Open Access Article
Open Access ArticleFructooligosaccharide supplementation alleviated the pathological immune response and prevented the impairment of intestinal barrier in DSS-induced acute colitis mice†
Minjing
Liao‡
 a,
Yuanfang
Zhang‡
a,
Yilan
Qiu
b,
Zhengchun
Wu
c,
Zhihong
Zhong
a,
Xiaoqi
Zeng
a,
Yiliang
Zeng
d,
Li
Xiong
*e,
Yu
Wen
*e and
Rushi
Liu
a,
Yuanfang
Zhang‡
a,
Yilan
Qiu
b,
Zhengchun
Wu
c,
Zhihong
Zhong
a,
Xiaoqi
Zeng
a,
Yiliang
Zeng
d,
Li
Xiong
*e,
Yu
Wen
*e and
Rushi
Liu
 *a
*a
aDepartment of Medical Laboratory, School of Medicine, Hunan Normal University, Changsha 410013, China. E-mail: liurushi@hunnu.edu.cn
bSchool of Life Science, Hunan Normal University, Changsha 410018, China
cHunan Cancer Hospital, Changsha 410013, China
dShaoshan Changbaitong Biotechnology Co., Ltd., Shaoshan 411100, China
eGeneral Surgery Department, Second Xiangya Hospital, Central South University, Changsha 410011, China. E-mail: lixionghn@csu.edu.cn; wenyu2861@csu.edu.cn
First published on 28th August 2021
Abstract
The dysbiosis of gut microbiota is closely related to the occurrence and development of inflammatory bowel disease (IBD). The manipulation of intestinal flora through prebiotics or probiotics is expected to induce and maintain the remission of IBD symptoms. 6-week-old C57BL/J mice were daily gavaged with fructooligosaccharides (FOS) or the synbiotic two weeks before the administration of dextran sulfate sodium (DSS). The supplementation of FOS or synbiotic could significantly ameliorate the body weight loss and colon histological damage in DSS-induced acute colitis mice. The altered composition of gut microbiota in acute colitis mice was reversed by FOS or Synbiotic supplementation, with a characteristic of decreased abundance of Mucispirillum. Both FOS and synbiotic mitigated DSS-induced loss of mucus protein (MUC2) and epithelium tight junction proteins (ZO-1, Occluding, Claudin1) in colon mucosa. The expression of pro-inflammatory cytokines (IL-6 and TNF-α) was decreased by FOS or synbiotic treatment, while the expression of Tbx21 and IL-10 was increased. The results suggested that the modulation of gut microbiota by FOS or synbiotic supplementation could decrease the inflammation potential of colonized commensals, which prevented the impairment of the intestinal barrier and induced a regulation of immune response in DSS-induced acute colitis mice.
1 Introduction
Inflammatory bowel disease (IBD) is a kind of chronic idiopathic intestinal inflammatory disease, mainly including ulcerative colitis and Crohn's disease. With the westernization of lifestyle, the incidence of IBD has increasingly raised in newly industrialized countries, such as China.1,2 It is characterized by early age of onset, frequent recurrence, and high risk of cancer. The routine strategy for IBD treatment mainly includes use of anti-inflammatory drugs, corticosteroids, immunosuppressive agents, and a compound of tumor necrosis factor (TNF) monoclonal antibodies to suppress intestinal inflammation. Although current therapies can ameliorate symptoms in the active phase of IBD, still some patients do not respond to these treatments or cannot tolerate them due to their significant side effects, and most patients may experience frequent recurrence because the disease is still incurable.3The interaction of gut microbiota, epithelial cells, and immune system was considered to play an important role in the pathogenesis of IBD.4 Although it is not clear whether the increase of potential pathogenic bacteria or the loss of protective strains has a bigger impact on IBD susceptibility and morbidity, the disease progression and gastrointestinal symptoms are closely related to the composition and function of intestinal flora.5,6 The widespread clinical experience and many preclinical studies suggested that modulation of gut microbiota through administration of beneficial bacterial species (probiotics), poorly absorbed dietary oligosaccharides (prebiotics), or combined prebiotics and probiotics (synbiotic) was a very promising option for treating IBD in combination with traditional anti-inflammatory and immunosuppressive agents.7,8
In the past decades, many studies focused on the effects of probiotics in the prevention or treatment of IBD, especially the Lactobacillus and Bifidobacterium strains.9–11Bifidobacterium lactis was found to inhibit NF-κB in intestinal epithelial cells.12Lactobacillus reuteri was found to maintain a functional mucosal barrier by decreasing the bacterial translocation during DSS treatment.13Lactobacillus fermentum and Bifidobacterium animal could aid in the regeneration of intestinal epithelium in DSS-treated mice.14 Current evidence indicated that the intervention effects of probiotics are strain specific, and their efficacy were more certain in the treatment of patients with ulcerative colitis or chemically induced colitis animal models.
Compared with probiotics, prebiotics are nontoxic and easy to store, can modulate a wide range of gut commensals, and won't transfer bacterial resistance gene. Many kinds of prebiotics were proved to favor the growth of bifidobacteria or the production of short-chain fatty acids such as butyrate, indicating their potential benefits to treat inflammatory bowel diseases.15 Fructooligosaccharides (FOS) is a widely used prebiotic, which might exert anti-inflammatory effects through immunomodulation or indirectly through the microbiota.16 A few studies had suggested the beneficial effects of FOS to suppress gut inflammation in chemically induced rodent models of colitis,17–19 while studies on the intervention effects of FOS in patients with Crohn's disease showed contradictory results.20,21
Recently, several studies suggested that the use of probiotic Lactobacillus or Bifidobacterium with prebiotic as a synbiotic combination was more efficacious than either the probiotic or prebiotic alone to alleviate the intestinal inflammation and prevent the dysbiosis of gut microbiota caused by DSS treatment.22–24 In present study, we explored the intervention effects and mechanisms of FOS and the synbiotic (FOS combined with several probiotics mainly from Bifibacterium and Lactobacillus) in DSS-induced colitis mice.
2 Materials and methods
2.1 Animals
The manipulation of animals was approved by Ethics Committee Board of Hunan Normal University, and performed according to the guidelines in accordance with National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications no. 8023, revised 1978). Six-week-old healthy male C57BL/6 mice were purchased from Hunan SJA laboratory animal company and maintained in specific-pathogen-free environment under normal conditions (at a temperature of 21–23 °C and a humidity of 40–60%, on a 12 h light/dark cycle). The mice were housed in groups of 4–5 per cage with free access to food and water.2.2 Prebiotic and synbiotic
The prebiotic used in present study is fructo-oligosaccharide (FOS). FOS (P95S) was manufactured by Quantum Hi-Tech Biological Company and gifted by Mr Xiaolin Chen. The synbiotic used in present study was composed of FOS and 8 probiotic strains, including Lactobacillus reuteri, Lactobacillus paracasei, Lactobacillus fermentum, Lactobacillus rhamnosus, Bifidobacterium lactis, Bifidobacterium breve, Bifidobacterium animal and Streptococcus thermophilus.2.3 Experimental design
After a week of adaptation to the laboratory conditions, the mice were assigned randomly to four groups, as Control group, DSS-induced colitis group (DSS), FOS-treated colitis group (DSS_FOS), and synbiotic-treated colitis group (DSS_Synbiotic). Each group included 8–10 mice (19 ± 1 g) and were fed with ordinary rodent fodder, while mice in group DSS_FOS and DSS_Synbiotic were daily administered with FOS (2 g per kg b.w.) or the synbiotic (FOS 2 g per kg b.w. + probiotic 109 CFU per kg b.w.) by gavage. Two weeks later, the mice were subjected to the procedure of DSS-induced colitis, except for Control group mice.2.4 Procedure of DSS-induced colitis
The mice in control group were free to drink sterilized water every day, while the mice in other groups were subjected to drink dextran sulfate sodium (DSS) solution to induce colitis. Mice were subjected to drink 3% DSS solution daily for 7 days to induce acute colitis. Mice that had underwent 3 rounds of DSS administration were considered to have chronic colitis. Each round included 7 days of 2% DSS solution drinking, and 7 days of sterilized water drinking.2.5 Mice colonoscopy and sample collection
The colorectal mucosa was observed directly under the mouse live enteroscope. The anesthetized mice were fixed in supine position on a clean operating table, and their enteric cavity were rinsed with normal saline through anus to remove feces. Insert the endoscope (Guangzhou Red Pine Medical Instrument Co., Ltd, China) through the anus, and gently reach the splenic flexure of colon. Inject the gas intermittently to ensure the intestinal cavity is filling to get a clear display of intestinal mucosa. Then draw back the endoscope slowly and record the images by software. 2 days after the completion of DSS modeling, all mice were anesthetized by intraperitoneal injection of pentobarbital sodium, then sacrificed by decapitation for sampling. The intestine of each mouse was quickly isolated, and the colon length was measured. 1 cm of distal colon segment was taken for tissue section and HE staining, and the pathological damage of colon tissue of mice in each group were evaluated. 2 cm of colonic segments adjacent to the caecum were collected to analyze the gene expression and gut microbiota.2.6 Histological analysis
The intestinal segments were cut longitudinally, rinsed with phosphate buffer, and fixed in 10% neutral formalin for 24 hours. Then the specimens were prepared to be embedded in paraffin for tissue section. Each group included 5 mice at least, and 3 randomly selected sections per mouse were used to evaluate the colon impairment. 3 μm-thick sections were de-paraffinized with xylene and rehydrated through graded alcohols, and then counterstained with hematoxylin and eosin. The slides were finally dehydrated with grade alcohols, soaked in xylene, and mounted with neutral balsam. The images were captured by a light microscope (Olympus CX41), and assessed by two independent researchers. The pathological score was assessed according to the degree of tissue damage and inflammatory cell infiltration, based on the criteria described by Wirtz et al.252.7 Amplicon sequencing and bioinformatics analysis
