Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Protein–saliva interactions: a systematic review†
Frances N.
Brown
a,
Alan R.
Mackie
 *a,
Qi
He
b,
Alison
Branch
b and
Anwesha
Sarkar
*a,
Qi
He
b,
Alison
Branch
b and
Anwesha
Sarkar
 *a
*a
aFood Colloids and Bioprocessing Group, School of Food Science and Nutrition, University of Leeds, Leeds, UK. E-mail: A.R.Mackie@leeds.ac.uk; A.Sarkar@leeds.ac.uk
bMondelēz International, Reading, UK
First published on 26th March 2021
Abstract
Food industries are challenged to reformulate foods and beverages with higher protein contents to lower fat and sugar content. However, increasing protein concentration can reduce sensory acceptability due to astringency perception. Since the properties of food–saliva mixtures govern mouthfeel perception, understanding how saliva and protein interact is key to guide development of future protein-rich reformulations with optimal sensory attributes. Hence, this systematic review investigated protein–saliva interaction using both model and real human saliva, including a quality assessment. A literature search of five databases (Medline, Pubmed, Embase, Scopus and Web of Science) was undertaken covering the last 20 years, yielding 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 articles. Using pre-defined criteria, this was reduced to a set of 33 articles with bulk protein solutions (n = 17), protein-stabilized emulsions (n = 13) and protein-rich food systems (n = 4). Interaction of dairy proteins, lysozyme and gelatine with model or human saliva dominated the literature. The pH was shown to have a strong effect on electrostatic interaction of proteins with negatively-charged salivary mucins, with greater interactions occurring below the isoelectric point of proteins. The effect of protein concentration was unclear due to the limited range of concentrations being studied. Most studies employed a 1
604 articles. Using pre-defined criteria, this was reduced to a set of 33 articles with bulk protein solutions (n = 17), protein-stabilized emulsions (n = 13) and protein-rich food systems (n = 4). Interaction of dairy proteins, lysozyme and gelatine with model or human saliva dominated the literature. The pH was shown to have a strong effect on electrostatic interaction of proteins with negatively-charged salivary mucins, with greater interactions occurring below the isoelectric point of proteins. The effect of protein concentration was unclear due to the limited range of concentrations being studied. Most studies employed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w protein
1 w/w protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) saliva ratio, which is not representative of true oral conditions. The interaction between protein and saliva appears to affect mouthfeel through aggregation and increased friction. The searches identified a gap in research on plant proteins. Accurate simulation of in vivo oral conditions should clarify understanding of protein–saliva interaction and its influence on sensory perception.
saliva ratio, which is not representative of true oral conditions. The interaction between protein and saliva appears to affect mouthfeel through aggregation and increased friction. The searches identified a gap in research on plant proteins. Accurate simulation of in vivo oral conditions should clarify understanding of protein–saliva interaction and its influence on sensory perception.
1. Introduction
The mouthfeel and subsequent sensory perception a food evokes undoubtedly govern consumer acceptance and prospective consumption.1 Food industries are under increasing pressure to reformulate foods and beverages to reduce fat and sugar while still maintaining desirable mouthfeel in order to address pressing global obesity challenges. However, both changes in formulations to reduce fat have been shown to result in reduced acceptability in texture and mouthfeel, which affects overall palatability.2–4 For example in ice-cream, when 6% fat was replaced with whey protein, there was a reduction in sensory scores for both smoothness and overall acceptability when compared to the full-fat counterpart.5 Protein is commonly used to modify texture and replace fat or as a bulking agent when sugar is substituted with a sweetener. However, this often generates undesirable textural changes such as grittiness and chalkiness6. Thus, understanding the physical mechanism behind mouthfeel is of paramount importance when re-designing food formulation with proteins.Although rather underestimated, a critical component of mouthfeel results from the interaction of food components with saliva. For the purpose of this review, mouthfeel includes sensory perception and after feel. Saliva is an inherent bio-lubricant, that coats all surfaces within the mouth and therefore it is implicated in all stages of food processing.7–9 Saliva is primarily responsible for providing lubrication in the mouth preventing wear and also interacts with food and beverages. These interactions have previously been shown to impact mouthfeel. For example, the astringency in tea and wine have often been linked to the interaction of polyphenols (a key component in tea and wine) with salivary proline-rich proteins (PRP's) as well as salivary mucins.10–12 Although some dietary protein alone has been shown to elicit astringency, the mechanisms behind such astringent perceptions are not so well understood.7 Therefore, a mechanistic understanding of the interactions of saliva with dietary proteins to understand those perceptions is important for reformulating food with higher protein content. This has received rare attention in literature to date.
Unstimulated whole human saliva is known for its high stretchability – a property aiding lubrication, coating and food bolus formation, subsequently enabling swallowing.13 Saliva wets and helps to cluster food particles and limits the friction between oral surfaces.9,14 It is a non-Newtonian fluid and exhibits a shear thinning behaviour.15 Saliva is a complex mixture, composed of predominantly water (99.5%) in addition to various proteins (0.3%), inorganic ions and trace substances (0.2%).16 It is the protein and ionic components of saliva which distinguish its properties from water.7 The proteins contained within the saliva are responsible for saliva's lubricating qualities. In particular, mucins (MUC5B), statherin, proline-rich glycoproteins, acidic protein-rich proteins and lactoferrin have been suggested to play primary roles in aiding lubrication.14,16–18
Specifically, self-assembly of high molecular weight, negatively-charged mucins together with small molecular weight positively-charged proteins such as lactoferrin has been recently proposed to be the main cause of salivary lubrication. In this case, mucin aids in viscous lubrication and the lactoferrin aids in boundary lubrication.19 During oral processing saliva mixes with food to form a bolus. This bolus is formed to increase the ease of swallowing.20 The subsequent perception of foods or beverages texture will depend on the transforming status of food–salivary film coating15,21,22 from a ‘rheology-dominant’ to a ‘tribology-dominant’ phase. It is postulated that rheology attributes which are based on how material flows and if/how it responds to stress initially dominate mouthfeel perception such as sensory thickness. However, as mastication and oral processing proceeds, tribological properties tend to dominate mouthfeel.3,4,23,24 Tribology is the study of friction and lubrication for interacting surfaces in relative motion. Therefore, in oral processing context, tribology elucidates how the tongue and palate interact with food and saliva coating the oral surfaces.
Although there has been extensive research on salivary interactions with food, a detailed review of how saliva interacts with dietary proteins is a necessary undertaking. Therefore, this review aims to combine current fundamental understanding of protein–saliva interaction in order to aid the increasing demand for the design of high protein formulations with pleasurable mouthfeel.
To the best of our knowledge, there has been no systematic review of protein–saliva interactions, although a narrative review exists.23 Systematic reviews originate from the field of medical science, where they were created to help refine the mass of research being produced in quick succession with often contradictory findings. They have now become a well-established high-quality method for assessing research and uncovering gaps in the literature and are used in a variety of fields including nursing, crime, transport, policy and social research.25 Systematic reviews are beginning to become popular within food science and have been conducted on a range of aspects, for example, the impact of food structure on appetite and satiety,26 consumer acceptance of reformulated products,27 as well as tribology-sensory relationship.3 The prior research used to inform a non-systematic review may be random, therefore, is at risk of selection bias with important articles omitted. Whereas systematic reviews use a developed search strategy which is stated to allow readers to replicate the search or evaluate and judge the search approach with greater transparency. Additionally, systematic reviews search a number of sources aiming to collate all of the currently available and relevant evidence. Grey literature areas such as reference lists may additionally be searched to increase rigour. For data analysis, systematic reviews utilise a precise method to appraise and summarize findings in addition to assessment of the quality of included research. By doing so, it provides a clearer synthesis of evidence and can indicate the strength and accuracy of the present research. Systematic reviews are particularly beneficial for identifying research gaps as well as areas of saturation, which do not require further investigation. Moreover, methodology can be critical to highlighting concerns and providing recommendations for methodological development. Although an elegant narrative review23 exists on protein–saliva interactions and summarizes relevant electrostatic, hydrophobic interactions and hydrogen bonding between some dietary proteins and salivary proteins, using a systematic approach may yield a more critical overview of the field. In addition, a systematic review would help to understand the type of experimental techniques and conditions used to report those interactions. Consequently, with this first systematic review on protein–saliva interactions, we aim to examine the key interactions between saliva and salivary components with food proteins focussing on protein type, protein concentration, pH, processing of protein, saliva type and saliva–protein ratios to inspire future research in this field. To examine the field effectively, we have covered proteins as bulk solution as well as protein in lipid emulsions and food systems.
1.1 Study identification
The systematic review aimed to summarise and synthesize evidence on saliva and protein interactions. The search strategy used synonyms of saliva as well as various salivary components, including mucins from bovine and porcine sources. In addition, protein as well as different types of proteins were added. Thirdly, terms used in relation to mouthfeel or instrumental characteristics with commonly used techniques for analyses of protein–saliva interactions was further included. Although instrumental characterization such as rheology and tribology may not measure mouthfeel, they can give indirect indications about mouthfeel, and thus were incorporated.12,15The search terms are included below: (saliva* OR amylase OR bovine-submaxillary OR BSM OR parotid OR porcine gastric OR proline-rich-protein* OR PRP OR PGM OR proline OR statherin OR stimulated OR unstimulated OR MUC5B OR MUC7) AND (protein OR casein* OR gelatin OR lactoferrin OR lupin OR pea OR potato OR soy OR whey OR dairy OR food OR gluten OR lysozyme OR milk OR plant OR protein OR skimmed-milk) AND (astringen* OR boli OR dry* OR friction OR lubric* OR mouth* OR mouthfeel OR oral processing OR perception OR SDS-Page OR sensor* OR sensory analysis OR surface* OR tribol* OR turbidity OR rheol*) AND (interact*).
The literature searching was an iterative process with search terms modified based on the search results. The developed search strategy was tested by checking if key studies identified in a previous review came up.23 In addition, the titles were screened to identify any new search terms. Based on this, the search terms ‘milk’ and ‘skimmed-milk’ were added. Additionally, as the initial search yielded an extremely large number of results (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000+), interact* was included to reduce the breadth of the results based on the literature search. The following four databases were searched; Medline or PubMed, Embase, Scopus and Web of Science. In adherence to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, hand searches of references lists in articles included for full text screening were undertaken.
000+), interact* was included to reduce the breadth of the results based on the literature search. The following four databases were searched; Medline or PubMed, Embase, Scopus and Web of Science. In adherence to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, hand searches of references lists in articles included for full text screening were undertaken.
1.2 Study selection
Articles were eligible if they were published in the last 20 years (between 2000 and 10.07.2020). Articles were only included if they were published in English. The first author (FB) performed the screening of potentially relevant studies based on title and abstract. Articles were independently checked by co-author (AS). Following the screening, full-text papers were evaluated using defined selection criteria by the first author and checked independently again by co-author (AS). Uncertainties regarding inclusion and exclusion were resolved involving discussion with another co-author (AM).For paper inclusion, the following criteria were chosen based on the PICO (Population, Intervention, Comparison and Outcome) inclusion criteria. Population. Only human studies were included. Live animal studies were excluded, only studies using commercially available animal saliva were included. For example, studies where protein was fed to animals to understand the influence of oral processing were excluded. However, bovine-submaxillary mucin and porcine gastric mucin studies were included as these are well-established sources of mucin for preparing model saliva.28 Furthermore, studies involving unhealthy (with oral or other diseases and conditions) were excluded. This was because salivary property can be affected by disease, which may also alter how it interacts with dietary protein.29,30 Furthermore, only adults from ages (18–64 in accordance with UK Office for National Statistic's age range) with no children or elderly were included, as salivary quantity and quality has been shown to change with age.19Intervention. Only studies in which the specific effect of protein was considered were included. This includes some studies using bulk solutions which were designed such that the effects of protein could be isolated. Studies with complex designs that do not allow the specific effect of protein to be identified were excluded. Comparison. If saliva (or related synonym) was not included, then studies were omitted. Outcome. Articles were excluded if they were published as opinions, reviews, theoretical studies with no measurable outcomes.
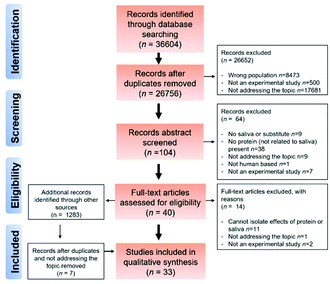
The process of obtaining the results is shown in the PRISMA diagram in Fig. 1. Initially, a total number of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 articles were identified using literature by searching the four electronic databases mentioned in the method section.
604 articles were identified using literature by searching the four electronic databases mentioned in the method section.
As can be seen from Fig. 1, 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652 studies were excluded based on the PICOS criteria. After removing duplicates, articles involving excluded population i.e. animal studies, or clinical studies involving patients, older adults and/or children (n = 8472) were excluded. Additionally, articles not addressing the topic of interest were excluded (17
652 studies were excluded based on the PICOS criteria. After removing duplicates, articles involving excluded population i.e. animal studies, or clinical studies involving patients, older adults and/or children (n = 8472) were excluded. Additionally, articles not addressing the topic of interest were excluded (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 681) or non-experimental studies were excluded (500).
681) or non-experimental studies were excluded (500).
The resulting 104 articles were then taken to the abstract screening stage where abstracts were screened by FB and AS. This resulted in the exclusion of an additional 64 articles (57 articles had no relevance to the topic (s) of the systematic review i.e. involving no dietary protein or using saliva, 56 had non-relevant outcome measures, 23 were new or validation of existing protocols, 1 was a non-human study with an additional 7 being non-eligible because of a lack of any original experimental work. Forty full-text articles, including 7 additional articles that were identified through supplementary approaches (e.g. manual searches of reference list of pre-screened articles) were screened independently by FB and AS. By mutual agreement, articles with inappropriate interventions and designs (e.g. cannot separate based on protein or salivary interaction) led to the exclusion of a further 14 articles. Finally, 33 articles were included in the qualitative synthesis.
1.3 Study characteristics and data extraction
For each study, study characteristics data were extracted in Table 1 which include author and year of publication, protein type (concentration and pH), saliva type (model or human, if model saliva: the type of mucin, if human saliva: stimulated or unstimulated, number of human donors), the ratio of saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein mixture, methods used, and the main findings.
protein mixture, methods used, and the main findings.
| Reference (author, year) | Protein | Saliva | Experimental set up | Main findings | Quality score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein type (pI) | pH | Protein concentration | Model or real human saliva | Type of saliva (or mucins) | Number of saliva donors, gender, age | Methods | Saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein solution ratio (w/w) protein solution ratio (w/w) |
|||
| 7.0*: ‘mentioned in the literature as neutral pH’, AS: artificial saliva (commercial formulation), BAA: bicinchoninic acid assay, β-lg: beta-lactoglobulin, BSM: bovine submaxillary mucin, CD: circular dichroism, DLS: dynamic light scattering, F: female, HS: human saliva, LD: laser diffraction, M: male, MS: model saliva, NA: not applicable, ND: not disclosed, NMR: nuclear magnetic resonance, pI: isoelectric point, PGM: porcine gastric mucin, SDS-PAGE: sodium dodecyl sulphate polyacrylamide gel electrophoresis, SEC: size exclusion chromatography, SWHS: stimulated whole human saliva, WPI: whey protein isolate, ZP: zeta potential. | ||||||||||
| Çelebioğlu, et al. (2016)51 | β-lg (5.2) | 3.0–7.4 | 1 mg mL−1 | MS | BSM or PGM | NA | BAA, tribology, ZP | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
β-lg + BSM solutions had reduced adsorbed masses, with β-lg dominating the surface adsorption at pH 3.5. At pH 7.4, BSM dominated the tribological interface. β-lg + BSM had lowest lubrication at pH 5.0 due to aggregation when β-lg was near isoelectric point (pI), pH-dependent lubricating effects are dominated by competitive absorption of the two proteins and β-lg + BSM does not form strong aggregates | 96% |
| Çelebioğlu, et al. (2015)52 | β-lg (5.2) | 3.0–7.4 | 1 or 10 mg mL−1 | MS | BSM | NA | CD, DLS, NMR, ZP | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
BSM dominated surface charged characteristics of β-lg + BSM mixtures. β-lg and BSM interaction caused a more compact conformation of BSM. Spectroscopy showed intermediate spectra in mixtures which were not present in either β-lg/BSM alone. NMR indicated polar interactions at pH 3.0 and 5.0 with no interaction visible at pH 7.4 | 96% |
| Celebioglu, et al. (2017)53 | β-lg (5.2) | 3.0–7.4 | 1 or 2 mg mL−1 | MS | BSM | NA | Rheology | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Elastic modulus was greater in β-lg than BSM and β-lg + BSM mixtures suggesting β-lg formed stronger viscoelastic layers in all pH conditions. At pH 7.4, greater electrostatic repulsions may be responsible for lower viscoelastic interactions and reduced interfacial modulus compared with pH 5.0. At pH closest to β-lg's pI, electrostatic repulsions between β-lg was reduced thus aiding the formation of a stable absorbed layer which had a high elastic modulus. β-lg + BSM mixtures appeared to be predominantly formed by adsorption of interfacial β-lg molecules with BSM molecules within the surface layer | 63% |
| Vardhanabhuti, et al. (2011)36 | β-lg (5.2) | 3.5 and 7.0 | 0.5–10% w/w | HS | SWHS | n = 1, F, ND | Sensory analysis (n = ND, ND), tribology | ND | Friction coefficient of SWHS increased more substantially when β-lg was added at pH 5.2 compared to pH 3.5. The increase in friction coefficient was unaffected by when β-lg was added concentration (0.5–4% w/w). Sensory analysis showed an increase in astringency ratings with increasing protein concentration (up to 4% w/w), after this point, ratings plateaued, astringency did not correlate with tribology | 74% |
| Withers, et al. (2013)40 | β-lg (5.2) or casein (4.6) | 7.4 | 8.7% w/w (rheology); 2% w/v (force and bioadhesion) | MS | AS | NA | Adhesion, fluorescence microscopy, rheology, thiol content analysis, retention, ZP | 0.088![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (bioadhesion) 1 (bioadhesion) |
Casein bound more efficiently than β-lg to the epithelial lining or porcine oral mucosa. At pH 9.0, both casein and β-lg were negatively-charged, with β-lg having higher ZP and casein having 2.3× greater viscosity. The force of bioadhesion measurements was greater for β-lg compared to casein suggesting β-lg but not casein interacts with mucin-rich AS | 83% |
| Vardhanabhuti, et al. (2010)54 | β-lg (5.2) or lactoferrin (8.7) | β-lg 3.4 or 6.0; lactoferrin 3.5 to 7.0 | 4% w/w (2% w/w for SDS-PAGE) | HS | ND | n = 2, ND, ND | SDS-PAGE, sensory analysis (β-lg; trained panel, n = 10; lactoferrin, trained panel, n = 12) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
At pH 7.0, β-lg had low astringency which increased with decreasing pH. Whereas lactoferrin was astringent at all pH values with no pH effect on the ratings. At pH 7.0, β-lg is negatively-charged whereas lactoferrin is positively charged-indicating electrostatic interaction between lactoferrin and HS proteins at pH 7.0 | 91% |
| Ye, et al. (2011)43 | β-lg (5.2), lactoferrin (8.7) or WPI (4.5) | 2.0–7.0 | WPI 5% w/w, β-lg 2% w/w or lactoferrin 2% w/w | HS | SWHS | n = 5, ND, ND | DLS, SDS-PAGE, sensory analysis (trained panel, n = 12), turbidity, ZP | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
β-lg + SWHS had increased particle size and turbidity (in both unheated and heated) at pH of 3.4. Turbidity decreased with further pH reductions until pH 2.5. Larger particle size was found between pH 3.0–4.0. Sensory analysis showed intense astringency between pH 3.0–4.0, which hypothesized β-lg–saliva interaction to be electrostatically-driven | 96% |
| Lactoferrin + SWHS mixtures were smaller in particle size range and turbidity than β-lg + SWHS mixtures between pH 8.3 and 3.0. At pH 2.0, particle size increased. Sensory analysis showed little/no astringency at neutral pH. Ratings were greatest at pH 3.4 and reduced with further decreases in pH | ||||||||||
| Ahmad, et al. (2020)55 | Gelatine (4.85) | 3.0 or 7.0 | 0–8 mM | MS | PGM | NA | Fluorescence spectroscopy, rheology, ZP | Various ratios (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 6 2, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 5 4, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 4 5, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and 2 6, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
At pH 7.0, there was aggregation and phase separation between PGM and gelatine. At pH 3.0, there was an indication of electrostatic interaction with binding regimes exhibited between PGM and gelatine at both pH 3.0 and 7.0 | 96% |
| Sano, et al. (2005)38 | Gelatine (4.85) or WPI (4.5) | 3.5 or 7.0 | In vitro experiments 50 mg mL−1, n vivo 0.13–5 mg mL−1 | HS | SWHS | n = 7, M, 28–37 years | BAA, sensory analysis | ND | WPI was rated more astringent than gelatine, latter was not rated astringent at pH 3.5. For WPI, astringency increased with protein concentration | 85% |
| Biegler, et al. (2016)61 | Lysozyme (10.7) | 7.0 | 0.25 mM | HS or MS | ND or BSM | n = 12, ND, 25–35 years | Rheology, SEC, tribology | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The addition of lysozyme to saliva (HS or MS) increased friction coefficient. Lysozyme had a low tendency to precipitate BSM (but still induced a loss of lubrication) | 73% |
| Ritzoulis, et al. (2012)58 | Sodium caseinate (4.2) | 1.0–7.0 | 0.00–0.05 wt% | MS | PGM | NA | LD, microscopy, ZP | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Sodium caseinate and PGM interacted around the pI of sodium caseinate (pH 3), whereas they repelled at pH 7.0. Interactions also occurred at pH 1.0 but aggregation was much smaller than at pH 3.0 | 88% |
| Ahmad, et al. (2020)44 | WPI (4.5) | 3.0–7.0 | 1% w/w | MS | PGM | NA | Fluorescence spectroscopy, rheology, ZP | Various ratios (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 6 2, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 5 4, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 4 5, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 2 6 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
At pH 3.0 and 7.0, there was phase separation between PGM and WPI. At pH 3.0, interactions where electrostatically driven. Conversely, at pH 7.0, interactions were entropically and enthalpically driven | 100% |
| Andrewes, et al. (2011)45 | WPI (4.5) | 3.0–4.0 | 1–10% w/w | MS or HS | PGM or SWHS | n = 5, ND, ND | pH, turbidity | Dynamic model with saliva added; 2 different conditions | Increasing protein concentration delayed time to reach maximum turbidity. At higher protein concentrations, there was not enough saliva to increase the pH to the point of aggregation – therefore had limited effects on turbidity. Increasing pH reduced turbidity duration and time to reach maximum turbidity, as less saliva was needed to raise the pH to the pI of WPI | 87% |
| Beecher, et al. (2008)46 | WPI (4.5) | 3.4–7.0* | 6% w/w | HS | ND | n = 3, ND, ND | Sensory analysis (trained, n = 8), turbidity | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
WPI interacted with HS proteins leading to increased turbidity. At neutral pH, the change in turbidity was small compared to pH 3.4. The increase in turbidity was correlated with levels of astringency (sensory analysis) | 74% |
| Hsein, et al. (2015)47 | WPI (4.5) | 3.5 or 6.8 | 0.25–10.8% w/w | MS | PGM | NA | Isothermal calorimetry. rheology, turbidity | Various ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1 4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1 2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 4 1, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
From pH 1.2 to 4.5, there was an increase in turbidity of all WPI + BSM, which did not occur at pH 6.8, where no interaction was found. Bio-adhesion forces were greatest for pH 6.8 for high concentration (10.8% w/w) denatured WPI. Forces were reduced at pH 1.2 for all WPI solutions. Use of chemical blockers demonstrated hydrogen bonding and disulfide bridges were the primary interaction mechanisms with PGM | 96% |
| Kelly, et al. (2010)48 | WPI (4.5) | 2.6–4.2 | 0.25–13% w/w | HS | SWHS | n = 3, F, ND | pH, salivary flow rate (n = 10), sensory analysis (trained panel, n = 10), turbidity | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Astringency increased with increasing protein concentration from 0.05–4% w/w and then a plateau occurred (4–13%w/w). Time to maximum astringency was indifferent between protein concentrations (p > 0.5). Increasing protein concentration also increased turbidity. Maximum turbidity occurred at pH 4.6–5.2 which is near the pI of WPI | 93% |
| Lee and Vickers (2008)49 | WPI (4.5) | 3.4 | 1 or 6% w/v | HS | SWHS | n = 20, 5M, 15F, ND | Sensory analysis (trained panel, n = 20) | ND | WPI was rated less astringent than acid-only solutions matched for total acidity. WPI was rated more astringent than controls matched for pH. Sourness ratings were reduced in WPI solutions. Collectively, this is indicative of astringency ratings resulting from high acidity not WPI in acidic solutions | 74% |
1.4 Assessment of risk of bias and reporting quality
Despite the method of systematic reviewing being created to assess research quality and reporting of potential bias; for previous systematic reviews that analysed in vitro methods were shown to have a lack of quality and risk of bias reporting. For example, a systematic review of in vitro studies reporting of quality found only 19 out of 65 systematic reviews included a risk of bias for each individual study and assessed studies quality.31 A range of tools exist to analyse study quality for systematic reviews for example; Cochrane risk of bias tool for randomised trials,32 Robins-1 tool for non-randomised studies of interventions33 and the JBI checklist for prevalence studies or the JBI checklist for qualitative research (Joanna Briggs Institute34). However, at present, there is no standard tool for assessing the quality and risk of bias employing in vitro studies.31,35Hence, for the present systematic review, a bias tool was developed based on a tool previously used for calcium homeostasis and low-frequency magnetic and electric field exposure.35 The bias tool assesses reporting quality, performance bias, selection bias and detection bias. Industry funding was not considered as a bias here as this review is about understanding interactions rather than focusing on any health claims. The tool is shown in (Table S1, ESI†) and is comprises of 15 items, for each of which articles were marked if they reported or not. If the article clearly disclosed the item (yes) 2 points were awarded, if they somewhat disclosed or it was ambiguous/not directly reported 1 point was awarded, and 0 points were awarded if no attempt was made. As some of the items did not apply to each study (i.e. human saliva description when no human saliva was used), the item was not included in overall score for that study. The weighted percentage total was calculated by equally weighting each score between the reporting quality, performance bias, selection bias and detection bias sections (i.e. 4 × 25%).
2. Results
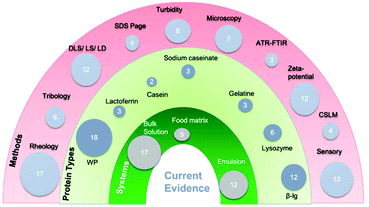
The systematic search of the literature over the last 20 years (2005 and 2020) identified 33 studies which met the inclusion/exclusion criteria from a total of 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 articles, as shown in Fig. 1. As illustrated in the demographics (Fig. 2), 17 analysed bulk protein solutions, 12 protein-stabilized emulsions, 5 protein-rich food systems with 1 incorporating both emulsion and bulk solution. In addition, a variety of methods were used to analyse different responses to possible interactions as demonstrated in Fig. 2. The majority of research has focussed on rheology, zeta-potential, turbidity and sensory analysis. More recently tribology has been used (first seen in 2011) specifically for protein–human saliva interaction.36 The earliest studies identified in the present search were published in 2005 and used the techniques of particle size37 and sensory analysis.38 Seven bulk solution studies included links to in vivo methods by including sensory analysis, whereas only two emulsion studies included sensory analysis.
604 articles, as shown in Fig. 1. As illustrated in the demographics (Fig. 2), 17 analysed bulk protein solutions, 12 protein-stabilized emulsions, 5 protein-rich food systems with 1 incorporating both emulsion and bulk solution. In addition, a variety of methods were used to analyse different responses to possible interactions as demonstrated in Fig. 2. The majority of research has focussed on rheology, zeta-potential, turbidity and sensory analysis. More recently tribology has been used (first seen in 2011) specifically for protein–human saliva interaction.36 The earliest studies identified in the present search were published in 2005 and used the techniques of particle size37 and sensory analysis.38 Seven bulk solution studies included links to in vivo methods by including sensory analysis, whereas only two emulsion studies included sensory analysis.
Study characteristics with quality assessment scores are shown in Tables 1 and 2 involving bulk solution, and emulsions and food systems, respectively. Emulsions are an important system to study as they contribute to a large proportion of food formulations. Understanding how emulsions behave in oral conditions is critical in the manipulation of the physical and sensorial attributes of colloidal systems,39 hence a separate table is allocated to include interaction of protein-stabilized emulsions with saliva along with protein-rich food systems. All studies shown in Tables 1 and 2 used animal-based protein, with the majority of studies focusing on dairy proteins. Of these, 18 studies investigated whey protein forms isolate or concentrate (WPI or WPC), 12 investigated the whey protein derivative i.e. β-lactoglobulin (β-lg), three investigated lactoferrin, three investigated sodium caseinate, two investigated casein. In addition, three investigated gelatine and six lysozyme (Fig. 2). Whey protein and β-lg are known for being globular glycoproteins whereas casein has a random coil structure and had different behaviour in presence of saliva (Table 1). Five out of six studies using lysozyme investigated it in emulsion systems (Table 2), which is a globular positively-charged protein at neutral pH. Gelatine which is a hydrophilic protein, with a high molecular weight is made by the thermal denaturation of collagen and has been used to measure interaction with saliva both in bulk phase as well as in emulsified form (Tables 1 and 2). All five studies using food matrices (Fig. 2) in formulating model foods and beverages or yoghurts, investigated whey protein either as WPI or WPC (Table 2). In addition, these studies were more recent, published between 2010 to 2017.
| Reference (author, year) | Protein | Saliva | Experimental set up | Main findings | Quality score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein type | pH | Protein concentration | Model or real human saliva | Type of saliva (or mucins) | Number of saliva donors, gender, age | Methods | Saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein solution ratio protein solution ratio |
|||
| 7.0*: ‘mentioned in the literature as neutral pH’, ATR-FTIR spectroscopy: attenuated total reflection–Fourier transform infrared spectroscopy, β-lg = beta-lactoglobulin, CLSM: confocal laser scanning microscopy, F: female, FG: focus group, FP: flash profiling, HS: human saliva, LS = light scattering, LTSEM: low-temperature scanning electron microscopy; M: male, MS: model saliva, NA: not applicable, ND: not disclosed, ODT: orthonasal detection threshold, OP: oral processing, O/W: oil in water, pI: isoelectric point, PGM: porcine gastric mucin, PNA: protein non-associated; SDS-PAGE: sodium dodecyl sulphate polyacrylamide gel electrophoresis, SWHS: stimulated whole human saliva, UWHS: unstimulated whole human saliva, WPH: whey protein hydrolysate, WPI: whey protein isolate, WPC: whey protein concentrate, ZP: zeta potential. | ||||||||||
| Food emulsions | ||||||||||
| Sarkar, et al. (2009)56 | β-lg or lactoferrin | 6.8 | Soy oil O/W (20% w/w) emulsion stabilized by 1.0% w/w protein | MS | PGM | NA | CSLM, LS, rheology, ZP | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, different concentrations of mucin used (0.0–3.0% w/w mucin) 1, different concentrations of mucin used (0.0–3.0% w/w mucin) |
There was reversible droplet aggregation between β-lg-stabilized emulsions and MS containing PGM, which was not observed in MS without mucin. β-lg emulsion + MS mixtures were stable when mucin was below 1.0 wt% due to repulsion. When mucin concentration was increased, flocculation and phase separation was observed with no significant alteration of droplet size – thus suggestive of depletion flocculation. β-lg-stabilized emulsions with mucin ≥1.0 wt% showed shear thinning behaviour. Lactoferrin showed droplet aggregation regardless of mucin presence with MS. When mucin concentration was increased (2.0 wt%), aggregation of oil droplets was found, with lower mucin concentrations aggregates were monodisperse (0.5 wt%). When lactoferrin was combined with MS (no mucin), pseudoplastic flow behaviour was found due to bridging flocculation, when mucin was added viscosity increased. ZP results showed a decrease from +27 mV to −27 mV when mucin concentration was increased from 0.1 to 1.5 wt%. Secondary surface coverage increased with mucin concentration-attributed to electrostatic interactions between lactoferrin and mucin | 100% |
| Silletti, et al. (2007,1 2007,60 2008,57 201041) | β-lg or lysozyme | 6.7 and 3.0 | Sunflower oil O/W emulsions (40% w/w) stabilized by 1% w/w protein | HS | SWHS or UWHS | n = 11, 5F, 6M, age 20–45 | CLSM, LS, rheology, ZP, microscopy, ATR-FTIR, SDS-PAGE, western blotting | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
When emulsions were mixed with saliva, pronounced flocculation was found for β-lg at pH 6.7 and at pH 3.0, as well as with lysozyme. The β-lg-stabilized emulsions at pH 6.7 had homogenously dispersed flocs whereas larger densely packed flocs were seen at at lower pH 3.0. Lysozyme had heterogenous, strongly packed long thread structures upon mixing with saliva. Flocculation was reversible for β-lg at pH 3.0 and lysozyme 6.7 suggesting flocculation is weak. β-lg stabilized emulsions at pH 3.0 had irreversible flocculation, as this pH was close to the pI of β-lg. ZP suggest flocculation is electrostatically-driven. Furthermore at pH 3.0, greater viscosity was found with all shear rates suggesting that the flocs were not completely disrupted by shear rate. Unstimulated saliva had a higher ratio of viscosity (ηmix/ηemul) and greater modulii (G′mix/G′emul) compared to stimulated for β-lg. Changes of results between saliva type were more limited for lysozyme emulsions. ATR-FTIR indicate PNA fractions are comparable with that of saliva. SDS-PAGE showed mucins present, with MUC5B bound to lysozyme stabilized emulsions and MUC7 as well as moderate levels of MUC5B associated with β-lg stabilized emulsions | 87%, 94%, 67%, 63% |
| Vingerhoeds, et al. (2005)37 | β-lg, casein, sodium caseinate or WPI | 6.7 | Sunflower oil O/W emulsions (40% w/w) stabilized by 1% w/w protein | HS or MS | UWHS, PGM | n = 6, 2M, 4F, age 28–43 | Demixing (turbiscan), LS, microscopy, and Rheology | UWHS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Parotid saliva (no mucin) only caused reversible aggregation with WPI- and β-lg stabilized emulsions. PGM induced flocculation of emulsions, and human saliva also resulted in aggregation of emulsions. All proteins when mixed with PGM concentration greater than 0.4 ± 0.1 wt% had phase separation suggesting interactions were depletion flocculation | 79% |
| Koukoura, et al. (2019)59 | Sodium caseinate | ND | Medium chain triglyceride O/W emulsions (30% w/w) stabilized by 2% w/w protein | MS | PGM | NA | CLSM, LS, ZP, | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Sodium caseinate-stabilized emulsions size were not affected by addition of saliva. Caseinate-stabilized emulsions initially had a highly negative zeta-potential which was reduced when mixed with saliva. As sodium caseinate-stabilized emulsions had a strong negative zeta-potential, and saliva had a negative (but not as strong) zeta potential; this reduction was expected | 96% |
| Ritzoulis, et al. (2012)58 | Sodium caseinate | 1.0–7.0 | n-Hexadecane O/W emulsions (30% w/w) stabilized by 1–1.25% w/w protein | MS | PGM | NA | LS, microscopy, ZP | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Caseinate-stabilized emulsions flocculated in the presence of model saliva. Flocculation was bridging and electrostatic at lower pH (pH 3.0) whereas at greater i.e. pH 5.0 and above, depletion flocculation occured. Average particle size increased with increasing pH, however at pH 7.0, flocs separated due to electrostatic repulsion. At pH 3.0 and 5.0, flocculation was irreversible | 88% |
| Fuhrmann, et al. (2019)66 | Gelatine or WPI | 5.0 or 7.0 | Sunflower oil O/W emulsions (20% w/w) stabilized by 0.0–0.6% w/w gelatine or 0.0–0.19% w/w WPI | HS | UWHS | n = 10 (ND, ND) | Rheology, LS, tribology, sensory analysis (n = 83, 62F, 21M, mean age 23.5 ± 3.8), ZP | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
For gelatine-stabilized emulsions, viscosity and droplet size increased from 1–2 mm to >25 mm likely because of flocculation. Emulsions with large clusters showed a reduction in effective cluster size when mixed with saliva attributed to the negative charge of proteins in saliva interacting with gelatine. This inhibited binding among oil droplets and reformed clusters. Friction for gelatine-stabilised emulsions was reduced marginally in the boundary regime but not in mixed/hydrodynamic regime. When WPI-stabilised emulsions was combined with saliva, no change in droplet size was observed. Sensory analysis showed correlations between thickness and consistency (viscosity) and friction properties correlated with creaminess when saliva was not present | 73% |
| Vingerhoeds, et al. (2009)42 | Lysozyme or WPI | 6.7 | Sunflower oil O/W emulsions (40% w/w) stabilized by 1% w/w WPI; sunflower oil O/W emulsions (20% w/w) stabilized by 1% w/w lyzozyme | HS | SWHS | Oral processed (OP) emulsions spitting out oral processed samples; sensory analysis (n = 9, 9F, age 30–60) | ATR-FTIR, LS, western blotting, SDS PAGE, OP emulsions spitting out oral processed samples, trained sensory analysis, rheology | NA | Lysozyme-stabilised emulsions had larger flocs than WPI-stabilised emulsions after oral processing. Before oral processing, both lysozyme and WPI non thickened emulsions had similar viscosity. After oral processing, viscosity of lysozyme-stabilized emulsions increased more than those of WPI; which was hypothesized to be because of irreversible flocculation between positively-charged lysozyme and negatively-charged saliva, whereas WPI showed reversible flocculation. Tongue oil retention was found to be greater for lysozyme-stabilized over WPI stabilized emulsions. SDS-PAGE indicated β-lg and lysozyme were retained on the tongue. MUC7 (not MUC5B) was retained more for lysozyme emulsions suggestive of greater clearance for WPI emulsions. Sensory analysis showed WPI were associated with greater thickness, creaminess, fattiness and slipperiness attributes compared to lysozyme, whereas, lysozyme was associated with dryness, roughness and astringency | 79% |
| Dresselhuis, et al. (2008)73 | WPI | ND | Sunflower oil O/W emulsions (40% w/w) stabilized by 0.3 or 1% w/w protein | HS | UWHS | n = 30, ND, ND | LS, microscopy, retention (pig tongue – CLSM), rheology, swallow and spit out | Raman spectroscopy and CSLM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Emulsions stabilized by 0.3% w/w WPI emulsions were less stable, with more fat remaining, which was harder to remove on the tongue surface than the ones stabilized by 1.0% w/w WPI. In addition, spectroscopy showed more emulsion droplets visible for higher protein concentrations (1.0 vs. 0.3% w/w) | 84% |
| Hu, et al. (2019)72 | WPI | pH 7.0* | Sunflower oil O/W emulsions (20% w/w) or orange oil O/W emulsions (0.0033% w/w) stabilized by 1.0% w/w protein | MS + HS | PGM or SWHS | n = 10, 6F, 4M, age range 20–25 | Optical microscopy, OP (in vitro), LS, rheology, turbidity | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
WPI emulsion mixed with model saliva in conditions with and without α-amylase. Flow analysis showed WPI + model saliva mixtures were shear thinning (non-Newtonian). There was no viscosity differences with and without α-amylase | 90% |
| Food matrices | ||||||||||
| Campbell, et al. (2017)68 | WPI | 6.1 to 6.9 | 11% w/w protein solutions formed into model foods by manipulating pH, ionic strength and heating time | HS | WSHS or UWHS | n = 4, ND, age range 23–31 | Rheology, tribology | ND | Different processing affected rheology and textures of the model food. Friction values were reduced for saliva alone compared to model foods. Viscosity of saliva-model foods was increased compared to model food alone with reduced friction. Variation in tribology methods (elliptical versus linear motion) had different results | 63% |
| Childs and Drake (2010)65 | WPI | 2.6 to 3.4 | Acidified whey beverage (0–6.0% w/w) with added citric acid (0.43–1.8% w/w) | HS | SWHS | Sensory, n = 49 (30F, 19M, age ≥25); ODT n = 25 (ND, ND), taste threholds, n = 25 (ND, ND) acceptance tests, n = 120 (ND, ND) | Sensory analysis (FG, ODT, taste threshold, consumer acceptance tests) | NA | FG 95% chose WP sports drink as their least favourite, and 40/49 suggest it was very astringent. Terrible aftertaste was also recorded. Consumer acceptance was reduced as protein concentration increased without nose clips | 63% |
| Morell, et al. (2017)63 | WPI | 4.5–4.6 | Milk powder or WPC yogurts; 10% w/w skimmed milk powder versus 4.3% w/w WPC | MS or HS | PGM or SWHS | n = 1, ND, ND | Sensory analysis (n = 13), tribology | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
The addition of saliva led to a reduction in yogurt friction values, with the reduction greater in yogurts without added starch. At low sliding speeds, WPC and milk powder had similar friction coefficient values (boundary lubrication). MS vs. SWHS had similar lubrication effects on yoghurt. WPC-based yoghurts were described as rough, gritty, grainy and astringent whereas milk powder yoghurts were described as smooth and creamy | 95% |
| Morell, et al. (2015)64 | WPI | 4.5–4.6 | 1.9% w/w of WPI or 10% w/w skimmed milk formulated into milk yogurts | MS or HS | PGM + SWHS | Sensory n = 2, 16F, 5M, age range 21–45 years; liking and satiating n = 121, ND, ND | LTSEM, microscopy, rheology, sensory analysis (FP and liking and satiating tests) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
After in vitro OP, channel like formations of saliva was observed in all samples, with aggregation of protein network forming dense and opaque areas. WPI formed dense and finely branched network hypothesized due to micelles forming individual entities around flocculated protein and aggregates thus increasing the number of bonds. Saliva reduced viscosity values in all samples. Milk protein samples were rated as creamy, thick and dense, whereas whey protein samples were rated as lumpy, grainy and gritty. WPI-rich yoghurts was the least popular, followed by milk protein and the control which had half the protein content | 96% |
| Wang, et al. (2016)50 | WPI | 2.2–3.9 | 21 g L−1 protein with 8% wt/wt sucrose, phosphoric acid 40% or potassium carbonate 20% | HS | SWHS | n = 10, ND, ND | Chemometric analysis, (ATR-FTIR spectrometer), quantitative sensory analysis | ND | Comparing WPC, WPI and WPH, Whey protein beverages had increased astringency when pH was reduced from 3.9 to 2.2. WPH had highest variability in astringency, and WPI had lowest variability of scores. pH affected WPC and WPH with lower pH (more acidic) correlated to greater astringency – whereas pH only affected 1/3 WPI samples. Infrared analysis indicated protein structure, in particular carboxylic functional groups (COO-) and amide 11 which alter the secondary structure may be associated with astringency as they correlated with sensory scores | 49% |
The demographics for the type of saliva were similar, with 23 using real human saliva and 16 using model saliva whereas 6 using both types of saliva (Tables 1 and 2) with limited number of studies using bovine submaxillary mucin (BSM) as the mucin source in case of model saliva. The quantitative assessment of each individual study's bias was conducted (see Table S1, ESI†). Collectively the average percentage was 84%, within general reporting quality and performance bias scores the lowest and detection bias the highest (see Tables 1 and 2 for individual scoring).
3. Discussion
All the protein types all showed indications of interaction with either model or human saliva. Shifting pH to around the isoelectric point (pI) of proteins indicated most proteins identified electrostatically interacted with mucin. Interactions observed however varied by protein type.Specifically, casein did not show any indications of interaction with mucin in bulk solutions40 (Table 1). However, aggregation was found in casein-based emulsified systems37 (Table 2). Despite lysozyme eliciting aggregation in presence of saliva, the parameters driving interactions are not well-established. Lysozyme has only been investigated at a limited range of conditions, although pH dependence of interactions with saliva does suggest electrostatic interactions are involved.1,41,42 For whey protein, interactions with saliva (model or real) appeared to be predominately electrostatically driven. However, in different conditions they can be entropically and enthalpically driven too38,43–50 (Table 1). Zeta-potential analysis again indicated that whey protein, β-lg, gelatine, and lactoferrin electrostatically interact with mucin.51–54 However, gelatine and WPI have also been shown to interact with mucin via non-electrostatic mechanisms (Table 1).55 Evidence supported entropically and enthalpically driven aggregation with formation of hydrogen bonds or hydrophobic interactions even at neutral pH where both whey protein, gelatine and mucins are negatively charged.44,55
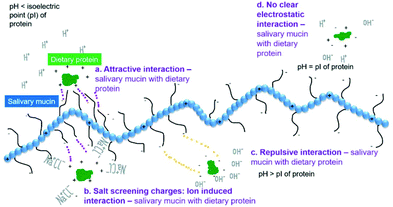
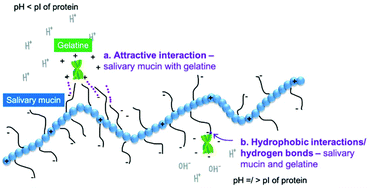
In the following sections, we discuss the effects of protein type (at neutral pH), variation of pH, protein concentration, saliva type, protein–saliva mixing ratio and heat treatments of proteins. Throughout the discussion, we have focussed on the proteins in bulk phase but included examples from emulsions. Examples from emulsions were included when interactions varied from bulk solutions. Interactions are shown schematically in Fig. 3a–d and Fig. 4a–b.
3.1 Protein type
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) was −38 mV, −15 mV and −28 mV, respectively.44 The authors theorized this happens because mucin contains positively charged patches despite its negative charge, which attract to WPI's negatively charged acidic amino acids at neutral pH.44 However, this is unlikely as the negative charge of WPI is so high (−38 mV). Temperature-dependent fluorescence spectroscopy and the Benesi–Hildebrand equation used to assess the thermodynamic stability of interactions revealed that WPI–mucin interactions and phase separation at pH 7.0 could not be explained by electrostatics. In fact, both endothermic with spontaneous binding and hydrophobic association appeared to influence interactions with mucin.44 Hydrophobic interactions with non-glycated terminal peptide regions also cannot be ignored44 (Table 1), which is likely if local charge repulsion is low. At neutral pH there was an increase in the viscosity of the whey protein–saliva mixture. This was hypothesized to originate from increased energy dissipation due to phase separation of WPI + mucin colloidal particles increasing the viscosity.44 However in whey protein-stabilised emulsions (Table 2) viscosity was only minimally affected by the addition of human saliva at near-neutral pH (6.7).42 Equivocal results were found for turbidity, with only small changes reported by one study for WPI-stabilized emulsion + mucin interaction at neutral pH46versus no changes in another study.47 Tongue retention analysis found β-lg, the main protein in WPI, retained on the tongue after oral processing of WPI emulsions.42 Separately, at pH 6.7 when parotid saliva containing no mucin was used, WPI reversibly aggregated highlighting the importance of non-mucinous salivary proteins in such aggregation.37
1 w/w) was −38 mV, −15 mV and −28 mV, respectively.44 The authors theorized this happens because mucin contains positively charged patches despite its negative charge, which attract to WPI's negatively charged acidic amino acids at neutral pH.44 However, this is unlikely as the negative charge of WPI is so high (−38 mV). Temperature-dependent fluorescence spectroscopy and the Benesi–Hildebrand equation used to assess the thermodynamic stability of interactions revealed that WPI–mucin interactions and phase separation at pH 7.0 could not be explained by electrostatics. In fact, both endothermic with spontaneous binding and hydrophobic association appeared to influence interactions with mucin.44 Hydrophobic interactions with non-glycated terminal peptide regions also cannot be ignored44 (Table 1), which is likely if local charge repulsion is low. At neutral pH there was an increase in the viscosity of the whey protein–saliva mixture. This was hypothesized to originate from increased energy dissipation due to phase separation of WPI + mucin colloidal particles increasing the viscosity.44 However in whey protein-stabilised emulsions (Table 2) viscosity was only minimally affected by the addition of human saliva at near-neutral pH (6.7).42 Equivocal results were found for turbidity, with only small changes reported by one study for WPI-stabilized emulsion + mucin interaction at neutral pH46versus no changes in another study.47 Tongue retention analysis found β-lg, the main protein in WPI, retained on the tongue after oral processing of WPI emulsions.42 Separately, at pH 6.7 when parotid saliva containing no mucin was used, WPI reversibly aggregated highlighting the importance of non-mucinous salivary proteins in such aggregation.37
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w mixing ratio, zeta-potential values and particle size were almost identical to lactoferrin alone (Table 1). SDS-PAGE further showed mixtures of heated lactoferrin and human saliva were predominantly lactoferrin.43 Of the two studies including sensory analyses, one reported little or no astringency,43 whereas the other showed intense astringency in lactoferrin solutions, although precipitation in mixtures of lactoferrin and saliva was limited.54 This may suggest that electrostatic interactions between lactoferrin and human saliva may not be the sole factor governing astringency of lactoferrin. On the other hand, when lactoferrin was analysed in emulsions (pH 6.8 using model PGM saliva, 1% w/w lactoferrin) there were clear signs of interactions with pronounced bridging flocculation (Table 2).56 Since lactoferrin has an isoelectric point of around 8.5, the attractive interaction between lactoferrin-stabilized droplets and anionic mucins as schematically shown in Fig. 3a led to aggregation. The zeta-potential went from +27 mV to −27 mV, when the mucin concentration was raised from 0.1 to 1.5 wt%. Zeta-potential measurements were screened when salivary salts (no mucin) were combined with emulsions.43 This indicates that besides electrostatic binding with mucin, charge screening effects by salts present in model saliva also caused aggregation in lactoferrin stabilized emulsion droplets (Fig. 3b). In addition, mucin coverage was greater in lactoferrin stabilized emulsion (compared to β-lg) which was further hypothesized to be because of electrostatic interaction.56
1 w/w mixing ratio, zeta-potential values and particle size were almost identical to lactoferrin alone (Table 1). SDS-PAGE further showed mixtures of heated lactoferrin and human saliva were predominantly lactoferrin.43 Of the two studies including sensory analyses, one reported little or no astringency,43 whereas the other showed intense astringency in lactoferrin solutions, although precipitation in mixtures of lactoferrin and saliva was limited.54 This may suggest that electrostatic interactions between lactoferrin and human saliva may not be the sole factor governing astringency of lactoferrin. On the other hand, when lactoferrin was analysed in emulsions (pH 6.8 using model PGM saliva, 1% w/w lactoferrin) there were clear signs of interactions with pronounced bridging flocculation (Table 2).56 Since lactoferrin has an isoelectric point of around 8.5, the attractive interaction between lactoferrin-stabilized droplets and anionic mucins as schematically shown in Fig. 3a led to aggregation. The zeta-potential went from +27 mV to −27 mV, when the mucin concentration was raised from 0.1 to 1.5 wt%. Zeta-potential measurements were screened when salivary salts (no mucin) were combined with emulsions.43 This indicates that besides electrostatic binding with mucin, charge screening effects by salts present in model saliva also caused aggregation in lactoferrin stabilized emulsion droplets (Fig. 3b). In addition, mucin coverage was greater in lactoferrin stabilized emulsion (compared to β-lg) which was further hypothesized to be because of electrostatic interaction.56
It is noteworthy that there has not been a single study performed to investigate the interaction of plant protein with saliva within the search date of this systematic review. A recent study on the interaction of pea proteins with BSM (published outside the inclusion dates)62 shows that the adsorption capacity of pea protein to a hydrophobic surface is reduced in the presence of BSM due to electrostatic repulsion between pea protein and BSM. Nevertheless, the extent and kinetics of adsorption of pea protein has been found to be significantly higher than WPI on BSM-coated surfaces. This suggests pea protein might give rise to astringency perception due to more binding to BSM-coated surface compared to that of WPI, however no sensory evaluation was conducted in this study.62 Thus, understanding the interaction of pea protein with saliva and salivary proteins seems to be a key knowledge gap. Particularly in view of the growing interest in sustainability and designing plant-based food formulations.
3.2 pH
In the following section the effect of changing pH is discussed. Since electrostatics appears to be the key mechanism driving protein–saliva interaction as schematically shown in Fig. 3. pH is an important factor that determines the attractive or repulsive nature of such interactions in presence of real or model saliva. The isoelectric points (pI) of proteins is referred to in Tables 1 and 2. When the pH, was around the pI of whey proteins (Table 1), turbidity increased (pH 4.6–5.2) indicating interactions between WPI and human saliva.48 Alternatively, as this is around the isoelectric point of WPI, hydrophobic interactions of WPI self-aggregation may have dominated (Fig. 3d). However, some attractive interactions between negatively-charged mucins and some positively-charged patches of WPI at the pI cannot be neglected.44 Regarding emulsion systems when pH was lowered to pH 3.0 i.e. below the pI of WPI (Fig. 3a), flocculation was no longer reversible. The saliva-induced flocs were also larger and densely packed (Table 2) thus clearly dominated by electrostatics. Moving on, pH was also varied in food matrixes using whey-based yoghurt63,64 (Table 2). The pH of the whey based yoghurts was set between 4.5–4.6 and when model saliva (PGM) was added, friction reduced compared to yoghurt alone.64 Importantly, the results from sensory analysis were linked as the whey-based yoghurts were described as ‘rough, gritty, and astringent’ at these pH values.63 Further, a comparison of whey protein to milk-based yoghurts was made. The milk protein yoghurt was rated as creamy and thick whereas whey protein yoghurts were rated as grainy, lumpy and thick.64 Similarly, whey-based sports drinks formulated at pH 2.6–3.4 were again rated very astringent in sensory analysis.65 Increased turbidity at pH 3.4 was correlated with higher sensory astringency scores.46 Interestingly, another study which also varied pH but also processing method reported no correlation between pH and astringency ratings for WPI beverages. However, whey protein concentrate and whey protein hydrolysate beverages had increased astringency with lower pH.50 As a whole these results highlight the importance of electrostatic attraction between positively charged whey protein at low pH (pH < pI) and anionic salivary mucins driving such astringency (Fig. 3a).Adjusting pH in β-lg, mirrored the results found for WPI which was expected as β-lg is the main component of WPI43 (Table 1). Zeta-potential analysis indicated β-lg was positively charged (+21 mV) at pH 4.3 and thus attracted to the negatively-charged saliva and eliciting pronounced flocculation1 (Fig. 3a). At pH 5.0, β-lg is near the isoelectric point43,48 and electrostatic repulsion will be minimal. Accordingly, at pH 5.0, attractive hydrophobic interactions led to β-lg aggregation and network formation1 (Fig. 3d), which overshadowed any β-lg–mucin interaction.53 Tribology showed that at pH 5.0, a reduction in lubrication in β-lg was observed compared to that at pH 3.5 and pH 7.451 and an increase in friction36 compared to pH 3.5 (Table 1). This is expected as β-lg aggregates were particulate in nature and were incapable of forming a continuous load bearing film at the tribo-contact surface as opposed to β-lg films or β-lg + mucin films. Sensory analysis showed that astringency increased as the pH was lowered, also observed in WPI.43,52,54
The results for lactoferrin do not appear to be affected by pH. As food-relevant pH are below the pI of lactoferrin and thus electrostatic interactions with salivary mucins remain irrespective of pH. Lactoferrin was shown to be astringent in all pH conditions (pH 3.5–7.0) and interactions were predominantly electrostatic in origin as discussed previously.54 Human saliva combined with lactoferrin between pH 2.0 to pH 7.0 had a net positive charge which was very similar to lactoferrin alone43 (Table 1). Particle size increased when pH was lowered to 2.0, and between pH 3.0 to pH 8.3 particle size was small, but no precipitation of lactoferrin + human saliva was found in any pH condition.43 Moreover, lactoferrin was investigated in an emulsion system but only at a single pH (pH 6.8) (Table 2), so could not give further indications of the effect of pH.56
When sodium caseinate was analysed at a range of pH (1.0–7.0), interactions were again postulated to be electrostatic. This was demonstrated through zeta-potential and microscopy analysis which showed electrostatic interactions at pH 3.0 eliciting bridging flocculation, whereas depletion flocculation was observed at pH 5.0 (Table 2). Both pH 3.0 and 5.0 led to irreversible flocculation.58 At pH 1.0, 75% of PGM was found in the serum with the remaining 25% observed to be bound to the droplet surface, which were hypothesized to be because of interactions with interfacial sodium caseinate.58 In addition, fast flocculation driven creaming occurred at pH 3.0 whereas at pH 7.0 creaming was limited and emulsions were stable58 (see Fig. 3c).
With non-dairy proteins, the pH of solutions was shown to also affect gelatine's interaction with PGM.55 Viscosity varied with pH, with the Trouton ratio (Tr) (ratio of extensional to shear viscosity) being relatively low at pH 3.0 (Tr = 200 for mucin and gelatine at a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio), and significantly higher at pH 7.0 (Tr = 1400).55 As Trouton ratio followed the same trend as extensional viscosity it was suggested the importance of binding regimes between gelatine and mucin for the extensional viscosity and hence on Trouton ratio. Fluorescence spectroscopy indicated binding between mucin and gelatine at both pH 3.0 and 7.055. Mucin (PGM) has a small net charge at pH 3.0 that was suggested not to be large enough to attract positively-charged gelatine, although electrostatic attraction cannot be ignored (Fig. 4a).55 As mentioned previously, it was postulated that interactions at neutral pH were caused by hydrogen bonds and hydrophobic interactions between gelatine and mucin (Fig. 4b). At pH 3.0, the interactions were weak,55 and sensory analysis for gelatine at pH 3.5 showed no astringency.38 When gelatine was investigated in emulsion format despite the study not specifically analysing differences in pH (Table 2), the data at pH 5.0 and 7.0 results do not appear to differ significantly. For instance, gelatine had a positive charge at pH 5.0 (+10 mV) and pH 7.0 (+7 mV). The oil droplet size was similar 1–2 μm and when mixed with human saliva increased to over 25 μm. Consistency indicates the viscosity of a fluid. When K is below 1 the fluid tends to be shear thinning, and above 1 shear thickening. Consistency increased in both conditions when saliva was added from 0.01 to 0.17 K (Pa sn) for single droplet pH 5.0 vs. 0.005 to 0.25 Pa s for pH 7.0. Friction in both pH 5 and pH 7 also decreased slightly in the boundary regime.66 Although the origin of interactions was not investigated, when comparing these emulsion result to results from bulk solution, the lack of difference between pH 5.0 and 7.0 in case of gelatine + saliva is surprising.
4 ratio), and significantly higher at pH 7.0 (Tr = 1400).55 As Trouton ratio followed the same trend as extensional viscosity it was suggested the importance of binding regimes between gelatine and mucin for the extensional viscosity and hence on Trouton ratio. Fluorescence spectroscopy indicated binding between mucin and gelatine at both pH 3.0 and 7.055. Mucin (PGM) has a small net charge at pH 3.0 that was suggested not to be large enough to attract positively-charged gelatine, although electrostatic attraction cannot be ignored (Fig. 4a).55 As mentioned previously, it was postulated that interactions at neutral pH were caused by hydrogen bonds and hydrophobic interactions between gelatine and mucin (Fig. 4b). At pH 3.0, the interactions were weak,55 and sensory analysis for gelatine at pH 3.5 showed no astringency.38 When gelatine was investigated in emulsion format despite the study not specifically analysing differences in pH (Table 2), the data at pH 5.0 and 7.0 results do not appear to differ significantly. For instance, gelatine had a positive charge at pH 5.0 (+10 mV) and pH 7.0 (+7 mV). The oil droplet size was similar 1–2 μm and when mixed with human saliva increased to over 25 μm. Consistency indicates the viscosity of a fluid. When K is below 1 the fluid tends to be shear thinning, and above 1 shear thickening. Consistency increased in both conditions when saliva was added from 0.01 to 0.17 K (Pa sn) for single droplet pH 5.0 vs. 0.005 to 0.25 Pa s for pH 7.0. Friction in both pH 5 and pH 7 also decreased slightly in the boundary regime.66 Although the origin of interactions was not investigated, when comparing these emulsion result to results from bulk solution, the lack of difference between pH 5.0 and 7.0 in case of gelatine + saliva is surprising.
Finally, for lysozyme, when pH was lowered to 3.0, pronounced flocculation between unstimulated saliva and lysozyme stabilized emulsions were apparent.60 Additionally, in low pH conditions flocs were larger and more densely packed. Contrastingly, at neutral pH flocs were homogeneously dispersed. Viscosity was also shown to be increased at lower pH. However, only one study using lysozyme varied the pH and only used two conditions pH 3.0 and pH 6.7,60 the remaining studies all used neutral pH. Therefore, the full impact of pH remains unknown and further research in a wider range of pH may provide useful insights. Additionally, sensory analysis should be included to see if potential physicochemical and rheological changes observed as a function of pH translate into mouthfeel differences.
3.3 Protein concentration
When thinking about formulating food with high protein content, it is important to understand how increased protein concentration affects oral perception. The only studies which investigated the effect of protein concentration used either whey protein or the whey protein component β-lg. Collectively, protein–saliva interactions appear to be a function of protein concentration in addition to pH.48 For example, two studies using bulk solution's and measuring turbidity reported a delayed time to reach maximum turbidity when protein concentration of WPI was increased from 0.5% w/w to 10% w/w45,48 (Table 1). This was hypothesized to arise from more saliva being required to interact with the greater amounts of protein consumed.45Interestingly, different results were found for different types of whey protein (i.e. native versus denatured) (Table 1). Increasing protein concentration (0.25 to 10.8% w/w) increased aggregate size and turbidity for denatured whey proteins (heated at 80 °C) at pH 6.8. Whereas the effect for native whey protein (rehydrated into deionized water) was much smaller. In addition, the viscosity increased in the denatured samples. It was hypothesized to be due to more opportunity for covalent bonding due to increased unfolding of chains inhibiting interpenetration of polymers and higher free thiol availability.47 Alternatively, heating proteins increases surface hydrophobicity, which can also drive aggregation.67 Friction in polydimethylsiloxane tribopairs in presence of protein was found to be unaffected by concentration (0.5–4% w/w), indicating that protein concentration in this low range did not affect the loss of lubrication of human saliva.48 However, similar to turbidity, a significant difference in friction coefficient was observed at higher concentration (10% w/w).36 By using a single sliding speed, entrainment of protein solutions at higher concentrations leads to an effective separation of contact bodies and therefore can contribute to lowering of friction. In contrast, in real-life situations, it is known that frictional conditions are dynamic in the mouth occurring at various speeds depending upon the type of food consumed during oral processing. Also swallowing will impact the amount of protein solutions retained on the tongue surfaces. Therefore, the frictional reduction due to higher concentration of protein at higher entrainment speed might not translate into sensory responses.36
Linking these concentration effects to sensory mouthfeel produced equivocal results. For example, Kelly et al. reported no effect of concentration on time-intensity sensory astringency analysis.48 Thereby suggesting the mechanism of astringency might not always be lubrication failure-linked. In another study, greater astringency was reported with higher concentrations up to 4% w/w, after this point, a plateau was observed.36,48,54
When protein concentration was varied in more complex food systems (Table 2), it was found that increasing whey protein concentration (0 to 6% w/w) reduced consumer acceptance. Similarly, the sports drink used was rated increasingly astringent.65 As only limited studies appeared to include a sensory link when analysing concentration, further evidence is needed to fully confirm protein concentration effects.
In addition to the individual effects of protein concentration, the effects of concentration alongside pH was also investigated in few studies. This is because increasing protein concentration increases the amount of acid required to shift the overall pH due to the buffering capacity of protein.48 When increasing protein concentration was investigated concurrently with pH, particularly in low pH conditions (pH 2.6) and when protein concentration was raised, the maximum intensity of astringency was reduced. Conversely, at pH 4.2, when the protein concentration was increased, the maximum intensity of astringency increased. Collectively indicating pH affects the relationship between concentration and astringency.48 In other words, the amount of protein governs the buffering capacity of the solution dictating the magnitude of saliva–protein interactions via electrostatics. Furthermore, the saliva flow rate was shown to be raised with increasing protein concentration (0.5 to 10% w/w). With increasing saliva flow, there will be a quicker rate of clearance for the astringent compounds.45 To summarize, concentration effects appear to be range specific (0.5–4% w/w); further analysis is needed to confirm and see if the same differences also apply to a wider range of protein. In addition, a clear gap exists in the literature for higher concentrations of proteins (>10% w/w) where viscosity and elastohydrodynamic lubrication will play a key role in driving sensorial perception. Such as when translating this to food formulation where at least 20% of the energy value of the food provided by protein is required to make a content claim of “high protein”.
3.4 Heat treatment of proteins
Few studies openly varied the preparation of the protein solution and the studies which did, used whey protein or its derivative β-lg. For instance, one study included denatured whey heated to 80 °C for 40 minutes. This was compared to whey protein samples that were re-hydrated in de-ionized water. Increased turbidity independent of pH and enhanced bio adhesion was recorded for denatured whey protein compared to native whey protein at pH 6.8.47 Similarly, β-lg was processed by heating to 90 °C for 10 minutes and immediately cooled in ice and compared to an unheated control but little difference was found in zeta-potential and SDS-page between the two conditions although turbidity was affected suggesting that interactions were hydrophobic in nature.The heated sample had two peaks in particle size, which also drove an increase in turbidity at both pH 3.4 and pH 5.0, whereas the unheated sample only had a peak at pH 3.4 – which is suggestive of a complexation between human saliva and β-lg.43 Sensory ratings of astringency were similar despite these differences, therefore although heat treatment may affect turbidity, it may not translate into mouthfeel differences. In a study that used model food systems heating was used to vary the viscosity of the fluid and elicited distinctly different textures. Although the effect of heating was not looked at in isolation, rheological thickness and descriptive sensory viscosity were highly correlated with fluid (heating time 5 minutes) and semisolid treatments (heating time 15 or 30 minutes).68
Moreover, a recent study on plant proteins and whey protein again (published outside the inclusion dates) used heat treatment. From this, it was shown that heat treatment increased viscosity of both pea protein and whey protein isolate solutions. This in turn reduced friction in mixed and elastohydrodynamic lubrication regimes but the boundary regime (where astringency is thought to arise) was unaffected62. Furthermore, pea protein isolate was not affected by heat treatment whereas WPI had significant structural changes.62 In general heat treatment of milk is a critical process used by the dairy industry. It is used to prolong stability and enhance quality through lowering microbial load or manipulating functionality of dairy proteins and strengthening the organoleptic properties.69 Therefore, further research on protein treatment effects, which can fundamentally change protein properties, is important for understanding the parameters protein related mouthfeel may operate.
3.5 Saliva type
There were a number of different saliva types used in the selected studies, with 17 using exclusively human saliva, 11 using exclusively model and 6 using both model and human saliva (Tables 1 and 2). A previous review comparing model saliva and human saliva studies concluded model saliva cannot fully replicate the physicochemical and biophysical properties of human saliva.28 It is not yet possible to fully simulate the intricate architecture that dictates the properties of human saliva. However, only very recently has lubrication performance been able to be simulated.14 Differences in behaviour are especially apparent when using more advanced methods to understand of how saliva and proteins interact. There will have been further variation in the studies that used human saliva, as human saliva is inherently variable. It is known to vary from different salivary gland, gender, age, diet, type of stimulations, circadian rhythm etc.28 The issues associated with these variables are discussed in the limitations section.The two main model saliva types are mucin based (PGM and BSM). Out of the 17 studies, which used model saliva (either exclusively or with human), 13 used PGM, 4 used BSM, 1 used a commercially available artificial saliva substitute and one of these studies using both PGM and BSM. Although mucin is often cited as being responsible for saliva's lubrication properties, it cannot replicate the lubrication performance of whole human saliva and its biophysical properties.28 For instance, other salivary components such as proline-rich–proteins have been proposed to play a role in the development of astringency for other astringents (such as polyphenols).70 The role of low molecular weight protein, such as lactoferrin has also been recently acknowledged14 as tending to bind mucin to itself and to the surface.
When model saliva containing PGM was compared against human saliva, results in terms of turbidity where similar. Following this, model saliva without mucins but still containing saliva salts was used.45 This model saliva with no mucin present did not increase turbidity. Similarly, a separate study used parotid saliva which contains salivary salts but no mucin71 found no aggregation measured by light microscopy.37 Collectively this indicates mucin is a key component of saliva which drives turbidity and aggregation45 (Table 1). Another study again comparing both model (BSM) and human saliva used more advanced methods by analysing lubrication.61 Although human and model saliva (BSM) produced similar friction coefficients, when the protein (lysozyme) was added incrementally, model and human saliva friction results differed (Table 1). Human saliva showed an increase in friction coefficient as lysozyme was added. Conversely, the increase in friction for BSM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein mixtures reached its peak when lysozyme was first added and then plateaued. Thus, model saliva may not fully represent oral conditions when protein is added continuously-like what happens in in vivo conditions. Furthermore, human saliva was more reproducible and had less variation between the repetitions.61 Thus, further work may be warranted to repeat studies using model saliva alone in methods beyond turbidity with human saliva to check for correlations.
protein mixtures reached its peak when lysozyme was first added and then plateaued. Thus, model saliva may not fully represent oral conditions when protein is added continuously-like what happens in in vivo conditions. Furthermore, human saliva was more reproducible and had less variation between the repetitions.61 Thus, further work may be warranted to repeat studies using model saliva alone in methods beyond turbidity with human saliva to check for correlations.
The type of mucin has been investigated in relation to surface adsorption and lubricity.23 Both mucins have similarity in composition such as being heavily glycosylated in the central region and both absorb onto hydrophobic surfaces likely due to hydrophobic interactions.15 Results comparing BSM and PGM showed PGM had greater absorbed mass (onto a PDMS surface).23 Lubrication was also different, with BSM having greater lubrication and elasticity.23 Similar results were shown by Lee and Vickers49 who showed BSM to be more efficient in reducing frictional forces than PGM using atomic force microscopy (AFM). The differences in PGM/BSM lubrication have been proposed to be related to the observed differences in adsorption.28 Moreover, it has been suggested BSM has greater pH sensitivity.28 This may have impacted results as a proposed mechanism of interactions especially with whey proteins is electrostatic which is influenced by pH. Looking at the two emulsion studies which used both human and model saliva, no discourse in results were found between the types37,72 of saliva (Table 2).
Besides model saliva, there are large number of studies that employed real human saliva to understand interaction with dietary proteins. The use of stimulated versus unstimulated tends to affect results as the latter has higher protein and consequently mucin concentration. One study used both stimulated and unstimulated saliva with β-lg and lysozyme-stabilized emulsions (Table 2). It was found that the unstimulated mixed with β-lg had different elastic properties compared to that of the stimulated saliva.57
There also appears to be a possible contribution from non-mucin components. Another component of saliva which can contribute to interactions but is at present understudied is the role of salivary salts. Salivary salts alone (with no mucin present) were found to elicit aggregation in lactoferrin stabilized emulsions but not for β-lg56 (Table 2). It was suggested the salivary salts screened the positive patches on lactoferrin molecules which reduces the overall positive charge (Fig. 3b). Salt was also varied in another study using WPI-based food systems.68 The study varied salt concentration from 0–30 mM NaCl to change the degree of aggregation. Higher salt conditions lead to greater protein aggregation and aggregate size which in turn affected turbidity. Although the study incorporated sensory analysis, you could not isolate the effect of salt in the results.68 Therefore, it would be useful to explore if salt-induced aggregation has similar sensory mouthfeel effects as electrostatic in terms of astringency perception.
3.6 Protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) saliva mixing ratio
saliva mixing ratio
Saliva to protein mixing ratio is another key parameter in understanding interactions of protein. Noticeably, the mixing ratio is directly related to the earlier discussed parameter of protein concentration however this section will also detail saliva's contribution. The majority of studies of bulk solutions used a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w ratio43,46,48,51–54,61 (Table 1) and for emulsion studies1,37,41,57–59,66,73,74 (Table 2), irrespective of the type of saliva employed. However, using this ratio has been suggested not to fully simulate real oral conditions, studies revealed that saliva mixes with food in more of a 1
1 w/w ratio43,46,48,51–54,61 (Table 1) and for emulsion studies1,37,41,57–59,66,73,74 (Table 2), irrespective of the type of saliva employed. However, using this ratio has been suggested not to fully simulate real oral conditions, studies revealed that saliva mixes with food in more of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w ratio.26,75 Of course, this depends upon the food type and the moisture content of the food. A limited number of studies have varied mucin
4 w/w ratio.26,75 Of course, this depends upon the food type and the moisture content of the food. A limited number of studies have varied mucin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein concentration; and in those that did, it was difficult to isolate the effects of this versus other confounding effects (e.g. pH, concentration). One study which did alter saliva
protein concentration; and in those that did, it was difficult to isolate the effects of this versus other confounding effects (e.g. pH, concentration). One study which did alter saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein ratio reported differences in zeta-potential between protein
protein ratio reported differences in zeta-potential between protein![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mucin ratios (Table 1). For example, when mucin (PGM) and WPI were mixed at 1
mucin ratios (Table 1). For example, when mucin (PGM) and WPI were mixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w ratio, zeta-potential was −28 mV, whereas, at 2
1 w/w ratio, zeta-potential was −28 mV, whereas, at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 w/w, the zeta-potential changed to −24 mV indicating a monotonic dependence on the mixing ratio.44 In terms of rheology, viscosity was increased by almost 20-folds from WPI alone to 2
8 w/w, the zeta-potential changed to −24 mV indicating a monotonic dependence on the mixing ratio.44 In terms of rheology, viscosity was increased by almost 20-folds from WPI alone to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 WPI
8 WPI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mucin mixtures. Mucin alone was the most viscous sample, and when mixed at 1
mucin mixtures. Mucin alone was the most viscous sample, and when mixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w indicated little change in viscosity between 6
1 w/w indicated little change in viscosity between 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 5
4, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 4
5 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 w/w WPI
6 w/w WPI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mucin mixing ratios (Table 1). It was suggested the higher viscosity is a result of increased energy dissipation during flow because of phase separation by the colloidal particles of WPI + mucin44 and would match the rheology dominated high molecular weight of mucin.76
mucin mixing ratios (Table 1). It was suggested the higher viscosity is a result of increased energy dissipation during flow because of phase separation by the colloidal particles of WPI + mucin44 and would match the rheology dominated high molecular weight of mucin.76
Similarly, when mixtures with different gelatine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mucin ratios were tested at pH 3.0, the Trouton ratio (ratio of extensional viscosity to shear viscosity) varied. Trouton ratios for mucin alone are c.100 and 239 for gelatine. As the ratio of mucin
mucin ratios were tested at pH 3.0, the Trouton ratio (ratio of extensional viscosity to shear viscosity) varied. Trouton ratios for mucin alone are c.100 and 239 for gelatine. As the ratio of mucin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gelatine decreases from 2
gelatine decreases from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 4
8, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 8
6 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, Trouton ratio values rise from 100, to 200 and finally to 500–700, respectively.55 The increase in Trouton ratio is a consequence of the assembly of mixtures and reflects increasing extensional viscosity thus, aggregation of gelatine–mucin appeared to reduce filament drainage which increased extensional viscosity.55 The human threshold have greater sensitivity in discriminating extensional over shear viscosity77 and these results indicate the self-assembly of mucin
2, Trouton ratio values rise from 100, to 200 and finally to 500–700, respectively.55 The increase in Trouton ratio is a consequence of the assembly of mixtures and reflects increasing extensional viscosity thus, aggregation of gelatine–mucin appeared to reduce filament drainage which increased extensional viscosity.55 The human threshold have greater sensitivity in discriminating extensional over shear viscosity77 and these results indicate the self-assembly of mucin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gelatine mixtures (which is reflected by the Trouton ratio, as explained above) will govern how thick foods combined with saliva flow. However, a key question to raise here is how much the mucin concentration varies in case of unstimulated or stimulated human saliva. Will that change depending upon the type or concentration of protein consumed? This definitely needs further exploration to clearly understand if the stimulation of salivary flow and mucin release is related to the protein being consumed.
gelatine mixtures (which is reflected by the Trouton ratio, as explained above) will govern how thick foods combined with saliva flow. However, a key question to raise here is how much the mucin concentration varies in case of unstimulated or stimulated human saliva. Will that change depending upon the type or concentration of protein consumed? This definitely needs further exploration to clearly understand if the stimulation of salivary flow and mucin release is related to the protein being consumed.
Besides the ratio of saliva itself, the way of adding saliva i.e. static versus dynamic can play an important role in its interaction with proteins. One elegant study varied the saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein ratio by adapting how saliva was added to the protein to simulate the dynamics of oral processing45 (Table 1). The study divided oral processing into two stages. The initial stage utilized a continuous flow method via a peristaltic pump delivering a continuous flow (1 mL min−1) of model saliva, which was gently stirred. Then WPI (5 or 10 mL) was poured on top of the container containing the model saliva to simulate sipping of a beverage, and afterwards to simulate swallowing, the solution was drained. Results of the continuous flow model revealed minimal changes in turbidity initially. Aggregates formed after a short period of time as the pH increased towards WPI's isoelectric point, increasing the turbidity. When protein concentration was varied (1–10% w/w), there was little change in turbidity. During this stage, there was not enough saliva to significantly alter the pH from the isoelectric point of the whey proteins. The second stage of stepwise mixing45 (Table 1) aimed to simulate residue and clearance. This method relied on two main assumptions. Firstly, that 1 mL of liquid (saliva + beverage residues) constantly covers the mouth; and secondly, that 250 mL of saliva + beverage is swallowed every 15 seconds. During stepwise mixing, turbidity further increased rapidly before a plateauing indicating maximum point was reached. The addition of more saliva caused the turbidity to quickly decline as residual whey proteins were diluted. When protein concentration was increased from 5 to 10 mL, the same trends occurred however maximum turbidity occurred later. Collectively, the amount of saliva affected both turbidity build up and turbidity decline through clearance. This emphasises the importance of using methods like these which simulate the dynamic in vivo conditions where saliva is added more continuously or in sequential steps rather than all at once. Although results further understanding of how astringency may develop temporally, the research was limited by the methods used. Only turbidity was evaluated, and the study did not account for the possible formation of aggregates and friction increase which may contribute to astringency.45
protein ratio by adapting how saliva was added to the protein to simulate the dynamics of oral processing45 (Table 1). The study divided oral processing into two stages. The initial stage utilized a continuous flow method via a peristaltic pump delivering a continuous flow (1 mL min−1) of model saliva, which was gently stirred. Then WPI (5 or 10 mL) was poured on top of the container containing the model saliva to simulate sipping of a beverage, and afterwards to simulate swallowing, the solution was drained. Results of the continuous flow model revealed minimal changes in turbidity initially. Aggregates formed after a short period of time as the pH increased towards WPI's isoelectric point, increasing the turbidity. When protein concentration was varied (1–10% w/w), there was little change in turbidity. During this stage, there was not enough saliva to significantly alter the pH from the isoelectric point of the whey proteins. The second stage of stepwise mixing45 (Table 1) aimed to simulate residue and clearance. This method relied on two main assumptions. Firstly, that 1 mL of liquid (saliva + beverage residues) constantly covers the mouth; and secondly, that 250 mL of saliva + beverage is swallowed every 15 seconds. During stepwise mixing, turbidity further increased rapidly before a plateauing indicating maximum point was reached. The addition of more saliva caused the turbidity to quickly decline as residual whey proteins were diluted. When protein concentration was increased from 5 to 10 mL, the same trends occurred however maximum turbidity occurred later. Collectively, the amount of saliva affected both turbidity build up and turbidity decline through clearance. This emphasises the importance of using methods like these which simulate the dynamic in vivo conditions where saliva is added more continuously or in sequential steps rather than all at once. Although results further understanding of how astringency may develop temporally, the research was limited by the methods used. Only turbidity was evaluated, and the study did not account for the possible formation of aggregates and friction increase which may contribute to astringency.45
4. Limitations
Disclosing limitations are an integral part of transparent reporting and crucial for developing scientific discourse, allowing readers to interpret each study accurately. Disclosing limitations helps to rationally develop future studies with accurate methodology, which can target addressing the disclosed limitations for future work,78 as well as communicating relevance79. Limitations are inherent within research79 and within the studies analysed, for example the use of model saliva reduces variability seen when using human saliva,28 but it will have limited applicability when comparing results to the mouthfeel. It has been suggested for scholarly inquiry that disclosing limitations is also an ethical element, and that by not reporting limitations each study included will have a risk of bias79 consequently it would be recommended to include limitations in future research. Similarly, the present review will have its own limitations. The review only included articles published in English, therefore relevant articles published in different languages may have been omitted. However, when scanning reference lists no additional articles published not in English were found.Looking at the quantitative bias assessment conducted (Table S1, ESI†), there was a range of scores from 49% to 100%. There was a high variance between studies and a standard deviation of 13% across all studies. The scores did not appear to change over time and the lowest three scores recorded were in the date range 2008–201741,57,68 whereas the highest two were published in 2009 and 2020.44,56 The two categories where scores were especially low were reporting quality (average 79%) and publication bias (average 79%). Whereas selection and detection bias had averages of 86% and 91% respectively. Specifically, the 23 studies using human saliva had high risk of bias by poor reporting quality. For example, 10 studies did not report age or sex, 5 studies reported one but not the other and 8 studies reported both. In addition, for health status, 7 studies reported health status and how it was obtained, 4 studies did not report health status and 9 studies reported ‘healthy’ but did not explain how. Ambiguity was again the problem for publication bias. For temperature control 7 studies reported exact temperature, 4 studies made no mention of temperature and 22 out of 33 studies used ambiguous terms such as ‘room temperature’. Thus, study quality especially when using human saliva was low, and in general study quality does not appear to have improved with advancing techniques over time. Therefore, a more conscious effort to consider areas of bias should be undertaken in future research.
5. Conclusions
To sum it all up, dairy protein interactions with saliva are dominated by electrostatics, and in low charge scenarios by hydrophobic interactions; which was also concluded in the similar narrative review.23 As electrostatics tends to drive the interactions, a strong influence of pH for whey protein and β-lactoglobulin is observed, with enhanced interactions being found at a pH below the isoelectric point of proteins. Due to a lack of studies using sensory analysis, it cannot be concluded whether electrostatic interactions always translate into sensorial differences for proteins other than whey protein. Incorporating protein into a food system rather than bulk solution was only conducted on whey protein. It showed increased turbidity, lower viscosity and higher friction in whey vs. control conditions which translated into increased astringency with increased electrostatic interactions. At neutral pH both whey protein and gelatine interacted non-electrostatically. However, further work is needed to see if these alternative mechanisms apply to other proteins as well. In addition, further incorporation of in vivo oral processing studies, bolus analysis and sensory analysis to see if they contribute to sensory mouthfeel in the same way as electrostatic interactions is warranted.Protein concentration appears to influence the development of saliva–protein interactions. However, the effect of concentration cannot be fully elucidated due to limited variability in protein type and concentration range. The present review is the first to identify a clear gap in research on protein–saliva interaction at higher protein concentration relating to mouthfeel perception relevant for claiming “high protein” source. Moreover, the present review identified concerns over methodology used in studies. Most studies analysed used a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w saliva
1 w/w saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) protein ratio, however, previous research has suggested a 1
protein ratio, however, previous research has suggested a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w ratio is more physiologically relevant and using this different ratio yielded different results in zeta-potential and viscosity compared to that of 1
4 w/w ratio is more physiologically relevant and using this different ratio yielded different results in zeta-potential and viscosity compared to that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w. For similar reasons, methodological development to accurately simulate saliva's continuous secretion with swallowing and oral residual analysis are warranted. Another methodological concern was the poor study similarity making comparison difficult because of inconsistency or missing information such as saliva type, protein processing method, temperature and lack of limitations disclosure. It would be recommended that future studies take more consideration into transparent reporting to improve quality and minimize bias. In addition, it is recommended that future research employ a variety of newer techniques such as tribology, QCM-D and work towards standardizing approaches to improve comparability between studies. Finally, there is a key gap in the literature for analysing plant protein–saliva interaction to predict mouthfeel perception, which is becoming increasingly popular due to environmental sustainability and support the rise in veganism.
1 w/w. For similar reasons, methodological development to accurately simulate saliva's continuous secretion with swallowing and oral residual analysis are warranted. Another methodological concern was the poor study similarity making comparison difficult because of inconsistency or missing information such as saliva type, protein processing method, temperature and lack of limitations disclosure. It would be recommended that future studies take more consideration into transparent reporting to improve quality and minimize bias. In addition, it is recommended that future research employ a variety of newer techniques such as tribology, QCM-D and work towards standardizing approaches to improve comparability between studies. Finally, there is a key gap in the literature for analysing plant protein–saliva interaction to predict mouthfeel perception, which is becoming increasingly popular due to environmental sustainability and support the rise in veganism.
Author contributions
FB: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing – original draft, ARM: funding acquisition, project administration, supervision, writing – review & editing, QH: writing – review & editing; AB: writing – review & editing; AS: conceptualization, methodology, project administration, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Biotechnology and Biological Sciences Research Council (BBSRC) funded Collaborative Training Partnership (CTP), Grant Ref. No. BB/S506813/1 as well as Mondelēz International (Reading, UK) for financial support.References
- E. Silletti, M. H. Vingerhoeds, W. Norde and G. A. van Aken, The role of electrostatics in saliva-induced emulsion flocculation, Food Hydrocolloids, 2007, 21, 596–606 CrossRef CAS.
- J. R. Stokes, M. W. Boehm and S. K. Baier, Oral processing, texture and mouthfeel: From rheology to tribology and beyond, Curr. Opin. Colloid Interface Sci., 2013, 18, 349–359 CrossRef CAS.
- A. Sarkar and E. M. Krop, Marrying oral tribology to sensory perception: a systematic review, Curr. Res. Food. Sci., 2019, 27, 64–73 Search PubMed.
- C. Pradal and J. R. Stokes, Oral tribology: bridging the gap between physical measurements and sensory experience, Curr. Res. Food. Sci., 2016, 9, 34–41 Search PubMed.
- T. Ö. Yilsay, L. Yilmaz and A. A. Bayizit, The effect of using a whey protein fat replacer on textural and sensory characteristics of low-fat vanilla ice cream, Eur. Food Res. Technol., 2006, 222, 171–175 CrossRef CAS.
- H. S. Joyner Melito, C. W. Pernell and C. R. Daubert, Impact of formulation and saliva on acid milk gel friction behavior, J. Food Sci., 2014, 79, 867–880 CrossRef PubMed.
- G. Carpenter, Role of Saliva in the Oral Processing of Food, in Food Oral Processing, 2012, pp. 45–60 Search PubMed.
- R. G. Schipper, E. Silletti and M. H. Vingerhoeds, Saliva as research material: Biochemical, physicochemical and practical aspects, Arch. Oral Biol., 2007, 52, 1114–1135 CrossRef CAS PubMed.
- A. C. Mosca and J. Chen, Food-saliva interactions: Mechanisms and implications, Trends Food Sci. Technol., 2017, 66, 125–134 CrossRef CAS.
- H. L. Gibbins and G. H. Carpenter, Alternative mechanisms of astringency – What is the role of saliva?, J. Texture Stud., 2013, 44, 364–375 CrossRef.
- L. Laguna Cruañes and A. Sarkar, Oral tribology: update on the relevance to study astringency in wines, Tribol.-Mater., Surf. Interfaces, 2017, 11, 116–123 CrossRef.
- R. Upadhyay, N. Brossard and J. Chen, Mechanisms underlying astringency: introduction to an oral tribology approach, J. Phys. D: Appl. Phys., 2016, 49, 104003 CrossRef.
- L. Mao, Y. H. Roos, D. J. O'Callaghan and S. Miao, Volatile release from whey protein isolate–pectin multilayer stabilized emulsions: Effect of pH, salt, and artificial aalivas, J. Agric. Food Chem., 2013, 61, 6231–6239 CrossRef CAS PubMed.
- F. Xu, E. Liamas, M. Bryant, A. F. Adedeji, E. Andablo-Reyes, M. Castronovo, R. Ettelaie, T. V. J. Charpentier and A. Sarkar, A self-assembled binary protein model explains high-performance salivary lubrication from macro to nanoscale, Adv. Mater. Interfaces, 2020, 7, 1901549 CrossRef.
- A. Sarkar, E. Andablo-Reyes, M. Bryant, D. Dowson and A. Neville, Lubrication of soft oral surfaces, Curr. Opin. Colloid Interface Sci., 2019, 39, 61–75 CrossRef CAS.
- R. G. Schipper, E. Silletti and M. H. Vingerhoeds, Saliva as research material: Biochemical, physicochemical and practical aspects, Arch. Oral Biol., 2007, 52, 114–1135 CrossRef PubMed.
- I. C. Hahn Berg, L. Lindh and T. Arnebrant, Intraoral lubrication of PRP-1, statherin and mucin as studied by AFM, Biofouling, 2004, 20, 65–70 CrossRef CAS PubMed.
- G. E. Yakubov, L. Macakova, S. Wilson, J. H. Windust and J. R. Stokes, Aqueous lubrication by fractionated salivary proteins: Synergistic interaction of mucin polymer brush with low molecular weight macromolecules, Tribol. Int., 2015, 89, 34–45 CrossRef CAS.
- F. Xu, L. Laguna and A. Sarkar, Aging-related changes in quantity and quality of saliva: Where do we stand in our understanding?, J. Texture Stud., 2019, 50, 27–35 CrossRef PubMed.
- S. Wada, T. Goto, K. Fujimoto, M. Watanabe, K. Nagao, A. Nakamichi and T. Ichikawa, Changes in food bolus texture during mastication, Journal of Texture Studies, J. Texture Stud., 2017, 48, 171–177 CrossRef PubMed.
- J. R. Stokes, M. W. Boehm and S. K. Baier, Oral processing, texture and mouthfeel: From rheology to tribology and beyond, Curr. Opin. Colloid Interface Sci., 2013, 18, 349–359 CrossRef CAS.
- J. Chen and J. R. Stokes, Rheology and tribology: Two distinctive regimes of food texture sensation, Trends Food Sci. Technol., 2012, 25, 4–12 CrossRef CAS.
- H. Y. Çelebioğlu, S. Lee and I. S. Chronakis, Interactions of salivary mucins and saliva with food proteins: a review, Critical Reviews in Food Science and Nutrition, Crit. Rev. Food Sci. Nutr., 2019, 60, 64–83 CrossRef PubMed.
- E. M. Krop, M. M. Hetherington, M. Holmes, S. Miquel and A. Sarkar, On relating rheology and oral tribology to sensory properties in hydrogels, Food Hydrocolloids, 2019, 88, 101–113 CrossRef CAS.
- D. Tranfield, D. Denyer and P. Smart, Towards a methodology for developing evidence–informed management knowledge by means of systematic review, Br. J. Manag., 2003, 14, 207–222 CrossRef.
- E. Stribiţcaia, E. M. Krop, R. Lewin, M. Holmes and A. Sarkar, Tribology and rheology of bead-layered hydrogels: Influence of bead size on sensory perception, Food Hydrocolloids, 2020, 104, 105692 CrossRef.
- R. Jaenke, F. Barzi, E. McMahon, J. Webster and J. Brimblecombe, Consumer acceptance of reformulated food products: A systematic review and meta-analysis of salt-reduced foods, Crit. Rev. Food Sci. Nutr., 2017, 57, 3357–3372 CrossRef CAS PubMed.
- A. Sarkar, F. Xu and S. Lee, Human saliva and model saliva at bulk to adsorbed phases – similarities and differences, Adv. Colloid Interface Sci., 2019, 273, 102034 CrossRef CAS PubMed.
- G. A. Sánchez, V. Miozza, A. Delgado and L. Busch, Determination of salivary levels of mucin and amylase in chronic periodontitis patients, J. Periodontal Res., 2011, 46, 221–227 CrossRef PubMed.
- J. Liu and Y. Duan, Saliva: A potential media for disease diagnostics and monitoring, Oral Oncol., 2012, 48, 569–577 CrossRef PubMed.
- A. Elshafay, E. S. Omran, M. Abdelkhalek, M. O. El-Badry, H. G. Eisa, S. Y. Fala, T. Dang, M. A. T. Ghanem, M. Elbadawy, M. T. Elhady, N. L. Vuong, K. Hirayama and N. T. Huy, Reporting quality in systematic reviews of in vitro studies: a systematic review, Curr. Med. Res. Opin., 2019, 35, 1631–1641 CrossRef PubMed.
- J. P. Higgins, D. G. Altman, P. C. Gøtzsche, P. Jüni, D. Moher, A. D. Oxman, J. Savović, K. F. Schulz, L. Weeks and J. A. Sterne, The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, Br. Med. J., 2011, 343, d5928 CrossRef PubMed.
- I. Hinneburg, ROBINS-1: A tool for asssessing risk of bias in non-randomised studies of interventions, Med. Monatsschr. Pharm., 2017, 40, 175–177 Search PubMed.
- K. Porritt, J. Gomersall and C. Lockwood, JBI's systematic reviews: study selection and critical appraisal, Am. J. Nurs., 2014, 114, 47–52 CrossRef PubMed.
- L. A. Golbach, L. A. Portelli, H. F. Savelkoul, S. R. Terwel, N. Kuster, R. B. de Vries and B. L. Verburg-van Kemenade, Calcium homeostasis and low-frequency magnetic and electric field exposure: A systematic review and meta-analysis of in vitro studies, Environ. int., 2016, 92, 695–706 CrossRef PubMed.
- B. Vardhanabhuti, P. W. Cox, I. T. Norton and E. A. Foegeding, Lubricating properties of human whole saliva as affected by β-lactoglobulin, Food Hydrocolloids, 2011, 25, 1499–1506 CrossRef CAS.
- M. H. Vingerhoeds, T. B. J. Blijdenstein, F. D. Zoet and G. A. van Aken, Emulsion flocculation induced by saliva and mucin, Food Hydrocolloids, 2005, 19, 915–922 CrossRef CAS.
- H. Sano, T. Egashira, Y. Kinekawa and N. Kitabatake, Astringency of Bovine Milk Whey Protein, J. Dairy Sci., 2005, 88, 2312–2317 CrossRef CAS PubMed.
- A. Sarkar and H. Singh, Oral behaviour of food emulsions, Food Oral Processing, Wiley-Blackwell, UK, 2012, 111–137 Search PubMed.
- C. A. Withers, M. T. Cook, L. Methven, M. A. Gosney and V. V. Khutoryanskiy, Investigation of milk proteins binding to the oral mucosa, Food Funct., 2013, 4, 1668–1674 RSC.
- E. Silletti, R. M. P. Vitorino, R. Schipper, F. M. L. Amado and M. H. Vingerhoeds, Identification of salivary proteins at oil-water interfaces stabilized by lysozyme and beta-lactoglobulin, Arch. Oral Biol., 2010, 55, 268–278 CrossRef CAS PubMed.
- M. H. Vingerhoeds, E. Silletti, J. de Groot, R. G. Schipper and G. A. van Aken, Relating the effect of saliva-induced emulsion flocculation on rheological properties and retention on the tongue surface with sensory perception, Food Hydrocolloids, 2009, 23, 773–785 CrossRef CAS.
- A. Ye, C. Streicher and H. Singh, Interactions between whey proteins and salivary proteins as related to astringency of whey protein beverages at low pH, J. Dairy Sci., 2011, 94, 5842–5850 CrossRef CAS PubMed.
- M. Ahmad, C. Ritzoulis, W. Pan and J. Chen, Chemical physics of whey protein isolate in the presence of mucin: From macromolecular interactions to functionality, Int. J. Biol. Macromol., 2020, 143, 573–581 CrossRef CAS PubMed.
- P. Andrewes, M. Kelly, B. Vardhanabhuti and E. A. Foegeding, Dynamic modelling of whey protein-saliva interactions in the mouth and relation to astringency in acidic beverages, Int. Dairy J., 2011, 21, 523–530 CrossRef CAS.
- J. W. Beecher, M. A. Drake, P. J. Luck and E. A. Foegeding, Factors Regulating Astringency of Whey Protein Beverages, J. Dairy Sci., 2008, 91, 2553–2560 CrossRef CAS PubMed.
- H. Hsein, G. Garrait, E. Beyssac and V. Hoffart, Whey protein mucoadhesive properties for oral drug delivery: Mucin-whey protein interaction and mucoadhesive bond strength, Colloids Surf., B, 2015, 136, 799–808 CrossRef CAS PubMed.
- M. Kelly, B. Vardhanabhuti, P. Luck, M. A. Drake, J. Osborne and E. A. Foegeding, Role of protein concentration and protein-saliva interactions in the astringency of whey proteins at low pH, J. Dairy Sci., 2010, 93, 1900–1909 CrossRef CAS PubMed.
- C. A. Lee and Z. M. Vickers, The astringency of whey protein beverages is caused by their acidity, Int. Dairy J., 2008, 18, 1153–1156 CrossRef CAS.
- T. Wang, S. Y. Tan, W. Mutilangi, M. Plans and L. Rodriguez-Saona, Application of infrared portable sensor technology for predicting perceived astringency of acidic whey protein beverages, J. Dairy Sci., 2016, 99, 9461–9470 CrossRef CAS PubMed.
- H. Y. Çelebioğlu, M. Gudjónsdóttir, I. S. Chronakis and S. Lee, Investigation of the interaction between mucins and β-lactoglobulin under tribological stress, Food Hydrocolloids, 2016, 54, 57–65 CrossRef.
- H. Y. Çelebioğlu, M. Gudjónsdóttir, S. Meier, J. O. Duus, S. Lee and I. S. Chronakis, Spectroscopic studies of the interactions between β-lactoglobulin and bovine submaxillary mucin, Food Hydrocolloids, 2015, 50, 203–210 CrossRef.
- H. Y. Celebioglu, J. Kmiecik-Palczewska, S. Lee and I. S. Chronakis, Interfacial shear rheology of beta-lactoglobulin-Bovine submaxillary mucin layers adsorbed at air/water interface, Int. J. Biol. Macromol., 2017, 102, 857–867 CrossRef CAS PubMed.
- B. Vardhanabhuti, M. A. Kelly, P. J. Luck, M. A. Drake and E. A. Foegeding, Roles of charge interactions on astringency of whey proteins at low pH, J. Dairy Sci., 2010, 93, 1890–1899 CrossRef CAS PubMed.
- M. Ahmad, C. Ritzoulis, W. C. Pan and J. S. Chen, Molecular interactions between gelatin and mucin: Phase behaviour, thermodynamics and rheological studies, Food Hydrocolloids, 2020, 102, 105585 CrossRef CAS.
- A. Sarkar, K. K. T. Goh and H. Singh, Colloidal stability and interactions of milk-protein-stabilized emulsions in an artificial saliva, Food Hydrocolloids, 2009, 23, 1270–1278 CrossRef CAS.
- E. Silletti, M. H. Vingerhoeds, G. A. Van Aken and W. Norde, Rheological behavior of food emulsions mixed with saliva: Effect of oil content, salivary protein content, and saliva type, Food Biophys., 2008, 3, 318–328 CrossRef.
- C. Ritzoulis, S. Siasios, K. D. Melikidou, C. Koukiotis, C. Vasiliadou and S. Lolakos, Interactions between pig gastric mucin and sodium caseinate in solutions and in emulsions, Food Hydrocolloids, 2012, 29, 382–388 CrossRef CAS.
- E. Koukoura, M. Panagiotopoulou, A. Pavlou, V. Karageorgiou, D. G. Fatouros, C. Vasiliadou and C. Ritzoulis, In Vitro Digestion of caseinate and Tween 20 Emulsions, Food Biophys., 2019, 14, 60–68 CrossRef.
- E. Silletti, M. H. Vingerhoeds, W. Norde and G. A. van Aken, Complex formation in mixtures of lysozyme-stabilized emulsions and human saliva, J. Colloid Interface Sci., 2007, 313, 485–493 CrossRef CAS PubMed.
- M. Biegler, J. Delius, B. T. Kasdorf, T. Hofmann and O. Lieleg, Cationic astringents alter the tribological and rheological properties of human saliva and salivary mucin solutions, Biotribology, 2016, 6, 12–20 CrossRef.
- M. Zembyla, E. Liamas, E. Andablo-Reyes, K. Gu, E. M. Krop, B. Kew and A. Sarkar, Surface adsorption and lubrication properties of plant and dairy proteins: A comparative study, Food Hydrocolloids, 2021, 111, 106364 CrossRef CAS PubMed.
- P. Morell, J. S. Chen and S. Fiszman, The role of starch and saliva in tribology studies and the sensory perception of protein-added yogurts, Food Funct., 2017, 8, 545–553 RSC.
- P. Morell, I. Hernando, E. Llorca and S. Fiszman, Yogurts with an increased protein content and physically modified starch: rheological, structural, oral digestion and sensory properties related to enhanced satiating capacity, Food Res. Int., 2015, 70, 64–73 CrossRef CAS.
- J. L. Childs and M. Drake, Consumer perception of astringency in clear acidic whey protein beverages, J. Food Sci., 2010, 75, S513–S521 CrossRef CAS PubMed.
- P. L. Fuhrmann, L. C. M. Kalisvaart, G. Sala, E. Scholten and M. Stieger, Clustering of oil droplets in o/w emulsions enhances perception of oil-related sensory attributes, Food Hydrocolloids, 2019, 97, 105215 CrossRef CAS.
- Y. Deng, C. Govers, S. Bastiaan-Net, N. Van Der Hulst, K. Hettinga and H. J. Wichers, Hydrophobicity and aggregation, but not glycation, are key determinants for uptake of thermally processed β-lactoglobulin by THP-1 macrophages, Food Res. Int., 2019, 120, 102–113 CrossRef CAS PubMed.
- C. L. Campbell, E. A. Foegeding and F. van de Velde, A comparison of the lubrication behavior of whey protein model foods using tribology in linear and elliptical movement, J. Texture Stud., 2017, 48, 335–341 CrossRef PubMed.
- V. Raikos, Effect of heat treatment on milk protein functionality at emulsion interfaces. A review, Food Hydrocolloids, 2010, 24, 259–265 CrossRef CAS.
- N. J. Baxter, T. H. Lilley, E. Haslam and M. P. Williamson, Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation, Biochemistry, 1997, 36, 5566–5577 CrossRef CAS PubMed.
- M. Larsen and E. Pearce, Saturation of human saliva with respect to calcium salts, Arch. Oral Biol., 2003, 48, 317–322 CrossRef CAS PubMed.
- X. Hu, P. Karthik and J. S. Chen, Manipulating oral behaviour of food emulsions using different emulsifiers, Int. J. Food Sci. Technol., 2019, 54, 2408–2415 CrossRef CAS.
- D. M. Dresselhuis, M. A. C. Stuart, G. A. van Aken, R. G. Schipper and E. H. A. de Hoog, Fat retention at the tongue and the role of saliva: Adhesion and spreading of ‘protein-poor’ versus ‘protein-rich’ emulsions, J. Colloid Interface Sci., 2008, 321, 21–29 CrossRef CAS PubMed.
- A. Madadlou, S. Zamani, Y. Lu and A. Abbaspourrad, Effect of surfactant addition on particle properties of whey proteins and their subsequent complexation with salivary proteins, Int. Dairy J., 2018, 87, 107–113 CrossRef CAS.
- M. Devezeaux de Lavergne, M. van Delft, F. van de Velde, M. A. J. S. van Boekel and M. Stieger, Dynamic texture perception and oral processing of semi-solid food gels: Part 1: Comparison between QDA, progressive profiling and TDS, Food Hydrocolloids, 2015, 43, 207–217 CrossRef CAS.
- S. J. Haward, J. A. Odell, M. Berry and T. Hall, Extensional rheology of human saliva, Rheol. Acta, 2011, 50, 869–879 CrossRef CAS.
- Z. Lv, J. Chen and M. Holmes, Human capability in the perception of extensional and shear viscosity, J. Texture Stud., 2017, 48, 463–469 CrossRef PubMed.
- M. A. Puhan, E. A. Akl, D. Bryant, F. Xie, G. Apolone and G. T. Riet, Discussing study limitations in reports of biomedical studies- the need for more transparency, Health Qual. Life Outcomes, 2012, 10, 23 CrossRef PubMed.
- P. T. Ross and N. L. Bibler Zaidi, Limited by our limitations, Perspect. Med. Educ., 2019, 8, 261–264 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0fo03180a |
| This journal is © The Royal Society of Chemistry 2021 |