Antimicrobial peptides: mechanism of action and lipid-mediated synergistic interactions within membranes
Abstract
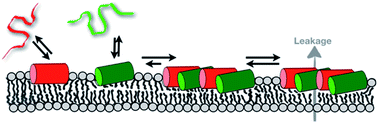
Biophysical and structural studies of peptide–lipid interactions, peptide topology and dynamics have changed our view of how antimicrobial peptides insert and interact with membranes. Clearly, both peptides and lipids are highly dynamic, and change and mutually adapt their conformation, membrane penetration and detailed morphology on a local and a global level. As a consequence, peptides and lipids can form a wide variety of supramolecular assemblies in which the more hydrophobic sequences preferentially, but not exclusively, adopt transmembrane alignments and have the potential to form oligomeric structures similar to those suggested by the transmembrane helical bundle model. In contrast, charged amphipathic sequences tend to stay intercalated at the membrane interface. Although the membranes are soft and can adapt, at increasing peptide density they cause pronounced disruptions of the phospholipid fatty acyl packing. At even higher local or global concentrations the peptides cause transient membrane openings, rupture and ultimately lysis. Interestingly, mixtures of peptides such as magainin 2 and PGLa, which are stored and secreted naturally as a cocktail, exhibit considerably enhanced antimicrobial activities when investigated together in antimicrobial assays and also in pore forming experiments applied to biophysical model systems. Our most recent investigations reveal that these peptides do not form stable complexes but act by specific lipid-mediated interactions and the nanoscale properties of phospholipid bilayers.
- This article is part of the themed collection: Peptide-membrane interactions


 Please wait while we load your content...
Please wait while we load your content...