Open Access Article
Open Access ArticleEntrapment of natural compounds in spray-dried and heat-dried iota-carrageenan matrices as functional ingredients in surimi gels
Daniel
Marín-Peñalver
,
Ailén
Alemán
,
M. Pilar
Montero
and
M. Carmen
Gómez-Guillén
 *
*
Institute of Food Science, Technology and Nutrition (ICTAN-CSIC), José Antonio Novais 10, 28040, Madrid, Spain. E-mail: mc.gomez@csic.es
First published on 20th January 2021
Abstract
Two drying methods (spray drying and heat drying) were used to entrap various natural compounds within a matrix of iota-carrageenan. The natural compounds were, namely, collagen hydrolysate (CH), pomegranate polyphenolic extract (PP) and shrimp lipid extract (SL). The resulting dry powders were compared in terms of water solubility, entrapment efficiency, hydrodynamic particle properties, ζ potential and antioxidant properties (ABTS radical scavenging capacity, ferric ion reducing power and Folin-reactive substances). Dry powders and plain compounds were incorporated into squid surimi gels, and after in vitro simulated gastrointestinal digestion (sGID), the residual antioxidant and angiotensin-converting enzyme (ACE)-inhibitory activities were evaluated. All powders showed antioxidant properties, electronegative ζ potential and great entrapment efficiency after rehydration (ranging from ∼70 to 97%). The heat-dried powders were composed of microparticles ranging from 177 to 380 μm resulting in low water solubility (21.6–36.1%), while the average particle size and solubility values of spray-dried preparations were 2.9–13.2 μm and >86%, respectively. In contrast to the plain compounds, the addition of any of the microparticle dried preparations allowed obtaining well-conformed surimi gels. The ACE-inhibitory capacity of the surimi gels after sGID was increased by the addition of any of the compounds studied, but to a lesser extent by their entrapment forms (except with the entrapped SL). The antioxidant activities of gels with the entrapped compounds were even lower than those of gels without bioactives in some cases. In conclusion, the addition of dried microparticles did not increase the biological activity as compared to the plain compounds; however, they were beneficial to ensure adequate gel consistency.
1. Introduction
The increasing awareness about the benefits of a healthy diet has attracted interest to the consumption of foods enriched with natural bioactive compounds. However, the biological properties of active compounds can be compromised due to their partial degradation by environmental factors during food processing and/or gastrointestinal digestion, or just by interacting with other food components.1 In this context, different strategies for the entrapment of active molecules (core) in the supporting matrix of a secondary (wall) material have been proposed to improve the stability and bioaccessibility of biological molecules in food systems.2,3 The spray drying method typically produces spherical microcapsules ranging approximately from 5 to 80 μm,4 and it is one of the most widely used encapsulation techniques for food ingredients.5 Its benefits include the ability to control the particle size, shape and morphology of microcapsules, high encapsulation efficiency and extended shelf life of the resulting powders, suitability for both hydrophilic and hydrophobic compounds, processing of heat-sensitive substances with low degradation risk, low cost, and fast and energy-efficient processing.6A variety of lipids, proteins, carbohydrates and complex biopolymer systems can be used as wall materials.3 Carrageenan, an anionic water-soluble galactose-based polysaccharide derived from the Rhodophyceae red algae, has been proposed as a microencapsulating matrix of living cells due to its capacity to form cross-linked three-dimensional networks induced by thermal/ionotropic gelation (hydrogels).7 Depending on the number and position of ester sulphate groups, carrageenan can be classified into three major types: i.e. iota, kappa and lambda. Iota-carrageenan in aqueous solution forms soft elastic gels, which are more resistant to syneresis than gels formed with kappa-carrageenan.8 Both kappa- and lambda-carrageenan have been widely used as pharmaceutical encapsulating matrices based on their gelling properties or their capacity to produce complexes with active molecules.9,10 Iota-carrageenan conjugated with soy-protein isolate through the Maillard reaction has been proposed as a bacterial encapsulating system obtained by a combination of spray drying and dry heating.11 However, unlike kappa-carrageenan, reports on iota-carrageenan as a microencapsulating agent of natural compounds for food applications are not available.
Carrageenans are commonly used as food ingredients due to their stabilizing, gelling, texturizing and water binding capacities.12 In particular, iota-carrageenan has been shown to form a reticular structure when included in heat-induced fish and squid mince gels, favouring protein gelation and water binding properties;13,14 however, there is no information regarding its incorporation into surimi gels in the form of microcapsules or complexes containing natural bioactive compounds. In this sense, surimi gels could be excellent matrices for the development of fish-derived functional foods, since they form highly digestible protein gel networks, which can incorporate a variety of natural bioactive compounds.15 In the previous work carried out by Marín et al.,15 various food waste compounds (collagen hydrolysate from squid tunics, polyphenolic extract from pomegranate peel and albedo, and shrimp lipid extract) were encapsulated in soy phosphatidylcholine liposomes and incorporated into squid surimi gels, providing a notable radical scavenging capacity after in vitro simulated gastrointestinal digestion. These natural compounds, which were chosen for their different chemical nature and biological properties, can show different susceptibilities to thermal degradation during gel processing, or to changes in pH and action of digestive enzymes after oral intake, as well as different entrapment abilities depending on the encapsulation method used.
As a follow-up to the work mentioned above, in the present study different microcapsules containing the above-mentioned natural compounds were prepared by spray-drying (SD) using iota-carrageenan as the wall material. Alternatively, based on the ability of iota-carrageenan to form soft gels at elevated temperature, complex dry powders were also obtained in a more simple way, by drying aqueous mixtures of iota-carrageenan and natural compounds by forced convection at 80 °C (HD). This method for entrapment of bioactives could be an interesting alternative due to its simplicity and ease of implementation in SME industries dedicated to on-demand food production. Both types of entrapment systems were evaluated in terms of water solubility, entrapment efficiency, hydrodynamic particle properties and antioxidant capacity. Then, both dry powders and plain compounds were incorporated into squid surimi gels and subjected to in vitro simulated gastrointestinal digestion, after which the remaining antioxidant and antihypertensive activities were evaluated.
2. Materials and methods
2.1. Materials
Iota-carrageenan was obtained from Hispanagar S.A. (Burgos, Spain). Pepsin, pancreatin and ACE (EC. 3.4.15.1.) enzymes were purchased from Sigma-Aldrich, Inc. (St Louis, MO, USA). Giant squid (D. gigas) surimi was purchased from PSK Oceanos (Spain). The collagen hydrolysate (CH) from giant squid tunics, the polyphenolic extract from pomegranate peel and albedo (PP), and the astaxanthin-containing lipid extract from shrimp cephalothorax and cuticles (SL) were produced, dried and stored at −20 °C, as reported in a previous work,15 in which their chemical compositions were also described. All reagents were of analytical grade. Milli-Q® Integral Water Purification System was used to produce pure and ultrapure water.2.2. Preparation of dry powders
Iota-carrageenan was previously dissolved (1% w/v) in warm pure water. Then, each natural compound, CH (fine powder), PP (coarse powder), or SL (oil), was added in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (compound/iota-carrageenan, w/w) and homogenized by vigorous magnetic stirring at room temperature for 30 min. This core/coating ratio was selected to ensure high entrapment efficiency and stability of the resulting dried entrapment powders, as well as a balanced proportion of both iota-carrageenan and natural compound in the surimi gel. Thus, for each natural compound, two types of dry powders were obtained following two different methodologies: (i) spray-drying (SD) using a B-290 Basic Mini Spray-Dryer (Büchi, Flawil, Switzerland) with an inlet temperature of 220 °C, an aspirator at 90%, a pump at 65% and a Q-flow rate of 12.3 L min−1, denominated SD-CH, SD-PP and SD-SL; and (ii) heat-drying (HD) using a heating/drying chamber with forced convection (FD 240, Binder, Tuttlingen, Germany) at 80 °C for 20 h (the resulting product was ground to a fine powder), denominated HD-CH, HD-PP and HD-SL. All powders were stored at 4 °C until use.
4 (compound/iota-carrageenan, w/w) and homogenized by vigorous magnetic stirring at room temperature for 30 min. This core/coating ratio was selected to ensure high entrapment efficiency and stability of the resulting dried entrapment powders, as well as a balanced proportion of both iota-carrageenan and natural compound in the surimi gel. Thus, for each natural compound, two types of dry powders were obtained following two different methodologies: (i) spray-drying (SD) using a B-290 Basic Mini Spray-Dryer (Büchi, Flawil, Switzerland) with an inlet temperature of 220 °C, an aspirator at 90%, a pump at 65% and a Q-flow rate of 12.3 L min−1, denominated SD-CH, SD-PP and SD-SL; and (ii) heat-drying (HD) using a heating/drying chamber with forced convection (FD 240, Binder, Tuttlingen, Germany) at 80 °C for 20 h (the resulting product was ground to a fine powder), denominated HD-CH, HD-PP and HD-SL. All powders were stored at 4 °C until use.
