The anti-allergic potential of tea: a review of its components, mechanisms and risks
Qing-Sheng
Li†
 ab,
Ying-Qi
Wang†
a,
Yue-Rong
Liang
a and
Jian-Liang
Lu
ab,
Ying-Qi
Wang†
a,
Yue-Rong
Liang
a and
Jian-Liang
Lu
 *a
*a
aTea Research Institute, Zhejiang University, China. E-mail: jllu@zju.edu.cn; Tel: +86 571 88982704
bInstitute of Sericulture and Tea, Zhejiang Academy of Agricultural Sciences, China
First published on 3rd November 2020
Abstract
Allergy is an immune-mediated disease with increasing prevalence worldwide. Regular treatment with glucocorticoids and antihistamine drugs for allergy patients is palliative rather than permanent. Daily use of dietary anti-allergic natural products is a superior way to prevent allergy and alleviate the threat. Tea, as a health-promoting beverage, has multiple compounds with immunomodulatory ability. Persuasive evidence has shown the anti-allergic ability of tea against asthma, food allergy, atopic dermatitis and anaphylaxis. Recent advances in potential anti-allergic ability of tea and anti-allergic compounds in tea have been reviewed in this paper. Tea exerts its anti-allergic effect mainly by reducing IgE and histamine levels, decreasing FcεRI expression, regulating the balance of Th1/Th2/Th17/Treg cells and inhibiting related transcription factors. Further research perspectives are also discussed.
1. Introduction
Allergy is an immune-mediated disease with high morbidity in the world and affects more than 20% of the population of western countries.1 As one of the four types of hypersensitivity reactions, allergy is formally called type I (or immediate) hypersensitivity mainly mediated by the interaction of immunoglobulin E (IgE) with a sudden release of mast cell- and basophil-derived mediators into the circulatory system.2 Based on allergen types, allergies can be classified into several types such as food allergies, drug allergies and insect allergies. In addition to ordinary allergies, a severe hypersensitivity named anaphylaxis is systemic and life threatening,3 causing respiratory symptoms, dizziness, unconsciousness, and gastrointestinal symptoms.4 The problem of anaphylaxis affects 1.21%–15.04% of the United States population with 1500 deaths per year, and calculated 0.3% of the population of Europe.5–7 Thus, allergy, as a worldwide public health problem, needs fundamental research studies (mechanism discovery and drug development), clinical therapeutics (proper diagnosis and immediate treatment) and preventive measures (immune regulation and functional edible product exploitation). Although allergic reactions can be clinically diagnosed and medicated effectively at present, people have misgivings about the side effects of allergy drugs and mounting medical expenses. There is also a demand for the development of functional foods to prevent allergies. Moreover, studies of certain crops, fruits and herbals have shown the potential of natural products to prevent and inhibit allergies.Tea, made from the tender shoots of the Camellia sinensis, is a worldwide-consumed herbal beverage with various pharmacological functions such as antioxidant,8 antidiabetic,9 anti-tumor,10 and anti-β-amyloid.11 In addition, tea also has anti-allergic ability because of its multiple bioactive compounds such as polyphenols, polysaccharides and saponins. Recent evidence has shown that leaves harvested from special tea cultivars with high content of methylated catechins have better effects in alleviating allergic symptoms. However, more fundamental research is still needed to prove the effects of tea on the prevention and treatment against allergy, and the anti-allergic bioactive compounds of tea need further characterization. This review summarizes recent research on the anti-allergic effects of tea and tea components, including in vivo and in vitro studies, and offers directions for further study.
2. Phases of the allergic response modulated by tea
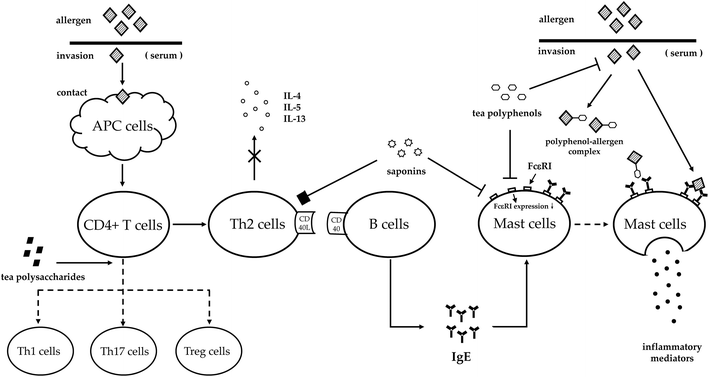
The words ‘allergy’ and ‘hypersensitivity’ are similar and inaccurately used occasionally. In 1960s, Gell and Coombs put forward the most recognized classification of hypersensitivity with four types based on the immunologic mechanisms related to the symptoms in organisms.12 After entering the 21st century, a new hypersensitivity type was appended in the classification to represent the immune disorders when the body deals with a class of infectious agents.13 Regardless of the two classification methods, we generally recognize ‘allergy’ as type I hypersensitivity with the most clear-cut immunopathological correlation, which is an IgE mediated allergic reaction. This allergic reaction comprises three phases: (1) inductive phase. An allergen enters the immune system and is taken up by antigen-presenting cells, the CD4 + T-cells differentiate into T-helper type 2 (Th2) cells and release cytokines. Due to the CD40L–CD40 interaction, allergen-specific IgE is secreted into the serum. Then, the Fc portion of the specific IgE binds to the high-affinity receptor FcεRI that is distributed on the surface of the mast cells and basophils. (2) Stimulation phase. Since the immune system re-exposes to the same allergen, the bound IgE will cross-link to the allergen. (3) Effector phase. Mast cells and basophils will be activated after the stimulation, and a robust secretion of inflammatory mediators into the serum triggers symptoms involving the skin, respiratory system or circulatory system.14 Modern therapeutic approaches towards allergy focus on modulating the sensitization in the inductive phase such as prevention, or the impact on allergic mediators released in the effector phase, thus alleviating allergy symptoms as treatment.15,16Tea, as a natural herbal beverage, exerts anti-allergic effects not only in allergy prevention, but also in allergy treatment. It has been proved that tea has the anti-allergic ability against food allergy, respiratory allergy, atopic dermatitis and anaphylaxis. Generally, the down-regulated ratios of IgE and histamine are main indexes to evaluate the anti-allergic ability of certain compounds such as polyphenols, saponins and polysaccharides. The essential allergy phases interfered with by compounds in tea are postulated as follows (Fig. 1): (1) inhibiting allergen–IgE complex formation; (2) reducing FcεRI expression or allergen–IgE complex binding to FcεRI; (3) balancing Th1/Th2/Th17/Treg systems; and (4) inhibiting cytokine release of Th2 cells.17–19 Among the four aspects, tea modulates the sensitization of the human body toward allergens through the former two ways, whilst the latter two means help the human body to alleviate allergy symptoms.
3. Anti-allergic compounds in tea
3.1. Polyphenols
Polyphenols in tea can mainly be classified into four types including catechins, flavone/flavonols, anthocyanidins/leucoanthocyanidin, and phenolic acids/depsides. The anti-allergic ability of tea is highly related with the compositions of catechins and flavonols, due to their predominant contents and physicochemical properties. Catechins are the most abundant polyphenols present in tea with four major components including (−)-epigallocatechingallate (EGCG), (−)-epicatechingallate (ECG), (−)-epigallocatechin (EGC), and (−)-epicatechin (EC).20 Among the catechin derivatives, methylated catechins such as (−)-epigallocatechin-3-O-(3-O-methyl) gallate (EGCG3′′Me) and (−)-epigallocatechin-3-O-(4-O-methyl) gallate (EGCG4′′Me) should be emphasized for their superior anti-allergic ability. Meanwhile, quercetin and kaempferol are mainly discussed in this paper as main flavonols in tea. The chemical structures of the major polyphenols in tea are shown in Fig. 2.Methylated catechins, a group of catechin derivatives, have drawn attention from researchers for their superior anti-allergic ability to regular catechins.25–27 Combined with the fact that methylated catechins can be detected as metabolites in human serum and urine after tea or catechin consumption, methylated catechins may contribute to the anti-allergic ability of tea more directly.28–30 Drinking tea with methylated catechins for a long term can significantly alleviate allergy symptoms, such as throat pain, nose-blowing and tears.31 Class I O-methyltransferase (CsOMT) isolated from tea cultivars can synthesize EGCG3′′Me, EGCG4′′Me, (−)-epigallocatechin-3-O-(3,5-O-dimethyl)-gallate (EGCG3′′,5′′diMe), and (−)-3-O-methyl-epigallocatechin-3-O-(3,5-O-dimethyl)-gallate (EGCG3′,3′′,5′′triMe) from EGCG.32,33 Among these methylated catechins, EGCG3′′Me and EGCG4′′Me, which are the major methylated catechins, have been found in multiple tea cultivars from Japan and China.34,35 The average content of EGCG3′′Me in regular tea cultivar is less than 0.45%, while it could reach 2.0% in specific cultivars such as ‘Benifuuki’.35–37 However, EGCG3′′Me can exhibit better anti-allergic effects even at low concentration because of its high bioavailability and bioactivity. The absolute quantity of EGCG3′′Me is only one fifth of that of EGCG in ‘Benifuuki’, but the anti-allergic effect of EGCG3′′Me is higher than that of EGCG.38–40 Because of the high bioavailability of the EGCG3′′Me, it could significantly relieve allergy symptoms by inhibiting mast cell activation, histamine release and cytokine production.39,41 One of the underlying causes of high efficiency of methylated catechins may be that the methyl groups added onto regular catechins increase their lipophilicity, thus enhancing the absorptivity of catechins.
Quercetin has anti-allergic abilities in inhibiting mast cell activation, histamine release, and eosinophilic inflammation. Quercetin can stabilize the membrane of mast cell, inhibit the release of histamine, leukotrienes (LTs), prostaglandin D2 (PGD2), and granulocyte macrophage-colony stimulating factor (GM-CSF).47 Meanwhile, quercetin can down-regulate the gene expression and decrease the production of the relevant cytokines such as tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-8 in vitro directly or collaboratively.48 Moreover, quercetin has better function than the typical mast cell stabilizer named cromolyn in inhibiting IL-8 and TNF-α release, which offers a strong evidence for preventing allergy by a natural product.49 Essential mechanisms of the action may be related to the fact that quercetin can decrease the cytosolic calcium level, inhibit the NF-κB activation and the MAPK activity.50,51 Besides, quercetin can also inhibit the synthesis of downstream enzymes such as tryptase, histidine decarboxylase, phospholipase A2 and eosinophil peroxidase.52–54 Briefly, quercetin is an inhibitor of human mast cell activation via the inhibition of Ca2+ influx and the prevention of histamine, LTs and PGs release.
Orally administration with kaempferol is effective to alleviate symptoms of OVA-induced murine model of food allergy and allergic asthma. As far as food allergy prevention, kaempferol can reduce diarrhea rate and anaphylaxis risk with decreased levels of IL-4, IL-5, and IL-13.55 In respiratory allergy, kaempferol can alleviate the infiltration of eosinophils and mast cells through inhibiting the anti-α-smooth muscle actin expression in the airways and blocking their degranulation in the lung tissue, as well as reducing IgE, histamine and IL-4 levels, and increasing interferon-γ (IFN-γ) content.56,57 A cytokine named IL-32 has been observed above normal level in the nasal mucosa of AR patients, and it significantly elevates the downstream thymic stromal lymphopoietin (TSLP) production in monocytes. Treating with kaempferol to murine model decreases the IL-32 and TSLP levels, thus inhibiting the TSLP-driven Th2 inflammatory cell infiltration, cytokine secretion, and IgE production.58 Hemeoxygenase (HO)-1 is generally known as a rate-limiting enzyme in degradation of heme, while it also has the ability to transfect mast cells to decrease degranulation.59 Kaempferol can improve HO-1 expression to inhibit histamine and β-hexosaminidase release.60 Those evidences suggest kaempferol has anti-allergic abilities in relaxing airway muscles and inhibiting histamine release.
3.2. Saponins
Tea saponins are natural non-ionic surfactants that are present in tea leaves, stem, flowers, and mostly in seeds.63–65 The content of saponins reaches more than 10%, up to 19.57%, of the dry weight in tea seeds.66,67 Saponins are widely recognized as constituents of cleaning products and are used in soil remediation.68,69 Besides, saponins have been also proved to show anti-allergic potential against asthma, PCA, atopic dermatitis and systemic anaphylactic shock.70–72 Saponins extracted from tea leaves can inhibit bronchospasm in a Guinea pig model and PCA reaction in a rat model in a dose-dependent manner, along with the inhibition of LT D4 release.70 Oral administration of saponins to mice sensitized with 2,4-dinitrochlorobenzene can reduce the thickness of the epidermis/dermis and dermal infiltration of inflammatory cells and mast cells in the ears.71 In addition, saponins can inhibit the β-hexosaminidase activity and histamine release, and block the production of TNF-α, IFN-γ, IL-4, IL-5, IL-6 and IL-13 in vivo/in vitro.71,73–753.3. Polysaccharides
Polysaccharides, important bioactive components of tea, are nonstarch protein-bound acidic polysaccharides.76,77 Tea polysaccharides can improve immunity by enhancing the activity of splenocytes or by decreasing the level of pro-inflammatory cytokines.78,79 Though little definite evidence can prove polysaccharides from tea have the ability to prevent allergy, polysaccharides from other species show the anti-allergic ability as circumstantial evidence to imply their functions in tea. The current studies have shown that polysaccharides have the ability to alleviate the symptoms of allergic rhinitis, food allergy and atopic dermatitis directly or collaboratively. Oral administration of 180 mg of cassis polysaccharides twice a day to Japanese cedar pollinosis patients, suffering from a type of allergic rhinitis, can moderate their symptoms of sneezing, itchy nose, itchy eye and watery eye.80 Treatment of OVA induced rats with allergic rhinitis with 3, 9 and 27 mg kg−1Cryptoporus polysaccharides decreased the sneezing rate of 27.4%, 38.4%, and 44.3%, and nasal rubbing rate of 27.5%, 34.9%, and 47.7% compared with the model group, respectively.81 Meanwhile, polysaccharides show their anti-food allergic ability in a mouse model. A daily dose of 5 mg/mouse of polysaccharides from Gracilaria lemaneiformis can decrease the anaphylactic response and diarrhea rates moderately, and diminish the increase of histamine and mast cell proteinase concentrations.82 In addition, polysaccharides can alleviate intestinal villi injury in a food-allergy mouse model by decreasing B cell and mast cell populations, IgE, histamine, mast cell protease-1, IL-4, and increased IFN-γ level in serum.83 However, polysaccharides alleviate atopic dermatitis symptoms with diverse causes. Polysaccharides can significantly increase the water content of the stratum corneum and decrease the epidermal thickness of skin besides Th2 system regulation.84,85 Oral treatments with polysaccharides can suppress allergy symptoms through the induction of galectin-9 in vivo, which can recognize β-galactoside and prevent IgE binding to mast cells.863.4. Other potential anti-allergic components
Besides polyphenols, polysaccharides and saponins, another bioactive compound in tea named theanine also has anti-allergic potential. Intragastric administration of L-theanine to OVA induced murine models of asthma will inhibit mucus production and inflammatory cell infiltration with significant decrease of IgE, monocyte chemoattractant protein-1 (MCP-1), IL-4, IL-5, IL-13 and TNF-α.87 Similar to the inhibition results of L-theanine to asthma, theanine can inhibit systemic anaphylactic shock and ear swelling responses of ICR mice by oral administration or injection. Theanine inhibits not only histamine release from mast cells, but also TNF-α, IL-1β, IL-6, and IL-8 secretions by blocking the activation of NF-κB and receptor interacting protein-2/caspase-1.884. Anti-allergic mechanisms of tea
4.1. General
The basic indexes to evaluate the anti-allergic properties of tea or natural products are the release content of IgE, histamine and β-hexosaminidase in serum. However, these indexes are the results of anti-allergic activity through different mechanisms such as inhibition of FcεRI expression, protein kinases and transcription factor activities, control of mediators and balance of Th1/Th2/Th17/Treg cells. Consequently, tea and the compounds in tea have a deep impact on several immune cells and immune mechanisms which are important in the allergic processes (Table 1).| Compounds | Methods | Models | Effects | Mechanisms | Ref. | |
|---|---|---|---|---|---|---|
| Note: The percentages in the brackets are the concentration ranges of selected compounds in dry tea leaves or seeds. | ||||||
| Polyphenols (18%–36%) | Catechins (12%–24%) | In vivo | BALB/c mice | Inhibit IgE, IgG1 and mMCP-1 release; reduce IL-13 and IL-12a expressions | Reduce T-bet and GATA-3 expressions | 21 |
| Inhibit IgE, histamine, IL-1β, IL-4, and IL-6 release | Inhibit the mRNA and protein expressions of COX-2 | 22 | ||||
| ddY mice | Inhibit the release of IL-4 from APC and Th2 cells; inhibit the release of IgE from B cells | 23 | ||||
| C3H/HeJ mice | Reduce IgG2a and mMCP-1 levels | Balance the Th1/Th2 system; increase Th17 cell populations | 24 | |||
| ICR mice | Reduce histamine and IL-4 levels | Bind to FcεRI | 94 | |||
| In vitro | KU812 cells | Reduce the expressions of α-chain and γ-chains of FcεRI | 17 | |||
| RBL-2H3 cells | Reduce mast cell degranulation | Inhibit the phosphorylation of Lyn, Syk, Akt and NF-κB | 94 | |||
| Methylated catechins (0.45%–2%) | Epidemiology | Alleviate symptoms of throat pain, nose blowing, itchy eyes and tears | 27, 31 and 39 | |||
| In vivo | ddY mice | Inhibit IgE, IL-4 and IL-10 release | 23 | |||
| Inhibit histamine and LT release; inhibit cytokine production and secretion | Inhibit Lyn, Syk, and Bruton's tyrosine kinase | 93 | ||||
| In vitro | KU812 cells | Reduce the expressions of α-chain and γ-chains of FcεRI | 18 | |||
| Associate lipid rafts to reduce FcεRI expression | Inhibit MRLC and ERK1/2 phosphorylation | 89–92 | ||||
| Quercetin (0.2%–0.5%) | in vivo | BALB/c mice | Reduce IL-4 production; increase IFN-γ secretion | Reduce T-bet and GATA-3 expressions | 97 | |
| Guinea pigs | Inhibit histamine, PLA2, and EPO productions; inhibit the recruitment of leukocytes | 52 and 53 | ||||
| In vitro | HMC-1 cells | Reduces TNF-α, IL-1β, IL-6, and IL-8 levels | Inhibits NF-κB and MAPK activation | 51 | ||
| LAD2 cells | Reduces histamine, LT, PGD2, IL-6, IL-8 and TNF levels | Inhibits cytosolic calcium increase and NF-κB activation | 49 | |||
| HCM cells | Inhibits histamine, LT, PGD2, and granulocyte release | Inhibits Ca2+ influx and PKC activation | 47 | |||
| Caco-2 cells | Suppresses TNF-α production and IL-8 expression; reduce β-hexosaminidase level | 48 | ||||
| Kaempferol (0.16%–0.35%) | In vivo | BALB/c mice | Reduces IgE, histamine, IL-4, IL-32 and TSLP levels | Reduces MIP-2, ICAM-1 and COX-2 levels | 58 | |
| Blunt eosinophil deposition and degranulation | Disturbs the NF-κB signaling pathway | 56 | ||||
| Reduces IL-4, IL-5, and IL-13 levels | 55 | |||||
| In vitro | Eol-1 cells | Reduces IL-32, IL-8 and caspase-1 levels | 56 | |||
| RBL-2H3 cells | Reduces anti-α-smooth muscle actin expression; reduce PGD2 and PGF2α levels | 57 | ||||
| Reduces histamine and β-hexosaminidase release | Increases HO-1 expression | 60 | ||||
| Saponins (10%–19.57%) | In vivo | Guinea pigs | Inhibits LT C4 release | 68 | ||
| Nc/Nga mice | Reduces IgE and TARC levels; reduce TARC, TNF-α, IFN-γ, IL-4, IL-5, and IL-13 expressions | Suppresses NF-κB and STAT-1; induce HO-1 expression | 69 | |||
| In vitro | RBL-2H3 cells | Inhibit β-hexosaminidase release | 61 and 71 | |||
| Inhibit β-hexosaminidase and histamine release; inhibit IL-4 and IL-6 expressions | Reduce the intracellular Ca2+ level; reduce the expression of α-chain of FcεRI | 73 | ||||
| Reduce β-hexosaminidase and histamine release; reduce IL-4 and IFN-γ levels | Suppress Syk, Akt and MAP phosphorylation | 72 | ||||
| Polysaccharides (0.4%–2.63%) | Epidemiology | Alleviate symptoms of sneezing, itchy nose, itchy eyes and watery eyes | 78 | |||
| In vivo | Sprague-Dawley rats | Decrease the sneezing and nasal rubbing rate; decrease the eotaxin expression | 79 | |||
| BALB/c mice | Reduce IL-4 and IL-13 levels; increase the TGF-β level | Suppress Th2 cell polarization; promote Treg cells; decrease GATA-3 expression; increase FOXP3 expression | 80 | |||
| Reduce IgE, histamine, mast cell protease-1 and IL-4 levels; increase IFN-γ, IL-10 and TGF-β levels | Promote Treg cells | 81 | ||||
| Reduce histamine and IgE levels; reduce IL-4,IL-5 and IL-13 expressions; increase IFN-γ and IL-10 expressions | Inhibit the JNK and JAK2 signaling pathways | 99 | ||||
| Reduce IgE level; decrease IL-4 and IL-17A expressions | 83 | |||||
| Increase galectin-9 expression | 84 | |||||
| ICR mice | Reduce histamine, IgE and TNF-α levels | 98 | ||||
| In vitro | KU812 cells | Inhibit cell activation | Suppress the MAPK signaling pathway | 80 | ||
| RBL-2H3 cells | Inhibit cell degranulation; reduce histamine, IL-4 and TNF-α levels | 80 | ||||
| Cells from BALB/c mice | Increase IFN-γ production; enhance Th1 cell differentiation; reduce IL-4 and IgE levels | 100 and 101 | ||||
4.2. Inhibiting IgE receptor FcεRI
The mechanisms underlying alleviation of allergy symptoms, particularly inhibition of histamine release by catechins, are blocking the production of protein tyrosine kinases (PTK) and reduction of FcεRI expression. FcεRI is an IgE high-affinity receptor which is distributed on the surface of mast cells and basophils with a tetrameric structure comprising one α-chain, one β-chain, and two γ-chains.89 The γ chains of FcεRI are critical for both membrane orientations of the whole receptor and signal transduction, while β chain can amplify the γ subunit-generated signals by associating constitutively with PTK and stabilize the receptor. The down-regulated expression of FcεRI can reduce histamine release from mast cells and basophils. EGCG3′′Me administration can reduce the transcript levels of FcεRI α and γ.18 EGCG and methylated EGCG can regulate basophils or mast cells through a cell-surface receptor named 67 kDa laminin receptor (67LR). Down-regulated FcεRI expression by EGCG3′′Me is due to the 67LR-mediated inhibition of myosin II regulatory light chain (MRLC) and extracellular signal-regulated kinase1/2 (ERK1/2) phosphorylation.90 It has been proved that EGCG can bind to plasma membrane microdomains as lipid rafts, and inhibit the phosphorylation of ERK1/2, and consequently suppress the expression of FcεRI,91–93 whilst EGCG3′′Me and EGCG4′′Me can inhibit PTK resulting in the decrease of histamine release.94 Moreover, a derivative from gallic acid (GA), which is a part of EGCG, is able to directly bind to the α-chain of FcεRI, thus reducing the possibility of IgE binding to FcεRI.95 These results indicate that catechins and methylated catechins reduce histamine release through two phases, but all relate to FcεRI.4.3. Balancing Th1/Th2/Th17/Treg systems
T-cells play crucial roles in the balance of adaptive immunity. Active T-cells differentiated from naïve T-cells can be classified into four main types namely Th1, Th2, Th17 and Treg cells.96 While T-cell response will be a Th2-dominated immune response in allergic patients, the chain reaction is that cytokine IL-4 induces the differentiation of naïve T-cells into Th2 cells, which produce more IL-4, IL-5 and IL-13, and eventually IgE production and eosinophil activation are stimulated.2 Balancing the T-cell model by decreasing Th2 cells is an appropriate way for alleviating allergy symptoms. The up-regulation of Th1, Th17 and Treg are immune responses to the reduction of CD4+ T-cell differentiation into Th2 cells. EGCG can increase Th1, Th17 and Treg cell population, along with their released cytokines.24,97 Quercetin can alter Th1/Th2 polarization via suppression of GATA-3 and increase of T-bet expression, and then reduce the increased levels of IL-4 and elevate the IFN-γ level in serum, thus inducing the CD4+ T-cell differentiation into Th1 cells.98 Polysaccharides are able to suppress Th2 cell polarization, and reduce IgE, histamine and Th2 cytokines including IL-2, IL-4, IL-5 and IL-13 levels in serum.82,85,99 In the meantime, polysaccharides can amplify IL-12 production to enhance antigen-primed naïve T cell transformation into Th1 cells with an increase of IFN-γ levels, and eventually modulate the imbalance of the Th1/Th2 immune response.100–102 Explaining that polysaccharides regulate allergy only according to the Th1/Th2 model is simplistic; it can also enhance the levels of IL-10, FOXP3, and transforming growth factor-β, which leads to the suppression of Th2 responses by the Treg cells.834.4 Activity inhibition of protein kinases and transcription factors
Protein kinases are involved in signal transduction during cell activation in allergic reaction. Compounds in tea can modulate protein kinases by the inhibition of transcription factors, such as NF-κB and T-bet. NF-κB is translocated from the cytoplasm to the nucleus where it induces the expression of multiple immune related genes. In addition, NF-κB and STAT-1 are thought to play pivotal roles in the regulation of inflammatory responses as TNF-α and IFN-γ.103,104 Saponins can inhibit TNF-α, IFN-γ and IL-4 production by attenuating the activation of NF-κB and STAT-1.71,74 Moreover, saponins can inhibit tyrosine phosphorylation of Syk resulting in the decrease of the expression of FcεRI.74,75Flavonoids can regulate master regulatory transcription factors for Th2 cytokines such as GATA-3, and signal transducer and activator of transcription 6 (STAT-6).105,106 Quercetin can reduce the eosinophil peroxidase activity and alter Th1/Th2 polarization via suppression of GATA-3 and increasing T-bet expression, and then decrease the IL-4 level and increase the IFN-γlevel.98 Kaempferol can suppress LPS-induced eotaxin-1 protein expression, which might be mediated likely via JAK2 signaling, and it can also attenuate the TNF-α induced expression of epithelial ICAM-1 and eosinophil integrin b2, and MCP-1 transcription encumbering eosinophil–epithelial cell interactions.56 Moreover, polysaccharides can suppress basophil cells via suppression of MAPK and decreasing eotaxin expression.81,82
5. Allergy caused by tea
In the previous sections of this paper, we totally focused on the ability of tea on attenuating the immune system and anti-allergies. However, some people might be allergic to tea in several medical cases. People who worked in tea manufacturing factories might have the possibility to develop green tea-induced asthma, particularly the workers who had a long term exposure history to tea powders.107,108 However, allergic respiratory symptoms caused by tea powders or its extract powders are rarely reported. Interestingly, Otera et al. reported a case of hypersensitivity pneumonitis caused by inhalation of catechin powders.109 The essential allergens of those cases may be dust, fluff or specks. Besides, a young woman had oropharyngeal and respiratory symptoms after drinking green tea, who could tolerate tea beverage before.110 In 2014, a 43-year-old firefighter had sudden flushing and slight facial itching followed by dry mouth and progressive dizziness after drinking a green tea infusion. Moreover, he experienced the same symptoms, as well as syncope and loss of vesical sphincter control after eating a green tea ice cream the next year.111 According to the SDS-PAGE and Western-blot results, the authors considered that an IgE-mediated hypersensitivity reaction to a 70 kDa protein from green tea extract might have caused the firefighter to suffer from such anaphylaxis. Nevertheless, the patient had positive test result of a specific black tea rather than regular black tea. The possibility of cross-reactivity dominates this case. Based on above rare cases, we cannot guarantee that tea is not a food allergen, but its risk is minimal.6. Further suggestions
6.1 Systematic epidemiological investigation
The current epidemiological studies of tea in preventing allergy and alleviating allergy symptoms are insufficiency with small sample scale. Most of them were performed in Japan to measure the anti-allergic ability of tea in reducing the symptoms of Japanese cedar pollinosis which is a regional respiratory allergy. Systematic epidemiological investigation in multiple regions on various types of allergy, particularly in high incidence areas, should be comprehensively performed to offer a preference for tea in anti-allergy.6.2 Binding sites and interaction effects of compounds
Recent research studies of natural products in preventing allergies usually focused on comparison and summarization of the anti-allergic functions of different products or their components. Thus, we have found plenty of products and compounds with anti-allergic potential. However, the AUCs and metabolites of such components in the circulatory system have been rarely investigated, let alone the interaction sites and transcriptional regulatory sites in cells. EGCG and methylated EGCG could regulate basophils or mast cells through a cell-surface receptor 67LR. Analyzing the structure of 67LR and figuring out the binding efficiency difference between EGCG and methylated EGCG would explain the mechanism of catechins in regulating the inductive phase of allergy. Moreover, we need to elucidate the interaction effects and synergistic functions of multiple compounds in vivo.6.3 The veracity of anti-allergy by polysaccharides
Polysaccharides have been proved to have several bioactive functions. The anti-allergic ability of polysaccharides extracted from multiple herbal species also has been confirmed in vivo and in vitro. Polysaccharides are mostly glycoconjugates in which a protein carries one or more carbohydrate chain(s) covalently attached to a polypeptide backbone. Most crude polysaccharides extracted from tea were usually contaminated with catechins and strictinin which might promote the formation of a catechin–polysaccharide complex. Thus, the immunomodulatory activity of polysaccharides might be the effects of the polysaccharide complexes rather than itself.112 Pinpointing the most effective polysaccharide monomer could help illustrate the action mechanism and direct the industrial production of allergy prevention natural products.6.4 Dietary mode development
As a traditional beverage, tea is usually drunk directly or mixed. Nowadays, tea is also produced as packaged beverage or food additives. With the popularity of tea drinks, it is easy to introduce tea into a lifestyle. Although the complete anti-allergic mechanism of tea is still unclear, its function of preventing allergy has been basically proved in vivo and in vitro. Studying and developing a dietary mode with tea products will offer the allergy patients with mild symptoms a supplementary option to ease the suffering and a preventive measure.7. Conclusions
Allergy is an immune response of the human body in a chain reaction manner. Though it is not a severe epidemic disease with clustering paroxysm, it is lethal occasionally with increasing prevalence. The functional components of tea and the effects and partial mechanisms of tea against allergy have been explored and verified. Although the epidemiological investigations on such points are insufficient, evidence supporting that tea has anti-allergic functions in vivo/in vitro is obvious. Knowledge of more precise and deeper mechanisms of tea against allergy is required. Overall, tea plays a potential role in preventing allergy.Abbreviations
| IgE | Immunoglobulin E |
| Th1 | T-helper type 1 cells |
| Th2 | T-helper type 2 cells |
| Th17 | T-helper type cells which produce IL-17A and IL-17F |
| Treg | Regulatory T cells |
| FcεRI | A receptor with Fc portion |
| EGCG | Epigallocatechingallate |
| ECG | Epicatechingallate |
| EGC | Epigallocatechin |
| EC | Epicatechin |
| EGCG3′′Me | Epigallocatechin-3-O-(3-O-methyl) gallate |
| EGCG4′′Me | Epigallocatechin-3-O-(4-O-methyl) gallate |
| OVA | Ovalbumin |
| IL | Interleukin |
| AUC | Area under the drug concentration time curves |
| Syk | Spleen tyrosine kinase |
| PKC | Protein kinase C |
| MAPK | p38 mitogen-activated protein kinase |
| PG | Prostaglandin |
| TNF | Tumor necrosis factor |
| IFN | Interferon |
| TSLP | Thymic stromal lymphopoietin |
| HO | Hemeoxygenase |
| LT | Leukotriene |
| MCP | Chemoattractant protein |
| ICR | Institute of cancer research |
| PTK | Protein tyrosine kinases |
| 67LR | A cell-surface receptor named 67 kDa laminin receptor |
| ERK1/2 | Extracellular signal-regulated kinase1/2 |
| GA | Gallic acid |
| FOXP3 | A member of forkhead/winged-helix |
| STAT-1 | Signal transducer and activator of transcription 1 |
| JNK | Jun N-terminal kinase |
| JAK2 | Janus kinase 2 |
| ICAM-1 | Intercellular cell adhesion molecule 1 |
| MRLC | Myosin II regulatory light chain. |
Author contributions
Q.-S.L.: Section 1: Introduction; Section 3: Anti-allergic compounds in tea; Modification of the manuscript; Table 1; Y.-Q.W.: Fig. 1; Section 2: Phases of the allergic response modulated by tea; Fig. 2; Y.-R.L.: Section 4: Anti-allergic mechanisms of tea; Section 5: Allergy caused by tea; J.-L.L.: Section 6: Further suggestions; Section 7: Conclusions; Modification of the manuscript.Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31770728) and Agriculture Research System of China (Tea) (No. CARS-19).Conflicts of interest
The authors declare no conflict of interest.References
- M. A. Kaliner and S. D. Giacco, In WAO white book on allergy: update 2013, ed. R. Pawankar, S. T. Holgate, G. W. Canonica, R. F. Lockey and M. S. Blaiss, The practice of allergology, World Allergy Organization, Milwaukee, Wisconsin, America, 1st ed., 2013, 23–26 Search PubMed.
- Z. S. Gao, H. H. Shen, M. Zheng, L. J. Frewer and L. J. W. J. Gilissen, Multidisciplinary approaches to allergies, Zhejiang University Press, Hangzhou, China, 2012 Search PubMed.
- S. G. Cohen and M. Zelaya-Quesada, Portier, Richet, and the discovery of anaphylaxis: a centennial, J. Allergy Clin. Immun., 2002, 110, 331–336 CrossRef.
- S. F. Kemp and R. F. Lockey, Anaphylaxis: a review of causes and mechanisms, J. Allergy Clin. Immun., 2002, 110, 341–348 CrossRef CAS.
- A. I. Neugut, A. T. Ghatak and R. L. Miller, Anaphylaxis in the United States - an investigation into its epidemiology, Arch. Intern. Med., 2001, 161, 15–21 CrossRef CAS.
- D. A. Moneret-Vautrin, M. Morisset, J. Flabbee, E. Beaudouin and G. Kanny, Epidemiology of life-threatening and lethal anaphylaxis: a review, Allergy, 2005, 60, 443–451 CrossRef CAS.
- S. S. Panesar, S. Javad, D. de Silva, B. I. Nwaru, L. Hickstein, A. Muraro, G. Roberts, M. Worm, M. B. Bilo, V. Cardona, A. E. J. Dubois, A. D. Galvin, P. Eigenmann, M. Fernandez-Rivas, S. Halken, G. Lack, B. Niggemann, A. F. Santos, B. J. Vlieg-Boerstra, Z. Q. Zolkipli, A. Sheikh and E. F. A. A. Grp, The epidemiology of anaphylaxis in Europe: a systematic review, Allergy, 2013, 68, 1353–1361 CrossRef CAS.
- J. V. Higdon and B. Frei, Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions, Crit. Rev. Food Sci., 2003, 43, 89–143 CrossRef CAS.
- Q. Y. Fu, Q. S. Li, X. M. Lin, R. Y. Qiao, R. Yang, X. M. Li, Z. B. Dong, L. P. Xiang, X. Q. Zheng, J. L. Lu, C. B. Yuan, J. H. Ye and Y. R. Liang, Antidiabetic effects of tea, Molecules, 2017, 22, 849 CrossRef.
- Y. Q. Wang, J. L. Lu, Y. R. Liang and Q. S. Li, Suppressive effects of EGCG on cervical cancer, Molecules, 2018, 23, 2334 CrossRef.
- C. A. Polito, Z. Y. Cai, Y. L. Shi, X. M. Li, R. Yang, M. Shi, Q. S. Li, S. C. Ma, L. P. Xiang, K. R. Wang, J. H. Ye, J. L. Lu, X. Q. Zheng and Y. R. Liang, Association of tea consumption with risk of Alzheimer's disease and anti-beta-amyloid effects of tea, Nutrients, 2018, 10, 655 CrossRef.
- P. G. H. Gell and R. R. A. Coombs, In Clinical aspects of immunology, ed. R. R. A. Coombs and P. G. H. Gell, The classification of allergic reactions underlying disease, Blackwell Scientific Publications, Oxford, UK, 1st ed., 1963, 317–337 Search PubMed.
- T. V. Rajan, The Gell-Coombs classification of hypersensitivity reactions: a re-interpretation, Trends Immunol., 2003, 24, 376–379 CrossRef CAS.
- I. Pali-Scholl and E. Jensen-Jarolim, In Allergy Frontiers: Classification and Pathomechanisms, ed. R. Pawankar, S. T. Holgate and L. J. Rosenwasser, Basic aspects of allergy and hypersensitivity reactions, Springer, Tokyo, Japan, 1st ed, 2009, 3–17 Search PubMed.
- S. L. Prescott and C. A. Jones, An update of immunotherapy for specific allergies, Curr. Drug Targets Inflamm. Allergy, 2002, 1, 65–75 CrossRef CAS.
- E. M. Varga, K. Nouri-Aria, S. J. Till and S. R. Durham, Immunomodulatory treatment strategies for allergic diseases, Curr. Drug Targets Inflamm. Allergy, 2003, 2, 31–46 CrossRef CAS.
- Y. Fujimura, H. Tachibana and K. Yamada, A tea catechin suppresses the expression of the high-affinity IgE receptor Fc epsilon RI in human basophilic KU812 cells, J. Agric. Food Chem., 2001, 49, 2527–2531 CrossRef CAS.
- Y. Fujimura, H. Tachibana, M. Maeda-Yamamoto, T. Miyase, M. Sano and K. Yamada, Antiallergic tea catechin, (-)-epigallocatechin-3-O-(3-O-methyl)-gallate, suppresses Fc epsilon RI expression in human basophilic KU812 cells, J. Agric. Food Chem., 2002, 50, 5729–5734 CrossRef CAS.
- T. Tokura, N. Nakano, T. Ito, H. Matsuda, Y. Nagasako-Akazome, T. Kanda, M. Ikeda, K. Okumura, H. Ogawa and C. Nishiyama, Inhibitory effect of polyphenol-enriched apple extracts on mast cell degranulation in vitro targeting the binding between IgE and Fc epsilon RI, Biosci. Biotech. Biochem., 2005, 69, 1974–1977 CrossRef CAS.
- Y. R. Liang, Q. Ye, J. Jin, H. Liang, J. L. Lu, Y. Y. Du and J. J. Dong, Chemical and instrumental assessment of green tea sensory preference, Int. J. Food Prop., 2008, 11, 258–272 CrossRef CAS.
- A. Singh, A. Demont, L. Actis-Goretta, S. Holvoet, A. Leveques, M. Lepage, S. Nutten and A. Mercenier, Identification of epicatechin as one of the key bioactive constituents of polyphenol-enriched extracts that demonstrate an anti-allergic effect in a murine model of food allergy, Brit. J. Nutr., 2014, 112, 358–368 CrossRef CAS.
- M. Fu, S. L. Fu, S. H. Ni, L. Y. Zou, Y. M. Liu and T. Hong, Anti-inflammatory effect of epigallocatechin gallate in a mouse model of ovalbumin-induced allergic rhinitis, Int. Immunopharmacol., 2017, 49, 102–108 CrossRef CAS.
- K. Yoshino, T. Miyase and M. Sano, Preventive effects of C-2 epimeric isomers of tea catechins on mouse type I allergy, J. Nutr. Sci. Vitaminol., 2010, 56, 211–215 CrossRef CAS.
- T. B. Pessato, N. C. de Carvalho, D. de Figueiredo, T. C. Colomeu, L. G. R. Fernandes, F. M. Netto and R. D. Zollner, Complexation of whey protein with caffeic acid or (-)-epigallocatechin-3-gallate as a strategy to induce oral tolerance to whey allergenic proteins, Int. Immunopharmacol., 2019, 68, 115–123 CrossRef CAS.
- M. Sano, M. Suzuki, T. Miyase, K. Yoshino and M. Maeda-Yamamoto, Novel antiallergic catechin derivatives isolated from oolong tea, J. Agric. Food Chem., 1999, 47, 1906–1910 CrossRef CAS.
- M. Suzuki, K. Yoshino, M. Maeda-Yamamoto, T. Miyase and M. Sano, Inhibitory effects of tea catechins and O-methylated derivatives of (-)-epigallocatechin-3-O-gallate on mouse type IV allergy, J. Agric. Food Chem., 2000, 48, 5649–5653 CrossRef CAS.
- M. Maeda-Yamamoto, K. Ema, M. Monobe, Y. Tokuda and H. Tachibana, Epicatechin-3-O-(3′′-O-methyl)-gallate content in various tea cultivars (Camellia sinensis L.) and its in vitro inhibitory effect on histamine release, J. Agric. Food Chem., 2012, 60, 2165–2170 CrossRef CAS.
- X. Meng, M. J. Lee, C. Li, S. Sheng, N. Zhu, S. Sang, C. T. Ho and C. S. Yang, Formation and identification of 4′-O-methyl-(-)-epigallocatechin in humans, Drug Metab. Dispos., 2001, 29, 789–793 CAS.
- J. M. Van Amelsvoort, K. H. Van Hof, J. N. Mathot, T. P. Mulder, A. Wiersma and L. B. Tijburg, Plasma concentrations of individual tea catechins after a single oral dose in humans, Xenobiotica, 2001, 31, 891–901 CrossRef CAS.
- X. F. Meng, S. M. Sang, N. Q. Zhu, H. Lu, S. Q. Sheng, M. J. Lee, C. T. Ho and C. S. Yang, Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats, Chem. Res. Toxicol., 2002, 15, 1042–1050 Search PubMed.
- M. Maeda-Yamamoto, K. Ema, M. Monobe, I. Shibuichi, Y. Shinoda, T. Yamamoto and T. Fujisawa, The efficacy of early treatment of seasonal allergic rhinitis with benifuuki green tea containing O-methylated catechin before pollen exposure: an open randomized study, Allergol. Int., 2009, 58, 437–444 CrossRef CAS.
- C. P. Joshi and V. L. Chiang, Conserved sequence motifs in plant S-adenosyl-L-methionine-dependent methyltransferases, Plant Mol. Biol., 1998, 37, 663–674 CrossRef CAS.
- M. Kirita, D. Honma, Y. Tanaka, S. Usui, T. Shoji, M. Sami, T. Yokota, M. Tagashira, A. Muranaka, M. Uchiyama, T. Kanda and M. Maeda-Yamamoto, Cloning of a novel O-methyltransferase from Camellia sinensis and synthesis of O-methylated EGCG and evaluation of their bioactivity, J. Agric. Food Chem., 2010, 58, 7196–7201 CrossRef CAS.
- H. P. Lv, T. Yang, C. Y. Ma, C. P. Wang, J. Shi, Y. Zhang, Q. H. Peng, J. F. Tan, L. Guo and Z. Lin, Analysis of naturally occurring 3-methyl-epigallocatechin gallate in 71 major tea cultivars grown in China and its processing characteristics, J. Funct. Foods, 2014, 7, 727–736 CrossRef CAS.
- S. C. Lee, R. H. Yan, H. Y. Cheng, S. S. Wu and S. Y. Liu, Screen and genetic assessment of tea germplasms with elevated methylated catechin, (-)-epigallocatechin-3-O-(3-O-methyl) gallate, J. Agric. Food Chem., 2009, 57, 8906–8912 CrossRef CAS.
- H. Nagai, M. Maeda-Yamamoto, Y. Suzuki, K. Sato and H. Mitsuda, The development of a suitable manufacturing process for ‘Benifuuki’ green tea beverage with anti-allergic effects, J. Sci. Food Agric., 2005, 85, 1606–1612 CrossRef CAS.
- M. Maeda-Yamamoto, H. Nagai, K. Asai, S. Moriwaki, H. Horie, K. Kohata, H. Tachibana, T. Miyase and M. Sano, Changes in epigallocatechin-3-O-(3-O-methyl) gallate and strictinin contents of tea (Camellia sinensis L.) cultivar ‘Benifuki’ in various degrees of maturity and leaf order, Food Sci. Technol. Res., 2004, 10, 186–190 CrossRef CAS.
- Y. Oritani, Y. Setoguchi, R. Ito, H. Maruki-Uchida, T. Ichiyanagi and T. Ito, Comparison of (-)-epigallocatechin-3-O-gallate (EGCG) and O-methyl EGCG bioavailability in rats, Biol. Pharm. Bull., 2013, 36, 1577–1582 CrossRef CAS.
- M. Maeda-Yamamoto, K. Ema and I. Shibuichi, In vitro and in vivo anti-allergic effects of ‘benifuuki’ green tea containing O-methylated catechin and ginger extract enhancement, Cytotechnology, 2007, 55, 135–142 CrossRef CAS.
- M. Maeda-Yamamoto, K. Ema, Y. Tokuda, M. Monobe, H. Tachibana, Y. Sameshima and S. Kuriyama, Effect of green tea powder (Camellia sinensis L. cv. Benifuuki) particle size on O-methylated EGCG absorption in rats; The Kakegawa study, Cytotechnology, 2011, 63, 171–179 CrossRef CAS.
- M. Maeda-Yamamoto and H. Tachibana, Anti-allergic action of O-methylated EGCG in green tea cultivar Benifuuki, J. Food Drug. Anal., 2012, 20, 313–317 CAS.
- S. J. Maleki, J. F. Crespo and B. Cabanillas, Anti-inflammatory effects of flavonoids, Food Chem., 2019, 299, 125124 CrossRef CAS.
- S. Chirumbolo, The role of quercetin, flavonols and flavones in modulating inflammatory cell function, Inflamm. Allergy Drug Targets, 2010, 9, 263–285 CrossRef CAS.
- C. Y. Wu, H. R. Xu, J. Heritier and W. Andlauer, Determination of catechins and flavonol glycosides in Chinese tea varieties, Food Chem., 2012, 132, 144–149 CrossRef CAS.
- D. H. Kim, W. S. Jung, M. E. Kim, H. W. Lee, H. Y. Youn, J. K. Seon, H. N. Lee and J. S. Lee, Genistein inhibits pro-inflammatory cytokines in human mast cell activation through the inhibition of the ERK pathway, Int. J. Mol. Med., 2014, 34, 1669–1674 CrossRef CAS.
- E. J. Lee, G. E. Ji and M. K. Sung, Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells, Inflamm. Res., 2010, 59, 847–854 CrossRef CAS.
- M. Kimata, M. Shichijo, T. Miura, I. Serizawa, N. Inagaki and H. Nagai, Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells, Clin. Exp. Allergy, 2000, 30, 501–508 CrossRef CAS.
- M. Mizuno, S. Yamashita and T. Hashimoto, Enhancement of anti-inflammatory and anti-allergic activities with combination of luteolin and quercetin in in vitro co-culture system, Food Sci. Technol. Res., 2017, 23, 811–818 CrossRef CAS.
- Z. Y. Weng, B. D. Zhang, S. Asadi, N. Sismanopoulos, A. Butcher, X. Y. Fu, A. Katsarou-Katsari, C. Antoniou and T. C. Theoharides, Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans, PLoS One, 2012, 7, e33805 CrossRef CAS.
- J. Mlcek, T. Jurikova, S. Skrovankova and J. Sochor, Quercetin and its anti-allergic immune response, Molecules, 2016, 21, 623 CrossRef.
- Y. D. Min, C. H. Choi, H. Bark, H. Y. Son, H. H. Park, S. Lee, J. W. Park, E. K. Park, H. I. Shin and S. H. Kim, Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappa B and p38 MAPK in HMC-1 human mast cell line, Inflamm. Res., 2007, 56, 210–215 CrossRef CAS.
- C. H. Jung, J. Y. Lee, C. H. Cho and C. J. Kim, Anti-asthmatic action of quercetin and rutin in conscious guinea-pigs challenged with aerosolized ovalbumin, Arch. Pharmacal Res., 2007, 30, 1599–1607 CrossRef CAS.
- H. Moon, H. H. Choi, J. Y. Lee, H. J. Moon, S. S. Sim and C. J. Kim, Quercetin inhalation inhibits the asthmatic responses by exposure to aerosolized-ovalbumin in conscious guinea-pigs, Arch. Pharmacal Res., 2008, 31, 771–778 CrossRef CAS.
- Y. B. Shaik, M. L. Castellani, A. Perrella, F. Conti, V. Salini, S. Tete, B. Madhappan, J. Vecchiet, M. A. De Lutiis, A. Caraffa and G. Cerulli, Role of quercetin (a natural herbal compound) in allergy and inflammation, J. Biol. Regul. Homeostatic Agents, 2006, 20, 47–52 CAS.
- M. Y. Chung, H. S. Shin, D. W. Choi and D. H. Shon, Citrus Tachibana leaf extract mitigates symptoms of food allergy by inhibiting Th2-associated responses, J. Food Sci., 2016, 81, H1537–H1545 CrossRef CAS.
- J. H. Gong, D. Shin, S. Y. Han, J. L. Kim and Y. H. Kang, Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma, J. Nutr., 2012, 142, 47–56 CrossRef CAS.
- D. Shin, S. H. Park, Y. J. Choi, Y. H. Kim, L. D. Antika, N. U. Habibah, M. K. Kang and Y. H. Kang, Dietary compound kaempferol inhibits airway thickening induced by allergic reaction in a bovine serum albumin-induced model of asthma, Int. J. Mol. Sci., 2015, 16, 29980–29995 CrossRef CAS.
- H. A. Oh, N. R. Han, M. J. Kim, H. M. Kim and H. J. Jeong, Evaluation of the effect of kaempferol in a murine allergic rhinitis model, Eur. J. Pharmacol., 2013, 718, 48–56 CrossRef CAS.
- R. Takamiya, M. Murakami, M. Kajimura, N. Goda, N. Makino, Y. Takamiya, T. Yamaguchi, Y. Ishimura, N. Hozumi and M. Suematsu, Stabilization of mast cells by heme oxygenase-1: an anti-inflammatory role, Am. J. Physiol-Heart. C, 2002, 283, H861–H870 CrossRef CAS.
- E. Hirose, M. Matsushima, K. Takagi, Y. Ota, K. Ishigami, T. Hirayama, Y. Hayashi, T. Nakamura, N. Hashimoto, K. Imaizumi, K. Baba, Y. Hasegawa and T. Kawabe, Involvement of heme oxygenase-1 in kaempferol-induced anti-allergic actions in RBL-2H3 cells, Inflammation, 2009, 32, 99–108 CrossRef CAS.
- H. F. He, Research progress on theaflavins: efficacy, formation, and preparation, Food Nutr. Res., 2017, 61, 1344521 CrossRef.
- K. Yoshino, K. Yamazaki and M. Sano, Preventive effects of black tea theaflavins against mouse type IV allergy, J. Sci. Food Agric., 2010, 90, 1983–1987 CAS.
- T. Morikawa, S. Nakamura, Y. Kato, O. Muraoka, H. Matsuda and M. Yoshikawa, Bioactive saponins and glycosides. XXVIII. New triterpene saponins, foliatheasaponins Ir and V, from Tencha (the leaves of Camellia sinensis), Chem. Pharm. Bull., 2007, 55, 293–298 CrossRef CAS.
- N. Guo, T. T. Tong, N. Ren, Y. Y. Tu and B. Li, Saponins from seeds of genus Camellia: phytochemistry and bioactivity, Phytochemistry, 2018, 149, 42–55 CrossRef CAS.
- X. L. Yu and Y. He, Optimization of tea-leaf saponins water extraction and relationships between their contents and tea (Camellia sinensis) tree varieties, Food Sci. Nutr., 2018, 6, 1734–1740 CrossRef CAS.
- W. L. Hu, J. X. Liu, J. A. Ye, Y. M. Wu and Y. Q. Guo, Effect of tea saponin on rumen fermentation in vitro, Anim. Feed Sci. Technol., 2005, 120, 333–339 CrossRef CAS.
- X. J. Wu, L. Y. Jia, J. F. Wu, Y. W. Liu, H. Kang, X. B. Liu, P. Li, P. M. He, Y. Y. Tu and B. Li, Simultaneous determination and quantification of triterpene saponins from Camellia sinensis seeds using UPLC-PDA-QTOF-MS/MS, Molecules, 2019, 24, 3794 CrossRef CAS.
- J. Feng, Y. Chen, X. Liu and S. B. Liu, Efficient improvement of surface activity of tea saponin through gemini-like modification by straightforward esterification, Food Chem., 2015, 171, 272–279 CrossRef CAS.
- X. Y. Liu, L. Y. Cao, Q. Wang, X. Y. Zhang and X. X. Hu, Effect of tea saponin on phytoremediation of Cd and pyrene in contaminated soils by Lolium multiflorum, Environ. Sci. Pollut. Res., 2017, 24, 18946–18952 CrossRef CAS.
- M. Akagi, N. Fukuishi, T. Kan, Y. M. Sagesaka and R. Akagi, Anti-allergic effect of tea-leaf saponin (TLS) from tea leaves (Camellia sinensis var. sinensis), Biol. Pharm. Bull., 1997, 20, 565–567 CrossRef CAS.
- J. H. Choi, S. W. Jin, E. H. Han, B. H. Park, H. G. Kim, T. Khanal, Y. P. Hwang, M. T. Do, H. S. Lee, Y. C. Chung, H. S. Kim, T. C. Jeong and H. G. Jeong, Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-kappa B and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1, Phytomedicine, 2014, 21, 1053–1061 CrossRef CAS.
- W. Sipos, B. Reutterer, M. Frank, H. Unger, A. Grassauer, E. Prieschl-Grassauer and P. Doerfler, Escin inhibits type I allergic dermatitis in a novel porcine model, Int. Arch. Allergy Immunol., 2013, 161, 44–52 CrossRef CAS.
- H. Matsuda, S. Nakamura, K. Fujimoto, R. Moriuchi, Y. Kimura, N. Ikoma, Y. Hata, O. Muraoka and M. Yoshikawa, Medicinal Flowers. XXXI. Acylated oleanane-type triterpene saponins, sasanquasaponins I-V, with antiallergic activity from the flower buds of Camellia sasanqua, Chem. Pharm. Bull., 2010, 58, 1617–1621 CrossRef CAS.
- E. H. Han, L. H. Park, J. Y. Kim, Y. C. Chung and H. G. Jeong, Inhibitory mechanism of saponins derived from roots of Platycodon grandiflorum on anaphylactic reaction and IgE-mediated allergic response in mast cells, Food Chem. Toxicol., 2009, 47, 1069–1075 CrossRef CAS.
- T. Zhang, C. B. Yang, P. Rupa, B. Jiang and Y. Mine, Inhibitory effects of Quillaja saponin on IgE-mediated degranulation of rat basophilic leukemia RBL-2H3 Cells, J. Funct. Foods, 2012, 4, 864–871 CrossRef CAS.
- H. Chen, M. Zhang, Z. Qu and B. Xie, Compositional analysis and preliminary toxicological evaluation of a tea polysaccharide conjugate, J. Agric. Food Chem., 2007, 55, 2256–2260 CrossRef CAS.
- L. L. Du, Q. Y. Fu, L. P. Xiang, X. Q. Zheng, J. L. Lu, J. H. Ye, Q. S. Li, C. A. Polito and Y. R. Liang, Tea polysaccharides and their bioactivities, Molecules, 2016, 21, 1449 CrossRef.
- Y. L. Fan, W. H. Wang, Y. S. Hu, Q. Rong, A. G. Teng and A. J. Liu, Comparison of the roles of tea polysaccharides and polyphenols in growth inhibition of hepatoma H22 cells in mice. Advanced Materials Research: 4th International Conference on Manufacturing Science and Engineering, Dalian, China, March 2013 Search PubMed.
- J. J. Yang, B. Chen and Y. Gu, Pharmacological evaluation of tea polysaccharides with antioxidant activity in gastric cancer mice, Carbohydr. Polym., 2012, 90, 943–947 CrossRef CAS.
- K. Dejima, A. Ohshima, T. Yanai, R. Yamamoto, R. Takata and T. Yoshikawa, Effects of polysaccharide derived from black currant on relieving clinical symptoms of Japanese cedar pollinosis: a randomized double-blind, placebo-controlled trial, Biosci. Biotechnol. Biochem., 2007, 71, 3019–3025 CrossRef CAS.
- Q. M. Xie, J. F. Deng, Y. M. Deng, C. S. Shao, H. Zhang and C. K. Ke, Effects of Cryptoporus polysaccharide on rat allergic rhinitis associated with inhibiting eotaxin mRNA expression, J. Ethnopharmacol., 2006, 107, 424–430 CrossRef CAS.
- Q. M. Liu, Y. Yang, S. J. Maleki, M. Alcocer, S. S. Xu, C. L. Shi, M. J. Cao and G. M. Liu, Anti-food allergic activity of sulfated polysaccharide from Gracilaria lemaneiformis is dependent on immunosuppression and inhibition of p38 MAPK, J. Agric. Food Chem., 2016, 64, 4536–4544 CrossRef CAS.
- J. Han, B. Liu, Q. M. Liu, Y. F. Zhang, Y. X. Liu, H. Liu, M. J. Cao and G. M. Liu, Red algae sulfated polysaccharides effervescent tablets attenuated ovalbumin-induced anaphylaxis by upregulating regulatory T cells in mouse models, J. Agric. Food Chem., 2019, 67, 11911–11921 CrossRef CAS.
- K. Motoyama, Y. Tanida, A. Sakai, T. Higashi, S. Kaneko and H. Arima, Anti-allergic effects of novel sulfated polysaccharide sacran on mouse model of 2,4-dinitro-1-fluorobenzene-induced atopic dermatitis, Int. J. Biol. Macromol., 2018, 108, 112–118 CrossRef CAS.
- K. Na, E. Lkhagva-Yondon, M. Kim, Y. R. Lim, E. Shin, C. K. Lee and M. S. Jeon, Oral treatment with aloe polysaccharide ameliorates ovalbumin-induced atopic dermatitis by restoring tight junctions in skin, Scand. J. Immunol., 2020, 91, e12856 Search PubMed.
- Y. Tanino, T. Hashimoto, T. Ojima and M. Mizuno, F-fucoidan from Saccharina japonica is a novel inducer of galectin-9 and exhibits anti-allergic activity, J. Clin. Biochem. Nutr., 2016, 59, 25–30 CrossRef CAS.
- Y. P. Hwang, S. W. Jin, J. H. Choi, C. Y. Choi, H. G. Kim, S. J. Kim, Y. Kim, K. J. Lee, Y. C. Chung and H. G. Jeong, Inhibitory effects of L-theanine on airway inflammation in ovalbumin-induced allergic asthma, Food Chem. Toxicol., 2017, 99, 162–169 CrossRef CAS.
- N. H. Kim, H. J. Jeong and H. M. Kim, Theanine is a candidate amino acid for pharmacological stabilization of mast cells, Amino Acids, 2012, 42, 1609–1618 CrossRef CAS.
- U. Blank, C. Ra, L. Miller, K. White, H. Metzger and J. P. Kinet, Complete structure and expression in transfected cells of high-affinity IgE receptor, Nature, 1989, 337, 187–189 CrossRef CAS.
- Y. Fujimura, D. Umeda, S. Yano, M. Maeda-Yamamoto, K. Yamada and H. Tachibana, The 67 kDa laminin receptor as a primary determinant of anti-allergic effects of O-methylated EGCG, Biochem. Biophys. Res. Commun., 2007, 364, 79–85 CrossRef CAS.
- Y. Fujimura, H. Tachibana, R. Kumai and K. Yamada, A difference between epigallocatechin-3-gallate and epicatechin-3-gallate on anti-allergic effect is dependent on their distinct distribution to lipid rafts, Biofactors, 2004, 21, 133–135 CrossRef CAS.
- H. Tachibana, Y. Fujimura and K. Yamada, Tea polyphenol epigallocatechin-3-gallate associates with plasma membrane lipid rafts: lipid rafts mediate anti-allergic action of the catechin, Biofactors, 2004, 21, 383–385 CrossRef CAS.
- Y. Fujimura, H. Tachibana and K. Yamada, Lipid raft-associated catechin suppresses the Fc epsilon RI expression by inhibiting phosphorylation of the extracellular signal-regulated kinase1/2, Febs Lett., 2004, 556, 204–210 CrossRef CAS.
- M. Maeda-Yamamoto, N. Inagaki, J. Kitaura, T. Chikumoto, H. Kawahara, Y. Kawakami, M. Sano, T. Miyase, H. Tachibana, H. Nagai and T. Kawakami, O-methylated catechins from tea leaves inhibit multiple protein kinases in mast cells, J. Immunol., 2004, 172, 4486–4492 CrossRef CAS.
- M. J. Kim, I. G. Je, J. Song, X. Fei, S. Lee, H. Yang, W. Kang, Y. H. Jang, S. Y. Seo and S. H. Kim, SG-SP1 suppresses mast cell-mediated allergic inflammation via inhibition of Fc epsilon RI signaling, Front. Immunol., 2020, 11, 50 CrossRef CAS.
- J. Zhu and W. E. Paul, Heterogeneity and plasticity of T helper cells, Cell Res., 2010, 20, 4–12 CrossRef.
- H. Wu, B. Zhu, Y. Shimoishi, Y. Murata and Y. Nakamura, (-)-Epigallocatechin-3-gallate induces up-regulation of Th1 and Th2 cytokine genes in Jurkat T cells, Arch. Biochem. Biophys., 2009, 483, 99–105 CrossRef CAS.
- H. J. Park, C. M. Lee, I. D. Jung, J. S. Lee, Y. I. Jeong, J. H. Chang, S. H. Chun, M. J. Kim, I. W. Choi, S. C. Ahn, Y. K. Shin, S. R. Yeom and Y. M. Park, Quercetin regulates Th1/Th2 balance in a murine model of asthma, Int. Immunopharmacol., 2009, 9, 261–267 CrossRef CAS.
- J. Tian, H. Che, D. Ha, Y. Wei and S. Zheng, Characterization and anti-allergic effect of a polysaccharide from the flower buds of Lonicera japonica, Carbohydr. Polym., 2012, 90, 1642–1647 CrossRef CAS.
- C. Shi, T. Pan, M. Cao, Q. Liu, L. Zhang and G. Liu, Suppression of Th2 immune responses by the sulfated polysaccharide from Porphyra haitanensis in tropomyosin-sensitized mice, Int. Immunopharmacol., 2015, 24, 211–218 CrossRef CAS.
- T. Kawashima, K. Murakami, I. Nishimura, T. Nakano and A. Obata, A sulfated polysaccharide, fucoidan, enhances the immunomodulatory effects of lactic acid bacteria, Int. J. Mol. Med., 2012, 29, 447–453 CAS.
- T. Yoshida, A. Hirano, H. Wada, K. Takahashi and M. Hattori, Alginic acid oligosaccharide suppresses Th2 development and IgE production by inducing IL-12 production, Int. Arch. Allergy Immunol., 2004, 133, 239–247 CrossRef CAS.
- P. Salamon, N. G. Shoham, R. Gavrieli, B. Wolach and Y. A. Mekori, Human mast cells release interleukin-8 and induce neutrophil chemotaxis on contact with activated T cells, Allergy, 2005, 60, 1316–1319 CrossRef CAS.
- D. E. Levy and J. E. Darnell Jr., Stats: transcriptional control and biological impact, Nat. Rev. Mol. Cell Biol., 2002, 3, 651–662 CrossRef CAS.
- F. Gao, D. Wei, T. Bian, P. Xie, J. Zou, H. J. Mu, B. Zhang and X. Y. Zhou, Genistein attenuated allergic airway inflammation by modulating the transcription factors T-bet, GATA-3 and STAT-6 in a murine model of asthma, Pharmacology, 2012, 89, 229–236 CrossRef CAS.
- C. D. Liu, D. Weir, P. Busse, N. Yang, Z. W. Zhou, C. Emala and X. M. Li, The flavonoid 7,4-dihydroxyflavone inhibits MUC5AC gene expression, production, and secretion via regulation of NF-B, STAT6, and HDAC2, Phytother. Res., 2015, 29, 925–932 CrossRef CAS.
- T. Shirai, A. Sato and Y. Hara, Epigallocatechin gallate. The major causative agent of green tea-induced asthma, Chest, 1994, 106, 1801–1805 CrossRef CAS.
- T. Shirai, K. Reshad, A. Yoshitomi, K. Chida, H. Nakamura and M. Taniguchi, Green tea-induced asthma: relationship between immunological reactivity, specific and non-specific bronchial responsiveness, Clin. Exp. Allergy, 2003, 33, 1252–1255 CrossRef CAS.
- H. Otera, K. Tada, T. Sakurai, K. Hashimoto and A. Ikeda, Hypersensitivity pneumonitis associated with inhalation of catechin-rich green tea extracts, Respiration, 2011, 82, 388–392 CrossRef.
- S. S. Wu, J. A. Johnson, B. Peppers, H. Tcheurekdjian and R. Hostoffer, A case of green tea (Camellia sinensis) imbibement causing possible anaphylaxis, Ann. Allergy Asthma Immunol., 2017, 118, 747–748 CrossRef.
- R. Mourelle, F. Pineda, I. Ojeda, G. Rubio, A. Yago, D. Baquero and P. Ojeda, Anaphylaxis caused by green tea: a case report, J. Invest. Allergy Clin., 2018, 28, 343–344 CrossRef CAS.
- M. Monobe, K. Ema, F. Kato and M. Maeda-Yamamoto, Immunostimulating activity of a crude polysaccharide derived from green tea (Camellia sinensis) extract, J. Agric. Food Chem., 2008, 56, 1423–1427 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |