.6% at T14, 3.7% at T24, and reached a level of 6.3% at T36 (Fig. 3B). Even though the relative abundance of Escherichia-Shigella was increased to 76.0% at T14, it kept decreasing to 70.8% (T24) and 61.3% (T36) with 3-FL (Fig. 3A).
LNT2 could have already induced a relative abundance of Bifidobacterium with 21.6% at T14 (Fig. 3A), but decreased slightly to 21.3% at T24 and 18.7% at T36. This might be due to the LNT2 effect on the increase of the relative abundance of Collinsella, which belonged to the same phylum of Actinobacteria as Bifidobacterium, by reaching a level of 10.1% at T36. The increase of Collinsella was unique for LNT2, because this effect was not observed with GOS/inulin or 3-FL. LNT2 also decreased the relative abundance of Escherichia-Shigella from 73.1% at T14 to 59.8% at T36.
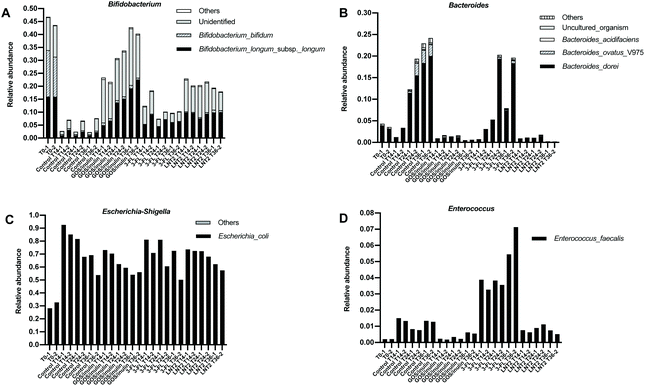
3.4 GOS/inulin, 3-FL, and LNT2 fermentation increase the relative abundance of Bifidobacterium_longum_subsp._longum, and 3-FL specifically increases Bacteroides_dorei
As we observed such a different effect on the microbiome compositions during the fermentation of GOS/inulin, 3-FL, and LNT2, especially on Bifidobacterium and Escherichia-Shigella, we next investigated which specific species contributed the most to the dominant genera including Bifidobacterium, Bacteroides, Escherichia-Shigella, and Enteroccus. To do so, a SIMPER test was performed. The species that got a contribution of >1% in microbiome compositions is shown in ESI Tables 3–6.†At the start of fermentation at T0 (Fig. 4A), the relative abundance of Bifidobacterium genus was 45.2%, which mainly consisted of two species, i.e., Bifidobacterium_bifidum, with a relative abundance of 16.7% and Bifidobacterium_longum_subsp._longum, with a relative abundance of 16.0. However, there was still a high abundance of unidentified species, with a relative abundance of 12.4%. The Bacteroides genus was dominated by the species of Bacteroides_dorei (Fig. 4B), which was 3.8% compared to the total relative abundance of 4.3%. Of the relative abundance of 30.4% of the Escherichia-Shigella genus in the gut microbiome, the species of Escherichia_coli was the most prevalent (Fig. 4C), with a relative abundance of 30.4%. Enterococcus_faecalis was the only species present in the 12-week-old baby microbiome belonging to the genus of Enterococcus (Fig. 4D).
Control fermentation in SIEM without GOS/inulin or hMO led to a dramatic decrease in the relative abundance of species of Bifidobacterium_bifidum from 16.7% to 0.56% at T14 (Fig. 4A), and remained below 0.5% during the fermentation. Bifidobacterium_longum_subsp._longum and other identified species were around 2%. During the fermentation in SIEM, the increased species belonging to Bacteroides genus (23.6% at T36) were dominated by Bacteroides_dorei and Bacteroides_ovatus_V975 (Fig. 4B), of which, the relative abundance of Bacteroides_dorei was increased up to 19.2%, and Bacteroides_ovatus_V975 was up to 2.8% at T36. The increased effects on Escherichia-Shigella and Enterococcus genera were attributed to Escherichia_coli and Enterococcus_faecalis (Fig. 4C and D).
The enhancing effect of GOS/inulin during fermentation on the Bifidobacterium genus was achieved through increasing the relative abundance of Bifidobacterium_longum_subsp._longum and other unidentified species, but not Bifidobacterium_bifidum (Fig. 4A). The relative abundance of Bifidobacterium_longum_subsp._longum was 14.5% at T24 and 20.9% at T36, which was even higher than T0, which was 16.0%. The effect of GOS/inulin on the unidentified species cannot be ignored, as the relative abundance was already up to 15.1% at T14, and reached a level of 19.2% at T36. Limited effects were observed on Bifidobacterium_bifidum, as the relative abundance during the fermentation was kept stable at 0.9% (T14), 0.9% (T24) and 0.1% (T36).