2.3. Characterization of dry powders
| EE = (entrapped compound/total compound) × 100. |
The entrapped compound was calculated from the difference between the total compound added and the non-entrapped compound, which was separated and quantified by different methods depending on the type of compound, following the procedure described by Marín et al.15
2.4. Preparation of surimi gels and in vitro gastrointestinal digestion
Giant squid surimi was thawed and homogenized with sodium chloride (1%, w/w) under refrigeration and vacuum conditions using a Stephan Universal Machine (UMC 5, Stephan Machinery GmbH, Germany). The moisture level in the final product was adjusted to 80% by adding the required amount of water (in the form of crushed ice). Dry powders were added to the salt-ground surimi paste (10% w/w, accounting for 2% of the bioactive compound with respect to surimi) and homogenized again for 2 min. Gels were obtained by stuffing the resulting pastes into the plastic casings with 3.5 cm diameter and heating at 90 °C for 45 min. They were stored at 4 °C until use. Gels with plain compounds (2% with respect to surimi) and a control gel (CG) without compounds or carrageenan were prepared under the same conditions. The in vitro simulated gastrointestinal digestion (sGID) of surimi gels was carried out following the procedure described by Marín et al.15 The water soluble fraction of non-digested gels (WS) was obtained by homogenizing 20 g of gel with distilled water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 w/v) using an Ultraturrax mixer (IKA-Werke, Germany) and centrifuging at 10
10 w/v) using an Ultraturrax mixer (IKA-Werke, Germany) and centrifuging at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and 4 °C for 45 min. The supernatant was freeze-dried and stored at −20 °C until use.
000g and 4 °C for 45 min. The supernatant was freeze-dried and stored at −20 °C until use.
2.5. Colour of surimi gels
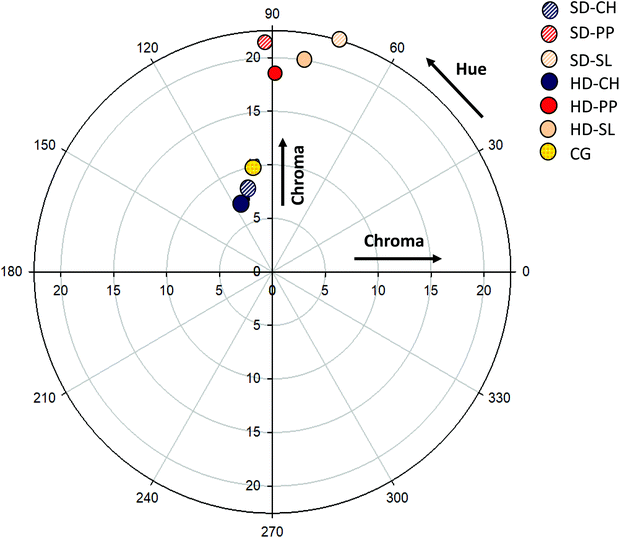
The Chroma (saturation) and Hue (tonality) parameters of gels were obtained as described by Marín et al.15 using a Konica Minolta CM-3500d colorimeter (Konica Minolta, Spain) equipped with a D65 illuminant and a D10 standard observer.2.6. Antioxidant activity
The antioxidant activity was determined in plain compounds, dry microparticle powders and surimi gels (water soluble fraction and after sGID). The ABTS radical scavenging capacity (ABTS) and ferric ion reducing antioxidant power (FRAP) were determined as described by Alemán et al.16 Folin-reactive substances (FRS) were determined following the procedure described by Slinkard and Singleton.17 The results of the ABTS assay were expressed as mg vitamin C equivalents (VCEAC) per g of sample, based on a standard curve of vitamin C. The results of FRAP were expressed as μM Fe2+ equivalents per g of sample, based on a standard curve of FeSO4·7H2O. The results of FRS were expressed as mg of gallic acid (GA) equivalents per g of sample, based on a standard curve of gallic acid. For appropriate comparison between the plain compounds and dry powders, the results were expressed per gram of bioactive compound and per g of dry powder.2.7. Antihypertensive activity
The antihypertensive capacity was measured according to the angiotensin-converting enzyme (ACE) inhibition method described by Alemán et al.16 using a High Performance Liquid Chromatography analyser (HPLC, model SPE-MA10AVP, Shimadzu, Kyota, Japan). All samples were dissolved in ultrapure Milli-Q water at 0.8 mg mL−1. The results were expressed as the percentage of ACE-inhibitory activity.2.8. Statistical analysis
