Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Clostridioides difficile toxin A-mediated Caco-2 cell barrier damage was attenuated by insect-derived fractions and corresponded to increased gene transcription of cell junctional and proliferation proteins†
Liyou
Dong
 ab,
Monic M.
Tomassen
a,
Renata M. C.
Ariëns
ab,
Monic M.
Tomassen
a,
Renata M. C.
Ariëns
 a,
Els
Oosterink
a,
Harry J.
Wichers
a,
Els
Oosterink
a,
Harry J.
Wichers
 ab,
Teun
Veldkamp
c,
Jurriaan J.
Mes
a and
Coen
Govers
ab,
Teun
Veldkamp
c,
Jurriaan J.
Mes
a and
Coen
Govers
 *ad
*ad
aWageningen Food and Biobased Research, Wageningen University & Research, Wageningen, The Netherlands. E-mail: coen.govers@wur.nl
bLaboratory of Food Chemistry, Wageningen University & Research, Wageningen, The Netherlands
cWageningen Livestock Research, Wageningen University & Research, Wageningen, The Netherlands
dLaboratory of Cell Biology and Immunology, Wageningen University & Research, Wageningen, The Netherlands
First published on 9th July 2021
Abstract
Pathogenesis of C. difficile in the intestine is associated with the secretion of toxins which can damage the intestinal epithelial layer and result in diseases such as diarrhoea. Treatment for C. difficile infections consists of antibiotics which, however, have non-specific microbiocidal effects and may cause intestinal dysbiosis which results in subsequent health issues. Therefore, alternative treatments to C. difficile infections are required. We investigated whether different black soldier fly- and mealworm-derived fractions, after applying the INFOGEST digestion protocol, could counteract C. difficile toxin A-mediated barrier damage of small intestinal Caco-2 cells. Treatment and pre-treatment with insect-derived fractions significantly (p < 0.05) mitigated the decrease of the transepithelial electrical resistance (TEER) of Caco-2 cells induced by C. difficile toxin A. In relation to these effects, RNA sequencing data showed an increased transcription of cell junctional and proliferation protein genes in Caco-2 cells. Furthermore, the transcription of genes regulating immune signalling was also increased. To identify whether this resulted in immune activation we used a Caco-2/THP-1 co-culture model where the cells were only separated by a permeable membrane. However, the insect-derived fractions did not change the basolateral secreted IL-8 levels in this model. To conclude, our findings suggest that black soldier fly- and mealworm-derived fractions can attenuate C. difficile induced intestinal barrier disruption and they might be promising tools to reduce the symptoms of C. difficile infections.
1. Introduction
Our intestinal homeostasis is dependent on interactions between host defensive mechanisms and commensals.1 This host defence mechanism is composed of various layers and includes the mucus, epithelium and local immune system.2 The epithelial layer is composed of secretory Paneth, goblet and enteroendocrine cells, antigen sampling microfold cells (M cells) and mainly absorptive enterocytes. Intestinal epithelial cells provide a physical and biochemical barrier to separate luminal microbiota from immune cells in the lamina propria. Whilst doing so, the luminal content is screened by enterocytes through receptor recognition3 and M cells that are specialised in transporting the luminal content for immune monitoring. This is necessary as the intestinal lumen is prone to enteral pathogenic entry.Some commensals contain pathogenic features, especially when overgrown. Clostridioides is such a genus that contains many opportunistic species including Clostridioides (C.) difficile.4,5C. difficile is a Gram-positive, obligate anaerobic and spore forming bacterium.6,7 In homeostasis, C. difficile naturally resides at a low frequency in the human intestinal tract. Upon dysbiosis of the microbiome, C. difficile frequencies can increase dramatically and induce intestinal diseases such as diarrhoea and intestinal necrotic enteritis.8 These pathologies are a direct result of the secretion of toxins during sporulation and facilitate dissemination of the spores.
C. difficile produces two main virulent toxins called toxin A and toxin B and these two toxins exhibit enterotoxicity and cytotoxicity, respectively.9 Toxin A and toxin B contain RHO and RAC glucosyl transferase domains at the amino terminus allowing them to inactivate host RHO and RAC GTPases in the cytosol through glycosylation.6 This disrupts the continuous (re)formation of the cytoskeleton and leads to the disassociation of tight junctions between the epithelial cells, resulting in an increased permeability of the intestinal epithelial barrier. The increased intestinal epithelial permeability results in exposure of intestinal microbiota and microbiota-associated molecular patterns to intestinal immune cells, which exacerbates intestinal symptoms with inflammation and contributes to many intestinal diseases such as inflammatory bowel disease.10 Even though some antibiotics such as cephamycin have been demonstrated to be effective for the treatment of recurrent C. difficile infections, the strong microbial inhibitive effect consequently induces dysbiosis of gut microbiota, resulting in adverse health effects.11 Therefore, alternatives to treat C. difficile infections are needed.
In animal studies, insects have been demonstrated to promote the proliferation of intestinal epithelial cells and prevent C. difficile-mediated mucosal damage.12 The underlying mechanism is attributed to the presence of anti-microbial peptides (AMPs) (i.e. defensins and cecropins) and fatty acids (i.e. lauric acid) that can aid in controlling the growth of pathogenic bacteria, including C. difficile12 and Streptococcus mitis.13 Moreover, insects contain fibres such as chitin that could be fermented by intestinal microbiota, leading to the production of SCFAs such as butyrate which support the proliferation of intestinal epithelial cells.14
In this study, we explored the C. difficile toxin A counteractive potency of four different insect-derived fractions with varying contents of AMPs, fatty acids and polysaccharides that were in vitro digested to mimic the intestinal bioactivity. Support of the barrier integrity of small intestinal human epithelial cells and the potential mechanism were investigated using black soldier fly larvae protein meal, mealworm larvae powder, chitin rich black soldier fly larvae protein meal, and black soldier fly cocoon meal.
2. Materials & methods
2.1 Nutritional composition of insect-derived fractions
Black soldier fly larvae protein meal (BP), mealworm larvae powder (MP), black soldier fly cocoon meal (BC) and chitin rich black soldier fly larvae protein meal (BchP) were provided by Protix (Dongen, The Netherlands). Detailed production processes and nutritional compositions of these insect-derived products have been described in a previous study.15 Briefly, BP contains 56% protein, 10% chitin and 15% fat; BC contains 63% protein, 20% chitin and 7% fat; BchP contains 50% protein, 15% chitin and 18% fat; and MP contains 56% protein, 8% chitin and 29% fat.2.2 In vitro digestion
In vitro digestion was performed according to the consensus INFOGEST standardized protocol with a minor modification.16 The enzyme concentration in the consensus INFOGEST protocol was decreased 10-fold to support the cell viability upon exposure to the digests without affecting the hydrolysis level. The in vitro digestion involved the exposure of insect-derived fractions to three successive digestive processes: oral, gastric and intestinal, each containing a dilution component with specific fluids. Simulated salivary fluid (SSF) was prepared by mixing 3.7 mM KH2PO4 (Merck, Darmstadt, Germany), 15.1 mM KCl (Merck), 13.6 mM NaHCO3 (Merck), 0.15 mM MgCl2(H2O)6 (BOOM, Meppel, The Netherlands) and 0.06 mM (NH4)2CO3 (Sigma-Aldrich, Zwijndrecht, The Netherlands) in 1 L of MilliQ (Biopak® polisher; Merck). Simulated gastric fluid (SGF) was prepared by dissolving 6.9 mM KCl, 0.9 mM KH2PO4, 25 mM NaHCO3, 47.2 mM NaCl (Sigma-Aldrich), 0.1 mM MgCl2(H2O)6 and 0.5 mM (NH4)2CO3 in 1L of MilliQ. Finally, simulated intestinal fluid (SIF) was prepared by dissolving 6.8 mM KCl, 0.8 mM KH2PO4, 123.4 mM NaCl and 0.33 mM MgCl2(H2O)6 in 1 L of MilliQ. The oral digestive phase involved the fractionation of insects using an IKA mill (model A11 B, IKA-Werke GmbH and Co. KG, Staufen, Germany) to mimic the chewing process, dilution of insect samples by mixing 1 gram of fractioned insect samples or MilliQ (empty digest control) with 9 grams of MilliQ water. Subsequently, 10 grams of insect suspension was mixed with 8 ml of SSF, 50 μl of 0.3 M CaCl2 (Sigma-Aldrich) and 1.95 ml of MilliQ reaching a total volume of 20 ml and the mixture was transferred to a thermal water jacket vessel to maintain a temperature of 37 °C through circulating water to complete the oral digestive phase. For the gastric digestive phase, 16 ml of SGF, 0.6 ml of pepsin (Sigma-Aldrich) solution (200 units per ml) and 10 μl of 0.3 M CaCl2 were added to the bottle. The pH of the total fluid was adjusted to 3 using 1 M HCl (Sigma-Aldrich) after which the total volume of the mixture was adjusted to 40 ml and incubated for 2 h at 37 °C. Next for the small intestinal digestive phase 27 ml of SIF, 4.02 ml of 199 mM bile extracts (Sigma-Aldrich), 80 μl of 0.3 M CaCl2 and 5 ml of pancreatin solution (10 units per ml) (Sigma-Aldrich) were added. The pH value was adjusted to 7 using 1 M NaOH (Sigma-Aldrich) after which the total volume was adjusted to 80 ml, reaching a final insect fraction concentration of 12.5 mg ml−1, and incubated for 2 h at 37 °C. The samples were continuously stirred at level 3 (on a scale from 1 to 8) during all steps of digestion and the pH of the total fluid was monitored and maintained at 3 for the gastric digestion phase or 7 for the small intestinal digestion phase using a pH-stat titration apparatus (model 888 Titrando, Metrohm AG, Switzerland). Immediately after in vitro digestion, digests of the control (empty digest (ED)) BP, BC, BchP and MP were aliquoted and stored at −80 °C.2.3 Caco-2 cell culture and treatment
Caco-2 cells (American Type Culture Collection, Rockville, USA) were seeded on the apical side of the transwell inserts of 33.6 mm2, 0.4 μm pore size and 1 × 108 pores per cm2 (Greiner Bio-one, Frickenhausen, Germany). The Caco-2 cells were added at a concentration of 3.375 × 104 cells to 150 μl of DMEM (Gibco, Bleiswijk, The Netherlands), and 700 μl of basolateral medium containing 4.5 g l−1D-glucose, L-glutamine, 25 mM HEPES and supplemented with 10% heat inactivated fetal bovine serum (FBS; Hyclone PerBio, Etten-Leur, The Netherlands) and cultured at 37 °C with 5% CO2. The cells were incubated for 21 days to allow differentiation into small intestinal-like epithelial cells. Both the apical and basolateral media were replaced three times per week and one day before exposure to the samples. Before addition to differentiated Caco-2 cells, the digests of all samples were diluted 4 times with medium (yielding a final concentration of 3.125 mg ml−1). This constitutes the minimal required dilution to prevent digestive enzymes from affecting the Caco-2 cell viability (data not shown). FITC-dextran 4 kDa (FD4, Sigma-Aldrich) was added to the apical compartment of the differentiated Caco-2 cells at a concentration of 250 μg ml−1 and translocation to the basolateral compartment was measured using a spectrophotometer at 490 nm excitation and 525 nm emission wavelengths (model Infinite 200 PRO, TECAN, Giessen, The Netherlands). The differentiated Caco-2 cells were exposed to 0.25 μg ml−1Clostridioides difficile toxin A (List Lab, Campbell, CA, USA) and/or 3.125 mg ml−1 insect-derived fraction. The integrity of the Caco-2 monolayer was measured by transepithelial electrical resistance (TEER) using a MilliCell-ERS (Millipore, Amsterdam-Zuidoost, The Netherlands) apparatus before and after the addition of toxin A or any of the stimuli. The TEER value was normalised to the medium control and the starting time point of individual transwells.2.3 RNA extraction and sequencing
RNA isolation was performed as described previously.17 Briefly, Caco-2 cells were lysed with 0.2 ml of TRIzol® (Invitrogen, Bleiswijk, The Netherlands) after 3 h of exposure to medium, ED, BPD, MPD, BCD or BchPD and RNA was isolated using a RNeasy mini kit (Qiagen, Germantown, MD, USA) according to the manufacturer's protocol. The isolated RNA was sent to Baseclear B.V. (Leiden, The Netherlands) for RNA sequencing. The cDNA library was sequenced on the NovaSeq 6000 system (Illumina, San Diego, CA, USA) to produce 150 bp paired-end reads. The raw paired-ends FASTQ files were trimmed and converted using the bcl2fastq2 Conversion Software (version 2.18, Illumina). In this step, the raw data were filtered to remove adapter and low-quality sequences and only reads with an average Q-score >30 were imported to the CLC Genomics Workbench (Microbial Genomics toolbox version 20.0, Qiagen) for further analysis. The paired-end reads were mapped to Homo sapiens HG38 reference genome (v99) to generate counts of genes and transcript hits as RPKM (reads per kilobase of exon model per million mapped reads) and TPM (transcripts per million). The counts were normalized by TMM (Trimmed Mean of M values) normalization according to the method of Robinson.18 The normalized counts in the treated and control samples were subjected to differential expression analysis which was determined using a negative binomial Generalized Linear Model.19 Differentially expressed genes with an absolute p value <0.05, an expression value >10.0 and a fold change ≥1 were uploaded into Ingenuity Pathway Analysis (IPA, Qiagen, Hilden, Germany) to perform gene and pathway analysis.2.4 Gene transcription and pathway analysis
Ingenuity Pathway Analysis (IPA, Qiagen, Hilden, Germany) was used to perform core analysis to analyse the significantly activated (−log10(p-value) > 1.3) canonical pathway in Caco-2 cells after exposure to insect-derived fractions when compared to that of the empty digest. For analysis, the limitations were set to “Human” in the “Species” category, and “Caco-2 cells” in the category of “Tissue & Cell lines”.2.5 THP-1 cell culture
The human monocytic leukaemia cell line (THP-1; American Type Culture Collection, Rockville, MD, USA) was cultured in RPMI 1640 medium (Lonza, Basel, Switzerland) containing 10% FBS (Hyclone PerBio) and 1% penicillin/streptomycin (Invitrogen) at 37 °C under a 5% CO2 atmosphere. THP-1 cells were passaged every 3 days at a concentration of 0.25 × 106 ml−1 in 20 ml of medium in a T75 culture flask (Corning®, Amsterdam, The Netherlands). To differentiate THP-1 cells into macrophages, THP-1 cells were seeded into a 24-well plate (Greiner Bio-one) at a concentration of 1 × 106 cells per ml in 0.5 ml of RPMI medium and exposed to 100 ng ml−1 phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) for 3 days. Following extensive washing to remove residual PMA, the cells were rested for 72 h before subsequent stimulations.2.6 Caco-2 and THP-1 co-cultured model
In the co-culture model, Caco-2 cells were cultured on the apical side of the membrane in transwell inserts and THP-1 macrophages were cultured on the basolateral side of the membrane (ESI, Fig. 1†). Transwells containing Caco-2 cells that were differentiated for 20 days were placed upside down in a container with sufficient surface area to contain up to 12 transwell inserts and sufficient volume to ensure the Caco-2 cells, now inverted, remained in continuous contact with DMEM containing additional 1% penicillin/streptomycin as described before as part of the generated M-cells.20 Of note, inverting the transwells is done in such a way that no air pocket is trapped with the Caco-2 cells. A silicon ring was attached to the transwells to create a compartment on the basolateral side of the membrane. In this compartment, 0.5 ml of THP-1 macrophages, harvested after 72 h of rest, at a concentration of 0.4 × 106 cells per ml were seeded onto the transwell membrane and cultured O/N at 37 °C under a 5% CO2 atmosphere to support cell adhesion. On the next day, the silicon ring was removed and the co-culture system was placed into a new 24-well plate with 150 μl and 700 μl of fresh DMEM medium in the apical and basolateral compartments, respectively, followed by a 2 h recovery period at 37 °C under a 5% CO2 atmosphere (ESI, Fig. 1†). Typically, 60% of the macrophages remain attached to the basolateral side of the membrane (data not shown). After recovery, the medium in the apical compartment was removed and replaced by 4× diluted digests of insect-derived fractions (3.125 mg ml−1) or fresh medium (control) and incubated at 37 °C under a 5% CO2 atmosphere for 24 h. The integrity of the monolayer was measured by TEER before and 24 h after the addition of stimuli. After 24 h of exposure, the basolateral medium was collected and stored at −80 °C.2.7 Measurement of IL-8 secretion
In the collected supernatants the secretion of IL-8 was measured using an IL-8 ELISA kit (BioLegend, Koblenz, Germany) according to the manufacturer's instructions.2.8 Statistical analysis
Data are presented as mean or mean + SD and statistically significant differences between parameters were analysed by one-way ANOVA (GraphPad Prism 8, La Jolla, CA, USA).3. Results
3.1 Digested insect-derived fractions did not affect the barrier integrity of small intestinal-like Caco-2 cells
Caco-2 cells, mimicking small intestinal epithelial cells, were exposed to digested insect-derived fractions including black soldier fly larvae protein meal digest (BPD), mealworm larvae powder digest (MPD), black soldier fly cocoon meal digest (BCD) and chitin rich black soldier fly larvae protein meal digest (BchPD). The insect-derived fractions were digested according to the consensus INFOGEST protocol, with the adaptation that the enzyme levels were reduced by a factor of 10. This did not result in a reduced protein hydrolysis following a full digest (i.e. 120 minutes of intestinal digestion) (data not shown). All applications of insect-derived fractions followed this digestion protocol.The changes in the barrier integrity of small intestinal-like Caco-2 cells were monitored by TEER analysis during a period of 24 h (Fig. 1A) and by determining the basolateral FD4 translocation over a period of 24 h (Fig. 1B). Neither the empty digest (ED), containing solely the enzymes added during digestion, nor any of the insect-derived fractions induced significant changes in TEER or FD4 translocation levels when compared to the medium control.
3.2 Insect-derived fractions mitigated toxin A-induced Caco-2 cell barrier disruption
Having established that the insect-derived fractions did not reduce the barrier integrity, we investigated whether they can counteract the harmful effects of C. difficile-derived toxins on the intestinal epithelium. To this end, we applied toxin A to the small intestinal-like Caco-2 cells, which is known to disrupt the barrier integrity.21 Although C. difficile produces both toxin A and toxin B, small intestinal-like Caco-2 cells were demonstrated to be only sensitive to toxin A.21 Indeed, toxin A significantly reduced the TEER values to approximately 50% after 3 hours, which reached approximately 75% after 24 hours of exposure (Fig. 2).Next, the differentiated Caco-2 cells were simultaneously exposed to toxin A and medium (no treatment) or insect-derived fractions or empty digest control (Fig. 2). The addition of ED or BCD did not alter the TEER values of Caco-2 cells when compared to exposure to toxin A alone. In contrast, the addition of BPD, MPD or BchPD to the toxin A challenged Caco-2 cells led to a significant (p < 0.05) increase in the TEER values. After 3 hours of incubation the impact of toxin A on TEER was reduced by 27.1%, 18.2% or 21.5% through the addition of BPD, MPD or BchPD, respectively. After 6 hours of incubation BPC, MPD or BchPD continued to reduce the impact of toxin A by 9.2%, 7.1% or 8.1%, respectively.
3.3 Insect-derived fractions demonstrated a preventive effect towards toxin A-mediated Caco-2 cell barrier disruption
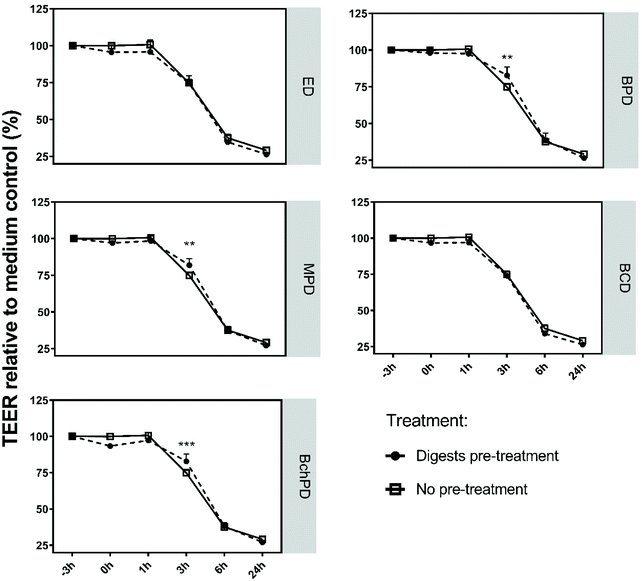
To study whether the pre-treatment of Caco-2 cells with insect-derived fractions could also limit the impact of toxin A on the Caco-2 monolayer, we exposed the Caco-2 cells to ED, BPD, MPD, BCD and BchPD for a period of 3 h prior to the addition of toxin A (Fig. 3). Before the addition of toxin A, the digests were removed through aspiration. As shown in Fig. 1, none of the pre-treatments altered the TEER values during the 3 hours before the addition of toxin A. In this preventive setting, again BPD, MPD or BchPD demonstrated significantly (p < 0.05) increased TEER values when compared to the medium treatment of toxin A. ED and BCD again did not counteract the impact of toxin A. In addition to pre-treatment, the effects of the insect-derived fractions were also tested in a curative setting. When added 2 h post the addition of toxin A, neither the medium nor any of the insect-derived fractions significantly mitigated the toxin A mediated Caco-2 cell barrier disruption (ESI, Fig. 2†).3.4 Insect-derived fractions induced significant changes in gene transcription of Caco-2 cells
To investigate the mechanism by which the insect-derived fractions counteract the toxin A-induced Caco-2 cell barrier damage, the transcriptional changes of Caco-2 cells following stimulation with the fractions were investigated. RNA was extracted from the cells treated for 3 h with ED, BPD, MPD, BCD or BchPD and analysed via RNA sequencing to provide a transcriptional overview. Gene expression was investigated as a fold change compared to the ED treated Caco-2 cells. The most significant (p < 0.001) changes in gene expression when compared to ED were observed following BchPD stimulation (i.e. 229) and for the least amount of genes following BCD stimulation (i.e. 78) (Fig. 4A). Among all the genes that were significantly increased or decreased in transcription by the individual stimuli, the expression of one gene was downregulated and the expression of 34 genes was upregulated by BPD, MPD, BCD and BchPD (data not shown). Of note, the transcription of 138 genes was altered by both BPD and BchPD, reflecting the homogeneity between these two samples (Fig. 4B). Genes whose transcription was commonly or stimulation-specifically activated are listed in ESI, Table 1.†3.5 Processes to support barrier integrity appeared to be stimulated by treatment of Caco-2 cells with insect-derived fractions
Genes coding for proteins involved in forming and maintaining the Caco-2 monolayer barrier integrity were investigated for changes in transcription following stimulation with the insect-derived fractions. Genes related to tight junctions, non-classical junctions (i.e. immunoglobulin superfamily cell adhesion molecules and tetraspanin-enriched microdomains), desmosomes and focal adhesions22 with significant (p < 0.05) changes in expression when compared to ED are listed in Table 1. In general, all insect-derived fractions demonstrated increased transcription of junction-related genes when compared to ED. Of note, only claudin-1 was found to be significantly (p < 0.05) increased in ED when compared with the medium control among all the epithelial cell junction-related genes (data not shown). Next, all genes that were significantly (p < 0.05) changed in transcription following the Caco-2 cell treatment with ED, BPD, MPD, BCD or BchPD were integrated and analysed by ingenuity pathway analysis (IPA). Analysis of the activated or inhibited canonical pathways (Fig. 5A) showed that only BPD and BchPD were predicted with high significance ((−log10(p-value) > 1.3) to activate the pathways related to barrier function. Both BPD and BchPD were predicted to activate the “signalling pathways of Sertoli cell–Sertoli cell junction” and “regulation of epithelial mesenchymal transition (EMT)” in Caco-2 cells, with BPD shown to be more active in the regulation of these pathways when compared to BchPD. Neither MPD nor BCD activated pathways related to barrier function, indicating that genes with a significantly altered transcription level insufficiency overlapped with genes involved in the barrier function-related pathways.| Gene acronym | Gene name | BPD | MPD | BCD | BchPD |
|---|---|---|---|---|---|
| Caco-2 cells were differentiated into small intestinal-like cells and exposed for 3 h to ED, BPD, MPD, BCD or BchPD after which gene transcription was analysed using RNA sequencing. Genes related to epithelial cell junctions were listed with fold changes following exposure to BPD, MPD, BCD or BchPD when compared to ED. BPD: digest of black soldier fly larvae protein meal; MPD: digest of mealworm larvae powder; BCD: digest of black soldier fly cocoon meal; BchPD: digest of chitin rich black soldier fly larvae protein meal; and N. D: not detected. | |||||
| CEACAM1 | CEA cell adhesion molecule 1 | 1.20 | 1.25 | N. D | N. D |
| CLDN1 | Claudin 1 | 1.56 | 1.25 | N. D | 1.36 |
| CLDN2 | Claudin 2 | N. D | 1.38 | N. D | N. D |
| CLDN4 | Claudin 4 | 1.55 | 1.29 | N. D | 1.48 |
| CLDN7 | Claudin 7 | N. D | N. D | −1.28 | −1.30 |
| CLDND1 | Claudin domain containing 1 | 1.56 | 1.31 | 1.38 | 1.69 |
| CXADR | CXADR Ig-like cell adhesion molecule | 1.37 | N. D | 1.27 | 1.57 |
| DSG2 | Desmoglein 2 | 1.21 | N. D | N. D | N. D |
| EPCAM | Epithelial cell adhesion molecule | N. D | 1.30 | N. D | 1.35 |
| ITGA6 | Integrin subunit alpha 6 | 1.30 | 1.29 | N. D | 1.40 |
| ITGAV | Integrin subunit alpha V | N. D | N. D | N. D | 1.30 |
| OCLN | Occludin | 1.28 | 1.34 | 1.27 | 1.31 |
| PVR | PVR cell adhesion molecule | N. D | −1.40 | −1.39 | −1.32 |
| TJP3 | Tight junction protein 3 | N. D | N. D | −1.34 | N. D |
| TSPAN12 | Tetraspanin 12 | 1.33 | 1.47 | 1.28 | 1.46 |
| TSPAN13 | Tetraspanin 13 | 1.28 | 1.31 | N. D | 1.38 |
In a similar fashion, ingenuity pathway analysis was performed to identify the activation of pathways related to cell proliferation, as this also represents a manner through which barrier integrity can be supported. The significantly (−log10(p-value) > 1.3) activated canonical pathways and genes in Caco-2 cells that are related to cell proliferation following the exposure to ED, BPD, MPD, BCD or BchPD were analysed (Fig. 5B and ESI Table 2†). This revealed that BPD was predicted to activate the “integrin-linked kinase (ILK) signalling” pathway and BchPD was predicted to activate the “p21-activated kinase (PAK) signalling”, “cell cycle control of chromosomal replication”, “aldosterone signalling in epithelial cells”, “ErbB2-ErbB3 signalling” and “extracellular signal-regulated kinases/mitogen-activated protein kinase (ERK/MAPK) signalling” pathways in Caco-2 cells. Neither the MPD nor BCD activated pathway related to cell proliferation.
3.6 Effect of insect-derived fractions on immune function-related gene expression and pathway analysis
Genes coding for proteins that are related to the immune response of Caco-2 cells were studied for transcriptional changes following the exposure to the insect-derived fractions. Genes that were significantly (p < 0.05) changed in expression when compared with ED and involved in immune signalling pathways, including chemokines and cytokines, cytokine receptors, and transcription factors that regulate inflammatory or anti-inflammatory responses, are listed in Table 2. In general, the expressions of most of these genes were increased after exposure to BPD, MPD or BchPD when compared with ED. Of note, expression of the listed genes was not significantly (p < 0.05) changed following ED exposure when compared with medium control (data not shown).| Gene acronym | Gene name | BPD | MPD | BCD | BchPD |
|---|---|---|---|---|---|
| Caco-2 cells were differentiated into small intestinal-like cells and exposed for 3 h to ED, BPD, MPD, BCD or BchPD after which gene transcription was analysed using RNA sequencing. Genes related to immunity were listed with fold changes following exposure to BPD, MPD, BCD or BchPD when compared to ED. BPD: digest of black soldier fly larvae protein meal; MPD: digest of mealworm larvae powder; BCD: digest of black soldier fly cocoon meal; BchPD: digest of chitin rich black soldier fly larvae protein meal; and N. D: not detected. | |||||
| CCL20 | C–C motif chemokine ligand 20 | 3.11 | 2.04 | 2.96 | 5.62 |
| IL17RB | interleukin 17 receptor B | −1.36 | −1.46 | N. D | N. D |
| IL22RA1 | interleukin 22 receptor subunit alpha 1 | N. D | −1.44 | −1.35 | −1.44 |
| IL6ST | interleukin 6 signal transducer | 1.28 | N. D | 1.42 | 1.35 |
| MYD88 | MYD88 innate immune signal transduction adaptor | 1.26 | 1.26 | N. D | N. D |
| NFKBIA | NFKB inhibitor alpha | 1.62 | N. D | 1.40 | 2.02 |
| TAB2 | TGF-beta activated kinase 1 (MAP3K7) binding protein 2 | 1.31 | 1.28 | 1.29 | 1.29 |
| TNFRSF1A | TNF receptor superfamily member 1A | 1.75 | 1.78 | N. D | 1.68 |
| TNFRSF21 | TNF receptor superfamily member 21 | 1.61 | 1.70 | N. D | 1.73 |
| TRAF7 | TNF receptor associated factor 7 | N. D | −1.36 | N. D | N. D |
Analysis of significantly (−log10(p-value) > 1.3) activated or inhibited canonical pathways related to immune responses revealed that BPD was predicted to activate cytokine signalling pathways “IL-2-, IL-6- and IL-17 signalling” and the chemokine signalling pathway “chemokine signalling” and BchPD was predicted to activate the chemokine signalling pathway “C–X–C chemokine receptor type 4 (CXCR4) signalling” and the transcription factor signalling pathway “nuclear factor activated T-cell (NFAT) signalling” (Fig. 6). Similar to the barrier function- and cell proliferation-related pathways, neither the MPD nor BCD activated pathway related to the immune function.
3.7 Testing the immunomodulatory effects of insect-derived fractions on a co-culture of Caco-2 cells and THP-1 macrophages
To further characterize the Caco-2 cell immune signalling, a transwell model was employed in which Caco-2 cells were co-cultured with THP-1 macrophages. THP-1 macrophages were adhered to the basolateral side of the membrane of small intestinal-like Caco-2 cells. As a result, the Caco-2 cells and THP-1 macrophages were physically separated only by a porous membrane, potentially allowing direct cell–cell contact. The model was stimulated with apical addition of medium, ED, BPD, MPD, BCD or BchPD and analysed for the basolateral presence of IL-8 (Fig. 7). The results revealed that neither ED exposure nor exposure to any of the insect-derived fractions induced significant changes in the basolateral IL-8 concentration of the co-cultured Caco-2 cells and THP-1 macrophages when compared with the medium control.4. Discussion
Pathogenesis of C. difficile is associated with enterotoxin secretion6,7 and for many years, studies towards mitigating these effects have mainly focused on the large intestine as C. difficile colonization was initially thought to occur only in the colon.23 However, C. difficile infection was also demonstrated in the small intestine.24 Here, in vitro models mimicking the small intestinal enterocytes were used to investigate whether insect-derived fractions can mitigate the barrier integrity reducing effect of C. difficile toxin A and to explore the underlying mechanisms. We tested four different insect-derived fractions, including black soldier fly larvae protein meal (BP), mealworm larvae powder (MP), chitin rich black soldier fly larvae protein meal (BchP) and black soldier fly cocoon meal (BC), after digestion according to the INFOGEST consensus protocol with lowered enzyme concentrations.16We have found that our insect-derived fractions did not impede the integrity of small intestinal-like Caco-2 cells (Fig. 1). Moreover, BPD, BchPD and MPD significantly attenuated the damage of the Caco-2 cell barrier integrity induced by C. difficile toxin A (Fig. 2). Such an effect might result from the insect-derived fractions which either change the Caco-2 cell activity or interact with toxin A and prevent Caco-2 cell damage as they contain a matrix of protein hydrolysates, fibres and fatty acids. To differentiate between both possibilities, we performed an assay in a preventive setting (Fig. 3). We found that BPD, BchPD and MPD reduced the detrimental effects of toxin A on the Caco-2 cell barrier integrity (Fig. 3), indicating that the insect-derived fractions alter the Caco-2 activity and thereby improve protection against subsequent toxin A activity. The intestinal epithelial barrier integrity is critically controlled by tightly connected junctional complexes including tight junctions, adhesion junctions, gap junctions, desmosomes and tetraspanin-enriched microdomain proteins.22 Maintenance of these junctions is associated with various signalling molecules such as protein kinase C, mitogen-activated protein kinase and Rho GTPases, which are targeted and rendered ineffective by post-translational modification by C. difficile toxins.6,25 Junctions are highly dynamic structures that are consistently shaped by both external (i.e. food compounds) and internal (i.e. secreted hormones and peptides by enteroendocrine cells) factors.25,26 To investigate the mechanisms by which the insect-derived fractions support the Caco-2 cell barrier, RNA sequencing was performed to map the transcriptome. This revealed that at the single gene level and the canonical pathway level, BPD, BchPD and MPD supported junctional complexes. In particular, CEACAM1, CLDN1, DSG2, OCLN, TJP3 and TSPAN12, involved in various junctional complexes, and the Sertoli cell-Sertoli cell junction canonical pathway were significantly upregulated and predicted to be activated, respectively, after Caco-2 cell exposure to BPD, BchPD or MPD (Table 1 and Fig. 5A). Lower levels of the tight junction protein 3 (TJP3) and claudin 1 and 2 (CLDN1, 2) were actually found in C. difficile toxin A-challenged Caco-2 cells using proteomics.27 This indicates that BPD, BchPD and MPD might directly counteract the toxin activity. Furthermore, a murine study reported a significantly decreased transcription of SLC20A1 in the cecum after exposure to C. difficile toxin A.28 As SLC20A1 (solute carrier family 20 member 1) encodes a transmembrane protein that can regulate cell proliferation,29 its reduced detection might not be surprising given the apoptosis-inducing activity of C. difficile toxin A.30 Another study demonstrated that an anti-microbial peptide, CopA3, isolated from the Korean dung beetle, prevented C. difficile mediated intestinal inflammation by enhancing the proliferation of colonic epithelial cells through reducing the expression of p21Cip1/Waf1, a cyclin-dependent kinase inhibitor that triggers cell cycle arrest and apoptosis, in colonic epithelial cells.31,32 Transcriptional analysis of Caco-2 cells demonstrated that BPD, BchPD, MPD and BCD significantly enhanced the transcriptional expression of SLC20A1 (ESI, Table 2†) and were predicted to activate various proliferation pathways after exposure to BPD or BchPD (Fig. 5B). In summary, our data reveal that Caco-2 cell treatment with BPD, BchPD or MPD increases the transcription of genes that support junctional complexes and cell proliferation, which might directly counteract the mechanisms through which C. difficile toxin A exerts its intestinal barrier integrity reducing activities.
The barrier function of intestinal epithelial cells is closely linked with intestinal immune homeostasis.2 A breakdown of intestinal immune homeostasis can lead to increased invasion of luminal microbial pathogens and microbiota-associated molecular patterns, resulting in inflammation and tissue damage. Intestinal epithelial cells developed defensive strategies, including immunomodulatory capabilities, to limit damage and promote effective barrier function.3 Their immunomodulatory capabilities are closely linked to the receptors expressed on both apical and basolateral sides. In particular, they can express the microbial pattern-recognition receptors including toll-like receptors (TLRs) (i.e. TLR2) and C-type lectin receptor (CLRs) (i.e. Dectin-1) on their membrane which allow intestinal epithelial cells to respond to their luminal environment33,34 and produce signalling molecules such as IL-1β or TGF-β to instruct lamina propria residing immune cells.2,35 To investigate the immunomodulatory effects of insect-derived fractions on Caco-2 cells, we studied the transcriptional changes of immune signalling genes of Caco-2 cells following exposure. Transcription of immune signalling genes, including CCL20, MYD88 and NFKBIA, was significantly increased upon exposure of Caco-2 cells to BPD, BchPD, MPD or BCD (Table 2). Moreover, the cytokine pathways of IL-2-, IL-6- and IL-12 signalling and the chemokine pathways were activated after exposure of Caco-2 cells to BPD and CXCR4 signalling and NFAT signalling pathways were activated after exposure to BchPD (Fig. 6). In general, these transcriptional changes are related to proinflammatory responses.36,37
In contrast, insect-derived components have demonstrated anti-inflammatory intestinal activities. CopA3, in addition to supporting proliferation, also significantly reduced LPS induced IL-6 and TNF-α production by murine macrophages through inactivating the transcription factors STAT1 and STAT5.38 Another anti-microbial peptide apidaecin, isolated from honeybees, inhibited LPS induced IL-6 and TNF-α production by human primary macrophages.39 Chitin, however, has also been extensively reported for its immune supportive effects.40 These conflicting findings in pro- and anti-inflammatory effects might be related to obvious differences, as we tested a homeostatic system and applied a matrix of bioactive compounds, where these studies focused on individual components in an inflammatory system. The tested insect-derived fractions also demonstrated a strong anti-microbial, and specifically an anti-C. perfringens, activity,15 which is in agreement with the immune activating properties as observed here. To determine the cumulative activity of the insect-derived fractions, in vivo testing is required.
To limit animal trials, in vitro complex cell system development has gained a lot of attention.41 In line with this, we employed a Caco-2 cell and THP-1 macrophage co-culture model in which intracellular signalling is not limited by spatial separation or dilution. None of the insect-derived fractions significantly changed the basolateral IL-8 levels in the co-culture model when compared to ED (Fig. 7). This suggests that the observed immunomodulatory signalling in Caco-2 cells did not result in THP-1 macrophage inflammation. However, analysing the impact of the insect-derived fractions in a physiological inflammatory setting in this model, similar to the CopA3 and apidaecin studies, would provide additional insights.
Taken together, the results of the in vitro studies on Caco-2 cells indicated that black soldier fly protein and mealworm powder effectively mitigate C. difficile toxin A-mediated damage in small intestinal-like Caco-2 cells, which might relieve the symptoms of C. difficile infections. The underlying mechanisms appear to be associated with the ability to enhance the transcription of cell junctional and proliferation genes in Caco-2 cells. Due to the generic nature of these mechanisms, these insect-derived fractions might therefore also mitigate barrier damage by other bacterial toxins or pathogens when permanently included in the diet.
Author contributions
L. D., C. G., J. J. M., T. V. and H. J. W. conceived and designed the experiments. L. D., M. T., E. O. and R. A. performed the experiments and data analysis. L. D., R. A., C. G., and J. J. M. wrote the manuscript. All the authors read and approved the final manuscript.Conflicts of interest
The authors report no conflict of interest.Acknowledgements
The authors would like to acknowledge the financial support from the China Scholarship Council, the Dutch Ministry of Economic Affairs (KB-23-001-015), and the Top Sector Alliance for Knowledge and Innovation (TKI, number: AF 16178) along with private partners ABZ Diervoeding, Nutrition Sciences, Protix and Provimi B.V. The authors would like to thank Aman Paul from Protix B.V for kindly providing the insect-derived fractions.References
- F. Malard, J. Dore, B. Gaugler and M. Mohty, Introduction to host microbiome symbiosis in health and disease, Mucosal Immunol., 2020, 1–8 Search PubMed.
- L. W. Peterson and D. Artis, Intestinal epithelial cells: regulators of barrier function and immune homeostasis, Nat. Rev. Immunol., 2014, 14, 141–153 CrossRef CAS PubMed.
- C. Pardo-Camacho, A. M. González-Castro, B. K. Rodiño-Janeiro, M. Pigrau and M. Vicario, Epithelial immunity: priming defensive responses in the intestinal mucosa, Am. J. Physiol.: Gastrointest. Liver Physiol., 2018, 314, G247–G255 CrossRef PubMed.
- M. H. Wright, C. J. Lee, C. E. Pollock, A. C. Greene and I. E. Cock, Growth Inhibitory Activity of Selected High Antioxidant Australian Syzygium Species Against the Food Poisoning and Tissue Necrotic Pathogen Clostridium perfringens, Pharmacogn. Commun., 2016, 7(3), 129–136 Search PubMed.
- V. Shankar, M. J. Hamilton, A. Khoruts, A. Kilburn, T. Unno, O. Paliy and M. J. Sadowsky, Species and genus level resolution analysis of gut microbiota in Clostridium difficile patients following fecal microbiota transplantation, Microbiome, 2014, 2, 1–10 CrossRef.
- M. C. Abt, P. T. McKenney and E. G. Pamer, Clostridium difficile colitis: pathogenesis and host defence, Nat. Rev. Microbiol., 2016, 14, 609–620 CrossRef CAS.
- R. Kiu and L. J. Hall, An update on the human and animal enteric pathogen Clostridium perfringens, Emerging Microbes Infect., 2018, 7, 1–15 CrossRef CAS PubMed.
- O. Sklenickova, J. Flesar, L. Kokoska, E. Vlkova, K. Halamova and J. Malik, Selective growth inhibitory effect of biochanin A against intestinal tract colonizing bacteria, Molecules, 2010, 15, 1270–1279 CrossRef CAS.
- M. Fallani, L. Rigottier-Gois, M. Aguilera, C. Bridonneau, A. Collignon, C. A. Edwards, G. Corthier and J. Doré, Clostridium difficile and Clostridium perfringens species detected in infant faecal microbiota using 16S rRNA targeted probes, J. Microbiol. Methods, 2006, 67, 150–161 CrossRef CAS.
- B. Lee, K. M. Moon and C. Y. Kim, Tight junction in the intestinal epithelium: its association with diseases and regulation by phytochemicals, J. Immunol. Res., 2018, 2018, 2645465 Search PubMed.
- M. H. Wilcox, Caution is warranted in using cephamycin antibiotics against recurrent Clostridioides difficile infection, Nat. Microbiol., 2020, 5, 236–236 CrossRef CAS.
- J. K. Kang, J. S. Hwang, H. J. Nam, K. J. Ahn, H. Seok, S.-K. Kim, E. Y. Yun, C. Pothoulakis, J. T. Lamont and H. Kim, The insect peptide coprisin prevents Clostridium difficile-mediated acute inflammation and mucosal damage through selective antimicrobial activity, Antimicrob. Agents Chemother., 2011, 55, 4850–4857 CrossRef.
- C. L. Fischer, D. R. Drake, D. V. Dawson, D. R. Blanchette, K. A. Brogden and P. W. Wertz, Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria, Antimicrob. Agents Chemother., 2012, 56, 1157–1161 CrossRef CAS PubMed.
- L. Borrelli, L. Coretti, L. Dipineto, F. Bovera, F. Menna, L. Chiariotti, A. Nizza, F. Lembo and A. Fioretti, Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens, Sci. Rep., 2017, 7, 1–11 CrossRef CAS PubMed.
- L. Dong, R. M. Ariëns, A. H. America, A. Paul, T. Veldkamp, J. J. Mes, H. J. Wichers and C. Govers, Clostridium perfringens suppressing activity in black soldier fly protein preparations, LWT–Food Sci. Technol., 2021, 149, 111806 CrossRef CAS.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig and D. Dupont, A standardised static in vitro digestion method suitable for food–an international consensus, Food Funct., 2014, 5, 1113–1124 RSC.
- L. Dong, R. M. Ariëns, M. M. Tomassen, H. J. Wichers and C. Govers, In Vitro Studies Toward the Use of Chitin as Nutraceutical: Impact on the Intestinal Epithelium, Macrophages, and Microbiota, Mol. Nutr. Food Res., 2020, 64, 2000324 CrossRef CAS.
- M. D. Robinson and A. Oshlack, A scaling normalization method for differential expression analysis of RNA-seq data, Genome Biol., 2010, 11, 1–9 CrossRef.
- D. J. McCarthy, Y. Chen and G. K. Smyth, Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation, Nucleic Acids Res., 2012, 40, 4288–4297 CrossRef CAS PubMed.
- A. des Rieux, V. Fievez, I. Théate, J. Mast, V. Préat and Y.-J. Schneider, An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells, Eur. J. Pharm. Sci., 2007, 30, 380–391 CrossRef CAS PubMed.
- P. B. Venkatasubramanian, E. Oosterink, M. M. Tomassen, M. Suarez-Diez, J. J. Mes, E. Saccenti and N. de Wit, Transcriptome-based identification of the beneficial role of blackcurrant, strawberry and yellow onion to attenuate the cytopathic effects of Clostridium difficile toxins, J. Berry Res., 2021, 11, 231–248 CAS.
- B. N. Giepmans and S. C. van IJzendoorn, Epithelial cell–cell junctions and plasma membrane domains, Biochim. Biophys. Acta, Biomembr., 2009, 1788, 820–831 CrossRef CAS PubMed.
- D. A. Leffler and J. T. Lamont, Clostridium difficile infection, N. Engl. J. Med., 2015, 372, 1539–1548 CrossRef CAS.
- S. Killeen, M. Mannion, A. Devaney and D. Winter, Complete mesocolic resection and extended lymphadenectomy for colon cancer: a systematic review, Colorectal Dis., 2014, 16, 577–594 CrossRef CAS PubMed.
- D. Ulluwishewa, R. C. Anderson, W. C. McNabb, P. J. Moughan, J. M. Wells and N. C. Roy, Regulation of tight junction permeability by intestinal bacteria and dietary components, J. Nutr., 2011, 141, 769–776 CrossRef CAS.
- P. D. Cani, A. Everard and T. Duparc, Gut microbiota, enteroendocrine functions and metabolism, Curr. Opin. Pharmacol., 2013, 13, 935–940 CrossRef CAS.
- J. Zeiser, R. Gerhard, I. Just and A. Pich, Substrate specificity of clostridial glucosylating toxins and their function on colonocytes analyzed by proteomics techniques, J. Proteome Res., 2013, 12, 1604–1618 CrossRef CAS PubMed.
- K. M. D'Auria, G. L. Kolling, G. M. Donato, C. A. Warren, M. C. Gray, E. L. Hewlett and J. A. Papin, In vivo physiological and transcriptional profiling reveals host responses to Clostridium difficile toxin A and toxin B, Infect. Immun., 2013, 81, 3814–3824 CrossRef.
- K. Byskov, N. Jensen, I. B. Kongsfelt, M. Wielsøe, L. E. Pedersen, C. Haldrup and L. Pedersen, Regulation of cell proliferation and cell density by the inorganic phosphate transporter PiT1, Cell Div., 2012, 7, 1–16 CrossRef.
- M. M. Awad, P. A. Johanesen, G. P. Carter, E. Rose and D. Lyras, Clostridium difficile virulence factors: insights into an anaerobic spore-forming pathogen, Gut Microbes, 2014, 5, 579–593 CrossRef PubMed.
- Y. Seomun, J.-T. Kim, H.-S. Kim, J.-Y. Park and C.-K. Joo, Induction of p21Cip1-mediated G2/M arrest in H2O2-treated lens epithelial cells, Mol. Vision, 2005, 11, 764–774 CAS.
- D. H. Kim, J. S. Hwang, I. H. Lee, S. T. Nam, J. Hong, P. Zhang, L. F. Lu, J. Lee, H. Seok and C. Pothoulakis, The insect peptide CopA3 increases colonic epithelial cell proliferation and mucosal barrier function to prevent inflammatory responses in the gut, J. Biol. Chem., 2016, 291, 3209–3223 CrossRef CAS.
- S. E. Hardison and G. D. Brown, C-type lectin receptors orchestrate antifungal immunity, Nat. Immunol., 2012, 13, 817–822 CrossRef CAS.
- M. T. Abreu, Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function, Nat. Rev. Immunol., 2010, 10, 131–144 CrossRef CAS PubMed.
- K. Atarashi, T. Tanoue, T. Shima, A. Imaoka, T. Kuwahara, Y. Momose, G. Cheng, S. Yamasaki, T. Saito and Y. Ohba, Induction of colonic regulatory T cells by indigenous Clostridium species, Science, 2011, 331, 337–341 CrossRef CAS.
- J. Egger, P. Bretscher, S. Freigang, M. Kopf and E. M. Carreira, Synthesis of epoxyisoprostanes: effects in reducing secretion of pro–inflammatory cytokines IL–6 and IL–12, Angew. Chem., Int. Ed., 2013, 52, 5382–5385 CrossRef CAS PubMed.
- N. Alaaeddine, J. Antoniou, M. Moussa, G. Hilal, G. Kreichaty, I. Ghanem, W. Abouchedid, E. Saghbini and J. A. Di Battista, The chemokine CCL20 induces proinflammatory and matrix degradative responses in cartilage, Inflammation Res., 2015, 64, 721–731 CrossRef CAS.
- H. J. Nam, A. R. Oh, S. T. Nam, J. K. Kang, J. S. Chang, D. H. Kim, J. H. Lee, J. S. Hwang, K. E. Shong and M. J. Park, The insect peptide CopA3 inhibits lipopolysaccharide–induced macrophage activation, J. Pept. Sci., 2012, 18, 650–656 CrossRef CAS.
- R. Tavano, D. Segat, M. Gobbo and E. Papini, The honeybee antimicrobial peptide apidaecin differentially immunomodulates human macrophages, monocytes and dendritic cells, J. Innate Immun., 2011, 3, 614–622 CrossRef CAS PubMed.
- L. Dong, H. J. Wichers and C. Govers, in Chitin and Chitosan: Properties and Applications, Wiley, Hoboken, NJ, 2019, ch. 6, pp. 145–167 Search PubMed.
- D. Papazian, P. A. Würtzen and S. W. Hansen, Polarized airway epithelial models for immunological co-culture studies, Int. Arch. Allergy Appl. Immunol., 2016, 170, 1–21 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo00673h |
| This journal is © The Royal Society of Chemistry 2021 |