Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of a rapid pre-concentration protocol and a magnetic beads-based RNA extraction method for SARS-CoV-2 detection in raw municipal wastewater†
A. L.
Parra-Guardado‡
 a,
C. L.
Sweeney‡
a,
C. L.
Sweeney‡
 a,
E. K.
Hayes
a,
E. K.
Hayes
 a,
B. F.
Trueman
a,
B. F.
Trueman
 a,
Y.
Huang
a,
Y.
Huang
 a,
R. C.
Jamieson
a,
R. C.
Jamieson
 a,
J. L.
Rand
a,
J. L.
Rand
 b,
G. A.
Gagnon
b,
G. A.
Gagnon
 a and
A. K.
Stoddart
a and
A. K.
Stoddart
 *a
*a
aCentre for Water Resources Studies, Department of Civil & Resource Engineering, Dalhousie University, 1360 Barrington Street, Halifax, Nova Scotia B3H 4R2, Canada. E-mail: amina.stoddart@dal.ca
bAcadia University, Ivan Curry School of Engineering, 15 University Avenue, Wolfville, Nova Scotia B4P 2R6, Canada
First published on 1st October 2021
Abstract
In this work, a rapid and simplified method for extracting SARS-CoV-2 RNA from whole wastewater using a magnetic beads-based protocol is presented. The described method involves the centrifugation of a 50-mL aliquot of raw wastewater influent for 5 min to obtain a 500-μL pellet, which is eluted with 2 mL of a Tween®20-based elution buffer; 1 mL of the elute is extracted for RNA using a direct magnetic bead-based extraction method. RNA recovery was examined in several bench-scale experiments using heat-inactivated SARS-CoV-2 (HI-SCV-2) spiked into raw wastewater to assess the effects of different solids pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratios, inhibition mitigation strategies, and varying levels of total suspended solids. When the method was assessed using an influent wastewater sample known to contain SARS-CoV-2, the viral signal was detected in all five biological replicates, whereas direct extraction of 1-mL aliquots of the raw wastewater resulted in a 40% viral detection rate. The experimental method limit of detection (MLOD) using HI-SCV-2 spiked into raw wastewater was 50 GU mL−1 with a 95% limit of detection. Using the described protocol, the presence of SARS-CoV-2 RNA was verified in wastewater collected from wastewater treatment facilities (WWTFs) in Atlantic Canada over a period of 15 weeks during the rise and fall of a COVID-19 outbreak. This method is effective and rapid and could provide potential application for laboratories with limited resources. Of approximately 50 methods that have been developed for measuring SARS-CoV-2 in wastewater referenced in the literature, this is the first to advance a robust magnetic beads-based RNA extraction technique from whole wastewater without extensive sample pre-treatment. The novel application of this method in the rapid extraction of SARS-CoV-2 RNA from municipal wastewater is an indispensable tool to potentially understand COVID-19 infection occurrence within communities.
buffer ratios, inhibition mitigation strategies, and varying levels of total suspended solids. When the method was assessed using an influent wastewater sample known to contain SARS-CoV-2, the viral signal was detected in all five biological replicates, whereas direct extraction of 1-mL aliquots of the raw wastewater resulted in a 40% viral detection rate. The experimental method limit of detection (MLOD) using HI-SCV-2 spiked into raw wastewater was 50 GU mL−1 with a 95% limit of detection. Using the described protocol, the presence of SARS-CoV-2 RNA was verified in wastewater collected from wastewater treatment facilities (WWTFs) in Atlantic Canada over a period of 15 weeks during the rise and fall of a COVID-19 outbreak. This method is effective and rapid and could provide potential application for laboratories with limited resources. Of approximately 50 methods that have been developed for measuring SARS-CoV-2 in wastewater referenced in the literature, this is the first to advance a robust magnetic beads-based RNA extraction technique from whole wastewater without extensive sample pre-treatment. The novel application of this method in the rapid extraction of SARS-CoV-2 RNA from municipal wastewater is an indispensable tool to potentially understand COVID-19 infection occurrence within communities.
Water impactWastewater surveillance is currently being explored around the world as a tool for monitoring SARS-CoV-2 to better understand COVID-19 prevalence in our communities. This study provides a simple and transferable method that allows rapid identification of SARS-CoV-2 RNA in whole wastewater without the need for extensive sample pre-treatment, thus offering advantageous application for laboratories that may have limited resources. |
1. Introduction
The coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to spread worldwide and has claimed the lives of over four million people as of July 2021.1 SARS-CoV-2 is an enveloped, positively charged, single-stranded ribonucleic acid (RNA) virus (60 to 140 nm) of the beta coronavirus genus.2,3 Although COVID-19 is characterized as a respiratory illness, many infected patients also present with gastrointestinal distress.4 Data from faecal and respiratory specimen analyses suggest that SARS-CoV-2 virus particles survive longer in the gastrointestinal tract than in the respiratory tract.2 Moreover, SARS-CoV-2 RNA has been detected in feces of both symptomatic and asymptomatic infected individuals.5 As such, the monitoring of wastewater for SARS-CoV-2 occurrence to investigate the prevalence of COVID-19 infections in a given population is an approach that has been rapidly adopted worldwide as the pandemic continues to spread.6–13Reliable tests for SARS-CoV-2 infections target the viral genome through quantitative reverse transcription PCR (RT-qPCR).14,15 The use of RT-qPCR led to the first report of SARS-CoV-2 RNA detection in untreated wastewater in a proof-of-concept study that demonstrated the applicability of wastewater surveillance for COVID-19 as a tool to understand infection rates within communities.5 To effectively monitor SARS-CoV-2 occurrence in wastewater, the development of an efficient and reliable methodology for viral fragment recovery from this complex matrix is paramount.
Several methods have been reported for measuring SARS-CoV-2 RNA in raw wastewater and are generally comprised of a combination of procedures for sample pre-treatment, concentration, extraction, and molecular analysis.16–18 For most techniques, SARS-CoV-2 analysis in wastewater requires pre-concentration of the sample. The most common methods of virus concentration in wastewater are ultrafiltration, polyethylene glycol (PEG) precipitation, and ultracentrifugation.5,15,19–23 Other methods include skimmed milk flocculation,24 glass wool filtration,25 and monolithic affinity filtration.26 The selection of a reliable and effective virus concentration method has a major impact on detection sensitivity, but many RNA concentration methods are cost-ineffective, time-consuming, and labour-intensive.19 Moreover, information on the recovery efficiencies of methods for measuring SARS-CoV-2 in wastewater is limited,19,27 as most studies use surrogates (e.g., murine hepatitis virus (MHV), MS2 (an F-specific RNA phage), and other coronaviruses), to study recovery efficiency.28
In the last decade, several studies have demonstrated the use of magnetic beads to extract nucleic acids from a variety of matrices, including serum,29 urine,30 sputum,31 whole blood,32 tissue,33 and wastewater.34,35 While conventional approaches involve both pre-concentration and extraction using commercially available kits, this work proposes a novel methodology for the extraction of SARS-CoV-2 RNA from wastewater using magnetic beads without the need for extensive sample preparation. The advantages of RNA extraction via magnetic beads are that the procedure is rapid, simple to perform, and does not require the use of specialized equipment. The sensitivity of the direct magnetic beads-based method was further increased by adding a simple step to recover SARS-CoV-2 from the solids fraction of a 50-mL wastewater sample using a buffer containing the surfactant, Tween®20. Indeed, surfactant compounds are commonly used in virology to separate viral aggregates and enhance the release of the viral particles attached to solids by reducing the hydrophobic interactions with the adsorbent.36,37 Recently, the use of a Tween®20-based elution buffer was reported in the elution of SARS-CoV-2 from passive sampling materials.38 This pre-extraction approach has been adapted to municipal wastewater samples which allows processing of larger volumes, potentially bridging the gap between pre-concentration sample volumes and traditional RNA extraction sample volumes. Such advantages allow for greater diversity in monitoring applications, which is valuable for laboratories with limited resources. This study advances the application of magnetic bead extraction approaches through the development of a new method for a rapid and simple extraction of SARS-CoV-2 RNA from whole wastewater samples.
2. Materials and methods
2.1 Reagents
Deionized (DI) water was produced by a Milli-Q system (Reference A+, Millipore) and had a total organic carbon (TOC) concentration <5 μg L−1 and a resistivity of 18.2 MΩ cm−1. Dispersive solid-phase extraction (dSPE) salts (SELECTRASORB™ endcapped octadecyl (C18) and ENVIRO-CLEAN primary secondary amine (PSA)) were obtained from Chromatographic Specialties (Mississauga, ON, CA). An elution buffer was made by adding 75 μL of Tween®20 and 250 μL of a 0.1 M Tris-HCl intermediate (both sourced from Sigma Aldrich, Ottawa, ON, CA) to DI water for a total volume of 100 mL. Ethanol (EtOH) and acetonitrile (ACN) were purchased from Fisher Scientific (Ottawa, ON, CA). Magnetic binding beads (50 g L−1), RNA isolation kits, and SARS-CoV-2 assay kits were obtained from LuminUltra Technologies Ltd (Fredericton, NB, CA). Bovine serum albumin (BSA), used to reduce inhibition in RT-qPCR reactions, was purchased from Alfa Aesar by Thermo Fisher Scientific (Tewksbury, MA, US) to make a 1 mg mL−1 BSA solution (10 mg lyophilized BSA in 10 mL DI water).Two viral surrogates were used for the development and validation of the methods described in this work. The surrogates were selected based on their availability and suitability (e.g., required sample concentration) for the different experiments. Accuplex SARS-CoV-2 positive reference material (ASCV-2) containing non-replicative recombinant virus particles with sequences from the SARS-CoV-2 genome (ORF1a, RdRp, E gene, and N gene) was purchased at ∼5000 copies per mL from Seracare Life Sciences Inc (Milford, MA, USA). Heat inactivated SARS-CoV-2 (HI-SCV-2) (ATCC® VR-1986HK™) was obtained at a concentration of ∼3.75 × 105 copies per mL from American Type Culture Collection (Virginia, USA). The HI-SCV-2 is a non-replicative preparation of the SARS-CoV-2 strain 2019-nCoV/USA-WA1/2020 that has been inactivated by incubation at 65 °C for 30 min.