Analysis of variance was performed using the SPSS® computer program (IBM SPSS Statistics 22 Software, Inc., Chicago, IL, USA). Differences among the samples were established using the Tukey test, with a significance level set at p ≤ 0.05. All results are expressed as the mean of at least three replicates.3. Results and discussion
3.1. Characterization of dry powders
| Sample | Size (μm) | ζ potential (mV) | Water solubility (%) | EE (%) |
|---|---|---|---|---|
| Different letters (a, b, and c) indicate significance differences (p ≤ 0.05) among the extracts for the same dried preparation and (x and y) between SD and HD preparations for the same extract. | ||||
| SD-CH | 3.60 ± 0.04b/x | −68.05 ± 4.20a/x | 90.92 ± 0.53a/x | 96.29 ± 0.81a/x |
| SD-PP | 13.21 ± 0.16a/x | −47.50 ± 3.49b/x | 86.49 ± 1.51a/x | 95.61 ± 1.18a/x |
| SD-SL | 2.87 ± 0.04c/x | −47.75 ± 2.22b/x | 88.08 ± 2.47a/x | 70.36 ± 1.54b/x |
| HD-CH | 177.3 ± 5.48c/y | −56.23 ± 1.56a/y | 33.63 ± 2.40a/y | 97.21 ± 2.36a/x |
| HD-PP | 280.3 ± 3.32b/y | −42.90 ± 2.24b/x | 36.14 ± 3.71a/y | 97.14 ± 1.49a/x |
| HD-SL | 380.1 ± 4.07a/y | −53.93 ± 4.82a/x | 21.55 ± 0.77b/y | 91.23 ± 1.02b/y |
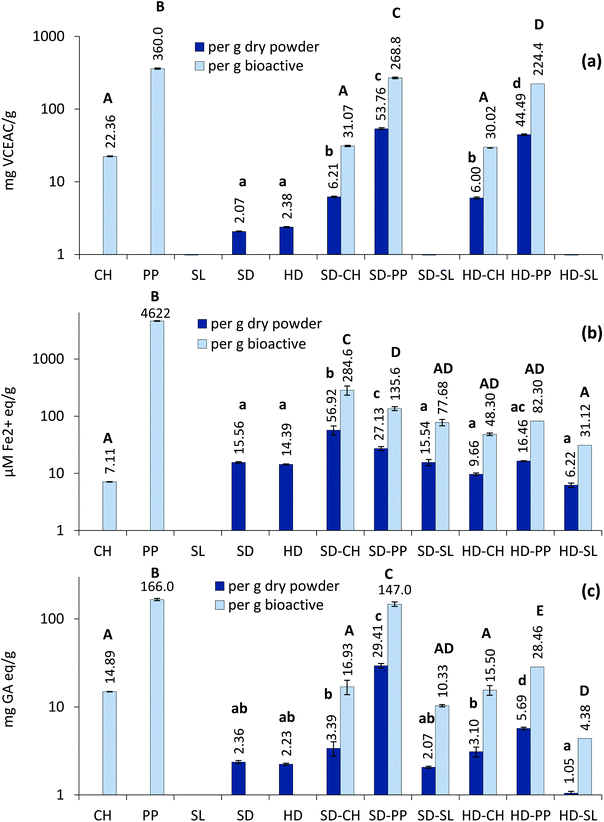
ζ potential refers to the aggregation ability of dispersed particles and is considered a key stability indicator of colloidal suspensions. The studied dispersions had the ζ potential values in the range of −42.9 and −56.2 mV for HD and −47.5 and −68.1 mV for SD samples (Table 1), all indicative of high particle stability. The negative charge is attributed to the anionic nature of iota-carrageenan, which typically presents a ζ potential value between −50 and −70 mV.21 Negative ζ potential for microcapsules obtained by Sun et al.22 was also attributed to the employment of κ-carrageenan as the wall material.
Regarding the mixture of bioactive compound and iota-carrageenan, the antioxidant activity of dried powders was generally lower than in both CH and PP (Fig. 1), which in part could be due to the diluting effect of the wall material. Such a diluting effect has been reported in terms of the total phenolic content for spray-dried maltodextrin microcapsules loaded with the pomegranate peel extract at different ratios.29 The only exception was SD-CH, which registered higher (p ≤ 0.05) FRAP values than the plain CH hydrolysate which indeed showed very low ferric ion reducing capacity. One possible explanation could be the capacity of iota-carrageenan to act as a reducing agent of ferric ions.30 This effect is clearly observable in Fig. 1b, where plain carrageenan SD and HD dried powders registered similar FRAP values than CH. It should be noted that the FRAP values for SD–CH were considerably higher than the sum of the values obtained for CH and SD separately; therefore, the possible conformational changes in peptides due to the interaction with iota-carrageenan during the atomization process should not be disregarded. Tkaczewska et al.31 also observed an increase in the FRAP values of a carp skin gelatin hydrolysate by microencapsulation with furcellaran (a polysaccharide structurally similar to κ-carrageenan). Carrageenan also contributed to the ABTS and FRS values, but much less than for FRAP. In fact, carrageenan was probably the main factor determining the FRAP and FRS measured values for SD-SL and HD-SL dried powders, the latter showing lower values probably as a result of its lower water solubility. In contrast to the present work, Zhao et al.26 reported high radical scavenging capacity in highly soluble spray-dried gelatin-maltodextrin microcapsules containing an H. pluvialis astaxanthin extract; this difference is probably attributed to the different wall materials used (gelatin-maltodextrin vs. iota-carrageenan) and the astaxanthin content of the original extract (9% vs. 0.7%).
