Open Access Article
Open Access ArticleEmulsions stabilised with pectin-based microgels: investigations into the effect of pH and ionic strength on emulsion stability†
G. I.
Saavedra Isusi
 *,
M.
Weilandt
,
I.
Majollari
,
H. P.
Karbstein
and
U. S.
van der Schaaf
*,
M.
Weilandt
,
I.
Majollari
,
H. P.
Karbstein
and
U. S.
van der Schaaf
Karlsruhe Institute of Technology, Institute of Process Engineering in Life Sciences – Chair of Food Process Engineering, Gotthard-Franz-Str. 3, Building 50.31, 76131 Karlsruhe, Germany. E-mail: gabriela.saavedra@kit.edu; Fax: +0049 721 608 45967; Tel: +0049 721 608 43614
First published on 2nd June 2021
Abstract
Pectin-based microgel particles (MGPs) are encouraging sustainable emulsifying agents for food-applications. Based on polyelectrolytes, pectin-based MGPs are assumed to be pH and ionic strength sensitive, in a similar manner to MGPs of synthetic polymers. Besides building a barrier around oil droplets, charged MGPs repulse each other. Thus the stabilisation mechanisms of pectin-based MGPs should be both steric and electrostatic. To investigate this, emulsions were homogenised with MGP concentrations ranging from 0.5 to 2 wt% MGPs. After emulsification, the pH of the emulsions was adjusted to 4, 3, or 2; and the resulting droplet sizes were measured. We found out that the droplet size and the appearance of agglomerates increased with decreasing pH values. This was caused by the loss of the MGP surface charge, as stated by their ζ-potential, showing an increase from −33.71 ± 4.1 mV for samples with pH 4 to −17 ± 0.6 mV, and −3.4 ± 0.6 mV for pH 3 and 2, respectively. However, the degree of coalescence was dependent on the MGP concentration, as samples with 0.5 wt% coalesced more readily than samples with 2 wt% MGP. These results help understand the emulsion stabilisation mechanisms of pectin-based MGPs and what effect formulation parameters have on the long-term stability of MGP-stabilised emulsions.
1. Introduction
Emulsion-based food products such as, drinks, salad dressings, desserts, and sauces, are part of our daily lives. These products are composed of two immiscible liquids (oil and water), and are thermodynamically unstable, thus, they require the use of emulsifying agents, and often thickening agents in order to guarantee stability, and avoid phase separation.1 In addition, most of these products have acidic pH values and high to moderate ion concentrations in order to preserve them and enhance flavour.2 There is a wide range of emulsifying agents useful for this purpose, such as proteins and hydrocolloids, but also particles and low molecular surfactants.3 This article draws focus to a relatively new kind of emulsifying agent: microgel particles (MGPs) based on biopolymers for food applications. Microgel particles are lyophilic, particulate polymer networks, whose properties are more complex than those of single polymer chains and particles.3,4 Due to their polymer-colloid duality, MGPs possess properties such as thermal and pH responsiveness, reversible swelling, deformability, and interfacial activity among other characteristics.3,5–7MGPs based on food biopolymers have been successfully produced.3 Similar to their synthetic counterparts, the biopolymer used for MGP formation can determine or influence the functionality and characteristics of the obtained microgel particle: aggregation, self-assembly, complexation or denaturation, and the long-term integrity of microgel particles.8 A variety of charged polysaccharides or proteins can form microgel particles. MGPs produced from proteins, such as whey, have great potential for foam9 and emulsion10 stabilisation. Researchers have investigated the pH-responsiveness of pea protein MGPs and whey protein MGPs.10,11 However, few have investigated the pH responsiveness of polysaccharide-based MGPs. There are two studies that focus on the release of encapsulated substances from polysaccharide beads as a response to pH changes.12,13 However, the influence of changes in pH and ionic strength on the emulsifying and interfacial properties of polysaccharide particles has been investigated scarcely. Many polysaccharides used for MGP formation, such as pectins or alginate, are polyelectrolytes. Hence, they are affected by the pH and ionic strength in a similar manner to protein-based MGPs. Therefore, a thorough understanding of the emulsion stabilisation mechanisms of complex charged polysaccharide-based MGPs is required in order to use such particles in food applications, where low pH values and high ionic strengths are dominant.
The pH sensitivity and response to the presence of counterions have been thoroughly investigated for poly(N-isopropylacrylamide) (PNIPAM) and PNIPAM-co-MAA (methylacryl acid) MGPs.5,14–17 Thus, we take these findings as the basis for our work on polysaccharide-based MGPs. Depending on the polymer used, the sensitivity of MGPs towards alterations in pH or ionic strength may vary. The presence of ionisable groups on the polymer can determine the MGP's surface or inner charge, depending on their location within the particle.18,19 Besides affecting MGP's charge, ionisable groups determine MGP interactions with the solvent, which are key for the swelling capability of MGPs.20
The swelling capability of MGPs is not only determined by the mere presence of ionisable groups. It also makes a difference whether these groups are basic or acidic,21 as they determine the pH-sensitivity of the MGPs, thus affecting MGP volume, charge, and stability.4 Since both MGP's volume and charge are affected by pH or ionic strength, one cannot assume that the stabilisation of droplets by MGPs is based solely on electrostatic repulsion.4 The presence of counterions and the deprotonation of acidic groups cause the deswelling of charged MGPs. This can reduce the oil surface coverage by MGPs, or even cause MGP detachment, rendering the oil droplets prone to coalescence.22–24 Liu et al.25 showed that swollen and charged poly(N-isopropylacrylamide-co-methacrylic acid) and poly(N-isopropylacrylamide-co-2-aminoethyl methacrylate) MGPs stabilise oil droplets better than in their uncharged state. The long-term stability of the investigated emulsions was prolonged when both MGPs were electrostatically charged. However, emulsions were also successfully prepared with uncharged MGPs as demonstrated by Schmidt et al.26 for core–shell microgels. Even at almost zero charge, MGPs in their swollen state could stabilise droplets in the presence of counterions, indicating that the volume/conformation of MGPs is as important as the electrostatic repulsion for droplet stabilisation. The literature suggests that the deformability and interpenetration capability of MGPs are key for droplet stabilisation.22 Therefore, it is assumed that the stabilisation mechanisms of MGPs are based on electrostatic and steric barriers. Although much has been published on MGPs based on synthetic polymers, their use in food applications is unthinkable. Hence, research focuses on biopolymer-based MGPs, which can be used in the food, pharma, and cosmetic industries, without losing consumer acceptance.
