Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of cranberry juice deacidification on its antibacterial activity against periodontal pathogens and its anti-inflammatory properties in an oral epithelial cell model
Geneviève
Pellerin
ab,
Laurent
Bazinet
 ab and
Daniel
Grenier
ab and
Daniel
Grenier
 *c
*c
aInstitute of Nutrition and Functional Foods (INAF) and Department of Food Sciences, Université Laval, Quebec City, QC, Canada G1V 0A6
bLaboratoire de Transformation Alimentaire et Procédés Électro-Membranaires (LTAPEM, Laboratory of Food Processing and Electromembrane Processes), Université Laval, Quebec City, QC, Canada G1V 0A6. E-mail: Laurent.Bazinet@fsaa.ulaval.ca
cOral Ecology Research Group, Faculty of Dentistry, Université Laval, Quebec City, QC, Canada G1V 0A6. E-mail: Daniel.Grenier@greb.ulaval.ca
First published on 15th September 2021
Abstract
Cranberries are widely recognized as a functional food that can promote oral health. However, the high concentration of organic acids in cranberry juice can cause tooth enamel erosion. Electrodialysis with bipolar membrane (EDBM) is a process used for the deacidification of cranberry juice. The present study investigated whether the removal of organic acids (0%, 19%, 42%, 60%, and 79%) from cranberry juice by EDBM affects its antibacterial activity against major periodontopathogens as well as its anti-inflammatory properties in an oral epithelial cell model. A deacidification rate ≥60% attenuated the bactericidal effect against planktonic and biofilm-embedded Aggregatibacter actinomycetemcomitans but had no impact on Porphyromonas gingivalis and Fusobacterium nucleatum. Cranberry juice increased the adherence of A. actinomycetemcomitans and P. gingivalis to oral epithelial cells, but reduced the adherence of F. nucleatum by half regardless of the deacidification rate. F. nucleatum produced more hydrogen sulfide when it was exposed to deacidified cranberry juice with a deacidification rate ≥42% compared to the raw beverage. Interestingly, the removal of organic acids from cranberry juice lowered the cytotoxicity of the beverage for oral epithelial cells. Deacidification attenuated the anti-inflammatory effect of cranberry juice in an in vitro oral epithelial cell model. The secretion of IL-6 by lipopolysaccharide (LPS)-stimulated oral epithelial cells exposed to cranberry juice increased proportionally with the deacidification rate. No such effect was observed with respect to the production of IL-8. This study provided evidence that organic acids, just like phenolic compounds, might contribute to the health benefits of cranberry juice against periodontitis.
1. Introduction
Although largely preventable, severe forms of periodontal diseases affect 5–15% of the population worldwide.1 Periodontitis is an affliction of the oral cavity characterized by gingival inflammation, destruction of the periodontal ligament, alveolar bone resorption, and, if left untreated, tooth loss.2 The recently reported strong association with cardiovascular diseases, diabetes, colorectal cancer, and Alzheimer's disease makes periodontitis a major health concern.3,4Periodontitis refers to two main forms (chronic and aggressive) of periodontal disease, which have distinct etiologies, clinical manifestations, and evolutions. Chronic periodontitis progresses slowly, with the accumulation of a dental biofilm in the subgingival region that mainly contains anaerobic Gram-negative bacteria.2,5Fusobacterium nucleatum, an oxygen-reducing pathogen, marks the transition from a healthy streptococci-dominated microbiota to an anaerobic pathogenic biofilm by bridging the two bacterial communities.6 This Gram-negative bacterium harbors a large array of adhesins that allow it to adhere to host tissue and to coaggregate with the pathogens responsible for destructive periodontitis.7,8 As a keystone pathogen in chronic periodontitis, Porphyromonas gingivalis is proficient at adhering to and invading oral epithelial cells as well as evading the host immune response.9 Aggressive periodontitis differs slightly from the chronic form in that it is associated with the presence of low amounts of dental plaque and a much faster rate of progression, and appears to rely on a number of genetic factors.2,5 Many studies point to Aggregatibacter actinomycetemcomitans as the key etiological agent of aggressive periodontitis.10
Irreversible damage to the periodontium involves the induction of an inflammatory response following the interaction between periodontopathogens and host mucosal cells.11 Among others, F. nucleatum, P. gingivalis, and A. actinomycetemcomitans trigger the production of pro-inflammatory cytokines by oral epithelial cells and fibroblasts.6,12–14 The over-production of IL-6, one of the main modulators of chronic immune defenses, leads to the disruption of bone homeostasis by stimulating osteoclastic activity, while IL-8 acts as a chemoattractant for leukocytes and neutrophils, which are prominent producers of matrix metalloproteinases (MMP) that contribute to soft and hard tissue breakdown.15 In addition, F. nucleatum and P. gingivalis metabolize sulfur-containing amino acids into volatile sulfur compounds (VSC) that accumulate in the oral cavity, causing halitosis and stimulating the production of IL-8 by gingival epithelial cells, thus promoting tissue destruction.16,17
Cranberry (Vaccinium macrocarpon) shows strong potential for the prevention of oral diseases due to its high polyphenol content, including A-type linkage proanthocyanidins (PAC).18,19 Phenolic extracts and by-products of this North American berry reduce the adherence of bacteria to inorganic surfaces and epithelial cells.20,21 They also interfere with bacterial co-aggregation and biofilm formation by periodontal pathogens.20–22 Cranberry polyphenols are also immunomodulatory compounds that attenuate the production of pro-inflammatory cytokines and MMP by mucosal cells.23,24 However, studies investigating high cranberry juice (CJ) intakes have shown that CJ can cause gastrointestinal discomfort among study participants, with up to 25% reporting adverse effects.25,26 These troubles have been ascribed to the high titratable acidity of raw CJ and, more precisely, to its citric acid (CA) content.27 In addition, the combination of high titratable acidity and low pH of CJ typifies an erosive beverage that can accelerate tooth enamel demineralization.28 Nevertheless, organic acids, in their non-dissociated form, may play a role in the prevention of infections since they exhibit antimicrobial activity against a broad array of periodontal pathogens.29,30
Recent studies have focused on the optimization of electrodialysis with bipolar membrane (EDBM) to remove organic acids from raw CJ in a sustainable way.31,32 For example, Serre et al.32 obtained a deacidification rate (DR) of 80% for CJ in a short period of time (6 h). EDBM has been successfully tested at a semi-industrial scale by Faucher et al.33 The process selectively removes citrate and malate from CJ, as well as quinate when the DR exceeds 40%, with no significant loss in total phenolic compounds, PAC, or anthocyanins.31,32 CJ deacidified by this process is poor in CA and, as such, preserves the integrity of the intestinal barrier in vitro, as recently reported by Serre et al.27 and Renaud et al.34 Organic anions removed from CJ can be recovered for use as preservatives in the food industry, making the deacidification of CJ by EDBM part of a circular economy.31
The aim of the present study was to determine how the DR of CJ achieved by EDBM impacts the potential benefits of the beverage with regard to the prevention of periodontal disease. The specific objectives of this study thus were to (1) investigate the antimicrobial activities of raw and deacidified CJs against A. actinomycetemcomitans, P. gingivalis, and F. nucleatum, and (2) assess how DR affects the anti-inflammatory properties of CJ.
2. Materials and methods
2.1. Cranberry juice
Pasteurized and clarified CJ was provided by Fruit d'Or (Plessisville, Quebec, Canada). The CJ was kept frozen at −30 °C, and was thawed at 4 °C prior to deacidification.2.1.2.1. Titratable acidity. The titratable acidity of the raw and deacidified CJs was determined as described in AOAC method 942.15.35 Briefly, 4 mL of CJ was mixed with 40 mL of degassed distilled water and was then titrated with 0.1 M NaOH until a pH of 8.2 was reached. Titratable acidity was expressed as g L−1 of citric acid monohydrate equivalents.
2.1.2.2. Organic acid content. Organic acids were extracted from the raw and deacidified CJs using C18-SPE cartridges (non endcapped 6 mL, 500 mg; Silicycle, Quebec City, QC, Canada). The cartridges were conditioned with 5 mL of methanol and were then rinsed with 5 mL of distilled water followed by 10 mL of a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile
1 acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solution. The cartridges were vacuum dried, and 10 mL of each CJ sample was passed through the cartridges. The concentrations of quinic acid (QA), citric acid (CA), and malic acid (MA) were determined by high-performance liquid chromatography (HPLC) analysis as described in AOAC method 986.13.36 Samples (10 μL) were injected on a Synergi Hydro-RP80A column (250 × 4.6 mm; Phenomenex, Torrance, CA, USA) at room temperature using a KH2PO4 solution (0.2 M; pH 2.4) as an isocratic mobile phase. An Agilent 1100 series HPLC system equipped with a UV detector (λ = 214 nm) was used to separate and detect the organic acids. Calibration curves and the retention times of authentic quinic, citric, and malic acid standards (Sigma Aldrich, Saint-Louis, MO, USA) were used to quantify and identify the organic acids.
water solution. The cartridges were vacuum dried, and 10 mL of each CJ sample was passed through the cartridges. The concentrations of quinic acid (QA), citric acid (CA), and malic acid (MA) were determined by high-performance liquid chromatography (HPLC) analysis as described in AOAC method 986.13.36 Samples (10 μL) were injected on a Synergi Hydro-RP80A column (250 × 4.6 mm; Phenomenex, Torrance, CA, USA) at room temperature using a KH2PO4 solution (0.2 M; pH 2.4) as an isocratic mobile phase. An Agilent 1100 series HPLC system equipped with a UV detector (λ = 214 nm) was used to separate and detect the organic acids. Calibration curves and the retention times of authentic quinic, citric, and malic acid standards (Sigma Aldrich, Saint-Louis, MO, USA) were used to quantify and identify the organic acids.
2.1.2.3. Anthocyanin content. The anthocyanin profile of the raw and deacidified CJs was determined as described by Wu and Prior.37 The CJ samples were passed through 0.45 μm pore size nylon filters, and 20 μL was injected on a Zorbax SB-C18 5 μm column (250 × 4.6 mm, Agilent, Santa Clara, CA, USA) at room temperature. Anthocyanins were eluted with 1 mL min−1 of two mobile phases (solvent A: 95% water/5% formic acid. Solvent B: 100% methanol). An Agilent 1100 series system equipped with a diode array detector (wavelength set at 520 nm) was used to quantify the anthocyanin content. Results are expressed as mg L−1 of cyanidin-3-glucoside equivalents.
2.1.2.4. Proanthocyanidin content. The PAC profile was determined as described by Khanal et al.38 The raw and deacidified CJ samples were passed through 0.45 μm nylon filters. An Agilent 1260 series HPLC system equipped with a fluorescence detector (emission wavelength: 321 nm, excitation wavelength: 230 nm) was used. Samples (5 μL) were injected on a Nomura chemical Develosil 100 Diol-5 column (250 × 4.6 mm; Phenomenex, Torrance, CA, USA) at 35 °C. The PAC were eluted based on their degree of polymerization using 0.8 mL min−1 of two solvents (solvent A: 98% acetonitrile/2% acetic acid. Solvent B: 95% methanol/3% water/2% acetic acid). The PAC were quantified using an epicatechin calibration curve with a correction factor to convert the different response factors of monomeric to polymeric PAC. Results are expressed as mg L−1 of epicatechin equivalents.
2.1.2.5. Total phenolic compounds. The concentration of total phenolic compounds was measured using the microscale Folin–Ciocalteu assay.39 Absorbance was measured using an xMark Microplate spectrophotometer (Bio-Rad Laboratories Inc., Mississauga, ON, Canada) with the wavelength set at 765 nm. Results are expressed as mg L−1 of gallic acid equivalents.
2.2. Bacteria, growth conditions, and lipopolysaccharide preparation
The periodontopathogenic species Aggregatibacter actinomycetemcomitans ATCC 29522, Porphyromonas gingivalis ATCC 33277, and Fusobacterium nucleatum ATCC 25586 were included in the study. A. actinomycetemcomitans was grown in Trypticase Soy Broth (Becton Dickinson and Co., Sparks, MD, USA) supplemented with 0.6% (w/v) yeast extract, 0.4% (w/v) sodium bicarbonate, and 0.8% (w/v) glucose (AAGM). F. nucleatum and P. gingivalis were grown in Todd-Hewitt Broth (Becton Dickinson and Co.) supplemented with 0.001% (w/v) hemin and 0.0001% (w/v) vitamin K (THB-HK). All the bacteria were grown in an anaerobic chamber (80% N2, 10% CO2, 10% H2) at 37 °C. A. actinomycetemcomitans lipopolysaccharide (LPS) was prepared using the protocol described by Darveau and Hancock,40 and was stored at −20 °C.2.3. Bactericidal activity against planktonic bacteria
The bactericidal activity of the raw and deacidified CJs against planktonic A. actinomycetemcomitans and F. nucleatum was assessed using a direct contact test similar to the NF EN 1040 standard for disinfectants and antiseptics.41 Contact times of 1 min and 15 min were chosen to simulate juice consumption. Briefly, 100 μL of a bacterial suspension (108 CFU mL−1) prepared from an overnight culture was added to 900 μL of CJ samples that had been previously incubated in an anaerobic chamber for 1 h. Chlorhexidine (CHX; 0.12% [w/v]) and dilution buffer (0.85% [w/v] NaCl and 0.01% [w/v] tryptone; pH 7.0) were used as positive and negative controls, respectively. After 0 min (initial count), 1 min, and 15 min, 100 μL samples were collected, and 10-fold serial dilutions to 10−7 were made in 900 μL of dilution buffer. A 100 μL volume of each serial dilution (10−3 to 10−7) was plated in triplicate on either AAGM agar (A. actinomycetemcomitans) or THB-HK agar (F. nucleatum). Following a 48 h incubation in an anaerobic chamber at 37 °C, colony-forming units (CFU) were counted. Only plates with between 25 and 250 CFU were taken into account to determine bacterial concentrations. The experiment was repeated in triplicate to ensure repeatability.2.4. Bactericidal activity against biofilm-embedded bacteria
The viability of biofilm-embedded bacteria following contact with the CJs was measured using a FilmTracer LIVE/DEAD Biofilm Viability kit (Life Technologies Corporation, Inc., Eugene, OR, USA) according to the manufacturer's protocol, with slight modifications. Briefly, 24 h preformed mono-species biofilms (A. actinomycetemcomitans, P. gingivalis, F. nucleatum) in 96-well clear bottom black wall microplates (Greiner Bio-One North America, Monroe, NC, USA) were incubated for 1 min or 15 min with 100 μL of raw or deacidified CJ that had been previously incubated in an anaerobic chamber for 1 h. The CJ was removed by aspiration, and the biofilms were washed once with Hanks’ Balanced Salt Solution (HBSS; pH 7.2; Life Technologies Inc., Burlington, ON, Canada). Reagent solution was prepared by diluting 3 μL mL−1 of SYTO 9 green (3.34 mM) and 3 μL mL−1 of propidium iodide (PI; 20 mM) in HBSS, and 50 μL of the reagent solution was added to each well. The microplate was incubated at room temperature for 30 min in the dark. The stained biofilms were then washed twice with distilled water, and 100 μL of distilled water was added to each well. Relative fluorescence units (Ex: 485/Em: 528 nm for SYTO 9 green, and Ex: 485/Em: 590 nm for PI) were recorded using a Synergy 2 microplate reader (BioTek Instruments, Winooski, VT, USA). The % viability of biofilm-embedded bacteria was calculated from the fluorescence units (FU) using the following equation: 100 × FUSYTO 9/(FUSYTO 9 + FUPI). A 100% value was attributed to the negative control (phosphate-buffered saline [PBS; pH 7.2]). CHX was used as a positive control. The assays were performed in triplicate in three independent experiments.2.5. Bacterial adherence to oral epithelial cells
A previously described protocol was used to determine the impact of CJ deacidification on bacterial adherence to epithelial cells.42 The immortalized human oral epithelial cell line GMSM-K, which was previously characterized by Gilchrist et al.,43 was grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 4.5 g L−1 of glucose, L-glutamine, and sodium pyruvate (Corning, Inc., Corning, NY, USA), 10% heat-inactivated fetal bovine serum (FBS) (VWR International, Radnor, PA, USA), 100 μg mL−1 of penicillin G/streptomycin, and 2.5 μg mL−1 of amphotericin B. The GMSM-K cells (1.5 × 106 cells per mL) were seeded in sterile 96-well clear bottom black microplates and were incubated for 24 h at 37 °C in a 5% CO2 atmosphere. Each well was then pretreated with 100 μL of raw or deacidified CJ for 5 min, after which the CJ was removed by aspiration. Cells from a 24 h bacterial culture were labeled with fluorescein isothiocyanate (FITC) (Sigma-Aldrich Canada, Oakville, ON, Canada). Briefly, the bacterial culture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min. The pellet was washed once and was then resuspended in 10 mL of fresh sodium bicarbonate buffer (0.5 M, pH 8.2) containing 0.03 mg mL−1 of FITC. The bacterial suspension was incubated for 30 min at 37 °C in the dark under constant agitation. The bacteria were then washed three times with PBS and were resuspended in antibiotic-free DMEM. A volume of 100 μL of FITC-labeled bacterial culture was added to the wells at a multiplicity of infection (MOI) of 103. Following a 4 h incubation in the dark at 37 °C in a 5% CO2 atmosphere, unbound bacteria were removed by aspiration, and the cells were washed twice with PBS. Relative FU were measured using a Synergy 2 microplate reader with the excitation and emission wavelengths set at 485 nm and 528 nm, respectively. Wells with no bacteria served as controls to measure basal autofluorescence. Antibiotic-free DMEM served as a negative control.