3-FL fermentation induced a relative abundance at T14 of Bifidobacterium genus of 15.4%, and it was dominated by Bifidobacterium_longum_subsp._longum and the unidentified species, with a relative abundance of 7.1% and 7.7% (Fig. 4A). During 3FL fermentation, the unidentified species of Bifidobacterium was reduced to 2.8% at T24, and 3.5% at T36, but the relative abundance of Bifidobacterium_longum_subsp._longum was stable, which was 6.2% at T36. 3-FL failed to impact Bifidobacterium_bifidum, and the relative abundance was only of 0.3% (T14), 0.1% (T24), and 0.1% (T36) during the fermentation. Meanwhile, the increasing effect of 3-FL on the Bacteroides genus was demonstrated as by increasing Bacteroides_dorei (Fig. 4B). The relative abundance of Bacteroides_dorei was up to 12.7% at T36, which was 93.3% of the relative abundance of Bacteroides genus (a level of 13.8% at T36).
LNT2 had a strong effect on the Bifidobacterium genus, which was 21.6% at T14 (Fig. 4A), and was dominated by Bifidobacterium_longum_subsp._longum and the unidentified species, with a level of 9.8% and 11.0%. The relative abundance of Bifidobacterium_bifidum was only 0.4% at T14. During fermentation, LNT2 induced a relatively stable abundance of Bifidobacterium_longum_subsp._longum, which was of 10.0% at T36, and slightly increased the relative abundance of Bifidobacterium_bifidum to 0.9% at T36, but the unidentified species was reduced to 7.6% at T36. As we explained in section 3.4, the reduction of the Bifidobacterium genus might be attributed to the increase of the Collinsella genus, but the species belonging to Collinsella was unidentified.
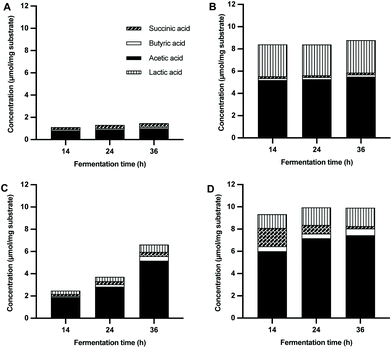
3.5 3-FL fermentation stimulates acetic acid, butyric acid and lactic acid production, and LNT2 also specifically induces succinic acid production
Next, we determined the production of SCFAs and organic acids by microbiota of 12-week-old babies during fermentation of GOS/inulin, 3-FL, and LNT2 at T14, T24, and T36. We took fermentation in the SIEM medium without added GOS/inulin, 3-FL, or LNT2 as a control. The fermentation of the control only resulted in a low amount of SCFAs and organic acids during the fermentation, i.e., 1.1–1.5 μmol mg−1 (Fig. 5A). Propionic acid was undetectable in the fermentation samples (data not shown).During the fermentation of GOS/inulin, we observed a total SCFAs and organic acid production of 7.3 μmol mg−1 at T14, which remained stable throughout the whole fermentation (Fig. 5B). SCFAs formed by GOS/inulin was dominated by acetic acid, with a level of 4.4 μmol mg−1 at T14, and remained stable until T36. GOS/inulin fermentation only led to a production of low amounts of butyric acid, with less than 0.1 μmol mg−1. Organic acid of lactic acid, but not succinic acid, was formed during fermentation of GOS/inulin, with a level of 2.9 μmol mg−1 at T14, which remained stable until T36.
During the fermentation of 3-FL, only minor amounts of acetic acid and lactic acid were produced (Fig. 5C). At T14, there was only 1.1 μmol mg−1 of acetic acid and 0.3 μmol mg−1 of lactic acid produced by fermentation of 3-FL. The acetic acid increased to 2.0 μmol mg−1 at T24. At this time point, we also observed 0.1 μmol mg−1 of butyric acid and 0.4 μmol mg−1 of lactic acid, which formed a total SCFAs and organic acid production of 2.4 μmol mg−1. The total SCFAs and organic acid production kept increasing to 5.1 μmol mg−1 at T36, with 4.2 μmol mg−1 of acetic acid, 0.3 μmol mg−1 of butyric acid, and 0.7 μmol mg−1 of lactic acid.
Fermentation of LNT2 resulted in a higher production of total SCFAs and organic acids (Fig. 5D). At T14, a level of 8.2 μmol mg−1 was reached, which consisted of acetic acid (5.2 μmol mg−1), butyric acid (0.3 μmol mg−1), succinic acid (1.5 μmol mg−1), and lactic acid (1.3 μmol mg−1). The production of succinic acid was distinct for LNT2, as it was not observed during fermentation of 3-FL. The total SCFAs and organic acids production was stable throughout the fermentation, but the composition did change. The succinic acid production decreased to 0.5 μmol mg−1 at T24, and was non-detectable at T36. At the same time, the production of acetic acid and lactic acid at T24 increased to 6.3 μmol mg−1 and 1.6 μmol mg−1. At T36, the production of acetic acid, butyric acid, and lactic acid increased to 6.4 μmol mg−1, 0.5 μmol mg−1, and 1.6 μmol mg−1, respectively, with a ratio of 75.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.38
5.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.21.
19.21.
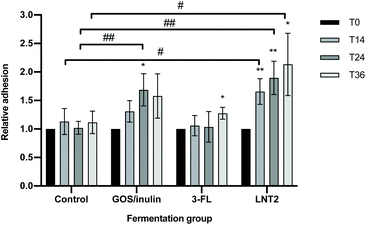
3.6 GOS/inulin and LNT2 fermentation products exposure enhance the adhesion of L. plantarum WCFS1 to intestine epithelial Caco-2 cells
To investigate whether the fermentation digesta of GOS/inulin, 3-FL, and LNT2 would have any impact on adhesion of a commensal bacterium to gut epithelial cells, we studied adhesion of L. plantarum WCFS1 to Caco-2 cells in the presence of the fermentation digesta. Digesta of the fermentation with only SIEM medium was taken as the control.Compared to the fermentation products of the control, exposure to the T24 digesta of GOS/inulin significantly increased the adhesion of L. plantarum WCFS1, with an increase of 68.7% (p < 0.01, Fig. 6). Compared to the control, the fermentation digesta of 3-FL did not influence the adhesion of L. plantarum WCFS1, but the T14, T24 and T36 digesta of the LNT2 fermentation significantly increased the adhesion of L. plantarum WCFS1 to Caco-2 cells, with 52.6% (p < 0.05), 87.7% (p < 0.01), and 1.0 (p < 0.05) fold, respectively (Fig. 6).
Within the fermentation of different carbohydrates, the effect on L. plantarum WCFS1 adhesion changed over time. Exposure to the T14, T24, and T36 digesta of the control fermentation did not influence the adhesion of L. plantarum WCFS1 on Caco-2 cells compared to T0 (Fig. 6). However, exposure to the T14 digesta of GOS/inulin showed the tendency to increase L. plantarum WCFS1 adhesion by 30.9% (p = 0.085) compared to T0. In addition, we found that the T24 digesta of this fermentation significantly increased the adhesion of L. plantarum WCFS1 with 68.7% (p < 0.05), and the T36 digesta reached a tendency by 57.9% (p = 0.108) compared to T0 digesta (Fig. 6). The T36 fermentation digesta of 3-FL also significantly increased the adhesion of L. plantarum WCFS1 (p < 0.05), compared to the T0 digesta (Fig. 6). The digesta taken at earlier time points during this fermentation did not change adherence. The T14 digesta of LNT2 could also significantly increase the adhesion of L. plantarum WCFS1 (p < 0.01), and the significant increase was also observed for the T24 digesta (p < 0.01) and the T36 digesta (p < 0.05), with an increase of 89.6% and 1.1-fold compared to T0 (Fig. 6).
4. Discussion
Human milk oligosaccharides (hMOs) and non-digestible carbohydrates (NDCs) in infant formula are considered to guide colonization by the gut microbiota and to support microbial metabolism.3,14 A well-established gut microbiota is crucial for gastrointestinal health in early life and prevents disease at later age.2 The NDCs GOS/inulin, and the hMOs 2′-FL and LNnT have been commonly applied in infant formula to mimic the functions of hMOs in mother's milk.12,33 In recent years, 3-FL and LNT2 have also been proposed for supplementation in infant formula because of the specific benefits they might have.25 Similar to 2′-FL, 3-FL has lactose as the core structure, and only differs in the fucosylation of fucose to lactose.26 LNT2 is the acid hydrolysate of LNnT that might be formed in vivo to some extent after passage through the stomach.34 3-FL and LNT2 have been shown to have even stronger beneficial effects than 2′-FL in many in vitro studies, such as on enhancing commensal bacteria adhesion to the gut epithelium,35 reducing inflammation,36 and enhancing intestinal epithelial barrier function.25Our data show that 3-FL was not as readily fermented by 12-week-old baby microbiota as LNT2 and GOS/inulin, but resulted in microbiota with a more diverse composition. Besides an increased relative abundance of the Bifidobacterium genus of the Actinobacteria phylum, the Enterococcus genus of the Firmicutes phylum was also increased compared to the control fermentation. The Bifidobacterium genus, dominated by B. longum subspecies longum, was stable during the full duration of the fermentation study. This may illustrate that B. longum subsp. longum cannot ferment 3-FL, which was also observed in a recent study.37 The fermentation of fucosylated hMOs by bifidobacteria is subspecies and strain specific, i.e., they were commonly utilized by B. longum subsp. infantis, B. longum subsp. bifidum, and B. breve strains,37–39 while only certain B. longum subsp. longum strain such as B. longum subsp. longum SC596 can take 2′-FL and 3-FL as a growth substrate.40 These strains might be responsible together with other species for the stimulation of the production of acetic acid and lactic acid.41,42 With longer fermentation time, the relative abundance of Bacteroides, which was specifically dominated by B. dorei, a species specifically present in vaginally born infants,43 was increased. The increase of B. dorei positively correlates with the degradation of 3-FL and with enhanced production of acetic acid, butyric acid, and lactic acid. This might be attributed to the α-ι-fucosidase produced by both Bifidobacterium and Bacteroides, which is responsible for the degradation of 3-FL.44
