Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aqueous extracts of lingonberry and blackberry leaves identified by high-content screening beneficially act on cholesterol metabolism†
Clemens
Röhrl
 *a,
Stefanie
Steinbauer
*a,
Stefanie
Steinbauer
 a,
Raimund
Bauer
a,
Raimund
Bauer
 b,
Eva
Roitinger
a,
Katharina
Otteneder
a,
Melanie
Wallner
b,
Eva
Roitinger
a,
Katharina
Otteneder
a,
Melanie
Wallner
 a,
Cathrina
Neuhauser
a,
Bettina
Schwarzinger
ac,
Clemens
Schwarzinger
d,
Herbert
Stangl
a,
Cathrina
Neuhauser
a,
Bettina
Schwarzinger
ac,
Clemens
Schwarzinger
d,
Herbert
Stangl
 b,
Marcus
Iken
e and
Julian
Weghuber
*ac
b,
Marcus
Iken
e and
Julian
Weghuber
*ac
aUniversity of Applied Sciences Upper Austria, Wels, Austria. E-mail: clemens.roehrl@fh-wels.at; julian.weghuber@fh-wels.at
bMedical University of Vienna, Center for Pathobiochemistry and Genetics, Vienna, Austria
cAustrian Competence Center for Feed and Food Quality, Safety and Innovation, Wels, Austria
dJohannes Kepler University, Institute for Chemical Technology of Organic Materials, Linz, Austria
ePM International AG, Schengen, Luxembourg
First published on 7th October 2021
Abstract
Decreasing circulating low-density lipoprotein (LDL) cholesterol levels leads to decreased risk of cardiovascular diseases. Natural compounds are capable of lowering LDL-cholesterol even on top of lifestyle modification or medication. To identify novel plant-derived compounds to lower plasma LDL cholesterol levels, we performed high-content screening based on the transcriptional activation of the promoter of the LDL receptor (LDLR). The identified hits were thoroughly validated in human hepatic cell lines in terms of increasing LDLR mRNA and protein levels, lowering cellular cholesterol levels and increasing cellular LDL uptake. By means of this incremental validation process in vitro, aqueous extracts prepared from leaves of lingonberries (Vaccinium vitis-idaea) as well as blackberries (Rubus fruticosus) were found to have effects comparable to lovastatin, a prototypic cholesterol-lowering drug. When applied in vivo in mice, both extracts induced subtle increases in hepatic LDLR expression. In addition, a significant increase in high-density lipoprotein (HDL) cholesterol was observed. Taken together, aqueous extracts from lingonberry or blackberry leaves were identified and characterized as strong candidates to provide cardiovascular protection.
Introduction
Cardiovascular diseases including ischemic heart disease and stroke are the major causes of mortality on a global scale.1 Low-density lipoprotein (LDL) is the main transport vehicle of cholesterol in humans. Unequivocal evidence from epidemiologic, genetic and clinical intervention studies has identified LDL as a principal driver in atherosclerosis leading to cardiovascular diseases.2 LDL—especially when oxidized in the highly reactive environment of atherosclerotic vessel lesions—is taken up by intima-resident macrophages in an unregulated manner, contributes to macrophage cell death, amplifies inflammation and additional monocyte invasion and thereby enhances atherosclerotic plaque formation.3 Therefore, maintaining adequate circulating LDL-cholesterol levels is atheroprotective.Desired LDL-cholesterol levels are dependent on the individual's risk for cardiovascular diseases. While excess LDL-cholesterol levels make pharmacological cholesterol-lowering therapy mandatory for many individuals, a variety of persons will profit from natural cholesterol-lowering compounds. Consuming plant sterols, for instance, might be advantageous for individuals (i) with borderline LDL-cholesterol levels at low or intermediate cardiovascular risk, who do not qualify for statin treatment; (ii) who are statin-intolerant; and (iii) who fail to achieve desired LDL-cholesterol levels on pharmacological treatment and aim to use natural compounds as adjuvant therapy.4 Indeed, the additive effects of several natural compounds on LDL-cholesterol decrease are observed on top of lipid lowering clinical drugs as well as on top of other lifestyle modifications.5
The lipid-lowering effects of soy protein, green tea, plant sterols, probiotic yogurt, marine-derived omega-3 fatty acids and lovastatin-containing red yeast rice in persons with dyslipidaemia were confirmed with strong evidence by the combination of in vitro, in vivo and clinical studies. In addition, seaweed, berberine, hawthorn and garlic are further promising natural compounds with lipid lowering effects in select individuals.6
The regulation of circulating LDL-cholesterol relies on its uptake into tissues—in particular into the liver—by the LDL receptor (LDLR). Therefore, hepatic LDLR levels are a major determinant of plasma LDL cholesterol levels.7 When cellular cholesterol levels decline, the endoplasmic reticulum-resident transcription factor sterol response element-binding protein 2 (SREBP2) translocates to the Golgi apparatus and further into the nucleus after proteolytic cleavage.8 As depicted in Fig. 1a, SREBP2 binds to specific DNA sequences (SREs) in the promoter regions of genes involved in cholesterol uptake (LDLR) and synthesis (HMGCR and HMGCS). Therefore, depletion of cellular cholesterol in hepatic cells is counteracted by induced LDL uptake via the LDLR, constituting a crucial step in lowering systemic LDL-cholesterol. This mechanism is also exploited by statins, the most frequently described cholesterol lowering drugs.3
We aimed to identify and characterize novel plant-derived inducers of LDLR expression in hepatic cells. Therefore, a luciferase-based screening assay was developed to assess the activation of SRE derived from the LDLR promoter in response to cellular cholesterol depletion in human hepatic cell lines (Fig. 1b). A high-content screen with medium throughput was performed using the PECKISH library as an extensive source of plant extracts.9 The particular strength in using this library is its broad diversity of plant extracts. It contains—but is not restricted to—edible and commonly eaten plants. An unbiased screening strategy without pre-selection of extracts was chosen in order not to hinder the identification of novel hits. We focused on aqueous extracts in order to facilitate the subsequent incorporation of identified cholesterol-lowering plant extracts into functional food or nutritional supplements. Initial screening results were confirmed by a secondary screening cycle and positive hits were thoroughly validated in terms of elevating LDLR mRNA and protein level, lowering cellular cholesterol levels and increasing the uptake of LDL.
Materials and methods
Plant extracts
The open access screening library “PECKISH” containing ∼4500 extracts from more than 800 different plant species was used as a starting point for screening. The library contains primarily aqueous and ethanolic extracts at an estimated concentration of 5–10 mg ml−1,9 which were diluted 1/1000 for screening and validation purposes.Plant extracts of interest identified in the screening approach were then prepared in-house as follows: Dried leaves of lingonberry (Vaccinium vitis-idaea), dried leaves of blackberry (Rubus fruticosus) and dried agrimony herb (Agrimonia eupatoria) were obtained from a local pharmacy. 90 ml of dH2O were boiled on a heat plate. Acetic acid (final concentration 1%) was added to inhibit swelling of the plant material. 10 g of plant material was added and the suspension was stirred on the heat plate for 20 min without further heating. Afterwards, the final volume was adjusted to 100 ml with dH2O and the suspension was sonicated in an ultrasonic water bath for 15 min. After centrifugation (1700 g, 10 min, RT), the supernatant was filtered through a folding filter followed by another clearing step by centrifugation (10000 g, 2 min, RT). The supernatants were sterile-filtered (PES-membrane, 0.45 μm) and stored in aliquots at −20 °C. The dry matter was determined using an MA160 Moisture Analyzer (Sartorius, Göttingen, Germany) and was found to be 12 mg ml−1 (agrimony extracts), 26.3 mg ml−1 (lingonberry extract) and 17.2 mg ml−1 (blackberry extract).
Cell culture
Cell lines were maintained under standard cultivation conditions and were routinely checked for mycoplasma infections. Cells were utilized within 15 passages throughout the experiments. HepG2 cells (ATCC: HB-8065) were cultivated in MEM supplemented with 10% FBS, 1% penicillin/streptomycin and 1% non-essential amino acids (all from Biochrom GmbH, Berlin, Germany). Huh-7 (ATCC: JCRB-0403) cells were cultivated in DMEM containing 10% FBS and 1% penicillin/streptomycin (Biochrom).Prior to experimental treatments, the cells were washed once with PBS containing calcium and magnesium (PBS+/+; Biochrom). Plant extracts and lovastatin (Sigma-Aldrich, Saint Louis, Missouri, US) were applied under serum-reduced conditions (media containing 1% FBS).
Huh-7 cells were seeded into 96-well plates at a density of 1.5 × 104 cells on day 0. The outer wells were omitted and filled with cell culture media only. On day 1, the cells were co-transfected with 100 ng per well pSRE-Luc and 0.1 ng per well pGL4.73 using TurboFect (0.25 μl per well; Thermo Fisher Scientific, Waltham, MA, US). On day 2, the cells were washed with PBS+/+ and treated with extracts of the PECKISH library, which were diluted to 1/1000 in media containing 1% FBS for 24 h. Total media volume was 200 μl per well; lovastatin (10 μM) was included as a positive control on every plate. On day 3, luciferase activity was measured by using the Dual-Glo Luciferase Assay System (Promega) according to the manufacturer's protocol with modifications: 150 μl of media was removed from the cells and discarded. 50 μl of the luciferase assay reagent (enabling cell lysis and detection of firefly luciferase) was directly added to the remaining 50 μl followed by incubation in an orbital shaker for 30 min. 100 μl of cell lysate was transferred to white microplates (Greiner Bio-One, Kremsmünster, Austria) and firefly luminescence was measured by using a POLARstar Omega microplate reader (BMG LABTECH, Ortenberg, Germany). Afterwards, 50 μl of Dual-Glo Stop & Glo reagent were added and Renilla luminescence was measured after 20 min of incubation. Firefly luminescence originating from pSRE-Luc was normalized to Renilla luminescence originating from the pGL4.73 control plasmid.
qRT-PCR and immunoblotting
Cells (Huh-7: 2 × 105 and HepG2 5 × 105 cells per well) were seeded into 6-well plates on day 0. On day 2, the cells were washed with PBS+/+ and treated with plant extracts or lovastatin under serum-reduced conditions (1% FBS). Extracts from the screening library were diluted to 1/1000 and extracts prepared in-house were applied at the indicated concentrations. Lovastatin (10 μM, diluted in DMSO) was used as a positive control. The final DMSO concentration was 0.1% and did not influence LDLR expression (data not shown). After 24 h, cells were processed for RNA isolation or protein isolation.RNA isolation, cDNA synthesis and qRT-PCR were performed according to standard methods using taqman-probes (Thermo Fisher Scientific) against LDLR (Hs00181192_m1) and ABCA1 (Hs00194045_m1). Expression was normalized to two housekeeping genes, ACTB (Hs99999903_m1) and GAPDH (Hs99999905_m1).
Proteins were isolated using a cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) according to the manufacturer's instructions. Protein concentrations were determined using a Micro BCA Protein Assay Kit (Thermo Fisher Scientific). Twenty μg of protein was mixed with the Laemmli sample buffer containing mercaptoethanol without boiling and was separated by SDS-PAGE (separation gel: 7.5% acrylamide). Afterwards, the samples were blotted onto a PVDF membrane (0.2 μm; Bio-Rad Laboratories; Hercules, CA, US). A western blocking reagent (Roche Diagnostics, Mannheim, Germany) was used as the reagent for blocking, washing and antibody dilution according to the manufacturer's instructions. The membranes were incubated with primary antibodies against LDLR11 [1/400] and β-actin (1/1000; Cell Signaling Technology #4967S, lot #11) at 4 °C overnight. The membranes were incubated with appropriate peroxidase-labeled secondary antibodies and staining was detected by chemiluminescence using the Clarity Max Western ECL substrate and a ChemiDoc MP imager (both from Bio-Rad Laboratories). Band intensities were semi-quantitatively analyzed by densitometry using Image J (NIH, USA; version 1.52d).
Gas chromatography
The content of free and esterified cholesterol was directly analyzed by gas chromatography.12 Huh-7 cells were seeded into 6 cm dishes at a density of 5 × 105 cells per dish on day 0. On day 2, the cells were washed with PBS+/+ and incubated with lovastatin or plant extracts at the indicated concentration under serum-reduced conditions for 24 h. Afterwards, the cells were washed, detached by trypsin/EDTA, re-suspended in PBS and centrifuged (4 °C, 200g, 5 min). The lipids were isolated from cell pellets by standard Folch extraction. An aliquot of the pellet was used for cell protein determination by the Bradford assay. The lipids were separated using a GC-2010 gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a programmed temperature vaporizer injector, a ZB-5HT capillary column (15 m × 0.32 mm × 0.1 μm; Phenomenex, Aschaffenburg, Germany) and an FID detector. Tridecanoyl glycerol and cholesteryl myristate (both from Sigma-Aldrich) were used as standards for free and esterified cholesterol, respectively. Chromatograms were analyzed using GC Solutions 2.3 (Shimadzu) and the values were normalized to cell protein.LDL uptake
To measure the cellular uptake of fluorescently labeled LDL, a previously published protocol13 was applied with modifications. First, LDL was isolated from healthy normolipidemic volunteers by sequential flotation ultracentrifugation.14 The isolation of LDL from human subjects was approved by the ethics committee of the Medical University of Vienna (#1414/2016). One mg of LDL (diluted to 2 mg ml−1 in PBS) was labeled with 50 μg DyLight 488 NHS ester (Thermo Fisher Scientific) in the presence of NaHCO3 (final concentration: 100 mM) at room temperature for 1 h. Then, the unbound dye was removed by extensive dialysis against PBS.Huh-7 cells were seeded in 24-well plates at a density of 5 × 104 cells per well on day 0. On day 2, the cells were washed with PBS+/+ and incubated with lovastatin or plant extracts at the indicated concentration under serum-reduced conditions for 24 h. On day 3, the cells were washed twice with PBS+/+ and incubated with 10 μg ml−1 DyLight-LDL in media containing 0.5% BSA for 2 h. Wells containing 200 μg ml−1 unlabeled LDL in addition to 10 μg ml−1 DyLight-LDL were included as controls to determine specific LDL uptake. After 2 h, the cells were washed, lyzed, and processed as described.13 Specific LDL uptake was calculated by subtracting the fluorescence of wells containing excess unlabeled LDL. The values were normalized to cell protein as determined by the Micro BCA Protein Assay Kit.
In vivo experiments
Animal experiments were conducted at the Medical University of Vienna in accordance with Austrian laws and guidelines (licence #BMBWF-66.009/0333-V/3b/2019 approved by the Animal Ethics Committee of the Medical University of Vienna and the Federal Ministry for Education, Science and Research). Male C57BL/6 mice (eight weeks of age, six mice per group) were obtained from the Center of Biomedical Research (Medical University of Vienna) and housed under standard laboratory conditions. The mice received water and rodent chow ad libitum. Plant extracts or water was applied by daily oral gavage (250 μl per mouse) for five days. After the last application of plant extracts, the animals were restricted from food intake for four hours. Afterwards, the animals were anesthetized, blood was collected by retro-orbital bleeding, animals were sacrificed by cervical dislocation and the liver was collected. Total cholesterol was determined from plasma using a CHOD-PAP kit (Greiner Bio-One). HDL-cholesterol was measured from plasma using an HDL-C Immuno FS Kit (DiaSys, Holzheim, Germany). Cholesterol present as VLDL or LDL was calculated by subtracting HDL-cholesterol from total cholesterol.HPLC-MS analysis
Extract analyses were performed by reversed-phase chromatography using a Surveyor HPLC (Thermo Fisher Scientific) equipped with an Accucore C18 column (150 mm × 3.0 mm i.d., 2.6 μm particle size; Thermo Fisher Scientific).15 The column temperature was set to 40 °C and the injection volume was 1 μl. The analytes were separated by gradient elution with mobile phase A containing 0.1% formic acid (FA) in water and mobile phase B containing 0.1% FA in acetonitrile at a flow rate of 0.5 ml min−1. The elution gradient starting conditions were 95% A and 5% B. After 5 min of equilibration time, the proportion of B was increased to 20% at 8 min and to 40% at 12 min, followed by 60% at 15 min and 80% at 17 min for 3 min. B was reduced to 5% at 20 min until 25 min.High-resolution mass spectra were recorded using an LTQ Orbitrap Velios (Thermo Fisher Scientific) with an APCI source operated in the positive and negative ionization modes. The resolution was set to 30000 and diisooctylphthalate (m/z = 391.2843) was used as the internal standard for mass calibration. The spectra were collected from 80–1000 m/z and MS2 spectra were automatically recorded from the most intense peaks. Data were analyzed using Xcalibur (Thermo Fisher Scientific; version 2.2 SP1.48. Chromatograms are depicted in Fig. S1 and S2.†
Statistics
If not otherwise indicated, data are expressed as the mean ± SD. Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA; version 8.0.2). Two-sided t-tests were applied to compare two experimental groups. ANOVA followed by Tukey's multiple testing corrections was used to compare more than two experimental groups. Significant p-values are indicated as * (≤0.05), ** (≤0.01), or *** (≤0.001).Results
Screening for natural extracts that increase SRE-activity
In order to identify novel plant-based cholesterol-lowering drugs, we established a screening assay based on the measurement of the activity of the LDLR promoter. SRE-containing regulatory regions in the 5′ UTR of the LDLR promoter were chosen for this study. These SREs are bound by SREBP2 in response to declining cellular cholesterol levels and mediate the induction of LDLR expression. This induction of LDLR expression for reducing circulating LDL is especially relevant in the liver. Therefore, human hepatocyte-derived Huh-7 cells that mimic native hepatocytes in various characteristics of lipid metabolism, for instance in the secretion of lipoproteins,16 were selected. The level of SRE-driven luciferase expression was utilized as a readout for cellular cholesterol depletion and increased LDLR expression (Fig. 1b).To this end, ∼200 aqueous plant extracts of the open source library PECKISH9 were screened for their ability to induce transcriptional activation of the LDLR promoter (Fig. 2a and Table S1†). Notably, 24 extracts exhibited increases in SRE-activity by 1.5-fold or higher. Validation of the top 34 extracts in a secondary screening cycle revealed that 20 extracts activated the LDLR-promoter by 1.5-fold or higher with 14 plant extracts being more effective than lovastatin (Fig. 2b and Table S1†). Six plant extracts being initially effective displayed low (<1.2-fold) activity in the second screening round, indicating that ∼80% of the initial hits could be confirmed.
Validation of the most promising extracts
The most effective extracts from the secondary screening rounds were chosen to analyze their potential to increase LDLR mRNA expression. Extracts of lingonberry leaves (Vaccinium vitis-idaea), blackberry leaves (Rubus fruticosus) and agrimony (Agrimonia eupatoria) increased LDLR mRNA expression in Huh-7 cells comparably to lovastatin or in a more pronounced way (Fig. 3a). Eight of the extracts tested increased LDL mRNA in a less pronounced way (1.1 to 1.3-fold). Of note, limited amounts of plant extract provided by the screening library did not allow for an independent repetition of this experiment and thus a proper statistical analysis of these effects was not feasible.Subsequently, LDLR protein expression was determined after incubation with the most effective extracts. Increased mRNA expression translated into considerably pronounced increases in LDLR protein expression for extracts of agrimony, lingonberry and blackberry (Fig. 3b).
Given the limited amounts of extracts in the screening library, aqueous extracts of lingonberry leaves, blackberry leaves and agrimony were prepared in-house. The extraction protocols (see methods section) were chosen to closely match the protocol applied to the extracts of the screening library. The extract prepared from agrimony dose-dependently induced the expression of LDLR mRNA as well as protein (Fig. 4a and d). The expression of ABCA1, which is positively regulated by increased cellular cholesterol levels and counteracts LDLR action by exporting cholesterol to extracellular acceptors,17 tended to be decreased. Lingonberry leaf extract applied at a concentration of 50 μg ml−1 induced LDLR mRNA and protein expression by 1.7-fold and 5.1-fold, respectively, while ABCA1 expression was decreased (Fig. 4b and e). Blackberry leaf extract likewise induced LDLR mRNA dose-dependently, whereas ABCA1 expression was unaltered (Fig. 4c). LDLR protein expression was increased 3.4-fold at 50 μg ml−1 (Fig. 4f).
To exclude the effects limited to a single cell line, the extracts were applied to HepG2 cells, another human hepatocyte-derived cell line that retains various aspects of cholesterol and lipoprotein metabolism.16 Comparably to Huh-7 cells, all the extracts tested significantly induced the expression of LDLR mRNA ∼3-fold, while ABCA1 expression was unchanged or slightly decreased (Fig. 4g). Altogether, these data indicated that extracts of agrimony, lingonberry leaves or blackberry leaves increase LDLR expression in hepatic cells.
Functional validation of cholesterol levels and LDL uptake
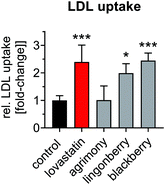
In a subsequent step, the identified extracts of interest were validated in terms of functionally altering cellular cholesterol homeostasis. Depletion of cellular cholesterol is the basis for compensatory induction of LDLR expression. We therefore quantitated cellular cholesterol levels in response to treatment with extracts of agrimony, blackberry leaves and lingonberry leaves. Free cholesterol, which is not esterified to fatty acids and is predominantly localized in cellular membranes, accounted for the majority of cellular cholesterol in Huh-7 cells under the experimental conditions (Fig. 5a–c). Cellular free cholesterol was significantly reduced by all the extracts tested to an extent comparable to or greater than lovastatin (Fig. 5a). Cholesteryl esters, which consist of cholesterol esterified to fatty acids and represent the storage form of cholesterol in lipid droplets, were reduced by agrimony and lingonberry extracts, but not by blackberry extracts (Fig. 5b). The total cholesterol levels (i.e. free cholesterol plus esterified cholesterol) decreased by ∼30% after incubation with plant extracts (Fig. 5c). These data suggest that the increase in LDLR expression observed after treatment with these extracts is in fact caused by the depletion of cellular cholesterol levels.Statins mediate increased LDLR-mediated clearance of circulating LDL into the liver, which constitutes their main atheroprotective effects. The uptake of human LDL was thus measured in Huh-7 cells after incubation with plant extracts. LDL uptake was increased ∼2.3-fold after treatment with lovastatin (Fig. 6). The extracts of lingonberry and blackberry leaves resulted in comparable increases in LDL uptake. However, incubation with the agrimony extract did not result in enhanced LDL uptake. Taken together, plant extracts prepared from lingonberry and blackberry leaves lower cellular cholesterol levels, increase LDLR mRNA and protein expression and increase LDL uptake comparably to lovastatin.
Biological effects of extracts in vivo
Next, a proof-of-concept study was conducted in mice to investigate whether these extracts exert biological activity in vivo. Extracts from lingonberry and blackberry leaves were fed to wild-type mice for five consecutive days. Unexpectedly, total plasma cholesterol was increased after treatment with both extracts (Fig. 7a). This increase was due to an increase in cholesterol transported via HDL (Fig. 7b), which is the main carrier of cholesterol in mice. The remaining cholesterol—i.e. cholesterol transported via VLDL and LDL—was not altered (Fig. 7c). Compared to the LDLR-increasing effect in vitro, both extracts only tended to increase LDLR mRNA expression in the murine liver (Fig. 7d). Similarly, LDLR protein was not significantly altered (Fig. S1†). However, the expression of hepatic ABCA1 was considerably augmented (Fig. 7e), which is consistent with the elevated HDL-cholesterol levels in mice treated with the extracts of lingonberry or blackberry leaves.Analysis of putative active compounds
Finally, we aimed to identify the biologically active compounds that exert cholesterol-lowering effects in the extracts of lingonberry and blackberry leaves. Therefore, HPLC-MS analyses were performed and the identified compounds are summarized in Table 1 (also see Tables S2 and S3 and Fig. S2 and S3† for more details). In lingonberry leaf extracts, various compounds identified could be attributed to flavonols. In particular, glycosides of quercetin, including quercetin rhamnosides (also referred to as quercitrin) were detected (Table 1). In addition, kaempferol-3-hydroxy-3-methylglutaroyl-rhamnoside was identified. The existence of these compounds as glycosides is consistent with the aqueous nature of the prepared extract.| Compound name | t R (UV) [min] | Exact mass m/z [MH]+ | Exact mass m/z [M − H]− | |
|---|---|---|---|---|
| Lingonberry | Quercetin-hexoside | 9.01 | 465.1015 | — |
| Quercetin-hexoside | 9.20 | 465.1015 | — | |
| Quercetin-pentoside | 9.57 | 435.0918 | — | |
| Quercetin-pentoside | 9.77 | 435.0918 | — | |
| Quercetin-pentoside | 9.96 | 435.0918 | — | |
| Quercetin-3-O-rhamnoside | 10.28 | 449.1072 | — | |
| Quercetin-3-O-4′′-(3-hydroxy-3-methylglutaroyl)-rhamnoside | 11.53 | 593.1433 | — | |
| Kaempferol-3-hydroxy-3-methylglutaroyl-rhamnoside | 12.25 | 577.1262 | — | |
| Blackberry | Caffeoylquinic acid isomer | 3.31 | 355.1025 | 353.0886 |
| Caffeic acid hexoside isomer | 4.65 | 343.1022 | 341.0887 | |
| Apigenin 7-O-hexuronide | 5.23 | 447.0560 | 445.0423 | |
| Caffeoylquinic acid isomer | 5.76 | 355.1024 | 353.0886 | |
| Caffeoylquinic acid isomer | 6.50 | 355.1024 | 353.0889 | |
| Apigenin 7-O-hexuronide | 7.15 | 445.0403 | 443.0272 | |
| Valoneic acid dilactone isomer | 7.82 | 471.0195 | 469.0559 | |
| Ellagic acid | 9.24 | 303.0136 | 300.9990 | |
| Quercetin-hexuronide | 9.64 | 479.0819 | 477.0669 | |
| Kaempferol 7-O-hexuronide | 9.90 | 463.0871 | 461.0749 | |
| Quercetin 3-O-pentoside | 10.24 | 435.0923 | 433.0792 | |
| Kaempferol 7-O-hexuronide | 10.58 | 463.0869 | 461.0725 | |
| Apigenin 7-O-hexuronide | 10.98 | 447.0822 | 445.0770 | |
| Kaempferol hexoside isomer | 12.53 | 595.1443 | 593.1321 |
A more versatile pattern of secondary plant compounds was identified in extracts prepared from blackberry leaves. Comparable to lingonberry, glycosides of quercetin and kaempferol were detected in blackberry extract (Table 1). In addition, apigenin glycosides, ellagic acid, caffeic acid, caffeoylquinic acids and valoneic acid isomers were identified.
In summary, we have characterized diverse as well as overlapping compounds (i.e. derivatives of quercetin and kaempferol) in lingonberry and blackberry leaf extracts.
Subsequently, quercetin, kaempferol and selected glycosides thereof were tested for their ability to mimic the biological effects of lingonberry and blackberry leaf extracts. However, only quercetin was found to significantly increase LDLR expression while kaempferol and glycosides of both compounds failed to do so (Fig. S4†).
Discussion
It is well established that lowering circulating LDL-cholesterol levels protects from atherosclerosis and cardiovascular diseases. Natural compounds have been shown to effectively lower circulating LDL-cholesterol either alone or in combination with lifestyle modifications or even on top of pharmaceutical intervention.5 Here, we identified novel aqueous, plant-derived extracts as modulators of the hepatic cholesterol metabolism in vitro.Extracts of both lingonberry and blackberry leaves robustly induced LDLR mRNA and protein expression, lowered cellular cholesterol and enhanced the uptake of LDL in a manner comparable to lovastatin, a member of the prototypical cholesterol-lowering drugs.
Identification of these extracts was enabled by a luciferase-based reporter gene assay applied in a 96-well format allowing for reliable screening under medium-throughput conditions. We anticipate that this assay will also be applicable for automated high-throughput screenings after minor adaptions. Through incremental validations, we have confirmed the screening results and identified the compounds that are most likely to be active in follow up in vivo studies. This screening assay was designed for high specificity as the reporter vector contains three repeats of SREs, which respond to declining cellular cholesterol levels followed by the activation of SREBP2. Indeed, it has been shown that this vector more actively responds to SREBPs than the native LDLR promoter.10 This is probably the reason why a large percentage of plant extracts was identified as positive after the initial screening assay. However, the increase in LDLR expression exerted by some of these positive extracts was rather low and likely translated into neglectable inductions of LDLR mRNA levels (compare Fig. 3). An alternative explanation for the high number of screening results is to identify the compounds that are most likely to be active in follow up in vivo studies. This screening assay was designed for high specificity as the reporter vector contains three repeats of SREs, which respond to declining cellular cholesterol levels followed by the activation of SREBP2. Indeed, it has been shown that this vector more actively responds to SREBPs than the native LDLR promoter.10 This is probably the reason why a large percentage of plant extracts was identified as positive after the initial screening assay. However, the increase in LDLR expression exerted by some of these positive extracts was rather low and likely translated into neglectable inductions of LDLR mRNA levels (compare Fig. 3). An alternative explanation for the high number of initially positive results might be that SREPB2 is also activated by factors other than the declining cellular cholesterol levels. For instance, others and ourselves have shown that the unfolded protein response enhances the transcriptional activity of SREBP2.18,19
Finally, we ended up with three plant extracts that robustly augmented LDLR mRNA and protein expression and lowered cellular cholesterol levels. However, while extracts of lingonberry and blackberry leaves increased cellular uptake of LDL, an extract of agrimony was inactive. This might be explained by the fact that LDL uptake is not only regulated by transcriptional and posttranscriptional mechanisms determining the expression of the LDLR but also by endosomal sorting of the LDLR. The multi-subunit protein complexes CCC and WASH, for instance, have been shown to regulate proper LDLR cell surface expression. An altered function of these complexes leads to reduced cell surface expression of the LDLR accompanied by reduced LDL uptake without changes in total cellular protein expression of the LDLR.20 Altogether, this highlights the importance of a stringent validation of screening results by functional assays, as performed in our study.
The fruits of lingonberries are known to beneficially affect cholesterol levels in animal experiments, exemplified by the findings that lingonberries reduced serum cholesterol in a mouse model of obesity21 and reduced atherosclerosis in apoE−/− mice.22 However, to the best of our knowledge, lingonberry leaves have never been investigated in the context of cholesterol lowering. Regarding blackberry leaf extracts, a study in rats focusing on non-alcoholic fatty liver disease showed that an ethanolic extract, besides affecting neutral lipid metabolism, also lowered LDL-cholesterol.23
Given the overlapping presence of quercetin and kaempferol glycosides in the extracts of both, lingonberry and blackberry leaf extracts, together with similar effects on LDLR expression, cellular cholesterol levels and LDL uptake, we initially hypothesized that quercetin and kaempferol glycosides are the biologically active compounds of both extracts. Quercetin and kaempferol have cardioprotective and hypertensive properties in animal studies.24 Quercetin as well as quercetin-3-O-glycoside (also known as isoquercitrin), increase the expression of LDLR in human hepatic cell lines.25,26 Interestingly, quercetin also increases the expression of paraoxonase 1, an enzyme inhibiting the oxidation of LDL.27
However, in our experiments, only the quercetin aglycon increased LDLR expression when applied as an isolated compound. Therefore, other secondary plant components likely contribute to the cholesterol-modifying effect of the identified extracts. It cannot be excluded that the analytical methodology applied in our study fails to detect certain compounds; for instance, high-molecular-weight saponins. Alternatively, additive or even synergistic actions of various polyphenols present in the extracts may lead to the observed biological effects.
Our in vivo studies in mice revealed that an increase in hepatic LDLR expression by both plant extracts was limited. These studies did not involve dietary modifications to increase plasma cholesterol. Notably, the application of isoquercitrin to mice increased the expression of the hepatic LDLR only when the mice were challenged with a high-cholesterol diet, but not a standard rodent diet.28 Moreover, mice are not ideal models for studying LDL-cholesterol lowering interventions because HDL is the main carrier of cholesterol in mice.29 Although mouse models with elevated LDL-cholesterol are commonly available, these models harbour mutations in the LDLR or its ligands and are therefore not suitable to study the regulation of LDLR per se. More complex model systems such as rabbits or primates would thus be necessary to definitely prove the LDL-cholesterol lowering properties of the plant extracts investigated in our study.
Most interestingly, our animal study revealed an increase in plasma cholesterol due to an increase in HDL-cholesterol, which is considered atheroprotective. Augmented HDL levels were accompanied by increased expressions of hepatic ABCA1, a key factor in HDL biogenesis.30 This increase in ABCA1 expression was not apparent from our in vitro studies. It should be noted that dietary polyphenols, including quercetin derivatives, undergo extensive modifications by human and microbiome-derived enzymes,31 which might explain the discrepancies between the in vivo and in vitro findings in terms of ABCA1 regulation.
Conclusions
Our findings derived from screening and thorough validation warrant the investigation of the cholesterol-modifying effects of lingonberry and blackberry leaf extracts in follow-up studies in pre-clinical models such as cholesterol-fed rabbits. The aqueous nature of the extracts identified in our study makes them easily accessible through simple extraction methods, for instance, to be applied as tea. Of note, berry leaves and teas thereof are widely used in traditional medicine.32 Taken together, we have identified aqueous lingonberry and blackberry leaf extracts as novel potential natural atheroprotective agents.Author contributions
Clemens Röhrl: conceptualization, methodology, investigation, writing – original draft, writing – review & editing, and supervision. Stefanie Steinbauer: investigation. Raimund Bauer: investigation. Eva Roitinger: investigation. Katharina Otteneder: methodology. Melanie Wallner: investigation. Cathrina Neuhauser: investigation. Bettina Schwarzinger: investigation and data curation. Clemens Schwarzinger: investigation, data curation, and writing – review & editing. Herbert Stangl: resources and writing – review & editing. Marcus Iken: conceptualization and writing – review & editing. Julian Weghuber: writing – review & editing, project administration, and funding acquisition.Conflicts of interest
Marcus Iken is employed by PM International AG, which provided the funding for the Josef Ressel Center for Phytogenic Drug Research. PM International AG had no influence on the study design or reporting of research. The other authors declare no conflict of interest.Acknowledgements
This research was funded by the Christian Doppler Forschungsgesellschaft (Josef Ressel Center for Phytogenic Drug Research). In addition, this work was created within a research project of the Austrian Competence Centre for Feed and Food Quality, Safety and Innovation (FFoQSI). The COMET-K1 Competence Centre FFoQSI is funded by the Austrian ministries BMVIT, BMDW and the Austrian provinces Lower Austria, Upper Austria and Vienna within the scope of COMET—Competence Centers for Excellent Technologies. The COMET programme is handled by the Austrian Research Promotion Agency FFG. We are grateful to Ingrid Hassl for excellent technical assistance.References
- T. Vos, et al., Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019, Lancet, 2020, 396, 1204–1222 CrossRef.
- J. Borén, et al., Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel, Eur. Heart J., 2020, 41, 2313–2330 CrossRef PubMed.
- D. J. Rader and A. Daugherty, Translating molecular discoveries into new therapies for atherosclerosis, Nature, 2008, 451, 904 CrossRef CAS PubMed.
- H. Gylling, et al., Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease, Atherosclerosis, 2014, 232, 346–360 CrossRef CAS PubMed.
- S. Baumgartner, et al., The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management, Atherosclerosis, 2020, 311, 116–123 CrossRef CAS PubMed.
- P. M. Hunter and R. A. Hegele, Functional foods and dietary supplements for the management of dyslipidaemia, Nat. Rev. Endocrinol., 2017, 13, 278–288 CrossRef CAS PubMed.
- M. J. Rudling, et al., Low density lipoprotein receptor-binding activity in human tissues: quantitative importance of hepatic receptors and evidence for regulation of their expression in vivo, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 3469–3473 CrossRef CAS PubMed.
- J. L. Goldstein and M. S. Brown, The LDL receptor, Arterioscler., Thromb., Vasc. Biol., 2009, 29, 431–438 CrossRef CAS PubMed.
- S. Onur, et al., The Plant Extract Collection Kiel in Schleswig-Holstein (PECKISH) Is an Open Access Screening Library, J. Food Res., 2013, 2, 101 CrossRef.
- X. Hua, et al., Sterol Resistance in CHO Cells Traced to Point Mutation in SREBP Cleavage–Activating Protein, Cell, 1996, 87, 415–426 CrossRef CAS PubMed.
- C. Wadsack, et al., Intrauterine growth restriction is associated with alterations in placental lipoprotein receptors and maternal lipoprotein composition, Am. J. Physiol., 2007, 292, E476–E484 CAS.
- S. Vondra, et al., Metabolism of cholesterol and progesterone is differentially regulated in primary trophoblastic subtypes and might be disturbed in recurrent miscarriages, J. Lipid Res., 2019, 60, 1922–1934 CrossRef CAS PubMed.
- A. Loregger, et al., Assaying Low-Density-Lipoprotein (LDL) Uptake into Cells, Methods Mol. Biol., 2017, 1583, 53–63 CrossRef CAS PubMed.
- V. N. Schumaker and D. L. Puppione, in Plasma Lipoproteins Part A: Preparation, Structure, and Molecular Biology, Elsevier, 1986, pp. 155–170 Search PubMed.
- A. Konig, et al., Guava (Psidium guajava) Fruit Extract Prepared by Supercritical CO2 Extraction Inhibits Intestinal Glucose Resorption in a Double-Blind, Randomized Clinical Study, Nutrients, 2019, 11, 1512 CrossRef PubMed.
- S. J. R. Meex, et al., Huh-7 or HepG2 cells: which is the better model for studying human apolipoprotein-B100 assembly and secretion?, J. Lipid Res., 2011, 52, 152–158 CrossRef CAS PubMed.
- A. Venkateswaran, et al., Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 12097–12102 CrossRef CAS PubMed.
- S. M. Colgan, et al., Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2, Int. J. Biochem. Cell Biol., 2007, 39, 1843–1851 CrossRef CAS PubMed.
- C. Rohrl, et al., Endoplasmic reticulum stress impairs cholesterol efflux and synthesis in hepatic cells, J. Lipid Res., 2014, 55, 94–103 CrossRef PubMed.
- P. Bartuzi, et al., CCC- and WASH-mediated endosomal sorting of LDLR is required for normal clearance of circulating LDL, Nat. Commun., 2016, 7, 10961 CrossRef CAS PubMed.
- R. Ryyti, et al., Beneficial effects of lingonberry (Vaccinium vitis-idaea L.) supplementation on metabolic and inflammatory adverse effects induced by high-fat diet in a mouse model of obesity, PLoS One, 2020, 15, e0232605 CrossRef CAS PubMed.
- C. Matziouridou, et al., Lingonberries reduce atherosclerosis in Apoe(-/-) mice in association with altered gut microbiota composition and improved lipid profile, Mol. Nutr. Food Res., 2016, 60, 1150–1160 CrossRef CAS PubMed.
- S. Park, et al., Mixture of blackberry leaf and fruit extracts alleviates non-alcoholic steatosis, enhances intestinal integrity, and increases Lactobacillus and Akkermansia in rats, Exp. Biol. Med., 2019, 244, 1629–1641 CrossRef PubMed.
- W. M. Dabeek and M. V. Marra, Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans, Nutrients, 2019, 11, 2288 CrossRef CAS PubMed.
- M. Mbikay, et al., Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh-7 human hepatocytes in culture, FEBS Open Bio, 2014, 4, 755–762 CrossRef CAS PubMed.
- J. Moon, et al., Quercetin up-regulates LDL receptor expression in HepG2 cells, Phytother. Res., 2012, 26, 1688–1694 CrossRef CAS PubMed.
- C. Boesch-Saadatmandi, et al., Effect of quercetin on paraoxonase 1 activity–studies in cultured cells, mice and humans, J. Physiol. Pharmacol., 2010, 61, 99–105 CAS.
- M. Mbikay, et al., Mice Fed a High-Cholesterol Diet Supplemented with Quercetin-3-Glucoside Show Attenuated Hyperlipidemia and Hyperinsulinemia Associated with Differential Regulation of PCSK9 and LDLR in their Liver and Pancreas, Mol. Nutr. Food Res., 2018, 62, e1700729 CrossRef PubMed.
- Y. T. Lee, et al., Mouse models of atherosclerosis: a historical perspective and recent advances, Lipids Health Dis., 2017, 16, 12 CrossRef PubMed.
- N. Wang and M. Westerterp, ABC Transporters, Cholesterol Efflux, and Implications for Cardiovascular Diseases, Adv. Exp. Med. Biol., 2020, 1276, 67–83 CrossRef CAS PubMed.
- M. R. Olthof, et al., Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans, J. Nutr., 2003, 133, 1806–1814 CrossRef CAS PubMed.
- A.-V. Ferlemi and F. N. Lamari, Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value, Antioxidants, 2016, 5, 17 CrossRef PubMed.
- A. Marsol-Vall, et al., Influence of enzymatic treatment on the chemical composition of lingonberry (Vaccinium vitis-idaea) juice, Food Chem., 2021, 339, 128052 CrossRef CAS PubMed.
- A. V. Pavlović, et al., Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia, Ind. Crops Prod., 2016, 87, 304–314 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo01169c |
| This journal is © The Royal Society of Chemistry 2021 |