 Open Access Article
Open Access ArticleMain drivers of (poly)phenol effects on human health: metabolite production and/or gut microbiota-associated metabotypes?
Carlos E.
Iglesias-Aguirre
 a,
Adrián
Cortés-Martín
a,
Adrián
Cortés-Martín
 a,
María Á.
Ávila-Gálvez
a,
María Á.
Ávila-Gálvez
 bc,
Juan A.
Giménez-Bastida
bc,
Juan A.
Giménez-Bastida
 a,
María V.
Selma
a,
María V.
Selma
 a,
Antonio
González-Sarrías
a,
Antonio
González-Sarrías
 a and
Juan Carlos
Espín
a and
Juan Carlos
Espín
 *a
*a
aLaboratory of Food & Health, Research Group on Quality, Safety and Bioactivity of Plant Foods, CEBAS-CSIC, 30100 Campus de Espinardo, Murcia, Spain. E-mail: jcespin@cebas.csic.es
bCEDOC, NOVA Medical School, Faculdade de Ciências Médicas, Universidade NOVA de Lisboa, Lisboa, Portugal
cInstituto de Biologia Experimental e Tecnológica (IBET), Apartado 12, 2781-901, Oeiras, Portugal
First published on 20th August 2021
Abstract
Despite the high human interindividual variability in response to (poly)phenol consumption, the cause-and-effect relationship between some dietary (poly)phenols (flavanols and olive oil phenolics) and health effects (endothelial function and prevention of LDL oxidation, respectively) has been well established. Most of the variables affecting this interindividual variability have been identified (food matrix, gut microbiota, single-nucleotide-polymorphisms, etc.). However, the final drivers for the health effects of (poly)phenol consumption have not been fully identified. At least partially, these drivers could be (i) the (poly)phenols ingested that exert their effect in the gastrointestinal tract, (ii) the bioavailable metabolites that exert their effects systemically and/or (iii) the gut microbial ecology associated with (poly)phenol metabolism (i.e., gut microbiota-associated metabotypes). However, statistical associations between health effects and the occurrence of circulating and/or excreted metabolites, as well as cross-sectional studies that correlate gut microbial ecologies and health, do not prove a causal role unequivocally. We provide a critical overview and perspective on the possible main drivers of the effects of (poly)phenols on human health and suggest possible actions to identify the putative actors responsible for the effects.
1. Introduction
Historically, dietary (poly)phenols, such as flavonoids, evolved from possible compounds with vitamin-like properties to non-essential nutrients1 (check the comprehensive and excellent review on the metabolism and bioactivity of flavonoids from a historical perspective by Williamson et al.2). Later, (poly)phenols were considered anti-nutrients with potential mutagen and carcinogenic activities.3 However, this perception changed when epidemiological studies showed an inverse relationship between the consumption of plant foods and cancer risk,4 and primarily cardiovascular disease (CVD) risk.5 Together with early experimental studies, these observations endorsed the health-promoting effects observed from the flavonoid content of fruits and vegetables, and the most plausible mechanism of action was attributed to the antioxidant activity of flavonoids.5–7 In the late 1990s and early 2000s, there was a massive output of in vitro studies describing the free radical scavenging capacity of (poly)phenol-rich plant extracts, establishing a potential health claim based merely on the content of (poly)phenols in foods.8–11 Later, ADME (absorption, distribution, metabolism, and excretion) studies showed that (poly)phenols are poorly bioavailable, i.e., the proportion of intact (poly)phenolic compounds ingested that reach the bloodstream is low.12–16 Upon absorption, phase-II enzymes extensively metabolise (poly)phenols, yielding conjugated metabolites that can show some activity but are much lower than their food-occurring phenolic precursors.17–20 This evidence questions the reliability of thousands of in vitro results based on non-physiological conditions, i.e., the assay of food-occurring (poly)phenols instead of their physiologically relevant conjugates.21 Unfortunately, this useless approach persists.To date, a substantial number of human studies and meta-analyses have discussed the preventive effects of (poly)phenol-containing foods against cardiometabolic diseases and, to a lesser extent, against some types of cancer and neurodegenerative diseases.22–33 However, there is controversy and not all the studies reach conclusive links between (poly)phenols and health, even for the same outcome, e.g., vascular function, performed by the same research group and assaying the same pure flavonoid, e.g. epicatechin.34,35 Overall, most systematic reviews and meta-analyses on (poly)phenols and health highlight the finding of some “promising” or “potential” health effects, but conclude with the same call of caution: “…more high-quality research is needed…”, “…further well-designed randomised and controlled trials are required…”
A well-established cause-and-effect relationship between most (poly)phenols consumed and their possible beneficial effects remains elusive. In this regard, many of their related health claims have been rejected by the European Food Safety Agency (EFSA). Indeed, except for a few examples of health benefits, including protection of low-density lipoprotein cholesterol (LDLc) particles from oxidative damage by olive oil phenolics such as hydroxytyrosol,36 and improvement of endothelial function by cocoa flavanols,37 most of the health effects of dietary (poly)phenols have not been unequivocally proven. Even in these cases, the final triggering metabolite for the effects is unknown. Beyond antioxidant activity, many mechanisms for the action of (poly)phenols have been described, including the modulation of inflammatory mediators, fat metabolism, and transcription factors,38–40 but the actual mechanisms behind the effects have not been fully established. We do not aim here to review possible determinants affecting (poly)phenols effects, an issue extensively reviewed in recent years under the COST Action POSITIVe activities.41–43 However, it is not yet clear whether the effects observed after polyphenol-rich food consumption are mainly exerted by: (i) the ingested phenolics, (ii) their phase-II derived metabolites, (iii) the greater or lesser amount of phenolic-derived metabolites produced by the microbiota (metabolite gradient), and/or (iv) the specific gut microbiota associated with the metabolism of (poly)phenols (the so-called gut microbiota metabotypes).
Therefore, we aim to provide an overview and perspective to address which player(s), i.e., metabolite production and/or gut microbiota-associated metabotypes, could be responsible for the observed health effects in humans upon (poly)phenol consumption.
2. Interindividual variability in response to (poly)phenol consumption: in search of the putative driver responsible for the effects
It has long been known that the clinical response to drug administration varies widely between individuals.44 Similarly, increasing evidence began to appear on interindividual variability in the metabolism of certain dietary (poly)phenols, such as isoflavones,45 flavanones,46 tea catechins,47 and ellagitannins,48 among others. In parallel, other studies also began to throw controversy about the statistical significance of the observed effects due to the large standard deviations of the results obtained.22 In this regard, the wide range of (poly)phenol structures was suggested to be a crucial factor that impacts their metabolism and could be behind the wide variability in the effects on biomarkers of CVD risk.49–51 However, despite dietary (poly)phenols being structurally different, many share the same multi-target action mechanisms.2 Finally, there is notable interindividual variability in response to (poly)phenol consumption. Overall, this avoids claiming that (poly)phenols exert health effects for the entire population, which could be behind the rejection by EFSA of many health claims for (poly)phenols. Instead, the participation of many specific variables leads us to personalised dietary recommendations that consider a complex mixture of individuals’ conditions (sex, age, genetic makeup, lifestyle, physiological status, and gut microbiota) and other aspects (food matrix and processing, dietary patterns, etc.).41,43,52–57 It seems that not all of these conditions and aspects necessarily contribute equally. However, the possible weight of importance for each contributor is not known.In the last decade, the two-way interaction between (poly)phenols and gut microbiota (modulation of the microbiota by (poly)phenols and metabolism of (poly)phenols by the microbiota) has attracted attention as a new piece in the puzzle of (poly)phenols and health.43,53,58,59 In the search for the main actor(s) involved in the final (poly)phenol health effects, growing evidence has identified their derived microbial metabolites as a possible connection to establishing the bioactivity of (poly)phenols. However, the two-way interaction between the gut microbiota and dietary (poly)phenols is also the main driver of the interindividual variation detected.43,59,60 In addition to the possible bioactivity exerted by the ingested (poly)phenols and/or their derived microbial derivatives, each individual's gut microbiota, including that involved in (poly)phenol metabolism, is also relevant to explain the final effects. For example, the daidzein-derived metabolite equol was suggested as being more bioactive than its daidzein precursor and seemed to be predominant in some individuals capable of producing equol (i.e., “equol producers”).61 Similarly, the presence of urolithins in the bloodstream was initially claimed as a plausible explanation behind the effects observed after consumption of foods containing the non-bioavailable urolithin precursors ellagitannins.48,62 However, not all individuals produce the same urolithins, nor harbour the same associated gut microbiota.43,63
While the determinants affecting the individuals’ response to (poly)phenol consumption have been comprehensively identified,41,42 the relationship between effects and the (poly)phenols ingested and/or their derived metabolites is not conclusive. The questions to ask are: Which is (are) currently accepted as the final molecule(s) responsible for the effects after (poly)phenol consumption? Is there a consensus? It is not clear if the effects are exerted by the ingested phenolics and/or their microbial metabolites, and in both cases, if the phase-II conjugates participate actively, or if the effects are mediated by indirect signalling cascades where it is not necessary for the direct interaction of the molecule with the systemic target. It seems that the studies that associate the observed activity with the simultaneous presence of circulating (or excreted) phenolic metabolites or their microbial derivatives may be affected by many variables that prevent a well-established cause-and-effect relationship. In this regard, although local effects can have an impact on a systemic level, and vice versa, the likely site of the action exerted by the (poly)phenol ingested, i.e., gastrointestinal or systemic, could be the first criterion to search for a possible cause-and-effect relationship.
2.1. Effects of dietary (poly)phenols on the gastrointestinal tract
Most (poly)phenols (90%) might exert their potential benefits in the gastrointestinal (GI) tract because they are poorly absorbed and are retained for a longer time than other food compounds in the gut, where they suffer microbial transformations.43,58 Several strategies have been developed to improve the solubility and transport of dietary (poly)phenols through the GI tract and their delivery efficiency to specific intestinal regions.64 The microbial conversion of (poly)phenols in the intestine often results in metabolites with greater bioavailability than their precursors and, therefore, with potential activity beyond the GI tract. These microbial transformations are different depending on the phenolic structure (flavonoids or non-flavonoids), polymerisation degree, the spatial configuration of the compound, and gut microbial composition of the host.43,58 Several mechanisms of action of the (poly)phenols and their microbial metabolites on intestinal health have been investigated and summarised in the last decade.65–70 Some of their molecular mechanisms of action against gut inflammation are the reduction of oxidative stress, the inhibition of the secretion of inflammatory cytokines (TNF-α, IL-6, IL-8 and IL-1β), suppression of NF-κB activation, positive regulation of Nrf2, modulation of immune function, and protection of the intestinal barrier.68,70,71 Furthermore, the intestinal barrier protection by (poly)phenols and their metabolites indirectly limits the translocation of antigens and bacterial pathogens and the infiltration of bacterial lipopolysaccharides (LPS) into the bloodstream, which could reduce metabolic endotoxemia and host inflammation. The molecular mechanisms (epigenetics, inflammation, mRNA expression, and gut microbes) of several (poly)phenols have also been reviewed, such as curcumin and resveratrol against colorectal cancer (CRC).66,67,69 Although (poly)phenols, their microbial metabolites, and short-chain fatty acids (SCFAs) co-exist in the colon, it is unknown if there are in vivo synergistic interactions that reduce inflammation and protect the gut barrier. It is also unknown in what proportion most (poly)phenols and their derived metabolites contribute to the protective effect on the GI tract because most intervention studies are performed with the food (poly)phenols. Therefore, both (precursors and microbial metabolites) co-exist in the colon. Furthermore, the ratio of precursor/derived metabolites varies among individuals depending on their gut microbiota, whose composition is specific at the individual level. The higher absorption rate and anti-inflammatory activity of (poly)phenol microbial metabolites suggest they could play a more crucial role than precursors in preserving intestinal health and mitigating gut inflammatory conditions. However, it could depend on the host's ability to produce these microbial metabolites, which differs from one individual to another depending on their gut microbiota composition, which in turn depends on many factors such as health, age, lifestyle, etc. According to this, some (poly)phenol microbial metabolites such as urolithin A (Uro-A) (a microbial metabolite of ellagic acid and ellagitannins) and hydrocaffeic acid (a microbial metabolite of caffeic acid and chlorogenic acid) have been reported to be effective in treating gut inflammation in dextran sodium sulfate (DSS)-induced colitis modelled in rats.72,73 Indeed, Uro-A supplementation was more effective than pomegranate extract for ameliorating the inflammation. Besides this, a clear effect was also observed on inflammatory markers, antioxidant status, microbiota, and gene expression in the DSS-pomegranate extract group. The authors suggested that the effect could be due to either the remaining ellagic acid or the ellagitannin fraction that could reach the colon together with the small amount of Uro-A produced by the gut microbiota.73 Similarly, the only study of the chemopreventive effect of ellagitannin-containing pomegranate extract in CRC patients has revealed that the pomegranate extract counterbalanced the expression of several CRC-related genes in cancerous colon tissues. However, this effect was not associated with the urolithin level in colon tissues or with urolithin metabotypes (UMs).74 In most cases, microbial metabolites (e.g., Uro-A) could be the main active intestinal anti-inflammatory and chemopreventive compounds related to the consumption of (poly)phenols (e.g., ellagitannins) in healthy people. However, in gut dysbiosis (e.g., inflammatory bowel diseases (IBD) and CRC) and producers of low quantity or no metabolites, the precursors together with a low amount of microbial metabolites could act synergistically.Most human studies do not unequivocally show the specific effect of metabolites in the GI tract because they were performed with their precursor (poly)phenols. However, the molecular mechanisms of some metabolites have been identified using in vitro and animal studies. In this regard, Uro-A decreased inflammation markers (iNOS, cycloxygenase-2, PTGES and PGE2) both in the colonic mucosa of rats after oral administration and in an intestinal inflammatory cell model, and upregulated tight junction proteins in HT-29 cells.73,75,76 Besides this, hydrocaffeic acid intake has been shown to attenuate colitis by reducing inflammatory cytokines, including TNF-α and IL-8, and dihydro ferulic acid inhibited lipid peroxidation and DNA damage in colon mucosa after carrageenan-induced colitis, diminishing the expression of TNF-α, IL-1β and IL-8 and oxidative DNA damage in the distal colon mucosa.72 Hydroxybenzoic acids are the common microbial degradation metabolites obtained in the gut from flavonoid and non-flavonoid phenolics and are commonly found in most fruits.43 Some metabolites’ potential mechanism of action, including 3,4-dihydroxyphenylpropionic acid and 3,4-dihydroxyphenylacetic acid, has been investigated. These metabolites significantly decreased the secretion of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in LPS-stimulated peripheral blood mononuclear cells obtained from healthy people, suggesting their usefulness to alleviate IBD.77 According to these studies, it was suggested that a diet rich in these metabolite precursors (i.e., pomegranate, artichoke, cocoa, apples, strawberries, etc.) could exert anti-inflammatory effects and attenuate intestinal inflammation in humans. However, in some cases, the physiological activities of microbial phenolic-derived metabolites could not be higher than those of their precursor (poly)phenols. In an in vitro colon model, flavonoids (flavanols, theaflavin, quercetin, rutin and hesperidin) of citrus, green and black tea were catabolised to 4-hydroxyphenylacetic acid, 3-methoxy-4-hydroxyphenylacetic acid, 3,4-dihydroxyphenylacetic acid, and other catabolites with lower anti-proliferation effects than the corresponding parent flavonoids on colon cancer cell lines.78
The gut microbial imbalance (i.e., gut dysbiosis) has been associated with IBD, irritable bowel syndrome, CRC, allergies, obesity, metabolic syndrome and neurodegenerative diseases, among others.79–81 A healthy, diverse, and stable gut microbiota is essential in maintaining the homeostasis of the gut barrier and regulating the gut-brain axis.82,83 Besides other gut bacteria such as Faecalibacterium prausnitzii and Bacteroides thethaiotamicron, lactobacilli and bifidobacteria are considered beneficial in human health, helping to improve gut barrier function and the host immune system, prevent diarrhoea or allergies, activate pro-vitamins, and modulate lipid metabolism.79 The impact of dietary (poly)phenols on the gut microbial ecology and the mechanism underlying the putative beneficial effects on the GI tract and extra-intestinal diseases have been recently reviewed.64,84 Several (poly)phenol-rich foods increase gut microbiota diversity, improve the relative abundance of beneficial bacteria such as lactobacilli and bifidobacteria, and inhibit the pathogenic species. However, the precise association of (poly)phenols with benefits for the GI tract through gut microbiota modulation has been suggested but not unequivocally proven. In a human intervention study, cocoa flavanols promoted the growth of lactobacilli and bifidobacteria (intestinal barrier protectors) and reduced C-reactive protein (CRP) concentrations, an inflammatory blood biomarker and a hallmark of the acute phase inflammatory response. Changes in CRP concentrations were significantly linked to changes in lactobacilli counts.85 Other human intervention studies showed that an ellagitannin-rich pomegranate extract decreased plasma LBP in overweight-obese subjects,86 and patients with newly diagnosed CRC.87 Changes in LBP were significantly associated with the increase of Faecalibacterium, one of the main SCFA-producing bacteria, especially butyrate.86 It is known that the gut microbiota interacts with the host through microbial metabolites such as SCFAs from dietary fibre and metabolites produced from dietary (poly)phenols. It has been well established that SCFAs are the primary candidates in the crosstalk between bacteria and the intestine.88 SCFAs are an important source of energy for colonocytes and play a significant role in regulating tight junction proteins (claudin-1, occluding and zonula occludens-1), critical in preserving the integrity of the intestinal barrier.79,89 Several bacteria contribute to SCFA production. Modulation of growth and/or metabolic activity of SCFA-producing bacteria and, therefore SCFA production, has been associated with fibre consumption and the presence of (poly)phenols in the diet. For instance, red wine (poly)phenols significantly increased the number of faecal lactobacilli, bifidobacteria, and butyrate-producing bacteria (F. prausnitzii and Roseburia) at the expense of less desirable groups of bacteria such as LPS producers (Escherichia coli and Enterobacter cloacae).90 Different studies have shown that some (poly)phenols either interfered with or enhanced these positive effects of fibre.84 For instance, a combination of apple (poly)phenolic fraction (epicatechin, procyanidins, and chlorogenic acid) with apple pectin showed an additive effect on SCFA production in rat cecum size.84,91 Conversely, strawberry ellagitannins thwarted the positive effects of dietary fructooligosaccharides, while anthocyanin-rich strawberry extracts enhanced the beneficial effects of a fructooligosaccharide-rich diet.92,93 Factors such as the state of health (composition and functionality) of the gut microbiota and its enormous complexity of interactions with the host show the difficulty of demonstrating the in vitro effects of dietary (poly)phenols in the human GI tract.
2.2. Effects of dietary (poly)phenols on systemic targets
Before entering the bloodstream, dietary (poly)phenols must pass from the gut lumen, which is a crucial bottleneck in their subsequent potential biological activity.2,94 Most (poly)phenols from plant foods are glycosylated, and the attached sugar moiety is usually released before absorption occurs. The bioaccessibility of (poly)phenols is closely related to their physicochemical structure and the food matrix that contains them, which could interfere with their release ability and intestinal absorption. Once in the enterocyte, the cytochrome P450 (CYP) family of enzymes catalyse phase-I reactions, involving oxidation, reduction, and hydrolysis. Later, the aglycones can undergo phase-II metabolism in enterocytes by enzymatic systems like UDP-glucuronosyltransferases (UGT), acetyltransferases, glutathione-S-transferases (GST), sulfotransferases (SULT), and catechol-O-methyltransferases. Besides this, members of the ATP-binding cassette (ABC) superfamily are considered gatekeepers governing (poly)phenol absorption, their excretion, and, in many cases, their entry into target organs.95,96 ABC transporters can limit the absorption of (poly)phenols, their aglycones or derived metabolites by driving efflux back into the lumen (Pgp, MRP2 and BCRP) or transporting them from the intracellular compartment to the bloodstream (MRP3).96–98 Once in the bloodstream, phenolic metabolites can be subjected to additional phase-II metabolism, mainly in the liver, where CYP enzymes and ABC transporters can also critically influence the metabolism and fate of (poly)phenol-derived metabolites before urinary excretion.16,98,99 Finally, the (poly)phenols not absorbed, which can be a substantial amount of those ingested, are metabolised by the gut microbiota, mainly in the colon. The microbial-derived metabolites can be subjected to the same restrictions and metabolic actions as their phenolic precursors (i.e., absorption, transport, etc.). The microbial metabolites are usually excreted in the urine in high quantities, usually over those phenolic metabolites that enter the bloodstream before gut microbial catabolism. Overall, systemic cells can be exposed to these gut microbial-derived phenolics rather than their parent (poly)phenols ingested through plant foods.2,15,59,99The metabolism of many (poly)phenols, including flavanones, lignans, prenylflavonoids, proanthocyanidins, anthocyanins and stilbenes, shows high interindividual variability, demonstrated by the presence of a metabolite production gradient that gives rise to the so-called “high producers” and “low producers” of certain metabolites.55,104–107 However, no gut microbiota-associated metabotypes have been unequivocally described in their metabolism.43
Flavanone metabolism, such as hesperidin, yields high and low metabolite (hesperetin) excreters, a process mainly governed by the rhamnosidase activity of the gut microbiota (Fig. 1).108,109 However, hesperetin is an intermediate, further metabolised to phenolic acids (phenylpropionic, phenylacetic, and hippuric acid derivatives), which are typical final catabolites for many flavonoids.
