Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecular changes among non-volatile disinfection by-products between drinking water treatment and consumer taps†
Anna
Andersson
 *a,
Michael
Gonsior
*a,
Michael
Gonsior
 b,
Mourad
Harir
cd,
Norbert
Hertkorn
c,
Philippe
Schmitt-Kopplin
cd,
Leanne
Powers
b,
Henrik
Kylin
b,
Mourad
Harir
cd,
Norbert
Hertkorn
c,
Philippe
Schmitt-Kopplin
cd,
Leanne
Powers
b,
Henrik
Kylin
 ae,
Daniel
Hellström
f,
Kerstin
Nilsson
g,
Ämma
Pettersson
h,
Helena
Stavklint
i and
David
Bastviken
ae,
Daniel
Hellström
f,
Kerstin
Nilsson
g,
Ämma
Pettersson
h,
Helena
Stavklint
i and
David
Bastviken
 a
a
aDepartment of Thematic Studies – Environmental Change, Linköping University, SE-581 83 Linköping, Sweden. E-mail: anna.e.andersson@liu.se
bUniversity of Maryland Center for Environmental Science, Chesapeake Biological Laboratory, Solomons, Maryland 20688, USA
cResearch Unit Analytical BioGeoChemistry, Helmholtz Zentrum München, German Research Center for Health and Environment, Ingolstaedter Landstraße 1, 85764 Neuherberg, Germany
dChair of Analytical Food Chemistry, Technische Universität München, 85354 Freising, Germany
eResearch Unit: Environmental Sciences and Management, North-West University, Potchefstroom, South Africa
fNorrvatten, Kvalitet och Utveckling, SE-169 02 Solna, Sweden
gVA SYD, Rännemästaregatan 3, SE-212 23 Malmö, Sweden
hNodra, Borgs vattenverk, SE-603 36 Norrköping, Sweden
iTekniska verken i Linköping AB (publ), SE-581 15 Linköping, Sweden
First published on 18th October 2021
Abstract
The formation of disinfection by-products (DBPs) during drinking water treatment has been associated with various health concerns but the total DBP exposure is still unknown. In this study, molecular level non-target analysis by Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) was used to study non-volatile DBPs, and how their composition changes during water distribution in four drinking water treatment plants (DWTPs) in Sweden using different types of raw water and disinfection processes. The largest portion of tap water DBP compositions were detected also at the DWTPs, highlighting that these DBP formulae were rather stable and contribute to human DBP exposure. Yet the number of detected DBPs decreased 14–48% between drinking water treatment and consumer taps in the three plants in which no mixing of water from other DWTPs in the distribution system occurred showing active DBP processing in the water distribution network. While considerable amounts of bromine-containing DBPs were detected upon chemical disinfection in some DWTPs, few of them were detected in the tap water samples, likely due to debromination by hydrolytic reactions. The overall fewer non-volatile DBPs detected in tap waters, along with changed distribution among chlorine and bromine DBPs, demonstrate that DBP mixtures are highly dynamic and that DBP measurements at DWTPs do not adequately reflect exposure at the point-of-use. Clearly, more knowledge about changes of DBP mixtures through the distribution system is needed to improve DBP exposure assessments.
Water impactTo mitigate health concerns from disinfection by-products (DBPs), potential changes to DBPs during distribution are important to account for. Non-target analysis demonstrates that the DBP mixture changes during distribution and that fewer bromine-containing DBPs were detected at consumer taps. |
1. Introduction
The production of drinking water of high quality is of great societal importance. Chemical disinfection, where, e.g., chlorine, chloramine, chlorine dioxide or ozone is added, leads to reactions with natural organic matter (NOM) and the formation of potentially toxic disinfection by-products (DBPs).1The formation of DBPs has been related to various health risks, such as bladder cancer and effects on adverse birth outcomes.2 A recent study based on ∼500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 births in Sweden found indications of a dose-dependent association between total trihalomethanes (TTHM) and adverse reproductive health effects (decreased fetal growth) in populations exposed to chlorinated drinking water, while no association was found in populations exposed to water treated with chloramine.3 Instead, human exposure to TTHM from chloraminated water was dose-dependently associated to other health risks connected to prenatal development, including malformations of the nervous system, urinary system, genitals and limbs.4 The differences in health risk associations between chlorinated and chloraminated water, while mutually based on TTHM exposure, shows that THMs are likely not the agents of these health concerns. In fact, which specific DBPs that cause the observed health risks are still not known.5 Recent findings indicate that nitrogen-containing DBPs, such as haloacetonitriles, constitute important components of toxicity in treated drinking water.6
000 births in Sweden found indications of a dose-dependent association between total trihalomethanes (TTHM) and adverse reproductive health effects (decreased fetal growth) in populations exposed to chlorinated drinking water, while no association was found in populations exposed to water treated with chloramine.3 Instead, human exposure to TTHM from chloraminated water was dose-dependently associated to other health risks connected to prenatal development, including malformations of the nervous system, urinary system, genitals and limbs.4 The differences in health risk associations between chlorinated and chloraminated water, while mutually based on TTHM exposure, shows that THMs are likely not the agents of these health concerns. In fact, which specific DBPs that cause the observed health risks are still not known.5 Recent findings indicate that nitrogen-containing DBPs, such as haloacetonitriles, constitute important components of toxicity in treated drinking water.6
To date, most efforts have been directed towards analyzing DBPs using gas chromatography (GC) and a range of different detectors, which is important due to the regulatory status of DBPs determined with GC, e.g., THMs and haloacetic acids (HAAs). However, bioassay assessments based on fractionation experiments show that the non-volatile fraction contribute higher to total toxicity from DBPs compared to the volatile fraction.7,8 Logically, attempts to reduce health risks from DBPs are directed at new treatments or adaptations of routines, however, potential shifts in DBP composition during water distribution is important to account for as such changes may affect DBP exposure and associated health effects.
A promising way to study complex DBP mixtures and to investigate specific changes of the molecular compositions of these mixtures is non-target analyses using ultrahigh-resolution Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS). FT-ICR MS enables the detection of thousands of distinct molecular formulae and has successfully been used to characterize individual elemental compositions in complex mixtures in, e.g., marine environments9–12 and boreal lakes.13 A multitude of DBPs have already been detected at drinking water treatment plants (DWTPs) through such non-targeted screening approaches.14–19 But the fate of these compounds in the distribution system remains uncertain, as well as if samples taken at the DWTP reflect exposure and risks to consumers at the point-of-use.
This study was undertaken to investigate potential changes to DBP composition between the DWTP effluent and the point-of-use (at consumer taps). By adopting a non-targeted approach using FT-ICR MS, DBPs of higher molecular weight, compared to commonly monitored DBPs, could be detected, offering a focus on a part of the DBP pool that is less known. This study comprises a multi-seasonal one-year sampling campaign at four Swedish DWTPs.