000g for 5 min. The pellet was washed once and was then resuspended in 10 mL of fresh sodium bicarbonate buffer (0.5 M, pH 8.2) containing 0.03 mg mL−1 of FITC. The bacterial suspension was incubated for 30 min at 37 °C in the dark under constant agitation. The bacteria were then washed three times with PBS and were resuspended in antibiotic-free DMEM. A volume of 100 μL of FITC-labeled bacterial culture was added to the wells at a multiplicity of infection (MOI) of 103. Following a 4 h incubation in the dark at 37 °C in a 5% CO2 atmosphere, unbound bacteria were removed by aspiration, and the cells were washed twice with PBS. Relative FU were measured using a Synergy 2 microplate reader with the excitation and emission wavelengths set at 485 nm and 528 nm, respectively. Wells with no bacteria served as controls to measure basal autofluorescence. Antibiotic-free DMEM served as a negative control.
2.6. Hydrogen sulfide production
The enzymatic production of hydrogen sulfide (H2S) using L-cysteine as a substrate was assessed by monitoring the precipitation of bismuth sulfide according to the procedure described by Yoshida et al.,44 with slight modifications. Biofilms (24 h) were preformed as described above in 96-well tissue culture microplates (Corning, Inc.). The CJ samples were left in an anaerobic chamber at room temperature for 1 h prior to the assay in order to remove oxygen from the samples. The biofilms were then pretreated with 100 μL of CJ for 1 min. The CJ was removed by aspiration, and 200 μL of degassed reaction mixture consisting of 400 mM triethanolamine–HCl, 20 μM pyridoxal 5′-phosphate, 10 mM bismuth trichloride, 20 mM L-cysteine, and 20 mM EDTA (pH 8.0) was added to each well. The microplate was sealed with a plastic sheet and was incubated at 37 °C in an xMark Microplate spectrophotometer. Absorbance was measured at 405 nm after 60 min. Wells with no bismuth trichloride were used as negative controls. Biofilms treated with PBS served as positive controls.2.7. Determination of cytotoxicity
The cytotoxicity of the raw and deacidified CJs was investigated using an MTT (3-[4,5-diethylthiazol-2-yl]-2,5diphenyltetra-zolim bromide) colorimetric cell proliferation kit (Cell Proliferation Kit I; Roche Diagnostics, Laval, QC, Canada) according to the manufacturer's protocol. GMSM-K cells were seeded in a 96-well tissue culture microplate at 1.0 × 106 cells per mL. The cells were left to adhere overnight at 37 °C in a 5% CO2 atmosphere and were then treated with CJ samples for 1 min, 5 min, 15 min, and 30 min. A 100% viability value was attributed to a treatment with culture medium. CJ samples resulting in a cell viability less than 80% were considered cytotoxic.2.8. Secretion of IL-6 and IL-8 by LPS-stimulated epithelial cells
To assess the anti-inflammatory properties of the CJs, GMSM-K cells were seeded (1.0 × 106 cells per mL) in 12-well tissue-culture treated microplates (Sarstedt, USA) and were incubated overnight in a 5% CO2 atmosphere. They were then treated with 1 mL of raw or deacidified CJ for 5 min at room temperature. The CJ was removed by aspiration, and the cells were incubated for a further 24 h in 1 mL of DMEM supplemented with 1% FBS and 1 μg mL−1 of A. actinomycetemcomitans LPS to induce an inflammatory response. The supernatants were collected, and the secretion of IL-6 and IL-8 was analyzed using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience Inc., San Diego, CA, USA) according to the manufacturer's protocols.2.9. Statistical analysis
Unless specified otherwise, all assays were performed in triplicate. Results are expressed as means ± standard deviations (SD). The physicochemical compositions of the CJ samples were analyzed using a one-way analysis of variance (ANOVA) with a post hoc Tukey test (p < 0.05). Statistical analyses of bactericidal activity, biofilm viability, and cytotoxicity were performed using a two-way analysis of variance with a post hoc Bonferroni multiple comparison test (p < 0.01) (GraphPad Software Inc., La Jolla, CA, USA). For the other assays, an ANOVA with a post hoc Bonferroni multiple comparison test (p < 0.01) was used.3. Results
3.1. Composition of raw and deacidified CJs
As expected with the EDBM process, the total phenolic, PAC, and anthocyanin content was similar for all the deacidification rates (DR) (Table 1). The process caused a slight increase in pH, which reached 3.2 for CJ at a DR of 79%. The increase in pH is normal with bipolar membranes, as previously reported in the literature.32 QA, CA, and MA were the main organic acids in raw CJ. There was a linear decrease in CA and MA levels as the deacidification process proceeded, while QA levels started to decrease when the DR reached ≥60%. Since EDBM allows the selective migration of charged and small molecules, the more marked migration of CA and MA with respect to QA can be explained by their respective pKa (CA: pKa1 = 3.13, pKa2 = 4.76, pKa3 = 6.39; MA: pKa1 = 3.46, pKa2 = 5.05; QA: pKa = 3.46), molecular weights (CA: 192.12 g mol−1; MA: 134.09 g mol−1; QA: 192.17 g mol−1), and molecular structures (unlike CA and MA, QA possesses a carboxycyclic ring that hinders diffusion through porous membranes), as previously shown by Serre et al.32 It was expected that the 1/4 dilution of raw CJ contained 25% of the total polyphenol and organic acid content of undiluted raw CJ.| Deacidification rate (%) | 0 (raw) | 19 | 42 | 60 | 79 |
|---|---|---|---|---|---|
| *Data on the same row with different letters for the same parameter are significantly different (Tukey, p < 0.05). | |||||
| pH | 2.59 ± 0.03a | 2.74 ± 0.01b | 2.71 ± 0.01b | 2.87 ± 0.01c | 3.24 ± 0.02d |
| Titratable acidity (g L−1 of citric acid monohydrate equivalents) | 9.25 ± 0.05a | 7.48 ± 0.02b | 5.40 ± 0.05c | 3.72 ± 0.02d | 1.91 ± 0.05e |
| Organic acids (g L−1) | |||||
| Quinic acid | 10.35 ± 0.31ab | 10.72 ± 0.04a | 10.49 ± 0.17ab | 10.11 ± 0.14b | 9.19 ± 0.11c |
| Citric acid | 11.59 ± 0.20a | 9.49 ± 0.15b | 6.88 ± 0.15c | 4.67 ± 0.09d | 2.35 ± 0.06e |
| Malic acid | 6.03 ± 0.10a | 4.44 ± 0.04b | 2.40 ± 0.11c | 1.34 ± 0.06d | 0.00 ± 0.00e |
| Anthocyanins (mg L−1 of cyanidin-3-glucoside equivalents) | |||||
| Cyanidin-3-galactoside | 65.14 ± 0.51a | 65.59 ± 0.67a | 64.70 ± 0.32a | 65.76 ± 1.22a | 61.89 ± 0.61b |
| Cyanidin-3-glucoside | 2.15 ± 0.12a | 2.97 ± 0.06b | 2.47 ± 0.10c | 2.06 ± 0.06a | 2.22 ± 0.19ac |
| Cyanidin-3-arabinoside | 51.12 ± 0.69a | 51.27 ± 0.15a | 50.99 ± 0.37a | 50.57 ± 0.89a | 48.24 ± 0.22b |
| Peonidin-3-galactoside | 84.74 ± 0.54a | 85.91 ± 0.95a | 83.76 ± 0.51a | 85.02 ± 1.21a | 80.74 ± 0.71b |
| Peonidin-3-glucoside | 8.50 ± 0.10a | 8.86 ± 0.09bc | 9.01 ± 0.12b | 8.71 ± 0.06abc | 8.53 ± 0.10ac |
| Peonidin-3-arabinoside | 37.94 ± 0.62a | 38.41 ± 0.39a | 37.13 ± 0.36a | 37.29 ± 0.34a | 35.98 ± 0.30b |
| Total | 249.58 ± 1.82a | 253.11 ± 0.94a | 248.05 ± 0.51a | 249.40 ± 2.75a | 237.60 ± 1.47b |
| Proanthocyanidins (mg L−1 of epicatechin equivalents) | |||||
| Monomers | 39.35 ± 0.64a | 40.43 ± 1.35a | 36.05 ± 2.74a | 36.67 ± 2.41a | 37.43 ± 1.97a |
| 2–3 mers | 148.36 ± 1.80a | 155.41 ± 6.66a | 142.12 ± 18.47a | 157.39 ± 3.86a | 159.30 ± 9.67a |
| 4–6 mers | 59.92 ± 1.24a | 62.94 ± 2.26a | 57.64 ± 7.06a | 62.55 ± 1.88a | 62.29 ± 3.58a |
| 7–10 mers | 4.28 ± 0.27a | 4.52 ± 0.35a | 4.06 ± 0.54a | 4.41 ± 0.48a | 4.53 ± 0.50a |
| Polymers | 5.55 ± 0.52a | 5.60 ± 0.35a | 5.90 ± 0.38a | 5.88 ± 0.05a | 5.78 ± 0.05a |
| Total | 257.46 ± 2.36a | 268.90 ± 10.34a | 245.78 ± 29.00a | 266.91 ± 8.35a | 269.33 ± 15.61a |
| Total phenolic compounds (mg L−1 of gallic acid equivalents) | 1074.79 ± 4.90a | 1039.65 ± 28.27a | 978.42 ± 47.79a | 1075.52 ± 34.87a | 984.21 ± 53.66a |
3.2. Bactericidal activity of raw and deacidified CJs
A direct contact test was used to determine whether CJ exerts antibacterial activity against Gram-negative periodontal pathogens and whether the removal of organic acids from the CJ has an impact on its antibacterial activity. P. gingivalis was excluded from this assay because its growth on agar plates was found to be erratic and non-reproducible. As shown in Fig. 1A, the antibacterial activity of CJ against A. actinomycetemcomitans was time- and DR-dependent. The bactericidal effect of CJ decreased when a DR ≥60% was reached, and the reduction in the mortality rate was significant only after a 15 min exposure. All the CJ samples killed planktonic F. nucleatum after 15 min, regardless of the DR. However, the bactericidal activity decreased with higher DRs, and the mortality induced by DRs ≥60% was not significantly different from the negative control after a 1 min exposure (Fig. 1B). Given that similar results were obtained with undiluted and diluted raw CJs, the reported antibacterial effect is more likely to be due to the acidic pH of the samples rather than a low organic acid concentration. Overall, the deacidification of CJ by EDBM reduced its antibacterial activity against the planktonic periodontal pathogens studied.3.3. Effect of deacidification on bactericidal activity against biofilm-embedded bacteria
