Stability limits of a red Carthamin–cellulose complex as a potential food colourant
Abstract
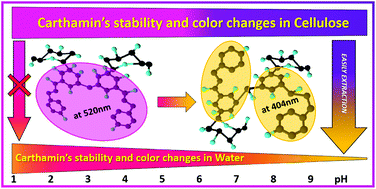
This study covers aspects of stability and colouration of Carthamin– a unique red chalcone extracted from Carthamus tinctorius L. Due to its fast degradation in aqueous solutions even at room temperature, Carthamin has no significant use in the food industry. Therefore, obtaining Carthamin in a stable form is of high interest. Comparing UV–Vis spectra of Carthamin solutions and RGB-data of Carthamin–cellulose complex in the wet state showed a predominant formation of stable Carthamin conformation on the cellulose phase. It was determined that the wet Carthamin–cellulose complex acquires a stable and rich magenta colour in the pH range of 1–5. In aqueous suspensions with pH >6, the Carthamin–cellulose complex gets a purple colour, which is absolutely uncharacteristic for pure Carthamin in an aqueous solution. IR spectra indicate the fixation of Carthamin molecules on the cellulose, which presumably causes hindrance of free internal rotation of Carthamin molecules in the cellulose phase. The reduction of water activity in the cellulosic phase represents an additional stabilizing factor. As a result, the Carthamin–cellulose complex withstands heating up to 70 °C for 15 min in the pH range of 2–5, showing up to 90% of stability. These conditions are typical for the preparation of a wide range of food products. High stability in a food-like environment and magenta colour make the Carthamin–cellulose complex a prospective natural food dye.


 Please wait while we load your content...
Please wait while we load your content...