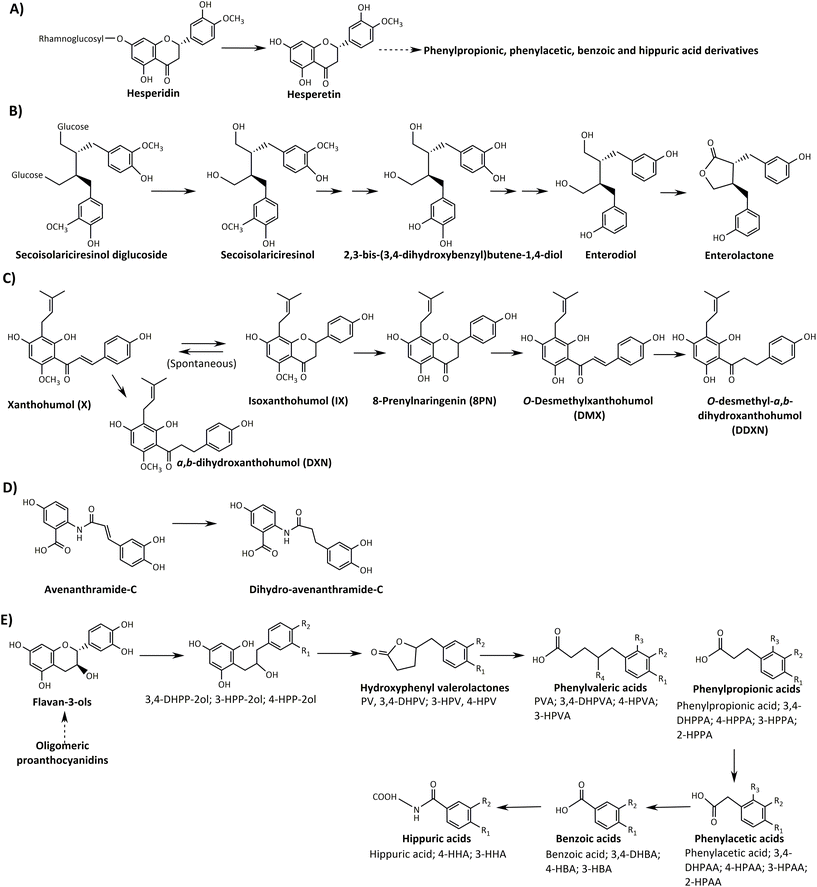 | ||
| Fig. 1 Summarised catabolic pathways of dietary (poly)phenols non-related to gut microbiota metabotypes. (A) Flavanones (hesperidin), (B) lignans (secoisolariciresinol), (C) prenyl-flavanones (xanthohumol), (D) avenanthramides (avenanthramide-C) and (E) proanthocyanidins and flavan-3-ols (catechin), HPP-2-ol, 1-(hydroxyphenyl)-3-(2′′,4′′,6′′-trihydroxyphenyl)-propan-2-ol; PV, phenylvalerolactone; HPV, hydroxyphenyl valerolactone; DHPV, dihydroxyphenylvalerolactone; PVA, phenylvaleric acid; HPVA, hydroxyphenylvaleric acid; DHPVA, dihydroxyphenylvaleric acid; HPPA, hydroxyphenylpropionic acid; DHPPA, dihydroxyphenylpropionic acid; HPAA, hydroxyphenylacetic acid; DHPAA, dihydroxyphenylacetic acid; HBA, hydroxybenzoic acid; DHBA, dihydroxybenzoic acid; HA, hippuric acid; HHA, hydroxyhippuric acid. Adapted from Cortés-Martín et al.43 with permission from Wiley, copyright March 19, 2020. | ||
Therefore, flavanone metabolism does not yield unique microbial metabolites but a metabolite production gradient, depending on the rhamnosidase activity of the individuals’ gut microbiota and the physicochemical properties of flavanones, especially their solubility under physiological conditions.53,104,107,108,110
Likewise, the metabolism of lignans such as the flaxseed secoisolariciresinol diglucoside does not produce specific metabolites but a gradient of intermediates to get enterodiol and enterolactone, which seems to be produced by the entire population to a greater or lesser extent.111,112 The presence of unique enterodiol and/or enterolactone-derived catabolites, which could be related to specific gut microbiota-associated metabotypes, has not been identified so far (Fig. 1).43
The possible gut microbiota metabotype associated with the metabolism of hops prenylflavonoids, such as xanthohumol and isoxanthohumol, has not been sufficiently demonstrated either. Xanthohumol can be either chemically or enzymatically converted into isoxanthohumol, which the gut microbial Eubacterium limosum can demethylate to yield the potent phytoestrogen 8-prenylnaringenin (8PN) (Fig. 1).113 However, several issues prevent a possible clustering of individuals according to a gut microbiota metabotype associated with the unique production of 8PN. Firstly, the gut microbial Eubacterium ramulus can metabolise 8PN to O-desmethylxanthohumol and O-desmethyl-α,β-dihydroxanthohumol (Fig. 1).113 Besides this, 8PN can already be present in food products (hops and beer), and finally, 8PN can also be formed by human cytochromes.114,115
Avenanthramides (AVAs) are phenolic alkaloids found mainly in whole-grain oat.116 Recently, Wang et al.117 observed interindividual variations in the metabolism of AVAs to dihydro-AVAs (DH-AVAs) in humans (Fig. 1). These authors identified F. prausnitzii as the individual bacterium to metabolise AVAs to DH-AVAs and proposed that the abundance of this species could be helpful to stratify individuals into AVA metabolisers and non-metabolisers after whole-grain oat intake.117 The authors claimed the term “metabotype” to illustrate the above interindividual variability. However, these results were based on a pharmacokinetic study with only 11 volunteers and 21 in vitro faecal fermentation experiments. F. prausnitzii is one of the most abundant bacterial species in the human intestinal microbiota of healthy adults, representing more than 5% of the total bacterial population.118 The chronic consumption of a (poly)phenolic substrate can increase the abundance of the microbial groups involved in its metabolism,58,119 implying that the continuous consumption of whole-oat could convert non-metabolisers into DH-AVA producers; in this case by the increase of F. prausnitzii. Besides this, many microbial groups could catalyse the reduction of the double-bond from AVAs to DH-AVAs in the hydroxycinnamic acid moiety since this is a relatively non-specific reaction (Fig. 1).58,105,120 Therefore, it is difficult to conceive that changes in the abundance of F. prausnitzii could be the limiting step in converting AVAs to DH-AVAs in healthy adults. Although there is interindividual variability in the metabolism of AVAs,117 the existence of a genuine gut microbiota-associated metabotype for AVA metabolism deserves further research.
Flavan-3-ols (flavanols) and proanthocyanidins from tea, cocoa, grapes, apples, etc., are catabolised into different phenylvalerolactone derivatives.47,121–123 Although valerolactone-derivatives are characteristic of flavanol metabolism, these metabolites are further transformed to phenolic acid derivatives, as in many other flavonoid metabolic pathways (Fig. 1).55,124,125
Cortés-Martín et al.55 reported several points that prevent the identification of gut microbiota metabotypes associated with the metabolism of flavanols. For example, the binary response of gut microbiota metabotypes is not accomplished in flavanols, i.e., the presence vs. absence of unique metabolites, which does not allow the population to be stratified as producers vs. non-producers. As in the case of other (poly)phenols, the production gradient (high and low producers of valerolactone-derived metabolites) gives rise to substantial interindividual variability, in which phase-II polymorphisms and other variables (food matrix, flavanols polymerisation degree, etc.) can contribute critically.55,126,127 Therefore, despite the opinion of other researchers, strictly speaking, there are no gut microbiota metabotypes associated with the metabolism of flavanols. As previously suggested, phase-II enzymes give rise to high interindividual variability and possible potential metabotypes (not associated with the gut microbiota).53,55,128 However, these possible metabotypes should provide a clear discriminant clustering of individuals and not an additional gradient (i.e., high and low levels) based on, for example, the typical glucuronidation/sulfation ratio.55,128 The question raised in any possible gradient is always the same: what is the cut-off to consider an individual from one or another “metabotype”? This issue is even more relevant when tentative “metabotypes” have been proposed after the analysis of in vitro or in vivo samples from a few volunteers (n ≈ 10–15), which is, unfortunately, the most common scenario in those studies that recurrently use the term “metabotype” in the context of (poly)phenol metabolism.117,128–130 Indeed, after analysing such small sample sizes, high and low producers could be tentatively identified by specifying an arbitrary limit to establish such a classification. However, in a large cohort, it could be possible that after applying the same cut-off, these same volunteers could be all grouped within the high producers or all of them within the low producers. The establishment of this cut-off is arbitrary and depends on many external factors, as discussed in a further section. For example, for identifying genuine metabotypes, the sensitivity of the analytical procedure is crucial. If the technique is not sensitive enough, especially when the detection limit of a specific metabolite is relatively high, it could lead to the erroneous interpretation that an individual is a non-producer. Besides this, identifying “new” metabotypes based on the absence of a specific metabolite in urine is questionable because polymorphisms in gut transporters involved in phenolic-derived metabolite absorption and excretion can affect the urine excretion profile of metabolites. Therefore, a comparative analysis between urine and faecal excretion is mandatory. Overall, large groups are needed to identify gut microbiota metabotypes associated with the metabolism of (poly)phenols.43,55,111,131
The stratification of individuals according to their gut microbiota (poly)phenol metabotypes has been proposed to understand individuals’ response to dietary (poly)phenols, which could be crucial in the context of personalised nutrition.43,119 To date, the metabotypes identified unequivocally are those involved in the metabolism of isoflavones (equol producers vs. non-producers), and ellagic acid (UMs, including producers of only Uro-A (UM-A), producers of Uro-A, isourolithin-A (IsoUro-A), and urolithin-B (Uro-B) (UM-B), and urolithin non-producers (UM-0)) (check Cortés-Martín et al.43 for a comprehensive description of these metabotypes). Of course, within each metabotype, a metabolite production gradient also defines tentatively “high” and “low” producers of metabolites. However, the same problem for establishing the boundary between high and low metabolite producers is also found within genuine metabotypes.43,111,132
Therefore, the concept of gut microbiota metabotypes associated with (poly)phenol metabolism involves a qualitative criterion with two possible putative players responsible for the (poly)phenol health effects: (i) (poly)phenol-derived metabolites with distinctive biological activity and specifically produced only for some individuals, and/or (ii) the particular gut microbial ecology in terms of composition and functionality, associated with (poly)phenol metabolism, and harboured by specific individuals.
2.2.1.1. Production of distinctive microbial phenolic-derived metabolites. There are two paradigmatic examples regarding the production of phenolic-derived postbiotics produced by specific gut microbiota ecologies: (i) equol-producing individuals (producers) and equol non-producers in the daidzein metabolism, and (ii) urolithin-producing individuals (UM-A and UM-B) vs. those individuals not capable of producing urolithins (urolithin non-producers, UM-0) after consuming ellagic acid (Fig. 2). Whether these phenolic-derived postbiotics are the final drivers in the health benefits observed upon isoflavone- or ellagitannin-rich foodstuff consumption deserves further research.43,59
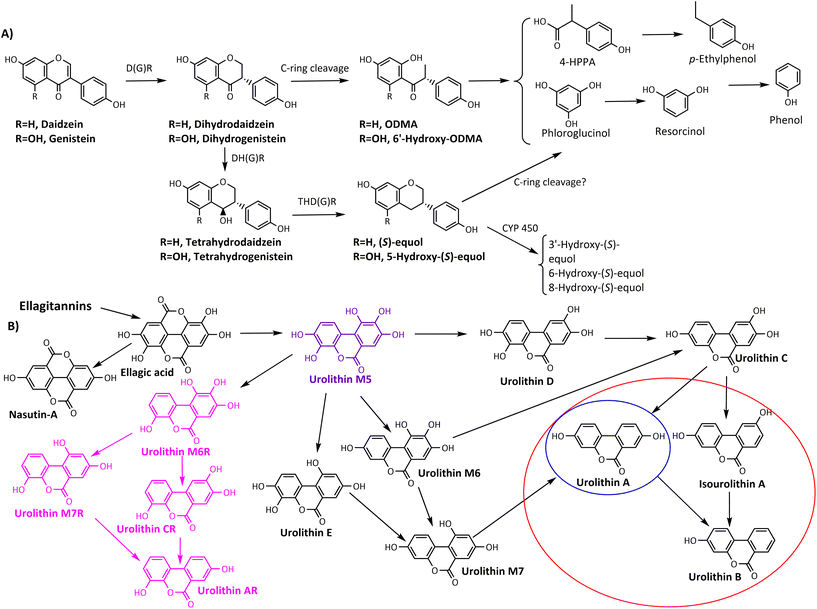 | ||
| Fig. 2 Summarised catabolic pathways of (A) isoflavones (daidzein and genistein) and (B) ellagic acid to urolithins. The circles specifically enclose the final urolithins for each metabotype (red, UM-B; blue, UM-A). Urolithin M5 (purple) is the only detected urolithin in UM-0. R-urolithins (pink) are those present in individuals with specific 3-dehydroxylase activity (they can be present in both UM-A and UM-B). D(G)R, daidzein/genistein reductase; DH(G)R, dihydrodaidzein/genistein reductase; THD(G)R, tetrahydrodaidzein/genistein reductase; CYP450, mammalian cytochrome P450; 4-HPPA, 4-hydroxyphenyl propionic acid. Adapted from Cortés-Martín et al.43 with permission from Wiley, copyright March 19, 2020. | ||
The evidence on the role of postbiotics in human health is well consolidated for some compounds, including SCFAs,133 but still weak for many others.43,134–137 To date, there is no evidence to support a specific health benefit from producing, qualitatively, one metabolite (e.g. equol or Uro-A) compared with others.
While the distribution of UM-A and UM-B is critically affected by ageing, the proportion of individuals with UM-0 (urolithin non-producers) remains constant, about 10%, from 5 to 90 years of age.131 Therefore, the relatively low number of volunteers with UM-0 in different human studies has prevented the drawing of any statistically significant conclusion regarding the possible impact of non-producing urolithins in the outcomes of the studies.74,119,138,139
Regarding the metabolism of isoflavones, the equol- and ODMA-producer metabotypes have been identified so far.111 It seems that both metabotypes are independent of each other.140 However, as discussed for flavanols, flavanones, and prenylflavanones, the catabolism of ODMA can also give rise to simpler and unspecific metabolites such as phloroglucinol, 4-hydroxyphenyl propionic acid, etc. (Fig. 2), which are common in the microbial catabolism of many other dietary phenolics. Therefore, this fact could compromise a genuine ODMA-metabotype in the population and might have contributed to the inconsistent results linking isoflavone metabotypes and human health outcomes.43 The percentage of equol producers has been estimated to be around 30% and 50–60% in the Caucasian and Asian populations, respectively.141,142 Therefore, it could be potentially more feasible to reach statistically significant differences when linking the presence or absence of equol in the results of each study. However, producing a specific microbial-derived metabolite such as equol is linked to harbouring a particular gut microbial ecology.111,143 Therefore, whether a specific microbial-derived metabolite exerts biological activity, dependently or not of the associated microbiota, requires evaluating the effects upon direct administration of the metabolite.
To date, equol is the most studied phenolic-derived postbiotic in humans. The main evidence suggests improvement of cardiometabolic biomarkers and, particularly, protection against menopausal symptoms.144–154 However, the precise mechanisms are not yet fully elucidated.43 A human trial (n = 49) described the improvement of some cardiometabolic risk biomarkers in overweight-obese women after S-equol administration (10 mg), showing more effects in the equol non-producer participants.149 In contrast, Hazim et al.150 described acute vascular effects in healthy equol-producing men (n = 14) after consuming isoflavones. The effects were associated with peak circulating equol concentrations (although the isoflavones supplement did not contain synthetic S-equol). Remarkably, equol administration (40 mg) did not exert any effect on equol non-producers. However, in the parallel assay, no synthetic equol was administered to the equol-producers or isoflavones to equol non-producers.150 This means that the acute vascular effects of equol (dependently or not of the equol-related metabotype) have not been demonstrated unequivocally. Two randomised placebo-controlled trials (RCT) specifically recruited equol-producing participants to evaluate isoflavone effects on blood pressure, vascular function,155 bone metabolism and inflammation.156 Remarkably, the authors did not observe a significant effect on any cardiovascular risk factor among 253 Chinese equol-producing postmenopausal women that consumed isoflavones for 6 months.156 Unfortunately, whether the same approach could have exerted some effects on equol non-producers remains unanswered. Recently, an 8.8-years prospective study described that serum isoflavones and equol were associated with reduced carotid intima-media thickness progression, mediated by sex hormone-binding globulins and systolic blood pressure in 2572 Chinese subjects (40–75 years old).157 However, a case-control study with pre- and postmenopausal Chinese women (n = 792) only found a statistically significant association between serum daidzein and reduced odds of breast cancer, but not with the rest of the isoflavones or equol.158
Urolithins, mainly Uro-A, can exert anti-inflammatory and anti-obesity activities, preserve the intestinal barrier, modulate the gut microbiota, and protect from oxidative stress, among many other activities, in animal models.73,132,159–161 Besides this, physiologically relevant mechanistic in vitro studies support the underlying molecular mechanisms involved.18–21,162–164 However, there are no human studies to confirm these potential effects.132 To date, there are two human studies conducted with a commercial oral synthetic Uro-A supplement, funded by a private company. One of them described cellular health improvement by regulating gene expression associated with cellular and mitochondrial function.165 The second one claimed the consumption of 500 mg of synthetic Uro-A as the solution to achieve more plasma Uro-A-derived conjugates than the single intake of a specific pomegranate juice and after only 6 h,166 not enough time to achieve a substantial conversion of ellagic acid into Uro-A, which can take around 48 h.48,167 Besides this, walnut intake would have yielded much more Uro-A production.168
Nevertheless, as in equol, future human trials dealing with urolithins should consider the individuals’ UMs to evaluate the possible role of the associated gut microbiota in the postbiotic effects. Overall, the in vivo activity of phenolic-derived postbiotics cannot be discarded, but the evidence supporting their genuine role in health is still too limited to be unquestionable. In this regard, they could be potential biomarkers of specific human gut microbiota metabotypes associated with the metabolism of (poly)phenols rather than irrefutably bioactive metabolites with differential impact on human health.169
2.2.1.2. Specific gut microbial ecologies, associated with the metabolism of (poly)phenols, as possible health drivers. An increasing number of studies encourage the clustering of subjects into known metabotypes (i.e., metabotyping) as a strategy to explain, at least partially, the different response of individuals to dietary compounds, including (poly)phenols.43,63,102,119,170–172 This approach would allow tailoring of specific (poly)phenol-rich diets for specific individuals by linking metabotypes to the concept of “personalised nutrition”, i.e., optimising and aiming the diet individually for the prevention of some diseases.59,119,173–176
The current evidence suggests that equol and/or ODMA producers may have a lower cardiovascular risk than non-producers.111 The capacity of individuals for producing equol seems to be stable at least for 2 years,177 and the proportion of equol production status might increase with age.178 However, despite the attempts to correlate the occurrence of the equol- or ODMA-producer metabotypes with dietary patterns and some sociodemographic characteristics of the population, including age, education level and ethnicity, no clear associations have been consistently found.111,179 The association of the ODMA non-producer metabotype with obesity in peri- and postmenopausal women has been previously suggested.180 These results agree with those observed by Reverri et al.,181 who reported that equol and ODMA non-producers were related to obesity. In the same line, the inverse association between the equol producer metabotype and obesity has been recently suggested in postmenopausal women, using logistic regression analysis with adjustment for lifestyle factors.182
More than 10 gut microbes have been reported to be involved in the equol production, including Adlercreutzia equolifaciens,183Asaccharobacter celatus (now Adlercreutzia equolifaciens subsp. celatus),184Bacteroides ovatus,185Finegoldia magna, Lactobacillus mucosae (now Limosilactobacillus mucosae),186Slackia equolifaciens, Slackia isoflavoniconvertens,187,188 and Streptococcus intermedius.185 In contrast, reports on gut microbes that specifically produce ODMA, but not equol, are less abundant. This is the case of Adlercreutzia equolifaciens IPLA37004, whose genome sequence suggested a deletion in a large part of the equol operon.189
Nakatsu et al.190 found a greater abundance of the genera Bifidobacterium and Eubacterium in equol-producers vs. non-producers in 17 postmenopausal women after consuming isoflavone-containing soy bars for one week. Later, Lino et al.178 compared the relative abundance of 8 gut microbes capable of producing equol in 1044 adult subjects. Interestingly, the equol producing intestinal bacteria were present in both equol producers (n = 458) and non-producers (n = 586). However, the species A. equolifaciens subsp. celatus and S. isoflavoniconvertens were significantly more abundant in equol-producers vs. non-producers. Overall, a clue from this study could be the existence of a possible threshold abundance of A. equolifaciens subsp. celatus and S. isoflavoniconvertens necessary to produce equol from daidzein.
A cross-sectional study described the difference in the gut microbiota between the equol-producer and non-producer metabotypes in Chinese people (n = 99).143 Unfortunately, this study did not consider the ODMA metabotype as an important feature of daidzein metabolism. No significant difference in bacterial richness was found between the equol-producer and non-producer metabotypes, and the equol-producer metabotype was not affected by the intake of isoflavones for 3 days. Although this is too short an isoflavone intervention to modify a metabotype status, this finding agrees with Yoshikata et al.,191 who reported that equol production might not depend on the quantity of equol-producing bacteria but the type of these bacteria since equol-producing bacteria were identified in 56 women but only 13 produced equol (i.e., not all strains within the genus identified as potential equol producers are actually capable of metabolising daidzein to produce equol). However, unlike Zheng et al.,143 Yoshikata et al.191 described a higher microbial diversity in equol producers. Besides this, a previous study reported that isoflavone consumption for one month did not induce the ability to produce equol in postmenopausal women.192 These results suggest that the conversion of non-producing individuals into equol producers seems to be unlikely.
The equol-producer metabotype shows a higher abundance of the species A. equolifaciens and Bifidobacterium bifidum than the non-producer.143 Besides this, the equol-producer metabotype showed a higher abundance of Prevotella, Megamonas, Allistipes, Desulfovibrio, Collinsella, and Eubacterium genera. In contrast, the equol non-producer metabotype was enriched in the family Lachnospiraceae, the genus Eggerthella and several species from Ruminococcus and Bacteroides. Also, the authors found statistically significant associations between the equol-producer metabotype and lower prevalence of dyslipidaemia vs. non-producers (27% vs. 50%).143 However, no associations between microbial composition and functionality with body mass index (BMI), smoking habit, age, and gender were found. Other cross-sectional studies in Chinese postmenopausal women193 and Japanese men194 have reported better cardiovascular health profiles for both equol and ODMA producers than non-producers. Sekikawa et al.195 found proportionally inverse associations between equol-producing status (non-producers, low and high) and white matter lesion, but not the amyloid-beta deposition, in 91 normal cognitive elderly Japanese. Unfortunately, the gut microbiota of the participants was not analysed.
A recent study compared faecal samples from 20 individuals with sporadic colorectal adenomas vs. 20 without proliferative lesions and observed the presence of Parabacteroides distasonis, Clostridium clostridioforme, and Pediococcus pentasaceus only in controls, while Bacteroides fragilis and Prevotella melaningenica were present only in those subjects with colorectal adenomas.196 Remarkably, the authors found undetectable or deficient equol levels in individuals with colorectal adenomas vs. control and suggested that equol production could determine a milieu able to contrast the development of colonic mucosa proliferative lesions.196 Obviously, it is not known whether precancerous signalling could modulate the gut microbiota or a specific gut microbiota played a possible role in the development of lesions, or both. Thus, as in other cross-sectional studies, the causality role of the gut microbial ecologies cannot be unambiguously assumed.
The specific response of individuals, according to their equol and/or ODMA producing status after dietary intervention with isoflavones, are less abundant. Recently, Hayashi et al.197 reported that the interaction of aerobic exercise and equol production status plays an essential role in improving central artery compliance in postmenopausal women. However, the assay was not a crossover, and the sample size was relatively small (27 females performed exercise vs. 16 females did not). Reverri et al.198 described the improvement of endothelial function, but not inflammatory biomarkers, independently of the equol and/or ODMA production status of 17 postmenopausal women and men over the age of 45 years at cardiometabolic risk after consuming isoflavones. The same authors distinguished ODMA-only and equol + ODMA producers from non-producers, according to the serum metabolome of the same volunteers, and found a lower metabolic risk in those producer individuals than non-producers.181 Although it was a randomized and crossover study, the sample size (n = 17) and the absence of a placebo could limit the scope of these results.
A RCT with soy protein supplementation (n = 50) vs. placebo (n = 43) for 2 years in men following radical prostatectomy has recently reported no effects on body weight, blood pressure, total serum cholesterol, iron status parameters, calcium, phosphorus, and thyroid hormones.199 Interestingly, the stratification of subjects on equol production status in the soy group revealed that body weight increased in equol producers compared with non-producers. Besides this, both systolic and diastolic blood pressure decreased only in the equol non-producers.199 Overall, these results suggest that the capacity to produce equol in middle-aged to older men seemed to be a disadvantage and agree with Usui et al.149, who observed higher cardioprotective effects in the subgroup of women equol non-producers after consuming 10 mg S-equol. However, it should be noted that most participating men were under medication (mainly taking lipid-lowering and anti-hypertensive drugs, not considered as covariates in the analyses),199 which might affect the gut microbiota and determine the soy effects and equol production. Overall, the possible influence of medication on equol production and isoflavone effects agree with recent results observed for urolithins in polymedicated metabolic syndrome patients after consuming pomegranate.139 While the percentage of equol producers in Caucasians has been estimated to be about 30%,141 the proportion of equol producers was much higher in the study of Bosland et al.199
As commented above, the metabolism of ellagitannins and ellagic acid to produce (or not) distinctive UMs is critically affected by ageing, as reported in a large cohort (n = 839), mainly Caucasians and aged from 5 to 90 years.131 The percentage (10%) of urolithin non-producers (UM-0) remains constant in the range from 5 to 90 years of age. In contrast, the proportion of UM-A at an early age (85%) progressively decreases up to 55% from 40 to 90 years of age, concomitant with an increase of UM-B from 15% up to 45%. The shift from UM-A to UM-B was more evident from 25 to 35 years of age, and from that age, the proportion of UM-A and UM-B (55% and 45%, respectively) remains approximately unaltered.131 Although UMs are stable within individuals at a certain age, a challenge of a high dose of ellagitannins for 3 weeks can shift some specific individuals from apparent UM-0 to either UM-A or UM-B.119 However, we think these individuals were not genuine UM-0 but very low urolithin producers, in which urolithin production was below the limit of detection or their gut microbiota was more sensitive to ellagitannin consumption than other real UM-0 individuals. To date, no clear association between UMs and diet, sex, or ethnicity has been reported. Despite preliminary observations associating UM-B with higher BMI and gut dysbiosis,200,201 this was not unequivocally confirmed in a large cohort (n = 839) that included healthy volunteers and patients.131 On the other hand, in a recent study, gut microbiota and UM distribution in mothers were changing through the 1-year follow-up postpartum to resemble the distribution in the general population previously described.138 The decrease in the percentage of overweight mothers with UM-B was concomitant with the increase of normoweight mothers with UM-A over time. Although the correlation between UM-B and obesity cannot be unequivocally established, the results of that study suggest that the UM-B dysbiosis-prone metabotype could be a potential contributor to obesity.
The interindividual variability in urolithin production has been related to some dissimilarity in the gut microbiota. Pure cultures of Gordonibacter species can metabolise ellagic acid into different urolithins202 and are positively associated with Uro-A and UM-A in faeces and urine.63,168 In contrast, the occurrence of IsoUro-A, Uro-B, and UM-B are inversely associated with faecal concentrations of Gordonibacter spp.63,170Ellagibacter isourolithinifaciens, another human gut bacteria of the family Eggerthellaceae, converts ellagic acid into IsoUro-A and is positively associated with IsoUro-A, Uro-B and UM-B.63,203,204Gordonibacter increase has been described in adults at cardiovascular risk (n = 42) that consumed a diet containing whole walnuts for 2 weeks compared with a standard Western diet in a crossover design.205 Although the authors suggested that this shift might be involved in the underlying mechanisms associated with the cardiovascular benefits of walnut consumption, no direct link between Gordonibacter and any risk marker was provided. In contrast, in another study, faecal Gordonibacter concentration positively correlated with HDLc and negatively with both plasma glucose and VLDLc levels in overweight-obese-metabolic syndrome volunteers consuming pomegranate extract or nuts.170 Lower abundance of Gordonibacter among other bacteria was also observed in active Crohn's disease.206 Du et al.207 reported a higher abundance of the genera Scardovia, Lactobacillus, Gordonibacter, and Phascolarctobacterium in 40 Chinese patients with multiple system atrophy compared with healthy controls (n = 40). The authors found a positive statistical association between Gordonibacter and the scale for Parkinson's disease autonomic dysfunction (r = 0.195, P = 0.011).207 However, the authors acknowledged that their cross-sectional study did not reveal any causal relationship between microbiota and multiple system atrophy. In the case of Ellagibacter, this genus has been reported to increase after consuming a symbiotic drink (probiotics plus dietary fibre) by healthy postmenopausal Korean women (n = 37) and was inversely associated with the participants’ BMI.208 However, the trial was not placebo-controlled nor crossover, limiting the scope of the results. Overall, there is still a low number of studies on the role of Gordonibacter and Ellagibacter in human health. Besides this, finding specific associations between some genera or species and some risk factors might not be enough evidence to prove the role of such microbial groups in health.
The comparison of the human gut microbial ecologies associated with UMs has been reported in healthy normoweight, overweight, and obese individuals (n = 249).63 Unlike the equol-producer and non-producer metabotypes that did not show a significant difference in bacterial richness in a recent study,143 UM-0 showed lower diversity and richness than UM-A and UM-B subjects.63 Besides this, UM-0 was also characterised by a lower abundance of the genera Phascolarctobacterium, Bilophila, Alistipes, and Butyricimonas than UM-B and UM-A. Remarkably, UM-B showed a higher abundance of the Coriobacteriia class compared with UM-A and UM-0, which was positively associated with total cholesterol (Tchol), LDLc, and BMI.63,209,210 For example, the genus Slackia (belonging to the Coriobacteriia class), whose abundance was increased in UM-B vs. UM-A, correlated with Tchol, LDLc, apolipoprotein-B, and non-HDL-cholesterol levels. In contrast, the family Eubacteriaceae, which was increased in UM-A vs. UM-B, was positively associated with apolipoprotein-A. UM-B individuals also presented a higher abundance of some pro-inflammatory microbial groups vs. UM-A, including Methanobrevibacter, Parvimonas, Gammaproteobacteria and Methanosphaera.63 These differences in the gut microbial ecologies between UM-A and UM-B support previous results showing higher cardiometabolic risk in overweight-obese UM-B individuals vs. UM-A and UM-0.119,170 Indeed, correlations between baseline CVD risk markers and urolithins were found in overweight-obese individuals. Uro-A (primarily present in UM-A) was positively correlated with apolipoprotein A-I and intermediate-HDLc, while Uro-B and IsoUro-A (characteristic from UM-B) were positively correlated with Tchol, LDLc, apolipoprotein B, VLDLc, IDLc, oxLDL and the apolipoprotein B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) apolipoprotein A-I ratio. In metabolic syndrome patients, Uro-A only correlated inversely with glucose.170
apolipoprotein A-I ratio. In metabolic syndrome patients, Uro-A only correlated inversely with glucose.170
It is known that the gut microbiota is significantly altered during pregnancy and after childbirth.211 Interestingly, Cortés-Martín et al.138 described that the restoration capacity of the gut microbiota and the anthropometric values of mothers up to 12 months after delivery depended on their UM. Through the 1-year follow-up postpartum, UM-A women normalised their gut microbiota and anthropometric values to a greater extent than UM-B. For example, Methanobrevibacter and Olsenella reduction were correlated to waist reduction, and reduction of Clostridiaceae, Clostridium sensu stricto, and Anaerobacter correlated to the reduction of waist-to-hip ratio, BMI, and waist.138 These results also suggest that UM-B, in contrast to UM-A, was associated with a dysbiotic-prone microbial ecology and more resilient to change the microbial and anthropometric profiles during postpartum.
Recently, Cortés-Martín et al.212 explored the possible participation of UMs, along with other factors, in the prevalence of obesity in a cohort of children and adolescents (n = 415). A statistical ordinal logistic model revealed that overweight-obesity prevalence was related to being a young boy (9–12 years old) with either UM-B or UM-0, low adherence to the Mediterranean diet (KIDMED score) and high contribution of a specific consortium of 24 single-nucleotide polymorphisms (SNPs) from a total of 53 SNPs related to obesity and cardiometabolic diseases. In contrast, every variable (sex, diet, UMs, SNPs, age, physical activity, etc.) was not independently associated with overweight-obesity.212
The gut microbial ecologies of UMs have been reported to be differentially modulated upon consumption of walnuts for just 3 days.213 After consumption, the genera Bifidobacterium, Blautia, and some gut microbes of the Coriobacteriia class, including the genus Gordonibacter, increased exclusively in UM-B. In contrast, UM-A was less sensitive to walnut consumption, and some members of the Lachnospiraceae family decreased only in UM-A individuals.213
In the line of the UM-depending response of individuals to (poly)phenol consumption, for the first time, González-Sarrías et al.119 described the improvement of a panel of cardiometabolic risk biomarkers in UM-B individuals, but not in UM-A. In fact, no statistically significant effects were observed before clustering individuals according to their UM. Furthermore, no significant correlations were found between faecal urolithins excretion and improvement of CVD risk markers. However, urinary excreted urolithin conjugates significantly correlated with improvement of CVD risk markers in UM-B individuals, i.e., reduction of Tchol, LDLc, and non-HDLc correlated with urinary excretion levels of Uro-A metabolites, and changes of LDLc also correlated with both Uro-A and IsoUro-A + Uro-B conjugates. However, in UM-A individuals, where CVD risk markers did not change, no correlations were found.
Despite these correlations with CVD risk markers, other studies failed to find a significant correlation between urolithins occurrence in plasma, urine, faeces or colonic tissues and cancer-related markers and metabolic endotoxemia in colorectal cancer patients (n = 45),74,87 metabolic endotoxemia in overweight-obese individuals (n = 49),86 and the blood lipid profile in healthy subjects (n = 32).214
Recently, Cortés-Martín et al.139 described that UMs distribution was altered in polymedicated metabolic syndrome patients. The gut microbiota of these patients was in dysbiosis, mainly in hypertensive patients with an overabundance of LPS-producing members of the Enterobacteriaceae family.215 A RCT and crossover trial showed that the polymedication of the patients determined the prebiotic effect of an ellagitannin-rich pomegranate extract as a crucial variable. The metabolic endotoxemia slightly but significantly improved in all the patients after consuming the extract. However, the soluble intercellular adhesion molecule-1 (sICAM-1) only improved in those patients consuming lipid-lowering drugs (LL-), and the patients’ medication clearly determined the modulation of the gut microbiota. In this regard, the genus Lactococcus increased in patients consuming antidiabetic (AD-), LL- and anti-hypertensive (HP-) drugs, Bifidobacterium increased in LL- and AD-consuming patients, and Clostridium cluster XIVa decreased in non-LL- and non-HP-consuming patients. Urolithin production (type and amount) was not associated with the effects observed.139
Overall, the current evidence, albeit still low, suggests that the gut microbial ecology of UM-A could be “protective”, while UM-B could be a potential dysbiotic-prone metabotype to cardiometabolic impairments.43,63 In general, the lack of clear associations between circulating or excreted urolithin derivatives (and other phenolic-derived metabolites) and specific effects can be somewhat logical due to the highly variable turnover of these metabolites in the bloodstream and other reservoirs.
| Source and (poly)phenol classes ingested | Design of the study | Health outcome | Phenolic-derived metabolites evaluated | Main correlations | Ref. |
|---|---|---|---|---|---|
| 8-OHdG, 8-hydroxydeoxyguanosine; AD-, patients under antidiabetic medication; ADP, adenosine-5′-diphosphate; BMI, body mass index; BDNF, brain-derived neurotrophic factor; CGA, chlorogenic acid; CoQ, coenzyme Q; CRP, C-reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; EGCG, epigallocatechin gallate; FMD, flow-mediated dilation; GAE, gallic acid equivalents; GLP-1, glucagon-like peptide-1; HDL, high density lipoprotein; HDLc, high density lipoprotein cholesterol; HGF, hepatocyte growth factor, HOMA-IR, homeostatic model assessment of insulin resistance; HP-, patients under anti-hypertensive medication; ISI, insulin sensitivity index; LBP, lipopolysaccharide-binding protein; LDL, low density lipoprotein; LDLc, low density lipoprotein cholesterol; LL-, patients under lipid-lowering medication; LPO, lipid peroxide; MCP-1, monocyte chemoattractant protein-1; NO, nitric oxide; oxLDL, oxidized LDL; PAI-1, plasminogen activator inhibitor type-1; PGF2α, prostaglandin F2 alpha; PWV, pulse-wave velocity; RCT, randomised control trial; SBP, systolic blood pressure; RBP4, retinol-binding protein-4; sICAM-1, soluble intercellular adhesion molecule-1; SOD, superoxide dismutase; sVCAM-1, soluble vascular adhesion molecule-1; TAP, total antioxidant potential; Tchol, total cholesterol; TNF-α; tumour necrosis factor-α; UM, urolithin metabotype; WC, waist circumference; WO, wash-out. | |||||
| Studies that did find a correlation | |||||
| Flavanol-rich cocoa drink (100 mL) with high (176 to 185 mg) or low (<11 mg) flavanol content. (Poly)phenol classes: flavan-3-ols and procyanidins | Randomised control trial (RCT), double-blind, crossover and dose–response study. Healthy adults (n = 11). Duration: acute (2 h). Wash out (WO): 1 day | ↑NO species and ↑FMD at 2 h after ingestion of 176 to 185 mg flavonols | Plasma flavanol metabolites | Epicatechin, catechin, epicatechin-7-β-D-glucuronide, 4′-O-methyl-epicatechin, and 4′-O-methyl-epicatechin-β-D-glucuronide correlated with ↑NO increase. ↑FMD correlated with ↑epicatechin and catechin | 220 |
| Black tea (5 cups per d of 250 mL). Detailed (poly)phenolic content not provided | RCT and crossover. Adults with mild hyperlipidaemia (n = 21). Duration: acute (5 h) and chronic (4 weeks). No WO period | Significant increase in FMD response after 4-week consumption of black tea, but not acutely | Urinary 4-O-methylgallic acid after 5 h and 4 weeks | ↑4-O-Methylgallic acid excretion was inversely associated with the change in FMD responses after 4 weeks | 221 |
| Flavanol-rich cocoa drinks (300 mL; high or low flavanol content, 917 and 37 mg of total flavanols, respectively). (Poly)phenol classes: flavan-3-ols and procyanidins | RCT, double-blind, crossover. Healthy male adults (n = 16). Duration: acute study (assessments at 1, 2, 3, 4, and 6 h after a single-dose). WO: 2 days | Acute significant transient increase of the FMD response at 1–4 h after oral ingestion of the high-flavanol-rich cocoa drink, but not after the low dose | Plasma flavanol metabolites: epicatechin, epicatechin-7-O-glucuronide, 4′-O-methyl-epicatechin, 4′-O-methyl-epicatechin-O-glucuronide, catechin | Epicatechin and epicatechin-7-O-glucuronide were independent predictors of FMD effects | 222 |
| Curcumin (1 or 4 g d−1) | RCT, double blind, placebo-controlled. Elderly subjects (n = 36). Duration: 1 and 6 months | Consumption of curcumin did not significantly affect triacylglycerols, Tchol, LDLc, and HDLc | Plasma curcumin metabolites | Curcumin metabolites were correlated with slight increases in Tchol after 1 month | 223 |
| Breakfasts rich in olive oils with different phenolic contents (80 or 400 ppm). (Poly)phenol classes: phenolic alcohols, phenolic acids and derivatives. Others: oleuropein, flavones and lignans | RCT, crossover. Hypercholesteraemic adults (n = 21). Duration: postprandial study (assessments at 1 and 2 h after a single-dose). WO: 1 week | Plasma concentrations of the procoagulant activated factor VII (FVIIa) increased less and PAI-1 activity decreased more 2 h after the high-phenol meal than after the low-phenol meal | Plasma tyrosol, hydroxytyrosol, and 3-O-methyl-hydroxytyrosol | ↑Plasma hydroxytyrosol correlated with ↓FVIIa concentrations after intake of the high phenol olive | 224 |
| Olives (approximately 100 g). (Poly)phenol classes: phenolic alcohols, phenolic acids and derivatives, oleuropein, flavones and lignans | Interventional study. Healthy male adults (n = 8). Duration: acute study (assessments at 1, 2, 3 and 4 h after a single-dose) | ↑Plasma total antioxidant potential (TAP) at 2 h | Total phenolic compounds in plasma | ↑TAP correlated with total (but no individual) phenolic compounds at 4 h | 225 |
| Quercetin, epicatechin, or EGCG (200 mg in 300 mL of water). | RCT, crossover. Healthy male adults (n = 12). Duration: acute study (assessments at 2 and 5 h after a single-dose). 1 week of WO | ↑Plasma S-nitrosothiols, plasma nitrite, and urinary nitrate after quercetin and epicatechin intake. EGCG did not alter any of the measures of NO production. ↓Plasma endothelin-1 concentration after quercetin and epicatechin intake | Plasma quercetin, 3′-O-methylquercetin, epicatechin, 3′-O-methyl-epicatechin, and EGCG (2 h), and 11 flavonoids and aromatic metabolites in urine (5 h) | ↑Plasma S-nitrosothiol correlated with ↑plasma quercetin and epicatechin. Plasma nitrite, urinary nitrate, and urinary endothelin-1 were not correlated with plasma flavonoids | 226 and 227 |
| Two types of olive oils (25 mL; 22 g d−1) distributed over 3 meals. (Poly)phenol classes: phenolic alcohols, phenolic acids and derivatives, oleuropein, flavones and lignans | RCT double-blind, crossover. Healthy non-smokers adults (n = 36). Duration: 3 weeks. WO: 2 weeks | ↓Oxidized LDL (oxLDL), conjugated dienes, and hydroxyl-fatty acids after both olive oil consumption | Phenolics in isolated LDL particles, including hydroxytyrosol and tyrosol derivatives and homovanillic acid | Plasma oxLDL, but not other oxidation markers, were negatively correlated with the sum of phenols in LDL | 228 |
| Orange juice or control drink plus hesperidin (500 mL containing 292 mg hesperidin and 47.5 mg narirutin). (Poly)phenol classes: flavanones and flavones | RCT, crossover. Healthy overweight male adults (n = 24). Duration: acute study (assessments at 6 h after a single-dose). WO: 3 days | Both orange juice and drink plus hesperidin ingestion significantly improved postprandial microvascular endothelial reactivity | Plasma hesperetin | Plasma hesperetin and changes in microvascular endothelial reactivity were significantly correlated | 229 |
| Cocoa (250 mL; containing 2, 5, 13, or 26 g of cocoa) with 420, 840 or 1470 mg of total (poly)phenols, respectively. (Poly)phenol classes: flavan-3-ols and procyanidins | RCT, double-blind, crossover. Healthy older adults (n = 23). Duration: acute study (assessments at 1 and 2 h after a single-dose). WO: 3 days | ↑FMD at 1 and 2 h, after the intake of 5, 13, and 26 g cocoa | Plasma epicatechin | ↑Serum total epicatechin correlated with ↑FMD at 1 and 2 h | 230 |