Obtained colon samples were delivered to the laboratory in an icebox and stored at −80 °C. Total genomic DNA was extracted using QIAGEN DNA Extraction kit following the manufacturer's instructions. Extracted DNA was diluted to a concentration of 1 ng μl−1 and used as template for PCR amplification of bacterial 16s rDNA with barcoded primers and Takara Ex Taq (Takara) for amplicon sequencing. For bacterial diversity analysis, V3–V4 variable regions of 16s rDNA was amplified with universal primers 343F and 798R. After 2 rounds of PCR, the final amplicon was purified with the AMPure XP beads (Agencourt) and quantified using Qubit dsDNA assay kit. Equal amounts of purified amplicon were pooled for subsequent sequencing on Illumina MiSeq, conducted by OE Biotech Company (Shanghai, China). The raw sequencing data were filtered to eliminate the adapter pollution and low quality to obtain clean reads by Trimmomatic software (version 0.35), and then paired-end reads were merged to tags by FLASH software (version 1.2.11). Clean tags were clustered into Operational Taxonomic Units (OTU) at 97% sequence similarity by Vsearch software (version 2.4.2). All representative reads were annotated and blasted against Greengens database using Ribosomal Database Project Classifier (0.60 confidence values as cutoff). Abundance differences of microbial communities between samples were assessed with the statistical methods based on OTU and taxonomic ranks.2.8 Transcriptome sequencing and bioinformatics analysis
Total RNA was extracted from colon tissues using the RNA Isolation KIT (Ambion, AM1561) following the manufacturer's protocol, and the samples with RNA integrity number ≥ 8 were subjected to the subsequent analysis. The libraries were constructed using TruSeq Stranded mRNA LTSample Prep Kit (Illumina, CA, USA). Then these libraries were sequenced on the Illumina sequencing platform (HiSeq X-ten) and 125 bp/150 bp paired-end reads were generated. The sequencing and transcriptome analysis were conducted by OE Biotech Company (Shanghai, China). The clean reads were mapped to reference genome using hisat2 software. FPKM (Fragments Per kb Per Million Reads) value of each gene was calculated using cufflinks software and the read counts of each gene were obtained by htseq-count software. Differentially expressed genes (DEGs) were identified using DESeq. P value < 0.05 and foldChange > 2 or foldChange < 0.5 was set as the threshold for significantly differential expression. GO enrichment and KEGG pathway enrichment analysis of DEGs were respectively performed based on the hypergeometric distribution. Hierarchical cluster analysis of DEGs was performed using the OECloud tools at https://cloud.oebiotech.cn.2.9 Real-time quantitative PCR (qPCR)
The colon tissue samples prepared for qPCR detection were homogenized with AG RNAex Pro reagent (AG21102) for total RNA extraction. Then total RNAs were reverse-transcripted to cDNA using Evo M-MLV RT Kit (AG11705). qPCR was performed with SYBR Premix Pro Taq HS qPCR kit (AG11701) on CFX Connect system (Bio-Rad Co. Ltd, United States). The reactions were all carried out with a two-step program: 30 seconds at 95 °C followed by 40 cycles of 5 seconds at 95 °C and 30 seconds at 60 °C. The reagents for RNA extraction and RT-PCR were all purchased from Hunan Accurate Biotech. Co. Ltd, China. The primers for reference and target genes were synthesized from Tsingke Biological Technology Company (Changsha, Hunan, China), and primer sequences were provided in ESI.†2.10 Western blotting (WB)
The colon tissue samples prepared for WB detection were homogenized with RIPA lysis buffer (CW2334S, CWBIO Co. Ltd, China) for protein extraction. Proteins were separated by sodium dodecyl sulfate poly-acrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene fluoride membrane. After blocking, the membranes were incubated with primary antibody (diluted as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) against at 4 °C overnight. After washing, the membranes were further incubated with HRP-conjugated second antibody at a dilution of 1
1000) against at 4 °C overnight. After washing, the membranes were further incubated with HRP-conjugated second antibody at a dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 (goat anti-rabbit IgG, AP132P, Millipore Co. Ltd) (goat anti-mouse IgG, abs20001, Absin Bioscience Co. Ltd, China). The immune complexes were detected with ECL reagent (130231, Monad Biotech. Co. Ltd, China), and the chemiluminescence signal was imaged by Tanon 5500 system (Tanon Co. Ltd, Shanghai, China). The quantitation analysis was performed using ImageJ software and normalized to β-actin band intensity on the same membrane. The primary antibodies against β-actin, Claudin1, Occludin, ZO-1, MUC2 and TNF-α were all purchased from Proteintech Group Inc., China.
5000 (goat anti-rabbit IgG, AP132P, Millipore Co. Ltd) (goat anti-mouse IgG, abs20001, Absin Bioscience Co. Ltd, China). The immune complexes were detected with ECL reagent (130231, Monad Biotech. Co. Ltd, China), and the chemiluminescence signal was imaged by Tanon 5500 system (Tanon Co. Ltd, Shanghai, China). The quantitation analysis was performed using ImageJ software and normalized to β-actin band intensity on the same membrane. The primary antibodies against β-actin, Claudin1, Occludin, ZO-1, MUC2 and TNF-α were all purchased from Proteintech Group Inc., China.
2.11 Immunohistochemistry (IHC) analysis
The expression of MUC2 and tight junction proteins (Claudin1, Occludin, ZO-1) were analyzed by immunohistochemistry assay as follows. The antibodies used for IHC were same as that for WB. 3 μm-thick sections were de-paraffinized with xylene and rehydrated through graded alcohols, and then treated with 0.01 M sodium citrate for antigen retrieval. The sections were incubated with 3% H2O2 in the dark for 12 minutes to quench endogenous peroxidase activity, and then incubated with goat serum at room temperature for 30 minutes for blocking. The sections were incubated with primary antibody (diluted as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) at 4 °C overnight. Then the slides were rinsed with PBST 4 times, and incubated with respective HRP-conjugated secondary antibody (diluted as 1
500) at 4 °C overnight. Then the slides were rinsed with PBST 4 times, and incubated with respective HRP-conjugated secondary antibody (diluted as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) at 37 °C for 45 minutes. The slides were rinsed with PBST 4 times, and then incubated with DAB (ZLI-9017, ZSGB Biological Co. Ltd, Beijing, China) for 1 minute and immediately washed under tap water after color development. Slides were counter stained with hematoxylin for 90 seconds. The sections were finally dehydrated with grade alcohols, soaked in xylene, and mounted with neutral balsam. The images were captured by a light microscope (Olympus CX41), and the optical density were analyzed by IPP 6.0 software.
1000) at 37 °C for 45 minutes. The slides were rinsed with PBST 4 times, and then incubated with DAB (ZLI-9017, ZSGB Biological Co. Ltd, Beijing, China) for 1 minute and immediately washed under tap water after color development. Slides were counter stained with hematoxylin for 90 seconds. The sections were finally dehydrated with grade alcohols, soaked in xylene, and mounted with neutral balsam. The images were captured by a light microscope (Olympus CX41), and the optical density were analyzed by IPP 6.0 software.
2.12 Statistical analysis
The statistical analyses were carried out by GraphPad Prism 7 (GraphPad Software, USA). Data were analyzed for normal distribution before any statistical analyses. The values were expressed as means ± standard deviation (SD), except for the individual values of bacterial relative abundance which were presented as scatter plots with column bar graphs or as box plots showing the interquartile range and median value. The statistical significance between two groups was determined with two-way unpaired Student's t test, Mann–Whitney test, or Wilcoxon Rank-Sum test. The significance in difference was assigned at the level of <5% probability (P < 0.05).3 Results and discussion
3.1 FOS supplementation ameliorated DSS-induced acute colitis in mice
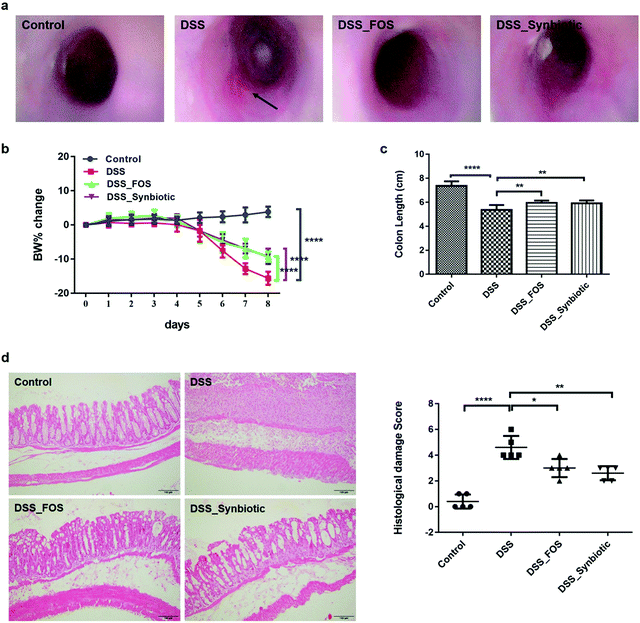
It was generally thought that the alleviation of colitis required the prebiotic given prior to DSS treatment.26 Therefore, the mice were supplemented with FOS or synbiotic two weeks before the DSS administration. Based on our experience in previous study,27 we administrated FOS at a daily dose of 2 g per kg b.w. in present study, which was about equivalent to the diet supplemented with 1% FOS. This dose was well-tolerated for weeks of administration in adult inbred mice and didn't exert significant effects on health condition.DSS-induced colitis in mice share many similarities with ulcerative colitis in human, characteristically with a significant decrease in body weight, bloody diarrhea, multiple ulcers, loss of intestinal epithelium cells and infiltration of inflammatory cells.25 As illustrated in Fig. 1, a week of DSS administration successfully induced acute colitis in C57BL/6 mice, while the supplementation of FOS and synbiotic significantly prevented the body weight loss and colon damage in acute colitis mice. The improvement of disease activity index or histological damage score in acute colitis mice presented no significant difference between FOS and synbiotic treatment.
We also examined the intervention effects of FOS and synbiotic in DSS-induced chronic colitis mice. As illustrated in ESI Fig. 1,† compared with untreated chronic colitis mice, FOS-treated mice presented less ulcers and obvious mucus accumulation on colon mucosa, and the DSS-induced alterations in colon length and histological damage score were attenuated in FOS-treated mice, suggesting that FOS could also partly prevent the colon damage in chronic colitis mice. Inflammatory bowel diseases are chronic inflammatory disorders. Although the usefulness of FOS needs to be further assessed in different animal models of colitis and clinical studies, the protective effects of FOS in DSS-induced acute and chronic colitis mice indicated it as a promising dietary supplement or functional food to treat IBD.
As illustrated in Fig. 1 and ESI Fig. 1,† the synbiotic only significantly attenuated the colon damage in DSS-induced acute colitis mice. Compared with untreated mice, the synbiotic-treated chronic colitis mice had an increased tendency of body weight loss. A previous study also found that the treatment of two probiotic strains (Lactobacillus rhamnosus and Bifidobacterium breve) exerted beneficial effects only in active phase of DSS-induced colitis.28 Clinical studies suggested Escherichia coli Nissle 1917 and VSL#3 shown strongest efficacy in the maintenance of remission in patients with ulcerative colitis.29,30 In present study, we also didn't find any synergistic effect between FOS and probiotic mixture. The use of synbiotic in inflammatory bowel diseases should take many factors such as disease course, probiotic strain, and dosage into consideration.31
3.2 The modulation of gut microbiota by FOS supplementation in acute colitis mice
Bacteria that ferment fibers and produce short chain fatty acids (SCFAs) are typically reduced in mucosa and feces of patient with IBD. The dysbiosis of gut microbiota has been considered to innitiate and aggravate gut inflammation in the course of IBD.5,8 The microbial composition in colon mucosa was analyzed by 16S rDNA sequencing after a week of DSS administration. As illustrated in Fig. 2a and b, compared with the control group, the diversity of gut microbiota in acute colitis mice was significantly decreased, and the microbial composition was significantly altered. DSS-induced decreased diversity of gut microbiota was partly attenuated by FOS or synbiotic supplementation in acute colitis mice (Fig. 2a). A previous study found that 28 days of chow diet supplemented with 10% FOS caused a decreased diversity of gut microbiota and aggravated the inflammation in ileocecal resection mice.32 Therefore, it was generally thought that the modulation of gut microbiota through prebiotics or probiotics should avoid exacerbating the loss of bacterial diversity in IBD patients.At the phulum level, the abundance of Deferribacteres was significantly increased in untreated acute colitis mice (ESI Fig. 2a and b†). At the genus level, the abundance of Allobaculum, Bacteroides, Intestinomonas, Mucispirillum and Oscillibacter was significantly increased, while the abundance of Alistipes and Lactobacillus was significantly decreased (ESI Fig. 2c†). DSS-induced increased abundance of Mucispirillum was significantly decreased by FOS or synbiotic supplementation (Fig. 2c), and its abundance was correlated to the index of disease activity (such as colon length) in acute colitis mice (Fig. 2d). Mucispirillum has been found to have specialized systems to handle oxidative stress during inflammation,33 and recognized as a bacterial indicator in DSS-induced colitis.34 In addition, we found the alleviation of gut inflammation by FOS or synbiotic treatment was accompanied with decreased abundance of Mucispirillum.
The abundance of Faecalibaculum was significantly increased in FOS-treated acute colitis mice (Fig. 2e), while the abundance of Lacotbacillus and Bifidobacterium had a tendency of increase (Fig. 2f and g). The abundance of Lacotbacillus was significantly increased in synbiotic-treated acute colitis mice (Fig. 2g). Many studies suggested the signatures of micriobial composition and metabolites might be used to predict the action mechanisms of prebiotic or probiotic.24,35–38 The presenting results suggested that FOS supplementation might prevent the DSS-induced dysbiosis of gut microbiota, by increasing the abundance of potentially beneficial bacteria (e.g., Faecalibaculum and Bifidobacterium) and decreasing the abundance of potentially harmful bacteria (e.g., Mucispirillum), but the mechanisms need to be further explored.
3.3 FOS supplementation prevented the impairment of intestinal barrier in acute colitis mice
The intestinal barrier plays a vital role to protect us from pathogens and commensals-induced inflammation.39 The physical structure of intestinal barrier mainly consists of a mucosal layer and a monolayer of epithelial cells. Mucin 2 (MUC2) was the major protein component of mucus, and its decrease was an early event in colitis pathogenesis.40 The epithelial cell layer is interconnected by tight junction (TJ) proteins such as Claudins, Occludin and Zonular occludens, which are crucial for the maintenance of epithelial barrier integrity.As illustrated in Fig. 3 and ESI Fig. 3,† the expression of MUC2 and TJ proteins (ZO-1, Occludin, Claudin-1) was significantly decreased after the administration of DSS solution, indicating a destruction of intestinal barrier. The supplementation of FOS or synbiotic could significantly prevented the loss of MUC2 and TJ proteins in DSS-induced acute colitis mice, indicating their protective effects on intestinal barrier. Many studies suggested the supplementation of prebiotic or probiotic could improve the integrity of gut barrier or strengthen the barrier function in animal models of colitis, indicating a therapeutical potential for IBD.41,42
3.4 FOS supplementation mitigated the pathological immune response in acute colitis mice
We analyzed the gene expression of colonic mucosa in acute colitis mice through transciptome sequencing. The administration of DSS caused a disastrous disturbance to intestinal environment. As illustrated in Fig. 4a, compared with control mice, the mRNA expression profile was significantly altered in other groups. The use of adjusted p-value will be more precise to identify the differentially expressed genes (DEGs). Since the sample size was not large enough in present study, we chose regular p-value <0.05 as one of criteria to perform DEG analysis. There were 782 DEGs between Control and DSS group, 51 DEGs between DSS and DSS_FOS group, 92 DEGs between DSS and DSS_Synbiotic group (data not shown).As illustrated in Fig. 4b, the majority of DEGs in DSS group were clustered to pathways of immune system and infectious diseases. Clinical studies had suggested the disordered immune responses driven by gut environment played a vital role in the pathogenesis of IBD.43 Although the mechanisms of its colitogenicity are not completely understood, it is widely believed that DSS could cause death of intestinal epithelial cells. The impaired intestinal barrier would allow the gut commensal bacteria to penetrate and reach epithelium,44 which might trigger immune responses in gut. As illustrated in Fig. 4c and d, compared with untreated DSS group, the mRNA expression profile occurred slight changes in FOS or synbiotic-treated mice, and most of DEGs were also clustered to pathways of immune system. The results suggested that supplementation of FOS or synbiotic was likely to alleviate the colitis through their immunomodulatory effects, but their effects were insufficient to revert the DSS-induced alterations to gene expression profile.
The DSS model of acute colitis is useful to investigate the roles of innate immune mechanisms. We chose several interested DEGs from immune pathways to perform hierarchical clustering analysis. As illustrated in Fig. 5a, the expression of genes which were involved with antigen processing and presentation (such as H2-DMb1 and Gm10499), T helpler cell differentiation (such as Stat1 and Tbx21), was down-regulated in untreated DSS group, while the expression of pro-inflammatory genes (such as Il6 and Tnf) was significantly up-regulated. The supplementation of FOS or synbiotic could partly mitigate above alterations.
Previous studies had found that patients receiving FOS supplementation had reduced proportion of IL-6 positive dentric cells (DCs) and increased proportion of IL-10 positive DCs.20,21 TNF-α is a key effector cytokine in IBD and its neutralizing Ab therapy is widely used in IBD treatment. IL-10 is a vital anti-inflammatory cytokine which could suppress the production of pro-inflammatory cytokines such as IL-6 and TNF-α. Thus, we further examined the colonic expression of inflammatory cytokines by qPCR and WB (Fig. 5b and c). Compared with untreated acute colitis mice, the expression of IL-6 and TNF-α was significantly decreased, and the expression of IL-10 was increased in FOS or synbiotic-treated mice. The results suggested that FOS or synbiotic supplementation could dampen the pathogenic immune responses in colonic mucosa of acute colitis mice.
T-BOX21 (Tbx21) or T-box factor expressed in T cells (T-bet) has been found to regulate the production of TNF-α in colon DCs,45 and considered to control the innate immune response across mucosal surfaces to avoid pro-inflammatory responses to commensal bacteria.46,47 As illustrated in Fig. 5b, we found that FOS or synbiotic supplementation could prevent the DSS-induced decrease of Tbx21 expression in acute colitis mice. The results suggested that FOS might exert immunomodulatory effects by regulating the expression of T-bet in innate immune system, which would be beneficial for the maintenance of intestinal barrier and thus prevented the DSS-induced acute colitis.
4 Conclusion
DSS model has been widely used to investigate the effects of gut microbiota and factors causing compositional changes (such as prebiotic) on the development of colitis. In present study, we found the supplementation of FOS or the synbiotic could significantly ameliorate the disease activity in DSS-induced acute colitis mice. The alleviation of colitis activity was correlated with the altered abundance of several gut commensals (such as Mucispirillum and Allobaculum). Both FOS and the synbiotic could significantly prevented DSS-induced loss of Muc2 and epithelium tight junction proteins at colonic mucosa, indicating the protection of intestinal barrier. Meanwhile, FOS and synbiotic up-regulated the colonic expression of T-bet in acute colitis mice, which was associated with the regulation of innate immune responses in colon. The expression of pro-inflammatory cytokines (such as TNF-α and IL-6) was decreased in FOS or synbiotic treated colitis mice, while the expression of anti-inflammatory cytokines (such as IL-10) was increased. In summary, the modulation of gut microbiota through FOS offers a bright prospect for the intervention of inflammatory bowel diseases, especially in the acute phase of disease. The application of metabolomics analysis and the determination of specific immune cell subpopulation at colonic mucosa might be helpful to further explore their action mechanisms in follow-up studies.Author contributions
Minjing Liao contributed to the conceptualization, funding acquisition and writing. Yuanfang Zhang, Yilan Qiu, Zhengchun Wu and Xiaoqi Zeng contributed to the investigation. Zhihong Zhong and Yiliang Zeng contributed to the resources. Li Xiong and Yu Wen contributed to the funding acquisition and resources. Rushi Liu contributed to the supervision and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the support of grants from Scientific Research Fund of Hunan Provincial Education Department (No. 18B033 and 2019A293), Hunan Provincial Key Research and Development Program (No. 2019SK2042), Hunan Provincial Natural Science Foundation of China (No. 2019JJ10002) and National Natural Science Foundation of China (No. 31072141, 81970569, 81970655, 81773293).References
- S. C. Ng, H. Y. Shi, N. Hamidi, F. E. Underwood, W. Tang and E. I. Benchimol, et al., Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies, Lancet, 2018, 390(10114), 2769–2778 CrossRef.
- J. Zhao, S. C. Ng, Y. Lei, F. Yi, J. Li and L. Yu, et al., First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of “western” disease, Inflammatory Bowel Dis., 2013, 19(9), 1839–1845 Search PubMed.
- K. O. Chudy-Onwugaje, K. E. Christian, F. A. Farraye and R. K. Cross, A State-of-the-Art Review of New and Emerging Therapies for the Treatment of IBD, Inflammatory Bowel Dis., 2019, 25(5), 820–830 CrossRef PubMed.
- A. Kaser, S. Zeissig and R. S. Blumberg, Inflammatory bowel disease, Annu. Rev. Immunol., 2010, 28, 573–621 CrossRef CAS PubMed.
- J. Halfvarson, C. J. Brislawn, R. Lamendella, Y. Vazquez-Baeza, W. A. Walters and L. M. Bramer, et al., Dynamics of the human gut microbiome in inflammatory bowel disease, Nat. Microbiol., 2017, 2, 17004 CrossRef CAS PubMed.
- A. D. Kostic, R. J. Xavier and D. Gevers, The microbiome in inflammatory bowel disease: current status and the future ahead, Gastroenterology, 2014, 146(6), 1489–1499 CrossRef CAS PubMed.
- R. B. Sartor, Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics, Gastroenterology, 2004, 126(6), 1620–1633 CrossRef PubMed.
- T. Zuo and S. C. Ng, The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease, Front. Microbiol., 2018, 9, 2247 CrossRef PubMed.
- M. J. Saez-Lara, C. Gomez-Llorente, J. Plaza-Diaz and A. Gil, The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials, BioMed Res. Int., 2015, 2015, 505878 Search PubMed.
- D. Jakubczyk, K. Leszczyńska and S. Górska, The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)-A Critical Review, Nutrients, 2020, 12(7), 1973 CrossRef CAS PubMed.
- M. S. Geier, R. N. Butler and G. S. Howarth, Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics, Int. J. Food Microbiol., 2007, 115(1), 1–11 CrossRef CAS PubMed.
- S. W. Kim, H. M. Kim, K. M. Yang, S. A. Kim, S. K. Kim and M. J. An, et al., Bifidobacterium lactis inhibits NF-kappaB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice, Inflammatory Bowel Dis., 2010, 16(9), 1514–1525 CrossRef PubMed.
- J. Dicksved, O. Schreiber, B. Willing, J. Petersson, S. Rang and M. Phillipson, et al., Lactobacillus reuteri maintains a functional mucosal barrier during DSS treatment despite mucus layer dysfunction, PLoS One, 2012, 7(9), e46399 CrossRef CAS PubMed.
- D. Paveljšek, P. Juvan, R. Košir, D. Rozman, B. Hacin and K. Ivičak-Kocjan, et al., Lactobacillus fermentum L930BB and Bifidobacterium animalis subsp. animalis IM386 initiate signalling pathways involved in intestinal epithelial barrier protection, Benefic. Microbes, 2018, 9(3), 515–525 CrossRef PubMed.
- C. Wong, P.J. Harris and L.R. Ferguson, Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease, Int. J. Mol. Sci., 2016, 17(6), 919 CrossRef PubMed.
- M. P. L. Guarino, A. Altomare, S. Emerenziani, C. Di Rosa, M. Ribolsi and P. Balestrieri, et al., Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults, Nutrients, 2020, 12(4), 1037 CrossRef CAS PubMed.
- J. Winkler, R. Butler and E. Symonds, Fructo-oligosaccharide reduces inflammation in a dextran sodium sulphate mouse model of colitis, Dig. Dis. Sci., 2007, 52(1), 52–58 CrossRef CAS PubMed.
- M. E. Rodríguez-Cabezas, D. Camuesco, B. Arribas, N. Garrido-Mesa, M. Comalada and E. Bailón, et al., The combination of fructooligosaccharides and resistant starch shows prebiotic additive effects in rats, Clin. Nutr., 2010, 29(6), 832–839 CrossRef PubMed.
- F. Capitán-Cañadas, B. Ocón, C. J. Aranda, A. Anzola, M. D. Suárez and A. Zarzuelo, et al., Fructooligosaccharides exert intestinal anti-inflammatory activity in the CD4+CD62L+T cell transfer model of colitis in C57BL/6J mice, Eur. J. Nutr., 2016, 55(4), 1445–1454 CrossRef PubMed.
- J. O. Lindsay, K. Whelan, A. J. Stagg, P. Gobin, H. O. Al-Hassi and N. Rayment, et al., Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn's disease, Gut, 2006, 55(3), 348–355 CrossRef CAS PubMed.
- J. L. Benjamin, C. R. Hedin, A. Koutsoumpas, S. C. Ng, N. E. McCarthy and A. L. Hart, et al., Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn's disease, Gut, 2011, 60(7), 923–929 CrossRef CAS PubMed.
- S. J. Son, J. H. Koh, M. R. Park, S. Ryu, W. J. Lee and B. Yun, et al., Effect of the Lactobacillus rhamnosus strain GG and tagatose as a synbiotic combination in a dextran sulfate sodium-induced colitis murine model, J. Dairy Sci., 2019, 102(4), 2844–2853 CrossRef CAS PubMed.
- K. Sheng, S. He, M. Sun, G. Zhang, X. Kong and J. Wang, et al., Synbiotic supplementation containing Bifidobacterium infantis and xylooligosaccharides alleviates dextran sulfate sodium-induced ulcerative colitis, Food Funct., 2020, 11(5), 3964–3974 RSC.
- Z. Liu, F. Liu, W. Wang, C. Sun, D. Gao and J. Ma, et al., Study of the alleviation effects of a combination of Lactobacillus rhamnosus and inulin on mice with colitis, Food Funct., 2020, 11(5), 3823–3837 RSC.
- S. Wirtz, V. Popp, M. Kindermann, K. Gerlach, B. Weigmann and S. Fichtner-Feigl, et al., Chemically induced mouse models of acute and chronic intestinal inflammation, Nat. Protoc., 2017, 12(7), 1295–1309 CrossRef CAS PubMed.
- A. L. M. Silveira, A. V. M. Ferreira, M. C. de Oliveira, M. A. Rachid, L. F. da Cunha Sousa and F. Dos Santos Martins, et al., Preventive rather than therapeutic treatment with high fiber diet attenuates clinical and inflammatory markers of acute and chronic DSS-induced colitis in mice, Eur. J. Nutr., 2017, 56(1), 179–191 CrossRef CAS PubMed.
- Y. Zhang, P. Wang, C. Xia, Z. Wu, Z. Zhong and Y. Xu, et al., Fructooligosaccharides supplementation mitigated chronic stress-induced intestinal barrier impairment and neuroinflammation in mice, J. Funct. Foods, 2020, 72, 104060 CrossRef CAS.
- B. Zheng, J. van Bergenhenegouwen, H. J. van de Kant, G. Folkerts, J. Garssen and A. P. Vos, et al., Specific probiotic dietary supplementation leads to different effects during remission and relapse in murine chronic colitis, Benefic. Microbes, 2016, 7(2), 205–213 CrossRef CAS PubMed.
- W. Kruis, P. Fric, J. Pokrotnieks, M. Lukás, B. Fixa and M. Kascák, et al., Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine, Gut, 2004, 53(11), 1617–1623 CrossRef CAS PubMed.
- T. M. Chapman, G. L. Plosker and D. P. Figgitt, VSL#3 probiotic mixture: a review of its use in chronic inflammatory bowel diseases, Drugs, 2006, 66(10), 1371–1387 CrossRef CAS PubMed.
- J. Plaza-Díaz, F. J. Ruiz-Ojeda, L. M. Vilchez-Padial and A. Gil, Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases, Nutrients, 2017, 9(6), 555 CrossRef PubMed.
- M. Laffin, T. Perry, H. Park, N. Hotte, R. N. Fedorak and A. Thiesen, et al., Prebiotic Supplementation Following Ileocecal Resection in a Murine Model is Associated With a Loss of Microbial Diversity and Increased Inflammation, Inflammatory Bowel Dis., 2017, 24(1), 101–110 CrossRef PubMed.
- A. Loy, C. Pfann, M. Steinberger, B. Hanson, S. Herp and S. Brugiroux, et al., Lifestyle and Horizontal Gene Transfer-Mediated Evolution of Mucispirillum schaedleri, a Core Member of the Murine Gut Microbiota, mSystems, 2017, 2(1), e00171–16 CrossRef CAS PubMed.
- D. Berry, C. Schwab, G. Milinovich, J. Reichert, K. Ben Mahfoudh and T. Decker, et al., Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis, ISME J., 2012, 6(11), 2091–2106 CrossRef CAS PubMed.
- Z. Xu, W. Chen, Q. Deng, Q. Huang, X. Wang and C. Yang, et al., Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice, Food Funct., 2020, 11(9), 8077–8088 RSC.
- J. Pujo, C. Petitfils, P. Le Faouder, V. Eeckhaut, G. Payros and S. Maurel, et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis, Gut, 2020, 70(6), 1088–1097 CrossRef PubMed.
- F. Liu, J. Liu, T. T. Y. Wang, Z. Liu, C. Xue and X. Mao, et al., Molecular and Microbial Signatures Predictive of Prebiotic Action of Neoagarotetraose in a Dextran Sulfate Sodium-Induced Murine Colitis Model, Microorganisms, 2020, 8(7), 995 CrossRef CAS PubMed.
- R. Valcheva, P. Koleva, I. Martínez, J. Walter, M. G. Gänzle and L. A. Dieleman, Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels, Gut Microbes, 2019, 10(3), 334–357 CrossRef CAS PubMed.
- C. Chelakkot, J. Ghim and S. H. Ryu, Mechanisms regulating intestinal barrier integrity and its pathological implications, Exp. Mol. Med., 2018, 50(8), 1–9 CrossRef CAS PubMed.
- S. van der Post, K. S. Jabbar, G. Birchenough, L. Arike, N. Akhtar and H. Sjovall, et al., Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis, Gut, 2019, 68(12), 2142–2151 CrossRef CAS PubMed.
- K. Hiippala, H. Jouhten, A. Ronkainen, A. Hartikainen, V. Kainulainen and J. Jalanka, et al., The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation, Nutrients, 2018, 10(8), 988 CrossRef PubMed.
- H. Wang, P. Shi, L. Zuo, J. Dong, J. Zhao and Q. Liu, et al., Dietary Non-digestible Polysaccharides Ameliorate Intestinal Epithelial Barrier Dysfunction in IL-10 Knockout Mice, Journal Crohn's Colitis, 2016, 10(9), 1076–1086 CrossRef PubMed.
- W. T. Uniken Venema, M. D. Voskuil, G. Dijkstra, R. K. Weersma and E. A. Festen, The genetic background of inflammatory bowel disease: from correlation to causality, J. Pathol., 2017, 241(2), 146–158 CrossRef PubMed.
- M. E. Johansson, J. K. Gustafsson, J. Holmén-Larsson, K. S. Jabbar, L. Xia and H. Xu, et al., Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis, Gut, 2014, 63(2), 281–291 CrossRef CAS PubMed.
- W. S. Garrett, G. M. Lord, S. Punit, G. Lugo-Villarino, S. K. Mazmanian and S. Ito, et al., Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system, Cell, 2007, 131(1), 33–45 CrossRef CAS PubMed.
- R. Mohamed and G. M. Lord, T-bet as a key regulator of mucosal immunity, Immunology, 2016, 147(4), 367–376 CrossRef CAS PubMed.
- N. Garrido-Mesa, J. H. Schroeder, E. Stolarczyk, A. L. Gallagher, J. W. Lo and C. Bailey, et al., T-bet controls intestinal mucosa immune responses via repression of type 2 innate lymphoid cell function, Mucosal Immunol., 2019, 12(1), 51–63 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo01147b |
| ‡ These authors make equal contribution to this paper. |
| This journal is © The Royal Society of Chemistry 2021 |