In order to factor out the diluting effect of carrageenan in dry powders, the antioxidant activities were also expressed per g of bioactive agent added (Fig. 1). The antioxidant activity of the collagen hydrolysate (CH) was maintained (by FRS and ABTS) or increased (by FRAP in SD–CH) when it was encapsulated. In contrast, HD preparations containing the pomegranate peel extract (HD-PP) registered a considerably lower (p ≤ 0.05) activity than the plain extract (Fig. 1), whether expressed as per g of dry powder or per g of bioactive. This effect could be due to the partial degradation of phenolic compounds during the drying process or to the possible polyphenol–polysaccharide interactions with the wall material, thus reducing the availability of the reactive groups. A similar loss of antioxidant activity (determined by ABTS) of PP was also reported in a previous work using soy phosphatidylcholine as the encapsulating agent.15 In spite of this, both SD-PP and HD-PP preparations showed the highest (p ≤ 0.05) activity in both ABTS and FRS assays, but the activity of SD-PP was higher (p ≤ 0.05) than that of HD-PP due to its higher water solubility. Çam et al.29 reported similar FRS values for spray-dried maltodextrin microcapsules with the pomegranate peel extract.
3.2. Properties of surimi gels
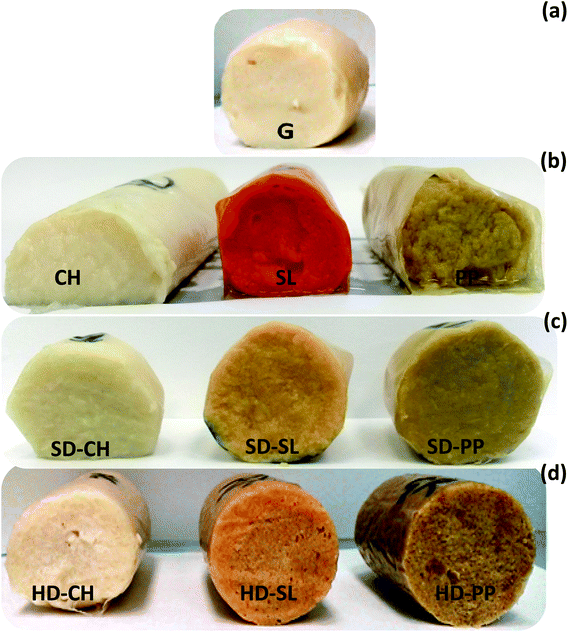
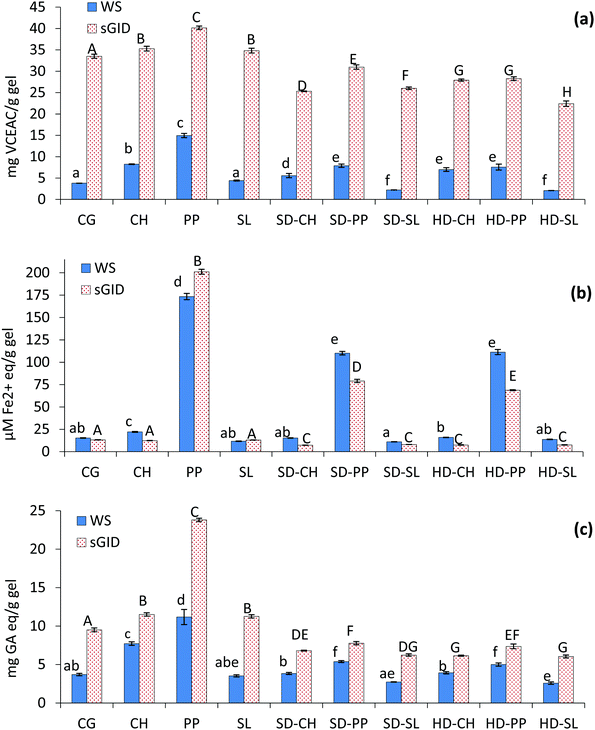
The addition of iota-carrageenan-based SD and HD powders containing the various natural compounds produced homogeneous and well-structured gel matrices similar to that obtained with the control gel without ingredients (Fig. 2). In contrast, the addition of plain compounds resulted in weak and friable gels, with a deformed structure, visible water syneresis and stronger coloration, especially with SL. The gels prepared with the addition of SD powders showed slightly more uniform cross-section than that prepared with the HD counterparts. This effect was attributed to the higher water solubility of the former, which favours homogenisation with the muscle protein. The improving effect of iota-carrageenan on the gel properties of fish mince or surimi has been well documented.13,14,32 The present work also reveals this effect by incorporating iota-carrageenan in the form of highly soluble (SD) or less soluble microparticles (HD).The results of ferric ion reducing capacity (FRAP) of both non-digested and digested gels are shown in Fig. 4b. Unlike ABTS, no general increase was observed in the FRAP values as a result of sGID, suggesting a negligible contribution of the digested muscle protein to the overall reducing capacity. In this case, the activity produced by PP in surimi gels was striking, especially when added as a plain extract. In contrast, CH and SL registered as low FRAP values as the control gel, and the values also slightly decreased when CH and SL were included in SD and HD preparations.