| Blueberry drinks (500 mL; containing 766, 1,278, or 1791 mg total blueberry polyphenols). (Poly)phenol classes: anthocyanins, flavanols, hydroxycinnamic acids and flavonols | RCT, double-blind, crossover. Healthy male adults (n = 10). Duration: acute study (assessments at 1, 2, 4, and 6 h after a 766 mg single-dose) | Biphasic time-dependent significant increase in FMD at 1–2 and 6 h after consumption of 766–1791 mg total blueberry polyphenols | Plasma phenolic acids and aromatic compounds (n = 32) | ↑Vanillic and benzoic acids at 1–2 h and hippuric, hydroxyhippuric, and homovanillic acids at 4–6 h were correlated with ↑FMD | 231 |
| Quercetin (200 or 400 mg d−1) | RCT, double-blind. Healthy adults (n = 15). Duration: acute study (assessments at 2 and 5 h after a single-dose). WO: 1 week | Time-dependent increase in brachial artery diameter after 400 mg of quercetin intake. No changes in blood pressure | Plasma quercetin, quercetin-3′-O-glucuronide and isorhamnetin | The change in diameter was correlated with quercetin-3′-O-glucuronide at 2 h | 232 |
| Strawberry pulp (50 g d−1). (Poly)phenol classes: anthocyanins, ellagitannins and procyanidins | Open-label, controlled, 2-phases study. Healthy adults (n = 31). Duration: 30 days each phase with a 10-day WO period | Strawberry consumption decreased median resting luminol enhanced whole blood chemiluminescence (LBCL), reflecting oxidants generation by circulating phagocytes after two phases | Caffeic acid, homovanillic acid, Uro-A and 4-hydroxyhippuric acid in urine and plasma | Resting LBCL correlated negatively with plasma 4-hydroxyhippuric acid after the first strawberry dose | 233 |
| Orange juice (containing either normal or high concentrations of polyphenols (299 and 745 mg d−1, respectively)). (Poly)phenol classes: flavanones and flavones | RCT, double-blind, crossover. Non-smoking overweight or obese adults (n = 100). Duration: 12 weeks. WO: 7 weeks | The intake of both orange juices significantly protected against DNA damage and lipid peroxidation, modified several antioxidant enzymes, and reduced body weight (BMI, WC, and leptin) | Urinary hesperetin and naringenin and their metabolites | ↑Urinary hesperetin and naringenin correlated with erythrocyte catalase activity, but not with CoQ9, LPO, SOD, BMI, WC, urinary 8-iso-PGF2α and 8-OHdG, and malondialdehyde | 234 |
| Mixed nuts (30 g d−1). (Poly)phenol classes: ellagitannins and procyanidins | RCT, 2-arms parallel. Adults with at least three metabolic syndrome risk factors (n = 50). Duration: 12 weeks | Nut consumption improved several cardiometabolic risk markers, including hyperlipidaemia and hypertension parameters | Uro-A glucuronide in plasma | Uro-A glucuronide was inversely correlated with basal abdominal adiposity (WC, waist-hip ratio) and impaired glycaemic control | 235 |
| Cocoa flavanol-containing drink (450 mg). (Poly)phenol classes: flavan-3-ols and procyanidins | RCT, double-blind, 2-arms parallel. Healthy adults (n = 100). Duration: acute study (assessments at 1 and 2 h; n = 5) and chronic (1 month) | Acute and chronic cocoa flavanol consumption → ↑FMD, and ↓blood pressure, vascular stiffness and cholesterol | Plasma flavanol metabolites, including epicatechin and its related metabolites | ↑Plasma flavanols at 2 h and after 1 month was correlated with ↑FMD | 236 |
| Cranberry juices (450 mL; containing 409, 787, 1238, 1534, or 1910 mg of (poly)phenols). (Poly)phenol classes: anthocyanidins, proanthocyanidins, flavanols and flavonols | RCT, double-blind, crossover. Healthy male adults (n = 10). Duration: acute study (assessments at 1, 2, 4, 6 and 8 h after a single-dose). WO: 1 week | Acute cranberry juice consumption → ↑FMD with a peak at 4 h and maximal effects with juice containing 1238 mg of total (poly)phenols | Plasma phenolic acids and metabolites (n = 60) from flavan-3-ols, proanthocyanidins, flavonols, and anthocyanins | Twelve metabolites, including dihydro isoferulic acid 3-O-sulfate, ferulic acid 4-O-sulfate and homovanillic acid sulfate correlated with ↑FMD (all time-points) | 237 |
| Soy isoflavones (80 mg aglycone equivalents) | RCT, double-blind, crossover. Healthy male adults equol and non-equol producers (n = 14 each group). Duration: acute study (assessments at 6 and 24 h after a single-dose). WO: 1 week | Carotid-femoral PWV was significantly improved in equol producers at 24 h, but not at 6 h or in equol non-producers | Plasma isoflavones (daidzein, genistein, and glycitein) and the metabolite equol | Carotid-femoral PWV change was significantly associated with plasma equol | 150 |
| Resveratrol (150 mg d−1) | RCT, double-blind, crossover. Adults with type 2 diabetes (n = 17). Duration: 30 days. WO: 30 days | Hepatic and peripheral insulin sensitivity and intrahepatic lipid content were not affected by resveratrol treatment, while intramyocellular lipid content increased in type 2 diabetes, muscle fibres and SBP tended to decrease | Plasma resveratrol and its metabolites | ↓Intrahepatic lipid content correlated with ↑plasma resveratrol | 238 |
| Resveratrol (75, 150 or 300 mg) | RCT, double-blind, crossover. Adults with type 2 diabetes (n = 36). Duration: acute study (assessments at 2 h). 1 week of WO period | Resveratrol (75 mg) significantly improved neurovascular coupling capacity | Plasma resveratrol and derived metabolites | ↑Neurovascular coupling capacity was correlated with ↑plasma total resveratrol | 239 |
| Coffee drink (50 mL; containing 89 or 310 mg of chlorogenic acid (CGA)). (Poly)phenol classes: hydroxycinnamic acids | RCT, crossover. Healthy male adults (n = 15). Duration: acute study (assessments at 1, 3 and 5 h after a single-dose). 1 week of WO period | Biphasic time-dependent significant increase in FMD at 1 and 5 h after low and high dose of CGA | Plasma CGA metabolites (n = 56) | ↑Total plasma CGA metabolites correlated with ↑FMD. Individual metabolites were correlated with FMD depending on the time frame | 240 |
| Pomegranate extract (450 g per capsule); 1st dose = 1 capsule per d (160 mg total phenolics) and 2nd dose = 4 capsules per d (640 mg total phenolics). (Poly)phenol classes: ellagitannins and ellagic acid derivatives | RCT, double-blind, dose–response, crossover. Healthy obese or overweight adults (n = 49). Duration: 3 weeks each dose. WO: 3 weeks | The consumption of pomegranate extract exerted a significant dose-dependent reduction of a range of CVD risk biomarkers, but only in UM-B subjects | Faecal, urinary and plasma urolithins | ↑Urolithins correlated with ↓CVD risk markers in UM-B individuals. ↓Tchol, LDLc, and non-HDLc correlated with ↑urinary Uro-A excretion, whereas ↓LDLc also correlated with ↑IsoUro-A + Uro-B | 119 |
| Aronia extract (500 mg d−1). (Poly)phenol classes: anthocyanins, hydroxycinnamic acids and proanthocyanidins | RCT. Healthy (former smokers) adults (n = 49). Duration: 12 weeks | Aronia consumption significantly reduced fasting plasma Tchol, LDLc, LDL receptor protein in peripheral blood mononuclear cells | Urinary anthocyanins metabolites (n = 9) | ↑Peonidin-3-O-galactoside, 3-(4-hydroxyphenyl) propionic acid, and cyanidin-3-O-galactoside were associated with ↓Tchol and LDLc | 241 |
| Cocoa chocolate (90%; 50 g). (Poly)phenol classes: flavan-3-ols and procyanidins | Interventional study. Healthy male adults (n = 18). Duration: acute study (assessments at 4 h after a single-dose) | The acute cocoa intake significantly increased collagen/ADP-induced platelet function closure time, but not collagen/epinephrine | Circulating (epi)catechin and phenyl-γ-valerolactone metabolites (n = 13) in plasma | ↑(Epi)catechin metabolites and the single (epi)catechin-sulfates significantly correlated with ↑collagen/ADP closure time. No correlations were found with phenyl-γ-valerolactones | 242 |
| Red raspberry drinks (200 or 400 g containing 201 or 403 mg of total (poly)phenols, respectively). (Poly)phenol classes: ellagitannins, anthocyanidins, flavonols, hydroxycinnamic acids, flavanols and hydroxybenzoic acid derivatives | RCT, double-blind, crossover. Healthy male adults (n = 10). Duration: acute study (assessments at 2 and 24 h after a single-dose). WO: 1 week | ↑FMD at 2 and 24 h after consumption of the 200 and 400 g red raspberry drinks | Circulating ellagitannins metabolites (n = 15) and other phenolic metabolites including benzaldehydes, catechols, pyrogallols, flavonols and propionic, benzoic cinnamic, phenylacetic, hippuric acids (n = 59) in plasma | Plasma ellagic acid (4.6 nM) at 2 h (after 200 and 400 g red raspberry), and Uro-A-3-glucuronide and Uro-A-sulfate at 24 h (41 nM) (only after 200 g) correlated with ↑FMD. No association was found between FMD and 67 circulating phenolic metabolites (120 μM at 24 h) | 243 |
| Wild blueberry drinks (11 g d−1 containing 150 mg of anthocyanins). (Poly)phenol classes: anthocyanins, flavanols, procyanidins, hydroxycinnamic acids and flavonols | RCT, 2-arms parallel, double-blind. Healthy adults (n = 20 in each arm). Duration: acute (2 h) and chronic (1 month) | Acute (2 h) and daily 1-month wild blueberry consumption significantly increased FMD. Chronic consumption significantly lowered 24-hour ambulatory SBP | Plasma anthocyanin metabolites (n = 63) | 14 and 21 anthocyanin plasma metabolites correlated with acute and chronic ↑FMD, respectively | 244 |
| Aronia (poly)phenol-rich extract (116 mg, 75 g berries) or whole fruit powder (12 mg, 10 g berries daily). (Poly)phenol classes: anthocyanidins, hydroxycinnamic acids, proanthocyanidins and hydroxybenzoic acid derivatives | RCT, double-blind, 3-arms parallel. Healthy male adults (n = 66). Duration: acute (2 h after single-dose) and chronic (12 weeks) | Acute and chronic consumption of aronia whole fruit and extract powder significantly increase in FMD | Plasma phenolic metabolites (n = 63) | 20 metabolites after Aronia extract consumption and 5 metabolites after consumption of the whole fruit correlated with acute and chronic ↑FMD | 245 |
| Red grape pomace drink (250 mL; containing 1562 g of total (poly)phenols as gallic acid equivalents (GAE). (Poly)phenol classes: anthocyanins, flavan-3-ols, procyanidins, flavonols, and gallic acid | RCT, crossover. Healthy male adults (n = 12). Duration: acute (3 h after single-dose), postprandial (5 h after the standard meal), and 24 h. WO: 1 week | Red grape pomace consumption → ↓Postprandial insulin incremental area and insulin secretion, and ↑insulin sensitivity index (ISI) | Circulating phenolic metabolites (n = 28) in plasma, including phenyl-γ-valerolactones, hydroxybenzoic acids and simple phenols | Only gallic acid correlated inversely with the insulin response and positively with the ISI | 246 |
| Blood orange juice or a sugar-matched control drink (200 mL twice daily). (Poly)phenol classes: flavanones, flavones and anthocyanins | RCT, single-blind, crossover trial. Overweight men and women (n = 15). Duration: 2 weeks. WO: 1 week | ↑FMD after blood orange juice consumption. Blood pressure, lipid profile, high-sensitivity CRP, and endothelin-1 were not affected | Urinary hesperetin glucuronide and sulfated metabolites | ↑Urinary hesperetin-3′-glucuronide and hesperetin-7-glucuronide were correlated with ↑FMD | 247 |
| Studies that did not find a correlation | |||||
| Black tea (900 mL; 12.9 and 13.3 mg dL−1 of total catechin and 150 and 163 mg dL−1 of total (poly)phenols for the freeze-dried and freshly brewed tea, respectively). (Poly)phenol classes: flavanols | RCT, crossover. Patients with stable coronary artery disease (n = 66). Duration: acute (450 mL) and chronic studies (assessments at 2 h after a single-dose and after 4 weeks with daily dose). No WO period | Black tea acute and chronic consumption did not improve plasma antioxidant capacity and did not reduce urinary 8-OHdG, or urinary 8-isoprostane levels | Circulating catechin metabolites in plasma: epicatechin, epicatechin gallate, epigallocatechin, and EGCG | Changes in catechin levels did not correlate with changes in endothelial function, plasma markers of oxidative stress, or CRP | 248 |
| EGCG (150 mg twice daily) | RCT, double blind, crossover. Patients with coronary artery disease (n = 42). Duration: acute and chronic studies (2 h after a single-dose and after 2 weeks with daily dose). WO: 1 week | ↑Brachial artery FMD two hours after the first dose of 300 mg of EGCG, but was similar to baseline after 2 weeks of treatment (14 h after the last dose) | Circulating EGCG in plasma | ↑Plasma EGCG concentration did not correlate with ↑FMD | 249 |
| Quercetin (150 mg d−1) | RCT, double-blind, crossover. Overweight or obese adults with metabolic syndrome traits (n = 93). Duration: 6 weeks. WO: 5 weeks | ↓SBP, pulse pressure, hs-TNF-α, serum HDLc, oxLDL and hs-CRP (but only in subjects with baseline concentrations >2 mg L−1) after quercetin consumption | Circulating flavonol metabolites in plasma: quercetin, isorhamnetin and kaempferol | There was no correlation between markers and plasma quercetin | 250 |
| Flavonoid-enriched chocolate (27 g d−1; containing 850 mg flavan-3-ols (90 mg epicatechin) + 100 mg isoflavones (aglycone equivalents)). (Poly)phenol classes: flavan-3-ols, procyanidins and isoflavones | RCT, double-blind, 2-arms parallel. Postmenopausal with type 2 diabetes mellitus patients (n = 93). Duration: 1 year | Equol producers (n = 17) had larger reductions in DBP, mean arterial pressure, and PWV, compared with non–equol producers (n = 30) after flavonoid intervention | Urinary total epicatechin, 3′-methyl epicatechin, 4′-methyl epicatechin, epicatechin sulfates, methyl epicatechin sulfates and isoflavones (daidzein, genistein, and equol) | Urinary equol concentrations tended to be inversely correlated with DBP only in the equol producer individuals (P = 0.08) | 251 |
| Pomegranate extract with high or low punicalagin/ellagic acid ratio (900 mg d−1). (Poly)phenol classes: ellagitannins and ellagic acid derivatives | RCT. Colorectal cancer patients (n = 52). Duration: intake of pomegranate extract for 7–30 days (pre-surgery period) | Pomegranate extract consumption modulated colorectal cancer markers expression (genes and miRNAs) | Ellagitannin-derived metabolites (ellagic acid and urolithins) in colorectal tissues | No correlation between miRNA changes and colorectal cancer marker expression with the urolithins detected in the tissues or with individuals’ UMs | 252 and 74 |
| Green tea beverage, green tea extract, or isolated EGCG (442 mL containing 200 mg of EGCG). (Poly)phenol classes: flavanols | RCT, crossover. Healthy male adults (n = 50). Duration: acute study (assessments at 2 h after a single dose). WO: 3 days | FMD significantly improved after consuming green tea containing 200 mg EGCG, but not after green tea extract or EGCG intake | Plasma catechin metabolites | No correlations between EGCG, epicatechin, epigallocatechin, epicatechin gallate, and the total catechin plasma levels and changes in FMD were observed | 253 |
| Pomegranate extract (450 g per capsule); 1st dose = 1 capsule per d (160 mg total phenolics) and 2nd dose = 4 capsules per d (640 mg total phenolics). (Poly)phenol classes: ellagitannins and ellagic acid derivatives | RCT, double-blind, dose–response, crossover. Healthy obese or overweight adults (n = 49). Duration: 3 weeks each dose. WO: 3 weeks | The highest pomegranate extract dose significantly reduced plasma LBP levels | Faecal, urinary and plasma urolithins | No correlation between urolithins and plasma LBP levels was found | 86 |
| Pomegranate extract with high or low punicalagin/ellagic acid ratio (900 mg d−1). (Poly)phenol classes: ellagitannins and ellagic acid derivatives | RCT, 2-arms, parallel. Colorectal cancer patients (n = 35). Duration: from 5 to 35 days before surgery | ↓LBP levels after daily consumption of pomegranate extract rich in punicalagin | Concentration of ellagic acid and urolithins in plasma, urine and colon tissues | No correlation with LBP levels and any specific urolithin in plasma, urine, or colon tissues | 87 |
| Pomegranate extract (320 mg phenolics per d). (Poly)phenol classes: ellagitannins and ellagic acid derivatives | RCT, double-blind and crossover. Metabolic syndrome patients under medication (n = 50). Duration: 4 weeks. WO: 4 weeks | Pomegranate effects depended on the patients’ medication. ↓Plasma sICAM levels was found only in LL-patients. ↑Lactococcus in AD-, LL- and HP-patients, ↑Bifidobacterium in LL- and AD-, while ↓Clostridium XIVa in non-LL- and non-HP-patients | Concentration of urolithins in plasma, urine and faeces | No urolithin was associated with any microbial group or plasma inflammatory-metabolic biomarker (sICAM-1, ghrelin, peptide YY TNF-α, leptin, adiponectin, sVCAM-1, RBP4, GLP-1, IL-6, PAI-1, resistin, BDNF, HGF, MCP-1, P-selectin, C-peptide and LBP) | 139 |
As commented before, the metabolism of some classes of polyphenols, including prenylflavonoids, flavanones, flavan-3-ols, lignans, ellagitannins and isoflavones, yields a high variability of metabolites. Therefore, the definition of “low and high producers” has been commonly used to stratify those individuals capable of producing a high or low amount of metabolites, respectively.43,55,62,104,105,166,195,199,216–218
However, as previously mentioned, this cut-off concentration is arbitrary and conditioned by external factors, including the lag period between the last intake of (poly)phenolic precursor and the sample analysis, the sensitivity of the analytical procedure and the food matrix. Regarding food matrix effects, flavanone metabolism is a paradigmatic example. The amount of phenolic-derived metabolites in flavanone metabolism is dramatically affected by their solubility. Encapsulation and micronisation of flavanones increase their excretion in subjects, including the so-called “low hesperetin producers”.219
Recently, the gut microbiota metabolism of eriocitrin, a soluble flavanone mainly present in lemon, has been reported to yield much higher plasma and urinary concentrations of metabolites (eriodyctiol, homoeriodyctiol, and hesperetin) than hesperidin.107 Therefore, the cut-off for being high or low producers will significantly depend on the solubility of the flavanone. Overall, the consumption of soluble flavanone-rich sources could be a strategy to enhance the metabolite production of those low-flavanone metabolite producers and provide a sufficient circulating concentration metabolite threshold to exert health benefits even in low producer individuals.107
There is a certain consensus regarding the intake of some specific (poly)phenols such as epicatechin, hydroxytyrosol and quercetin, among others, and health effects. However, the final driver in the health effects (a specific phenolic-derived metabolite or a mixture) has not been clearly identified. Besides this, and very importantly, the minimum concentration necessary for a specific (poly)phenol to exert health benefits, and even less in the case of its derived metabolites, is unknown. In this line, many trials, mainly based on the presence-absence of phenolic and dose–response studies, suggest that phenolic-derived metabolites that, as expected, are increased in plasma (or urine) after intake could be related to the health outcomes observed. However, among the vast number of studies dealing with (poly)phenols and health, only a few human studies have explored correlations and/or associations between the reached concentrations of these circulating or excreted metabolites and health outcomes (Table 1). Most of these studies that have observed a significant correlation with specific circulating phenolic-derived metabolites belonging to acute studies where the variation in the concentration-time profile of these circulating metabolites is more controlled than chronic studies.
Thus, to date, improvements in flow-mediated dilation (FMD) have been correlated with changes in plasma flavanols and derived metabolites such as epicatechin and its glucuronides following flavanol-rich cocoa consumption.220,222,230,236 On the contrary, significant increases in plasma catechin metabolite levels did not correlate with changes in endothelial function, plasma markers of oxidative stress, or CRP after acute and chronic black tea or pure EGCG consumption.248,249 These findings suggest that other (poly)phenolic components of tea may influence vascular health or, perhaps, it is another example of the food matrix effect. In this regard, the increase in urinary 4-O-methyl gallic acid was inversely associated with the change in FMD responses after 4-weeks of black tea consumption.221
Other studies have also shown acute correlations between FMD and several phenolic acid metabolites such as vanillic, benzoic and hippuric acids, and ferulic and caffeic acid sulfates, among others, after anthocyanin-rich berry intake.231,237,244,245 Surprisingly, the same authors associated the improvement of FMD in 10 subjects after red raspberry intake with the presence of plasma ellagic acid (4.6 nM) and Uro-A conjugates (41 nM), but not with the pool of plasma phenolic acid metabolites that reached 120 μM.243 Overall, it is likely that associations will be found between plasma metabolites that increase after consuming the corresponding (poly)phenolic precursor and acute specific effects such as FMD improvements. However, these associations are usually lost in longer studies, even if the effects are also observed. Statistical associations do not necessarily involve causality or clinical relevance. In this regard, the approach of these authors to prove the link between FMD effects and circulating metabolites should be highlighted. They injected the equivalent dose of the pool of metabolites found in human plasma into mice and observed an improvement of FMD in mice, proving the causality role of anthocyanin-derived metabolites.244
Urinary excreted flavanone metabolites such as hesperetin 3′-O-glucuronide, and hesperetin 7-O-glucuronide have also been correlated with relative FMD improvements in overweight subjects after drinking orange juice.247 As expected, flavanone metabolite excretion increased after orange juice intake. However, whereas orange juice might improve FMD, there is no proven causality between urinary flavanone metabolite excretion and observed effects.
Other acute or postprandial studies have reported correlations between some health outcomes and circulating metabolites such as hesperetin concentrations and changes in microvascular endothelial reactivity after both orange juice and control drink plus hesperidin intake,229 quercetin-3′-O-glucuronide concentrations and an increase in brachial artery diameter after 400 mg of quercetin intake,232 hydroxytyrosol concentrations with the reduction of the pro-coagulant activated factor VII (FVIIa) concentrations after intake of the olive oil,224 and gallic acid and the insulin response and insulin sensitivity after red grape pomace intake.246
Finally, other studies have not identified the main driver (or drivers) of beneficial effects despite reporting positive correlations. Thus, Wong et al.239 reported in a postprandial study that resveratrol intake improved neurovascular coupling capacity, and the effect was correlated with the total metabolites (free form plus conjugates).239 A similar aspect was found in an acute interventional study in healthy males that consumed olives, finding a significant correlation between plasma total phenolics and plasma total antioxidant potential (TAP) but not with individual phenolic-derived metabolites, including hydroxytyrosol.225 Besides this, another RCT reported that equol concentrations were correlated with the change in carotid-femoral pulse-wave velocity (PWV) after soy isoflavones intake, but no effect was observed after free equol intake.150
In contrast, correlations between phenolic-derived metabolites and health outcomes are less evident in chronic studies. This fact is remarkable with the lack of studies that correlated improvements in FMD and/or blood pressure, even with flavanol-derived metabolites such as epicatechin. In this regard, one RCT conducted in postmenopausal with type 2 diabetes mellitus patients that consumed flavonoid-enriched chocolate (27 g d−1) plus 100 mg isoflavones for 1 year reported that urinary equol concentrations, but not epicatechin and its metabolites, tended to be inversely correlated with the reduction in diastolic blood pressure, although only in the equol producer individuals.251 On the contrary, a certain correlation has been found with other cardiometabolic markers such as hyperlipidaemia. Thus, urinary anthocyanin metabolites such as peonidin-3-O-galactoside, 3-(4-hydroxyphenyl) propionic acid, and cyanidin-3-O-galactoside were correlated with lower plasma Tchol and LDLc in a RCT conducted with 49 healthy former smokers after 12-weeks Aronia consumption.241 In the same line, another RCT described that the plasma oxLDL concentrations were negatively correlated with the sum of phenolics including hydroxytyrosol and tyrosol derivatives and the homovanillic acid metabolite in LDL particles from 36 healthy volunteers that consumed olive oil for 3 weeks.228 However, no correlation was found with antioxidant markers. Similarly, in another RCT conducted in 100 overweight or obese adults that consumed either normal or (poly)phenol-enriched orange juice for 12 weeks, no correlation was found with urinary hesperetin and naringenin or their conjugated metabolites and several antioxidant markers and reduction of anthropometric parameters, except for an inverse correlation with erythrocyte catalase activity.234
Several RCT trials conducted with pomegranate extracts have tried to correlate different plasma cardiometabolic and inflammatory risk biomarkers regarding urolithins. However, most studies did not find correlations between concentration changes in urine and plasma of any conjugated urolithin and the evaluated biomarkers.86,87,139 Besides this, the occurrence of urolithin levels in human CRC tissues was not correlated with the modulation of different miRNAs and CRC marker expression.74,252 González-Sarrías et al.119 reported that total and single urinary urolithins (Uro-A and B and IsoUro-A) in subjects that belong to UM-B significantly correlated with a reduction in LDLc levels, and specifically for Uro-A with total cholesterol and non-HDLc, after 3-weeks consumption of pomegranate extract. Besides this, the intake of other ellagitannin-rich sources has yielded contradictory results. In this regard, Uro-A glucuronide was inversely correlated with basal abdominal adiposity (WC, waist-hip ratio) and impaired glycaemic control (fasting insulin, HOMA-IR) after 12-weeks consumption of mixed nuts (30 g d−1).235 However, no correlation was found after 1-month strawberry pulp consumption (50 g d−1) between Uro-A and several antioxidant markers, being the effects negatively correlated with plasma 4-hydroxyhippuric acid concentrations.233
Two RCT studies found a correlation between the sum of circulating curcumin metabolites and the change in Tchol,223 and the increase of intrahepatic lipid content with the plasma resveratrol levels238 after consuming curcumin and resveratrol for one month, respectively. On the contrary, in another RCT conducted in patients with metabolic syndrome that consumed quercetin for 6 weeks, no correlation was found between improvements in cardiometabolic and inflammatory risk biomarkers with plasma concentrations of quercetin.250
3. Conclusions and roadmap
A consistent cause-and-effect relationship has been observed between consuming a few dietary (poly)phenols, including flavanols and phenolics in olive oil, and health effects (endothelial function and prevention of LDL oxidation, respectively). However, this cause-and-effect relationship has not been well established for the rest of the dietary phenolics.To date, the possible final drivers of the effects, at least partially, i.e., the specifically produced metabolite(s) and/or the gut microbial ecology associated with (poly)phenol metabolism (gut microbiota-associated metabotypes), have not been fully identified. In this regard, several studies have found statistical associations between plasma metabolites and acute effects such as FMD improvements. This association is likely to happen if the precursor (pure (poly)phenol or phenolic-containing food) exerts an effect. In this case, as it is logical to observe the concomitant increase in plasma of the derived phenolic metabolites, the statistical possibility of finding a correlation between effect and metabolite concentration will be high. Likewise, a non-targeted plasma analysis could also yield many possible associations between FMD (or other acute determinations) and the concomitant presence of other plasma metabolites during the assay (e.g., vitamins, amino acids, and many others).
However, these associations are usually lost in longer studies due to the high turnover of phenolic metabolites. Besides this, significant statistical associations do not necessarily involve causality or clinical relevance.
Cross-sectional associations to observe differential health effects depending on the gut microbial ecology are insufficient to prove both causality and temporality in the effects. Due to the vast amount of interfering variables that affect the individuals’ response to (poly)phenol consumption, randomised and placebo-control trials are mandatory to establish a causal role between specific gut microbial ecologies (gut microbiota metabotypes) and health effects. In this regard, several points like clustering subjects according to their gut microbiota-associated metabotypes, production of metabolite level, age (since metabotypes might change with age) or phase-II polymorphisms, as well as qualitative and quantitative assessments of the circulating (and excreted) metabolites to find strong correlations should be considered in further RCT studies. No single associations are sufficient to prove causality (even from a RCT). Therefore, RCTs should include a crossover and dose–response design as a first step in demonstrating causality vs. apparent statistical associations of circulating metabolites and effects.
The genetic makeup can contribute to defining the impact of dietary (poly)phenols on human health. Therefore, there is a need for investigating the role of polymorphisms of transporters and enzymes involved in the ADME of (poly)phenols. The contribution of these polymorphisms on the existence of specific polyphenol-related metabotypes is uncertain. However, they could modulate the bioavailability of dietary (poly)phenols critically and consequently affect the health effects.
Instead of animal to human, we should perform human to animal translational approaches to prove in animals the causal role of specific phenolic-derived metabolites produced by humans and/or their gut microbiota metabotypes. For example, after consuming dietary (poly)phenols, the human plasmatic phenolic signature should be administered (intravenously or intraperitoneally) to animal models to check a systemic cause-and-effect relationship. Similarly, the qualitative and quantitative profile of phenolic-derived metabolites found in human tumours after consuming dietary (poly)phenols could be injected chronically into tumours of xenograft animal models to verify their specific response to the challenge of phenolic-derived metabolites individually or as a mix.
In the same line, specific gut microbes, a consortium of microbes or faecal transplants characteristic of specific human gut microbiota metabotypes, should be assayed in animal models to demonstrate their causal role in health effects.
Identifying the actual metabolites ultimately responsible for the health effects after (poly)phenol consumption remains elusive. Nevertheless, we believe that the approaches given above, in combination with growing evidence for the biological effects of circulating metabolites, thanks to physiologically relevant mechanistic studies using circulating phenolic-derived metabolites, but not the forms present in foods, will contribute to identifying the putative drivers responsible for the health effects attributed to (poly)phenols.
Author contributions
Conceptualisation, supervision and validation: J.C.E.; funding acquisition: J.C.E., M.V.S.; investigation: all the authors; writing – original draft: J.C.E., M.V.S., A.G.-S.; writing – review & editing: all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Project PID2019-103914RB-I00 from the Ministry of Science and Innovation (MICINN, Spain) and by Fundación Séneca de la Región de Murcia (Spain), grant number 20880/PI/18. J.A.G.-B. was supported by a Standard European Marie Curie Fellowship from the European Commission. This project has received funding from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 838991. A.C.M. and C.E.I.-A. are the holders of predoctoral grants from MINECO (grant number BES-2016-078098) and MICINN (grant number FPU18/03961) (Spain), respectively.References
- W. N. Pearson, Flavonoids in human nutrition and medicine, J. Am. Med. Assoc., 1957, 164, 1675–1678, DOI:10.1001/jama.1957.62980150012011.
- G. Williamson, C. D. Kay and A. Crozier, The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective, Compr. Rev. Food Sci. Food Saf., 2018, 17, 1054–1112, DOI:10.1111/1541-4337.12351.
- T. Sugimura and M. Nagao, Mutagenic factors in cooked foods, CRC Crit. Rev. Toxicol., 1979, 6, 189–209, DOI:10.3109/10408447909037483.
- G. Block, B. Patterson and A. Subar, Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence, Nutr. Cancer, 1992, 18, 1–29, DOI:10.1080/01635589209514201.
- M. G. L. Hertog, Epidemiological evidence on potential health properties of flavonoids, Proc. Nutr. Soc., 1996, 55, 385–397, DOI:10.1079/pns19960037.
- L. Bravo, Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance, Nutr. Rev., 1998, 56, 317–333, DOI:10.1111/j.1753-4887.1998.tb01670.x.
- I. Urquiaga and F. Leighton, Plant polyphenol antioxidants and oxidative stress, Biol. Res., 2000, 33, 55–64, DOI:10.4067/s0716-97602000000200004.
- I. E. Dreosti, Antioxidant polyphenols in tea, cocoa, and wine, Nutrition, 2000, 16, 692–694, DOI:10.1016/s0899-9007(00)00304-x.
- M. I. Gil, F. A. Tomás-Barberán, B. Hess-Pierce, D. M. Holcroft and A. A. Kader, Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing, J. Agric. Food Chem., 2000, 48, 4581–4589, DOI:10.1021/jf000404a.
- M. I. Gil, F. A. Tomás-Barberán, B. Hess-Pierce and A. A. Kader, Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California, J. Agric. Food Chem., 2002, 50, 4976–4982, DOI:10.1021/jf020136b.
- R. Llorach, J. C. Espín, F. A. Tomás-Barberán and F. Ferreres, Valorization of cauliflower (Brassica oleracea L. var. botrytis) by-products as a source of antioxidant phenolics, J. Agric. Food Chem., 2003, 51, 2181–2187, DOI:10.1021/jf021056a.
- A. Scalbert, C. Morand, C. Manach and C. Rémésy, Absorption and metabolism of polyphenols in the gut and impact on health, Biomed. Pharmacother., 2002, 56, 276–282, DOI:10.1016/s0753-3322(02)00205-6.
- C. Manach, G. Williamson, C. Morand, A. Scalbert and C. Rémésy, Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies, Am. J. Clin. Nutr., 2005, 81, 230S–242S, DOI:10.1093/ajcn/81.1.230S.
- G. Williamson, The role of polyphenols in modern nutrition, Nutr. Bull., 2017, 42, 226–235, DOI:10.1111/nbu.12278.
- T. Ruskovska, V. Maksimova and D. Milenkovic, Polyphenols in human nutrition: from the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability - an overview and perspective, Br. J. Nutr., 2020, 123, 241–254, DOI:10.1017/S0007114519002733.
- S. Galmés, B. Reynés, M. Palou, A. Palou-March and A. Palou, Absorption, distribution, metabolism, and excretion of the main olive tree phenols and polyphenols: A literature review, J. Agric. Food Chem., 2021, 69, 5281–5296, DOI:10.1021/acs.jafc.1c00737.
- A. González-Sarrías, J. A. Giménez-Bastida, M. Á. Núñez-Sánchez, M. Larrosa, M. T. García-Conesa, F. A. Tomás-Barberán and J. C. Espín, Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells, Eur. J. Nutr., 2014, 53, 853–864, DOI:10.1007/s00394-013-0589-4.
- M. Á. Ávila-Gálvez, A. González-Sarrías, F. Martínez-Díaz, B. Abellán, A. J. Martínez-Torrano, A. J. Fernández-López, J. A. Giménez-Bastida and J. C. Espín, Disposition of dietary polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin, Mol. Nutr. Food Res., 2021, e2100163, DOI:10.1002/mnfr.202100163.
- M. Á. Ávila-Gálvez, R. García-Villalba, F. Martínez-Díaz, B. Ocaña-Castillo, T. Monedero-Saiz, A. Torrecillas-Sánchez, B. Abellán, A. González-Sarrías and J. C. Espín, Metabolic profiling of dietary polyphenols and methylxanthines in normal and malignant mammary tissues from breast cancer patients, Mol. Nutr. Food Res., 2019, 63, e1801239, DOI:10.1002/mnfr.201801239.
- M. Á. Ávila-Gálvez, J. C. Espín and A. González-Sarrías, Physiological relevance of the antiproliferative and estrogenic effects of dietary polyphenol aglycones versus their phase-II metabolites on breast cancer cells: A call of caution, J. Agric. Food Chem., 2018, 66, 8547–8555, DOI:10.1021/acs.jafc.8b03100.
- M. Á. Ávila-Gálvez, A. González-Sarrías and J. C. Espín, In vitro research on dietary polyphenols and health: A call of caution and a guide on how to proceed, J. Agric. Food Chem., 2018, 66, 7857–7858, DOI:10.1021/acs.jafc.8b03377.
- L. Hooper, C. Kay, A. Abdelhamid, P. A. Kroon, J. S. Cohn, E. B. Rimm and A. Cassidy, Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials, Am. J. Clin. Nutr., 2012, 95, 740–751, DOI:10.3945/ajcn.111.023457.
- J. Tomé-Carneiro, M. Larrosa, A. González-Sarrías, F. A. Tomás-Barberán, M. García-Conesa and J. C. Espín, Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence, Curr. Pharm. Des., 2013, 19, 6064–6093, DOI:10.2174/13816128113199990407.
- A. González-Sarrías, E. Combet, P. Pinto, P. Mena, M. Dall'Asta, M. Garcia-Aloy, A. Rodríguez-Mateos, E. R. Gibney, J. Dumont, M. Massaro, J. Sánchez-Meca, C. Morand and M. T. García-Conesa, A systematic review and meta-analysis of the effects of flavanol-containing tea, cocoa and apple products on body composition and blood lipids: Exploring the factors responsible for variability in their efficacy, Nutrients, 2017, 9, 746, DOI:10.3390/nu9070746.
- G. Grosso, J. Godos, R. Lamuela-Raventos, S. Ray, A. Micek, A. Pajak, S. Sciacca, N. D'Orazio, D. Del Rio and F. Galvano, A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations, Mol. Nutr. Food Res., 2017, 61, 1600930, DOI:10.1002/mnfr.201600930.
- J. A. Rothwell, V. Knaze and R. Zamora-Ros, Polyphenols: dietary assessment and role in the prevention of cancers, Curr. Opin. Clin. Nutr. Metab. Care, 2017, 20, 512–521, DOI:10.1097/mco.0000000000000424.
- M. T. García-Conesa, K. Chambers, E. Combet, P. Pinto, M. Garcia-Aloy, C. Andrés-Lacueva, S. De Pascual-Teresa, P. Mena, A. K. Ristic, W. J. Hollands, P. A. Kroon, A. Rodríguez-Mateos, G. Istas, C. A. Kontogiorgis, D. K. Rai, E. R. Gibney, C. Morand, J. C. Espín and A. González-Sarrías, Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: Analysis of factors influencing variability of the individual responses, Int. J. Mol. Sci., 2018, 19, 694, DOI:10.3390/ijms19030694.
- A. Rees, G. Dodd and J. Spencer, The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function, Nutrients, 2018, 10, 1852, DOI:10.3390/nu10121852.
- M. Akbari, O. R. Tamtaji, K. B. Lankarani, R. Tabrizi, E. Dadgostar, F. Kolahdooz, M. Jamilian, H. Mirzaei and Z. Asemi, The effects of resveratrol supplementation on endothelial function and blood pressures among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials, High Blood Press. Cardiovasc. Prev., 2019, 26, 305–319, DOI:10.1007/s40292-019-00324-6.
- F. Potì, D. Santi, G. Spaggiari, F. Zimetti and I. Zanotti, Polyphenol health effects on cardiovascular and neurodegenerative disorders: A review and meta-analysis, Int. J. Mol. Sci., 2019, 20, 351, DOI:10.3390/ijms20020351.
- R. Giacco, G. Costabile, G. Fatati, L. Frittitta, M. I. Maiorino, G. Marelli, M. Parillo, D. Pistis, C. Tubili, C. Vetrani and M. Vitale, Effects of polyphenols on cardio-metabolic risk factors and risk of type 2 diabetes. A joint position statement of the Diabetes and Nutrition Study Group of the Italian Society of Diabetology (SID), the Italian Association of Dietetics and Clinical Nutrition (ADI) and the Italian Association of Medical Diabetologists (AMD), Nutr. Metab. Cardiovasc. Dis., 2020, 30, 355–367, DOI:10.1016/j.numecd.2019.11.015.
- S. V. Luca, I. Macovei, A. Bujor, A. Miron, K. Skalicka-Woźniak, A. C. Aprotosoaie and A. Trifan, Bioactivity of dietary polyphenols: The role of metabolites, Crit. Rev. Food Sci. Nutr., 2020, 60, 626–659, DOI:10.1080/10408398.2018.1546669.
- V. A. do Rosario, C. Chang, J. Spencer, T. Alahakone, S. Roodenrys, M. Francois, K. Weston-Green, N. Hölzel, D. S. Nichols, K. Kent, D. Williams, I. M. R. Wright and K. Charlton, Anthocyanins attenuate vascular and inflammatory responses to a high fat high energy meal challenge in overweight older adults: A cross-over, randomized, double-blind clinical trial, Clin. Nutr., 2021, 40, 879–889, DOI:10.1016/j.clnu.2020.09.041.
- J. I. Dower, J. M. Geleijnse, L. Gijsbers, P. L. Zock, D. Kromhout and P. C. Hollman, Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial, Am. J. Clin. Nutr., 2015, 101, 914–921, DOI:10.3945/ajcn.114.098590.
- J. I. Dower, J. M. Geleijnse, L. Gijsbers, C. Schalkwijk, D. Kromhout and P. C. Hollman, Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: A randomized double-blind, placebo-controlled, crossover trial, J. Nutr., 2015, 145, 1459–1463, DOI:10.3945/jn.115.211888.
- EFSA Panel on Dietetic Products and Nutrition and Allergies (NDA), Scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006, EFSA J., 2011, 9, 2033, DOI:10.2903/j.efsa.2011.2033.
- EFSA Panel on Dietetic Products and Nutrition and Allergies (NDA), Scientific opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006, EFSA J., 2012, 10, 2809, DOI:10.2903/j.efsa.2012.2809.
- M. Á. Ávila-Gálvez, J. A. Giménez-Bastida, J. C. Espín and A. González-Sarrías, Dietary phenolics against breast cancer. A critical evidence-based review and future perspectives, Int. J. Mol. Sci., 2020, 21, 5718, DOI:10.3390/ijms21165718.
- A. Sanches-Silva, L. Testai, S. F. Nabavi, M. Battino, K. Pandima Devi, S. Tejada, A. Sureda, S. Xu, B. Yousefi, M. Majidinia, G. L. Russo, T. Efferth, S. M. Nabavi and M. H. Farzaei, Therapeutic potential of polyphenols in cardiovascular diseases: Regulation of mTOR signaling pathway, Pharmacol. Res., 2020, 152, 104626, DOI:10.1016/j.phrs.2019.104626.
- L. Monfoulet, T. Ruskovska, V. Ajdzanovic, J. Havlik, D. Vauzour, B. Bayram, I. Krga, C. J. K. Fabiola, E. Kistanova, D. Abadjieva, M. Massaro, E. Scodetti, E. Deligiannidou, C. Kontogiorgis, A. Arola-Arnal, E. M. Schothorst, C. Morand and D. Milenkovic, Molecular determinants of the cardiometabolic improvements of dietary flavanols identified by an integrative analysis of nutrigenomic data from a systematic review of animal studies, Mol. Nutr. Food Res., 2021, 28, e2100227, DOI:10.1002/mnfr.202100227.
- D. Milenkovic, C. Morand, C. Aedin, A. Konic-Ristic, F. A. Tomás-Barberán, J. M. Ordovas, P. Kroon, R. De Caterina and A. Rodríguez-Mateos, Interindividual variability in biomarkers of cardiometabolic health after consumption of major plant-food bioactive compounds and the determinants involved, Adv. Nutr., 2017, 8, 558–570, DOI:10.3945/an.116.013623.
- E. R. Gibney, D. Milenkovic, E. Combet, T. Ruskovska, A. Greyling, A. González-Sarrías, B. de Roos, F. A. Tomás-Barberán, C. Morand and A. Rodríguez-Mateos, Factors influencing the cardiometabolic response to (poly)phenols and phytosterols: a review of the COST Action POSITIVe activities, Eur. J. Nutr., 2019, 58, 37–47, DOI:10.1007/s00394-019-02066-6.
- A. Cortés-Martín, M. V. Selma, F. A. Tomás-Barberán, A. González-Sarrías and J. C. Espín, Where to look into the puzzle of polyphenols and health ? The postbiotics and gut microbiota associated with human metabotypes, Mol. Nutr. Food Res., 2020, 64, e1900952, DOI:10.1002/mnfr.201900952.
- B. Marchant, Pharmacokinetic factors influencing variability in human drug response, Scand. J. Rheumatol., 1981, 39, 5–14, DOI:10.3109/03009748109095328.
- L. J. Lu and K. E. Anderson, Sex and long-term soy diets affect the metabolism and excretion of soy isoflavones in humans, Am. J. Clin. Nutr., 1998, 68, 1500S–1504S, DOI:10.1093/ajcn/68.6.1500S.
- M. Bourian, M. Runkel, A. Krisp, M. Tegtmeier, J. Freudenstein and W. Legrum, Naringenin and interindividual variability in interaction of coumarin with grapefruit juice, Exp. Toxicol. Pathol., 1999, 51, 289–293, DOI:10.1016/S0940-2993(99)80008-6.
- M. J. Lee, P. Maliakal, L. Chen, X. Meng, F. Y. Bondoc, S. Prabhu, G. Lambert, S. Mohr and C. S. Yang, Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability, Cancer Epidemiol. Biomarkers Prev., 2002, 11, 1025–1032 CAS.
- B. Cerdá, F. A. Tomás-Barberán and J. C. Espín, Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability, J. Agric. Food Chem., 2005, 53, 227–235, DOI:10.1021/jf049144d.
- R. Menezes, A. Rodríguez-Mateos, A. Kaltsatou, A. González-Sarrías, A. Greyling, C. Giannaki, C. Andres-Lacueva, D. Milenkovic, E. Gibney, J. Dumont, M. Schär, M. García-Aloy, S. Palma-Duran, T. Ruskovska, V. Maksimova, E. Combet and P. Pinto, Impact of flavonols on cardiometabolic biomarkers: A meta-analysis of randomized controlled human trials to explore the role of inter-individual variability, Nutrients, 2017, 9, 117, DOI:10.3390/nu9020117.
- G. Raman, E. E. Avendano, S. Chen, J. Wang, J. Matson, B. Gayer, J. A. Novotny and A. Cassidy, Dietary intakes of flavan-3-ols and cardiometabolic health: systematic review and meta-analysis of randomized trials and prospective cohort studies, Am. J. Clin. Nutr., 2019, 110, 1067–1078, DOI:10.1093/ajcn/nqz178.
- I. Mozos, C. Flangea, D. C. Vlad, C. Gug, C. Mozos, D. Stoian, C. T. Luca, J. O. Horbańczuk, O. K. Horbańczuk and A. G. Atanasov, Effects of anthocyanins on vascular health, Biomolecules, 2021, 11, 811, DOI:10.3390/biom11060811.
- T. Bohn, G. J. McDougall, A. Alegría, M. Alminger, E. Arrigoni, A. Aura, C. Brito, A. Cilla, S. N. El, S. Karakaya, M. C. Martínez-Cuesta and C. N. Santos, Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites- a position paper focusing on carotenoids and polyphenols, Mol. Nutr. Food Res., 2015, 59, 1307–1323, DOI:10.1002/mnfr.201400745.
- A. Cassidy and A. M. Minihane, The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids, Am. J. Clin. Nutr., 2017, 105, 10–22, DOI:10.3945/ajcn.116.136051.
- C. Scholl, A. Lepper, T. Lehr, N. Hanke, K. L. Schneider, J. Brockmöller, T. Seufferlein and J. C. Stingl, Population nutrikinetics of green tea extract, PLoS One, 2018, 13, e0193074, DOI:10.1371/journal.pone.0193074.
- A. Cortés-Martín, M. V. Selma, J. C. Espín and R. García-Villalba, The human metabolism of nuts proanthocyanidins does not reveal urinary metabolites consistent with distinctive gut microbiota metabotypes, Mol. Nutr. Food Res., 2019, 63, e1800819, DOI:10.1002/mnfr.201800819.
- F. A. Tomás-Barberán and J. C. Espín, Effect of food structure and processing on (poly)phenol-gut microbiota interactions and the effects on human health, Annu. Rev. Food Sci. Technol., 2019, 10, 221–238, DOI:10.1146/annurev-food-032818-121615.
- C. Zhao, Y. Wang, B. Zhang, Y. Yue and J. Zhang, Genetic variations in catechol-O-methyltransferase gene are associated with levodopa response variability in chinese patients with Parkinson's disease, Sci. Rep., 2020, 10, 9521, DOI:10.1038/s41598-020-65332-2.
- M. V. Selma, J. C. Espín and F. A. Tomás-Barberán, Interaction between phenolics and gut microbiota: Role in human health, J. Agric. Food Chem., 2009, 57, 6485–6501, DOI:10.1021/JF902107D.
- J. C. Espín, A. González-Sarrías and F. A. Tomás-Barberán, The gut microbiota: A key factor in the therapeutic effects of (poly)phenols, Biochem. Pharmacol., 2017, 139, 82–93, DOI:10.1016/j.bcp.2017.04.033.
- A. Kerimi, N. U. Kraut, J. A. da Encarnacao and G. Williamson, The gut microbiome drives inter- and intra-individual differences in metabolism of bioactive small molecules, Sci. Rep., 2020, 10, 19590, DOI:10.1038/s41598-020-76558-5.
- K. D. R. Setchell, N. M. Brown and E. Lydeking-Olsen, The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones, J. Nutr., 2002, 132, 3577–3584, DOI:10.1093/jn/132.12.3577.
- B. Cerdá, J. C. Espín, S. Parra, P. Martínez and F. A. Tomás-Barberán, The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans, Eur. J. Nutr., 2004, 43, 205–220, DOI:10.1007/s00394-004-0461-7.
- M. Romo-Vaquero, A. Cortés-Martín, V. Loria-Kohen, A. Ramírez-de-Molina, I. García-Mantrana, M. C. Collado, J. C. Espín and M. V. Selma, Deciphering the Human Gut Microbiome of Urolithin Metabotypes: Association with Enterotypes and Potential Cardiometabolic Health Implications, Mol. Nutr. Food Res., 2019, 63, e1800958, DOI:10.1002/mnfr.201800958.
- A. K. Singh, C. Cabral, R. Kumar, R. Ganguly, H. K. Rana, A. Gupta, M. R. Lauro, C. Carbone, F. Reis and A. K. Pandey, Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency, Nutrients, 2019, 11, 2216, DOI:10.3390/NU11092216.
- A. González-Sarrías, J. C. Espín and F. A. Tomás-Barberán, Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects, Trends Food Sci. Technol., 2017, 69, 281–288, DOI:10.1016/j.tifs.2017.07.010.
- M. N. Alam, M. Almoyad and F. Huq, Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action, Biomed Res. Int., 2018, 15, 4154185, DOI:10.1155/2018/4154185.
- S. Ding, S. Xu, J. Fang and H. Jiang, The Protective Effect of Polyphenols for Colorectal Cancer, Front. Immunol., 2020, 11, 1407, DOI:10.3389/FIMMU.2020.01407.
- H. Li, L. M. Christman, R. Li and L. Gu, Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases, Food Funct., 2020, 11, 4878–4891, 10.1039/D0FO00713G.
- M. Á. Núñez-Sánchez, A. González-Sarrías, M. Romo-Vaquero, R. García-Villalba, M. V. Selma, F. A. Tomás-Barberán and J. C. Espín, Dietary phenolics against colorectal cancer–From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs, Mol. Nutr. Food Res., 2015, 59, 1274–1291, DOI:10.1002/MNFR.201400866.
- M. Shimizu, Multifunctions of dietary polyphenols in the regulation of intestinal inflammation, J. Food Drug Anal., 2017, 25, 93–99, DOI:10.1016/J.JFDA.2016.12.003.
- M. H. Farzaei, R. Rahimi and M. Abdollahi, The Role of Dietary Polyphenols in the Management of Inflammatory Bowel Disease, Curr. Pharm. Biotechnol., 2015, 16, 196–210, DOI:10.2174/1389201016666150118131704.
- M. Larrosa, C. Luceri, E. Vivoli, C. Pagliuca, M. Lodovici, G. Moneti and P. Dolara, Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models, Mol. Nutr. Food Res., 2009, 53, 1044–1054, DOI:10.1002/MNFR.200800446.
- M. Larrosa, A. González-Sarrías, M. J. Yáñez-Gascón, M. V. Selma, M. Azorín-Ortuño, S. Toti, F. A. Tomás-Barberán, P. Dolara and J. C. Espín, Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism, J. Nutr. Biochem., 2010, 21, 717–725, DOI:10.1016/J.JNUTBIO.2009.04.012.
- M. Á. Núñez-Sánchez, A. González-Sarrías, R. García-Villalba, T. Monedero-Saiz, N. V. García-Talavera, M. B. Gómez-Sánchez, C. Sánchez-Álvarez, A. M. García-Albert, F. J. Rodríguez-Gil, M. Ruiz-Marín, F. A. Pastor-Quirante, F. Martínez-Díaz, F. A. Tomás-Barberán, J. C. Espín and M. T. García-Conesa, Gene expression changes in colon tissues from colorectal cancer patients following the intake of an ellagitannin-containing pomegranate extract: a randomized clinical trial, J. Nutr. Biochem., 2017, 42, 126–133, DOI:10.1016/J.JNUTBIO.2017.01.014.
- A. González-Sarrías, M. Larrosa, F. A. Tomás-Barberán, P. Dolara and J. C. Espín, NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts, Br. J. Nutr., 2010, 104, 503–512, DOI:10.1017/S0007114510000826.
- R. Singh, S. Chandrashekharappa, S. R. Bodduluri, B. V. Baby, B. Hegde, N. G. Kotla, A. A. Hiwale, T. Saiyed, P. Patel, M. Vijay-Kumar, M. G. I. Langille, G. M. Douglas, X. Cheng, E. C. Rouchka, S. J. Waigel, G. W. Dryden, H. Alatassi, H. G. Zhang, B. Haribabu, P. K. Vemula and V. R. Jala, Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway, Nat. Commun., 2019, 10, 89, DOI:10.1038/S41467-018-07859-7.
- M. Monagas, N. Khan, C. Andrés-Lacueva, M. Urpí-Sardá, M. Vázquez-Agell, R. M. Lamuela-Raventós and R. Estruch, Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells, Br. J. Nutr., 2009, 102, 201–206, DOI:10.1017/S0007114508162110.
- K. Gao, A. Xu, C. Krul, K. Venema, Y. Liu, Y. Niu, J. Lu, L. Bensoussan, N. P. Seeram, D. Heber and S. M. Henning, Of the major phenolic acids formed during human microbial fermentation of tea, citrus, and soy flavonoid supplements, only 3,4-dihydroxyphenylacetic acid has antiproliferative activity, J. Nutr., 2006, 136, 52–57, DOI:10.1093/JN/136.1.52.
- C. L. Boulangé, A. L. Neves, J. Chilloux, J. K. Nicholson and M. E. Dumas, Impact of the gut microbiota on inflammation, obesity, and metabolic disease, Genome Med., 2016, 8, 42, DOI:10.1186/S13073-016-0303-2.
- J. P. Haran, S. K. Bhattarai, S. E. Foley, P. Dutta, D. V. Ward, V. Bucci and B. A. McCormick, Alzheimer's Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway, mBio, 2019, 10, e00632–e00619, DOI:10.1128/mBio.00632-19.
- M. F. Sun and Y. Q. Shen, Dysbiosis of gut microbiota and microbial metabolites in Parkinson's Disease, Ageing Res. Rev., 2018, 45, 53–61, DOI:10.1016/J.ARR.2018.04.004.
- K. J. Portune, A. M. Davila, D. Tomé, F. Blachier, M. Beaumont and Y. Sanz, Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin, Trends Food Sci. Technol., 2016, 57, 213–232, DOI:10.1016/J.TIFS.2016.08.011.
- I. Rowland, G. Gibson, A. Heinken, K. Scott, J. Swann, I. Thiele and K. Tuohy, Gut microbiota functions: metabolism of nutrients and other food components, Eur. J. Nutr., 2018, 57, 1–24, DOI:10.1007/S00394-017-1445-8.
- K. Kawabata, Y. Yoshioka and J. Terao, Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols, Molecules, 2019, 24, 370, DOI:10.3390/molecules24020370.
- X. Tzounis, A. Rodríguez-Mateos, J. Vulevic, G. R. Gibson, C. Kwik-Uribe and J. P. E. Spencer, Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study, Am. J. Clin. Nutr., 2011, 93, 62–72, DOI:10.3945/ajcn.110.000075.
- A. González-Sarrías, M. Romo-Vaquero, R. García-Villalba, A. Cortés-Martín, M. V. Selma and J. C. Espín, The Endotoxemia Marker Lipopolysaccharide-Binding Protein is Reduced in Overweight-Obese Subjects Consuming Pomegranate Extract by Modulating the Gut Microbiota: A Randomized Clinical Trial, Mol. Nutr. Food Res., 2018, 62, e1800160, DOI:10.1002/mnfr.201800160.
- A. González-Sarrías, M. Á. Núñez-Sánchez, M. Á. Ávila-Gálvez, T. Monedero-Saiz, F. J. Rodríguez-Gil, F. Martínez-Díaz, M. V. Selma and J. C. Espín, Consumption of pomegranate decreases plasma lipopolysaccharide-binding protein levels, a marker of metabolic endotoxemia, in patients with newly diagnosed colorectal cancer: A randomized controlled clinical trial, Food Funct., 2018, 9, 2617–2622, 10.1039/C8FO00264A.
- M. Covasa, R. W. Stephens, R. Toderean and C. Cobuz, Intestinal Sensing by Gut Microbiota: Targeting Gut Peptides, Front. Endocrinol., 2019, 10, 82, DOI:10.3389/FENDO.2019.00082.
- A. Rivière, M. Selak, D. Lantin, F. Leroy and L. De Vuyst, Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut, Front. Microbiol., 2016, 7, 979, DOI:10.3389/FMICB.2016.00979.
- I. Moreno-Indias, L. Sánchez-Alcoholado, P. Pérez-Martínez, C. Andrés-Lacueva, F. Cardona, F. Tinahones and M. I. Queipo-Ortuño, Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients, Food Funct., 2016, 7, 1775–1787, 10.1039/C5FO00886G.
- O. Aprikian, V. Duclos, S. Guyot, C. Besson, C. Manach, A. Bernalier, C. Morand, C. Rémésy and C. Demigné, Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats, J. Nutr., 2003, 133, 1860–1865, DOI:10.1093/JN/133.6.1860.
- B. Fotschki, J. Milala, A. Jurgoński, E. Karlińska, Z. Zduńczyk and J. Juśkiewicz, Strawberry ellagitannins thwarted the positive effects of dietary fructooligosaccharides in rat cecum, J. Agric. Food Chem., 2014, 62, 5871–5880, DOI:10.1021/JF405612A.
- B. Fotschki, J. Juśkiewicz, A. Jurgoński, K. Kołodziejczyk, J. Milala, M. Kosmala and Z. Zduńczyk, Anthocyanins in strawberry polyphenolic extract enhance the beneficial effects of diets with fructooligosaccharides in the rat cecal environment, PLoS One, 2016, 11, e0149081, DOI:10.1371/JOURNAL.PONE.0149081.
- J. A. Domínguez-Ávila, A. Wall-Medrano, G. R. Velderrain-Rodríguez, C. Y. O. Chen, N. J. Salazar-López, M. Robles-Sánchez and G. A. González-Aguilar, Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds, Food Funct., 2017, 8, 15–38, 10.1039/c6fo01475e.
- A. I. Álvarez, R. Real, M. Pérez, G. Mendoza, J. G. Prieto and G. Merino, Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response, J. Pharm. Sci., 2010, 99, 598–617, DOI:10.1002/jps.21851.
- J. M. Planas, I. Alfaras, H. Colom and M. E. Juan, The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters, Arch. Biochem. Biophys., 2012, 527, 67–73, DOI:10.1016/j.abb.2012.06.004.
- Y. Li and J. W. Paxton, The effects of flavonoids on the ABC transporters: consequences for the pharmacokinetics of substrate drugs, Expert Opin. Drug Metab. Toxicol., 2013, 9, 267–285, DOI:10.1517/17425255.2013.749858.
- A. Miron, A. C. Aprotosoaie, A. Trifan and J. Xiao, Flavonoids as modulators of metabolic enzymes and drug transporters, Ann. N. Y. Acad. Sci., 2017, 1398, 152–167, DOI:10.1111/nyas.13384.
- C. Di Lorenzo, F. Colombo, S. Biella, C. Stockley and P. Restani, Polyphenols and Human Health: The Role of Bioavailability, Nutrients, 2021, 13, 273, DOI:10.3390/nu13010273.
- S. Bolca, T. Van de Wiele and S. Possemiers, Gut metabotypes govern health effects of dietary polyphenols, Curr. Opin. Biotechnol., 2013, 24, 220–225, DOI:10.1016/j.copbio.2012.09.009.
- S. G. Camps, H. R. Koh, N. X. Wang and C. J. Henry, A fructose-based meal challenge to assess metabotypes and their metabolic risk profile: A randomized, crossover, controlled trial, Nutrition, 2020, 78, 110799, DOI:10.1016/j.nut.2020.110799.
- M. Palmnäs, C. Brunius, L. Shi, A. Rostgaard-Hansen, N. E. Torres, R. González-Domínguez, R. Zamora-Ros, Y. L. Ye, J. Halkjær, A. Tjønneland, G. Riccardi, R. Giacco, G. Costabile, C. Vetrani, J. Nielsen, C. Andres-Lacueva and R. Landberg, Perspective: Metabotyping-A Potential Personalized Nutrition Strategy for Precision Prevention of Cardiometabolic Disease, Adv. Nutr., 2020, 11, 524–532, DOI:10.1093/advances/nmz121.
- N. Wawro, G. Pestoni, A. Riedl, T. A. Breuninger, A. Peters, W. Rathmann, W. Koenig, C. Huth, C. Meisinger, S. Rohrmann and J. Linseisen, Association of Dietary Patterns and Type-2 Diabetes Mellitus in Metabolically Homogeneous Subgroups in the KORA FF4 Study, Nutrients, 2020, 12, 1684, DOI:10.3390/nu12061684.
- F. Vallejo, M. Larrosa, E. Escudero, M. P. Zafrilla, B. Cerdá, J. Boza, M. T. García-Conesa, J. C. Espín and F. A. Tomás-Barberán, Concentration and solubility of flavanones in orange beverages affect their bioavailability in humans, J. Agric. Food Chem., 2010, 58, 6516–6524, DOI:10.1021/jf100752j.
- L. M. Bode, D. Bunzel, M. Huch, G. S. Cho, D. Ruhland, M. Bunzel, A. Bub, C. M. Franz and S. E. Kulling, In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota, Am. J. Clin. Nutr., 2013, 97, 295–309, DOI:10.3945/ajcn.112.049379.
- C. Favari, P. Mena, C. Curti, G. Istas, C. Heiss, D. Del Rio and A. Rodríguez-Mateos, Kinetic profile and urinary excretion of phenyl-γ-valerolactones upon consumption of cranberry: a dose-response relationship, Food Funct., 2020, 11, 3975–3985, 10.1039/d0fo00806k.
- M. Á. Ávila-Gálvez, J. A. Giménez-Bastida, A. González-Sarrías and J. C. Espín, New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study, Antioxidants, 2021, 10, 435, DOI:10.3390/antiox10030435.
- A. Mas-Capdevila, J. Teichenne, C. Domenech-Coca, A. Caimari, J. M. Del Bas, X. Escoté and A. Crescenti, Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability, Nutrients, 2020, 12, 1488, DOI:10.3390/nu12051488.
- M. Zhang, S. Zhu, W. Yang, Q. Huang and C. T. Ho, The biological fate and bioefficacy of citrus flavonoids: bioavailability, biotransformation, and delivery systems, Food Funct., 2021, 12, 3307–3323, 10.1039/d0fo03403g.
- A. Nishioka, E. C. Tobaruela, L. N. Fraga, F. A. Tomás-Barberán, F. M. Lajolo and N. M. A. Hassimotto, Stratification of Volunteers According to Flavanone Metabolite Excretion and Phase II Metabolism Profile after Single Doses of ‘Pera’ Orange and ‘Moro’ Blood Orange Juices, Nutrients, 2021, 13, 473, DOI:10.3390/nu13020473.
- C. L. Frankenfeld, Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes, Mol. Nutr. Food Res., 2017, 61, 1500900, DOI:10.1002/mnfr.201500900.
- E. Hålldin, A. K. Eriksen, C. Brunius, A. B. da Silva, M. Bronze, K. Hanhineva, A. M. Aura and R. Landberg, Factors Explaining Interpersonal Variation in Plasma Enterolactone Concentrations in Humans, Mol. Nutr. Food Res., 2019, 63, e1801159, DOI:10.1002/mnfr.201801159.
- I. L. Paraíso, L. S. Plagmann, L. Yang, R. Zielke, A. F. Gombart, C. S. Maier, A. E. Sikora, P. R. Blakemore and J. F. Stevens, Reductive Metabolism of Xanthohumol and 8-Prenylnaringenin by the Intestinal Bacterium Eubacterium ramulus, Mol. Nutr. Food Res., 2019, 63, e1800923, DOI:10.1002/mnfr.201800923.
- J. Guo, D. Nikolic, L. R. Chadwick, G. F. Pauli and R. B. van Breemen, Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.), Drug Metab. Dispos., 2006, 34, 1152–1159, DOI:10.1124/dmd.105.008250.
- A. Boronat, N. Soldevila-Domenech, J. Rodríguez-Morató, M. Martínez-Huélamo, R. M. Lamuela-Raventós and R. de la Torre, Beer Phenolic Composition of Simple Phenols, Prenylated Flavonoids and Alkylresorcinols, Molecules, 2020, 25, 2582, DOI:10.3390/molecules25112582.
- S. Sang and Y. Chu, Whole grain oats, more than just a fiber: Role of unique phytochemicals, Mol. Nutr. Food Res., 2017, 61, 1600715, DOI:10.1002/mnfr.201600715.
- P. Wang, S. Zhang, A. Yerke, C. L. Ohland, R. Z. Gharaibeh, F. Fouladi, A. A. Fodor, C. Jobin and S. Sang, Avenanthramide Metabotype from Whole-Grain Oat Intake is Influenced by Faecalibacterium prausnitzii in Healthy Adults, J. Nutr., 2021, 151, 1426–1435, DOI:10.1093/jn/nxab006.
- S. Miquel, R. Martín, O. Rossi, L. G. Bermúdez-Humarán, J. M. Chatel, H. Sokol, M. Thomas, J. M. Wells and P. Langella, Faecalibacterium prausnitzii and human intestinal health, Curr. Opin. Microbiol., 2013, 16, 255–261, DOI:10.1016/j.mib.2013.06.003.
- A. González-Sarrías, R. García-Villalba, M. Romo-Vaquero, C. Alasalvar, A. Örem, P. Zafrilla, F. A. Tomás-Barberán, M. V. Selma and J. C. Espín, Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial, Mol. Nutr. Food Res., 2017, 61, DOI:10.1002/mnfr.201600830.
- R. García-Villalba, D. Beltrán, M. D. Frutos, M. V. Selma, J. C. Espín and F. A. Tomás-Barberán, Metabolism of different dietary phenolic compounds by the urolithin-producing human-gut bacteria Gordonibacter urolithinfaciens and Ellagibacter isourolithinifaciens, Food Funct., 2020, 11, 7012–7022, 10.1039/d0fo01649g.
- C. Li, M. J. Lee, S. Q. Sheng, X. F. Meng, S. Prabhu, B. Winnik, B. M. Huang, J. Y. Chung, S. Q. Yan, C. T. Ho and C. S. Yang, Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion, Chem. Res. Toxicol., 2000, 13, 177–184, DOI:10.1021/tx9901837.
- M. Urpí-Sardá, I. Garrido, M. Monagas, C. Gómez-Cordovés, A. Medina-Remón, C. Andrés-Lacueva and B. Bartolomé, Profile of plasma and urine metabolites after the intake of almond [Prunus dulcis (Mill.) D.A. Webb] polyphenols in humans, J. Agric. Food Chem., 2009, 57, 10134–10142, DOI:10.1021/jf901450z.
- P. Mena, L. Bresciani, N. Brindani, I. A. Ludwig, G. Pereira-Caro, D. Angelino, R. Llorach, L. Calani, F. Brighenti, M. N. Clifford, C. I. R. Gill, A. Crozier, C. Curti and D. Del Rio, Phenyl-gamma-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: synthesis, analysis, bioavailability, and bioactivity, Nat. Prod. Rep., 2019, 36, 714–752, 10.1039/c8np00062j.
- J. I. Mosele, A. Macià and M. J. Motilva, Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review, Molecules, 2015, 20, 17429–17468, DOI:10.3390/molecules200917429.
- L. Zhang, Y. Wang, D. Li, C. T. Ho, J. Li and X. Wan, The absorption, distribution, metabolism and excretion of procyanidins, Food Funct., 2016, 7, 1273–1281, 10.1039/c5fo01244a.
- A. L. Mayorga-Gross and P. Esquivel, Impact of Cocoa Products Intake on Plasma and Urine Metabolites: A Review of Targeted and Non-Targeted Studies in Humans, Nutrients, 2019, 11, 1163, DOI:10.3390/nu11051163.
- W. J. Hollands, M. Philo, N. Perez-Moral, P. W. Needs, G. M. Savva and P. A. Kroon, Monomeric Flavanols Are More Efficient Substrates for Gut Microbiota Conversion to Hydroxyphenyl-γ-Valerolactone Metabolites Than Oligomeric Procyanidins: A Randomized, Placebo-Controlled Human Intervention Trial, Mol. Nutr. Food Res., 2020, 64, 1901135, DOI:10.1002/mnfr.201901135.
- A. Anesi, P. Mena, A. Bub, M. Ulaszewska, D. Del Rio, S. E. Kulling and F. Mattivi, Quantification of Urinary Phenyl-γ-Valerolactones and Related Valeric Acids in Human Urine on Consumption of Apples, Metabolites, 2019, 9, 254, DOI:10.3390/metabo9110254.
- P. Mena, I. A. Ludwig, V. B. Tomatis, A. Acharjee, L. Calani, A. Rosi, F. Brighenti, S. Ray, J. L. Griffin, L. J. Bluck and D. Del Rio, Inter-individual variability in the production of flavan-3-ol colonic metabolites: preliminary elucidation of urinary metabotypes, Eur. J. Nutr., 2019, 58, 1529–1543, DOI:10.1007/s00394-018-1683-4.
- C. Liu, J. Vervoort, K. Beekmann, M. Baccaro, L. Kamelia, S. Wesseling and I. M. C. M. Rietjens, Interindividual Differences in Human Intestinal Microbial Conversion of (-)-Epicatechin to Bioactive Phenolic Compounds, J. Agric. Food Chem., 2020, 68, 14168–14181, DOI:10.1021/acs.jafc.0c05890.
- A. Cortés-Martín, R. García-Villalba, A. González-Sarrías, M. Romo-Vaquero, V. Loria-Kohen, A. Ramírez-de-Molina, F. A. Tomás-Barberán, M. V. Selma and J. C. Espín, The gut microbiota urolithin metabotypes revisited: the human metabolism of ellagic acid is mainly determined by aging, Food Funct., 2018, 9, 4100–4106, 10.1039/c8fo00956b.
- F. A. Tomás-Barberán, A. González-Sarrías, R. García-Villalba, M. A. Núñez-Sánchez, M. V. Selma, M. T. García-Conesa and J. C. Espín, Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status, Mol. Nutr. Food Res., 2017, 61, 150090, DOI:10.1002/mnfr.201500901.
- S. Salminen, M. C. Collado, A. Endo, C. Hill, S. Lebeer, E. M. M. Quigley, M. E. Sanders, R. Shamir, J. R. Swann, H. Szajewska and G. Vinderola, The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics, Nat. Rev. Gastroenterol. Hepatol., 2021, 18, 649–667, DOI:10.1038/s41575-021-00440-6.
- F. F. Anhê, B. S. Y. Choi, J. R. B. Dyck, J. D. Schertzer and A. Marette, Host-Microbe Interplay in the Cardiometabolic Benefits of Dietary Polyphenols, Trends Endocrinol. Metab., 2019, 30, 384–395, DOI:10.1016/j.tem.2019.04.002.
- S. Mills, C. Stanton, J. A. Lane, G. J. Smith and R. P. Ross, Precision Nutrition and the Microbiome, Part I: Current State of the Science, Nutrition, 2019, 11, 923, DOI:10.3390/nu11040923.
- M. L. Marco, D. Heeney, S. Binda, C. J. Cifelli, P. D. Cotter, B. Foligné, M. Gänzle, R. Kort, G. Pasin, A. Pihlanto, E. J. Smid and R. Hutkins, Health benefits of fermented foods: microbiota and beyond, Curr. Opin. Biotechnol., 2017, 44, 94–102, DOI:10.1016/j.copbio.2016.11.010.
- J. E. Aguilar-Toalá, R. García-Varela, H. S. García, V. Mata-Haro, A. F. González-Córdova, B. Vallejo-Córdoba and A. Hernández-Mendoza, Postbiotics: An evolving term within the functional foods field, Trends Food Sci. Technol., 2018, 75, 105–114, DOI:10.1016/j.tifs.2018.03.009.
- A. Cortés-Martín, M. Romo-Vaquero, I. García-Mantrana, A. Rodríguez-Varela, M. C. Collado, J. C. Espín and M. V. Selma, Urolithin Metabotypes can Anticipate the Different Restoration of the Gut Microbiota and Anthropometric Profiles during the First Year Postpartum, Nutrition, 2019, 11, 2079, DOI:10.3390/nu11092079.
- A. Cortés-Martín, C. E. Iglesias-Aguirre, A. Meoro, M. V. Selma and J. C. Espín, Pharmacological Therapy Determines the Gut Microbiota Modulation by a Pomegranate Extract Nutraceutical in Metabolic Syndrome: A Randomized Clinical Trial, Mol. Nutr. Food Res., 2021, 65, 2001048, DOI:10.1002/mnfr.202001048.
- C. L. Frankenfeld, O-desmethylangolensin: the importance of equol's lesser known cousin to human health, Adv. Nutr., 2011, 2, 317–324, DOI:10.3945/an.111.000539.
- C. L. Frankenfeld, C. Atkinson, K. Wähälä and J. W. Lampe, Obesity prevalence in relation to gut microbial environments capable of producing equol or O-desmethylangolensin from the isoflavone daidzein, Eur. J. Clin. Nutr., 2014, 68, 526–530, DOI:10.1038/ejcn.2014.23.
- N. Li, X. Wu, W. Zhuang, L. Xia, Y. Chen, R. Zhao, M. Yi, Q. Wan, L. Du and Y. Zhou, Soy and Isoflavone Consumption and Multiple Health Outcomes: Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies and Randomized Trials in Humans, Mol. Nutr. Food Res., 2020, 64, 1900751, DOI:10.1002/mnfr.201900751.
- W. Zheng, Y. Ma, A. Zhao, T. He, N. Lyu, Z. Pan, G. Mao, Y. Liu, J. Li, P. Wang, J. Wang, B. Zhu and Y. Zhang, Compositional and functional differences in human gut microbiome with respect to equol production and its association with blood lipid level: a cross-sectional study, Gut Pathog., 2019, 11, 20, DOI:10.1186/s13099-019-0297-6.
- N. Ishiwata, M. K. Melby, S. Mizuno and S. Watanabe, New equol supplement for relieving menopausal symptoms: randomized, placebo-controlled trial of Japanese women, Menopause, 2009, 16, 141–148, DOI:10.1097/gme.0b013e31818379fa.
- Y. Tousen, J. Ezaki, Y. Fujii, T. Ueno, M. Nishimuta and Y. Ishimi, Natural S-equol decreases bone resorption in postmenopausal, non-equol-producing Japanese women: a pilot randomized, placebo-controlled trial, Menopause, 2011, 18, 563–574, DOI:10.1097/gme.0b013e3181f85aa7.
- T. Aso, S. Uchiyama, Y. Matsumura, M. Taguchi, M. Nozaki, K. Takamatsu, B. Ishizuka, T. Kubota, H. Mizunuma and H. Ohta, A natural S-equol supplement alleviates hot flushes and other menopausal symptoms in equol nonproducing postmenopausal Japanese women, J. Women's Health, 2012, 21, 92–100, DOI:10.1089/jwh.2011.2753.
- B. H. Jenks, S. Iwashita, Y. Nakagawa, K. Ragland, J. Lee, W. H. Carson, T. Ueno and S. Uchiyama, A pilot study on the effects of S-equol compared to soy isoflavones on menopausal hot flash frequency, J. Women's Health, 2012, 21, 674–682, DOI:10.1089/jwh.2011.3153.
- A. Oyama, T. Ueno, S. Uchiyama, T. Aihara, A. Miyake, S. Kondo and K. Matsunaga, The effects of natural S-equol supplementation on skin aging in postmenopausal women: a pilot randomized placebo-controlled trial, Menopause, 2012, 19, 202–210, DOI:10.1097/gme.0b013e318227427b.
- T. Usui, M. Tochiya, Y. Sasaki, K. Muranaka, H. Yamakage, A. Himeno, A. Shimatsu, A. Inaguma, T. Ueno, S. Uchiyama and N. Satoh-Asahara, Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status, Clin. Endocrinol., 2013, 78, 365–372, DOI:10.1111/j.1365-2265.2012.04400.x.
- S. Hazim, P. J. Curtis, M. Y. Schär, L. M. Ostertag, C. D. Kay, A. M. Minihane and A. Cassidy, Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: a double-blind randomized controlled trial, Am. J. Clin. Nutr., 2016, 103, 694–702, DOI:10.3945/ajcn.115.125690.
- R. Yoshikata, K. Z. Y. Myint and H. Ohta, Effects of Equol Supplement on Bone and Cardiovascular Parameters in Middle-Aged Japanese Women: A Prospective Observational Study, J. Altern. Complement. Med., 2018, 24, 701–708, DOI:10.1089/acm.2018.0050.
- J. W. Daily, B. S. Ko, J. Ryuk, M. Liu, W. Zhang and S. Park, Equol Decreases Hot Flashes in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Clinical Trials, J. Med. Food, 2019, 22, 127–139, DOI:10.1089/jmf.2018.4265.
- B. Mayo, L. Vázquez and A. B. Flórez, Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects, Nutrients, 2019, 11, 2231, DOI:10.3390/nu11092231.
- A. Fatima, M. S. Khan and M. W. Ahmad, Therapeutic Potential of Equol: A Comprehensive Review, Curr. Pharm. Des., 2020, 26, 5837–5843, DOI:10.2174/1381612826999201117122915.
- Z. M. Liu, S. C. Ho, Y. M. Chen, B. Tomlinson, S. Ho, K. To and J. Woo, Effect of whole soy and purified daidzein on ambulatory blood pressure and endothelial function–a 6-month double-blind, randomized controlled trial among Chinese postmenopausal women with prehypertension, Eur. J. Clin. Nutr., 2015, 69, 1161–1168, DOI:10.1038/ejcn.2015.24.
- Z. Liu, B. Chen, S. Li, G. Li, D. Zhang, S. C. Ho, Y. Chen, J. Ma, H. Qi and W. Ling, Effect of whole soy and isoflavones daidzein on bone turnover and inflammatory markers: a 6-month double-blind, randomized controlled trial in Chinese postmenopausal women who are equol producers, Ther. Adv. Endocrinol. Metab., 2020, 11, 2042018820920555, DOI:10.1177/2042018820920555.
- L. S. Y. Zuo, X. Y. Tang, F. Xiong, Y. P. Liu, M. Liu, C. W. Ling, T. Y. Sun, W. Ling, Z. Q. Zhang and Y. M. Chen, Isoflavone biomarkers are inversely associated with atherosclerosis progression in adults: a prospective study, Am. J. Clin. Nutr., 2021, nqab008, DOI:10.1093/ajcn/nqab008.
- X. L. Feng, S. C. Ho, X. X. Zhan, L. S. Y. Zuo, X. F. Mo, X. Zhang, A. Abulimiti, C. Y. Huang and C. X. Zhang, Serum isoflavones and lignans and odds of breast cancer in pre- and postmenopausal Chinese women, Menopause, 2021, 28, 413–422, DOI:10.1097/GME.0000000000001715.
- J. C. Espín, M. Larrosa, M. T. García-Conesa and F. A. Tomás-Barberán, Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far, J. Evidence-Based Complementary Altern. Med., 2013, 2013, 270418, DOI:10.1155/2013/270418.
- J. Djedjibegovic, A. Marjanovic, E. Panieri and L. Saso, Ellagic Acid-Derived Urolithins as Modulators of Oxidative Stress, Oxid. Med. Cell. Longev., 2020, 2020, 5194508, DOI:10.1155/2020/5194508.
- M. Kujawska and J. Jodynis-Liebert, Potential of the ellagic acid-derived gut microbiota metabolite - Urolithin A in gastrointestinal protection, World J. Gastroenterol., 2020, 26, 3170–3181, DOI:10.3748/wjg.v26.i23.3170.
- A. González-Sarrías, M. Á. Núñez-Sánchez, F. A. Tomás-Barberán and J. C. Espín, Neuroprotective Effects of Bioavailable Polyphenol-Derived Metabolites against Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells, J. Agric. Food Chem., 2017, 65, 752–758, DOI:10.1021/acs.jafc.6b04538.
- J. A. Giménez-Bastida, M. Á. Ávila-Gálvez, J. C. Espín and A. González-Sarrías, Conjugated Physiological Resveratrol Metabolites Induce Senescence in Breast Cancer Cells: Role of p53/p21 and p16/Rb Pathways, and ABC Transporters, Mol. Nutr. Food Res., 2019, 63, 1900629, DOI:10.1002/mnfr.201900629.
- J. A. Giménez-Bastida, M. Á. Ávila-Gálvez, J. C. Espín and A. González-Sarrías, The gut microbiota metabolite urolithin A, but not other relevant urolithins, induces p53-dependent cellular senescence in human colon cancer cells, Food Chem. Toxicol., 2020, 139, 111260, DOI:10.1016/j.fct.2020.111260.
- P. A. Andreux, W. Blanco-Bose, D. Ryu, F. Burdet, M. Ibberson, P. Aebischer, J. Auwerx, A. Singh and C. Rinsch, The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans, Nat. Metab., 2019, 1, 595–603, DOI:10.1038/s42255-019-0073-4.
- A. Singh, D. D'Amico, P. A. Andreux, G. Dunngalvin, T. Kern, W. Blanco-Bose, J. Auwerx, P. Aebischer and C. Rinsch, Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population, Eur. J. Clin. Nutr., 2021 DOI:10.1038/s41430-021-00950-1.
- N. P. Seeram, S. M. Henning, Y. Zhang, M. Suchard, Z. Li and D. Heber, Pomegranate Juice Ellagitannin Metabolites Are Present in Human Plasma and Some Persist in Urine for Up to 48 Hours, J. Nutr., 2006, 136, 2481–2485, DOI:10.1093/jn/136.10.2481.
- M. Romo-Vaquero, R. García-Villalba, A. González-Sarrías, D. Beltrán, F. A. Tomás-Barberán, J. C. Espín and M. V. Selma, Interindividual variability in the human metabolism of ellagic acid: Contribution of Gordonibacter to urolithin production, J. Funct. Foods, 2015, 17, 785–791, DOI:10.1016/j.jff.2015.06.040.
- F. A. Tomás-Barberán, M. V. Selma and J. C. Espín, Polyphenols’ Gut Microbiota Metabolites: Bioactives or Biomarkers?, J. Agric. Food Chem., 2018, 66, 3593–3594, DOI:10.1021/acs.jafc.8b00827.
- M. V. Selma, A. González-Sarrías, J. Salas-Salvadó, C. Andrés-Lacueva, C. Alasalvar, A. Örem, F. A. Tomás-Barberán and J. C. Espín, The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: Comparison between normoweight, overweight-obesity and metabolic syndrome, Clin. Nutr., 2018, 37, 897–905, DOI:10.1016/j.clnu.2017.03.012.
- C. Morand and F. A. Tomás-Barberán, Interindividual Variability in Absorption, Distribution, Metabolism, and Excretion of Food Phytochemicals Should Be Reported, J. Agric. Food Chem., 2019, 67, 3843–3844, DOI:10.1021/acs.jafc.9b01175.
- C. Kamrath, M. F. Hartmann, J. Pons-Kühnemann and S. A. Wudy, Urinary GC-MS steroid metabotyping in treated children with congenital adrenal hyperplasia, Metabolism, 2020, 112, 154354, DOI:10.1016/j.metabol.2020.154354.
- P. Perez-Martinez, A. Garcia-Rios, J. Delgado-Lista, F. Perez-Jimenez and J. Lopez-Miranda, Metabolic syndrome: evidences for a personalized nutrition, Mol. Nutr. Food Res., 2012, 56, 67–76, DOI:10.1002/mnfr.201100531.
- A. A. Kolodziejczyk, D. Zheng and E. Elinav, Diet-microbiota interactions and personalized nutrition, Nat. Rev. Microbiol., 2019, 17, 742–753, DOI:10.1038/s41579-019-0256-8.
- E. Hillesheim, M. F. Ryan, E. Gibney, H. M. Roche and L. Brennan, Optimisation of a metabotype approach to deliver targeted dietary advice, Nutr. Metab., 2020, 17, 82, DOI:10.1186/s12986-020-00499-z.
- S. Noerman, M. Kolehmainen and K. Hanhineva, Profiling of Endogenous and Gut Microbial Metabolites to Indicate Metabotype-Specific Dietary Responses: A Systematic Review, Adv. Nutr., 2020, 11, 1237–1254, DOI:10.1093/advances/nmaa031.
- C. L. Frankenfeld, C. Atkinson, W. K. Thomas, A. Gonzalez, T. Jokela, K. Wähälä, S. M. Schwartz, S. S. Li and J. W. Lampe, High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart, Br. J. Nutr., 2005, 94, 873–876, DOI:10.1079/BJN20051565.
- C. Lino, T. Shimoyama, K. Iino, Y. Yokoyama, D. Chinda, H. Sakuraba, S. Fukuda and S. Nakaji, Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production, Nutrition, 2019, 11, 433, DOI:10.3390/nu11020433.
- C. Atkinson, K. M. Newton, E. J. A. Bowles, M. Yong and J. W. Lampe, Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States, Am. J. Clin. Nutr., 2008, 87, 679–687, DOI:10.1093/ajcn/87.3.679.
- L. M. Miller, J. W. Lampe, K. M. Newton, G. Gundersen, S. Fuller, S. D. Reed and C. L. Frankenfeld, Being overweight or obese is associated with harboring a gut microbial community not capable of metabolizing the soy isoflavone daidzein to O-desmethylangolensin in peri- and post-menopausal women, Maturitas, 2017, 99, 37–42, DOI:10.1016/j.maturitas.2017.02.006.
- E. J. Reverri, C. M. Slupsky, D. O. Mishchuk and F. M. Steinberg, Metabolomics reveals differences between three daidzein metabolizing phenotypes in adults with cardiometabolic risk factors, Mol. Nutr. Food Res., 2017, 61, 1600132, DOI:10.1002/mnfr.201600132.
- A. Takahashi, Y. Anzai, N. Tanji, H. Imaizumi, M. Fujita, M. Hayashi, K. Abe and H. Ohira, Association of equol with obesity in postmenopausal women, Menopause, 2021, 28, 807–810, DOI:10.1097/GME.0000000000001761.
- T. Maruo, M. Sakamoto, C. Ito, T. Toda and Y. Benno, Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella, Int. J. Syst. Evol. Microbiol., 2008, 58, 1221–1227, DOI:10.1099/ijs.0.65404-0.
- K. Minamida, K. Ota, M. Nishimukai, M. Tanaka, A. Abe, T. Sone, F. Tomita, H. Hara and K. Asano, Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum, Int. J. Syst. Evol. Microbiol., 2008, 58, 1238–1240, DOI:10.1099/ijs.0.64894-0.
- T. Ueno and S. Uchiyama, Identification of the specific intestinal bacteria capable of metabolising soy isoflavone to equol, Ann. Nutr. Metab., 2002, 45, 114 Search PubMed.
- K. Decroos, S. Vanhemmens, S. Cattoir, N. Boon and W. Verstraete, Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions, Arch. Microbiol., 2005, 183, 45–55, DOI:10.1007/s00203-004-0747-4.
- J. S. Jin, M. Kitahara, M. Sakamoto, M. Hattori and Y. Benno, Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol, Int. J. Syst. Evol. Microbiol., 2010, 60, 1721–1724, DOI:10.1099/ijs.0.016774-0.
- A. Matthies, M. Blaut and A. Braune, Isolation of a human intestinal bacterium capable of daidzein and genistein conversion, Appl. Environ. Microbiol., 2009, 75, 1740–1744, DOI:10.1128/AEM.01795-08.
- L. Vázquez, A. B. Flórez, B. Redruello and B. Mayo, Metabolism of Soy Isoflavones by Intestinal Bacteria: Genome Analysis of an Adlercreutzia Equolifaciens Strain That Does Not Produce Equol, Biomolecules, 2020, 10, 950, DOI:10.3390/biom10060950.
- C. H. Nakatsu, A. Armstrong, A. P. Clavijo, B. R. Martin, S. Barnes and C. M. Weaver, Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption, PLoS One, 2014, 9, e108924, DOI:10.1371/journal.pone.0108924.
- R. Yoshikata, K. Z. Myint, H. Ohta and Y. Ishigaki, Inter-relationship between diet, lifestyle habits, gut microflora, and the equol-producer phenotype: baseline findings from a placebo-controlled intervention trial, Menopause, 2019, 26, 273–285, DOI:10.1097/GME.0000000000001202.
- N. Védrine, J. Mathey, C. Morand, M. Brandolini, M. J. Davicco, L. Guy, C. Rémésy, V. Coxam and C. Manach, One-month exposure to soy isoflavones did not induce the ability to produce equol in postmenopausal women, Eur. J. Clin. Nutr., 2006, 60, 1039–1045, DOI:10.1038/sj.ejcn.1602415.
- Z. M. Liu, S. C. Ho, Y. M. Chen, J. Liu and J. Woo, Cardiovascular risks in relation to daidzein metabolizing phenotypes among Chinese postmenopausal women, PLoS One, 2014, 9, e87861, DOI:10.1371/journal.pone.0087861.
- V. Ahuja, K. Miura, A. Vishnu, A. Fujiyoshi, R. Evans, M. Zaid, N. Miyagawa, T. Hisamatsu, A. Kadota, T. Okamura, H. Ueshima and A. Sekikawa, Significant inverse association of equol-producer status with coronary artery calcification but not dietary isoflavones in healthy Japanese men, Br. J. Nutr., 2017, 117, 260–266, DOI:10.1017/S000711451600458X.
- A. Sekikawa, A. Higashiyama, B. J. Lopresti, M. Ihara, H. Aizenstein, M. Watanabe, Y. Chang, C. Kakuta, Z. Yu, C. Mathis, Y. Kokubo, W. Klunk, O. L. Lopez, L. H. Kuller, Y. Miyamoto and C. Cui, Associations of equol-producing status with white matter lesion and amyloid-beta deposition in cognitively normal elderly Japanese, Alzheimers Dement., 2020, 6, e12089, DOI:10.1002/trc2.12089.
- L. Polimeno, M. Barone, A. Mosca, M. T. Viggiani, F. Joukar, F. Mansour-Ghanaei, S. Mavaddati, A. Daniele, L. Debellis, M. Bilancia, L. Santacroce and A. Di Leo, Soy Metabolism by Gut Microbiota from Patients with Precancerous Intestinal Lesions, Microorganisms, 2020, 8, 469, DOI:10.3390/microorganisms8040469.
- K. Hayashi, H. Yamaguchi, H. Amaoka, T. Takahara, S. Kunisa, N. Tamai, N. Maejima, N. Watanabe, Y. Kobayashi and H. Tanaka, Equol-producing status affects exercise training-induced improvement in arterial compliance in postmenopausal women, J. Appl. Physiol., 2021, 130, 827–835, DOI:10.1152/japplphysiol.00651.2020.
- E. J. Reverri, C. D. LaSalle, A. A. Franke and F. M. Steinberg, Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk, Mol. Nutr. Food Res., 2015, 59, 323–333, DOI:10.1002/mnfr.201400270.
- M. C. Bosland, E. Enk, J. Schmoll, M. J. Schlicht, C. Randolph, R. J. Deaton, H. Xie, A. Zeleniuch-Jacquotte and I. Kato, Soy protein supplementation in men following radical prostatectomy: a 2-year randomized, placebo-controlled clinical trial, Am. J. Clin. Nutr., 2021, 113, 821–831, DOI:10.1093/ajcn/nqaa390.
- F. A. Tomás-Barberán, R. García-Villalba, A. González-Sarrías, M. V. Selma and J. C. Espín, Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status, J. Agric. Food Chem., 2014, 62, 6535–6538, DOI:10.1021/jf5024615.
- M. V. Selma, M. Romo-Vaquero, R. García-Villalba, A. González-Sarrías, F. A. Tomás-Barberán and J. C. Espín, The human gut microbial ecology associated with overweight and obesity determines ellagic acid metabolism, Food Funct., 2016, 7, 1769–1774, 10.1039/c5fo01100k.
- M. V. Selma, D. Beltrán, R. García-Villalba, J. C. Espín and F. A. Tomás-Barberán, Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter, species, Food Funct., 2014, 5, 1779–1784, 10.1039/c4fo00092g.
- D. Beltrán, M. Romo-Vaquero, J. C. Espín, F. A. Tomás-Barberán and M. V. Selma, Ellagibacter isourolithinifaciens gen. nov., sp. nov., a new member of the family Eggerthellaceae, isolated from human gut, Int. J. Syst. Evol. Microbiol., 2018, 68, 1707–1712, DOI:10.1099/ijsem.0.002735.
- M. V. Selma, D. Beltrán, M. C. Luna, M. Romo-Vaquero, R. García-Villalba, A. Mira, J. C. Espín and F. A. Tomás-Barberán, Isolation of Human Intestinal Bacteria Capable of Producing the Bioactive Metabolite Isourolithin A from Ellagic Acid, Front. Microbiol., 2017, 8, 1521, DOI:10.3389/fmicb.2017.01521.
- A. M. Tindall, C. J. McLimans, K. S. Petersen, P. M. Kris-Etherton and R. Lamendella, Walnuts and Vegetable Oils Containing Oleic Acid Differentially Affect the Gut Microbiota and Associations with Cardiovascular Risk Factors: Follow-up of a Randomized, Controlled, Feeding Trial in Adults at Risk for Cardiovascular Disease, J. Nutr., 2020, 150, 806–817, DOI:10.1093/jn/nxz289.
- J. D. Lewis, E. Z. Chen, R. N. Baldassano, A. R. Otley, A. M. Griffiths, D. Lee, K. Bittinger, A. Bailey, E. S. Friedman, C. Hoffmann, L. Albenberg, R. Sinha, C. Compher, E. Gilroy, L. Nessel, A. Grant, C. Chehoud, H. Li, G. D. Wu and F. D. Bushman, Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn's Disease, Cell Host Microbe, 2015, 18, 489–500, DOI:10.1016/j.chom.2015.09.008.
- J. Du, P. Huang, Y. Qian, X. Yang, S. Cui, Y. Lin, C. Gao, P. Zhang, Y. He, Q. Xiao and S. Chen, Fecal and Blood Microbial 16s rRNA Gene Alterations in Chinese Patients with Multiple System Atrophy and Its Subtypes, J. Parkinson's Dis., 2019, 9, 711–721, DOI:10.3233/JPD-191612.
- S. H. Lee, H. S. You, H. G. Kang, S. S. Kang and S. H. Hyun, Association between Altered Blood Parameters and Gut Microbiota after Synbiotic Intake in Healthy, Elderly Korean Women, Nutrients, 2020, 12, 3112, DOI:10.3390/nu12103112.
- I. Martínez, D. J. Perdicaro, A. W. Brown, S. Hammons, T. J. Carden, T. P. Carr, K. M. Eskridge and J. Walter, Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters, Appl. Environ. Microbiol., 2013, 79, 516–524, DOI:10.1128/AEM.03046-12.
- T. Clavel, C. Desmarchelier, D. Haller, P. Gérard, S. Rohn, P. Lepage and H. Daniel, Intestinal microbiota in metabolic diseases: from bacterial community structure and functions to species of pathophysiological relevance, Gut Microbes, 2014, 5, 544–551, DOI:10.4161/gmic.29331.
- O. Koren, J. K. Goodrich, T. C. Cullender, A. Spor, K. Laitinen, H. K. Bäckhed, A. González, J. J. Werner, L. T. Angenent, R. Knight, F. Bäckhed, E. Isolauri, S. Salminen and R. E. Ley, Host remodeling of the gut microbiome and metabolic changes during pregnancy, Cell, 2012, 150, 470–480, DOI:10.1016/j.cell.2012.07.008.
- A. Cortés-Martín, G. Colmenarejo, M. V. Selma and J.C Espín, Genetic Polymorphisms, Mediterranean Diet and Microbiota-Associated Urolithin Metabotypes can Predict Obesity in Childhood-Adolescence, Sci. Rep., 2020, 10, 7850, DOI:10.1038/s41598-020-64833-4.
- I. García-Mantrana, M. Calatayud, M. Romo-Vaquero, J. C. Espín, M. V. Selma and M. C. Collado, Urolithin Metabotypes Can Determine the Modulation of Gut Microbiota in Healthy Individuals by Tracking Walnuts Consumption over Three Days, Nutrients, 2019, 11, 2483, DOI:10.3390/nu11102483.
- R. Puupponen-Pimiä, T. Seppänen-Laakso, M. Kankainen, J. Maukonen, R. Törrönen, M. Kolehmainen, T. Leppänen, E. Moilanen, L. Nohynek, A. M. Aura, K. Poutanen, F. A. Tómas-Barberán, J. C. Espín and K. M. Oksman-Caldentey, Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome, Mol. Nutr. Food Res., 2013, 57, 2258–2263, DOI:10.1002/mnfr.201300280.
- A. Cortés-Martín, C. E. Iglesias-Aguirre, A. Meoro, M. V. Selma and J. C. Espín, There is No Distinctive Gut Microbiota Signature in the Metabolic Syndrome: Contribution of Cardiovascular Disease Risk Factors and Associated Medication, Microorganisms, 2020, 8, 416, DOI:10.3390/microorganisms8030416.
- A. M. Hutchins, J. L. Slavin and J. W. Lampe, Urinary isoflavonoid phytoestrogen and lignan excretion after consumption of fermented and unfermented soy products, J. Am. Diet Assoc., 1995, 95, 545–551, DOI:10.1016/S0002-8223(95)00149-2.
- B. Cerdá, C. Soto, M. D. Albaladejo, P. Martínez, F. Sánchez-Gascón, F. A. Tomás-Barberán and J. C. Espín, Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial, Eur. J. Clin. Nutr., 2006, 60, 245–253, DOI:10.1038/sj.ejcn.1602309.
- A. Senizza, G. Rocchetti, J. I. Mosele, V. Patrone, M. L. Callegari, L. Morelli and L. Lucini, Lignans and Gut Microbiota: An Interplay Revealing Potential Health Implications, Molecules, 2020, 25, 5709, DOI:10.3390/molecules25235709.
- M. Tomás-Navarro, F. Vallejo, F. Borrego and F. A. Tomás-Barberán, Encapsulation and micronization effectively improve orange beverage flavanone bioavailability in humans, J. Agric. Food Chem., 2014, 62, 9458–9462, DOI:10.1021/jf502933v.
- C. Heiss, P. Kleinbongard, A. Dejam, S. Perré, H. Schroeter, H. Sies and M. Kelm, Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers, J. Am. Coll. Cardiol., 2005, 46, 1276–1283, DOI:10.1016/j.jacc.2005.06.055.
- J. M. Hodgson, I. B. Puddey, V. Burke and K. D. Croft, Is reversal of endothelial dysfunction by tea related to flavonoid metabolism?, Br. J. Nutr., 2006, 95, 14–17, DOI:10.1079/BJN20051621.
- H. Schroeter, C. Heiss, J. Balzer, P. Kleinbongard, C. L. Keen, N. K. Hollenberg, H. Sies, C. Kwik-Uribe, H. H. Schmitz and M. Kelm, (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 1024–1029, DOI:10.1073/pnas.0510168103.
- L. Baum, S. K. K. Cheung, V. C. T. Mok, L. C. W. Lam, V. P. Y. Leung, E. Hui, C. C. Y. Ng, M. Chow, P. C. Ho, S. Lam, J. Woo, H. F. K. Chiu, W. Goggins, B. Zee, A. Wong, H. Mok, W. K. F. Cheng, C. Fong, J. S. W. Lee, M. H. Chan, S. S. L. Szeto, V. W. C. Lui, J. Tsoh, T. C. Y. Kwok, I. H. S. Chan and C. W. K. Lam, Curcumin effects on blood lipid profile in a 6-month human study, Pharmacol. Res., 2007, 56, 509–514, DOI:10.1016/j.phrs.2007.09.013.
- J. Ruano, J. López-Miranda, R. de la Torre, J. Delgado-Lista, J. Fernández, J. Caballero, M. I. Covas, Y. Jiménez, P. Pérez-Martínez, C. Marín, F. Fuentes and F. Pérez-Jiménez, Intake of phenol-rich virgin olive oil improves the postprandial prothrombotic profile in hypercholesterolemic patients, Am. J. Clin. Nutr., 2007, 86, 341–346, DOI:10.1093/ajcn/86.2.341.
- A. M. Kountouri, A. Mylona, A. C. Kaliora and N. K. Andrikopoulos, Bioavailability of the phenolic compounds of the fruits (drupes) of Olea europaea (olives): impact on plasma antioxidant status in humans, Phytomedicine, 2007, 14, 659–667, DOI:10.1016/j.phymed.2007.06.001.
- W. M. Loke, J. M. Hodgson, J. M. Proudfoot, A. J. McKinley, I. B. Puddey and K. D. Croft, Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men, Am. J. Clin. Nutr., 2008, 88, 1018–1025, DOI:10.1093/AJCN/88.4.1018.
- W. M. Loke, A. M. Jenner, J. M. Proudfoot, A. J. McKinley, J. M. Hodgson, B. Halliwell and K. D. Croft, A metabolite profiling approach to identify biomarkers of flavonoid intake in humans, J. Nutr., 2009, 139, 2309–2314, DOI:10.3945/jn.109.113613.
- K. de la Torre-Carbot, J. L. Chávez-Servín, O. Jaúregui, A. I. Castellote, R. M. Lamuela-Raventós, T. Nurmi, H. E. Poulsen, A. V. Gaddi, J. Kaikkonen, H. F. Zunft, H. Kiesewetter, M. Fitó, M. I. Covas and M. C. López-Sabater, Elevated circulating LDL phenol levels in men who consumed virgin rather than refined olive oil are associated with less oxidation of plasma LDL, J. Nutr., 2010, 140, 501–508, DOI:10.3945/jn.109.112912.
- C. Morand, C. Dubray, D. Milenkovic, D. Lioger, J. F. Martin, A. Scalbert and A. Mazur, Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers, Am. J. Clin. Nutr., 2011, 93, 73–80, DOI:10.3945/ajcn.110.004945.
- K. D. Monahan, R. P. Feehan, A. R. Kunselman, A. G. Preston, D. L. Miller and M. E. J. Lott, Dose-dependent increases in flow-mediated dilation following acute cocoa ingestion in healthy older adults, J. Appl. Physiol., 2011, 111, 1568–1574, DOI:10.1152/japplphysiol.00865.2011.
- A. Rodríguez-Mateos, C. Rendeiro, T. Bergillos-Meca, S. Tabatabaee, T. W. George, C. Heiss and J. P. Spencer, Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity, Am. J. Clin. Nutr., 2013, 98, 1179–1191, DOI:10.3945/ajcn.113.066639.
- A. Pérez, S. González-Manzano, R. Jiménez, R. Pérez-Abud, J. M. Haro, A. Osuna, C. Santos-Buelga, J. Duarte and F. Pérez-Vizcaíno, The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: correlation with beta-glucuronidase activity, Pharmacol. Res., 2014, 89, 11–18, DOI:10.1016/j.phrs.2014.07.005.
- P. Bialasiewicz, A. Prymont-Przyminska, A. Zwolinska, A. Sarniak, A. Wlodarczyk, M. Krol, J. Glusac, P. Nowak, J. Markowski, K. P. Rutkowski and D. Nowak, Addition of strawberries to the usual diet decreases resting chemiluminescence of fasting blood in healthy subjects-possible health-promoting effect of these fruits consumption, J. Am. Coll. Nutr., 2014, 33, 274–287, DOI:10.1080/07315724.2013.870502.
- O. D. Rangel-Huerta, C. M. Aguilera, M. V. Martin, M. J. Soto, M. C. Rico, F. Vallejo, F. A. Tomás-Barberán, A. J. Perez-de-la-Cruz, A. Gil and M. D. Mesa, Normal or High Polyphenol Concentration in Orange Juice Affects Antioxidant Activity, Blood Pressure, and Body Weight in Obese or Overweight Adults, J. Nutr., 2015, 145, 1808–1816, DOI:10.3945/jn.115.213660.
- X. Mora-Cubillos, S. Tulipani, M. Garcia-Aloy, M. Bulló, F. J. Tinahones and C. Andres-Lacueva, Plasma metabolomic biomarkers of mixed nuts exposure inversely correlate with severity of metabolic syndrome, Mol. Nutr. Food Res., 2015, 59, 2480–2490, DOI:10.1002/mnfr.201500549.
- R. Sansone, A. Rodríguez-Mateos, J. Heuel, D. Falk, D. Schuler, R. Wagstaff, G. G. C. Kuhnle, J. P. E. Spencer, H. Schroeter, M. W. Merx, M. Kelm, C. Heiss and Flaviola Consortium, European Union 7th Framework Program, Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study, Br. J. Nutr., 2015, 114, 1246–1255, DOI:10.1017/S0007114515002822.
- A. Rodríguez-Mateos, R. P. Feliciano, A. Boeres, T. Weber, C. N. Dos Santos, M. R. Ventura and C. Heiss, Cranberry (poly)phenol metabolites correlate with improvements in vascular function: a double-blind, randomized, controlled, dose-response, crossover study, Mol. Nutr. Food Res., 2016, 60, 2130–2140, DOI:10.1002/mnfr.201600250.
- S. Timmers, M. de Ligt, E. Phielix, T. van de Weijer, J. Hansen, E. Moonen-Kornips, G. Schaart, I. Kunz, M. K. C. Hesselink, V. B. Schrauwen-Hinderling and P. Schrauwen, Resveratrol as Add-on Therapy in Subjects With Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial, Diabetes Care, 2016, 39, 2211–2217, DOI:10.2337/dc16-0499.
- R. H. X. Wong, D. Raederstorff and P. R. C. Howe, Acute Resveratrol Consumption Improves Neurovascular Coupling Capacity in Adults with Type 2 Diabetes Mellitus, Nutrients, 2016, 8, 425, DOI:10.3390/nu8070425.
- C. E. Mills, A. Flury, C. Marmet, L. Poquet, S. F. Rimoldi, C. Sartori, E. Rexhaj, R. Brenner, Y. Allemann, D. Zimmermann, G. R. Gibson, D. S. Mottram, M. J. Oruna-Concha, L. Actis-Goretta and J. P. E. Spencer, Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: two randomized, controlled, crossover intervention trials, Clin. Nutr., 2017, 36, 1520–1529, DOI:10.1016/j.clnu.2016.11.013.
- L. Xie, T. Vance, B. Kim, S. G. Lee, C. Caceres, Y. Wang, P. A. Hubert, J. Y. Lee, O. K. Chun and B. W. Bolling, Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: a randomized controlled trial, Nutr. Res., 2017, 37, 67–77, DOI:10.1016/j.nutres.2016.12.007.
- M. Montagnana, E. Danese, D. Angelino, P. Mena, A. Rosi, M. Benati, M. Gelati, G. L. Salvagno, E. J. Favaloro, D. Del Rio and G. Lippi, Dark chocolate modulates platelet function with a mechanism mediated by flavan-3-ol metabolites, Medicine, 2018, 97, e13432, DOI:10.1097/MD.0000000000013432.
- G. Istas, R. P. Feliciano, T. Weber, R. García-Villalba, F. A. Tomás-Barberán, C. Heiss and A. Rodríguez-Mateos, Plasma urolithin metabolites correlate with improvements in endothelial function after red raspberry consumption: A double-blind randomized controlled trial, Arch. Biochem. Biophys., 2018, 651, 43–51, DOI:10.1016/j.abb.2018.05.016.
- A. Rodríguez-Mateos, G. Istas, L. Boschek, R. P. Feliciano, C. E. Mills, C. Boby, S. Gomez-Alonso, D. Milenkovic and C. Heiss, Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights From Randomized Controlled Trials, Metabolomics, and Nutrigenomics, J. Gerontol., Ser. A, 2019, 74, 967–976, DOI:10.1093/gerona/glz047.
- G. Istas, E. Wood, M. Le Sayec, C. Rawlings, J. Yoon, V. Dandavate, D. Cera, S. Rampelli, A. Costabile, E. Fromentin and A. Rodríguez-Mateos, Effects of aronia berry (poly)phenols on vascular function and gut microbiota: a double-blind randomized controlled trial in adult men, Am. J. Clin. Nutr., 2019, 110, 316–329, DOI:10.1093/ajcn/nqz075.
- G. Costabile, M. Vitale, D. Luongo, D. Naviglio, C. Vetrani, P. Ciciola, A. Tura, F. Castello, P. Mena, D. Del Rio, B. Capaldo, A. A. Rivellese, G. Riccardi and R. Giacco, Grape pomace polyphenols improve insulin response to a standard meal in healthy individuals: A pilot study, Clin. Nutr., 2019, 38, 2727–2734, DOI:10.1016/j.clnu.2018.11.028.
- L. Li, G. K. Lyall, J. A. Martinez-Blazquez, F. Vallejo, F. A. Tomás-Barberán, K. M. Birch and C. Boesch, Blood Orange Juice Consumption Increases Flow-Mediated Dilation in Adults with Overweight and Obesity: A Randomized Controlled Trial, J. Nutr., 2020, 150, 2287–2294, DOI:10.1093/jn/nxaa158.
- M. E. Widlansky, S. J. Duffy, N. M. Hamburg, N. Gokce, B. A. Warden, S. Wiseman, J. F. Keaney, B. Frei and J. A. Vita, Effects of black tea consumption on plasma catechins and markers of oxidative stress and inflammation in patients with coronary artery disease, Free Radical Biol. Med., 2005, 38, 499–506, DOI:10.1016/j.freeradbiomed.2004.11.013.
- M. E. Widlansky, N. M. Hamburg, E. Anter, M. Holbrook, D. F. Kahn, J. G. Elliott, J. F. Keaney and J. A. Vita, Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease, J. Am. Coll. Nutr., 2007, 26, 95–102, DOI:10.1080/07315724.2007.10719590.
- S. Egert, A. Bosy-Westphal, J. Seiberl, C. Kürbitz, U. Settler, S. Plachta-Danielzik, A. E. Wagner, J. Frank, J. Schrezenmeir, G. Rimbach, S. Wolffram and M. J. Müller, Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study, Br. J. Nutr., 2009, 102, 1065–1074, DOI:10.1017/S0007114509359127.
- P. J. Curtis, J. Potter, P. A. Kroon, P. Wilson, K. Dhatariya, M. Sampson and A. Cassidy, Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: a double-blind randomized controlled trial, Am. J. Clin. Nutr., 2013, 97, 936–942, DOI:10.3945/ajcn.112.043745.
- M. A. Nuñez-Sánchez, A. Dávalos, A. González-Sarrías, P. Casas-Agustench, F. Visioli, T. Monedero-Saiz, N. V. García-Talavera, M. B. Gómez-Sánchez, C. Sánchez-Álvarez, A. M. García-Albert, F. J. Rodríguez-Gil, M. Ruiz-Marín, F. A. Pastor-Quirante, F. Martínez-Díaz, F. A. Tomás-Barberán, M. T. García-Conesa and J. C. Espín, MicroRNAs expression in normal and malignant colon tissues as biomarkers of colorectal cancer and in response to pomegranate extracts consumption: Critical issues to discern between modulatory effects and potential artefacts, Mol. Nutr. Food Res., 2015, 59, 1973–1986, DOI:10.1002/mnfr.201500357.
- M. Lorenz, F. Rauhut, C. Hofer, S. Gwosc, E. Müller, D. Praeger, B. F. Zimmermann, K. D. Wernecke, G. Baumann, K. Stangl and V. Stangl, Tea-induced improvement of endothelial function in humans: No role for epigallocatechin gallate (EGCG), Sci. Rep., 2017, 7, 2279, DOI:10.1038/s41598-017-02384-x.
| This journal is © The Royal Society of Chemistry 2021 |