In this work, we focus on pectin-based MGPs. Pectin as a charged polysaccharide serves as a model for various food grade hydrophilic biopolymers. Furthermore, pectin-based MGPs have already been successfully used as emulsifying agents.27 Pectin is found in higher plants, where it gives firmness and structure to plant tissues. Pectin is gained from side streams of the food industry, e.g. from juice or sugar production. This makes pectin a sustainable plant polysaccharide from natural sources28 and a food ingredient well-accepted by consumers.29 Pectin is a hetero block-copolymer, composed of three different polysaccharide structures forming a single pectin molecule: homogalacturonan (HG), rhamnogalacturonan I (RGI), and rhamnogalacturonan II (RGII). Low methyl-esterified pectin chains form physical gels in the presences of divalent cations.28,30 Pectin's charge can fluctuate depending on its degree of methyl-esterification, amidation, acetylation, and protein content, thus making pectin a polyelectrolyte, whose charge depends on the extraction source and environmental conditions. All of these parameters impact pectin's emulsifying properties as a single polymer chain,31 that is the droplet size that can be obtained when various pectin polymers are used for emulsion stabilisation at equal concentrations.
In contrast to pectin polymers, pectin-based MGPs uphold the same emulsifying properties regardless of possible variabilities in pectin origin and molecular structures.27 Therefore, pectin-based MGPs have the potential to overcome variations in the polymer raw material. Previous work has focused on the choice of pectin type for MGP formation, the impact of the process parameter on the MGP integrity, and the effect of MGP concentration on the resulting emulsion properties.27,32,33 However, the underlying mechanisms responsible for pectin MGP emulsifying properties are still unclear. As they are made from a polyelectrolyte, pectin-based MGPs are assumed to be pH and ionic strength sensitive, as reported for MGPs from synthetic polyelectrolytes. Thus, our underlying hypothesis states that the stabilisation mechanism of pectin-based MGP is of both steric and electrostatic in nature. Changes in pH and ion concentration might affect the MGP charge and size, possibly leading to droplet or MGP flocculation. Nevertheless, we assume that if the oil droplet coverage is sufficient, droplet coalescence might be hindered.
2. Materials and methods
2.1. Materials
Amidated apple pomace pectin was gifted by Herbstreith & Fox (Neuenbürg, Germany). The pectin had a molecular weight of 63 kDa, a degree of esterification of 24%, a degree of amidation of 24%, and a galacturonic acid content of 91% according to supplier information. Calcium chloride di-hydrate was obtained from Merck KGaA (Darmstadt, Germany). CaCO3 powder (98.5% purity) was purchased from Carl Roth GmbH & Co. KG (Karlsruhe, Germany). D-(+)-Glucono-1,5-lacton (GDL) with 99% purity was acquired from Alfa Aesar GmbH & Co. KG (Karlsruhe, Germany). Medium chain triglyceride (MCT) oil with C8 and C10 chains with a 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ratio was purchased from IOI Oleo GmbH (Hamburg, Germany). In order to increase the density of the MCT oil and delay the creaming of emulsions, 8 wt% of ester gum provided by Symrise AG (Holzminden, Germany) was added to the oil. The mixture was heated to 50 °C under constant stirring until dissolution. The MCT oil then had a density of 0.96 kg L−1 at room temperature.
40 ratio was purchased from IOI Oleo GmbH (Hamburg, Germany). In order to increase the density of the MCT oil and delay the creaming of emulsions, 8 wt% of ester gum provided by Symrise AG (Holzminden, Germany) was added to the oil. The mixture was heated to 50 °C under constant stirring until dissolution. The MCT oil then had a density of 0.96 kg L−1 at room temperature.
2.2. Preparations of pectin solution
Amidated pectin solutions, with a pectin mass concentration of 2 wt%, were prepared by dissolving 4 g of pectin in 196 g of demineralised water in a 600 mL beaker at 60 °C, using a high-shear mixer Ultraturrax T-25 digital (IKA® Werke GmbH & Co. KG, Staufen, Germany) at a rotational speed of 10.000 rpm for 30 s. Then solutions were left to cool down to room temperature.2.3. Preparation of pectin MGP suspensions
The pectin solutions prepared as described above were used for the preparation of MGP suspensions with a 50 wt% MGP concentration according to the method described by Saavedra Isusi et al.27 Gelation was achieved by adding a 40 mM CaCl2 solution to the pectin solution under constant shearing, followed by a homogenising step using a high-pressure homogeniser (HPH) at 400 bar. The HPH used was a Microfluidizer MF 110 EH (Microfluidics Corporation, Newton, MA, USA). It possesses a Y-type interaction chamber with a microchannel diameter of 75 μm and an auxiliary processing module (APM) with a diameter of 200 μm. The MGP suspension was then was diluted with demineralised water to obtain MGP suspensions with 0.5; 1 or 2 wt% MGP concentration. These MGP suspensions were used as the continuous phase of the investigated emulsions. The pH of the MGP suspensions was not adjusted prior to the emulsification step, and was equal to 4.2 at room temperature.2.4. Determination of MGP size and zeta-potential
The hydrodynamic diameter (z-average) of MGPs in suspension was determined by dynamic light scattering with a particle size analyser Horiba Nanopartica SZ-100 (Horiba Scientific, Kyoto, Japan). Samples were measured at least 10 times. Measurements were conducted at a scattering angle of 173° and at 22.0 ± 1 °C. The particle had a z-average of 77.0 ± 12.8 nm and a polydispersity index of 2.64 ± 0.59.The zeta-potential of MGP suspensions containing 1 wt% MGPs was determined using the particle size analyser Horiba Nanopartica SZ-100 (Horiba Scientific, Kyoto, Japan), at pH-values 4, 3, and 2 without adjusting the electric conductivity (EC) of the water phase. Additionally, the EC MGP suspensions at pH 4 were adjusted to 0.65 mS cm−1 and 6.5 mS cm−1 with a saturated NaCl solution prior to the zeta-potential measurements. The zeta-potential of MGP suspensions at pH 3 and EC 6.5 mS cm−1 was also determined. Three measurements of 10 runs were conducted at 25 °C for each solution.
2.5. Determination of gel's volume change depending on the pH-value
Cylindrical shaped gels with a radius of 40 mm and a height of 20 mm were synthesised according to the method described in ref. 34. Pectin solutions, prepared as previously described, were mixed with GDL and CaCO3. The CaCO3 concentration was chosen depending on the molar ratio R = 2 × [Ca2+]/[COO−] = 1.35–38 The GDL amount was chosen to fit the stoichiometric ratio [GDL] = 2 × [Ca2+], as described by Ström et al. (2003).39 After mixing, the solution was poured into silicon forms and was left to gel for a minimum of 20 h at room temperature.After gelation, the cylindrical shaped gels were submersed in demineralised water. The pH of the water was adjusted to pH 2 and pH 3 using a 1 M HCl solution, respectively. The gels remained submersed in the water phase for 72 h and at 5 °C. After this time, their volume V72 h was determined by submersing the gel blocks in oil and measuring the displaced oil volume. The relative volume change ΔV was then calculated using eqn (1).
 | (1) |
2.6. Preparation of pectin MGP-stabilised emulsions
Emulsions, containing 5 vol% MCT oil (disperse phase), were prepared by dispersing oil into either a 0.5, 1 or 2 wt% MGP suspension (continuous phase). The MCT oil was dispersed into the continuous phase under constant mixing with a high-shear mixer Ultraturrax T-25 digital (IKA® Werke GmbH & Co. KG, Staufen, Germany) at a rotational speed of 15.000 rpm over 30 s in a 600 mL beaker. Afterwards, the emulsion premixes were dispersed for another minute at the same rotational speed. Fine emulsions were obtained by homogenising the coarse emulsions twice at 400 bar using a HPH Microfluidizer MF 110 EH (Microfluidics Corporation, Newton, MA, USA). The pH of the emulsions after their preparation was equal to 4.4 ± 0.2 at room temperature.The fine emulsions were then separated into three samples of equal volume. The pH of the samples was adjusted to a pH-value of 4 (reference value), 3 or 2, respectively. The reference value was chosen as emulsions without any pH-adjustment had pH-values ranging from 4.2 to 4.4. The pH adjustment was performed using less than 0.5 vol% of 1 mol HCl to avoid pectin degradations.
The EC of the investigated samples was determined after pH adjustment. In order to investigate the effect of ionic strength and pH separately, emulsion samples with adjusted electric conductivity were prepared. The emulsification process was kept constant. The electric conductivity (EC) of emulsions with pH 3 was measured and was equal to 0.65 mS cm−1. A sample with reference pH (pH 4) was then prepared with the same EC (0.65 mS cm−1). For this, a saturated NaCl solution was used. Furthermore, samples with pH 3 and pH 4 and an increased EC equal to 6.5 mS cm−1 were also produced. Each emulsion type was prepared in triplicate if not stated otherwise.
2.7. Measurement of oil droplet size distribution
The droplet size distribution (DSD) of the prepared emulsions was determined by static laser light scattering using a HORIBA LA-950 Particle analyser (Retsch Technology, Haan, Germany). The results are shown as the cumulative volume distribution Q3. The characteristic mean droplet diameter x50,3 was selected for comparison of the changes in droplet size caused by pH or EC. The refractive indices were set at n = 1.4494 for MCT oil and n = 1.333 for water for all emulsions. The determination of the droplet sizes was made following the Fraunhofer theory. All measurements were conducted in triplicate at room temperature.All emulsion samples were observed under an Eclipse LV100ND microscope (Nikon GmbH, Düsseldorf, Germany), equipped with a DS-Fi1c camera. Micrographs of the samples were taken with 10 or 20-fold magnification lenses.
2.8. Statistical analysis
Each sample preparation was made in triplicate. If not specified otherwise, all analyses were conducted at least three times per independent test. All data was assessed by a multifactorial analysis of variance (ANOVA) and a Tukey test as the post-hoc test. Dissimilarities in samples were considered statistically relevant at a level of p ≤ 0.05. The software OriginPro 2019 (OriginLab Corp., Northampton, MA, USA) was used for the statistical analysis, calculation of averages, and standard deviations.3. Results and discussion
3.1. Influence of pH and ionic strength on MGP charge and size
Both pH and ionic strength affect the MGP charge and size, so they can influence the emulsion stability. The pH adjustment by adding HCl not only decreases the pH of the outer phase but also increases the ion content, resulting in a higher ionic strength. Thus, the influence of pH and ionic strength on the emulsion stability cannot be decoupled from one another. In order to still be able to differentiate between the effects of pH-value and ionic strength, we determined the zeta-potential of the MGP suspension (MGP in water) at pH 2, 3 and 4, and at two different electric conductivities at pH 3 and 4. The ionic strength of the dispersion (either MGP suspension or emulsion), can be determined indirectly by measuring the dispersion's electric conductivity EC. The values chosen for the EC were 0.65 and 6.50 mS cm−1. The first value was determined in preliminary experiments and corresponds to the EC of an emulsion after pH adjustment to pH 3. The second value, 10-fold of the first value, was chosen so that possible effects caused by high ionic strength could be magnified. The results are shown in Table 1.| pH | Electric conductivity EC [mS cm−1] | Zeta-potential [mV] |
|---|---|---|
| 4 (reference) | 0.28 | −33.7 ± 4.1 |
| 4 | 0.65 | −30.9 ± 0.2 |
| 4 | 6.50 | −12.6 ± 2.2 |
| 3 | 0.65 | −17 ± 0.6 |
| 3 | 6.50 | −8.2 ± 1.6 |
| 2 | 4.83 | −3.4 ± 0.6 |
As seen from Table 1, the zeta-potential (ZP) of MGPs in aqueous solutions is affected by changes in pH and ionic strength (determined indirectly as the electric conductivity). The reference samples show a negative charge, with a ZP of −33.7 mV. Increasing the electric conductivity of the watery phase at pH 4 leads to an increase in ZP (−12.6 ± 2.2 mV), as seen in Table 1. An increase in the ZP is also noticeable when the pH decreases. Samples with pH 3 possess a higher ZP-value as samples with the same EC at pH 4 (−17 ± 0.6 mV for 0.65 mS cm−1 and −8.2 ± 1.6 mV for 6.50 mS cm−1). Here again, the addition of ions decreases the particle's charge. At pH 2, MGPs have a ZP closest to zero (−3.4 ± 0.6 mV), which translates to uncharged particles. Under these conditions a very limited electrostatic repulsion among particles is expected.
The changes in the MGP charge under acidic conditions, as measured from their ZP, could be explained by the charge state of the MGP's functional groups. The functional groups responsible for surface charge in pectin-based MGPs are probably carboxyl groups (due to pectin's low degree of methyl-esterification). Although these groups are also involved in pectin gelation, not all carboxyl groups formed junction zones with calcium ions. Free carboxyl groups could still remain in the MGP providing them with a strongly negative surface charge (−33 mV). A decrease in the pH-value of the suspension decreases MGPs’ electrostatic surface charge, thus increasing their zeta-potential.
The addition of ions also causes an increase of the potential, as seen in Table 1. The salt used for the adjustment of the sample's electric conductivity was NaCl. These ions do not interact with pectin's functional groups, and hence they do not directly affect the particle's surface charge. Nevertheless, ions can alter the properties of the diffuse double layer, which surrounds the particle (Debye–Hückel-length). An increase in the electrolyte concentration of the samples results in the compression of the diffuse double layer, which reduces MGP's electrostatic barrier, thus increasing the particle's zeta-potential and the chance of particle aggregation.
However, the effect of the pH value on the zeta-potential is larger than that of the addition of ions. This is especially noticeable on samples with pH 2. In this sample an EC of 4.83 mS cm−1 was measured, which is lower than the highest investigated EC of samples at pH 3 and 4. Yet, this sample possessed the highest zeta-potential of all formulations. This means that the changes on the MGP's surface charge affect the electrostatic repulsion in a greater way than the compression of the diffuse double layer does.
Not only is the zeta-potential of the particles affected by the pH of the surrounding phase, as described above, but also the gel undergoes volume changes if the environmental conditions lead to changes in the osmotic pressure or in the charge of the polymer's functional groups. The determination of the volume changes in microgel particles is however problematic, as they might agglomerate. Thus, the determination of an increase/decrease in the volume of a single gel particle is rather difficult. For this reason, the volume changes caused by the pH were examined on macroscopic pectin gels, prepared with the same pectin type as the investigated MGPs. After 72 h, gels prepared with amidated pectin showed a relative volume decrease of −13% and −5% at pH 2 and pH 3, respectively. The fact that at pH 2 the gels decreased more in volume than at pH 3 concords with the zeta-potential measurements. At pH 2, the functional groups lose more charge than at pH 3, hence, they do not repel each other as much. This could lead to a contraction of the gel network. Moreover, at the lower pH, more HCl was added to the watery phase to reach the desired pH-value. Therefore, the osmotic pressure and ionic strength of water with pH 2 is higher than the one with pH 3. This can also lead to greater gel deswelling.
Although the macroscopic gels are not MGPs, we assume that MGPs can follow the same trend, as the polymer type used for the preparation of both gel types was the same. The difference would then lie on the kinetics in which the volume changes would occur and on the determined absolute values. However, the obtained data is only a qualitative approach and can help clarify the behaviour of MGPs. Hence, it can be said that MGPs would also shrink under acidic conditions and that the shrinkage percentage would be pH dependent, as well.
3.2. Assessment of the influence MGP concentration on emulsion stability
MGPs from charged polymers react to changes in the environment such as increase/decrease in the pH-value, osmotic pressure, and the presence of ions. This can result in MGP deswelling and/or changes in MGP surface charge. MGP deswelling can consequently result in insufficient surface coverage and droplet coalescence. In order to avoid this, one would intuitively use higher particle concentrations for emulsion preparation to guarantee that enough particles cover the interface, even in the case of particle shrinkage. However, the lack of electrostatic repulsion might lead to MGP agglomeration. In this case, a high MGP concentration could be contra-productive, as the agglomeration probability increases with the amount of particles.40 Higher agglomeration probability could accelerate the oil droplet coalesce process and render the emulsion unstable.To test the assumption that an increasing amount of MGP might provide better steric stabilisation to emulsions, and could promote droplet agglomeration depending on their surface charge, emulsions with three different MGP concentrations were prepared. However, before evaluating the effect of charge on droplet size and/or aggregation, it was important that all investigated emulsions possessed the same initial droplet size distribution. In this manner, one could trace back any observed differences solely to the pH or ionic strength.
The DSD of emulsions with the reference pH value (pH 4), prepared with 0.5; 1 and 2 wt% MGP particles is found in Fig. 1. As seen in the diagram, all formulations have similar oil droplet size distributions (x50,3 equal to 1.28 ± 0.02 μm for 0.5 wt%, 1.25 ± 0.18 μm for 1 wt%, and 1.65 ± 0.21 μm for 2 wt%). Therefore, these formulations are regarded as suitable for further investigation. Even though the 2 wt% MGP formulation is significantly different to the other two, the difference is only of 0.1 μm. An explanation why the MGP concentration does not strongly influence the resulting droplet size was discussed in previous publications:32 our own experiments had already demonstrated that the MGP concentration used for emulsion stabilisation does not affect the resulting oil droplet size, if the MGP content is kept below 5 wt%. Below this value, the ternary system composed of pectin-based MGP particles, oil, and water forms emulsions. Moreover, these previously investigated emulsions possessed identical viscosity curves and Newtonian flow behaviour. We have demonstrated that the key parameter to control droplet sizes in pectin-based MGP-stabilised emulsions is the mechanical energy input, i.e. process conditions.33 Hence, as the emulsification conditions in this study were kept constant, the particle concentration has little effect on the droplet size and viscosity prior to pH adjustment.
Coalescence and other emulsion instabilities are time-dependent and can occur over a period of minutes to hours, and even weeks.41 However, as we planned to assess the stability of the investigated emulsions and the effect of environmental conditions on emulsion stability (pH and I), the reference formulations should be stable at least over a period of three weeks. Therefore, we measured the droplet size distribution of each sample weekly, for a period of four weeks. The DSD of emulsions prepared with 1 wt% MGP and pH 4 is depicted in Fig. 2. The shown distributions do not change significantly from day 1 to day 14 (x50,3 = 1.39 ± 0.02 μm after 14 days). The curve measured after 21 days displays smaller droplet sizes (x50,3 = 0.98 ± 0.01 μm) than the initial emulsion. This could be attributed to the creaming of the largest droplets found in the emulsion. These droplets are then no longer detected by the static light scattering method, which translates into seemingly smaller droplet sizes.
Emulsions stabilised with 0.5 and 2 wt% MGP concentration at pH 4 showed a similar trend as the results presented in Fig. 2. The DSD of these formulations are found in the ESI S1.† After the evaluation of the long-term stability of the emulsions and the effect of MGP on the initial droplet size at the reference pH-value, these formulations were deemed representative for further investigation.
3.3. Influence of pH on emulsion stability
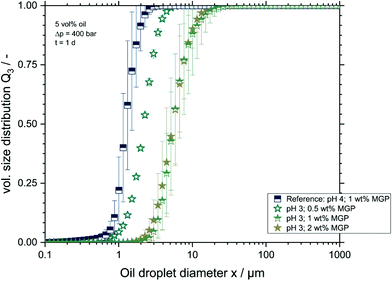
It is proposed in the literature that zeta-potentials over −30 mV or under +30 mV are insufficient to stabilise dispersions electrostatically as the repulsion among particles is not enough to avoid interactions.42 As seen from results depicted in Table 1, the investigated MGPs had potentials well over −30 mV. This means, in terms of droplet stabilisation, that at pH values under 4, most formulations are expected to be prone to aggregation. However, the zeta-potential measurements were conducted only on MGP dispersions in water. In order to test the influence of pH on droplet stability, the pH of the emulsions described above was reduced to 3 after emulsification. The DSD of emulsions prepared with 0.5; 1, and 2 wt% MGPs at pH 3 is depicted in Fig. 4. For a better comparison, the distribution of an emulsion with 1 wt% MGP at the reference pH value (pH 4) is also shown in the diagram.As seen in Fig. 3, the decrease of pH in the emulsions after the emulsification process increased the droplet sizes. The investigated samples had thereafter mean x50,3 diameters equal to 2.19 ± 0.02 μm, 6.02 ± 1.79 μm, and 5.65 ± 1.30 μm, for emulsions prepared with 0.5, 1, and 2 wt% MGPs, respectively. This could indicate that the loss of the electrostatic repulsion of the MGP triggered the coalescence of oil droplets. However, the detection of larger droplet sizes can also result from agglomeration: agglomerates can be detected as single droplets by the laser scattering method, shifting the droplet size distribution towards larger particles. This could also explain the appearance of larger droplets in the measurements. Therefore, microscope images of all samples were taken to allow for the differentiation between agglomerates and large oil droplets. The obtained micrographs are found in Fig. 4.
Fig. 4A (1 wt% MGP, pH4) shows evenly distributed uniform small emulsion droplets. Fig. 4B (0.5 wt% MGP, pH3) also shows evenly distributed emulsion droplets. However, several larger droplets can be observed, indicating coalescence. In Fig. 4C and D (1 wt% and 2 wt% MGP, pH 3) large agglomerates are depicted. In Fig. 4C, large droplets can be observed as well, whereas in Fig. 4D (the highest MGP concentration), there are no large oil droplets present.
Comparing Fig. 4A (pH 4, 1 wt% MGP) and 4B (pH 3, 0.5 wt% MGP) one can see that at pH 3 droplet sizes are larger than at the reference pH. This supports the measurements discussed in Fig. 3. As soon as the pH-value decreases, MGP's charge and size can change, which could cause changes in the oil droplet surface coverage, leaving the droplet surface free of MGPs.22 This could lead to the immediate droplet coalescence after an initial droplet encounter. Additionally, MGP deswelling will allow oil droplets to approach each other closer further, facilitating droplet coalescence.43,44
Micrographs of samples with higher microgel concentrations (1 wt% MGP and 2 wt% MGP, Fig. 4C and D) show that these samples are prone towards agglomeration. Although MGPs should have the same charge at a constant pH-value, the degree of droplet agglomeration increases with the MGP concentration. This could be due to the amount of particles available for agglomeration. In our studies, the MGPs are found to be adsorbed onto the surface of droplets. Therefore, we assume that the particle interactions translate to droplet interactions, and consequently to droplet agglomeration. Moreover, Fig. 4D (2 wt% MGP) displays larger agglomerates than 4B (1 wt% MGP), supporting the fact that the agglomerate size depends on the initial particle concentration.
The agglomerates (both particle and droplet agglomerates) appear to be larger in the micrographs than in the depicted DSD. Thus, we can conclude that they are easily broken up during the static laser scattering measurement by the recirculation of the measuring device. For this reason, the shown DSD curves of formulations with 1 or 2 wt% MGPs at pH 3 appear to be the identical. Nevertheless, the actual mean droplet size of single oil droplets is smaller than the measurement results suggest, as seen in Fig. 4C and D. This indicates that the steric stabilisation mechanism of MGP was able to prevent total droplet coalescence.
Fig. 5 shows the DSD of emulsions at pH 2. As is evident from Fig. 5, regardless of the MGP concentration used for emulsion stabilisation, the droplet size distribution is affected by the decrease of the pH. The mean droplet diameters of the investigated emulsions were 112.46 ± 0.05 μm, 60.77 ± 14.30 μm, and 25.48 ± 10.02 μm for 0.5; 1 and 2 wt% MGPs at pH 2, respectively. All diameters are significantly different from one another. All samples displayed larger particle sizes than the reference samples.
As seen in Table 1, MGPs at pH 2 had a zeta-potential close to 0 mV. Therefore, MGP-stabilised droplets were prone to form agglomerates upon droplet collision. Then, these agglomerates may coalesce if the steric barrier formed by MGP is insufficient. Fig. 5 shows that particle sizes at pH 2 are larger in samples with a lower MGP concentration. This relationship is opposite to the one determined at pH 3 where emulsions stabilised with 0.5 wt% had the smallest droplet sizes. In order to clarify whether the changes in droplet size are caused by agglomerate formation or droplet coalescence, micrographs were taken of all investigated samples at pH 2. These are depicted in Fig. 6.
Fig. 6 clearly shows that there are agglomerates and large coalesced droplets in all emulsions, regardless of the MGP concentration used for droplet stabilisation. Nonetheless, the aggregate size and droplet size do depend on the MGP concentration. Fig. 6A displays an emulsion stabilised with 0.5 wt% MGP. As seen from this micrograph, the agglomerates are made up of large oil droplets, probably held together by MGPs.10,32,45 In agreement with the data presented in Fig. 5, this formulation possessed larger oil droplets than the others. However, not only the oil droplets are measured in the distribution, but also the agglomerates. This could explain why diameters over 100 μm are detected. Here again, it is possible that agglomerates are ruptured during the DSD measurement.
Compared to Fig. 6A–D display smaller oil droplets, which are bound into agglomerates. For both these formulations, the quantity of MGPs used appears to have an influence on the coalescence prevention. Even though there are some large droplets, the majority of them are smaller than those shown in Fig. 6A. This indicates that higher MGP concentrations are better suited to stabilise oil droplets at pH 2, supporting the data shown in Fig. 5. Nevertheless, even the small droplets shown in Fig. 5 are larger than the initial droplet size found in the reference formulation (Fig. 1). This means that droplet coalescence still takes place even though a MGP steric barrier should prevent its occurrence. In summary, we can conclude that the stabilisation mechanism of pectin-based MGPs is predominately electrostatic. The steric barrier acts as secondary support, but is not able to stabilise oil droplets sufficiently on its own.
3.4. Influence of ionic strength on emulsion stabilisation
Having seen that electrostatic repulsion is the main stabilisation mechanism by which charged MGP particles stabilise oil-in-water emulsions, it becomes obvious that also the influence of ionic strength on emulsion stabilisation requires deeper investigation. In order to discriminate between the effect of pH and ionic strength, emulsions were prepared with 1 wt% MGP, and their pH value and EC were adjusted after the emulsification process. The investigated formulations at pH 3 and 4 were 0.65 mS cm−1 and 6.50 mS cm−1. The DSD of these formulations is shown in Fig. 7, alongside the DSD of the reference sample. The droplet sizes were determined one day after the samples’ preparation.Fig. 7 shows the volumetric size distributions of the investigated samples. It is clear that the increase in ionic strength (indirectly measured as EC) leads to an additional shift in droplet diameters. This is noticeable in the mean diameters, as seen for x50,3 equals to 40.94 ± 25.80 μm for 6.5 mS cm−1, and 6.02 ± 1.79 μm for 0.65 mS cm−1 at pH 3. The same trend is observed for samples with pH 4: 1.25 ± 0.18 μm for 0.28 mS cm−1, 1.08 ± 0.05 μm for 0.65 mS cm−1, and 2.48 ± 0.37 μm for 6.5 mS cm−1.
As shown in Fig. 7, the DSD generally correlates with the data obtained from the zeta-potential measurements. The reference sample and emulsions with pH 4 and an EC of 0.65 mS cm−1 have identical size distributions without any significant variance. In both these samples, the MGP had a zeta-potential of around −30 mV. However, the sample with pH 4 and an EC of 6.5 mS cm−1 displayed smaller droplets than the sample with pH 3 and EC 0.65 mS cm−1, even though the former had a greater ZP than the latter.
Despite having the same EC, emulsions display a larger droplet at pH 3 than at pH 4 due to a reduced surface charge at low pH. Moreover, at a constant pH-value, the effect of the added electrolytes is noticeable. For both investigated pH-values, the formulations with a higher electrolyte concentration showed larger droplets/or agglomerates due to a reduction of the Debye length. This is supported by the ZP-value, −8.2 ± 0.1 mV and −12.6 ± 0.1 mV for pH 3 and pH 4 at an EC of 6.5 mS cm−1, respectively.
In terms of particle size distribution, the effects on surface charge and on the length of the diffuse double layer add up and lead to large droplets or agglomerates. Emulsions with the highest EC and the lowest pH value (pH 3, 6.5 mS cm−1) possess the largest droplets (x50,3 of 40.94 ± 25.80 μm). Nevertheless, the effect of pH dominates over the effect of ionic strength on the increase of droplet size or aggregation.
These findings are not only valid for MGP from amidated pectin but are independent of the pectin type as long as the pectin used for MGP production bears significant charge. This was shown by preparing and characterising comparable emulsions with MGPs from different pectin types. The results for emulsions prepared with MGP from pectinic acid can be found in the ESI S2.†
In summary, one can conclude that the addition of ions to the emulsion lead to an increase in droplet size, as the length diffuse double layer surrounding the droplet might decrease. However this effect is not as predominant as the effect the pH has on the charge of MGPs. Stronger coalescence is expected under acidic conditions than in milieus of high ion concentration. Nevertheless, both effects add up, rending emulsion stabilised with pectin-based MGP bimodal and probably unstable.
3.5. Effect of pH on emulsion's long-term stability
The long-term stability of the investigated emulsions was also monitored. The long-term stability of reference emulsions is depicted in Fig. 2. The emulsions did not change their DSD or their appearance over a period of 3 weeks. However, as portrayed by the results above, emulsions at pH 3 and 2 were not stable. The observed agglomerates formed within minutes after pH adjustment. Immediately, strong agglomerate formation and creaming occurred, as is shown exemplarily in Fig. 8. The observations were comparable in all samples at pH 3, and 2, with emulsions at pH 2 displaying complete phase separation within minutes. Therefore, none of these samples were subjected to any further DSD characterisation. Emulsions stabilised by charged MGP can therefore be regarded as unstable at pH values lower than their pKa.4. Conclusions
Due to the fact that pectin is a polyelectrolyte, pectin-based MGPs were found to be pH and ionic strength sensitive in a similar manner to MGPs of charged synthetic polymers. The effect of the pH and ionic strength on the MGP charge was noticeable on the zeta-potential of the investigated MGPs. Under acidic conditions, MGPs approach a zero charge state. This effect was enhanced by the addition of counterions, as the MGP ZP decreased more pronouncedly at low pH-values and high EC.The sensitivity of pectin-based MGP towards changes in pH and ionic strength was also expected to influence the stability of emulsions. To investigate this, emulsions were homogenised with MGP concentrations ranging from 0.5 to 2 wt% MGP. After emulsification, the pH of the emulsions was adjusted from pH 4.2 to 4, 3 or 2, and the resulting droplet sizes were measured.
Directly after homogenisation, all emulsions had the same droplet size distribution regardless of the employed MGP concentration. They remained stable for at least three weeks. However, the droplet sizes changed upon pH-adjustment. All formulations displayed larger droplets at pH 3 and even larger at pH 2, but the degree of coalescence was dependent on the MGP concentration. The emulsion with the lowest MGP concentration showed coalesced droplets. Increasing MGP concentration did not prevent coalescence and agglomeration was noticed. High MGP concentrations caused particle bridging and even the formation of small particle networks with entrapped oil droplets within.
Similar effects were found when the electrolyte concentration was increased: at higher ionic strength droplet sizes increased and agglomerates were observed. However, compared to the effect of pH, the presence of counter ions did not cause as pronounced changes in the droplet size distribution. Therefore, one can conclude that the effect of reduced surface charge by pH shifts dominates over the effect of reduced Debye length by increasing ionic strength. Nevertheless, both effects can also add up leading to extremely unstable emulsions with complete phase separation at pH < pKa and high ionic strength. These findings were confirmed for other pectin types, such as pectinic acid. These results deepen the understanding of emulsion stabilisation by pectin-based MGPs. This knowledge can be used to control the stability of emulsions prepared with charged MGPs by formulation parameters.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This IGF Project (no. 19306 N) of the Förderkreis der Ernährungsindustrie e.V. (FEI) was supported via Arbeitsgemeinschaft industrieller Forschungsvereinigungen “Otto von Guericke” e.V. (AiF) within the program for promoting the Industrial Collective Research (IGF) of the German Ministry of Economic Affairs and Energy (BMWi), based on a resolution of the German Parliament.References
-
Food emulsions. Principles, practices, and techniques, ed. D. J. McClements, CRC Press, Boca Raton, 3rd edn, 2016, ISBN 9781498726689 Search PubMed
.
- L. Leistner, Food preservation by combined methods, Food Res. Int., 1992, 25, 151–158, DOI:10.1016/0963-9969(92)90158-2
.
- E. Dickinson, Microgels - An alternative colloidal ingredient for stabilization of food emulsions, Trends Food Sci. Technol., 2015, 43, 178–188 CrossRef CAS
.
- W. Richtering, Responsive emulsions stabilized by stimuli-sensitive microgels: emulsions with special non-Pickering properties, Langmuir, 2012, 28, 17218–17229, DOI:10.1021/la302331s
.
- T. Ngai, H. Auweter and S. H. Behrens, Environmental Responsiveness of Microgel Particles and Particle-Stabilized Emulsions, Macromolecules, 2006, 39, 8171–8177, DOI:10.1021/ma061366k
.
- L. A. Lyon and A. Fernandez-Nieves, The polymer/colloid duality of microgel suspensions, Annu. Rev. Phys. Chem., 2012, 63, 25–43 CrossRef CAS PubMed
.
- B. Brugger and W. Richtering, Emulsions stabilized by stimuli-sensitive poly(N-isopropylacrylamide)-co-methacrylic acid polymers: microgels versus low molecular weight polymers, Langmuir, 2008, 24, 7769–7777, DOI:10.1021/la800522h
.
- E. Dickinson, Hydrocolloids as emulsifiers and emulsion stabilizers, Food Hydrocolloids, 2009, 23, 1473–1482 CrossRef CAS
.
- C. Schmitt, C. Bovay and M. Rouvet, Bulk self-aggregation drives foam stabilization properties of whey protein microgels, Food Hydrocolloids, 2014, 42, 139–148, DOI:10.1016/j.foodhyd.2014.03.010
.
- M. Destribats, M. Rouvet, C. Gehin-Delval, C. Schmitt and B. P. Binks, Emulsions stabilised by whey protein microgel particles: towards food-grade Pickering emulsions, Soft Matter, 2014, 10, 6941–6954, 10.1039/c4sm00179f
.
- S. Zhang, M. Holmes, R. Ettelaie and A. Sarkar, Pea protein microgel particles as Pickering stabilisers of oil-in-water emulsions: Responsiveness to pH and ionic strength, Food Hydrocolloids, 2020, 102, 105583, DOI:10.1016/j.foodhyd.2019.105583
.
- Z. Zhang, R. Zhang, L. Zou and D. J. McClements, Protein encapsulation in alginate hydrogel beads: Effect of pH on microgel stability, protein retention and protein release, Food Hydrocolloids, 2016, 58, 308–315, DOI:10.1016/j.foodhyd.2016.03.015
.
- O. Torres, B. Murray and A. Sarkar, Emulsion microgel particles: Novel encapsulation strategy for lipophilic molecules, Trends Food Sci. Technol., 2016, 55, 98–108, DOI:10.1016/j.tifs.2016.07.006
.
- Z. Li and T. Ngai, Stimuli-responsive gel emulsions stabilized by microgel particles, Colloid Polym. Sci., 2011, 289, 489–496, DOI:10.1007/s00396-010-2362-z
.
- B. Brugger, B. A. Rosen and W. Richtering, Microgels as stimuli-responsive stabilizers for emulsions, Langmuir, 2008, 24, 12202–12208, DOI:10.1021/la8015854
.
- B. Brugger, S. Rütten, K. H. Phan, M. Möller and W. Richtering, The colloidal suprastructure of smart microgels at oil-water interfaces, Angew. Chem., Int. Ed., 2009, 48, 3978–3981, DOI:10.1002/anie.200900239
.
- T. Ngai, S. H. Behrens and H. Auweter, Novel emulsions stabilized by pH and temperature sensitive microgels, Chem. Commun., 2005, 331–333, 10.1039/b412330a
.
- K. M. Ho, W. Y. Li, C. H. Wong and P. Li, Amphiphilic polymeric particles with core–shell nanostructures: emulsion-based syntheses and potential applications, Colloid Polym. Sci., 2010, 288, 1503–1523, DOI:10.1007/s00396-010-2276-9
.
- S. Schachschal, A. Balaceanu, C. Melian, D. E. Demco, T. Eckert, W. Richtering and A. Pich, Polyampholyte Microgels with Anionic Core and Cationic Shell, Macromolecules, 2010, 43, 4331–4339, DOI:10.1021/ma100184h
.
- C. D. Jones and L. A. Lyon, Synthesis and Characterization of Multiresponsive Core–Shell Microgels, Macromolecules, 2000, 33, 8301–8306, DOI:10.1021/ma001398m
.
- A. Pich, A. Tessier, V. Boyko, Y. Lu and H.-J. P. Adler, Synthesis and Characterization of Poly(vinylcaprolactam)-Based Microgels Exhibiting Temperature and pH-Sensitive Properties, Macromolecules, 2006, 39, 7701–7707, DOI:10.1021/ma060985q
.
- M. Destribats, V. Lapeyre, M. Wolfs, E. Sellier, F. Leal-Calderon, V. Ravaine and V. Schmitt, Soft microgels as Pickering emulsion stabilisers: role of particle deformability, Soft Matter, 2011, 7, 7689, 10.1039/c1sm05240c
.
- C. G. Lopez, T. Lohmeier, J. E. Wong and W. Richtering, Electrostatic expansion of polyelectrolyte microgels: Effect of solvent quality and added salt, J. Colloid Interface Sci., 2020, 558, 200–210, DOI:10.1016/j.jcis.2019.07.042
.
- A. A. Gavrilov, W. Richtering and I. I. Potemkin, Polyelectrolyte Microgels at a Liquid-Liquid Interface: Swelling and Long-Range Ordering, J. Phys. Chem. B, 2019, 123, 8590–8598, DOI:10.1021/acs.jpcb.9b07725
.
- T. Liu, S. Seiffert, J. Thiele, A. R. Abate, D. A. Weitz and W. Richtering, Non-coalescence of oppositely charged droplets in pH-sensitive emulsions, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 384–389, DOI:10.1073/pnas.1019196109
.
- S. Schmidt, T. Liu, S. Rütten, K. Phan, M. Möller and W. Richtering, Influence of microgel architecture and oil polarity on stabilization of emulsions by stimuli-sensitive core-shell poly(N-isopropylacrylamide-co-methacrylic acid) microgels: Mickering versus Pickering behavior?, Langmuir, 2011, 27, 9801–9806, DOI:10.1021/la201823b
.
- G. I. Saavedra Isusi, B. Bindereif, H. P. Karbstein and U. S. van der Schaaf, Polymer or microgel particle: differences in emulsifying properties of pectin as microgel or as individual polymer chains, Colloids Surf., A, 2020, 124793, DOI:10.1016/j.colsurfa.2020.124793
.
- B. R. Thakur, R. K. Singh, A. K. Handa and M. A. Rao, Chemistry and uses of pectin. A Review, Crit. Rev. Food Sci. Nutr., 1997, 37, 47–73 CrossRef CAS PubMed
.
- P. Varela and S. M. Fiszman, Exploring consumers’ knowledge and perceptions of hydrocolloids used as food additives and ingredients, Food Hydrocolloids, 2013, 30, 477–484, DOI:10.1016/j.foodhyd.2012.07.001
.
- E. R. Morris, D. A. Powell, M. J. Gidley and D. A. Rees, Conformation and Interactions of Pectin: I. Polymorphism between Gel and Solid States of Calcium Polygalactunoate, J. Mol. Biol., 1982, 155, 507–516 CrossRef CAS PubMed
.
- U. S. Schmidt, K. Schmidt, T. Kurz, H.-U. Endreß and H. P. Schuchmann, Pectins of different origin and their performance in forming and stabilizing oil-in-water-emulsions, Food Hydrocolloids, 2015, 46, 59–66 CrossRef CAS
.
- G. I. Saavedra Isusi, L. B. Madlindl, H. P. Karbstein and U. S. van der Schaaf, Microstructures and conformational arrangement in emulsions caused by concentration ratios of pectin-based microgels and oil, Colloids Surf., A, 2020, 125166, DOI:10.1016/j.colsurfa.2020.125166
.
- G. I. Saavedra Isusi, N. Lohner, H. P. Karbstein and U. S. van der Schaaf, Emulsions stabilised with pectin-based microgels: Investigations into the break-up of droplets in the presence of microgels, J. Food Eng., 2021, 294, 110421, DOI:10.1016/j.jfoodeng.2020.110421
.
- M. A. K. Williams, R. R. Vincent, D. N. Pinder and Y. Hemar, Microrheological studies offer insights into polysaccharide gels, J. Non-Newtonian Fluid Mech., 2008, 149, 63–70 CrossRef CAS
.
- J. F. Thibault and M. Rinaudo, Chain Association of Pectic Molecules during calcium-induced gelation, Biopolymers, 1986, 25, 455–468 CrossRef CAS
.
- J. F. Thibault and M. Rinaudo, Interactions of Mono- and Divalent Counterions with Alkali- und Enzyme-Deesterified Pectins in Salt-Free Solutions, Biopolymers, 1985, 24, 2131–2143 CrossRef CAS
.
- M. Garnier, M. A. V. Axelos and J. F. Thibault, Selectivity and cooperation in the binding of calcium ions by pectins, Carbohydr. Res., 1971, 256, 71–81 CrossRef
.
- E. R. Morris, D. A. Powell, M. J. Gidley and D. A. Rees, Conformation and Interactions of Pectin: I. Polymorphism between Gel and Solid States of Calcium Polygalactunoate, J. Mol. Biol., 1982, 155, 507–516 CrossRef CAS PubMed
.
- A. Ström and M. A. K. Williams, Controlled Calcium Release in the Absence and Presence of an Ion-Binding Polymer, J. Phys. Chem. B, 2003, 107, 10995–10999 CrossRef
.
- C. P. Whitby, H. Khairul Anwar and J. Hughes, Destabilising Pickering emulsions by drop flocculation and adhesion, J. Colloid Interface Sci., 2016, 158–164, DOI:10.1016/j.jcis.2015.11.063
.
- M. Smoluchowski, Drei Vorträge über Diffusion, Brownsche Molekularbewegung und Koagulation von Kolloidteilchen, Phys. Z., 1916, 17, 557–585 Search PubMed
.
-
T. M. Riddick, Control of colloid stability through zeta potential, Livingston Pub. CO, Wynnewood, PA, 1968 Search PubMed
.
- A. K. Chesters, The modelling of coalescence processes in fluid-liquid dispersions: a review of current understanding, Chem. Eng. Res. Des., 1991, 69, 259–270 CAS
.
- J. Kamp, J. Villwock and M. Kraume, Drop coalescence in technical liquid/liquid applications: a review on experimental techniques and modeling approaches, Rev. Chem. Eng., 2017, 33, 1–47, DOI:10.1515/revce-2015-0071
.
- J. W. J. de Folter, M. W. M. van Ruijven and K. P. Velikov, Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein, Soft Matter, 2012, 8, 6807, 10.1039/c2sm07417f
.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/d1fo00891a |
| This journal is © The Royal Society of Chemistry 2021 |