The antioxidant activity expressed as Folin reactive substances (FRS) of the different gels (Fig. 4c) showed a similar trend to that of ABTS radical scavenging activity, although in this case the rise in FRS following sGDI was less pronounced. There was a considerable increase of FRS in the gel with plain PP after sGID, so that the specific differential effect of PP was observable over and above the outcome of the digested muscle protein. This effect, however, was not evident in the corresponding dried PP preparations, where differences with respect to their HC and SL counterparts were minimal. Therefore, depending on the antioxidant mechanism studied, the activity results in gels with different compounds may vary depending on how much was contributed by the hydrolysed muscle protein.
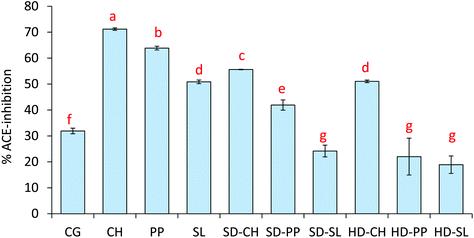
The ACE-inhibitory capacity of the surimi gels after sGID is shown in Fig. 5. The control gel (CG) presented 32% of ACE-inhibitory capacity, which is attributed to the release of active peptides by the hydrolysis of surimi proteins. The addition of any of the plain compounds led to a significant increase in the ACE-inhibition percentage, the gel with plain CH being the sample with highest activity (71.2%). It is worth noting the augmented (p ≤ 0.05) activity in the gels containing plain PP (63.87%) and SL (50.9%) compared to the control gel. These extracts did not stand out for their high ACE-inhibition, and thus the measured activity, especially with SL, should be attributed to their interference in protein gelation, causing the muscle protein to be more exposed to digestive enzymes. As mentioned before, good gels were not obtained by the addition of plain compounds. The ACE-inhibitory activity of gels containing SD-CH and HD-CH was lower than that of gels with the plain hydrolysate, but higher than that of the control gel, despite the diluting effect and gelling capacity of iota-carrageenan. A similar finding, but with lower values, was also observed with SD-PP. The presence of iota-carrageenan in the gel matrix would also affect the protein–protein interactions, and therefore muscle protein digestion. However, the resulting ACE-inhibitory activity varied considerably depending on the dried preparation, since HD-PP, SD-SL and HD-SL led to gels with ACE-inhibition values lower than the control gel. So, the observed differences could be attributed to the activity provided by the natural compounds, which was considerably higher with CH, especially when entrapped in the more soluble form (SD-CH). In the case of SD-PP, the encapsulation process could have protected the activity of PP from digestion, while the low solubility and/or lack of activity in HD-PP, SD-SL and HD-SL preparations might have contributed to the decrease of the ACE-inhibitory potential of the hydrolysed myofibrillar protein.
4. Conclusion
Both spray-drying (SD) and heat-drying (HD) of the aqueous dispersions of iota-carrageenan and natural food waste compounds resulted in antioxidant microparticle dry powders, which showed good miscibility with surimi protein to obtain well-conformed thermal gels, regardless of different solubilities of both types of preparations. Both types of dry powders were then suitable for addition as technological ingredients to surimi gel formulations. The noticeable high antioxidant capacity of the pomegranate extract (PP) was considerably decreased when included via SD or HD preparations, while the entrapped SL extract presented a limited antioxidant power, despite becoming highly soluble in the SD preparation. In general, the antioxidant properties and ACE-inhibitory capacity of the surimi gels determined after in vitro gastrointestinal digestion were noticeably lower when the natural compounds were entrapped in the iota-carrageenan matrices regardless of the drying method used, suggesting that the technological functionality of the dry powders could be superior to the bioactive itself. In vivo studies would be necessary to overcome possible limitations derived from the presence of iota-carrageenan, as well as its possible functionality as a dietary fibre.CRediT author statement
Daniel Marín-Peñalver: Formal analysis; investigation; methodology; writing original draft; Ailén Alemán: Formal analysis; investigation; methodology; M. Pilar Montero: Conceptualization; methodology; resources; supervision; project administration; funding acquisition; and M. Carmen Gómez-Guillén: Conceptualization; methodology; resources; supervision; project administration; funding acquisition; writing review and editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors are grateful for the financial support provided by Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER) through the project NANOALIVAL AGL2017-84161 and by CSIC through the project 202070E218. We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).References
- M. G. Bah, H. M. Bilal and J. Wang, Fabrication and application of complex microcapsules: A review, Soft Matter, 2020, 16(3), 570–590 RSC.
- M. Saifullah, M. R. I. Shishir, R. Ferdowsi, M. R. T. Rahman and Q. V. Vuong, Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review, Trends Food Sci. Technol., 2019, 86, 230–251 CrossRef CAS.
- J.-H. Ye and M. A. Augustin, Nano- and micro-particles for delivery of catechins: Physical and biological performance, Crit. Rev. Food Sci. Nutr., 2019, 59(10), 1563–1579 CrossRef CAS.
- F. Nazzaro, P. Orlando, F. Fratianni and F. Coppola, Microencapsulation in food science and technology, Curr. Opin. Biotechnol., 2012, 23, 182–186 CrossRef CAS.
- C. Encina, C. Vergara, B. Giménez, F. Oyarzún-Ampuero and P. Robert, Conventional spray-drying and future trends for the microencapsulation of fish oil, Trends Food Sci. Technol., 2016, 56, 46–60 CrossRef CAS.
- E. Assadpour and S. M. Jarari, Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules, Annu. Rev. Food Sci. Technol., 2019, 10, 103–131 CrossRef CAS.
- L. Gasperini, J. F. Mano and R. L. Reis, Natural polymers for the microencapsulation of cells, J. R. Soc., Interface, 2014, 11(100), 0817 CrossRef.
- T. R. Thrimawithana, S. Young, D. E. Dunstan and R. G. Alany, Texture and rheological characterization of kappa and iota carrageenan in the presence of counter ions, Carbohydr. Polym., 2010, 82(1), 69–77 CrossRef CAS.
- H. He, J. Ye, X. Zhang, Y. Huang, X. Li and M. Xiao, κ-Carrageenan/locust bean gum as hard capsule gelling agents, Carbohydr. Polym., 2017, 175, 417–424 CrossRef CAS.
- M. C. Bonferoni, S. Rossi, F. Ferrari, G. P. Bettinetti and C. Caramella, Characterization of a diltiazem-lambda carrageenan complex, Int. J. Pharm., 2000, 200, 207–216 CrossRef CAS.
- L. Mao, Q. Pan, Z. Hou, F. Yuan and Y. Gao, Development of soy protein isolate-carrageenan conjugates through Maillard reaction for the microencapsulation of Bifidobacterium longum, Food Hydrocolloids, 2018, 84, 489–497 CrossRef CAS.
- S. Ścieszka and E. Klewicka, Algae in food: a general review, Crit. Rev. Food Sci. Nutr., 2019, 59(21), 3538–3547 CrossRef.
- M. Pérez-Mateos, T. Solas and P. Montero, Carrageenans and alginate effects on properties of combined pressure and temperature in fish mince gels, Food Hydrocolloids, 2002, 16(3), 225–233 CrossRef.
- C. Gómez-Guillen, T. Solas, J. Borderías and P. Montero, Effect of heating temperature and sodium chloride concentration on ultrastructure and texture of gels made from giant squid (Dosidicusgigas) with addition of starch, ι-carrageenan and egg white, Z. Lebensm.-Unters. Forsch., 1996, 202(3), 221–227 CrossRef.
- D. Marín, A. Alemán, A. Sánchez-Faure, P. Montero and M. C. Gómez-Guillén, Freeze-dried phophatidylcholine liposomes encapsulating various antioxidant extracts from natural waste as functional ingredients in surimi gels, Food Chem., 2018, 245, 525–535 CrossRef.
- A. Alemán, B. Giménez, E. Pérez-Santín, M. C. Gómez-Guillén and P. Montero, Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate, Food Chem., 2011, 125, 334–341 CrossRef.
- K. Slinkard and V. L. Singleton, Total phenol analysis: automation and comparison with manual methods, Am. J. Enol. Vitic., 1977, 28(1), 49–55 CAS.
- R. E. Cian, A. Campos-Soldini, L. Chel-Guerrero, S. R. Drago and D. Betancur-Ancona, Bioactive Phaseoluslunatus peptides release from maltodextrin/gum arabic microcapsules obtained by spray drying after simulated gastrointestinal digestion, Int. J. Food Sci. Technol., 2019, 54, 2002–2009 CrossRef CAS.