2. Methods
2.1 Description of water sources and treatment processes
The four Swedish DWTPs investigated were Berggården in Linköping (LIN), Borg in Norrköping (NOR), Görväln in Stockholm (STO) and Bulltofta in Malmö (MAL). Together, these plants supply approximately 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 consumers with drinking water. These DWTPs were chosen to cover some of the diversity in Swedish drinking water production. For MAL, there were no points in the distribution system featuring drinking water from the studied DWTP only, i.e., the MAL tap water point represented mixed drinking water from three DWTPs, one of which is MAL. The resulting dilution of MAL water in the distribution system should be considered throughout this study. MAL was included as some comparisons still are relevant.
000 consumers with drinking water. These DWTPs were chosen to cover some of the diversity in Swedish drinking water production. For MAL, there were no points in the distribution system featuring drinking water from the studied DWTP only, i.e., the MAL tap water point represented mixed drinking water from three DWTPs, one of which is MAL. The resulting dilution of MAL water in the distribution system should be considered throughout this study. MAL was included as some comparisons still are relevant.
The water sources used by each DWTP, named raw water in tables and figures, differ. At LIN, surface water from the river Motala Ström is used. This water originates from Vättern, a deep clear-water lake. NOR also takes water from Motala Ström, but at a point about 50 km further downstream, after the water has passed two large lakes surrounded by agricultural areas and forests, adding particles and dissolved organic matter. STO uses water from lake Mälaren, a water source with rather high levels of organic material, utilized by multiple DWTPs in the Stockholm area making Mälaren one of the most important raw water sources in Sweden. Finally, MAL uses a groundwater source, which has been utilized for drinking water production since the DWTP was built in 1879.
These raw waters were treated in different ways (Table 1). LIN was operated with rapid and slow sand filtration, followed by UV-disinfection and chlorination. At NOR, aluminum sulphate (Al2(SO4)3) coagulation was followed by granular activated carbon filtration (GAC), slow sand filtration, and dosing of hypochlorite and ammonia to the water stream with hypochlorite added slightly in excess, forming monochloramine in the water stream. The GAC filter reduces taste and odor and is regenerated every four years, hence, it mostly operates as a biological filter (BAC). At STO, Al2(SO4)3 coagulation was followed by rapid sand filtration to remove remaining particulates from the water, GAC, UV-disinfection and dosing of preformed monochloramine. In MAL (groundwater), aeration and softening removed dissolved iron and hardness, i.e., calcium and magnesium ions, followed by rapid sand filtration, UV-disinfection, and dosing of chloramine. At MAL, hypochlorite and ammonia were added to the water stream in theoretically equivalent proportions to form monochloramine, i.e., hypochlorite was not added in excess.
2.2 Sample collection
Water samples were collected repeatedly at the four DWTPs, during five sampling campaigns throughout a one year cycle, in March, May, August, and November 2016 and in January 2017. On each occasion, the following samples were collected: raw water, water after each treatment step (described in 2.1 and Table 1), finished water (drinking water leaving the DWTP) and water from a tap water point connected to the DWTP. This study focuses on the sampling points chemical disinfection, finished water, and tap water; some background information from the other sampling points, such as temperature, pH and various bulk DOM characteristics is presented in the supplement (Fig. S1–S8†). In NOR, the available sampling point after chemical disinfection did not represent well-mixed water and, thus, this sampling point was excluded at NOR. Samples were collected in duplicates in pre-washed amber 5 L glass bottles.2.3 Water characteristics
Temperature was measured during sample collection. pH and conductivity were measured at room temperature within six hours after sample collection using a HACH HQ 40 (Hach, Stockholm). Total nitrogen (TN) was measured for duplicate samples of filtered water (Whatman GF/F, 0.7 μm porosity) using a Shimadzu TOC-VCSH TOC analyzer.20 The level of TN represents the sum of nitrate, nitrite, organic nitrogen, and ammonia. Total chlorine was measured on-line at the DWTPs and for tap water samples, an eXact idip photometer (Scantec Nordic, Jonsered) was used.Dissolved organic carbon (DOC) and absorbance at 254 nm (UVA254) were determined in filtered water (Whatman GF/F, 0.7 μm porosity). DOC was measured at the accredited lab of each DWTP using the nPOC method.21 UVA254 was analyzed with an Ultrospec 2100 pro (Biochrom, Cambridge) using a 5 cm quartz cuvette and was reported as the absorbance per centimeter (cm−1). Based on UVA254 and DOC, SUVA was computed by dividing the absorbance at 254 nm (cm−1) by the DOC (mg C L−1) and was reported in the unit L mg−1 m−1. UVA254 provides information on the abundance of organic molecules absorbing light at this wavelength, which depend on the overall organic content and the number of π-bonds and delocalized electrons in the organic molecules. SUVA is a proxy for DOC-normalized aromatic content based on correlations between % aromatic content and SUVA values for reference dissolved organic matter (DOM) samples.22
2.4 Sample preparation for FT-ICR MS
For detailed DOM characterization, the organic material was isolated from the water by solid phase extraction (SPE).23 The water samples were filtered through pre-combusted (5 hours at 450 °C) glass fiber filters (Whatman GF/F, pore size 0.7 μm, Whatman) into a second set of glass bottles and pH was adjusted to ∼2.5 using 3 M HCl (prepared by 32% HCl, p.a. and ultrapure water, spectrophotometric grade). At this lower pH, residual chlorine was not detected, and quenching agents were avoided to prevent potential interferences upon FT-ICR MS analysis. DOM was extracted using Bond Elut PPL cartridges (1 g, 6 mL, Agilent Technologies), a modified styrene and divinylbenzene co-polymer adsorbent, providing ∼60% DOC extraction efficiency.23 The cartridges were conditioned with methanol (10 mL, LC-MS ultra CHROMASOLV®) and acidified ultrapure water (10 mL, pH 2.5, spectrophotometric grade, acidified with 32% HCl, p.a.). Filtered water (2 L for NOR and STO raw waters due to high DOC content, 4 L for the other sampling points) was passed through the SPE cartridges (flow rate kept below 20 mL min−1) using Teflon tubing that connected each water sample bottle and cartridge. The cartridges were positioned on a vacuum manifold (Standard 24-port, 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250-U, Sigma-Aldrich) and the extraction was driven by a peristaltic pump (Vantage 3000 C S10, Svenska pump AB). After the whole sample had passed through, cartridges were washed with 0.1% formic acid water (10 mL, LC-MS ultra CHROMASOLV®) to remove ions that might interfere during FT-ICR MS analysis.
250-U, Sigma-Aldrich) and the extraction was driven by a peristaltic pump (Vantage 3000 C S10, Svenska pump AB). After the whole sample had passed through, cartridges were washed with 0.1% formic acid water (10 mL, LC-MS ultra CHROMASOLV®) to remove ions that might interfere during FT-ICR MS analysis.