The breakdown products produced by Bifidobacterium and Bacteroides after 3-FL fermentation may cross feed species from Enterococcus genus, which in this study was dominated by only one species, i.e., E. faecalis. This suggestion is supported by a study by Wang et al. who showed that Enterococcus is not able to directly degrade hMO, and is dependent on breakdown products from hMO degrading Bifidobacterium and Bacteroides spp.45 This cross feeding might contribute to the establishment of an adult-like gut microbiome, which helps infants to get through the transition from breastfeeding to infant formula or solid food.3,45 The results may imply that the need for 3-FL increases with age during gut microbiota maturation, which is corroborated with the observation that 3-FL increases in mother's milk as lactation progresses.3
LNT2 was more readily fermented than 3-FL and induced a similar composition of acetic acid as induced by GOS/inulin after 14 h. At this time point, we also observed a high production of succinic acid and butyric acid besides the production of acetic acid and lactic acid, as observed during fermentation of GOS/inulin and 3-FL. There is only limited research available on the degradation of LNT2. Recently, a Bifidobacterium strain, i.e., B. longum subsp. infantis belonging to the phylum of Actinobacteria was reported to grow on LNT2, resulting in the production of acetic acid,37 which corroborates our observation that fermentation of LNT2 induces a high relative abundance of species belonging to the Bifidobacterium genus, as well as continuous production of acetic acid. However, we only observed enhanced enrichment of B. longum subsp. longum in this study, and a high relative abundance of unidentified Bifidobacterium species. Studies towards LNT2 fermentation and impact on microbiota are urgently needed as it is formed when LNnT, which is already applied in infant formula, passes the stomach.
The production of succinic acid during the fermentation of LNT2 may suggest a potential anti-inflammatory effect of LNT2.25 Succinic acid formation is associated with an increased number of intestinal tuft cells, which can trigger anti-inflammatory immune responses.46 Succinate was gradually consumed at later stages of fermentation, and correlated with an increased abundance of Collinsella.47 A high abundance of Collinsella was also found in the T0 samples. Collinsella has been reported to be a commensal with ability to produce butyrate,48 which is the most bioactive SCFA in controlling inflammatory responses.22 LNT2 might also confer the anti-inflammatory effects through lowering the abundance of Escherichia-Shigella, a genus that is closely linked to acute necrotizing pancreatitis and other systemic inflammation.49 Thus, the addition of LNT2 to infant formula might beneficially influence microbiota compositions and strengthen the immune system.
Fermentation of GOS/inulin was more complex. We found that GOS was completely fermented after 14 h, while for inulin, DP8-DP14 was predominantly used by the fecal microbiota of 12-week-old infants. It supported the growth of Bifidobacterium, which is in line with previous findings.5,31Bifidobacterium is a signature organism of the gut microbiota in early life of breastfed infants. It is considered to provide substantial health benefits, which is the reason why enhancement of Bifidobacterium is a hallmark for efficacy of NDCs and hMOs in infant formula.31 Our results confirm a bifidogenic effect of GOS/inulin,12 as well as of 3-FL and LNT2, which all stimulated B. longum subsp. longum. B. longum subspecies express enzymes including α-fucosidases, β-galactosidases, and β-hexosaminidase. These enzymes are essential for the utilization of hMOs and NDCs.50 β-Galactosidases can degrade the β1–4 linkages within GOS structures, leading to the formation of acetic acid and lactic acid during the subsequent fermentation.31,50 Acetic acid is the most abundant SCFA and may serve as the intermediate product for the synthesis of butyrate.51 Together with lactic acid, butyrate will promote a lower pH environment and support the growth of Bifidobacterium.52 The production of acetic acid and lactic acid is also important for inhibition of, e.g., E. coli infections and for improving gut barrier function.53,54 We found experimental proof as we observed a reduction in the relative abundance of Escherichia_coli belonging to the Escherichia-Shigella genus. The delayed degradation of inulin may have contributed to a continuous level of acetic acid and lactic acid. Inulin needs a longer time to be degraded and may stay in the colon for an average duration of 30–40 h,55 and serve as a stock to guarantee a continuously supply of dietary fibers for the gut microbiota.
Not much is known about how the fermentation digesta of hMOs, and NDCs contribute to adhesion and colonization of commensal bacteria to the gut epithelium, which is essential to confer health effects.56 We demonstrated that the fermentation digesta of the different tested NDCs could modulate the adhesion of the commensal bacterium L. plantarum WCFS1 to intestine epithelial Caco-2 cells, but that the efficacy is highly dependent on the fermented hMO or GOS/inulin. Compared to the control, the fermentation digesta of LNT2 at T14 could already significantly increase the adhesion of L. plantarum WCFS1 to Caco-2 cells, which might be explained by the high concentration of different SCFAs and other organic acids. The T24 and T36 fermentation digesta of LNT2 also increased the adhesion of L. plantarum WCFS1 to Caco-2 cells, but this seems to correlate with the continuous production of SCFAs and other organic acids during the fermentation of LNT2. The fermentation digesta of 3-FL did not increase the adhesion of L. plantarum WCFS1. This could possibly be explained by the relatively low fermentability, and consequently lower production of SCFAs and other organic acids. The fermentation digesta of the reference, GOS/inulin at T24, increased the adhesion of L. plantarum WCFS1 to Caco-2 cells.
The enhanced adhesion of commensal bacterium L. plantarum WCFS1 by the fermentation products of LNT2 and GOS/inulin might be induced in multiple ways. It is possible that SCFAs, lactic acid, and succinic acid formed during the fermentation increased the expression of adhesion molecules, such as lectin-binding adhesin or glyceraldehyde-3-phosphate dehydrogenase, on the bacteria cell membrane.56,57 It is also possible that the SCFAs, lactic acid, and succinic acid modulate the glycosylation of the intestine epithelial cells to support bacteria adhesion.58 The enhanced commensal bacteria adhesion to the intestinal epithelial cells is important for many processes, but also eg. to exclude pathogens adhesion and infection.59,60
5. Conclusions
In summary, we show here that the hMO molecules 3-FL and LNT2 were fermented by fecal microbiota of 12-week-old infants. However, the degradation pattern, gut microbiota changes, and SCFAs production were hMO structure specific. Especially, LNT2 was rapidly utilized, and increased the relative abundance of Bifidobacterium and Collinsella, as well as the production of SCFAs. The LNT2 fermentation digesta significantly enhanced the adhesion of the commensal bacterium L. plantarum WCFS1 to intestinal epithelial cells. In addition, fermentation digesta of GOS/inulin enhanced adhesion of L. plantarum WCFS1. 3-FL was gradually degraded, and specifically increased bacteria of Bacteroides and Enterococcus genus. Our findings demonstrate positive effects of 3-FL and LNT2 on gut microbiome microbial metabolic products. This knowledge may contribute to the design of tailored infant formula containing specific hMO molecules to meet the healthy development of infants during the transition from breastfeeding to formula or solid food feeding.Author contributions
C. K., R. A., and P. d. V. conceived and designed the experiments. C. K., R. A., C. E. K., M. B., and M. M. P. O. performed the experiments. C. K., C. E. K., and M. B. analyzed data. C. K., H. A. S., and P. d. V. wrote the paper.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
C. K. is supported by the China Scholarship Council (CSC) under Grant No. 201600090212.References
- M. K. Lee and C. Binns, Breastfeeding and the risk of infant illness in asia: A review, Int. J. Environ. Res. Public Health, 2020, 17, 186 CrossRef.
- F. Turroni, C. Milani, S. Duranti, G. A. Lugli, S. Bernasconi and A. Margolles, et al., The infant gut microbiome as a microbial organ influencing host well-being, Ital. J. Pediatr., 2020, 46, 16 CrossRef.
- K. Borewicz, F. Gu, E. Saccenti, C. Hechler, R. Beijers and C. de Weerth, et al., The association between breastmilk oligosaccharides and faecal microbiota in healthy breastfed infants at two, six, and twelve weeks of age, Sci. Rep., 2020, 10, 4270 CrossRef CAS PubMed.
- S. B. Bering, Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates, Nutrients, 2018, 10, 1461 CrossRef.
- O. Perdijk, P. van Baarlen, M. M. Fernandez-Gutierrez, E. van den Brink, F. H. J. Schuren and S. Brugman, et al., Sialyllactose and galactooligosaccharides promote epithelial barrier functioning and distinctly modulate microbiota composition and short chain fatty acid production in vitro, Front. Immunol., 2019, 10, 94 CrossRef CAS PubMed.
- C. R. Martin, P. R. Ling and G. L. Blackburn, Review of infant feeding: Key features of breast milk and infant formula, Nutrients, 2016, 8, 279 CrossRef PubMed.
- N. M. Koropatkin, E. A. Cameron and E. C. Martens, How glycan metabolism shapes the human gut microbiota, Nat. Rev. Microbiol., 2012, 10, 323 CrossRef CAS.
- R. Akkerman, M. M. Faas and P. de Vos, Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation, Crit. Rev. Food Sci. Nutr., 2018, 59, 1486 CrossRef PubMed.
- Y. Vandenplas, B. Berger, V. P. Carnielli, J. Ksiazyk, H. Lagström, M. S. Luna and N. Migacheva, et al., Human milk oligosaccharides: 2′-fucosyllactose (2′-FL) and lacto-n-neotetraose (LNnT) in infant formula, Nutrients, 2018, 10, 1161 CrossRef.
- L. Cheng, R. Akkerman, C. Kong, M. T. C. Walvoort and P. de Vos, More than sugar in the milk: human milk oligosaccharides as essential bioactive molecules in breast milk and current insight in beneficial effects, Crit. Rev. Food Sci. Nutr., 2021, 61, 1184 CrossRef PubMed.
- A. M. Bakker-Zierikzee, M. S. Alles, J. Knol, F. J. Kok, J. J. M. Tolboom and J. G. Bindels, Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium, animalis on the intestinal microflora during the first 4 months of life, Br. J. Nutr., 2005, 94, 783 CrossRef PubMed.
- D. L. Ackerman, K. M. Craft and S. D. Townsend, Infant food applications of complex carbohydrates: Structure, synthesis, and function, Carbohydr. Res., 2017, 437, 16 CrossRef PubMed.
- F. Enam and T. J. Mansell, Prebiotics: tools to manipulate the gut microbiome and metabolome, J. Ind. Microbiol. Biotechnol., 2019, 46, 1445 CrossRef.
- C. Kong, M. M. Faas, P. de Vos and R. Akkerman, Impact of dietary fibers in infant formulas on gut microbiota and the intestinal immune barrier, Food Funct., 2020, 11, 9445 RSC.
- J. L. Carlson, J. M. Erickson, J. M. Hess, T. J. Gould and J. L. Slavin, Prebiotic dietary fiber and gut health: Comparing the in vitro fermentations of beta-glucan, inulin and xylooligosaccharide, Nutrients, 2017, 9, 1361 CrossRef PubMed.
- K. Salli, H. Anglenius, J. Hirvonen, A. A. Hibberd, I. Ahonen and M. T. Saarinen, et al., The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites; a pilot study in comparison to GOS and lactose, Sci. Rep., 2019, 9, 13232 CrossRef PubMed.
- M. B. Engfer, B. Stahl, B. Finke, G. Sawatzki and H. Daniel, Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract, Am. J. Clin. Nutr., 2000, 71, 1589 CrossRef.
- D. Paganini, M. A. Uyoga, C. I. Cercamondi, D. Moretti, E. Mwasi, C. Schwab and S. Bechtler, et al., Consumption of galacto-oligosaccharides increases iron absorption from a micronutrient powder containing ferrous fumarate and sodium iron EDTA: A stable-isotope study in Kenyan infants, Am. J. Clin. Nutr., 2017, 106, 1020 CrossRef.
- A. L. Morrow, G. M. Ruiz-Palacios, X. Jiang and D. S. Newburg, Human-milk glycans that inhibit pathogen binding protect breast-feeding Infants against Infectious Diarrhea, J. Nutr., 2005, 135, 1304 CrossRef.
- M. J. Logtenberg, R. Akkerman, R. An, G. D. A. Hermes, B. J. de Haan and M. M. Faas, et al., Fermentation of chicory fructo–oligosaccharides and native inulin by infant faecal microbiota attenuates pro–inflammatory responses in immature dendritic cells in an infant–age dependent and fructan–specific way, Mol. Nutr. Food Res., 2020, 64, e2000068 CrossRef PubMed.
- J. Langhorst, A. K. Koch, P. Voiss, G. J. Dobos and A. Rueffer, Distinct patterns of short-chain fatty acids during flare in patients with ulcerative colitis under treatment with mesalamine or a herbal combination of myrrh, chamomile flowers, and coffee charcoal: secondary analysis of a randomized controlled trial, Eur. J. Gastroenterol. Hepatol., 2020, 32, 175 CrossRef PubMed.
- S. Xiao, S. Jiang, D. Qian and J. Duan, Modulation of microbially derived short-chain fatty acids on intestinal homeostasis, metabolism, and neuropsychiatric disorder, Appl. Microbiol. Biotechnol., 2020, 104, 589 CrossRef PubMed.
- F. Bianchi, N. Larsen, T. de Mello Tieghi, M. A. T. Adorno, W. Kot and S. M. I. Saad, et al., Modulation of gut microbiota from obese individuals by in vitro, fermentation of citrus pectin in combination with Bifidobacterium longum BB-46, Appl. Microbiol. Biotechnol., 2018, 102, 8827 CrossRef CAS PubMed.
- K. M. Craft, H. C. Thomas and S. D. Townsend, Interrogation of human milk oligosaccharide fucosylation patterns for antimicrobial and antibiofilm trends in group B Streptococcus, ACS Infect. Dis., 2018, 4, 1755 CrossRef CAS PubMed.
- L. Cheng, C. Kong, M. T. C. Walvoort, M. M. Faas and P. de Vos, Human milk oligosaccharides differently modulate goblet cells under homeostatic, proinflammatory conditions and ER Stress, Mol. Nutr. Food Res., 2020, 64, e1900976 CrossRef PubMed.
- G. A. Sprenger, F. Baumgärtner and C. Albermann, Production of human milk oligosaccharides by enzymatic and whole-cell microbial biotransformations, J. Biotechnol., 2017, 258, 79 CrossRef.
- F. Gu, K. Borewicz, B. Richter, P. H. van der Zaal, H. Smidt and P. L. Buwalda, et al., In vitro, fermentation behavior of isomalto/malto-polysaccharides using human fecal inoculum indicates prebiotic potential, Mol. Nutr. Food Res., 2018, 62, 1800232 CrossRef.
- M. P. Jarlenski, W. L. Bennett, S. N. Bleich, C. L. Barry and E. A. Stuart, Effects of breastfeeding on postpartum weight loss among U.S. women, Prev. Med., 2014, 69, 146 CrossRef.
- A. G. M. Leijdekkers, M. Aguirre, K. Venema, G. Bosch, H. Gruppen and H. A. Schols, In vitro fermentability of sugar beet pulp derived oligosaccharides using human and pig fecal inocula, J. Agric. Food Chem., 2014, 62, 1079 CrossRef PubMed.
- H. R. Cardarelli, R. C. R. Martinez, S. Albrecht, H. Schols, B. D. G. M. Franco and S. M. I. Saad, et al., In vitro fermentation of prebiotic carbohydrates by intestinal microbiota in the presence of Lactobacillus amylovorus DSM 16998, Benef. Microbes, 2016, 7, 119 CrossRef PubMed.
- T. Matsuki, S. Tajima, T. Hara, K. Yahagi, E. Ogawa and H. Kodama, Infant formula with galacto-oligosaccharides (OM55N) stimulates the growth of indigenous bifidobacteria in healthy term infants, Benef. Microbes, 2016, 7, 453 CrossRef PubMed.
- G. T. Macfarlane, H. Steed and S. Macfarlane, Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics, J. Appl. Microbiol., 2008, 104, 305 CAS.
- S. Austin, D. Cuany, J. Michaud, B. Diehl and B. Casado, Determination of 2′-fucosyllactose and lacto-N-neotetraose in infant formula, Molecules, 2018, 23, 2650 CrossRef PubMed.
- M. J. Gnoth, C. Kunz, E. Kinne-Saffran and S. Rudloff, Human milk oligosaccharides are minimally digested in vitro, J. Nutr., 2000, 130, 3014 CrossRef CAS PubMed.
- C. Kong, L. Cheng, G. Krenning, J. Fledderus, B. J. de Haan, M. T. C. Walvoort and P. de Vos, Human milk oligosaccharides mediate the crosstalk between intestinal epithelial Caco-2 cells and Lactobacillus plantarum WCFS1 in an in vitro model with intestinal peristaltic shear force, J. Nutr., 2020, 150, 2077 CrossRef PubMed.
- L. Cheng, M. B. G. Kiewiet, A. Groeneveld, A. Nauta and P. de Vos, Human milk oligosaccharides and its acid hydrolysate LNT2 show immunomodulatory effects via TLRs in a dose and structure-dependent way, J. Funct. Foods, 2019, 59, 174 CrossRef.
- L. Cheng, M. B. G. Kiewiet, M. J. Logtenberg, A. Groeneveld, A. Nauta and H. A. Schols, et al., Effects of different human milk oligosaccharides on growth of bifidobacteria in monoculture and co-culture with Faecalibacterium prausnitzii, Front. Microbiol., 2020, 11, 569700 CrossRef.
- S. Asakuma, E. Hatakeyama, T. Urashima, E. Yoshida, T. Katayama and K. Yamamoto, et al., Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria, J. Biol. Chem., 2011, 286, 34583 CrossRef.
- S. Ruiz-Moyano, S. M. Totten, D. A. Garrido, J. T. Smilowitz, J. B. German and C. B. Lebrilla, et al., Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve, Appl. Environ. Microbiol., 2013, 79, 6040 CrossRef.
- D. Garrido, S. Ruiz-Moyano, N. Kirmiz, J. C. Davis, S. M. Totten and D. G. Lemay, et al., A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum, subsp. longum SC596, Sci. Rep., 2016, 6, 35045 CrossRef.
- S. M. Jandhyala, R. Talukdar, C. Subramanyam, H. Vuyyuru, M. Sasikala and D. N. Reddy, Role of the normal gut microbiota, World J. Gastroenterol., 2015, 21, 8787 CrossRef CAS PubMed.
- S. Verkhnyatskaya, M. Ferrari, P. de Vos and M. T. C. Walvoort, Shaping the infant microbiome with non-digestible carbohydrates, Front. Microbiol., 2019, 10, 343 CrossRef PubMed.
- F. Bäckhed, J. Roswall, Y. Peng, Q. Feng, H. Jia and P. Kovatcheva-Datchary, Dynamics and stabilization of the human gut microbiome during the first year of life, Cell Host Microbe, 2015, 17, 852 CrossRef.
- Z. T. Yu, C. Chen and D. S. Newburg, Utilization of major fucosylated and sialylated human milk oligosaccharides by isolated human gut microbes, Glycobiology, 2013, 23, 1281 CrossRef CAS.
- M. Wang, M. Li, S. Wu, C. B. Lebrilla, R. S. Chapkin and I. Ivanov, et al., Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed, J. Pediatr. Gastroenterol. Nutr., 2015, 60, 825 CrossRef CAS.
- C. A. Cowardin, P. P. Ahern, V. L. Kung, M. C. Hibberd, J. Cheng and J. L. Guruge, et al., Mechanisms by which sialylated milk oligosaccharides impact bone biology in a gnotobiotic mouse model of infant undernutrition, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11988 CAS.
- R. Takagi, K. Sasaki, D. Sasaki, I. Fukuda, K. Tanaka and K. Yoshida, et al., A single-batch fermentation system to simulate human colonic microbiota for high-throughput evaluation of prebiotics, PLoS One, 2016, 11, e0160533 CrossRef PubMed.
- P. Qin, Y. Zou, Y. Dai, G. Luo, X. Zhang and L. Xiao, Characterization a novel butyric acid-producing bacterium Collinsella aerofaciens subsp. Shenzhenensis, subsp. nov, Microorganisms, 2019, 7, 78 CrossRef CAS.
- J. Chen, C. Huang, J. Wang, H. Zhou, Y. Lu and L. Lou, et al., Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats, PLoS One, 2017, 12, e0176583 CrossRef PubMed.
- P. Thomson, D. A. Medina and D. Garrido, Human milk oligosaccharides and infant gut bifidobacteria: Molecular strategies for their utilization, Food Microbiol., 2018, 75, 37 CrossRef CAS.
- E. Boets, S. V. Gomand, L. Deroover, T. Preston, K. Vermeulen and V. de Preter, et al., Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study, J. Physiol., 2017, 595, 541 CrossRef CAS.
- S. L. Bridgman, M. B. Azad, C. J. Field, A. M. Haqq, A. B. Becker and P. J. Mandhane, et al., Fecal short-chain fatty acid variations by breastfeeding status in infants at 4 Months: Differences in relative versus absolute concentrations, Front. Nutr., 2017, 4, 11 Search PubMed.
- S. Fukuda, H. Toh, K. Hase, K. Oshima, Y. Nakanishi and K. Yoshimura, et al., bifidobacteria can protect from enteropathogenic infection through production of acetate, Nature, 2011, 469, 543 CrossRef CAS PubMed.
- E. C. Martens, M. Neumann and M. S. Desai, Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier, Nat. Rev. Microbiol., 2018, 16, 457 CrossRef CAS.
- U. C. Ghoshal, V. Sengar and D. Srivastava, Colonic transit study technique and interpretation: Can these be uniform globally in different populations with non-uniform colon transit time?, J. Neurogastroenterol. Motil., 2012, 18, 227 CrossRef PubMed.
- E. G. Kravtsov, A. V. Yermolayev, I. V. Anokhina, N. V. Yashina, V. L. Chesnokova and M. V. Dalin, Adhesion characteristics of Lactobacillus, is a criterion of the probiotic choice, Bull. Exp. Biol. Med., 2008, 145, 232 CrossRef CAS.
- G. Wang, M. Zhang, J. Zhao, Y. Xia, P. F. Lai and L. Ai, A surface protein from Lactobacillus plantarumincreases the adhesion of Lactobacillus strains to human epithelial cells, Front. Microbiol., 2018, 9, 2858 CrossRef PubMed.
- L. Bode, The functional biology of human milk oligosaccharides, Early Hum. Dev., 2015, 91, 619 CrossRef CAS PubMed.
- M. L. van Tassell and M. J. Miller, Lactobacillus, adhesion to mucus, Nutrients, 2011, 3, 613 CrossRef CAS.
- H. A. Seddik, F. Bendali, B. Cudennec and D. Drider, Anti-pathogenic and probiotic attributes of Lactobacillus salivarius and Lactobacillus plantarum, strains isolated from feces of Algerian infants and adults, Res. Microbiol., 2017, 168, 244 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo02563e |
| This journal is © The Royal Society of Chemistry 2021 |