The bactericidal activity of raw and deacidified CJs against the three biofilm-embedded periodontal pathogens was investigated. The reduction in viability was time-dependent for biofilm-embedded A. actinomycetemcomitans, with slower decreases for CJs with higher DRs, thus a lower organic acid content. There was no significant difference in the survival of biofilm-embedded A. actinomycetemcomitans for DRs between 0% and 60% after a 15 min contact, with the relative viability plateauing between 57% and 62% (Fig. 2A). The 1/4 diluted raw juice was more bactericidal than the CJ with a DR of 79%. The relative viability of biofilm-embedded P. gingivalis never dropped below 73%, indicating that the CJs were less lethal to biofilm-embedded P. gingivalis (Fig. 2B). Lastly, F. nucleatum was also resistant to the effects of the CJs, with an average viability remaining over 90% for all the CJ samples and contact times tested (Fig. 2C). The CJs were not as effective against the three periodontal pathogens as CHX (positive control), which lowered the relative viability to 18%, 13%, and 83% after 15 min for biofilm-embedded A. actinomycetemcomitans, P. gingivalis, and F. nucleatum, respectively.3.4. Effect of CJ on bacterial adherence to oral epithelial cells
As the first step in the colonization of gingival tissue by periodontopathogens involves their ability to adhere to oral epithelial cells, a suitable strategy to prevent periodontitis would be to consume products that limit bacterial adherence. To simulate juice consumption, epithelial cells were subjected to a brief 5 min contact with the CJ samples before being incubated with FITC-labeled bacteria. The results obtained differed depending on the bacterial species used. The fluorescence measurements revealed that a 5 min conditioning of cell surfaces with CJs with a DR ≤42% facilitated the adherence of A. actinomycetemcomitans to epithelial cells compared to the control (Fig. 3A). Indeed, the relative fluorescence was 1.4-fold higher than the control for CJs with a DR ≤42%, and 1.7-fold higher than the control for the 1/4 dilution of raw CJ. However, increasing the DR further did not result in any significant differences in bacterial adherence compared to the control. Similar results were obtained for P. gingivalis, whose adherence increased approximatively 2-fold following a pretreatment with CJs, regardless of the DR (Fig. 3B). The 1/4 dilution of raw CJ tripled the capacity of P. gingivalis to adhere to the epithelial compared to the control and increased it by 52% compared to the undiluted CJ. Overall, conditioning the oral epithelial cells with CJ favored the adherence of A. actinomycetemcomitans and P. gingivalis. On the other hand, conditioning the epithelial cells with raw and deacidified CJs reduced the adherence of F. nucleatum by 49% to 56% compared to a pretreatment with growth medium (Fig. 3C). Interestingly, the 1/4 dilution of raw CJ led to an average 33% higher relative fluorescence than the undiluted raw CJ after a 4 h incubation, meaning that more bacteria adhered to the epithelial cells. In addition, the deacidification of CJ by EDBM, which leaves the concentrations of phenolic compounds unchanged, did not alter the anti-adherence properties of the juice, since there was no significant difference in bacterial adherence among the CJ samples. Altogether, these results indicate that cranberry phenolic compounds, which are well-known for their anti-adherence properties, hindered the adherence of F. nucleatum.3.5. Effect of CJ deacidification on the production of H2S
Several periodontopathogens are able to metabolize sulfur-containing amino acids to produce VSC, including H2S, an important virulence factor. We thus evaluated the enzymatic production of H2S from L-cysteine by bacterial biofilms following a 1 min contact with raw or deacidified CJs. Fig. 4A and B show that the CJs, regardless of their DRs, had no impact on the production of H2S by A. actinomycetemcomitans and P. gingivalis biofilms. There was no significant difference in the absorbance values of the negative and the positive controls of either bacteria, indicating that they do not produce significant amounts of H2S. In the case of P. gingivalis, CJs with a DR ≥42% caused the precipitation of higher amounts of bismuth sulfide than PBS. In addition, for F. nucleatum biofilms, larger amounts of H2S were produced as the DR increased (Fig. 4C). Absorbance was 4-fold higher for wells treated with CJ with a DR of 79% compared to raw CJ 60 min following the treatment. Moreover, a treatment with 1/4 diluted raw CJ led to a 3.7-fold increase in absorbance compared to undiluted raw CJ. The absorbance following a treatment with undiluted raw CJ was not significantly different than the absorbance measured following a treatment with PBS. These results cannot be explained by biofilm desorption as assessed by crystal violet staining prior to the assay (data not shown).3.6. Effect of the CJ deacidification rate on cytotoxicity toward oral epithelial cells
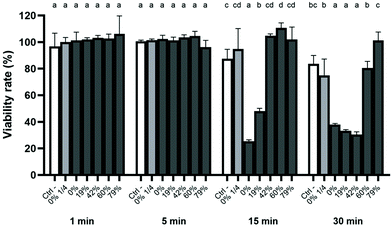
The results with respect to the cytotoxicity of the CJ samples are given in Fig. 5. None of the CJs reduced the viability of GMSM-K cells after a 1 min or 5 min contact compared to the control. However, following a 15 min contact with raw CJ, cell viability decreased to 25%, whereas it decreased to 48% for epithelial cells challenged with CJ with a DR of 19%. A 30 min contact with CJs with DRs of 0% (raw), 19%, and 42%, decreased cell viability to 38%, 33%, and 30%, respectively. The viability threshold remained above 80% for the other CJ samples after a 30 min contact, indicating that they were not cytotoxic. It should be noted that a raw CJ sample whose pH was adjusted to 7.0 with HCl and a PBS solution whose pH was adjusted to pH 2.6 did not significantly decrease cell viability compared to the control (Table 2). Also, the raw CJ exhibited no cytotoxicity when it was diluted 1/4 in distilled water, indicating that acidic pH alone was not responsible for the cytotoxic effect and suggesting that there that was a correlation between non-dissociated organic acids and cytotoxicity.| Treatment | pH | Viability rate (%) |
|---|---|---|
| *Data with different letters are significantly different (Bonferroni, p < 0.01). | ||
| Raw CJ | 2.6 | 28.6 ± 1.7a |
| 7.0 | 91.0 ± 6.0b | |
| PBS | 2.6 | 67.6 ± 1.3c |
| 7.2 | 83.7 ± 6.3bc | |
3.7. Effect of CJ deacidification on IL-6 and IL-8 production by oral epithelial cells
The effect of DR on the anti-inflammatory properties of CJ was investigated in an oral epithelial cell model stimulated with A. actinomycetemcomitans LPS. A 5 min contact with raw CJ significantly reduced the secretion of IL-6 (4.8-fold lower) and IL-8 (3.1-fold lower) by LPS-treated epithelial cells compared to the control (Fig. 6). Moreover, the concentration of IL-6 in the supernatant was directly correlated with the DR of the CJ, that is, the more organic acids were removed, the more IL-6 was produced by the cells. On the other hand, no significant differences were reported in the production of IL-8 with different DRs. Raw diluted CJ led to the production of IL-8 at a concentration similar to that of the control, suggesting that phenolic compounds are responsible for the anti-inflammatory properties of CJ.4. Discussion
4.1. Effect of the CJ deacidification rate on bactericidal activity
The CJ samples exhibited marked bactericidal activity against planktonic bacteria for both Gram-negative periodontal pathogens tested (A. actinomycetemcomitans, F. nucleatum), although this property was slightly less marked at higher DRs. Undiluted raw CJ completely eliminated the bacterial population of both pathogenic species included in the study after a 1 min (F. nucleatum) or a 15 min (A. actinomycetemcomitans) contact. These results are in agreement with those of Kranz et al.,45 who investigated the antimicrobial effect of various berry juices. They reported that F. nucleatum is completely eliminated and that there is a 5-log reduction in the A. actinomycetemcomitans population following a 60 s direct contact with CJ. The reduction of antibacterial activity noted for A. actinomycetemcomitans for the CJ with a DR of 79% might have been due to the removal of organic acids by EDMB. Jensen et al.46 attributed the antibacterial effect of CJ against uropathogenic Escherichia coli in an experimental mouse model of urinary tract infection to a synergistic effect of organic acids, namely MA/CA, MA/QA, and MA/CA/QA/shikimic acid. Suzuki et al.,47 who used a method similar to the one used in the present study, showed that a 15 min immersion in a 10% CA solution (pH 3.2) reduces the survival of Enterococcus faecalis, a major endodontic pathogen, by 60%. In addition, several reports indicate that CA exhibits marked antibacterial activity against periodontopathogens,29,30 while MA, either added to fruit juices or extracted from fruit peels, is effective in reducing the viability of planktonic Gram-positive and Gram-negative bacteria.48,49Bradshaw et al. showed that F. nucleatum has a very low tolerance to an acidic pH (3.8) and to organic acids.50 Given this, the pH of CJ, which, in the present study, remained below 3.8 regardless of the DR, may have been responsible for the bactericidal effect on F. nucleatum. However, it is also possible that the phenolic compounds in the CJ contributed to the eradication of this bacterial species as Sánchez et al. reported that the minimal bactericidal concentration (MBC) of a cranberry phenolic extract is 220 μg mL−1 for F. nucleatum,51 which is a 4.5-fold lower concentration than the total phenolic content of the undiluted CJ samples. No MBC was reported by Sánchez et al. for A. actinomycetemcomitans.51
In biofilms, bacteria are embedded in an extracellular matrix (ECM) consisting of proteins, polysaccharides, and nucleic acids that provides enhanced resistance to antimicrobial agents and environmental stresses.52 Reports in the literature indicate that cranberry phenolic extracts exhibit no significant bactericidal activity against A. actinomycetemcomitans, F. nucleatum, or P. gingivalis in a mixed biofilm.20,51 Furthermore, Sánchez et al. observed a greater viable count reduction in periopathogenic biofilms treated with red wine (with or without alcohol) compared to wine phenolic extracts.53 This difference could be due to the organic acid composition of wine, which has a low CA content and a high MA content.54 The ECM forms a mesh that can slow down the diffusion of high molecular weight molecules and hinder the diffusion of charged compounds due to electrostatic interactions.55,56 Given that MA has a higher pKa1, a smaller molecular weight, and fewer carboxyl groups than CA,32 it may have been able to infiltrate the biofilm and exert antibacterial activity. This possibility is supported by the fact that the CJ with a DR of 79%, which did not contain any MA, did not significantly affect the viability of the three biofilm-embedded periodontal pathogens tested compared to the control. On the other hand, CA has been reported to be less bactericidal for biofilm-embedded bacteria than for planktonic bacteria.57,58 As such, the removal of MA from CJ by EDBM, coupled with the slight increase in pH that leads to a higher proportion of organic acids being in their dissociated forms, could explain the results observed for the killing of biofilm-embedded A. actinomycetemcomitans.
4.2. Effect of CJ deacidification on bacterial adherence to epithelial cells
The mechanism driving the increase in the adherence of A. actinomycetemcomitans and P. gingivalis to oral epithelial cells is unknown. Assessments of the anti-adherence properties of cranberry products typically use non-dialyzable material (NDM) that is devoid of organic acids and carbohydrates or that is pH-neutralized.59–61 It is thus possible that other constituents of CJ in an acidic environment may counteract the bioactivity of the phenolic compounds. Belton et al. observed that P. gingivalis can invade epithelial cells within 12 min of infection and can remain in the intracellular environment for 24 h.62 Lamont et al. also reported that P. gingivalis invasions of primary cultures of epithelial cells peak after 90 min63 Similar findings have been described for A. actinomycetemcomitans after a 30 min incubation, although this bacterium does not remain in the cells and is more likely to reach the basal region of the monolayer.64 Results obtained in our laboratory show that there was a decrease in transepithelial electrical resistance (TER) in a keratinocyte cell line (B11) monolayer exposed to undiluted CJ samples with a DR ≤60%. This could, in part, be attributed to the disruption of the expression of tight junction (TJ) proteins.27,34,65 A weakened monolayer may facilitate bacterial invasion, as the correlation between enhanced bacterial invasion and TJ protein disruption reported by Choi et al. in mice suggests.66,67 The increased fluorescence obtained with CJ treatments compared to the negative control for A. actinomycetemcomitans and P. gingivalis could thus be due, at least in part, to bacterial internalization by and penetration of epithelial cells rather than increased adherence to the epithelial cells. Further work is required to explain these results.However, the outcome for F. nucleatum differed from the other two bacterial species studied. Indeed, F. nucleatum adherence decreased following a pretreatment of GMSM-K cells with the CJ samples. This may be due to interactions between the F. nucleatum fap2 adhesin, which is inhibited by galactose, and cyanidin-3-galactoside and peonidin-3-galactoside, two major anthocyanins in CJ that both carry a galactosyl substituent.68
4.3. Effect of CJ deacidification on bacterial H2S production
Halitosis is mainly caused by the production of VSC by anaerobic bacteria colonizing the dorsum of the tongue.69 The accumulation of malodorous compounds can induce a host inflammatory response.16 Plant products and extracts have yielded promising results in the treatment of halitosis by preventing the production of VSC, including H2S, by oral bacteria.42,70 The effect of CJ deacidification on bacterial H2S production is thus of interest. Our results showed that A. actinomycetemcomitans is a very poor producer of H2S. Although Salako and Philip reported that this bacterium produces large amounts of H2S from L-cysteine,71 other studies have failed to find a link between this bacterium and the production of VSC.72–74 Similarly, we observed that P. gingivalis causes a weak precipitation of bismuth sulfide, since the absorbance of the positive control was 0.06 ± 0.02, which was not significantly different from the negative control. This result contradicts reports in the literature indicating that P. gingivalis produces large amounts of H2S in vitro.17,71,73,75 This discrepancy may have been caused by the presence of some O2 in the biofilm, despite the effort taken to remove it all. It is worth mentioning that the microplate was prepared in the presence of O2 and that P. gingivalis biofilms are not thick, which could have facilitated the diffusion of O2 diffusion through the biofilm. Since VSC are only produced in the total absence of O2,76 small amounts of O2 could have hindered the production of H2S by P. gingivalis.Compared to P. gingivalis, F. nucleatum has a much higher tolerance for O2 and a capacity to deplete O2,77 meaning that an F. nucleatum biofilm can quickly become anaerobic after aerobic manipulations. The reason behind why DR correlates with an increase in H2S production by F. nucleatum requires further research, but our results point to a decrease in metabolic activity following a brief contact with a CJ that is richer in organic acids. Indeed, F. nucleatum produces H2S from L-cysteine by three different enzymatic pathways.78 A study on Veillonella ssp. showed that, at an acidic pH, the accumulation of lactate decreases the enzymatic production of H2S.79 Other reports suggest that organic acids and the concurring low pH derived from sugar consumption impede bacterial production of VSC.80
4.4. Effect of CJ deacidification on epithelial cell cytotoxicity
We showed that it takes over 5 min for CJ to exhibit cytotoxic activity toward GMSM-K epithelial cells. This is in agreement with Kranz et al.,45 who reported that CJ has no cytotoxic effect on human gingival fibroblasts following a 1 min contact. In addition, the results of Sakurazawa and Ohkusa suggest that epithelial cells are more sensitive to organic acids in their non-dissociated form than their anionic form,81 which is in line with our results for 15 min and 30 min exposure times. Furthermore, a concentration-dependent effect on the cytotoxicity of organic acid solutions and fruit juices has been reported in several studies for a wide variety of human cell lines.82–85 For instance, Xu showed that 30% concentrations of lime juice and of lemon juice lower Caco-2 intestinal epithelial cell viability to 66% and 75%, respectively, whereas 50% concentrations of the same juices lower viability to 58% and 41%.85 Interestingly, the cytotoxic effect remained when the pH of the lime and lemon juices was adjusted to 7.4.82 This contradicts the results obtained in the present study regarding the elimination of the cytotoxic properties of raw CJ at pH 7.0. However, it should be noted that Lim and Lim exposed the Caco-2 cells to citrus juices for 4 h,82 whereas we exposed GMSM-K oral epithelial cells to CJ for only 30 min. This may be because non-acidic CJ takes longer to express cytotoxic activity or other compounds in citrus juices may affect cell viability.4.5. Effect of CJ deacidification on the inflammatory response of oral epithelial cells
Over-activation of the host inflammatory response is a crucial aspect of periodontitis that can modulate tissue destruction.86 Studies using in vitro models have shown that berry phenolic compounds can modulate inflammation by attenuating the secretion of pro-inflammatory cytokines such as IL-6 and IL-8.87 Bodet et al. observed a significant reduction in the secretion of IL-6 by gingival fibroblasts stimulated with LPS from A. actinomycetemcomitans when treated with cranberry NDM (16.3 μg mL−1 of PAC, which is approximately 16-fold less than the concentration in the CJ used in the present study).23 We showed that even a short contact with CJ is enough for the anti-inflammatory properties of phenolic compounds to manifest, as did Soares et al.,88 who reported that a 10 min contact with pitanga juice reduces the production of IL-8 by LPS-stimulated gingival epithelial cells by half.However, the positive, linear trend that we observed between DR and IL-6 production indicated that organic acids can influence the host inflammatory response. Organic acids can exhibit anti-inflammatory properties through the reduction of cytokine gene activation in cells stimulated with LPS. This has been observed for cervicovaginal epithelial cells incubated with non-dissociated lactic acid prior to or simultaneously with exposure to bacterial LPS.89 A simultaneous lactic acid treatment led to a decrease in IL-6 and IL-8 secretion, which may be due to a significant inhibition of cytokine gene expression. Pre-exposure to lactic acid for 30 min produced the same effect. Citrate also inhibits the inflammatory response in a macrophage model.90 It should be mentioned that there was no significant difference in key volatile compounds between raw CJ and CJ with a DR of 79% (p < 0.01), indicating that the loss of anti-inflammatory monoterpenes during EDBM did not contribute to the increase in IL-6 secretion (data not shown).91
5. Conclusion
The adverse findings observed with respect to both the bacteriological and the immunological etiological factors of periodontitis indicated that the removal of organic acids (mostly CA and MA) from CJ by EDBM does not confer an advantage in terms of the prevention of periodontal diseases. Although the deacidification process decreased the cytotoxicity of CJ over a prolonged period of time, undiluted raw CJ was more effective at killing periodontopathogens and reducing bacterial adherence as well as attenuating the secretion of pro-inflammatory cytokines by oral epithelial cells compared to deacidified CJs. It is noteworthy that raw CJ exhibited antibacterial and anti-inflammatory properties, hence having the potential to counteract both etiological factors of periodontitis, unlike antibiotics that only improve periodontal health in regards to the bacteriological factor of the disease. When the recommendation by the manufacturer to dilute raw CJ before drinking to avoid gastro-intestinal discomfort is followed, it appears that the consumption of deacidified CJ compared to diluted CJ is more beneficial due to the reduction of the adherence of F. nucleatum and P. gingivalis and the decrease in the production of IL-6 and IL-8 by LPS-challenged oral epithelial cells. This is likely due to the maintenance of high concentrations of phenolic compounds in CJ that has been deacidified by EDBM.Care must be taken with in vitro studies as they do not take the environmental complexity of the oral cavity into account. It is important that the results obtained in the present study be reproduced using ex vivo dental biofilm sampling and in vivo assays. In addition, extensive studies are required to assess the effect of CJ on bacterial and cellular gene expression.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors thank V. Murrah (University of North Carolina at Chapel Hill) for providing the GMSM-K oral epithelial cell line. The technical assistance of A. Ben Lagha and K. Vaillancourt is gratefully acknowledged. G. Pellerin was financially supported by the Canada Graduate Scholarships-Master's program of the Natural Sciences and Engineering Research Council of Canada (NSERC) and by the Master's Research Scholarships of Fonds de recherche du Québec - Nature et technologies (FRQNT). This study was funded by the Laboratoire de Contrôle Microbiologique of Université Laval [2019–2020 to D. Grenier], the NSERC Industrial Research Chair on Electromembrane Processes aiming the ecoefficiency improvement of biofood production lines [IRCPJ 492889–15 to L. Bazinet], and Consortium de Recherche et Innovations en Bioprocédés Industriels au Québec [2017-072-C34 to L. Bazinet].References
- B. A. Dye, Global periodontal disease epidemiology, Periodontol. 2000, 2012, 58, 10–25 CrossRef PubMed.
- G. C. Armitage and M. P. Cullinan, Comparison of the clinical features of chronic and aggressive periodontitis, Periodontol. 2000, 2010, 53, 12–27 CrossRef PubMed.
- K. Abbayya, N. Y. Puthanakar, S. Naduwinmani and Y. S. Chidambar, Association between periodontitis and Alzheimer's Disease, North Am. J. Med. Sci., 2015, 7, 241–246 CrossRef PubMed.
- F. Q. Bui, C. L. C. Almeida-da-Silva, B. Huynh, A. Trinh, J. Liu, J. Woodward, H. Asadi and D. M. Ojcius, Association between periodontal pathogens and systemic disease, Biomed. J., 2019, 42, 27–35 CrossRef PubMed.
- D. H. Fine, G. C. Armitage, R. J. Genco, A. L. Griffen and S. R. Diehl, Unique etiologic, demographic, and pathologic characteristics of localized aggressive periodontitis support classification as a distinct subcategory of periodontitis, J. Am. Dent. Assoc., 2019, 150, 922–931 CrossRef PubMed.
- B. Signat, C. Roques, P. Poulet and D. Duffaut, Fusobacterium nucleatum in periodontal health and disease, Curr. Issues Mol. Biol., 2011, 13, 25–36 CAS.
- D. J. Bradshaw, P. D. Marsh, G. K. Watson and C. Allison, Role of Fusobacterium nucleatum and coaggregation in anaerobe survival in planktonic and biofilm oral microbial communities during aeration, Infect. Immun., 1998, 66, 4729–4732 CrossRef CAS PubMed.
- M. Xu, M. Yamada, M. Li, H. Liu, S. G. Chen and Y. W. Han, FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells, J. Biol. Chem., 2007, 282, 25000–25009 CrossRef CAS PubMed.
- K. Y. How, K. P. Song, K. G. Chan and C. C. Caldwell, Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line, Front. Microbiol., 2016, 7, 53 Search PubMed.
- C. H. Åberg, P. Kelk and A. Johansson, Aggregatibacter actinomycetemcomitans: virulence of its leukotoxin and association with aggressive periodontitis, Virulence, 2015, 6, 188–195 CrossRef PubMed.
- N. Tawfig, Proinflammatory cytokines and periodontal disease, J. Dent. Probl. Solutions, 2016, 3, 12–17 CrossRef.
- Y. W. Han, W. Shi, G. T.-J. Huang, S. K. Haake, N.-H. Park, H. Kuramitsu and R. J. Genco, Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells, Infect. Immun., 2000, 68, 3140–3146 CrossRef CAS PubMed.
- N. Pahumunto, P. Chotjumlong, A. Makeudom, S. Krisanaprakornkit, G. Dahlen and R. Teanpaisan, Pro-inflammatory cytokine responses in human gingival epithelial cells after stimulation with cell wall extract of Aggregatibacter actinomycetemcomitans subtypes, Anaerobe, 2017, 48, 103–109 CrossRef CAS PubMed.
- M. Yee, S. Kim, P. Sethi, N. Düzgünes and K. Konopka, K. Porphyromonas gingivalis stimulates IL-6 and IL-8 secretion in GMSM-K, HSC-3 and H413 oral epithelial cells, Anaerobe, 2014, 28, 62–67 CrossRef CAS PubMed.
- W. Pan, Q. Wang and Q. Chen, The cytokine network involved in the host immune response to periodontitis, Int. J. Oral Sci., 2019, 11, 1–13 CrossRef PubMed.
- W. Chen, M. Kajiya, G. Giro, K. Ouhara, H. E. Mackler, H. Mawardi, H. Boisvert, M. J. Duncan, K. Sato and T. Kawai, Bacteria-derived hydrogen sulfide promotes IL-8 production from epithelial cells, Biochem. Biophys. Res. Commun., 2010, 391, 645–650 CrossRef CAS PubMed.
- S. Persson, M.-B. Edlund, R. Claesson and J. Carlsson, The formation of hydrogen sulfide and methyl mercaptan by oral bacteria, Oral Microbiol. Immunol., 1990, 5, 195–201 CrossRef CAS PubMed.
- K. Feghali, M. Feldman, V. D. La, J. Santos and D. Grenier, Cranberry proanthocyanidins : natural weapons against periodontal diseases, J. Agric. Food Chem., 2011, 60, 5728–5735 CrossRef PubMed.
- E. Pappas and K. M. Schaich, Phytochemicals of cranberries and cranberry products : characterization, potential health effects, and processing stability, Crit. Rev. Food Sci. Nutr., 2009, 49, 741–781 CrossRef CAS PubMed.
- J. Labrecque, C. Bodet, F. Chandad and D. Grenier, Effects of a high-molecular- weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis, J. Antimicrob. Chemother., 2006, 58, 439–443 CrossRef CAS PubMed.
- H. R. Rajeshwari, D. Dhamecha, S. Jagwani, D. Patil, S. Hegde, R. Potdar, R. Metgud, S. Jalalpure, S. Roy, K. Jadhav, N. Kumar, S. Koduru, S. Hugar and S. Dodamani, Formulation of thermoreversible gel of cranberry juice concentrate: evaluation, biocompatibility studies and its antimicrobial activity against periodontal pathogens, Mater. Sci. Eng., 2017, 75, 1506–1514 CrossRef CAS PubMed.
- D. Polak, R. Naddaf, L. Shapira, E. I. Weiss and Y. Houri-Haddad, Protective potential of non-dialyzable material fraction of cranberry juice on the virulence of P. gingivalis and F. nucleatum mixed infection, J. Periodontol., 2013, 84, 1019–1025 CrossRef PubMed.
- C. Bodet, F. Chandad and D. Grenier, Cranberry components inhibit interleukin-6, interleukin-8, and prostaglandin E2 production by lipopolysaccharide-activated gingival fibroblasts, Eur. J. Oral Sci., 2007, 115, 64–70 CrossRef CAS PubMed.
- D. A. Tipton, T. B. Carter and M. K. Dabbous, Inhibition of interleukin 1β– stimulated interleukin-6 production by cranberry components in human gingival epithelial cells : effects on nuclear factor κB and activator protein 1 activation pathways, J. Periodontal Res., 2014, 49, 437–447 CrossRef CAS PubMed.
- A. E. Stapleton, J. Dziura, T. M. Hooton, M. E. Cox, Y. Yarova-Yarovaya, S. Chen and K. Gupta, Recurrent urinary tract infection and urinary Escherichia coli in women ingesting cranberry juice daily: a randomized controlled trial, Mayo Clin. Proc., 2012, 87, 143–150 CrossRef PubMed.
- D. A. Wing, P. J. Rumney, C. Preslicka and J. H. Chung, Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: a randomized, controlled pilot study, J. Urol., 2008, 180, 1367–1372 CrossRef PubMed.
- E. Serre, Y. Boutin, M.-E. Langevin, F. Lutin, K. Pedneault, S. Lacour and L. Bazinet, Deacidification of cranberry juice protects against disruption of in vitro intestinal cell barrier integrity, J. Funct. Foods, 2016, 26, 208–216 CrossRef CAS.
- A. Lussi, B. Megert, R. P. Shellis and X. Wang, Analysis of the erosive effect of different dietary substances and medications, Br. J. Nutr., 2012, 107, 252–262 CrossRef CAS PubMed.
- M. Georgopoulou, E. Kontakiotis and M. Nakou, Evaluation of the antimicrobial effectiveness of citric acid and sodium hypochlorite on the anaerobic flora of the infected root canal, Int. Endod. J., 1994, 27, 139–143 CrossRef CAS PubMed.
- J. F. Siqueira, M. Batista, R. C. Fraga and M. de Uzeda, Antibacterial effects of endodontic irrigants on black-pigmented Gram-negative anaerobes and facultative bacteria, J. Endod., 1998, 24, 414–416 CrossRef PubMed.
- M. Faucher, L. Henaux, C. Chaudron, S. Mikhaylin, M. Margni and L. Bazinet, Electromembrane approach to substantially improve the ecoefficiency of deacidified cranberry juice production: physicochemical properties, life cycle assessment and ecoefficiency score, J. Food Eng., 2020, 273, 109802 CrossRef CAS.
- E. Serre, E. Rozoy, K. Pedneault, S. Lacour and L. Bazinet, Deacidification of cranberry juice by electrodialysis: impact of membrane types and configurations on acid migration and juice physicochemical characteristics, Sep. Purif. Technol., 2016, 163, 228–237 CrossRef CAS.
- M. Faucher, E. Serre, M.-E. Langevin, S. Mikhaylin, F. Lutin and L. Bazinet, Drastic energy consumption reduction and ecoefficiency improvement of cranberry juice deacidification by electrodialysis with bipolar membranes at semi- industrial scale: reuse of the recovery solution, J. Membr. Sci., 2018, 555, 105–114 CrossRef CAS.
- V. Renaud, M. Faucher, V. Perreault, E. Serre, P. Dubé, Y. Boutin and L. Bazinet, Evolution of cranberry juice compounds during in vitro digestion and identification of the organic acid responsible for the disruption of in vitro intestinal cell barrier integrity, J. Food Sci. Technol., 2020, 57, 2329–2342 CrossRef CAS PubMed.
- AOAC, Acidity (titratable) of fruit products, in AOAC Official Methods of Analysis, 2000 Search PubMed.
- AOAC, Quinic, malic, and citric acids in cranberry juice cocktail and apple juice, in AOAC Official Methods of Analysis, 2010 Search PubMed.
- X. Wu and R. L. Prior, Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States : fruits and berries, J. Agric. Food Chem., 2005, 53, 2589–2599 CrossRef CAS PubMed.
- R. C. Khanal, L. R. Howard, C. R. Brownmiller and R. L. Prior, Influence of extrusion processing on procyanidin composition and total anthocyanin contents of blueberry pomace, J. Food Sci., 2009, 74, 52–58 CrossRef PubMed.
- V. L. Singleton and J. A. Rossi, Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, Am. J. Enol. Vitic., 1965, 16, 144–158 CAS.
- R. P. Darveau and R. E. W. Hancock, Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains, J. Bacteriol., 1983, 155, 831–838 CrossRef CAS PubMed.
- AFNOR, NF EN 1040 Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Basic Bactericidal Activity of Chemical Disinfectants and Antiseptics—Test method and Requirements (Phase 1), 2006 Search PubMed.
- A. Ben Lagha, B. Haas and D. Grenier, Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum, Sci. Rep., 2017, 7, 1–10 CrossRef PubMed.
- E. P. Gilchrist, P. Moyer, E. J. Shillitoe, N. Clare and V. A. Murrah, Establishment of a human polyclonal oral epithelial cell line, Oral Surg., Oral Med., Oral Pathol., Oral Radiol., Endod., 2000, 90, 340–347 CrossRef CAS PubMed.
- A. Yoshida, M. Yoshimura, N. Ohara, S. Yoshimura, S. Nagashima, T. Takehara and K. Nakayama, Hydrogen sulfide production from cysteine and homocysteine by periodontal and oral bacteria, J. Periodontol., 2009, 80, 1845–1851 CrossRef CAS PubMed.
- S. Kranz, A. Guellmar, P. Olschowsky, S. Tonndorf-Martini, M. Heyder, W. Pfister, M. Reise and B. Sigusch, Antimicrobial effect of natural berry juices on common oral pathogenic bacteria, Antibiotics, 2020, 9, 533 CrossRef CAS PubMed.
- H. D. Jensen, C. Struve, S. B. Christensen and K. A. Krogfelt, Cranberry juice and combinations of its organic acids are effective against experimental urinary tract infection, Front. Microbiol., 2017, 8, 542 Search PubMed.
- S. Suzuki, Y. Masuda, H. Morisaki, Y. Yamada, H. Kuwata and T. Miyazaki, The study of chitosan-citrate solution as a root canal irrigant : a preliminary report, Oral Hyg. Health, 2014, 2, 2–5 Search PubMed.
- R. M. U. S. K. Rathnayaka, Antibacterial effect of malic acid against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli in mango, pineapple and papaya juices, Am. J. Food Technol., 2013, 8, 74–82 CrossRef.
- M. S. Mokbel and F. Hashinaga, Antibacterial and antioxidant activities of banana (Musa, AAA cv. Cavendish) fruits peel, Am. J. Biochem. Biotechnol., 2005, 1, 125–131 CrossRef.
- D. J. Bradshaw, A. S. McKee and P. D. Marsh, Effects of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro, J. Dent. Res., 1989, 68, 1298–1302 CrossRef CAS PubMed.
- M. C. Sánchez, H. Ribeiro-Vidal, B. Bartolomé, E. Figuero, M. V. Moreno- Arribas, M. Sanz and D. Herrera, New evidences of antibacterial effects of cranberry against periodontal pathogens, Foods, 2020, 9, 246 CrossRef PubMed.
- J. W. Costerton, P. S. Stewart and E. P. Greenberg, Bacterial biofilms : a common cause of persistent infections, Science, 1999, 284, 1318–1322 CrossRef CAS PubMed.
- M. C. Sánchez, H. Ribeiro-Vidal, A. Esteban-Fernández, B. Bartolomé, E. Figuero, M. V. Moreno-Arribas, M. Sanz and D. Herrera, Antimicrobial activity of red wine and oenological extracts against periodontal pathogens in a validated oral biofilm model, BMC Complementary Altern. Med., 2019, 19, 145 CrossRef PubMed.
- M. Daglia, A. Papetti, P. Grisoli, C. Aceti, C. Dacarro and G. Gazzani, Antibacterial activity of red and white wine against oral streptococci, J. Agric. Food Chem., 2007, 55, 5038–5042 CrossRef CAS PubMed.
- S. G. McNee, D. A. M. Geddes and D. A. Weetman, Diffusion of sugars and acids in human dental plaque in vitro, Arch. Oral Biol., 1982, 27, 975–979 CrossRef CAS PubMed.
- P. S. Stewart, A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms, Biotechnol. Bioeng., 1998, 59, 261–272 CrossRef CAS PubMed.
- R. Ordinola-Zapata, C. M. Bramante, B. Cavenago, M. S. Z. Graeff, I. Gomes de Moraes, M. Marciano and M. A. H. Duarte, Antimicrobial effect of endodontic solutions used as final irrigants on a dentine biofilm model, Int. Endod. J., 2012, 45, 162–168 CrossRef CAS PubMed.
- M. T. Arias-Moliz, C. M. Ferrer-Luque, M. Espigares-Garcıa and P. Baca, Enterococcus faecalis biofilms eradication by root canal irrigants, J. Endod., 2009, 35, 711–714 CrossRef PubMed.
- V. D. La, A. B. Howell and D. Grenier, Anti-Porphyromonas gingivalis and anti- inflammatory activities of A-type cranberry proanthocyanidins, Antimicrob. Agents Chemother., 2010, 54, 1778–1784 CrossRef CAS PubMed.
- Y. Liu, M. A. Black, L. Caron and T. A. Camesano, Role of cranberry juice on molecular-scale surface characteristics and adhesion behavior of Escherichia coli, Biotechnol. Bioeng., 2005, 93, 297–305 CrossRef PubMed.
- A. Yamanaka, R. Kimizuka, T. Kato and K. Okuda, Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation, Oral Microbiol. Immunol., 2004, 19, 150–154 CrossRef CAS PubMed.
- C. M. Belton, K. T. Izutsu, P. C. Goodwin, Y. Park and R. J. Lamont, Fluorescence image analysis of the association between Porphyromonas gingivalis and gingival epithelial cells, Cell. Microbiol., 1999, 1, 215–223 CrossRef CAS PubMed.
- R. J. Lamont, A. Chan, C. M. Belton, K. T. Izutsu, D. Vasel and A. Weinberg, Porphyromonas gingivalis invasion of gingival epithelial cells, Infect. Immun., 1995, 63, 3878–3885 CrossRef CAS PubMed.
- D. H. Meyer, J. E. Lippmann and P. M. Fives-Taylor, Invasion of epithelial cells by Actinobacillus actinomycetemcomitans: a dynamic, multistep process, Infect. Immun., 1996, 64, 2988–2997 CrossRef CAS PubMed.
- G. Pellerin, L. Bazinet and D. Grenier, Deacidification of cranberry juice reduces its antibacterial properties against oral streptococci but preserves barrier function and attenuates the inflammatory response of oral epithelial cells, Foods, 2021, 10, 1634 CrossRef PubMed.
- Y. S. Choi, Y. C. I. Kim, A. R. Jo, S. Ji, K.-T. Koo, Y. Ko and Y. Choi, Porphyromonas gingivalis and dextran sulfate sodium induce periodontitis through the disruption of physical barriers in mice, Eur. J. Inflammation, 2013, 11, 419–431 CrossRef.
- Y. S. Choi, Y. C. Kim, S. Ji and Y. Choi, Increased bacterial invasion and proteins, growth factors, and growth factor receptors in periodontal lesions, J. Periodontol., 2014, 85, 313–322 CrossRef PubMed.
- S. Coppenhagen-Glazer, A. Sol, J. Abed, R. Naor, X. Zhang, Y. W. Han and G. Bachrach, Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth, Infect. Immun., 2015, 83, 1104–1113 CrossRef CAS PubMed.
- K. Yaegaki and K. Sanada, Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease, J. Periodontal Res., 1992, 27, 233–238 CrossRef CAS PubMed.
- S. Akkaoui and O. K. Ennibi, Use of traditional plants in management of halitosis in a Moroccan population, J. Intercult. Ethnopharmacol., 2017, 6, 267–273 CAS.
- N. O. Salako and L. Philip, Comparison of the use of the halimeter and the oral chroma in the assessment of the ability of common cultivable oral anaerobic bacteria to produce malodorous volatile sulfur compounds from cysteine and methionine, Med. Princ. Pract., 2011, 20, 75–79 CrossRef PubMed.
- A. H. Adedapo, B. Kolude, H. O. Dada-Adegbola, J. O. Lawoyin and H. A. Adeola, Targeted polymerase chain reaction-based expression of putative halitogenic bacteria and volatile sulphur compound analysis among halitosis patients at a tertiary hospital in Nigeria, Odontology, 2020, 108, 450–461 CrossRef PubMed.
- S. Awano, K. Gohara, E. Kurihara, T. Ansai and T. Takehara, The relationship between the presence of periodontopathogenic bacteria in saliva and halitosis, Int. Dent. J., 2002, 52, 212–216 CrossRef PubMed.
- N. T. Hashim, G. J. Linden, L. Winning, M. E. Ibrahim, B. G. Gismalla, F. T. Lundy and I. A. El Karim, Putative periodontal pathogens in the subgingival plaque of Sudanese subjects with aggressive periodontitis, Arch. Oral Biol., 2017, 81, 97–102 CrossRef CAS PubMed.
- A. Basic and G. Dhalén, Hydrogen sulfide production from subgingival plaque samples, Anaerobe, 2015, 35, 21–27 CrossRef CAS PubMed.
- J. Hess, J. Greenman and J. Duffield, Modelling oral malodour from a tongue biofilm, J. Breath Res., 2008, 2, 017003 CrossRef PubMed.
- P. I. Diaz, P. S. Zilm and A. H. Rogers, Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environment, Microbiology, 2002, 148, 467–472 CrossRef CAS PubMed.
- K. Suwabe, Y. Yoshida, K. Nagano and F. Yoshimura, Identification of an L- methionine γ-lyase involved in the production of hydrogen sulfide from L- cysteine in Fusobacterium nucleatum subsp. nucleatum ATCC 25586, Microbiology, 2011, 157, 2992–3000 CrossRef CAS PubMed.
- J. Washio, Y. Shimada, M. Yamada, R. Sakamaki and N. Takahashi, Effects of pH and lactate on hydrogen sulfide production by oral Veillonella spp., Appl. Environ. Microbiol., 2014, 80, 4184–4188 CrossRef PubMed.
- I. Kleinberg and G. Westbay, Salivary and metabolic factors involved in oral malodor formation, J. Periodontol., 1992, 63, 768–775 CrossRef CAS PubMed.
- T. Sakurazawa and T. Ohkusa, Cytotoxicity of organic acids produced by anaerobic intestinal bacteria on cultured epithelial cells, J. Gastroenterol., 2005, 40, 600–609 CrossRef CAS PubMed.
- S.-L. Lim and L.-Y. Lim, Effects of citrus fruit juices on cytotoxicity and drug transport pathways of Caco-2 cell monolayers, Int. J. Pharm., 2006, 307, 42–50 CrossRef CAS PubMed.
- A. Ljevar, N. Ćurko, M. Tomaševic, K. Radoševic, V. G. Srček and K. K. Ganić, Phenolic composition, antioxidant capacity and in vitro cytotoxicity assessment of fruit wines, Food Technol. Biotechnol., 2016, 54, 145–155 CAS.
- E. Navarro-Escobar, M.-P. González-Rodriguez and C. M. Ferrer-Luque, Cytotoxic effects of two acid solutions and 2.5% sodium hypochlorite used in endodontic therapy, Med. Oral Patol. Oral Cir. Bucal, 2010, 15, 90–94 Search PubMed.
- J. Xu, M. L. Go and L. Lim, Modulation of digoxin transport across Caco-2 cell monolayers by citrus fruit juices: lime, lemon, grapefruit, and pummelo, Pharm. Res., 2003, 20, 169–176 CrossRef CAS PubMed.
- A. Cekici, A. Kantarci, H. Hasturk and T. E. Van Dyke, Inflammatory and immune pathways in the pathogenesis of periodontal disease, Periodontol. 2000, 2014, 64, 57–80 CrossRef PubMed.
- K. Bunte, A. Hensel and T. Beikler, Polyphenols in the prevention and treatment of periodontal disease : a systematic review of in vivo, ex vivo and in vitro studies, Fitoterapia, 2019, 132, 30–39 CrossRef CAS PubMed.
- D. J. Soares, J. Walker, M. Pignitter, M. Walker, J. M. Imboeck, M. M. Ehrnhoefer-Ressler, I. M. Brasil and V. Somoza, Pitanga (Eugenia uniflora L.) fruit juice and two major constituents thereof exhibit anti-inflammatory properties in human gingival and oral gum epithelial cells, Food Funct., 2014, 5, 2981–2988 RSC.
- A. C. Hearps, D. Tyssen, D. Srbinovski, L. Bayigga, D. J. D. Diaz, M. Aldunate, R. A. Cone, R. Gugasyan, D. J. Anderson and G. Tachedjian, Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition, Mucosal Immunol., 2017, 10, 1480–1490 CrossRef CAS PubMed.
- E.-Y. Choi, H.-J. Kim and J.-S. Han, Anti-inflammatory effects of calcium citrate in RAW 264.7 cells via suppression of NF-κB activation, Environ. Toxicol. Pharmacol., 2015, 39, 27–34 CrossRef CAS PubMed.
- K. Moore, L. Howard, C. Brownmiller, I. Gu, S.-O. Lee and A. Mauromoustakos, Inhibitory effects of cranberry polyphenol and volatile extracts on nitric oxide production in LPS activated RAW 264.7 macrophages, Food Funct., 2019, 10, 7091–7102 RSC.
| This journal is © The Royal Society of Chemistry 2021 |