2.2 Wastewater sample collection for method development
Influent 24-h composite wastewater samples were obtained from a wastewater treatment facility (WWTF) in a municipality located in Atlantic Canada during a period of low COVID-19 prevalence. Wastewater samples were collected in volumes of at least 250 mL and transported to Dalhousie University on ice. For preliminary RNA extraction method development, the wastewater matrix was spiked with Accuplex SARS-CoV-2 Positive Reference Material to a final theoretical concentration of 1 × 103 genomic units per mL (GU mL−1). The spiked sample was mixed thoroughly and incubated at 4 °C for 30 min prior to RNA extraction. For subsequent bench-scale experiments carried out to improve the sensitivity of the RNA extraction method, 50-mL aliquots of wastewater were spiked to 1 × 103 GU mL−1 with HI-SCV-2 RNA and stirred continuously at room temperature for 60 min prior to sample processing and analysis. Additional water quality parameters measured (e.g., total suspended solids (TSS), temperature, flow, biological oxygen demand (BOD), and ammoniacal nitrogen (NH 3-N)) are listed in Table S1 of the ESI.†2.3 Determination of preliminary RNA extraction conditions
A screening statistical design was used to select the preliminary conditions for the direct magnetic beads-based RNA extraction method. Information regarding the statistical design and evaluation of the method recovery efficiency using the ASCV-2 RNA are detailed in the ESI.† All RNA extractions were performed with reagents provided by LuminUltra Technologies Ltd (Fredericton, NB, CA) according to the manufacturer's instructions. Briefly, in a 15-mL centrifuge tube, 6 mL of a lysis buffer concentrate and 250 μL of Lysis Supplement 1A were added to 1 mL of wastewater sample. The mixture was gently inverted five times and immediately incubated at 30 °C for 10 min. After incubation, 3.5 mL EtOH was added to the lysed sample; the tube was gently inverted five times to mix thoroughly and then spiked with 40 μL of magnetic beads. The mixture was gently inverted five times and incubated again at 30 °C for 10 min. The magnetic beads were precipitated by applying a magnet, and the supernatant was discarded.The magnetic beads were washed three times with 1 mL of wash I solution. A second wash step using 1 mL of wash II solution was carried out twice, followed by a final wash with 1 mL of EtOH. For each wash, the magnetic beads were swirled 10 times to mix, and the supernatant was discarded after magnet precipitation of the beads (a free magnet or magnetic stand may be utilized). Once the beads were washed, excess EtOH was pipetted from the tubes, and the samples were placed at room temperature with the caps off for at least 2 min to evaporate residual EtOH. Then, 50 μL of elution buffer (preheated to 60 °C) was added to the magnetic beads. The tube was swirled 10 times and incubated at 60 °C for 5 min. Finally, the magnet was applied to ensure separation, and the elution buffer was collected into a sterile tube for analysis. The experimental setup for the magnetic beads-based RNA extraction method is shown in ESI† Fig. S2.
2.4 Enhanced extraction protocol to increase the analytical sensitivity of the direct RNA extraction method
To increase the analytical sensitivity of the direct magnetic bead extraction protocol, experiments were carried out with a larger volume of wastewater that was concentrated prior to RNA extraction. For bench-scale experiments, 500 mL of an influent wastewater sample (that tested negative for SARS-CoV-2 RNA using the optimized RNA extraction method) was spiked to a theoretical concentration of 1 × 103 GU mL−1 with HI-SCV-2 and mixed continuously on a stir plate at room temperature for 1 h. Three biological replicates of 50 mL were centrifuged for 5 min at 5000 rpm and the supernatant discarded, leaving approximately 500 μL of solids-rich sample. The pellet was resuspended in 2 mL of the Tween®20-based buffer. The sample tube was shaken by hand for 5 s and allowed to sit at room temperature for 5 min to allow most of the solids to settle. From the uppermost layer of the sample, 1 mL was transferred to a new tube for RNA extraction using the magnetic bead-based protocol. Three 1-mL biological replicates were also collected from the spiked 500-mL wastewater sample and directly extracted for RNA using the magnetic bead-based protocol to compare RNA recovery efficiency as an indicator of increased analytical sensitivity.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 pellet
4 pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratio, respectively). Sample processing was carried out as described in section 2.4 Enhanced extraction protocol to increase the analytical sensitivity of the direct RNA extraction method prior to RNA extraction using magnetic beads; RNA was analyzed via RT-qPCR.
buffer ratio, respectively). Sample processing was carried out as described in section 2.4 Enhanced extraction protocol to increase the analytical sensitivity of the direct RNA extraction method prior to RNA extraction using magnetic beads; RNA was analyzed via RT-qPCR.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with DI water for a total volume of 1 mL, which was used for RNA extraction with the magnetic bead-based protocol. For the second group of samples, the 500-μL pellet was resuspended in 2 mL of the buffer and centrifuged for 1 min at 5000 rpm. A 1-mL aliquot of the supernatant was extracted for RNA. For the dSPE clean-up group, a salt mixture containing 135 mg of both PSA and C18, was added to each sample prior to RNA extraction. The samples were vortexed for 10 s, shaken by hand for 1 min, and centrifuged at room temperature for 5 min at 5000 rpm. Without disturbing the dSPE salts, 1 mL of the supernatant was transferred to a clean tube and used for RNA extraction. For each experimental group, three biological replicates were processed and analyzed via RT-qPCR following RNA extraction.
1 with DI water for a total volume of 1 mL, which was used for RNA extraction with the magnetic bead-based protocol. For the second group of samples, the 500-μL pellet was resuspended in 2 mL of the buffer and centrifuged for 1 min at 5000 rpm. A 1-mL aliquot of the supernatant was extracted for RNA. For the dSPE clean-up group, a salt mixture containing 135 mg of both PSA and C18, was added to each sample prior to RNA extraction. The samples were vortexed for 10 s, shaken by hand for 1 min, and centrifuged at room temperature for 5 min at 5000 rpm. Without disturbing the dSPE salts, 1 mL of the supernatant was transferred to a clean tube and used for RNA extraction. For each experimental group, three biological replicates were processed and analyzed via RT-qPCR following RNA extraction.
2.5 Assessing method performance in municipal wastewater with varying levels of total suspended solids
To assess the impact of total suspended solids (TSS) on the recovery of HI-SCV-2 RNA from wastewater using the enhanced extraction protocol, three different wastewater matrices with varying levels of total suspended solid concentrations were selected (ESI† Fig. S1). TSS concentrations were measured by filtering each wastewater sample through a standard glass fiber filter and drying the residue retained on the filter at 103 to 105 °C for 12 h.40 The increase in the filter mass represented the amount of suspended solids in each sample. TSS concentrations of the three different wastewater samples used in this study were measured as 77, 175, and 332 mg L−1. Each of the three wastewater matrices was spiked to a theoretical concentration of 1 × 103 GU mL−1 with HI-SCV-2 and analyzed in biological triplicates.2.6 Method limit of detection (MLOD) determination
The method limit of detection (MLOD) of the enhanced extraction protocol was evaluated using HI-SCV-2 spiked into raw influent wastewater. Prior to spiking, the wastewater matrix was analyzed for SARS-CoV-2 RNA to ensure background levels of the virus were not detected. After seeding, the wastewater was mixed continuously on a stir plate at room temperature for 1 h. HI-SCV-2 RNA was spiked into the wastewater samples at six different theoretical concentrations ranging from 1 × 101 to 1 × 103 GU mL−1. For each concentration level, six biological replicates were processed using the enhanced extraction protocol and analyzed by RT-qPCR. To mitigate inhibition, extracted RNA samples that resulted in non-detects were diluted and analyzed via RT-qPCR. The fraction of replicates resulting in a positive detection was related to the corresponding spiked virus concentration via a logistic regression model.41 The concentration yielding positive detections in an estimated 95% of replicates was designated as the MLOD.2.7 RT-qPCR assay
RNA samples were processed by RT-qPCR on a GeneCount® Q16 instrument (LuminUltra Technologies Ltd, Fredericton, CA). The sequences for primers and probes published by US CDC used in this study are shown in the ESI† (Table S3).42 For the detection of SARS-CoV-2, the RT-qPCR amplifications were performed in 20-μL reactions using the GeneCount SARS-CoV-2 Screening kit (LuminUltra Technologies Ltd, Fredericton, CA), which contained 15 μL of Master Mix (667 nM of forward primer, 667 nM of reverse primer and 167 nM of probe) and 5 μL of template RNA. Thermal cycling reactions were carried out as follows: a pre-denaturation step at 55 °C for 10 min followed by a second pre-denaturation step at 95 °C for 1 min; 45 cycles of 95 °C for 10 s and 55 °C for 45 s; and a final hold step at 50 °C for 1 min. Due to the limited capacity of the GeneCount® Q16 instrument (16 wells), all RT-qPCR analyses included at least one no-template control (NTC) containing molecular grade water. For bench-scale experiments, samples were analyzed in a least three replicates. RNA samples for the wastewater monitoring program were assayed without replication. For samples that resulted in non-detects, RNA dilution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) using a 1 mg mL−1 BSA solution was utilized to help alleviate inhibition and exclude the occurrence of false-negative results. Reactions were considered positive when cycle threshold (Ct) values were below 40, and the levels of SARS-CoV-2 in wastewater were reported in GU mL−1. The upper Ct value detection threshold for the RT-qPCR was 40 cycles corresponding to 1.4 copies per reaction.
10) using a 1 mg mL−1 BSA solution was utilized to help alleviate inhibition and exclude the occurrence of false-negative results. Reactions were considered positive when cycle threshold (Ct) values were below 40, and the levels of SARS-CoV-2 in wastewater were reported in GU mL−1. The upper Ct value detection threshold for the RT-qPCR was 40 cycles corresponding to 1.4 copies per reaction.
The LuminUltra Q-16 qPCR system utilizes a master standard curve incorporated into the software based on the average of six standard curves. The standard curves ranging from 1 × 104 to 1 × 101 copies per reaction (each 10-fold dilution points run in duplicate) were constructed using SARS-CoV-2 RNA reference material (ZeptoMetrix, Buffalo, USA) to convert Ct values to GU per reaction. The R2 value of the standard curve utilized in this work is 0.948 and the efficiency is ∼85%. Due to factors like lyophilization, the qPCR efficiency may be impacted. However, the Q-16 assay has shown consistent sensitivity and reproducible findings that would indicate its effectiveness.
2.8 Comparing the performance of the direct and enhanced RNA extraction protocols using wastewater samples known to contain SARS-CoV-2
The performance of the direct and enhanced RNA extraction protocols was evaluated with influent composite wastewater known to contain of SARS-CoV-2 collected from a region with high COVID-19 prevalence in Ontario. A 24-h composite influent wastewater sample was collected at a WWTF into an HDPE bottle and shipped to Dalhousie University on ice. TSS was measured as 103.7 mg L−1. The sample was stored at 4 °C prior to RNA extraction. Five 1-mL biological replicates were processed using the direct RNA extraction protocol. Five 50-mL aliquots were collected and processed using the pre-concentration step in combination with the magnetic bead extraction protocol. Following RNA extraction, all samples were analyzed via RT-qPCR.2.9 Application of the enhanced RNA extraction protocol for the detection and quantification of SARS-CoV-2 in wastewater
The improved magnetic beads-based RNA extraction method which includes the pre-concentration step was evaluated for the detection of SARS-CoV-2 using raw wastewater samples from four locations with low COVID-19 prevalence in Atlantic Canada. Influent 24-h composite wastewater samples were collected from WWTFs in four communities in Nova Scotia, Canada over a 15-week sampling period, during which the province experienced a third wave of COVID-19 cases. The WWTFs located in Halifax, Dartmouth, Mill Cove, and Eastern Passage serve estimated populations of 117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 64
000, 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 55
000, 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 35
000, and 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 211, respectively.43 The four WWTFs are part of a combined sewer system that collects the discharged wastewater from households as well as commercial and industrial buildings located in the service area. The mean influent flow rate for the Halifax, Dartmouth, Mill Cove, and Eastern Passage WWTFs is approximately 108
211, respectively.43 The four WWTFs are part of a combined sewer system that collects the discharged wastewater from households as well as commercial and industrial buildings located in the service area. The mean influent flow rate for the Halifax, Dartmouth, Mill Cove, and Eastern Passage WWTFs is approximately 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 58
000, 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 33
000, 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 30
000, and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 per day, respectively. From each WWTF, samples were collected at least three times per week in volumes of at least 250 mL and transported to Dalhousie University on ice. Within 24 h, a 50-mL aliquot from each sample was processed using the enhanced extraction protocol and analyzed via RT-qPCR.
000 m3 per day, respectively. From each WWTF, samples were collected at least three times per week in volumes of at least 250 mL and transported to Dalhousie University on ice. Within 24 h, a 50-mL aliquot from each sample was processed using the enhanced extraction protocol and analyzed via RT-qPCR.
All wastewater used in this monitoring program were collected from WWTFs located in the central zone, one of the Nova Scotia Health Authority's four management zones. Census data from 2016 show that the population in the central zone was 424![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 037.43 Daily new and active COVID-19 case data for the central zone were obtained by Health Canada.44 At the beginning of this sampling period (April 1st, 2021), there were 19 active COVID-19 cases in the central zone. The number of active cases peaked at 1415 by May 10th, 2021, and gradually decreased to less than 100 at the end of the study period. Moreover, a vaccination program in the province began in mid-December 2020. At the time of this publication, approximately 60% of the province's population had received one dose of a COVID-19 vaccine and approximately 5% had received both doses.
037.43 Daily new and active COVID-19 case data for the central zone were obtained by Health Canada.44 At the beginning of this sampling period (April 1st, 2021), there were 19 active COVID-19 cases in the central zone. The number of active cases peaked at 1415 by May 10th, 2021, and gradually decreased to less than 100 at the end of the study period. Moreover, a vaccination program in the province began in mid-December 2020. At the time of this publication, approximately 60% of the province's population had received one dose of a COVID-19 vaccine and approximately 5% had received both doses.
2.10 Quantitative analysis of HI-SCV-2 and SARS-CoV-2 RNA concentrations for the enhanced RNA extraction protocol
RNA concentrations that reflect the amount of viral RNA recovered from the original 50-mL wastewater sample (total genomic units per mL) were calculated using eqn (1), where sample concentration (GU mL−1) refers to the RNA copies quantified by RT-qPCR in the 1-mL sample taken from the Tween®20-based buffer eluate for RNA extraction. The buffer volume (mL) is the volume of the Tween®20-based buffer added to the solids-rich wastewater pellet for samples processed using the enhanced extraction protocol. Recovery of HI-SCV-2 was calculated using eqn (2), where the spiked concentration (GU mL−1) is the theoretical viral RNA gene units of HI-SCV-2 seeded in the 50-mL wastewater aliquot. | (1) |
 | (2) |
2.11 Quality control
To minimize contamination, RNA extraction and RT-qPCR assays were carried out in separate laboratories and were performed in a Thermo Scientific 1300 Series A2 biosafety cabinet. A method blank consisting of 1 mL DI water was included during RNA extraction to account for any contamination during sample processing. All extraction blanks evaluated during the method development stages and monitoring program did not present detectable levels of SARS-CoV-2. Extracted RNA samples were stored at −76 °C and analyzed via RT-qPCR within the same day of extraction. Standards outlined in minimum information for publication of quantitative real-time PCR experiments (MIQE) guidelines45 and environmental microbiology minimum information (EMMI) guidelines46 were consulted for evaluating RT-qPCR experiments. For instance, all RT-qPCR assays contained passing NTCs. The RT-qPCR master mix contains the MS-2 bacteriophage as an internal amplification control (IAC) that serves as an additional indicator for results validation. SARS-CoV-2 results were accepted if the IAC for each individual sample passed, which was verified by observing the amplification curve plot amplifying properly with Ct values ranging from 20 to 36. In the case of IAC failure, results for samples that were below the limit of detection would be considered invalid and the samples would be re-analyzed by RT-qPCR.2.12 Statistical analysis
All analyses for method development were performed using at least three biological replicates. Results were expressed as mean RNA concentrations ± standard deviation (eqn (1)). To evaluate the statistical significance between mean RNA concentrations obtained from experiments carried out under different conditions, two-sample t-tests on mean Ct values (two-tailed, α = 0.05; 95% confidence level) were carried out as per MIQE guidelines.45 For statistical analysis of experiments involving spiked viral surrogates, replicates that resulted in non-detects were assigned a Ct value of 40, as per Goni et al. (2009).47 All statistical analyses were performed using Microsoft® Excel for Mac version 16.50 (2021).48 The MLOD was defined here as the analyte concentration that yielded a positive detection in 95% of replicates. It was estimated using a logistic regression model, with the fraction of replicates yielding detections as the response and known spiked HI-SCV-2 concentrations in a series of test wastewater samples as the predictor. This approach is detailed in Forootan et al. (2017),41 and the logistic regression curve is described by eqn (3), where ŷ is the prediction and the![[small beta, Greek, circumflex]](https://www.rsc.org/images/entities/i_char_e114.gif) i are model parameters. The model was fit via iteratively reweighted least squares using the glm.fit() function in R.48 All statistical analyses were performed using R v. 3.6.2 (and the tidyverse family of packages).48,49
i are model parameters. The model was fit via iteratively reweighted least squares using the glm.fit() function in R.48 All statistical analyses were performed using R v. 3.6.2 (and the tidyverse family of packages).48,49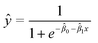 | (3) |
3. Results and discussion
3.1 Assessing the performance of the enhanced RNA extraction protocol in preliminary bench-scale experiments
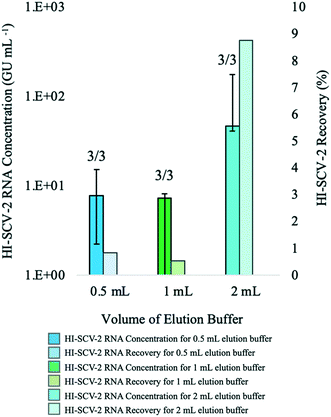
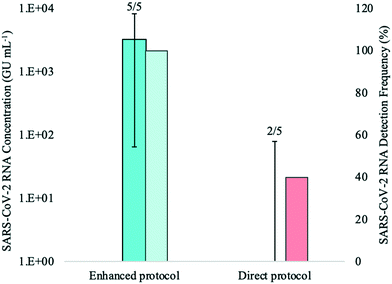
In the first of this series of preliminary experiments to assess the increase in sensitivity of the direct RNA extraction method (ESI†), influent 24-h composite wastewater samples were spiked with HI-SCV-2 to 1 × 103 GU mL−1 (theoretical concentration) and analyzed using the magnetic bead-based RNA extraction protocol with and without the pre-concentration step in biological triplicates to determine whether RNA detection rate and recovery were higher using the enhanced extraction protocol. HI-SCV-2 was detected in only one of three replicates at a concentration of 3.02 × 102 GU mL−1 using the direct extraction protocol. In contrast, HI-SCV-2 was detected in all three biological replicates processed using the enhanced extraction protocol with a mean RNA concentration of 8.77 × 101 ± 7.65 × 101 GU mL−1 (mean HI-SCV-2 RNA recovery = 8.8%). Comparisons of mean recovery between methods were difficult to make as viral RNA was detected in only one of three replicates for the direct extraction method. These preliminary results demonstrate that the pre-concentration step in combination with the magnetic beads-based RNA extraction method (3/3 detections) was more effective than the direct extraction method (1/3 detections) in detecting spiked viral HI-SCV-2 in the influent wastewater samples. This improvement in method performance was anticipated, as the enhanced extraction protocol (50 mL initial wastewater volume and 2 mL Tween®20 elution buffer) offers a 25-fold concentration factor over the direct extraction method and incorporates pre-treatment of the solids-rich fraction of wastewater to which SARS-CoV-2 partitions.Despite the 100% detection rate for the enhanced extraction protocol in this experiment, the co-concentration of inhibitors present in the wastewater sample during the added centrifugation step may impact RNA recovery through qPCR inhibition. There is an intrinsic trade-off between recovery and initial sample volume; while the enhanced extraction protocol offers an increase in sensitivity with its 25-fold concentration factor, it produces RNA extracts susceptible to RT-qPCR amplification inhibition, which may result in false-negative results or inaccurate quantitation. In contrast, while the direct extraction method is less prone to inhibitory effects, it has reduced sensitivity due to its limited volume. As with any method, dilution of RNA extracted from wastewater may increase the viral signal, as it reduces the concentration and impact of inhibitors that may be present in the complex sample.50 The effect of RNA dilution on maximum viral recovery was assessed in subsequent experiments.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 pellet
4 pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratios (Fig. 1). For the 1
buffer ratios (Fig. 1). For the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pellet
1 pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratio, HI-SCV-2 was detected in all three biological replicates at a mean RNA concentration of 8.38 × 100 ± 6.45 × 100 GU mL−1 (mean RNA recovery <1%). At a pellet
buffer ratio, HI-SCV-2 was detected in all three biological replicates at a mean RNA concentration of 8.38 × 100 ± 6.45 × 100 GU mL−1 (mean RNA recovery <1%). At a pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratio of 1
buffer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, HI-SCV-2 was detected in all three biological replicates (one of which required a 1
2, HI-SCV-2 was detected in all three biological replicates (one of which required a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BSA dilution) at a mean RNA concentration of 5.28 × 100 ± 4.25 × 100 GU mL−1. As with the 1
1 BSA dilution) at a mean RNA concentration of 5.28 × 100 ± 4.25 × 100 GU mL−1. As with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pellet
1 pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratio, mean RNA recovery for this experimental group was less than 1%. Although there were no significant differences in Ct values between the 1
buffer ratio, mean RNA recovery for this experimental group was less than 1%. Although there were no significant differences in Ct values between the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratios (p = 0.286) and the 1
4 ratios (p = 0.286) and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratios (p = 0.228), all subsequent analyses were carried out using 2 mL buffer with a 500-μL pellet, as the highest HI-SCV-2 RNA concentration (8.77 × 101 ± 7.65 × 101 GU mL−1) was achieved with the 1
4 ratios (p = 0.228), all subsequent analyses were carried out using 2 mL buffer with a 500-μL pellet, as the highest HI-SCV-2 RNA concentration (8.77 × 101 ± 7.65 × 101 GU mL−1) was achieved with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 pellet
4 pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratio.
buffer ratio.
In a recent study comparing approaches to quantifying SARS-CoV-2 in wastewater using RT-qPCR, it was reported that some viral surrogates were generally detected in supernatants rather than solids phases, indicating that they may not partition to solids as SARS-CoV-2 does in real samples.51 If a majority of the surrogate used in this study remains in the liquid fraction, a significant quantity of HI-SCV-2 spiked into these samples would be discarded in the supernatant following the centrifugation step. At the lowest buffer volume, the remaining HI-SCV-2 in the pellet would be less diluted but most impacted by inhibitory compounds in the sample. In contrast, the larger buffer volume would result in a lower viral recovery due to greater dilution of the pellet but would be less susceptible to inhibition. It is also possible that the lower viral RNA recoveries in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 pellet
2 pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratios were caused by a reduction in viral elution from the solids-rich pellet. The buffer volume in these experimental groups may have been too low to ensure sufficient saturation, mixing, and elution of the pellet. Although increasing the elution buffer volume to a 1
buffer ratios were caused by a reduction in viral elution from the solids-rich pellet. The buffer volume in these experimental groups may have been too low to ensure sufficient saturation, mixing, and elution of the pellet. Although increasing the elution buffer volume to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 pellet
4 pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratio showed highest HI-SCV-2 recoveries, greater elution buffer volumes were not explored, as adding larger volumes of the buffer would further dilute viral RNA in the sample. Depending on wastewater sample characteristics, the pellet volume and composition is expected to vary among different wastewater matrices and could impact SARS-CoV-2 RNA recovery.
buffer ratio showed highest HI-SCV-2 recoveries, greater elution buffer volumes were not explored, as adding larger volumes of the buffer would further dilute viral RNA in the sample. Depending on wastewater sample characteristics, the pellet volume and composition is expected to vary among different wastewater matrices and could impact SARS-CoV-2 RNA recovery.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
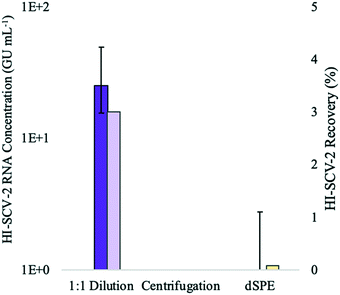
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio prior to the addition of the buffer in the enhanced extraction protocol resulted in both the highest detection rate (3/3 detections) and recovery (∼3%). This marked decrease in recovery compared to that achieved without dilution in preliminary experiments (∼9%) is likely attributed to the dilution of the solids-rich pellet prior to RNA extraction. When using dilution as a strategy to mitigate inhibition, dilution after RNA extraction allows amplification of the viral signal more effectively by harnessing the sensitivity of the qPCR, as the concentration of inhibitors relative to nucleic acid is reduced.50
1 ratio prior to the addition of the buffer in the enhanced extraction protocol resulted in both the highest detection rate (3/3 detections) and recovery (∼3%). This marked decrease in recovery compared to that achieved without dilution in preliminary experiments (∼9%) is likely attributed to the dilution of the solids-rich pellet prior to RNA extraction. When using dilution as a strategy to mitigate inhibition, dilution after RNA extraction allows amplification of the viral signal more effectively by harnessing the sensitivity of the qPCR, as the concentration of inhibitors relative to nucleic acid is reduced.50
For the second treatment group, none of the biological triplicates that were centrifuged for 1 min at 5000 rpm after the addition of the buffer in the enhanced extraction protocol resulted in detection of HI-SCV-2. This may be a result of centrifugal forces pulling viral particles adsorbed to the solids down into the pellet, which was not incorporated into the sample aliquot used for RNA extraction. Generally, ultracentrifugation is required for sufficient gravitational force to pellet free viruses,52,53 and the time and force required to sediment free virus from water samples are much higher than those used in this study. Therefore, it is unlikely that a significant proportion of the surrogate in its free form was left behind in the pellet after centrifugation.
In the experimental group that tested the clean-up of the 1-mL elute prior to RNA extraction using a dSPE salt mixture, only one of three replicates resulted in a positive detection for HI-SCV-2 RNA. Although dSPE (using PSA and C18) has been successful in the clean-up of complex wastewater matrices for the analysis of antibiotic residues54 and illicit drugs,55 PSA may have interfered with the HI-SCV-2 surrogate. For example, murine norovirus has been found to adhere to beads coated with PSA, and the degree of adhesion varied with pH.56 Moreover, the use of dSPE salts in combination with vortexing may have caused physical and/or chemical disruption of the viral envelope,57 resulting in premature lysis and subsequent release of HI-SCV-2 RNA. The release of viral RNA makes it vulnerable to RNases readily present in the sample and shearing forces caused by vortexing and centrifugation. Therefore, it is reasonable to assume that the low recovery observed in the dSPE experiment may be due to the degradation of viral RNA in samples following premature cell lysis.
In general, pre-extraction methods for combatting inhibition are favoured based on the rationale that the viral capsid of SARS-CoV-2 will protect the nucleic acids while removing unwanted inhibitory compounds.27 However, the results presented in this experiment suggest the contrary, as pre-extraction sample dilution yielded a lower mean HI-SCV-2 RNA recovery while centrifugation after the addition of the elution buffer and dSPE clean-up resulted in fewer RNA detections and diminished recoveries. Another method of assessing inhibition is the addition of a pre-extraction process control. However, Kantor et al. (2021) discontinued the use of a foreign spike-in RNA control for analyzing wastewater samples when inhibition of the control did not correlate with the inhibition of the SARS-CoV-2 N1 target; as such inhibition was subsequently accounted for by running undiluted and five-fold diluted RNA for all samples.58 From this point forward, the mitigation of inhibitory compounds was carried out using post-extract RNA dilutions with BSA for RT-qPCR reactions.
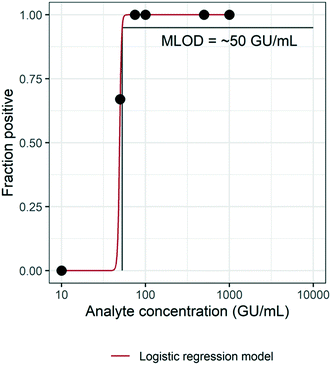
3.2 Method limit of detection (MLOD)
The MLOD was evaluated by spiking HI-SCV-2 RNA in raw wastewater over a theoretical concentration range of 1 × 101 to 1 × 103 GU mL−1 and estimated using a logistic regression model (Fig. 3). The enhanced extraction protocol was able to detect 100% of the replicates when the virus was present in concentrations at or above 7.5 × 101 GU mL−1. However, the percentage of positive replicates decreased as HI-SCV-2 RNA concentration decreased. The experimentally determined MLOD for the detection of HI-SCV-2 RNA in wastewater was approximately 5 × 101 GU mL−1. This value is within the range of theoretical limits of detection (100 to 103 GU mL−1) reported for 36 different methods used to quantify SARS-CoV-2 in raw wastewater.18 The theoretical LOD reported by Pecson et al. (2021) is calculated by correlating the PCR instrument detection limit, method concentration factor, and recovery efficiency of a surrogate spike detected by the method.18 Using the same approach, the theoretical LOD of the enhanced magnetic beads-based RNA extraction method is 2.3 GU mL−1, considering the assay detection limit of 5 copies per reaction, a 25-fold concentration factor, and a maximum recovery efficiency observed of 86.9%. The theoretical LOD is about 22 times lower than the experimentally determined MLOD and closer to the lowest range reported for the 36 methods evaluated in Pecson's work.3.3 Assessing method performance in municipal wastewater with varying levels of solids
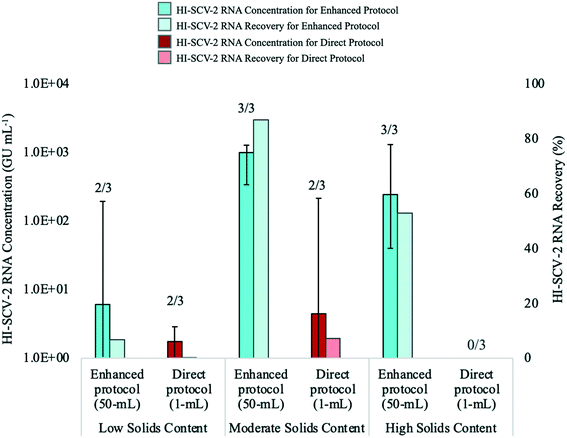
The performance of the direct RNA extraction method (using a 1-mL sample volume) and the enhanced extraction protocol, which includes the pre-concentration step, was assessed using wastewater with low, moderate, and high solids content (Fig. 4). The enhanced extraction protocol was carried out using a 50-mL sample volume centrifuged for 5 min at 5000 rpm to obtain a 500-μL pellet, which was eluted with 2 mL of the Tween®20-based buffer. To mitigate the effects of inhibition, all extracted RNA samples for both methods were analyzed undiluted via RT-qPCR and at the following dilutions with 1 mg mL−1 BSA: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1
1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; and 1
5; and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Mean RNA concentrations for each experimental group (e.g., direct (1 mL); moderate solids content) were calculated from the highest concentration reported for each sample among the four RNA dilutions.
10. Mean RNA concentrations for each experimental group (e.g., direct (1 mL); moderate solids content) were calculated from the highest concentration reported for each sample among the four RNA dilutions.
The recovery of HI-SCV-2 RNA was notably higher using the enhanced extraction protocol in all three wastewater matrices. In the low solids matrix (TSS = 77 mg L−1), both methods resulted in positive detections in two of three biological replicates. However, RNA concentrations were higher using the pre-concentration step prior the RNA extraction method (66.8 ± 110.6 GU mL−1; Ct values 34.15 and 37.01) than for the direct method (1.5 ± 1.4 GU mL−1; Ct values 38.23 and 39.04). There was no significant difference in mean Ct values between the two approaches (p = 0.300). Similarly, there was a noticeable difference in the performance of the methods in the moderate solids matrix (TSS = 175 mg L−1); the enhanced extraction protocol resulted in three of three positive detections and a mean concentration of 868.8 ± 484.1 GU mL−1 (Ct values ranged from 30.10 to 32.27) with an increase in RNA recovery from 6.7 (low solids matrix) to 86.9% (moderate solids matrix). In contrast, the direct method resulted in a viral detection in only two of three biological replicates, with a mean RNA concentration of 72.3 ± 121.4 GU mL−1 (Ct values 34.00 and 37.53) and a recovery of 7.2%. As with the low solids matrix, there was no significant difference in mean Ct values between the two approaches for the moderate solids matrix (p = 0.116). For the high solids matrix (TSS = 332 mg L−1), the enhanced extraction protocol resulted in detections in all three biological replicates, a mean RNA concentration of 529.3 ± 678.0 GU mL−1, and a recovery of 52.9% (Ct values ranged from 30.07 and 35.74). HI-SCV-2 RNA was not detected in any of the biological replicates using the direct method in the matrix with high solids content. Similar to findings by Feng et al. (2021), samples with higher TSS did not consistently result in higher viral RNA concentrations.59
It is widely known that the recovery of SARS-CoV-2 may be impacted by the presence of solids in wastewater, which can inhibit qPCR reactions, decrease assay sensitivity, and produce false negative results.60 However, the inclusion of the solids fraction of wastewater is a common approach to SARS-CoV-2 wastewater surveillance, as the virus tends to partition to solids in the wastewater.28,61,62 The detection of HI-SCV-2 in wastewater matrices with varying degrees of solids demonstrates that although the enhanced extraction protocol involves a concentration factor of 25, the co-concentration of inhibitors does not impede the detection of HI-SCV-2 RNA in these matrices. However, as mentioned earlier, it is unlikely that viral surrogates partition to solids as does SARS-CoV-2 in real wastewater samples.51 It is possible that a significant proportion of the HI-SCV-2 surrogate used in these experiments remained in the liquid fraction to be discarded in the supernatant following the centrifugation step of the enhanced extraction protocol, as the centrifugation force and time used in the method is insufficient to pellet free viruses.52,53 As such, follow-up experiments using wastewater known to contain SARS-CoV-2 were carried out to compare the performance of the methods in real world samples.
3.4 Performance of the direct and enhanced RNA extraction protocols using wastewater samples known to contain SARS-CoV-2
Influent composite wastewater known to contain SARS-CoV-2 was analyzed to assess the performance of the enhanced extraction protocol. As with the bench-scale experiments in wastewater with varying levels of solids, all extracted RNA samples for both methods were analyzed undiluted via RT-qPCR and at the following dilutions with BSA: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1
1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; and 1
5; and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Mean RNA concentrations for each experimental group were calculated from the highest concentration reported for each sample among the four RNA dilutions (Fig. 5).
10. Mean RNA concentrations for each experimental group were calculated from the highest concentration reported for each sample among the four RNA dilutions (Fig. 5).
SARS-CoV-2 was detected in all five biological replicates processed using the enhanced extraction protocol, with Ct values ranging from 28.05 to 34.94. Maximum RNA concentrations using this method were achieved through either 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 or 1
5 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 RNA dilution with BSA. In contrast, the viral signal was detected in only two of five replicates (RNA diluted with 1
10 RNA dilution with BSA. In contrast, the viral signal was detected in only two of five replicates (RNA diluted with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BSA) using the 1-mL direct extraction method and both detections showed Ct values >38. SARS-CoV-2 was not detected in any of the five biological replicates when undiluted RNA was analyzed or when diluted at 1
1 BSA) using the 1-mL direct extraction method and both detections showed Ct values >38. SARS-CoV-2 was not detected in any of the five biological replicates when undiluted RNA was analyzed or when diluted at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 BSA.
10 BSA.
The mean SARS-CoV-2 concentrations detected in the wastewater samples were 3.11 × 103 ± 3.26 × 103 GU mL−1 using the enhanced extraction protocol and 2.41 × 101 ± 3.54 × 101 GU mL−1 for the direct extraction method. These marked differences in RNA concentrations (over two orders of magnitude) and detection rates demonstrate that the pre-concentration step in combination with the magnetic beads-based RNA extraction method is more sensitive and reliable than the direct extraction method for monitoring SARS-CoV-2 in wastewater. The higher detection rate (100%) observed with the enhanced extraction protocol in this experiment is critical, as composite samples collected from WWTFs are not always homogeneous and SARS-CoV-2 detection may vary among smaller sample aliquots. While the 1-mL sample volume required for the direct extraction method is practical and amenable to high-throughput, the inconsistent detection and lower RNA concentration yields demonstrated in this experiment suggests that the direct extraction method may not be suitable for wastewater surveillance without the addition of the rapid pre-concentration step, especially during periods of low COVID-19 prevalence.
This experiment highlights several important features of the improved magnetic beads-based RNA extraction method with the addition of the pre-concentration step. First, the 25-fold concentration factor allows sufficient concentration of SARS-CoV-2 for reliable detection and quantitation without impacting RT-qPCR analysis through increased inhibition. Second, the larger initial sample volume (50 mL) is more representative of a larger composite sample than is the 1-mL aliquot used in the direct extraction method. Third, the low Ct values achieved by the method (<30) may present an opportunity for different applications such as environmental genomic sequencing. The results of this experiment suggest that the implementation of the enhanced extraction protocol may be a rapid and effective approach for the consistent and accurate routine monitoring of SARS-CoV-2 in wastewater.
3.5 Application of the enhanced RNA extraction protocol for the detection and quantification of SARS-CoV-2 in wastewater from four communities in Nova Scotia
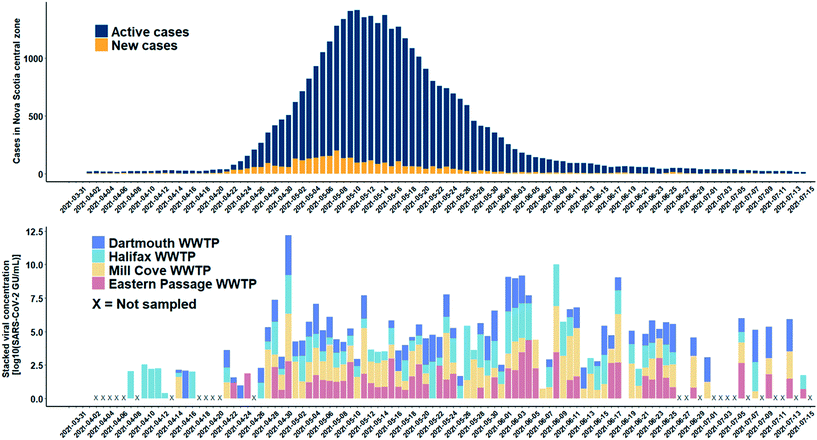
A total of 165 influent 24-h composite wastewater samples were collected from four WWTFs in Nova Scotia over a 15-week study period during which the province experienced a third wave of COVID-19 cases. The detection levels of SARS-CoV-2 for the WWTFs monitored are shown in Fig. 6. The results of this monitoring program show the clear persistent signal about two weeks in advance of the spike in COVID-19 cases at the Halifax WWTF. It is unknown if the viral signal was present in the wastewater between April 17th and 20th, 2021, as samples were not collected from any of the WWTFs during that time. A persistent viral signal was observed in wastewater from at least two of the WWTFs located within the central zone during the rise in COVID-19 caseloads from April 26th to May 10th, 2021, when the number of active COVID-19 cases peaked at 1415. Similarly, the signal was consistently present in wastewater samples from at least two of the WWTFs for the remainder of the study period even though caseloads decreased to 253 at the end of the study period. Moreover, the SARS-CoV-2 signal in the wastewater peaked on April 30th, while the reported case data peaked nine days later on May 9th, 2021. Although the location of the reported COVID-19 cases in the catchment areas within the central zone during the study period are unknown, our data provide information about the catchment areas that appeared to be contributing to the wastewater signal at different points in time throughout the third wave.There are several reasons why the wastewater data do not more closely match the COVID-19 case data for the central zone. First, the combined contributing population of the four WWTFs is approximately 271![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people, about 64% of the total population in the central zone. It is possible that active cases that may have been present in the remaining 36% of the population in the central zone were not contributing to the viral signal in the wastewater collected at the four WWTFs sampled in the study. Second, there were days within the sampling period when no samples were collected at any of the WWTFs (e.g., April 2 to 6, 8, 13, 17 to 20, and 25) and other days on which samples were collected, but not from all WWTFs (Fig. 6). Third, in our bench-scale experiments, different SARS-CoV-2 concentrations were observed for the same sample at different RNA dilution factors, and it is unknown if higher concentrations could have been achieved in the monitoring program at different dilution factors. In addition, hospitalizations and transfer of patients out of the central zone during the study period may have also affected the relationship between the wastewater viral signal and number of cases in the area. Moreover, while it is possible that prolonged shedding may be contributing to the persistent viral signal observed in the WWTF samples after caseloads decreased, further research is required to better understand shedding rates of infected individuals. Careful consideration of these factors is important when comparing the raw wastewater data presented here to the COVID-19 case data for the central zone.
000 people, about 64% of the total population in the central zone. It is possible that active cases that may have been present in the remaining 36% of the population in the central zone were not contributing to the viral signal in the wastewater collected at the four WWTFs sampled in the study. Second, there were days within the sampling period when no samples were collected at any of the WWTFs (e.g., April 2 to 6, 8, 13, 17 to 20, and 25) and other days on which samples were collected, but not from all WWTFs (Fig. 6). Third, in our bench-scale experiments, different SARS-CoV-2 concentrations were observed for the same sample at different RNA dilution factors, and it is unknown if higher concentrations could have been achieved in the monitoring program at different dilution factors. In addition, hospitalizations and transfer of patients out of the central zone during the study period may have also affected the relationship between the wastewater viral signal and number of cases in the area. Moreover, while it is possible that prolonged shedding may be contributing to the persistent viral signal observed in the WWTF samples after caseloads decreased, further research is required to better understand shedding rates of infected individuals. Careful consideration of these factors is important when comparing the raw wastewater data presented here to the COVID-19 case data for the central zone.
Of the 165 samples analyzed, there were 144 detections, 96 of which were below the experimentally determined MLOD of 50 GU mL−1, and the method allowed the detection of SARS-CoV-2 RNA as low as 1.7 GU mL−1. The experimentally determined MLOD of the enhanced extraction protocol using HI-SCV-2 is likely an overestimation of the MLOD for SARS-CoV-2 (as suggested by the calculated theoretical LOD), which may be attributed to a difference in partitioning behaviour of the surrogate when spiked into wastewater compared to that of SARS-CoV-2 in real wastewater samples. As with the series of bench-scale experiments carried out in this study, the 25-fold concentration step does not appear to increase inhibition to the point where detection and quantitation of SARS-CoV-2 is hindered in raw wastewater samples. This feature is a critical advantage and highlights the potential of applying this simple and rapid method to wastewater surveillance of SARS-CoV-2 RNA as a predictive tool for subsequent waves of infection in communities. These results demonstrate that the enhanced extraction protocol is an effective approach for the wastewater surveillance of SARS-CoV-2 in communities with low COVID-19 prevalence.
Pre-concentration of wastewater samples does not always result in higher viral recoveries. For example, Gonzalez et al., (2020) compared the recovery efficiency of bovine coronavirus (BCoV) and bovine respiratory syncytial virus (BRSV) from concentrated and unconcentrated wastewater samples.8 Two concentration techniques were used: concentrating pipette with centrifugation and electronegative filtration. Interestingly, recoveries of the surrogates without concentration were 59 and 75% for BCoV and BRSV, respectively, while neither concentration technique resulted in recoveries above 7.6% for either surrogate. Moreover, Green et al. (2020) reported only a 12% recovery of inactive SARS-CoV-2 from 20 mL samples of wastewater using ultracentrifugation.63 In a survey of surveillance methods for SARS-CoV-2 detection in wastewater released by the Water Research Foundation, 127 respondents from 35 countries participated; 71.4% of respondents indicated that they removed solids from the wastewater samples, 86.5% performed a pre-concentration step, and 46.9% incorporated a secondary concentration step.64 Of the vast array of methods reviewed, this is the first to advance a robust magnetic beads-based RNA extraction technique from whole wastewater without extensive sample pre-treatment such as ultracentrifugation or ultrafiltration.
SARS-CoV-2 concentrations may be normalized to wastewater flow, human fecal markers, or recovery of surrogates in wastewater surveillance programs.59 One of the limitations of this study is that the SARS-CoV-2 concentrations generated from the monitoring program were not normalized. However, Kantor et al. (2021) illustrated that the application of a normalization factor does not account for losses during RNA extraction and recommended reporting directly measured SARS-CoV-2 concentrations while stating the method's recovery efficiency instead of attempting to correct the RNA concentration for recovery efficiency.65 Moreover, Feng et al. (2021) demonstrated decreased correlations of SARS-CoV-2 concentrations in influent wastewater to COVID-19 case data when normalizing to wastewater flow, fecal indicators (pepper mild mottle virus and human Bacteroides HF183), and a spiked recovery control (bovine coronavirus).59 Another limitation of this study was that the HI-SCV-2 RNA may not represent the true behaviour of SARS-CoV-2 in wastewater. As such, experimentally determined MLOD and recovery efficiency values using the surrogate may not be representative of SARS-COV-2 in wastewater. Furthermore, as there is high variability in wastewater composition among samples and aliquots, the extent of how SARS-CoV-2 RNA recovery efficiency varies with different water quality parameters is largely unknown. Although recoveries of up to 87% were observed for HI-SCV-2 in one of the matrices of the bench-scale experiments in this study, overall recovery of SARS-CoV-2 from wastewater appears to be dependent on matrix characteristics and RNA dilution factor needed to overcome inhibition.
The 25-fold concentration step added to the magnetic beads-based RNA extraction protocol is required to detect low viral concentrations present in wastewater. This feature is especially important as SARS-CoV-2 concentrations may decrease as vaccinations programs roll out and COVID-19 prevalence diminishes in communities. Many viral concentration methods typically involve many tedious, time consuming and expensive steps for the analysis of wastewater samples. A simple and sensitive approach such as the sample pre-concentration step in combination with the magnetic beads-based RNA extraction method would therefore be highly favourable for a SARS-CoV-2 wastewater monitoring programs for the early detection of COVID-19 outbreaks in communities.
4. Conclusions
This work outlines the development of a rapid and effective pre-concentration protocol and a magnetic bead-based extraction method in the detection of SARS-COV-2 in complex wastewater samples. In bench-scale experiments, the enhanced extraction protocol (with an additional pre-concentration step) was more effective than the direct extraction method in recovering spiked viral HI-SCV-2 RNA from influent wastewater samples containing varying levels of solids. In an assessment of the optimal volume of the Tween-based buffer, the highest HI-SCV-2 RNA recovery was obtained with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 solids pellet
4 solids pellet![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer ratio. In experiments carried out to mitigate inhibition, pre-extraction sample dilution yielded a lower mean HI-SCV-2 RNA recovery while centrifugation after the addition of the elution buffer and dSPE clean-up resulted in fewer RNA detections and diminished recoveries. In field experiments, the pre-concentration step in combination with the magnetic beads-based RNA extraction method was consistently more effective than the direct extraction method in detecting SARS-CoV-2 RNA in influent wastewater known to contain SARS-CoV-2 and resulted in a 100% detection rate.
buffer ratio. In experiments carried out to mitigate inhibition, pre-extraction sample dilution yielded a lower mean HI-SCV-2 RNA recovery while centrifugation after the addition of the elution buffer and dSPE clean-up resulted in fewer RNA detections and diminished recoveries. In field experiments, the pre-concentration step in combination with the magnetic beads-based RNA extraction method was consistently more effective than the direct extraction method in detecting SARS-CoV-2 RNA in influent wastewater known to contain SARS-CoV-2 and resulted in a 100% detection rate.
When implemented into a wastewater surveillance program, the enhanced extraction protocol provided a clear persistent signal approximately two weeks in advance of a spike in COVID-19 cases in the region. In addition, a constant viral signal was observed in wastewater during the rise and fall in COVID-19 caseloads during the study period. Over half of the SARS-CoV-2 detections in wastewater samples were below the experimentally determined MLOD, indicating that the actual SARS-CoV-2 detection limit is likely lower than the experimentally determined value using the HI-SCV-2 surrogate. This study shows that the enhanced extraction protocol is an effective approach for the wastewater surveillance of SARS-CoV-2 and may be used as an early warning tool in communities with low COVID-19 prevalence. Although magnetic beads have previously been employed in the extraction of nucleic acids from a variety of matrices including wastewater, this work advances previous research through the development of a magnetic beads-based protocol for the rapid extraction of SARS-CoV-2 RNA from raw municipal wastewater. The described method is simple, transferable, and rapid, producing results in under three hours from receiving the sample. These features provide an advantageous application of the enhanced magnetic beads-based RNA extraction protocol for laboratories that may have limited resources.
5. Research ethics statement for wastewater surveillance studies
In consultation with the Research Ethics Board (REB) at Dalhousie University, it was determined that REB review was not required for research that involves analysis of anonymous human biological materials (such as municipal waste) without generating identifiable information. This research complies with article 2.4 described in the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS 2, 2018).Author contributions
Ana Parra: conceptualization, investigation, methodology, visualization, writing original draft, reviewing and editing. Crystal Sweeney: conceptualization, methodology, project administration, visualization, writing original draft, reviewing and editing. Emalie Hayes: investigation, writing original draft, reviewing and editing. Benjamin Trueman: formal analysis, manuscript reviewing and editing. Yannan Huang: investigation, manuscript reviewing and editing. Rob Jamieson: resources, manuscript reviewing and editing. Jennie Rand: resources, manuscript reviewing and editing. Graham Gagnon: funding acquisition, supervision, manuscript reviewing and editing. Amina Stoddart: funding acquisition, supervision, manuscript reviewing and editing.Conflicts of interest
The authors declare that LuminUltra Technologies Ltd. provided equipment, some reagents and technical support to assist in this work. The authors declare that they do not have any financial interest in LuminUltra Technologies Ltd.Acknowledgements
This study was funded through support from an NSERC COVID-19 Alliance Grant [grant number ALLRP 554503-20], an NSERC Collaborative Research and Development Grant in partnership with Halifax Water [grant number CRDPJ 539387-19] and the NSERC/Halifax Water Industrial Research Chair program [grant number IRCPJ: 349838-16]. Wastewater used for preliminary method development was spiked at the National Microbiology Laboratory in Winnipeg with GI-SCV-2 and HCV-229E acquired from the University of Alberta through the Canadian Water Network COVID-19 Coalition. The authors thank LuminUltra Technologies for providing qPCR technology and for technical support that was led through Dr. Jordan Schmidt throughout the project, and Halifax Water municipal staff for assisting with sample collection. The authors would like to extend thanks to Genevieve Erjavec and Madison Gouthro for wastewater sample collection and analysis in Nova Scotia, and Samina Hayat and Nivetha Srikanthan for wastewater sample collection and shipment from Ontario. As well, the authors would like to extend thanks to researchers Dr. Bofu Li, Paul Bjorndahl and Sebastian Munoz from the Centre for Water Resources Studies at Dalhousie University for providing technical reviews, data analysis, and data visualization during the study. The table of contents entry figure was adapted from “Quantifying SARS-CoV-2 Virions in City Wastewater”, by http://BioRender.com (2020); retrieved from https://app.biorender.com/templates.References
- World Health Organization, WHO Coronavirus Disease (COVID-19) Dashboard [Internet], 2021 [cited 2021 Jul 19], Available from: https://covid19.who.int/.
- Y. Tian, L. Rong, W. Nian and Y. He, Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission, Aliment. Pharmacol. Ther., 2020, 51(9), 843–851 CrossRef CAS.
- N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang and J. Song, et al., A Novel Coronavirus from Patients with Pneumonia in China, 2019, N. Engl. J. Med., 2020, 382(8), 727–733 CrossRef CAS.
- H. A. Rothan and S. N. Byrareddy, The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak, J. Autoimmun., 2020, 109, 102433 CrossRef CAS.
- W. Ahmed, N. Angel, J. Edson, K. Bibby, A. Bivins and J. W. O'Brien, et al., First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community, Sci. Total Environ., 2020, 728, 138764 CrossRef CAS PubMed.
- A. Carducci, I. Federigi, D. Liu, J. R. Thompson and M. Verani, Making Waves: Coronavirus detection, presence and persistence in the water environment: State of the art and knowledge needs for public health, Water Res., 2020, 179, 115907 CrossRef PubMed.
- P. Foladori, F. Cutrupi, N. Segata, S. Manara, F. Pinto and F. Malpei, et al., SARS-CoV-2 from faeces to wastewater treatment: What do we know? A review, Sci. Total Environ., 2020, 743, 140444 CrossRef CAS.
- R. Gonzalez, K. Curtis, A. Bivins, K. Bibby, M. H. Weir and K. Yetka, et al., COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology, Water Res., 2020, 186, 116296 CrossRef CAS PubMed.
- M. Kumar, A. K. Patel, A. V. Shah, J. Raval, N. Rajpara and M. Joshi, et al., First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2, Sci. Total Environ., 2020, 746, 141326 CrossRef CAS.
- W. Ahmed, B. Tscharke, P. M. Bertsch, K. Bibby, A. Bivins and P. Choi, et al., SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: A temporal case study, Sci. Total Environ., 2021, 761, 144216 CrossRef CAS.
- B. Li, D. Y. W. Di, P. Saingam, M. K. Jeon and T. Yan, Fine-Scale Temporal Dynamics of SARS-CoV-2 RNA Abundance in Wastewater during A COVID-19 Lockdown, Water Res., 2021, 197, 117093 CrossRef CAS PubMed.
- T. Baldovin, I. Amoruso, M. Fonzo, A. Buja, V. Baldo and S. Cocchio, et al., SARS-CoV-2 RNA detection and persistence in wastewater samples: An experimental network for COVID-19 environmental surveillance in Padua, Veneto Region (NE Italy), Sci. Total Environ., 2021, 760, 143329 CrossRef CAS.
- A. Hata, H. Hara-Yamamura, Y. Meuchi, S. Imai and R. Honda, Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak, Sci. Total Environ., 2021, 758, 143578 CrossRef CAS PubMed.
- S. A. Bustin and T. Nolan, RT-qPCR Testing of SARS-CoV-2: A Primer, Int. J. Mol. Sci., 2020, 21(8), 3004 CrossRef CAS.
- W. Randazzo, P. Truchado, E. Cuevas-Ferrando, P. Simón, A. Allende and G. Sánchez, SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area, Water Res., 2020, 181, 115942 CrossRef CAS.
- P. A. Barril, L. A. Pianciola, M. Mazzeo, M. J. Ousset, M. V. Jaureguiberry and M. Alessandrello, et al., Evaluation of viral concentration methods for SARS-CoV-2 recovery from wastewaters, Sci. Total Environ., 2021, 756, 144105 CrossRef CAS PubMed.
- P. Cervantes-Avilés, I. Moreno-Andrade and J. Carrillo-Reyes, Approaches applied to detect SARS-CoV-2 in wastewater and perspectives post-COVID-19, J. Water Process Eng., 2021, 40, 101947 CrossRef.
- B. M. Pecson, E. Darby, C. N. Haas, Y. M. Amha, M. Bartolo and R. Danielson, et al., Reproducibility and sensitivity of 36 methods to quantify the SARS-CoV-2 genetic signal in raw wastewater: findings from an interlaboratory methods evaluation in the U.S., Environ. Sci.: Water Res. Technol., 2021, 7(3), 504–520 RSC , Available from: https://pubs.rsc.org/en/content/articlelanding/2021/ew/d0ew00946f.
- W. Ahmed, P. M. Bertsch, A. Bivins, K. Bibby, K. Farkas and A. Gathercole, et al., Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater, Sci. Total Environ., 2020, 739, 139960 CrossRef CAS PubMed.
- G. La Rosa, M. Iaconelli, P. Mancini, G. Bonanno Ferraro, C. Veneri and L. Bonadonna, et al., First detection of SARS-CoV-2 in untreated wastewaters in Italy, Sci. Total Environ., 2020, 736, 139652 CrossRef CAS.
- G. Medema, L. Heijnen, G. Elsinga, R. Italiaander and A. Brouwer, Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands, Environ. Sci. Technol. Lett., 2020, 7(7), 511–516 CrossRef CAS.
- F. Wu, J. Zhang, A. Xiao, X. Gu, W. L. Lee and F. Armas, et al., SARS-CoV-2 Titers in Wastewater Are Higher than Expected from Clinically Confirmed Cases, mSystems, 2020, 5(4), e00614-20 Search PubMed , Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7566278/.
- S. Wurtzer, V. Marechal, J. Mouchel, Y. Maday, R. Teyssou and E. Richard, et al., Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters, 2020, medRxiv, 2020.04.12.20062679.
- S. E. Philo, E. K. Keim, R. Swanstrom, A. Q. W. Ong, E. A. Burnor and A. L. Kossik, et al., A comparison of SARS-CoV-2 wastewater concentration methods for environmental surveillance, Sci. Total Environ., 2021, 760, 144215 CrossRef CAS.
- A. Blanco, I. Abid, N. Al-Otaibi, F. J. Pérez-Rodríguez, C. Fuentes and S. Guix, et al., Glass Wool Concentration Optimization for the Detection of Enveloped and Non-enveloped Waterborne Viruses, Food Environ. Virol., 2019, 11(2), 184–192 CrossRef CAS.
- L. Pei, M. Rieger, S. Lengger, S. Ott, C. Zawadsky and N. M. Hartmann, et al., Combination of Crossflow Ultrafiltration, Monolithic Affinity Filtration, and Quantitative Reverse Transcriptase PCR for Rapid Concentration and Quantification of Model Viruses in Water, Environ. Sci. Technol., 2012, 46(18), 10073–10080 CrossRef CAS PubMed.
- I. Michael-Kordatou, P. Karaolia and D. Fatta-Kassinos, Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification, J. Environ. Chem. Eng., 2020, 8(5), 104306 CrossRef CAS PubMed.
- Y. Ye, R. M. Ellenberg, K. E. Graham and K. R. Wigginton, Survivability, Partitioning, and Recovery of Enveloped Viruses in Untreated Municipal Wastewater, Environ. Sci. Technol., 2016, 50(10), 5077–5085 CrossRef CAS PubMed.
- N. Sun, C. Deng, Y. Liu, X. Zhao, Y. Tang and R. Liu, et al., Optimization of influencing factors of nucleic acid adsorption onto silica-coated magnetic particles: Application to viral nucleic acid extraction from serum, J. Chromatogr. A, 2014, 1325, 31–39 CrossRef CAS PubMed.
- Z. Shan, Z. Zhou, H. Chen, Z. Zhang, Y. Zhou and A. Wen, et al., PCR-ready human DNA extraction from urine samples using magnetic nanoparticles, J. Chromatogr., B, 2012, 881–882, 63–68 CrossRef CAS PubMed.
- H. He, R. Li, Y. Chen, P. Pan, W. Tong and X. Dong, et al., Integrated DNA and RNA extraction using magnetic beads from viral pathogens causing acute respiratory infections, Sci. Rep., 2017, 7(1), 45199 CrossRef CAS PubMed.
- G. A. Albertoni, C. P. Arnoni, P. R. Barboza Araujo, S. S. Andrade, F. O. Carvalho and M. J. B. Castello Girão, et al., Magnetic bead technology for viral RNA extraction from serum in blood bank screening, Braz. J. Infect. Dis., 2011, 15(6), 547–552 CrossRef CAS PubMed.
- L. Mathot, M. Lindman and T. Sjöblom, Efficient and scalable serial extraction of DNA and RNA from frozen tissue samples, Chem. Commun., 2011, 47(1), 547–549 RSC.
- G. La Rosa, M. Pourshaban, M. Iaconelli and M. Muscillo, Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy, Ann. Ist. Super. Sanità, 2010, 46, 266–273 CAS.
- Q.-B. Yuan, Y.-M. Huang, W.-B. Wu, P. Zuo, N. Hu and Y.-Z. Zhou, et al., Redistribution of intracellular and extracellular free & adsorbed antibiotic resistance genes through a wastewater treatment plant by an enhanced extracellular DNA extraction method with magnetic beads, Environ. Int., 2019, 131, 104986 CrossRef CAS.
- L. Pisharody, S. Suresh and S. Mukherji, Evaluation of adsorbents and eluents for application in virus concentration and adsorption-desorption isotherms for coliphages, Chem. Eng. J., 2021, 403, 126267 CrossRef CAS.
- B. R. McMinn, A. Korajkic, J. Kelleher, M. P. Herrmann, A. C. Pemberton and W. Ahmed, et al., Development of a large volume concentration method for recovery of coronavirus from wastewater, Sci. Total Environ., 2021, 774, 145727 CrossRef CAS PubMed.
- E. K. Hayes, C. L. Sweeney, L. E. Anderson, B. Li, G. B. Erjavec and M. T. Gouthro, et al., A novel passive sampling approach for SARS-CoV-2 in wastewater in a Canadian province with low prevalence of COVID-19, Environ. Sci.: Water Res. Technol., 2021, 7(9), 1576–1586 RSC.
- A. Lawal, R. C. S. Wong, G. H. Tan, L. B. Abdulra'uf and A. M. A. Alsharif, Recent Modifications and Validation of QuEChERS-dSPE Coupled to LC–MS and GC–MS Instruments for Determination of Pesticide/Agrochemical Residues in Fruits and Vegetables: Review, J. Chromatogr. Sci., 2018, 56(7), 656–669 CAS.
- American Public Health Association, American Water Works Association and Water Environment Federation, Standard methods for the examination of water and wastewater, APHA-AWWA-WEF, Washington, D.C., 1998 Search PubMed.
- A. Forootan, R. Sjöback, J. Björkman, B. Sjögreen, L. Linz and M. Kubista, Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR), Biomol. Detect. Quantif., 2017, 12, 1–6 CrossRef CAS PubMed.
- CDC, CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel: CDC-006-00019, Revision: 05 [Internet], CDC/DDID/NCIRD/Division of Viral Disease, 2020 [cited 2020 Sep 29], Available from: https://www.fda.gov/media/134922/download Search PubMed.
- Government of Canada SC, Census Profile, 2016 Census - Zone 4 - Central [Health region, December 2017], Nova Scotia and Nova Scotia [Province] [Internet], 2017 [cited 2021 Jun 13], Available from: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=HR&Code1=1204&Geo2=PR&Code2=12&SearchText=Zone%204%20-%20Central&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=1204&TABID=1&type=0 Search PubMed.
- Health Canada, Interactive Data Visualization of COVID-19 in Canada - Public Health Infobase|Public Health Agency of Canada [Internet], Interactive data visualizations of COVID-19, 2021 [cited 2021 Mar 19], Available from: https://health-infobase.canada.ca/covid-19/.
- S. A. Bustin, V. Benes, J. A. Garson, J. Hellemans, J. Huggett and M. Kubista, et al., The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments, Clin. Chem., 2009, 55(4), 611–622 CrossRef CAS.
- M. A. Borchardt, A. B. Boehm, M. Salit, S. K. Spencer, K. R. Wigginton and R. T. Noble, The Environmental Microbiology Minimum Information (EMMI) Guidelines: qPCR and dPCR Quality and Reporting for Environmental Microbiology, Environ. Sci. Technol., 2021, acs.est.1c01767 Search PubMed.
- R. Goni, P. García and S. Foissac, The qPCR data statistical analysis, 2009, p. 9 Search PubMed.
- R Core Team, R: A language and environment for statistical computing [Internet], Vienna, Austria, 2020, Available from: https://www.r-project.org/ Search PubMed.
- H. Wickham, M. Averick, J. Bryan, W. Chang, L. D. McGowan and R. François, et al., Welcome to the Tidyverse, J. Open Source Softw., 2019, 4(43), 1686 CrossRef.
- I. G. Wilson, Inhibition and facilitation of nucleic acid amplification, Appl. Environ. Microbiol., 1997, 63(10), 3741–3751 CrossRef CAS PubMed.
- A. H. S. Chik, M. B. Glier, M. Servos, C. S. Mangat, X.-L. Pang and Y. Qiu, et al., Comparison of approaches to quantify SARS-CoV-2 in wastewater using RT-qPCR: Results and implications from a collaborative inter-laboratory study in Canada, J. Environ. Sci., 2021, 107, 218–229 CrossRef PubMed.
- O. Evans, I. Paul-Pont, P. Hick and R. J. Whittington, A simple centrifugation method for improving the detection of Ostreid herpesvirus-1 (OsHV-1) in natural seawater samples with an assessment of the potential for particulate attachment, J. Virol. Methods, 2014, 210, 59–66 CrossRef CAS PubMed.
- J. E. Lawrence and G. F. Steward, Purification of viruses by centrifugation, in Manual of Aquatic Viral Ecology [Internet], ed. S. Wilhelm, M. Weinbauer and C. Suttle, American Society of Limnology and Oceanography, 2010, pp. 166–181, Available from: http://www.aslo.org/books/mave/166.html Search PubMed.
- Z. Wang, X. Wang, H. Tian, Q. Wei, B. Liu and G. Bao, et al., High through-put determination of 28 veterinary antibiotic residues in swine wastewater by one-step dispersive solid phase extraction sample cleanup coupled with ultra-performance liquid chromatography-tandem mass spectrometry, Chemosphere, 2019, 230, 337–346 CrossRef CAS PubMed.
- L. Bijlsma, E. Beltrán, C. Boix, J. V. Sancho and F. Hernández, Improvements in analytical methodology for the determination of frequently consumed illicit drugs in urban wastewater, Anal. Bioanal. Chem., 2014, 406(17), 4261–4272 CrossRef CAS.
- A. Brié, R. Razafimahefa, J. Loutreul, A. Robert, C. Gantzer and N. Boudaud, et al., The Effect of Heat and Free Chlorine Treatments on the Surface Properties of Murine Norovirus, Food Environ. Virol., 2017, 9(2), 149–158 CrossRef.
- S. A. Thatcher, DNA/RNA Preparation for Molecular Detection, Clin. Chem., 2015, 61(1), 89–99 CrossRef CAS PubMed.
- R. S. Kantor, H. D. Greenwald, L. C. Kennedy, A. Hinkle, S. Harris-Lovett, M. Metzger, M. M. Thornton, J. M. Paluba and K. L. Nelson, 2021, medRxiv, 2021.06.06.21258431.
- S. Feng, A. Roguet, J. S. McClary-Gutierrez, R. J. Newton, N. Kloczko and J. G. Meiman, et al., Evaluation of sampling frequency and normalization of SARS-CoV-2 wastewater concentrations for capturing COVID-19 burdens in the community, 2021, medRxiv, 2021.02.17.21251867.
- C. Schrader, A. Schielke, L. Ellerbroek and R. Johne, PCR inhibitors – occurrence, properties and removal, J. Appl. Microbiol., 2012, 113(5), 1014–1026 CrossRef CAS PubMed.
- K. E. Graham, S. K. Loeb, M. K. Wolfe, D. Catoe, N. Sinnott-Armstrong, S. Kim, K. M. Yamahara, L. M. Sassoubre, L. M. Mendoza Grijalva, L. Roldan-Hernandez, K. Langenfeld, K. R. Wigginton and A. B. Boehm, SARS-CoV-2 RNA in Wastewater Settled Solids Is Associated with COVID-19 Cases in a Large Urban Sewershed, Environ. Sci. Technol., 2021, 55, 488–498 CrossRef CAS.
- J. Peccia, A. Zulli, D. E. Brackney, N. D. Grubaugh, E. H. Kaplan and A. Casanovas-Massana, et al., Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics, Nat. Biotechnol., 2020, 38(10), 1164–1167 CrossRef CAS.
- H. Green, M. Wilder, F. A. Middleton, M. Collins, A. Fenty and K. Gentile, et al., Quantification of SARS-CoV-2 and cross-assembly phage (crAssphage) from wastewater to monitor coronavirus transmission within communities, 2020, medRxiv, 2020.05.21.20109181.
- N. A. Zhou, C. Tharpe, J. S. Meschke and C. M. Ferguson, Survey of rapid development of environmental surveillance methods for SARS-CoV-2 detection in wastewater, Sci. Total Environ., 2021, 769, 144852 CrossRef CAS PubMed.
- R. S. Kantor, K. L. Nelson, H. D. Greenwald and L. C. Kennedy, Challenges in Measuring the Recovery of SARS-CoV-2 from Wastewater, Environ. Sci. Technol., 2021, 55(6), 3514–3519 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ew00539a |
| ‡ Authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2022 |