A previous analysis of extracted DOM samples from multiple boreal lakes and streams that were washed with pH adjusted water using HCl instead of formic acid, showed the consistent formation of chloride adducts in FT-ICR MS (Fig. S9†), which would bias the interpretation of DBP formation. Parallel sample preparation and the usage of formic acid water for washing avoided this effect. Finally, the cartridges were dried for ∼15 seconds using nitrogen gas (except for STO where air with a hydrocarbon trap was used, because nitrogen gas was not available) and DOM was eluted with methanol (10 mL). In previous work by the authors, a lower volume of 6 mL of methanol has been shown to not quantitatively desorb DOM from the cartridge and hence the volume of 10 mL was chosen to quantitatively elute DOM adsorbed to the PPL resin. Extractions were performed <4 hours after sample collection and for every extraction, a blank using 100 mL 0.1% formic acid water (LC-MS ultra CHROMASOLV®) was run. The extracts were stored at −20 °C until analysis.
2.5 FT-ICR MS analysis
Analysis was performed using a Bruker Solarix 12 T FT-ICR MS operating with electrospray ionization in negative mode, ESI(−). Negative mode was chosen to allow largest possible diversity of compositions detected.12 FT-ICR MS measurements and data processing including formula assignments, filtration and verification (for halogenated DOM compositions) were conducted using protocols described previously.16 The elements included in formula assignment were: 12C0–100, 1H0–∞, 16O0–80, 14N0–3, 32S0–2, 35Cl0–3 and 79Br0–3. The assigned formulae were filtered based on peak amplitude (total ion count >3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), mass error (error <0.2 ppm between the experimental and theoretical mass) and the so-called nitrogen rule. A subsequent filtration procedure removed chemically unrealistic formulae, keeping formulae with C, O and H > 0, O/C ratio ≤ 1, H/C ratio ≤ 2.5, and double bond equivalences (DBE) ≥ 0. Furthermore, the number of nitrogen and sulfur atoms were limited to one during data analysis. A specific verification procedure was adopted as a quality control in the assignment of halogenated compounds. In short, a Matlab script was developed to identify halogenated compositions (assigned based on the presence of 35Cl or 79Br), for which a mass peak for the second stable isotope (37Cl or 81Br) was computed and searched for in the mass lists. Halogenated formulae determined in this study were limited to those that comprised both 35/37Cl or 79/81Br variants, using this procedure. Therefore, compounds for which the less abundant halogen isotope was below the detection limit were not included. From the molecular formulae, elemental ratios and various indices were computed. The indices used were double bond equivalences (DBE), DBE/C ratios, a modified aromaticity index (AImod), the average carbon oxidation state (COS) and modified Kendrick mass defects (−KMD/z*).16,24–26 Average weighted values were obtained by computing the ratio of individual mass peak amplitudes by the consolidated intensities of all mass peaks in a spectrum.
000), mass error (error <0.2 ppm between the experimental and theoretical mass) and the so-called nitrogen rule. A subsequent filtration procedure removed chemically unrealistic formulae, keeping formulae with C, O and H > 0, O/C ratio ≤ 1, H/C ratio ≤ 2.5, and double bond equivalences (DBE) ≥ 0. Furthermore, the number of nitrogen and sulfur atoms were limited to one during data analysis. A specific verification procedure was adopted as a quality control in the assignment of halogenated compounds. In short, a Matlab script was developed to identify halogenated compositions (assigned based on the presence of 35Cl or 79Br), for which a mass peak for the second stable isotope (37Cl or 81Br) was computed and searched for in the mass lists. Halogenated formulae determined in this study were limited to those that comprised both 35/37Cl or 79/81Br variants, using this procedure. Therefore, compounds for which the less abundant halogen isotope was below the detection limit were not included. From the molecular formulae, elemental ratios and various indices were computed. The indices used were double bond equivalences (DBE), DBE/C ratios, a modified aromaticity index (AImod), the average carbon oxidation state (COS) and modified Kendrick mass defects (−KMD/z*).16,24–26 Average weighted values were obtained by computing the ratio of individual mass peak amplitudes by the consolidated intensities of all mass peaks in a spectrum.
2.6 Approach for data analysis
Two replicate samples were analyzed and in general, the coherence between them were high. However, for a few samples the spectral intensities of one replicate were significantly lower and to enable homogenized treatment of data, the replicate with the highest overall abundance, representing the highest level of molecular information, was chosen for data analysis. Data analysis focused on compositional changes among DBPs between DWTPs and taps at an aggregated level, i.e., a qualitative, rather than a quantitative analytical approach was adopted.3. Results and discussion
3.1 Differences in raw water characteristics
Water characteristics that were clearly different between the groundwater source (MAL) and the surface water sources (LIN, NOR, STO) regarded conductivity and TN. The higher conductivity in MAL (Table S1†) was explained primarily by the presence of chloride, sulphate, calcium, magnesium and sodium. The higher TN concentration in MAL (Table S1†), was primarily explained by ammonium ions. Water temperatures were most stable in the groundwater (MAL) and varied most in LIN (from 1.2 °C in January to 23.2 °C in August). Variations of pH were rather small (7.2–8.0).The raw water of STO had the highest DOC concentrations (7.0–7.9 mg L−1), followed by the raw water of NOR (5.6–7.0 mg L−1) (Table S1†). The DOC in LIN and MAL raw waters ranged between 2.2–3.0 mg L−1. STO and NOR had similar UVA254, indicating a similarity in optical bulk DOM characteristics. In LIN, UVA254 and SUVA were low, demonstrating a low abundance of aromatic DOM.
FT-ICR MS compositional data of raw water DOM formulae consolidated for all five sampling months, are shown in Table S2.† Molecules containing carbon, hydrogen, and oxygen (CHO) dominated (69–75%), followed by molecules with an additional nitrogen (CHNO) (16–22%), sulfur (CHOS) (8–11%), and a few molecules containing both nitrogen and sulfur (CHNOS) (<1%). LIN CHO formulae were more saturated (higher H/C ratio, lower double bond equivalences (DBE) and modified aromaticity index (AImod), compared to the other water sources, which is in accordance with the low absorbance observed (Table S1†). Nitrogen-containing DOM had higher DBE, compared to the CHO compositions, suggesting a higher abundance of unsaturated compounds among the nitrogen-containing DOM. The sulfur-containing DOM instead had lower weighted average of DBE and relatively elevated H/C ratio, suggesting higher relative proportions of near saturated aliphatic compounds. Few halogenated formulae were detected in the raw water (n = 16, 14, 10, 2) at LIN, NOR, STO and MAL, respectively, when summarizing formulae from the five sampling occasions, including both chlorine- and/or bromine-containing compounds.
3.2 Overall treatment-induced changes
Temperature did not change significantly during treatments while conductivity increased slightly from source to tap, except in MAL, where softening reduced conductivity by ∼50% (Fig. S1–S4†). pH was adjusted at the DWTPs to optimize certain treatments, such as coagulation (pH 6.4–6.9) in NOR and STO (Fig. S2 and S3†) and softening (pH ∼9) in MAL (Fig. S4†). pH was also adjusted before distribution (to above 8) to avoid corrosion in the distribution network. TN was slightly affected by coagulation (NOR and STO) and chloramine addition (STO) (Fig. S2 and S3†) and showed great variation at the consumer tap in MAL (Fig. S4†). Of the treatments (Table 1), coagulation, used in NOR and STO, showed the largest effect on DOM properties, including decreases in both DOC concentration (2.8–3.7 mg L−1 in NOR and 2.9–3.5 mg L−1 in STO), UVA254 (0.11–0.19 cm−1 in NOR and 0.11–0.15 cm−1 in STO) and SUVA (0.9–1.8 L mg−1 m−1 in NOR and 0.8–1.2 L mg−1 m−1 in STO). Tables S3- and S4† summarize relevant water characteristics at the point right before chemical disinfection.3.3 Halogenated DOM from source to tap
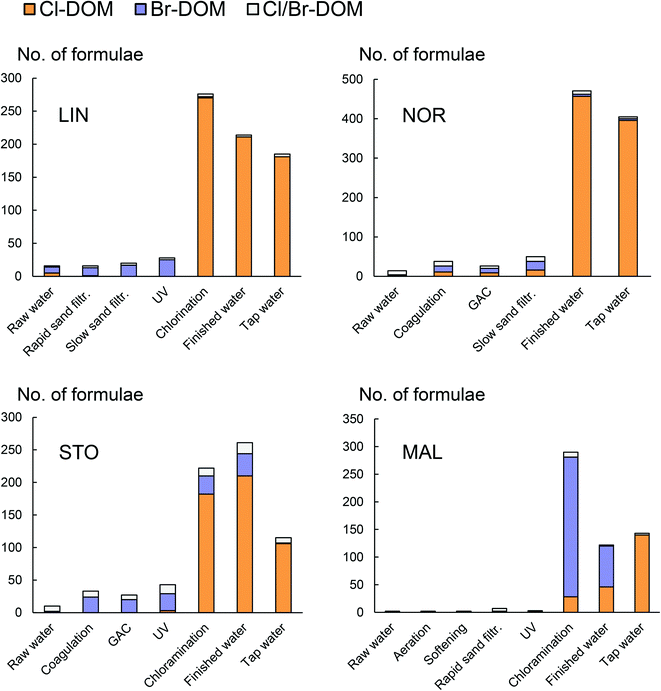
Some halogenated organic molecules, which were mostly brominated were detected and verified in all raw waters, but a major change was observed after chemical disinfection (Fig. 1), explained by the formation of DBPs.1 CHO-type molecules were the most significant precursors to halogenated DBPs but verified CHNO- and CHOS-based DBPs were also detected (Fig. S10 and S11†). The DBPs were mostly chlorinated, but at STO and especially at MAL, significant numbers of brominated compounds were detected, likely due to the relatively high concentration of bromide (∼0.28 mg L−1, analyzed at Eurofins accredited laboratory) in the source water of MAL. When including all variants of CHO-, CHNO- and CHOS-based brominated DBPs (Br-DBPs) detected at MAL, 18% had AImod > 0.50, which has been used as a threshold for aromatic structures,24 while 11% of the chlorinated DBPs (Cl-DBPs) were above that threshold. At STO, aromatic DBPs were found in similar proportions among Cl-DBPs (9%) and Br-DBPs (8%).3.4 Changes in the distribution system
From the point of disinfection to consumer taps, the abundance of halogenated compounds detected by FT-ICR MS decreased in LIN (33%), NOR (14%) and STO (48%), representing the DWTPs for which outgoing water is directly comparable with the tap water sample (Fig. 1). The observed molecular formulae changes indicate a dynamic DBP pool that changes in the distribution system and the observed trend might be explained by (1) transformation to DBPs not detectable with FT-ICR MS, e.g., volatile DBPs, such as THMs or (2) decomposition reactions, e.g., biodegradation leading to release of chloride or bromide ions and compounds lacking organically bound halogens,27,28 or (3) transformation to DBPs detected both at the DWTP and at the tap.In previous work, an overall decrease among phenolic DBPs during distribution of chlorinated and chloraminated water was observed29 and the pattern might be explained by hydrolytic reactions of these larger DBP molecules forming low molecular mass volatile compounds, such as THMs, which usually increase in number with increased contact times.30 In particular, this is likely for water treated with chlorine.31 However, for the DWTPs using chloramine in this study, such volatile DBPs were rarely detected.17,32 Through model-based experiments, aimed to explain changes in total organic halogen balances, the pathway that was suggested to lead to significant decay during chloramination (i.e., ∼15% of Cl-DBPs and ∼33% of Br-DBPs), was hydrolytic reactions leading to decomposition,27i.e., resulting in an altogether decrease in halogenated organic compounds. Such reactions can be catalyzed by dehalogenase enzymes during biodegradation, the rates of which increase with higher total organic carbon levels and availability of nutrients, such as phosphate, as observed for haloacetic acids.28
Changes to overall DOM composition during distribution was small (Fig. S5–S8 and Tables S5 and S6†) and most of the individual DOM formulae were detected both at the DWTPs and in the tap waters (Fig. S12†). Thus, changes among detected CHO-, CHNO- and CHOS-molecular formulae could not explain the DBP shifts observed. Contact times in the system may be indicated by differences in total chorine between DWTPs and consumer taps (Table 2). For example, total chlorine levels at NOR were rather unchanged between finished water and the tap (Table 2), which can be linked to a shorter contact time between DWTP and the tap point, compared to the other locations, possibly also explaining the smaller changes of halogenated compounds at NOR (Fig. 1). The exact contact times in the distribution system are difficult to estimate and vary with water use but were expected to be relatively similar for LIN and NOR (<24 hours), while being longer in STO (up to 4 days). In MAL, a higher number of DBPs was detected at the tap than at the DWTP (Fig. 1), likely due to the contribution of DBPs from other DWTPs in the distribution system. However, from the point of chloramine addition to the tap water point, the number of detected DBP formulae decreased by 51%.
| Total Cl2 (mg L−1) | Mar | May | Aug | Nov | Jan | |
|---|---|---|---|---|---|---|
| LIN | Chlorination | 0.26 | 0.27 | 0.44 | 0.26 | 0.22 |
| Finished water | 0.12 | 0.11 | 0.12 | 0.11 | 0.11 | |
| Tap water | ND | ND | ND | ND | ND | |
| NOR | Finished water | 0.32 | 0.27 | 0.29 | 0.38 | 0.31 |
| Tap water | 0.27 | 0.22 | 0.22 | 0.27 | 0.27 | |
| STO | Chloramination | 0.21 | 0.26 | 0.29 | 0.34 | 0.19 |
| Finished water | 0.16 | 0.26 | 0.31 | 0.34 | 0.20 | |
| Tap water | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | |
| MAL | Chloramination | 0.26 | 0.27 | 0.26 | 0.27 | 0.29 |
| Finished water | 0.11 | 0.16 | 0.22 | 0.22 | 0.16 | |
| Tap water | ND | ND | ND | 0.11 | ND |
3.5 The fate of Br-DBPs
The fate and contribution of Br-DBPs in consumer drinking water are particularly relevant, since they are generally 10–100 times more cytotoxic than their chlorine-containing analogues.33 The number of detected bromine-containing formulae decreased from the point of chloramine addition to tap (STO) and from the point of chloramine addition to finished water (MAL, comparison to tap not included for MAL because of the mixing of water from other DWTPs in the distribution system). This indicates changes in the balance between Cl-DBPs and Br-DBPs during distribution to consumers, potentially explained by the different stabilities of Cl-DBPs and Br-DBPs, where Br-DBPs are less stable.27,34,35 Debromination by hydrolytic reaction is faster than dechlorination as the Br–C bond is longer and its dissociation energy lower,36,37i.e., the C–Br bond is weaker. The degradation rate increases under alkaline conditions (Fig. S1–S4†).34Br in a phenolic Br-DBP can be substituted by Cl to form its Cl-DBP analogue38 and during chlorination, up to 30% of the organically bound bromine was estimated to be transformed to organically bound chlorine.27 However, such replacement reactions involving chloramine have not been considered likely, due to the lower reactivity of chloramine, compared to chlorine.39 The potential influence on DBP speciation of the biofilm inside the pipes in the distribution system, which can be considered an additional source of DOM, has been investigated, both for chlorine and chloramine residuals,40 however, for the low residual levels of disinfectant reported in this study (Table 2), such reactions are not expected to contribute significantly to the shift in the Br- and Cl-DBP balance. Altogether, the more likely reasons for the Br-DBP decrease in the distribution system is attributed to their faster hydrolysis reactions.
3.6 Characterization of tap water DBPs
The higher weighted average oxidation state of carbon (COS) among tap water DBPs (except in STO) compared to DBPs detected at the point of disinfection (Table 3) are likely linked to the continuous oxidation reactions with the residual disinfectant in the distribution system. The opposite trend observed in STO was linked to an overall lower oxygen and higher hydrogen content among tap water DBPs, caused by the presence of these formulae (of which many had high mass peak amplitude): C15H28O3ClBr, C15H30O2ClBr, C16H32O2ClBr, C19H34OSClBr (detected both in DWTP and tap, Tables S7 and S8†) and C14H28O4NBr, C16H32O3ClBr, C17H30OClBr, C21H20OCl2 (detected in tap water only, Tables S7 and S9†). These formulae are suggested to be halogenated fatty acids and their elevated contribution among tap water DBPs in STO might be explained by biofilm microbial activity41 or DBP formation42 involving biofilm-originated precursors.| DBP characteristics | LIN | NOR | STO | MAL | ||||
|---|---|---|---|---|---|---|---|---|
| Disinf. | Tap | Disinf. | Tap | Disinf. | Tap | Disinf. | Tap | |
| Number of DBP formulae | 276 | 185 | 471 | 405 | 222 | 115 | 290 | 143 |
| Mass weighted average [Da] | 382.5 | 373.6 | 372.7 | 375.1 | 377.0 | 369.9 | 414.9 | 384.4 |
| Average DBE | 7.6 | 7.8 | 7.7 | 7.8 | 7.4 | 6.8 | 8.4 | 7.8 |
| Average AImod | 0.35 | 0.38 | 0.38 | 0.38 | 0.36 | 0.32 | 0.43 | 0.37 |
| Average carbon oxidation state (COS) | 0.070 | 0.130 | 0.036 | 0.063 | −0.082 | −0.292 | 0.007 | 0.028 |
| Average DBE/C | 0.48 | 0.51 | 0.49 | 0.50 | 0.46 | 0.42 | 0.52 | 0.48 |
| Average H [%] | 40.5 | 39.2 | 40.3 | 39.9 | 42.1 | 44.8 | 40.0 | 40.5 |
| Average C [%] | 37.1 | 37.7 | 37.8 | 37.8 | 37.5 | 36.9 | 39.1 | 37.7 |
| Average O [%] | 19.7 | 20.2 | 19.1 | 19.5 | 17.6 | 15.6 | 18.1 | 18.7 |
| Average N [%] | 0.00 | 0.03 | 0.00 | 0.00 | 0.03 | 0.01 | 0.04 | 0.03 |
| Average S [%] | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.02 | 0.09 | 0.02 |
| Average Cl [%] | 2.7 | 2.8 | 2.7 | 2.8 | 2.4 | 2.3 | 0.3 | 3.0 |
| Average Br [%] | 0.0 | 0.0 | 0.1 | 0.0 | 0.4 | 0.4 | 2.3 | 0.0 |
| Computed average H/C ratio | 1.09 | 1.04 | 1.07 | 1.05 | 1.12 | 1.21 | 1.02 | 1.07 |
| Computed average O/C ratio | 0.53 | 0.54 | 0.51 | 0.52 | 0.47 | 0.42 | 0.46 | 0.50 |
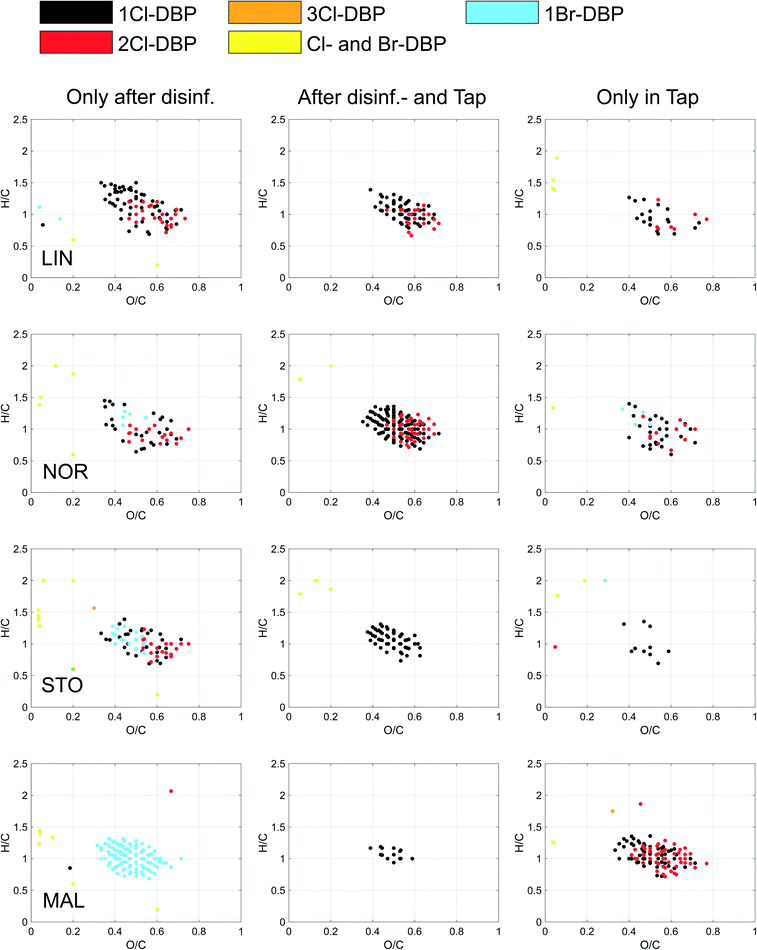
Other trends from disinfection to tap were observed, e.g., for average DBE, AImod and DBE/C where DBPs detected in tap water altogether had stronger aromatic character in LIN, while the opposite trend was seen in STO, partly explained by the increased contribution of halogenated fatty acids. These results highlight that changes occur in the distribution system, that the pool of available DBPs is changing and that these changes can be different for various DWTPs and distribution systems. Of the individual DBP formulae detected in LIN, NOR and STO tap waters, 71–81% were also detected at the point of disinfection (Fig. 2 and S13, Table S7,† left column), indicating that these DBP compositions were rather stable. These DBP formulae (Table S8†) were part of the DBPs to which consumers are exposed. At STO, the proportion of DBPs containing two chlorine atoms decreased from ∼18% after disinfection to <1% at the tap, possibly connected to differences in dehalogenation rates depending on the number of halogen substituents.36
DBPs solely detected in tap waters probably formed after longer contact times and were characterized by lower average O/C ratios (LIN and STO) and higher average mass (all DWTPs) (Table S7,† right column) and are listed in Table S9.† At MAL, the DBPs detected at the tap were substantially different from those detected at the DWTP (Fig. 2 and S13†) and not a single Br-DBP of those formed after chloramine addition were detected in the tap water (Fig. 1 and 2 and S13†). This was probably a result both of mixing in of water from other DWTPs in the system and potential transformations of MAL DBPs and highlights the challenges of addressing end-user DBP exposure via water from multiple DWTPs. DBPs detected after disinfection only are summarized in Table S10.†
3.7 Dynamic DBP mixtures
The patterns observed through non-target DBP analysis at the different stages, i.e., directly after chemical disinfection, finished water leaving the DWTP and tap water, demonstrate that the mixture of DBPs in drinking water leaving a DWTP is not the same as the DBP mixture consumers are exposed to. A perspective of dynamic transformations of DBPs, where the contribution of different DBP classes to the total DBP pool decrease or increase during drinking water distribution is in contrast to the commonly adopted static perspective, assuming that each DBP class contributes equally over time.43 The dynamic perspective suggests that high molecular weight DBPs decrease in contribution to the DBP pool with increased chlorine/chloramine residual contact time, while the typically monitored DBPs, including THMs and haloacetic acids (HAAs), and other volatile DBPs increase in contribution with increased contact time.43 This is likely a consequence of the continuous halogenation which finally results in ring opening (in case of aromatic precursors) and release of THMs.44Because brominated DBPs are often more toxic than chlorinated analogues,45 the lower counts of Br-DBPs detected by FT-ICR MS in tap waters seem favorable in terms of toxicity considering this group of DBPs only, but effects on overall toxicity is unclear as potential shifts to non-detected DBPs is not assessed.
The fate of different DBP groups vary; at specific conditions some will accumulate, while others degrade.39,46 If original DBP mixtures are transformed to less toxic mixtures over time it might have implications for the operation of drinking water distribution systems, e.g., optimizing water age of distributed drinking water as means to reduce health effects caused by DBPs. It seems logical to follow up this work using, e.g., TIC-TOX approaches,6 or effect-based methods47–49 to further investigate how the combined toxic effects from DBP mixtures change in distribution systems.
While the non-target FT-ICR-MS approach used here offers broadest coverage of molecular compositions and highest sensitivity among currently available chemical screening methods, it is still subject to ionization selectivity, which makes considerable parts of the DBP pool undetectable; the used extraction procedure was also selective.50 Moreover, the verification process was highly conservative and likely missed many DBP formulae. To improve FT-ICR-MS-based DBP detection including isotope verification, a database with verified DBP formulae, to which actual mass spectra could be matched, would be a useful asset. Halogenated DBPs, for which the 37Cl/81Br isotope is not detected but has been previously verified, could then be indirectly confirmed.
4. Conclusions
Despite an expected dehalogenation or transformation of DBPs to smaller, volatile compounds during distribution, FT-ICR MS detected DBPs were found in various tap water samples, showing that these oxygen-rich aromatic compounds, are relevant for human exposure. The dynamic changes of DBP mixtures between DWTPs and consumer taps highlight that DBP exposure cannot be accurately assessed by measurements at the DWTP alone but require DBP monitoring at points-of-use. Better understanding of the variability and changes (from DWTP to tap) of DBP mixtures is needed. Given that specific characteristics, unique for each distribution system, seem important for the local spatial variability and dynamics of DBPs, collaborations between researchers and engineers specialized on the water distribution systems will be beneficial to better assess and manage future DBP exposures.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by FORMAS, the Swedish Research Council for Sustainable Development [grant no. 2013-1077]. This is contribution [no. 6057] of the University of Maryland Center for Environmental Science. We warmly thank the staff at Berggården, Borg, Görväln and Bulltofta for the assistance and support during sample collection and data analysis; special thanks go to Peter Collin, Bodil Widell, Kristina Dahlberg, Lena Cedergren and Martin Sandell.References
- S. D. Richardson, M. J. Plewa, E. D. Wagner, R. Schoeny and D. M. DeMarini, Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research, Mutat. Res., 2007, 636, 178–242 CAS.
- C. M. Villanueva, S. Cordier, L. Font-Ribera, L. A. Salas and P. Levallois, Overview of Disinfection By-products and Associated Health Effects, Curr. Environ. Health Rep., 2015, 2, 107–115 CrossRef CAS PubMed.
- M. Säve-Söderbergh, J. Toljander, C. Donat-Vargas, M. Berglund and A. Åkesson, Exposure to drinking water chlorination by-products and fetal growth and prematurity: A nation wide register-based prospective study, Environ. Health Perspect., 2020, 128, 057006 CrossRef PubMed.
- M. Säve-Söderbergh, Risks and benefits of drinking water treatment - focusing on child health and prenatal development, Karolinska Institutet, 2021 Search PubMed.
- S. E. Hrudey, L. C. Backer, A. R. Humpage, S. W. Krasner, D. S. Michaud, L. E. Moore, P. C. Singer and B. D. Stanford, Evaluating Evidence for Association of Human Bladder Cancer with Drinking-Water Chlorination Disinfection By-Products, J. Toxicol. Environ. Health B Crit. Rev., 2015, 18, 213–241 CrossRef CAS PubMed.
- M. J. Plewa, E. D. Wagner and S. D. Richardson, TIC-Tox: A preliminary discussion on identifying the forcing agents of DBP-mediated toxicity of disinfected water, J. Environ. Sci., 2017, 58, 208–216 CrossRef CAS PubMed.
- D. Stalter, L. I. Peters, E. O'Malley, J. Y. M. Tang, M. Revalor, M. J. Farré, K. Watson, U. Von Gunten and B. I. Escher, Sample Enrichment for Bioanalytical Assessment of Disinfected Drinking Water: Concentrating the Polar, the Volatiles, and the Unknowns, Environ. Sci. Technol., 2016, 50, 6495–6505 CrossRef CAS PubMed.
- A. Hebert, C. Feliers, C. Lecarpentier, P. A. Neale, R. Schlichting, S. Thibert and B. I. Escher, Bioanalytical assessment of adaptive stress responses in drinking water: A predictive tool to differentiate between micropollutants and disinfection by-products, Water Res., 2018, 132, 340–349 CrossRef CAS PubMed.
- N. Hertkorn, R. Benner, M. Frommberger, P. Schmitt-Kopplin, M. Witt, K. Kaiser, A. Kettrup and J. I. Hedges, Characterization of a major refractory component of marine dissolved organic matter, Geochim. Cosmochim. Acta, 2006, 70, 2990–3010 CrossRef CAS.
- M. Gonsior, B. M. Peake, W. T. Cooper, D. C. Podgorski, J. D'Andrilli, T. Dittmar and W. J. Cooper, Characterization of dissolved organic matter across the Subtropical Convergence off the South Island, New Zealand, Mar. Chem., 2011, 123, 99–110 CrossRef CAS.
- R. Flerus, O. J. Lechtenfeld, B. P. Koch, S. L. McCallister, P. Schmitt-Kopplin, R. Benner, K. Kaiser and G. Kattner, A molecular perspective on the ageing of marine dissolved organic matter, Biogeosciences, 2012, 9, 1935–1955 CrossRef CAS.
- N. Hertkorn, M. Frommberger, M. Witt, B. P. Koch, P. Schmitt-Kopplin and E. M. Perdue, Natural organic matter and the event horizon of mass spectrometry, Anal. Chem., 2008, 80, 8908–8919 CrossRef CAS PubMed.
- J. Valle, M. Harir, M. Gonsior, A. Enrich-Prast, P. Schmitt-Kopplin, D. Bastviken and N. Hertkorn, Molecular differences between water column and sediment pore water SPE-DOM in ten Swedish boreal lakes, Water Res., 2020, 170, 115320 CrossRef CAS PubMed.
- E. E. Lavonen, M. Gonsior, L. J. Tranvik, P. Schmitt-Kopplin and S. J. Köhler, Selective chlorination of natural organic matter: Identification of previously unknown disinfection byproducts, Environ. Sci. Technol., 2013, 47, 2264–2271 CrossRef CAS PubMed.
- M. Gonsior, P. Schmitt-Kopplin, H. Stavklint, S. D. Richardson, N. Hertkorn and D. Bastviken, Changes in Dissolved Organic Matter during the Treatment Processes of a Drinking Water Plant in Sweden and Formation of Previously Unknown Disinfection Byproducts, Environ. Sci. Technol., 2014, 48, 12714–12722 CrossRef CAS PubMed.
- A. Andersson, M. Harir, M. Gonsior, N. Hertkorn, P. Schmitt-Kopplin, H. Kylin, S. Karlsson, M. J. Ashiq, E. Lavonen, K. Nilsson, Ä. Pettersson, H. Stavklint and D. Bastviken, Waterworks-specific composition of drinking water disinfection by-products, Environ. Sci.: Water Res. Technol., 2019, 5, 861–872 RSC.
- C. Postigo, A. Andersson, M. Harir, D. Bastviken, M. Gonsior, P. Schmitt-Kopplin, P. Gago-Ferrero, L. Ahrens, L. Ahrens and K. Wiberg, Unraveling the chemodiversity of halogenated disinfection by-products formed during drinking water treatment using target and non-target screening tools, J. Hazard. Mater., 2021, 401, 123681 CrossRef CAS PubMed.
- L. C. Powers, A. Conway, C. L. Mitchelmore, S. J. Fleischacker, M. Harir, D. C. Westerman, J. P. Croue, P. Schmitt-Kopplin, S. D. Richardson and M. Gonsior, Tracking the formation of new brominated disinfection by-products during the seawater desalination process, Environ. Sci.: Water Res. Technol., 2020, 6, 2521–2541 RSC.
- H. Zhang, Y. Zhang, Q. Shi, S. Ren, J. Yu, F. Ji, W. Luo and M. Yang, Characterization of low molecular weight dissolved natural organic matter along the treatment trait of a waterworks using Fourier transform ion cyclotron resonance mass spectrometry, Water Res., 2012, 46, 5197–5204 CrossRef CAS PubMed.
- J. H. Sharp, A. Y. Beauregard, D. Burdige, G. Cauwet, S. E. Curless, R. Lauck, K. Nagel, H. Ogawa, A. E. Parker, O. Primm, M. Pujo-Pay, W. B. Savidge, S. Seitzinger, G. Spyres and R. Styles, A direct instrument comparison for measurement of total dissolved nitrogen in seawater, Mar. Chem., 2004, 84, 181–193 CrossRef CAS.
- R. Benner and J. I. Hedges, A test of the accuracy of freshwater DOC measurements by high-temperature catalytic oxidation and UV-promoted persulfate oxidation, Mar. Chem., 1993, 41, 161–165 CrossRef CAS.
- J. L. Weishaar, G. R. Aiken, B. A. Bergamaschi, M. S. Fram, R. Fujii and K. Mopper, Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon, Environ. Sci. Technol., 2003, 37, 4702–4708 CrossRef CAS PubMed.
- T. Dittmar, B. Koch, N. Hertkorn and G. Kattner, A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater, Limnol. Oceanogr.: Methods, 2008, 6, 230–235 CrossRef CAS.
- B. P. Koch and T. Dittmar, From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter, Rapid Commun. Mass Spectrom., 2006, 20, 926–932 CrossRef CAS.
- S. Shakeri Yekta, M. Gonsior, P. Schmitt-Kopplin and B. H. Svensson, Characterization of dissolved organic matter in full scale continuous stirred tank biogas reactors using ultrahigh resolution mass spectrometry: A qualitative overview, Environ. Sci. Technol., 2012, 46, 12711–12719 CrossRef CAS PubMed.
- C. A. Hughey, C. L. Hendrickson, R. P. Rodgers, A. G. Marshall and K. Qian, Kendrick mass defect spectrum: A compact visual analysis for ultrahigh-resolution broadband mass spectra, Anal. Chem., 2001, 73, 4676–4681 CrossRef CAS PubMed.
- X. Zhu and X. Zhang, Modeling the formation of TOCl, TOBr and TOI during chlor(am)ination of drinking water, Water Res., 2016, 96, 166–176 CrossRef CAS PubMed.
- M. Behbahani, B. Lin, T. L. Phares and Y. Seo, Understanding the impact of water distribution system conditions on the biodegradation of haloacetic acids and expression of bacterial dehalogenase genes, J. Hazard. Mater., 2018, 351, 293–300 CrossRef CAS PubMed.
- Y. Pan, Y. Wang, A. Li, B. Xu, Q. Xian, C. Shuang, P. Shi and Q. Zhou, Detection, formation and occurrence of 13 new polar phenolic chlorinated and brominated disinfection byproducts in drinking water, Water Res., 2017, 112, 129–136 CrossRef CAS PubMed.
- M. Serrano, I. Montesinos, M. J. Cardador, M. Silva and M. Gallego, Seasonal evaluation of the presence of 46 disinfection by-products throughout a drinking water treatment plant, Sci. Total Environ., 2015, 517, 246–258 CrossRef CAS PubMed.
- W. W. Wu, P. A. Chadik and J. J. Delfino, The relationship between disinfection by-product formation and structural characteristics of humic substances in chloramination, Environ. Toxicol. Chem., 2003, 22, 2845–2852 CrossRef CAS PubMed.
- A. Andersson, M. J. Ashiq, M. Shoeb, S. Karlsson, D. Bastviken and H. Kylin, Evaluating gas chromatography with a halogen-specific detector for the determination of disinfection by-products in drinking water, Environ. Sci. Pollut. Res., 2019, 26, 7305–7314 CrossRef CAS PubMed.
- E. D. Wagner and M. J. Plewa, CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: An updated review, J. Environ. Sci., 2017, 58, 64–76 CrossRef CAS PubMed.
- G. Hua and D. A. Reckhow, Effect of alkaline pH on the stability of halogenated DBPs, J. - Am. Water Works Assoc., 2012, 104, 49–50 CrossRef CAS.
- B. Chen, Hydrolytic stabilities of halogenated disinfection byproducts: Review and rate constant quantitative structure-property relationship analysis, Environ. Eng. Sci., 2011, 28, 385–394 CrossRef CAS.
- S. Ding, Y. Deng, T. Bond, C. Fang, Z. Cao and W. Chu, Disinfection byproduct formation during drinking water treatment and distribution: A review of unintended effects of engineering agents and materials, Water Res., 2019, 160, 313–329 CrossRef CAS PubMed.
- L. Wang, B. Chen and T. Zhang, Predicting hydrolysis kinetics for multiple types of halogenated disinfection byproducts via QSAR models, Chem. Eng. J., 2018, 342, 372–385 CrossRef CAS.
- H. Zhai and X. Zhang, Formation and decomposition of new and unknown polar brominated disinfection byproducts during chlorination, Environ. Sci. Technol., 2011, 45, 2194–2201 CrossRef CAS PubMed.
- H. Zhai, X. Zhang, X. Zhu, J. Liu and M. Ji, Formation of brominated disinfection byproducts during chloramination of drinking water: New polar species and overall kinetics, Environ. Sci. Technol., 2014, 48, 2579–2588 CrossRef CAS PubMed.
- Z. Wang, L. Li, R. W. Ariss, K. M. Coburn, M. Behbahani, Z. Xue and Y. Seo, The role of biofilms on the formation and decay of disinfection by-products in chlor(am)inated water distribution systems, Sci. Total Environ., 2021, 753, 141606 CrossRef CAS PubMed.
- V. M. Dembitsky and M. Srebnik, Natural halogenated fatty acids: Their analogues and derivatives, Prog. Lipid Res., 2002, 41, 315–367 CrossRef CAS PubMed.
- M. Gonsior, L. C. Powers, E. Williams, A. Place, F. Chen, A. Ruf, N. Hertkorn and P. Schmitt-Kopplin, The chemodiversity of algal dissolved organic matter from lysed Microcystis aeruginosa cells and its ability to form disinfection by-products during chlorination, Water Res., 2019, 155, 300–309 CrossRef CAS PubMed.
- X. F. Li and W. A. Mitch, Drinking Water Disinfection Byproducts (DBPs) and Human Health Effects: Multidisciplinary Challenges and Opportunities, Environ. Sci. Technol., 2018, 52, 1681–1689 CrossRef CAS PubMed.
- M. B. Heeb, J. Criquet, S. G. Zimmermann-Steffens and U. Von Gunten, Oxidative treatment of bromide-containing waters: Formation of bromine and its reactions with inorganic and organic compounds—A critical review, Water Res., 2014, 48, 15–42 CrossRef CAS PubMed.
- M. J. Plewa, E. D. Wagner, M. G. Muellner, K.-M. Hsu and S. D. Richardson, Comparative mammalian cell toxicity of N-DBPs and C-DBPs, Urbana, 2008, vol. 51, p. 61801 Search PubMed.
- Y. Yu and D. A. Reckhow, Kinetic Analysis of Haloacetonitrile Stability in Drinking Waters, Environ. Sci. Technol., 2015, 49, 11028–11036 CrossRef CAS PubMed.
- B. I. Escher, C. Van Daele, M. Dutt, J. Y. M. Tang and R. Altenburger, Most oxidative stress response in water samples comes from unknown chemicals: The need for effect-based water quality trigger values, Environ. Sci. Technol., 2013, 47, 7002–7011 CrossRef CAS PubMed.
- B. I. Escher, M. Dutt, E. Maylin, J. Y. M. Tang, S. Toze, C. R. Wolf and M. Lang, Water quality assessment using the AREc32 reporter gene assay indicative of the oxidative stress response pathway, J. Environ. Monit., 2012, 14, 2877–2885 RSC.
- D. Stalter, E. O'Malley, U. Von Gunten and B. I. Escher, Mixture effects of drinking water disinfection by-products: Implications for risk assessment, Environ. Sci.: Water Res. Technol., 2020, 6, 2341–2351 RSC.
- Y. Li, M. Harir, J. Uhl, B. Kanawati, M. Lucio, K. S. Smirnov, B. P. Koch, P. Schmitt-Kopplin and N. Hertkorn, How representative are dissolved organic matter (DOM) extracts? A comprehensive study of sorbent selectivity for DOM isolation, Water Res., 2017, 116, 316–323 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ew00389e |
| This journal is © The Royal Society of Chemistry 2021 |