- P. Montero, M. M. Calvo, M. C. Gómez-Guillén and J. Gómez-Estaca, Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient: Characterization, stability and bioaccessibility, LWT–Food Sci. Technol., 2016, 70, 229–236 CrossRef CAS.
- A. M. Goula and K. G. Adamopoulos, A method for pomegranate seed application in food industries: Seed oil encapsulation, Food Bioprod. Process., 2012, 90, 639–652 CrossRef CAS.
- S. David, A. Wojciechowska, R. Portmann, A. Shpigelman and U. Lesmes, The impact of food-grade carrageenans and consumer age on the in vitro proteolysis of whey proteins, Food Res. Int., 2020, 108964 CrossRef CAS.
- X. Sun, C. Pan, Z. Ying, D. Yu, X. Duan, F. Huang, J. Ling and X. K. Ouyang, Stabilization of zein nanoparticles with κ-carrageenan and tween 80 for encapsulation of curcumin, Int. J. Biol. Macromol., 2020, 146, 549–559 CrossRef CAS.
- Jayanudin, M. Fahrurrozi and S. K. Wirawan, Rochmadi. Antioxidant activity and controlled release analysis of red ginger oleoresin (Zingiber officinale var. Rubrum) encapsulated in chitosan cross-linked by glutaraldehyde saturated toluene, Sustainable Chem. Pharm., 2019, 12, 100132 CrossRef.
- H. C. F. Carneiro, R. V. Tonon, C. R. F. Grosso and M. D. Hubinger, Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials, J. Food Eng., 2013, 115(4), 443–451 CrossRef CAS.
- J. Gómez-Estaca, T. A. Comunian, P. Montero, R. Ferro-Furtado and C. S. Favaro-Trindade, Encapsulation of an astaxanthin-containing lipid extract from shrimp waste by complex coacervation using a novel gelatin-cashew gum complex, Food Hydrocolloids, 2016, 61, 155–162 CrossRef.
- X. Zhao, H. Liu, X. Zhang, G. Zhang and H. Zhu, Astaxanthin from Haematococcuspluvialis Microencapsulated by Spray Drying: Characterization and Antioxidant Activity, J. Am. Oil Chem. Soc., 2019, 96, 93–102 CrossRef CAS.
- C. K. Venil, A. R. Khasim, C. A. Aruldass and W. A. Ahmad, Microencapsulation of flexirubin-type pigment by spray drying: Characterization and antioxidant activity, Int. Biodeterior. Biodegrad., 2016, 113, 350–356 CrossRef CAS.
- S. W. Chan, H. Mirhosseini, F. S. Taip, T. C. Ling, I. A. Nehdi and C. P. Tan, Emulsion Formulation Optimization and Characterization of Spray-dried κ-Carrageenan Microparticles for the Encapsulation of CoQ10, Food Technol. Biotechnol., 2016, 25(S), 53–62 CrossRef CAS.
- M. Çam, N. C. İçyer and F. Erdoğan, Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development, LWT–Food Sci. Technol., 2014, 55, 117–123 CrossRef.
- E. Sokolova, A. Barabanova, R. Bogdanovich, V. Khomenko, T. Soloveva and I. Yermak, In vitro antioxidant properties of red algal polysaccharides, Biomed. Pharmacother., 2011, 1, 161–167 Search PubMed.
- J. Tkaczewska, E. Jamróz, E. Piątkowska, B. Borczak, J. Kapusta-Duch and M. Morawska, Furcellaran-Coated Microcapsules as Carriers of Cyprinuscarpio Skin-Derived Antioxidant Hydrolysate: An In Vitro, and In Vivo Study, Nutrients, 2019, 11, 2502 CrossRef CAS.
- A. Hunt and J. W. Park, Alaska Pollock Fish Protein Gels as Affected by Refined Carrageenan and Various Salts, J. Food Qual., 2013, 36(1), 51–58 CrossRef CAS.
- N. Ueki, J. Wan and S. Watabe, The Pepsin Digestibility of Thermal Gel Products Made from White Croaker (Pennahia argentata) Muscle in Associating with Myosin Polymerization Levels, J. Food Sci., 2014, 79(12), C2427–C2433 CrossRef CAS.
- A. Alemán, E. Pérez-Santín, S. Bordenave-Juchereau, I. Arnaudin, M. C. Gómez-Guillén and P. Montero, Squid gelatin hydrolysate with antihypertensive, anticancer and antioxidant activity, Food Res. Int., 2011b, 44(4), 1044–1051 Search PubMed.
- A. Alemán, M. C. Gómez-Guillén and P. Montero, Identification of ace-inhibitory peptides from squid skin collagen after in vitro gastrointestinal digestion, Food Res. Int., 2013, 54(1), 790–795 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |