Open Access Article
Open Access ArticleThe bioaccumulation testing strategy for manufactured nanomaterials: physico-chemical triggers and read across from earthworms in a meta-analysis†
R. D.
Handy
 *a,
N. J.
Clark
*a,
N. J.
Clark
 a,
J.
Vassallo
a,
C.
Green
b,
F.
Nasser
b,
K.
Tatsi
a,
T. H.
Hutchinson
c,
D.
Boyle
a,
J.
Vassallo
a,
C.
Green
b,
F.
Nasser
b,
K.
Tatsi
a,
T. H.
Hutchinson
c,
D.
Boyle
 a,
M.
Baccaro
a,
M.
Baccaro
 d,
N.
van den Brink
d,
N.
van den Brink
 d and
C.
Svendsen
e
d and
C.
Svendsen
e
aSchool of Biological and Marine Sciences, University of Plymouth, PL4 8AA, UK. E-mail: r.handy@plymouth.ac.uk
bDepartment for Environment, Food and Rural Affairs (Defra), UK
cSchool of Geography, Earth and Environmental Sciences, University of Plymouth, PL4 8AA, UK
dWageningen University & Research, 6708 PB, Wageningen, Netherlands
eUK Centre for Ecology and Hydrology, Wallingford, OX10 8BB, UK
First published on 4th October 2021
Abstract
Little is known about the bioaccumulation potential of manufactured nanomaterials (MNs). For traditional chemicals, the Organisation for Economic Co-operation and Development (OECD) Test Guideline (TG) 305, bioaccumulation in fish is often used. However, for MNs, there are no approved processes to trigger or waive this test, or consider alternatives to vertebrate animals. The aim of the present study was to conduct a meta-analysis of existing data sets on particle properties and bioaccumulation in earthworms to understand what particle metrics could be used as a trigger for bioaccumulation testing. An apparent steady state tissue concentration of metal from MNs exposure in the earthworm (Eisenia fetida) was evident following exposures to Ag nanoparticles (NPs), CuO NPs and CdTe quantum dots (QDs). This allowed the derivation of nano bioaccumulation factors (nBAFs), calculated using soil and earthworm tissue metal concentrations. A prediction equation using all the particle metrics correlated with BAFs was possible. Similarly, nano biomagnification factors (nBMFs) were calculated in the rainbow trout (Oncorhynchus mykiss) tissue, relative to the concentration of total metals in the fish diet. Pearson's correlations were found to be significant, with p < 0.05 for nBMFs for the liver, mid intestine, hind intestine and kidney relative to the earthworm tissue nBAFs. Together these data indicate that bioaccumulation measurements in earthworms for metallic MNs could be predictive of those values in fish, and that there is scope to predict the bioaccumulation potential of MNs with confidence from a few simple particle metrics.
Environmental significanceDetermining the bioaccumulation potential of manufactured nanomaterials is a key part of environmental risk assessment for chemicals. A proposed tiered approach to bioaccumulation testing is important for the provision of safety information about chemicals, without necessarily needing vertebrate animal testing. A meta-analysis was undertaken, using existing data for silver, cupric oxide and cadmium telluride quantum dots, in order to show the feasibility of using physico-chemical triggers and validated invertebrate protocols as initial tools to inform decision makers of any bioaccumulation concerns. The results with silver and copper nanomaterials showed that the earthworm bioaccumulation test is predictive of the fish bioaccumulation test; thus offering immediate value for regulatory testing. |
Introduction
Manufactured nanomaterials (MNs) are now finding numerous applications in commercial products and industrial processes including: electronics, textiles, industrial coating and paints, cosmetics, medicines and medical devices, and the agri-food sector.1–5 For the latter, the range of applications includes food and drink, food packaging, nano-encapsulated crop protection products and fertilisers.2,5 The use of nano-enhanced agricultural products, such as nano pesticides, involves the direct application of the MN-containing product to biota, soil or water. In addition, the disposal of sewage sludge containing incidental nanomaterials to land is a major concern for the fate and behaviour of MNs in soils.6Arguably, the release of MNs into soil and surface waters has the potential to contaminate both terrestrial and aquatic food chains. Soils are estimated to contain μg kg−1 amounts of MNs,7 and surface waters contain ng L−1 to μg L−1 concentrations depending on the type of material.8 The ecotoxicity of MNs have been given considerable attention (e.g., review Lead et al.8). However, there is also concern regarding the bioaccumulation potential of MNs in wildlife.9 The precise definition of ‘bioaccumulation’ is debated, but originally it was intended as a steady-state determination of tissue concentration or whole body concentration of the test substance relative to the external medium.9 This approach has been applied to organic chemicals in fishes,10 to metal accumulation by soil invertebrates,11 and recently to MNs and their environmental transformations.12
For the purposes of regulatory testing, bioaccumulation is defined as the increase in concentration of a test chemical in or on an organism (or specified tissue thereof) relative to the concentration of the test substance in the surrounding medium.13 Laboratory studies with metal-containing MNs have demonstrated that total metal, nano form unknown inside the tissue, can accumulate in soil organisms including earthworms (CuO14), isopods (Ag NPs15), and nematodes (ZnO16). Manufactured nanomaterials also accumulate in some crops such as wheat and rice,17,18 and with the uptake mechanisms in plants partly elucidated.19 Similarly, soil mesocosm studies have raised concerns about the transfer of MNs through terrestrial food webs.20 It is therefore prudent to protect organisms in the environment from bioaccumulation hazards.
For chemicals, including MNs, the bioaccumulation potential is a key aspect of concern for environmental risk assessment. The European regulation on Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Annexes have been adapted by the Commission Regulation (EU) 2018/1881 to better address the safety of MNs. For traditional chemicals and MNs, bioaccumulation potential is measured using the Organisation for Economic Co-operation and Development (OECD) Test Guideline 305 for bioaccumulation in fish.21 For organic chemicals, the n-octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) has been used as a ‘chemical trigger’ of concern for bioaccumulation testing,9 and this is founded on the relationship between the lipid solubility of the substance (the log
Kow) has been used as a ‘chemical trigger’ of concern for bioaccumulation testing,9 and this is founded on the relationship between the lipid solubility of the substance (the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow value) and the measured bioaccumulation factor (BAF) in animal tissue.10,22 However, there are conceptual problems with applying the log
Kow value) and the measured bioaccumulation factor (BAF) in animal tissue.10,22 However, there are conceptual problems with applying the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow measurement to MNs that are not solutes and do not form steady-state equilibria.23 There are also practical difficulties in conducting the log
Kow measurement to MNs that are not solutes and do not form steady-state equilibria.23 There are also practical difficulties in conducting the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow method,24 such as the aggregation of the MNs at the oil–water interface, suggesting that log
Kow method,24 such as the aggregation of the MNs at the oil–water interface, suggesting that log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow may not be reliable as a trigger for the bioaccumulation testing of many MNs. The current default in the guidance if the log
Kow may not be reliable as a trigger for the bioaccumulation testing of many MNs. The current default in the guidance if the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow cannot be determined for a substance is to proceed directly to the in vivo fish test, TG 305.25
Kow cannot be determined for a substance is to proceed directly to the in vivo fish test, TG 305.25
For metals and in the EU, the appendix R7.13-2 of the REACH guidance applies,25 where instead of using log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow, a weight of evidence should be sought for a metal bioaccumulation concern. This mainly uses the scientific literature or existing data on dietary studies with fish, terrestrial organisms such as earthworms, evidence from aquatic and terrestrial food web studies, calculated oral probable no effect concentrations (PNECoral) in birds and mammals, as well as considering metal bioavailability in any bioaccumulation factor. Similar approaches including modelling bioaccumulation are suggested for the USA.26 However, it is not clear how such guidance for dissolved metals should apply to metallic MNs, and in the absence of existing bioaccumulation studies on the metallic MN of concern, then the default will be to conduct TG 305. This situation has unintended consequences for animal welfare, with mandatory vertebrate animal testing in TG 305 for MNs. In the EU alone, there were around 117 substances with forms in the nanoscale with a production volume over 100 tonnes per year expected to be registered under REACH,27 and with a fish bioaccumulation test per registration, this would potentially equate to an annual cost of ∼€10–15 million and 17
Kow, a weight of evidence should be sought for a metal bioaccumulation concern. This mainly uses the scientific literature or existing data on dietary studies with fish, terrestrial organisms such as earthworms, evidence from aquatic and terrestrial food web studies, calculated oral probable no effect concentrations (PNECoral) in birds and mammals, as well as considering metal bioavailability in any bioaccumulation factor. Similar approaches including modelling bioaccumulation are suggested for the USA.26 However, it is not clear how such guidance for dissolved metals should apply to metallic MNs, and in the absence of existing bioaccumulation studies on the metallic MN of concern, then the default will be to conduct TG 305. This situation has unintended consequences for animal welfare, with mandatory vertebrate animal testing in TG 305 for MNs. In the EU alone, there were around 117 substances with forms in the nanoscale with a production volume over 100 tonnes per year expected to be registered under REACH,27 and with a fish bioaccumulation test per registration, this would potentially equate to an annual cost of ∼€10–15 million and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 fish used for in vivo testing. In 2020, only around 136 unique registration updates covering 52 chemicals had been received by the European Chemicals Agency (ECHA), partly because of difficulty and confusion in the implementation of new nano-specific guidance in REACH.28 Innovation with new MNs is still growing rapidly, and there is an urgent need to overhaul the bioaccumulation testing strategy for MNs to rationalise the workload to testing only the materials of most concern while also minimising the use of vertebrate animals.
550 fish used for in vivo testing. In 2020, only around 136 unique registration updates covering 52 chemicals had been received by the European Chemicals Agency (ECHA), partly because of difficulty and confusion in the implementation of new nano-specific guidance in REACH.28 Innovation with new MNs is still growing rapidly, and there is an urgent need to overhaul the bioaccumulation testing strategy for MNs to rationalise the workload to testing only the materials of most concern while also minimising the use of vertebrate animals.
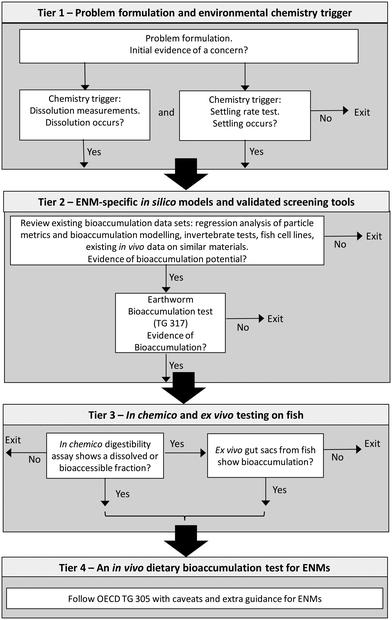
With these goals in mind, an alternative tiered approach to testing was proposed.9 This included four tiers, starting with a new physico-chemical trigger of concern and progressing towards selected in vivo testing with TG 305. The proposed tiers included: (i) particle settling and/or dissolution tests as alternative triggers to the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow test that are more relevant to the behaviour of MNs; (ii) the inclusion of data from in silico modelling, invertebrate tests, and/or cell cultures to provide a weight of evidence for a bioaccumulation concern; (iii) an in vitro tier using fish gut tissue; and finally tier (iv), the dietary method of TG 305. However, for regulatory acceptance of any such integrated approach to testing, it is vitally important to show the evidence-base that demonstrates the logic for tiers in the strategy and the links between the tiers. It is also crucial to show how any tests or measurements included in the first tier of the strategy are predictive of a bioaccumulation potential outcome. The relationship between the physico-chemical properties of MNs and their bioaccumulation potential in organisms is poorly understood, in part due to limited data sets on the latter.29 It is also unclear whether bioaccumulation measurements on terrestrial invertebrates such as earthworms, or other alternatives to vertebrate animal testing, would be predictive of bioaccumulation in fish and acceptable to replace, or decrease the use, of TG 305. Any scheme would also need to be precautionary and especially avoid false negatives.
Kow test that are more relevant to the behaviour of MNs; (ii) the inclusion of data from in silico modelling, invertebrate tests, and/or cell cultures to provide a weight of evidence for a bioaccumulation concern; (iii) an in vitro tier using fish gut tissue; and finally tier (iv), the dietary method of TG 305. However, for regulatory acceptance of any such integrated approach to testing, it is vitally important to show the evidence-base that demonstrates the logic for tiers in the strategy and the links between the tiers. It is also crucial to show how any tests or measurements included in the first tier of the strategy are predictive of a bioaccumulation potential outcome. The relationship between the physico-chemical properties of MNs and their bioaccumulation potential in organisms is poorly understood, in part due to limited data sets on the latter.29 It is also unclear whether bioaccumulation measurements on terrestrial invertebrates such as earthworms, or other alternatives to vertebrate animal testing, would be predictive of bioaccumulation in fish and acceptable to replace, or decrease the use, of TG 305. Any scheme would also need to be precautionary and especially avoid false negatives.
The overall aim of the current study was to use a meta-analysis approach to provide data to support the first two tiers of the testing strategy proposed by Handy et al.9 and to determine whether or not the scheme could be simplified to avoid vertebrate animal testing. The study necessarily focused on metal-containing MNs, where data sets on the total metal concentrations in tissues or whole animals are available. The specific objectives included to: (i) determine whether key particle metrics such as primary particle size, the hydrodynamic diameters of dispersions, settling behaviour, or dissolution rate, could be predictive of bioaccumulation observed in earthworms; and (ii) show whether bioaccumulation data from earthworms was predictive of bioaccumulation in fish for MNs following a dietary TG 305 protocol, with a view to exploring invertebrate alternatives to the fish bioaccumulation test.
Materials characterisation and data sources
The data sources used in this study were primary experimental data sets collected at the University of Plymouth during the following EU projects: NANOSOLUTIONS (https://nanosolutionsfp7.com/), Sustainable Nanotechnologies (SUN, http://www.sun-fp7.eu/) and NanoFASE (http://nanofase.eu/). The details of methodology are published in peer reviewed articles from these projects. This included detailed information on the MN characterisation with data on dissolution for all the materials in NANOSOLUTIONS,30 the CuO nanoparticles (NPs) used in NANOSOLUTIONS and SUN,14 and the Ag NPs and Ag2S NPs used in NanoFASE.31A summary of physico-chemical characteristics of the particles and materials used throughout this study are shown in Table 1; including details of purity and primary particle sizes, as well as data about the particles dispersion and settling in water. Only metal-containing MNs were considered where total metal could be measured in the tissues of organisms by inductively coupled plasma optical emission spectroscopy (ICP-OES) or mass spectrometry (ICP-MS) in order to infer bioaccumulation. Carbon-based MNs such as single walled carbon nanotubes (SWCNTs) were not considered because of the absence of routine methods of measuring the uptake of such materials in invertebrates. Raw data on total metal accumulation from MN exposures of earthworms were obtained from the following studies: CuO NPs;14 CdTe quantum dots (QDs);32 and Ag and Ag2S NPs.33
| Material | Manufacturer's information | Primary particle size (nm) | Hydrodynamic diameter (nm) | Metal dissolution rate (μg min−1) | Settling rate in ultrapure water (mg min−1) |
|---|---|---|---|---|---|
| a Supplied by Applied Nanoparticles (Barcelona) as part of the EU NanoFASE project. b Supplied by PlasmaChem GmbH as dry powder, with bespoke design as part of the EU NANOSOLUTIONS project. c Brunauer–Emmett–Teller (BET) surface area values (mean ± one standard deviation, n = 3) from EU NANOSOLUTIONS project. d Unless, otherwise stated based on transmission electron microscopy (TEM) images of material stocks in ultrapure water (18.2 MΩ, ELGA, UK) with data as mean ± standard error of the mean (S.E.M) and n = ≥60 measurements at University of Plymouth. e Unable to detect QDs using electron microscopy, an estimate is provided by Denmark Technical University. f Particle size distribution measurements (mean ± one standard deviation, n = 3) by nanoparticle tracking analysis (NTA) on the material stocks in ultrapure water at University of Plymouth. g Maximum slope from rectangular hyperbola function of curve fit of the metal rate of dissolution from the material stocks in ultrapure water during dialysis experiments (n = 3) at University of Plymouth. h Maximum particle settling calculated from an exponential decay curve fit of the material stocks in ultrapure water (calibration curves n = 3) at University of Plymouth. - Data not applicable to the test material. -- Not determined. | |||||
| Ag NPs | Diameter, 50 nm; concentration 10.4 g L−1 | 55 ± 3 | 66 ± 4 | 0.03 | -- |
| Ag2S NPs | Diameter, 20 nm; concentration 9.6 g L−1 | 37 ± 19 | 135 ± 7 | 0.00 | -- |
| AgNO3, Sigma-Aldrich | Purity, >99.00% | - | - | -- | - |
| CdTe bulk, Sigma-Aldrich | Diameter, <250 μm; purity, ≥99.99% trace metal basis | -- | 172 ± 28 | 0.003 Cd | -- |
| 0.002 Te | |||||
| CdTe QDs COOH-coated | Diameter, 3–5 nm | <4 | 84 ± 58 | 0.058 Cd | 0.002 |
| 0.021 Te | |||||
| CdTe QDs NH4+-coated | Diameter, 3–5 nm | <4 | 75 ± 50 | 0.494 Cd | 0.011 |
| 0.249 Te | |||||
| CdTe QDs PEG-coated | Diameter, 3–5 nm | <4 | 159 ± 72 | 0.107 Cd | 0.007 |
| 0.038 Te | |||||
| CuO bulk, British Drug Houses Ltd | Analar grade | -- | -- | -- | 0.344 |
| CuO NPs uncoated | Diameter, 10–20 nm; csurface area, 42 ± 2 m2 g−1 | 12.00 ± 0.37 | 41 ± 28 | 0.028 | 0.152 |
| CuO NPs COOH-coated | Diameter, 10–20 nm; csurface area, 7.4 ± 0.5 m2 g−1 | 6.45 ± 0.16 | 121 ± 91 | 1.152 | 0.016 |
| CuO NPs NH4+-coated | Diameter, 10–20 nm; csurface area, 6.1 ± 0.5 m2 g−1 | 9.53 ± 0.22 | 46 ± 36 | 0.31 | 0.043 |
| CuO NPs PEG-coated | Diameter, 10–20 nm | 7.46 ± 0.42 | 100 ± 36 | 0.867 | 0.000 |
| CuSO4·5H2O, Sigma-Aldrich | Purity, 99–102% | - | - | -- | - |
Chemistry triggers for nanomaterials of concern (tier 1)
The key concern is that the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow test is not appropriate for many MNs and so an alternative trigger for the testing strategy is needed, and preferably relating specific physico-chemical properties of the MNs with bioaccumulation potential (see Handy et al.9 for discussion). There are many possible particle metrics that could be measured, but pragmatically, the approach here in the meta-analysis was to consider metrics that are usually reported in bioaccumulation studies in the scientific literature. These include the primary particle size determined by electron microscopy, the hydrodynamic diameters of dispersions in Milli-Q ultrapure water (18.2 MΩ, ELGA, UK) determined by Nanoparticle Tracking Analysis (NTA), and the maximum dissolution rate determined by dialysis, as reported in the articles cited above. In addition, the meta-analysis also considered the particle settling rate. The latter was determined by measurements of optical density using an ultraviolet-visible spectrophotometer (Jenway 7315) at 220 nm. This optimal wavelength had been previously determined from preliminary experiments (data not shown). Stock suspensions at a concentration of 4 g L−1 were prepared in ultrapure water and sonicated (35 kHz frequency, Fisherbrand FB 11010, Germany) for 4 h to disperse the particles, and after that, immediately diluted to a concentration of 100 mg L−1. These suspensions were then homogenised by inverting the bottles vigorously ten times. Samples (n = 3), were taken from each bottle at 0, 10, 20, 30, 40, 50, 60, 90, 120, 150, 180, 210 and 240 min and analysed. The settling of particles in the test suspensions was presented as the total metal concentration plotted against time. The concentrations were calculated from the optical density values using calibration curves at 0, 5, 10, 20, 40, 60, 80 and 100 mg L−1.
Kow test is not appropriate for many MNs and so an alternative trigger for the testing strategy is needed, and preferably relating specific physico-chemical properties of the MNs with bioaccumulation potential (see Handy et al.9 for discussion). There are many possible particle metrics that could be measured, but pragmatically, the approach here in the meta-analysis was to consider metrics that are usually reported in bioaccumulation studies in the scientific literature. These include the primary particle size determined by electron microscopy, the hydrodynamic diameters of dispersions in Milli-Q ultrapure water (18.2 MΩ, ELGA, UK) determined by Nanoparticle Tracking Analysis (NTA), and the maximum dissolution rate determined by dialysis, as reported in the articles cited above. In addition, the meta-analysis also considered the particle settling rate. The latter was determined by measurements of optical density using an ultraviolet-visible spectrophotometer (Jenway 7315) at 220 nm. This optimal wavelength had been previously determined from preliminary experiments (data not shown). Stock suspensions at a concentration of 4 g L−1 were prepared in ultrapure water and sonicated (35 kHz frequency, Fisherbrand FB 11010, Germany) for 4 h to disperse the particles, and after that, immediately diluted to a concentration of 100 mg L−1. These suspensions were then homogenised by inverting the bottles vigorously ten times. Samples (n = 3), were taken from each bottle at 0, 10, 20, 30, 40, 50, 60, 90, 120, 150, 180, 210 and 240 min and analysed. The settling of particles in the test suspensions was presented as the total metal concentration plotted against time. The concentrations were calculated from the optical density values using calibration curves at 0, 5, 10, 20, 40, 60, 80 and 100 mg L−1.
Handy et al.9 proposed the dissolution of MNs and settling rate measurements as the chemical triggers in tier 1 to initiate further testing. The tiers are illustrated with a decision tree (Fig. 1), and with some refinements to show the exit points from strategy. In the first tier, the particle behaviours are of relevance to bioaccumulation in wildlife. For example, a metal or metal oxide MN that shows dissolution in water will release dissolved metals that are readily taken up by organisms, and it is well-known that non-essential metals such as Cd are bioaccumulative, and even nutritionally required metals such as Cu can accumulate if given in excess.34 While the focus here is on metal-containing MNs, similar dissolution measurements could be made with organic MNs in a lipid phase, such as corn oil, to infer if any persistent organic chemical might be released from the MNs.9 Settling rates are also relevant to bioaccumulation, with the assumption, for example, that any material that settles from the water column would contaminate the sediment and therefore the base of the aquatic food web. The meta-analysis here uses all the above metrics to calculate the correlation coefficients to define which metric, or rather, combination of metrics, gives the best prediction equation for bioaccumulation potential. Some of the methods for these metrics are also undergoing standardisation at the OECD. For example, the recent Guidance Document No. 318 (ref. 35) on testing of dissolution and dispersion stability of MNs is available. Whatever physico-chemical trigger(s) are selected, a key aspect for decision making is what value of dissolution rate or settling rate, for example, should trigger moving to the next tier. Here consensus building is needed within the scientific community to decide on threshold values, but for example, a decision to move to tier 2 might occur if the dissolution shows the release of a toxic metal at more than the current environmental quality standard, or if the metal is known to bioaccumulate.
A similar thinking could be applied to settling rates, or an arbitrary cut off applied, such as if more than 50% of the material settles during the test, then one would proceed to the next tier. A negative result from both the physico-chemical tests would exit the scheme and no further testing would be required. Thus, a MN that remains in the particulate form without dissolving and forms stable dispersions in water (i.e., no settling) would be regarded as a low bioaccumulation risk. The thinking here is that freshwater fish do not readily take up intact MNs across the gills and into the internal organs from a waterborne exposure in vivo.36 Instead, the emphasis in the scheme is on the dietary bioaccumulation and biomagnification risks. However, there is one caveat to consider regarding marine fishes. Seawater-adapted teleosts drink the surrounding media as part of their osmoregulatory strategy, and could be exposed by ingesting the MN dispersed in the water. This scenario is unlikely to lead to a negative result of the settling rate test because the high ionic strength of seawater would tend to cause particle settling by aggregation. The very saline conditions on the gut lumen would likely limit the bioavailability of any aggregates in the gut lumen of marine fish.37
Particle settling and/or aspects of dissolution are influenced by many factors in the media, including the pH value, the ionic strength, the presence of divalent ions, additions of natural organic matter (NOM), the level of anoxia, as well as viscosity and temperature.8,38,39 Of course, in the research laboratory, it is possible to make detailed experimental investigations of those parameters in order to understand particle settling or dissolution in natural water.39–41 However, for the international standardisation of methodology, and with regulatory use in mind, a simplified approach to the media composition is needed. In the meta-analysis here, we chose to illustrate the arguments using the behaviour of the MNs in ultrapure water. This was a pragmatic decision that enabled comparisons across different materials, and with the scientific literature where ultrapure water is often used as a reference point or control. Indeed, the existing guidance, TG 105, on the ‘water solubility’ of chemicals uses pure water.42 However, the dilemma of simplification of methodology for standardisation versus the complexity of environmental realism is recognised for MNs. In tier 1, the recently approved TG 318 for dissolution and dispersion stability of MNs43 could be used. This method uses synthetic natural water (i.e., a media of defined chemical composition), but with a simple matrix of chemistry variables (e.g., pH 4, 7 and 9; 0, 1 and 10 mM Ca2+, with and without NOM) that spans the likely concerns in freshwater at least. The TG 318 also includes a decision tree on how to categorise the results of the tests (e.g., dissolution present or absent). The TG 318 would be applicable, as written with synthetic natural water, in tier 1, when a waterborne bioaccumulation test with fish is intended within TG 305. However, there is an artificial saline that represents the trout gut lumen, and it is used in gut sac studies to measure bioaccumulation from MNs.31 This gut saline could be adopted into TG 318 to compliment any proposed dietary bioaccumulation test in fish using TG 305 at tier 4.
Using invertebrate bioaccumulation tests as an alternative to fish (tier 2)
Bioaccumulation studies with aquatic invertebrates in the presence of MNs are becoming available, with the prospect of using aquatic invertebrates in a tiered approach to testing (review, Kuehr et al.44). Studies on bivalves, gastropods, amphipods and brachiopods show that bioaccumulation can be measured,44 and in organisms relevant to the fate and behaviour of MNs in aquatic systems. For example, filter feeders and animals living on/in sediments. Metal uptake and elimination of Ag NPs in the estuarine snail utilised ICP-MS to measure total metal concentrations in the tissues.45 Studies using freshwater snails have measured total metals from Ag NPs and Ag2S NPs exposures using atomic absorption spectrometry,46 and total Cu from CuO NPs using ICP-MS coupled with an isotope tracing technique.47 The bioaccumulation potential of TiO2 NPs and SWCNTs was also assessed in a sediment dwelling marine polychaete using ICP-OES.48 More recently the technique of single particle ICP-MS (spICP-MS) has being used, in addition to total metal concentrations measurements of Ag NPs and TiO2 NPs, to determine particle concentration during metal uptake and elimination from a freshwater bivalve,49 and from an amphipod, Hyalella azteca.50The methods used in these available data sets on aquatic invertebrates might require further validation to enable a standardised protocol for the tissue detection of MNs in a technical guidance document. For example, filter feeding by aquatic invertebrates can show some size selection in the micron range for food particles, and this, as well as the filtration efficiency, may depend on the concentration of food particles in the media.51 There is also some evidence of selective filtration by size of MNs in clams.52 So, whether or not to feed the animals, and standardising the particle number concentration in the media, may be crucial aspects to the ingested dose of MNs in filter feeders and therefore any test method on bioaccumulation with these types of animals. There are also practical considerations for standardising washing procedures that remove excess media from delicate invertebrates, when the integument anatomy of invertebrates is so diverse. For invertebrates with a moulting cycle, a standardised protocol might also consider whether to include the carapace in the bioaccumulation measurement if moulting is likely during the test, or to only use animals in the inter-moult stage. Nonetheless, for the metal MNs at least, accumulation might be predicted from total metal concentration in the organisms. Of course, from a hazard perspective, it may seem logical to replace an aquatic test on fish with an aquatic test on an invertebrate. However, there is no requirement under REACH to focus on only aquatic data, and many of the freshwater invertebrate assays that are available for research purposes on bioaccumulation can provide data as part of a weight of evidence approach, despite those methods not yet being validated as OECD or similar guidance documents.
In contrast, bioaccumulation testing is well established with earthworms for metals and organic chemicals. There is an internationally agreed protocol, OECD TG 317, for testing the bioaccumulation of new substances in earthworms.13 In 2015, eighteen published studies had looked at the bioaccumulation potential of MNs in earthworms.53 The techniques used included ICP-OES, or sometimes flame atomic absorption spectrophotometry for total metals, as well as X-ray fluorescence spectrometry or gamma spectrometry for other MNs.53 Arguably, earthworm bioaccumulation studies show the most promise in their utility as a replacement for vertebrate fish tests with MNs. Earthworms are ecologically important detritivores that have been used in studies on the bioavailability of MNs,54,55 and also provide the ecosystem service of improving soil quality.56 Earthworms are also able to withstand relative high concentrations of substances in the environment and are easy to maintain in laboratory cultures.57 From the viewpoint of kinetics, it is possible to show net uptake and excretion of total metal or particles in earthworms so that accumulation factors can be calculated,33 and this would be fundamentally similar to whole body uptake kinetics as measured using TG 305 using fish. Indeed, some of the uptake mechanisms for MNs in the gut are highly conserve across the invertebrate species to fish.37
Table 2 shows some example studies where data on the bioaccumulation of MNs has been collected using the earthworm species (Eisenia fetida, Eisenia andrei, Lumbricus rubellus). The criteria used to identify these peer reviewed studies were: (i) appropriate particle characterisation through measurements of primary particle size and/or hydrodynamic diameter; (ii) measured metal concentrations in the test media to confirm the exposure; (iii) measured metal concentrations in the test organism that were detectable above the tissue background; (iv) evidence of quality assurance in the procedures for metal analysis, such as procedural blanks, spike recoveries, analysis of certified reference materials; (v) the experimental design was replicated, at least n = 3 vessels per treatment; and (vi) the experimental design had unexposed controls, and metal salt controls or bulk material controls as appropriate for a MN study design. In these studies, OECD, International Organization for Standardization (ISO) or similar test guidelines were essentially followed. The test materials studied were CuO, ZnO, Al2O3, Ag NPs, CdSe QDs and CNTs, with appropriate micro-scale and salt controls, as applicable. Crucially, these studies provide enough information to estimate bioaccumulation factors (BAF) for earthworms. The data shows (Table 2) that some non-essential metals, as expected, are very bioaccumulative, such as Cd in the nano form. There is also some evidence that the BAF values may be size-dependent within Ag NPs, but overall it is unclear if bulk forms of metal-containing materials have different BAFs to the nano form. Comparison with the metal salts are confounded by the necessity to use much lower concentrations of dissolved metals to avoid acute toxicity in the test design (Table 2).
| Material | Primary particle size | Nominal exposure concentration (mg kg−1) | Bioaccumulation factora | Species | Method | Exposure duration | Authors |
|---|---|---|---|---|---|---|---|
| a Bioaccumulation factor used as a general term with no specific distinction being made here between diet uptake and uptake from direct environmental contact. - Data not applicable to the test material. | |||||||
| AgNO3 | - | 100 | 0.05 | Eisenia fetida | OECD73 | 28 d | Shoults-Wilson et al.55 |
| Ag NPs | 30–50 nm | 0.01–0.02 | |||||
| AgNO3 | - | 15 | 7.8 | E. fetida | OECD13 | 28 d | Baccaro et al.33 |
| Ag NPs | 50 nm | 9.5 | |||||
| Ag2S NPs | 20 nm | 0.8 | |||||
| AgNO3 | - | 1.09 | 0.74 | Eisenia andrei | OECD13 | 21 d | Velicogna et al.74 |
| Ag NPs | 20 nm | 3.9 | 0.89 | ||||
| Ag NPs | 5 nm | 50 | 0.096 | E. fetida | OECD73 | 28 d | Garcia-Velasco et al.75 |
| AgNO3 | - | 15 | 0.033 | Lumbricus rubellus | ISO76 | 28 d | Makama et al.77 |
| Ag NPs | 50 nm | 250 | 0.002 | ||||
| AgNO3 | - | 154 | 0.200 | L. rubellus | ISO76 | 28 d | van der Ploeg et al.78 |
| Ag NPs | 15 nm | 15.4 | 0.023 | ||||
| AgNO3 | - | 200 | 0.030 | E. andrei | OECD73 | 28 d | Schlich et al.79 |
| Ag NPs | 15 nm | 0.060 | |||||
| Al2O3 bulk | 50–200 μm | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.008 | E. fetida | ASTM80 | 28 d | Coleman et al.81 |
| Al2O3 NPs | 11 nm | 0.011 | |||||
| Cd(NO3)2 | - | 1 | 17 | E. andrei | OECD73 | 28 d | Stewart et al.57 |
| H2SeO3 | - | 0.4 | 11 | ||||
| CdSe QDs | 10–20 nm | 1 | 9.6 Cd | ||||
| 0.4 | 6.2 Se | ||||||
| MWCNTs | 30–50 nm | 3000 | 0.015 | E. fetida | ASTM82 | 28 d | Li et al.83 |
| SWCNTs | 1–2 nm | 100 | 0.0078 | E. fetida | ASTM82 | 14 d | Petersen et al.84 |
| MWCNTs | 30–70 nm | 300 | 0.023 | ||||
| ZnO bulk | 5 μm | 10 | 1.99 | E. fetida | OECD61 | 28 d | Jośko et al.85 |
| ZnCl2 | - | 2.07 | |||||
| ZnO NPs | 50 nm | 2.09 | |||||
| CuO bulk | 10 μm | 0.56 | |||||
| CuCl2 | - | 0.82 | |||||
| CuO NPs | 100 nm | 0.94 | |||||
| ZnO bulk | 0.2 μm | 238–2500 | 0.36 | E. fetida | OECD73 | 28 d | Heggelund et al.86 |
| ZnO NPs | 30 nm | 0.48 | |||||
| ZnCl2 | - | 0.39 | |||||
| ZnCl2 | - | 5 | 1.53 | L. rubellus | OECD13 | 72 h | Laycock et al.60 |
| ZnO NPs | 7.8 nm | 1.56 | |||||
| ZnCl2 | - | 500 | 0.44 | E. andrei | OECD73 | 28 d | Romero-Freire et al.87 |
| ZnO NPs | 20–40 nm | 1000 | 0.26 | ||||
| ZnO bulk | <5 μm | 1000 | 0.150 | E. fetida | OECD88 | 28 d | García-Gómez et al.89 |
| ZnCl2 | - | 0.130 | |||||
| ZnO NPs | 58 nm | 0.210 | |||||
The tradition in the scientific literature for earthworms has been to use the phrase bioaccumulation factor (BAF), and while this is associated with dietary exposure through the ingested soil (i.e., like a BMF), some small or incidental dermal uptake through cutaneous contact with the soil cannot be excluded (e.g., for dissolved metals,58). However, in the case of both Ag and ZnO MNs, dietary uptake seems to dominate for earthworms59,60 and so these ‘BAF’ values might be considered as equivalent to the BMF in a dietary TG 305 test. For practical purposes of the decision tree (Fig. 1), this mechanistic detail is secondary, the idea is that a whole body bioaccumulation measurement in earthworms is broadly analogous to that in a fish using TG 305. In addition, given that MNs do not form steady-state equilibria like solutes, it is proposed that the prefix ‘nano’ or ‘n’ (e.g., nBAF for earthworms) is included to infer the value has come from an experiment on the nano form.9 The earthworm bioaccumulation test has two phases; an exposure phase (i.e., for ‘uptake’) of up to 21 days, and then a ‘depuration’ phase where the animals are transferred to clean soil for up to a further 21 days, with worms removed at time points during both phases for tissue analysis. The TG 317 protocol for earthworms13 works for both phases of the test for Ag NPs.33 BAFs can then be determined from the uptake and excretion kinetics. One suggestion is that data from TG 317 could be used as part of a weight of evidence approach for a bioaccumulation concern in REACH assessments for MNs.9 Evidence could also come from the earthworm reproduction test, OECD TG 222,61 which is essentially the same as the exposure phase in OECD TG 317 but lasting four weeks, where earthworms can also show metal accumulation from MNs exposures: CuO MNs14 and CdTe QDs.32
In the earthworm bioaccumulation test, like in the fish bioaccumulation test, the first concern is whether or not an apparent steady state can be achieved in order to validate any nBAF (earthworms) or (nBMF, dietary in fish) approach. Fig. S1† shows that this is indeed the case, with an apparent steady tissue concentration of metal from MNs exposures in the earthworm (Eisenia fetida) following exposure to different Ag-containing materials. Similar observations were made for CuO NPs with different surface coating (Fig. 2), and for the Cd concentrations (Fig. 3A–D) and Te concentrations (Fig. 3E–H) in earthworms exposed to CdTe QDs with different surface coating. The nBAFs determined for earthworms are shown in the respective figures (Fig. 2 and 3).
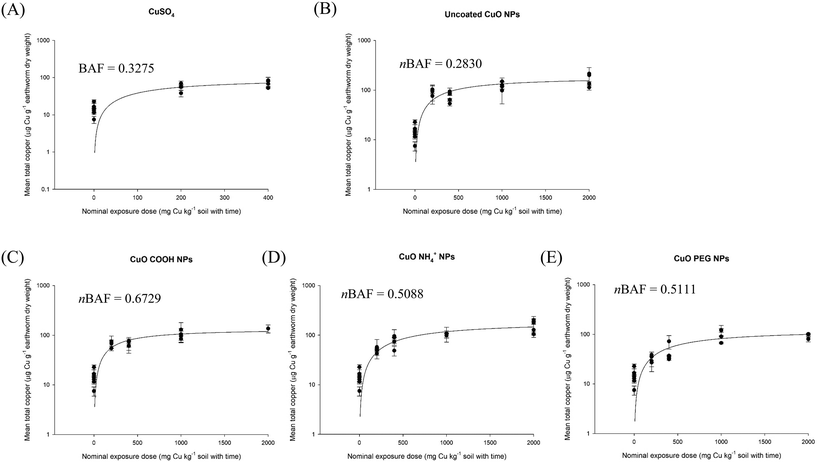 | ||
| Fig. 2 Nominal chemical dose exposure in soil with copper materials (A) CuSO4 (B) uncoated CuO NPs (C) CuO–COOH (D) CuO–NH4+ (E) CuO-PEG plotted against measured total metal concentration in the earthworm (Eisenia fetida) tissue (mean ± S.E.M, n = 4). Data are from Tatsi et al.14 | ||
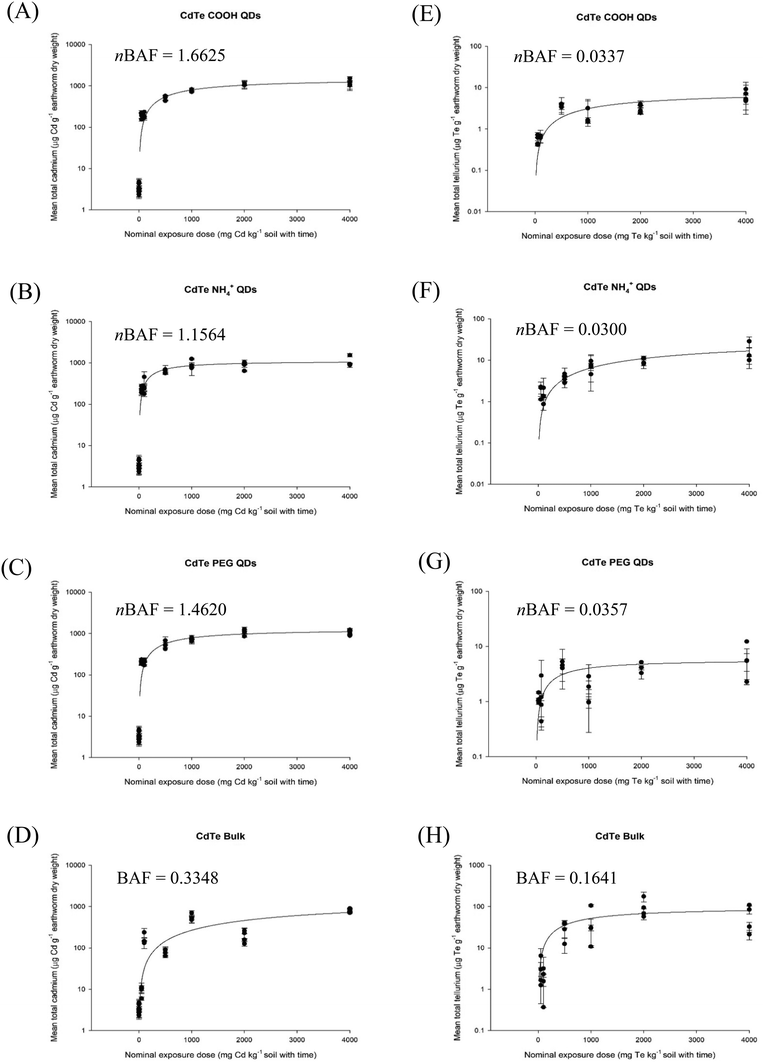 | ||
| Fig. 3 Nominal Cd (left panels) or Te (right panels) dose exposure in soil with CdTe QDs materials (A and E) CdTe–COOH, (B and F) CdTe–NH4+, (C and G) CdTe-PEG, (D and H) CdTe bulk material, plotted against measured total cadmium concentration in the earthworm (Eisenia fetida) tissue. Data are mean ± S.E.M, n = 4, from Tatsi et al.32 | ||
Can nanomaterial metrics be used to trigger an earthworm bioaccumulation test?
Given that the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow measurement is problematic for MNs, and that MNs do not behave in the same way as dissolved metals, it is useful to understand if particle properties could be used as a chemical trigger for conducting the earthworm bioaccumulation test (TG 317). Fig. 4 shows the physico-chemical parameters of particle size, hydrodynamic diameter, metal dissolution and particle settling plotted against the calculated nBAFs for the E. fetida, following soil exposure to uncoated CuO NPs and with negative, positive, or neutral particle coatings present (CuO–COOH, CuO–NH4+ and CuO-PEG respectively). Soil and tissue metal concentration data were taken from the last time point in the exposure (week 4) of Tatsi et al.14 The r2 values for the linear plots were 0.88, 0.65, 0.80 and 0.76 for primary particle size, hydrodynamic diameter, maximum metal dissolution rate, and particle settling rate respectively. However, due to the limited data set in the plots with just the CuO MNs, the Pearson's correlations were not statistically significant (p > 0.05). Nonetheless, the approach does show that the nBAF can be determined for earthworms.
Kow measurement is problematic for MNs, and that MNs do not behave in the same way as dissolved metals, it is useful to understand if particle properties could be used as a chemical trigger for conducting the earthworm bioaccumulation test (TG 317). Fig. 4 shows the physico-chemical parameters of particle size, hydrodynamic diameter, metal dissolution and particle settling plotted against the calculated nBAFs for the E. fetida, following soil exposure to uncoated CuO NPs and with negative, positive, or neutral particle coatings present (CuO–COOH, CuO–NH4+ and CuO-PEG respectively). Soil and tissue metal concentration data were taken from the last time point in the exposure (week 4) of Tatsi et al.14 The r2 values for the linear plots were 0.88, 0.65, 0.80 and 0.76 for primary particle size, hydrodynamic diameter, maximum metal dissolution rate, and particle settling rate respectively. However, due to the limited data set in the plots with just the CuO MNs, the Pearson's correlations were not statistically significant (p > 0.05). Nonetheless, the approach does show that the nBAF can be determined for earthworms.
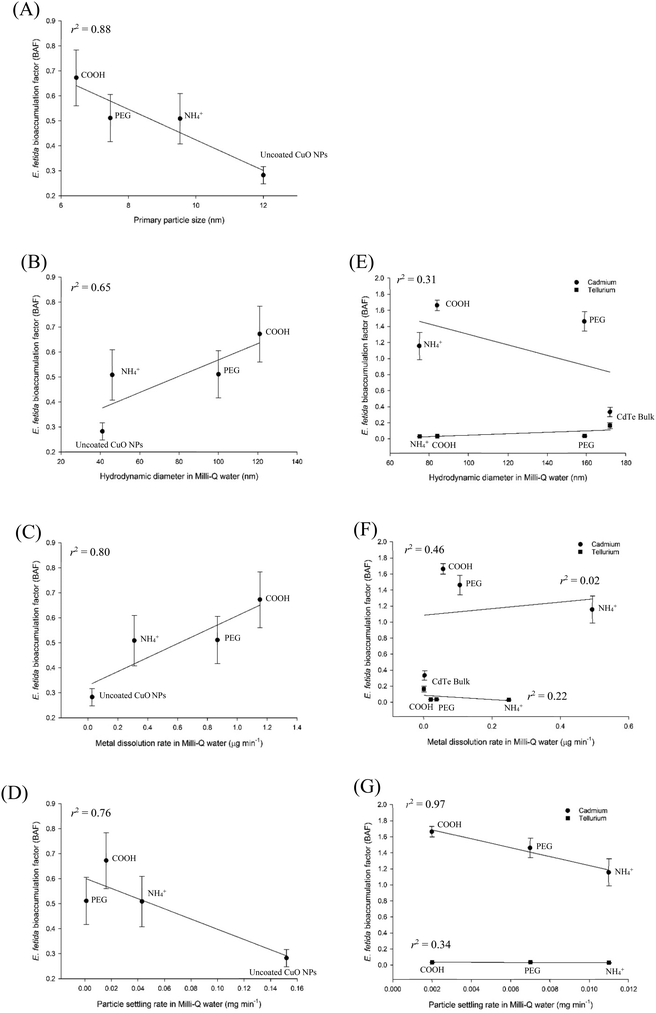 | ||
| Fig. 4 Physico-chemical parameters plotted against calculated (mean ± S.E.M, n = 4) bioaccumulation factors (BAFs) for the earthworm Eisenia fetida following exposure to copper- (left panels) or CdTe-based (right panels) materials. Properties of (A) particle size, (B and E) hydrodynamic diameter, (C and F) metal dissolution and (D and G) particle settling were considered against the calculated BAFs, following soil exposure. The copper nanoparticles were uncoated CuO, CuO–COOH, CuO–NH4+ and CuO-PEG. The CdTe treatments were CdTe bulk, CdTe QDs COOH, CdTe QDs NH4+ or CdTe QDs PEG. All Pearson's correlations were found to be insignificant (p > 0.05). Equations for the curve fits are in Table S1.† Data sourced from Tatsi et al.14,32 | ||
Similar plots are drawn (Fig. 4) for the calculated nBAFs for the total Cd or Te in Eisenia fetida, following soil exposure to various CdTe QDs (CdTe QD-COOH, CdTe QD-NH4+ and CdTe-PEG) and a bulk CdTe material using the last time point of exposure in the experiment (week 4,32). The r2 values for the cadmium plots were 0.31, 0.02 and 0.97 for hydrodynamic diameter, maximum metal dissolution rate and particle settling rate respectively. The r2 values for the tellurium plots were 0.46, 0.22 and 0.34 for hydrodynamic diameter, maximum metal dissolution rate and particle settling rate respectively. Notably, because of the uncertainty in the thickness of the coatings on the CdTe QD materials, the primary size of individual particles was not possible to measure; and this might be a problem for many materials with organic coatings. Again, because the plots were limited to one metal from a few materials, all the Pearson's correlation coefficients were not statistically significant (p > 0.05).
A multiple linear regression of the metal BAFs for all the materials (Ag, Cd, Te and Cu) against the particle metrics was carried out to reveal if together the particle metrics could better explain the observed calculated BAFs. The primary particle size, hydrodynamic diameter, dissolution rate and settling rate were used as independent variables, and the earthworm calculated BAFs as the dependant variable. Additionally, the ionic radii and charge densities of the metals in the different materials were included in the model (Table S2†). In the initial regression model, the mean calculated BAF values gave an r2 value of 0.99 and a good fit of the data (p < 0.002); suggesting that the independent variables explained 99% of the variability. To improve the accuracy, the model was re-run with all the calculated BAF values (n = 53), yielding an r2 of 0.87, with particle size and dissolution rate (p < 0.05) statistically significantly accounting for the ability to predict the bioaccumulation potential (Table S3†). A regression prediction equation was then fit to the data using eqn (1);
| y = 2.306 − (0.348 × a) + (1.064 × b) − (0.435 × c) − (0.00157 × d) − (1.090 × e) + (4.933 × f) | (1) |
This approach of iterative multiple regressions of particle properties against BAF values in earthworms is best included as part of the bioaccumulation modelling exercise at the start of tier 2 (Fig. 1). This is for practical reasons. At the present time, it is likely that the dissolution and settling rate measurements will need to be made in tier 1, and along with the manufacturer's information on particle size, or additional measurements of hydrodynamic diameters, the regression analysis may then be conducted to derive a prediction equation for the MNs being considered. In the future, as data sets on particle properties are accrued, it might be possible to conduct such regression analysis as part of the ‘problem formulation’ in tier 1. It will be for the scientific community to decide on thresholds for the ‘acceptability’ of the regression analysis, so that it is used in a standardised way as a tool for predicting bioaccumulation. However, there are widely accepted conventions used by statisticians in regression analysis, such as considering the residuals and the tests for the significance of the regression coefficient or slope, as well as estimates of the lack-of-fit and effect-size differences in meta-analysis as part of the data interpretation.62 We adhered to those conventions here, with the multiple regression equation above for all the MNs in the meta-analysis offering an r2 of 0.87 using 53 data points. Values exceeding an r2 of 0.8 seem very robust, given that traditional models for predicting bioaccumulation factors in fish from log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow often had r2 values around 0.75, and even with refinements and hundreds of data points for organic chemicals after years of research, outliers remain, with r2 not exceeding 0.95 (e.g.,63). Thus for MNs, provided the r2 values are around 0.75 or more, and the multiple regression equation is statistically significant, then BAF predictions exceeding 1.0 (i.e., bioaccumulative) would trigger the need for the earthworm bioaccumulation test in the latter part of tier 2 in the scheme.
Kow often had r2 values around 0.75, and even with refinements and hundreds of data points for organic chemicals after years of research, outliers remain, with r2 not exceeding 0.95 (e.g.,63). Thus for MNs, provided the r2 values are around 0.75 or more, and the multiple regression equation is statistically significant, then BAF predictions exceeding 1.0 (i.e., bioaccumulative) would trigger the need for the earthworm bioaccumulation test in the latter part of tier 2 in the scheme.
Are earthworm bioaccumulation tests (tier 2) predictive of the in vitro finding in fish (tier 3)?
The proposal for a tiered approach testing put forward by Handy et al.9 included a third tier that was intended to represent ‘in vitro fish’ alternatives that might help to decide to include or waive the final tier, the in vivo fish test (TG 305). The tier 3 include an in chemico digestibility assay for simulating the bioaccessible fractions of MNs in the gut lumen, and gut sac studies to show uptake to the gut tissue (Fig. 1). The tier 3 therefore enabled the implementation of the 3Rs (replacement, reduction, refinement); that is replacement of the in vivo test with ‘in vitro’ fish alternatives, and/or a reduction in the use of the TG 305 test. It was considered that the regulatory acceptance of any proposal to replace the dietary in vivo fish test would need an alternative that still used fish tissue, or was relevant to fish gut.9 However, with the benefit of new data here (see below), it is apparent that the earthworm bioaccumulation test is not only predictive of the in vivo fish test, but also predictive of the fish alternatives in tier 3. The latter will give confidence in the decision making to move from tier 2 (invertebrates) to tier 3 (‘in vitro’ fish) or not.While there is some logic to using alternative methods in fish gut to support an in vivo fish dietary bioaccumulation test. From the view of comparative physiology, a dietary bioaccumulation study on an earthworm is not conceptually different from that on a fish. In terms of colloid chemistry, the guts of both organisms have an ionic strength that would promote particle settling to exposure in the epithelium.64 The REACH annexes and the US EPA procedures for metals also allow bioaccumulation data on species other than fish, and any subsequent risk analysis would include safety factors for species differences and/or the amount of data on different organisms in the risk assessment. Ultimately, a path could be found through the regulations to waive studies on fish in favour of earthworm, as long as there was confidence that earthworm data would predict bioaccumulation in other biota.
Nonetheless, the tier 3 on ‘in vitro’ fish included the use of isolated gut sacs from rainbow trout (Oncorhynchus mykiss) intestine. The gut sac method has been used for many years in research on gut physiology and to determine the uptake mechanisms of chemicals,9 now including MNs e.g., TiO2;65 Ag and Ag2S NPs.31 Experiments have also been completed for trout gut sacs on CuO NPs compared to CuSO4 (ref. 66) and on the same CdTe QDs used in the earthworm studies (Clark et al., unpublished data). In these gut sac studies, the mid and hind intestine usually showed more metal accumulation than other regions of the gut, as expected for the absorptive functions of the gut. During the processing of the tissue samples, the underlying muscularis was separated from the gut mucosa, and both anatomical portions were analysed for total metals in the gut sac method. The muscularis is especially interesting as this may represent total metal that has been internalised (i.e., crossed the gut epithelium to arrive in the underlying vasculature and lymphatics). Therefore, the muscularis of the mid intestine was chosen for comparison with the total metal accumulation by earthworms, since both would be indicative of an internal dose.
Fig. 5 shows the data for the CdTe QDs, and plots the total Cd or Te in the earthworm against the muscularis of the mid intestine in fish. In both the CdTe QD-COOH and CdTe QD-PEG treatments, both Cd and Te accumulation in the earthworm was well correlated with that in the muscularis of the intestine (r2 > 0.78 for all), with each showing a statistically significant relationship: correlation coefficients with p values: 0.907 (p < 0.01), 0.882 (p < 0.01), 0.933 (p = 0.02) and 0.961 (p < 0.01) for the Cd in CdTe QD-COOH, Cd in CdTe QD-PEG, Te in CdTe QD-COOH and Te in CdTe QD-PEG treatments, respectively, using the Pearson's or Spearman's correlation. However, some of the linearity of the relationship is lost in the CdTe QD-NH4+ treatment; for instance, the Te tissue concentrations (Fig. 5E). For CuSO4 and CuO NPs, the relationship between total metal in the earthworm and fish gut sac is curvilinear (Fig. S2†), and this might be expected for an essential nutrient that is regulated by both organisms. Despite this, all treatments show a significant predictive relationship between earthworms and fish tissue. Importantly, the measurements are made at an apparent steady-state and with a sub-lethal exposure concentration so that the data are comparable. In any event, the key point is that the earthworm data are generally predictive of the gut sac results, so arguably, the tier 3 proposed9 could be omitted, and the scheme instead, rely on the invertebrate data in tier 2.
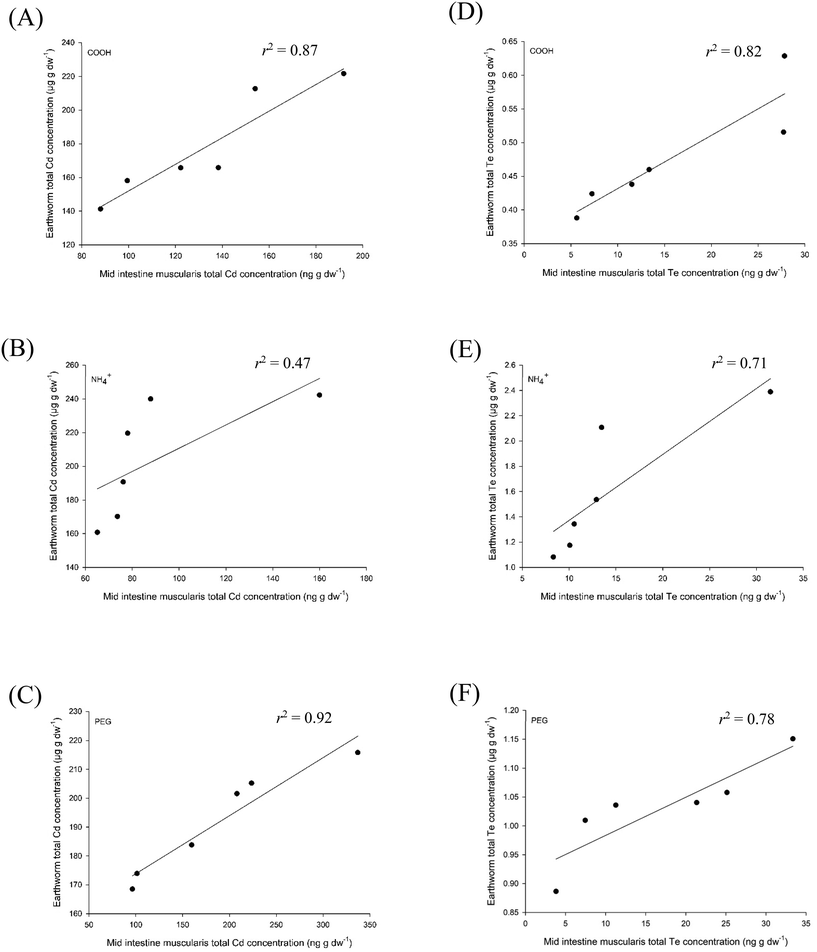 | ||
| Fig. 5 Correlations between tier 2 (earthworms) and tier 3 (gut sacs) exposed to COOH-, NH4+- or PEG-coated cadmium quantum dots. The left hand panels (A–C) are total Cd concentrations, whereas the right hand panels are the associated total Te concentrations. The earthworms were exposed to 50 mg kg−1 of the CdTe materials for 28 days, and the fish gut was exposed to 1 mg L−1 for 4 h. Data were ranked and then correlated. The equations of the lines are (A) y = 0.7899x + 72.997, (B) y = 0.6905x + 141.69, (C) y = 0.201x + 153.74, (D) y = 0.0079x + 0.3529, (E) y = 0.0522x + 0.85 and (F) y = 0.0066x + 0.9173. The CdTe earthworm data from Tatsi et al.,32 and the CdTe fish data is in preparation (Clark et al., unpublished data). | ||
Is bioaccumulation in earthworms predictive of bioaccumulation in fish?
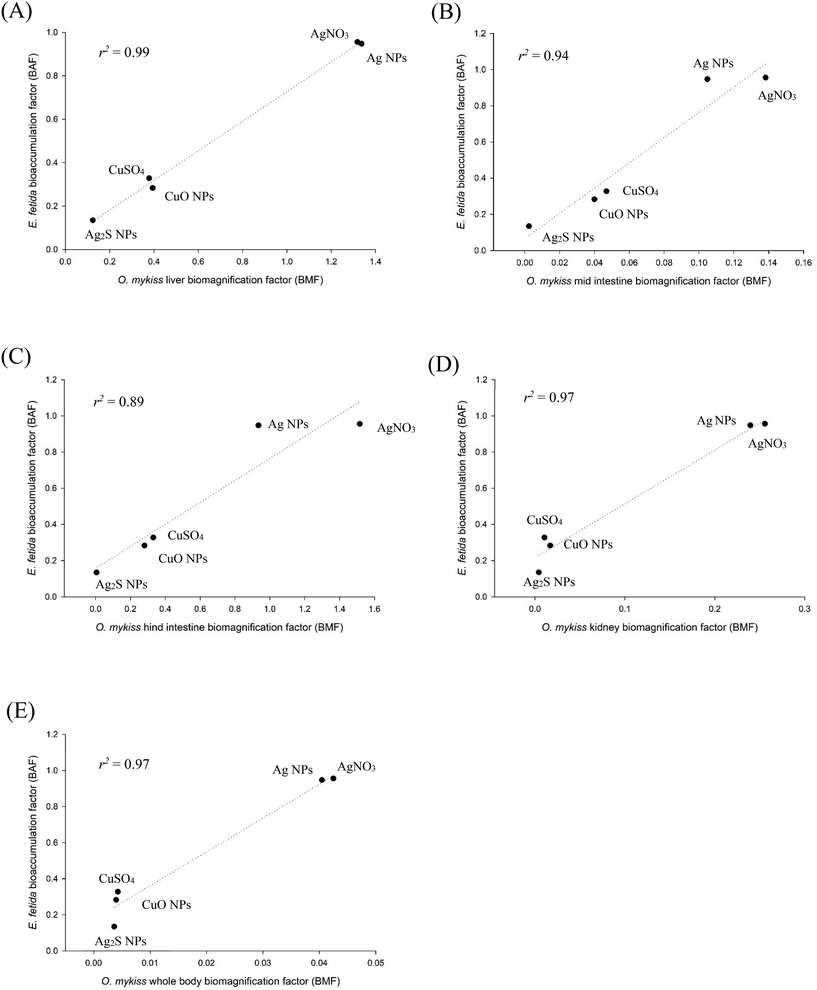
In keeping with the 3Rs and the desire to replace vertebrate animal testing entirely, it is apparent that TG 317 on earthworms might replace the fish test (TG 305) in a REACH assessment in cases where there is predictive data. This would be a positive step-change for the regulatory community with respect to animal welfare, and while a mandate from the regulatory authorities to formally discontinue TG 305 would be required, in principle, in vivo testing on fish may not be needed at all for metallic MNs. This thinking might even be extended to organic MNs, and in time, to chemicals generally, with considerable financial savings in the overall testing strategy and with respect to the animal welfare of fish. However, an incremental first step would be to show that the metal concentrations in the internal organs of fish correlate with those in the earthworm; and then to determine if the nBAF values in the earthworm correlate with the nBMFs in the fish test. For this aspect of data analysis, the fish liver was chosen as the central internal compartment in metal metabolism, and plotted against the whole body metal concentration in the earthworms (Fig. S3†).Following the exposure to AgNO3, Ag NPs or Ag2S NPs (Fig. S3A–C†), there was a significant relationship between the fish liver total Ag concentration and the respective earthworm total Ag concentration (AgNO3, Spearman's correlation coefficient 0.888; Ag NPs and Ag2S NPs, Pearson's correlation coefficient of 0.728 and 0.866 respectively, both p < 0.01). This was also observed in both the CuSO4 and CuO NPs treatments for total metal concentrations (Fig. S3D and E;† Pearson's correlation coefficients for CuSO4 and CuO NPs were 0.924 and 0.944, respectively, both p < 0.01).
Given the similarities in the biogeochemistry of Cu and Ag, the nBMF of the organs of fish was plotted against the nBAF of the earthworm for both Cu and Ag together; and ranking the pairs of data points by exposure concentration to accounts for concentration differences between studies (Fig. 6). When these variables were plotted against each other, the r2 values for a linear fit were 0.99, 0.94, 0.89, 0.97, and 0.97 for the liver, mid intestine, hind intestine, kidney, and fish whole body, respectively. Pearson's correlation values were also found to be significant for fish organs and fish whole body relative to the earthworm tissue (p = 0.000 for liver; 0.007 for mid intestine; 0.016 for hind intestine; 0.002 for kidney; and 0.002 for fish whole body). This demonstrates that nBMF from the fish bioaccumulation test could be replaced with the nBAF from the earthworm test in a REACH assessment for both the silver and copper-containing MNs. Whether this relationship exists for the CdTe QDs with different surface coatings is yet to be determined because the in vivo fish tests (TG 305) with these materials have not yet been conducted.
There is a mechanistic logic as to why nBAFs in earthworms might correlate with nBMF in fish for metals. For essential metals such as Cu, the solute transporters are highly conserved across species67 and this is no surprise as all animals need to homeostatic control their nutritive metal uptake. Conversely, for non-essential metals such as Ag, Cd, Pb, etc., no metal-specific endogenous uptake or excretion pathways have evolved in eukaryotes, as the metals have no biological functions and are therefore not needed inside the tissues.68 So, whether one examines invertebrates or vertebrate animals, the non-essential metals are not regulated and tend to be bioaccumulative. Logically, these arguments should also apply to minerals at the nanoscale since organisms have evolved in a nanoparticle-rich biosphere. Indeed, we now understand that some nutritionally required metals are taken up through high affinity vesicular trafficking systems (e.g., Cu,69), and/or taken up in the particulate form (e.g., iron,70). More data sets on MNs made from essential and non-essential metals are needed to prove this point and to refine any prediction equations that might be used. For organic chemicals, the idea of lipid solubility is universal, since all organisms have biological membranes made from lipids and so data from many species fit on the same line of bioaccumulation factor versus the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow measurement.63 However, for organic MNs such as dendrimers or carbon nanotubes with organic coatings, the uptake and therefore bioaccumulation potential is dependent on the affinity of the endocytosis-related mechanisms involved.71 More data are needed, but for example, it may also be the case that MNs with coatings or compositions found in nature (e.g., carboxylated or sulphated colloids,72) are taken up more readily, than entirely novel synthetic chemical structures. Of course, the notion of safe-by-design MNs would argue that they are made of naturally occurring ligands that might therefore be biodegradable, or at least not accumulated by organisms.
Kow measurement.63 However, for organic MNs such as dendrimers or carbon nanotubes with organic coatings, the uptake and therefore bioaccumulation potential is dependent on the affinity of the endocytosis-related mechanisms involved.71 More data are needed, but for example, it may also be the case that MNs with coatings or compositions found in nature (e.g., carboxylated or sulphated colloids,72) are taken up more readily, than entirely novel synthetic chemical structures. Of course, the notion of safe-by-design MNs would argue that they are made of naturally occurring ligands that might therefore be biodegradable, or at least not accumulated by organisms.
Decision tree and exit points from the testing strategy
A decision tree is needed in order to guide the user through the tiers in the testing strategy and to offer clear guidance on implementation. This is discussed in detail elsewhere,9 but the commentary here focusses on the utility of the invertebrate aspects and lower tiers. Fig. 1 shows the decision tree, with some additional refinements modified from Handy et al.9 to illustrate the exit points from the strategy and how to move forward from one tier to the next. The scheme starts with the problem formulation and initial evidence of concern, as would normally be the case in any hazard assessment process. For example, it might be that the intended use of the MN gives rise to a bioaccumulation concern; such as the use of MNs in crop protection products, or is designed to be persistent/durable (i.e., not biodegradable), or has a chemical composition that includes a substance known to bioaccumulate (e.g., MNs made with Cd).In tier 1, environmental chemistry triggers could be obtained from existing chemistry data sets, or as part of the initial data collection on the properties of the MN. New dissolution and particle settling measurements could be made using the recently approved TG 318 on dispersion stability of nanomaterials in simulated environmental media.43 Importantly, both measurements would be needed to decide on exiting the strategy, minimising the risk of a false negative (predicting no bioaccumulation when a bioaccumulation concern exists) so the scheme remains precautionary with respect to chemical safety in tier 1. For example, if dissolution was negligible (e.g., no ‘free metal ion’ bioaccumulation concern) and the MN showed no evidence of particle settling to contaminate the base of the food web, then the bioaccumulation concerns would be low (i.e., a reasonably inert MN that remains in the water column). In this case, one could exit the strategy without needing to proceed to a TG 305 test with fish, albeit with any caveats or exceptions about the likelihood of waterborne MN uptake through the gills. For example, a lipid soluble MN that might be taken up by diffusion, or a MN with a coating specifically designed to permeate cell membranes may still be a concern. Such aspects could be reported in the problem formulation step. If either one of the chemistry trigger(s) are positive for a concern, then one would move to tier 2. The thresholds for those chemical triggers need to be decided by consensus building within the scientific community, but for example, if the metallic MN entirely dissolved, as might be the case at pH 2 using an artificial saline to represent the stomach of a trout, then existing metals risk assessments would apply. Alternatively, if the MN remained predominantly in the nano form and showed particle settling, then there would be a bioaccumulation concern. The strategy aims to minimise the use of any animal testing, and so the first step in tier 2 is a mandatory review of existing data on bioaccumulation potential. Of course, some of this may have been done in the initial problem formulation, but this step is intended to be in more detail. This will include the use of existing bioaccumulation models for MNs in invertebrate species, such as that available from the NanoFASE EU project11 to predict BAFs in earthworms, as well as any measured BAFs from the scientific literature. Existing data from the literature on fish cell lines (gill, gut or liver cell lines) could also inform on bioaccumulation potential if those cells show bioaccumulation from the MN exposure.9
Similarly, existing data from digestibility assays could be used, from gut sacs, or from existing data from in vivo studies on fish and oral exposure studies in rodents for bulk materials and MNs. Existing data sets on the earthworm bioaccumulation test TG 317 would be especially welcome, as this could waive the need for doing this test in tier 2. If the review of existing data does not support a concern for bioaccumulation potential, then one can exit the strategy with no further testing required. However, if a concern is present and there is no existing data on TG 317 for the MN, then the earthworm bioaccumulation test should be conducted. If the nBAF for earthworms from that test raises a concern, then proceed to tier 3, if not, exit the testing strategy. This assumes that a very low nBAF value in the earthworms would equate to a low nBMF or no accumulation in the fish. This is certainly the case for Ag2S NPs (Fig. 6), but more data on MNs that yield negative results are needed. Note that tier 2 contains several steps and data from different sources, again this minimises the risk a false negative in the earlier tiers so that the tier remains precautionary.
The in vitro fish (tier 3) and the in vivo fish (tier 4) are discussed in detail elsewhere.9 However, one aspect to agree is the threshold that constitutes a bioavailable fraction of concern in the gut lumen of fish. The in chemico digestibility assay measures the release of dissolved metal in the case of metallic MNs, but does not exclude the possibility that the particle remains intact, and yet also bioavailable. Therefore, it would also be prudent to conduct the gut sac studies to confirm uptake of total metal, regardless of the chemical form suspected in the gut lumen. Thus, both the in chemico digestibility assay and gut sacs would show a concern in order to move to tier 4 on in vivo fish. For the gut sacs, a key decision to move to tier 4 would be the presence of the test substance in the muscularis (i.e., confirmed translocation across the gut to the internal compartment). However, the accumulation into the gut mucosa might also be considered. For example, CuO NPs dissolve in the acid conditions expected in the stomach (i.e., a positive results on ‘digestibility’), but in the gut sacs also show accumulation in/on the gut mucosa rather than muscularis over 4 h.66 Clearly, the total Cu was bioavailable to the mucosa, but may not yet have had time to translocate to high enough concentration in the muscularis in such a short test to infer an internalised dose. In such circumstances, the precautionary principle could be applied, and one might move to tier 4 to be sure of the outcome.
The correlations here with nBAFs show that the results from earthworm tests are predictive of the fish bioaccumulation test, and one would therefore argue the scheme could stop at tier 2 to eliminate vertebrate animal testing all together. Whether or not tier 3 could be omitted, and instead to rely primarily on evidence from earthworm bioaccumulation tests, or similar tests with other invertebrates, will depend on attitudes towards using alternatives to vertebrate animals, as well as the providing sufficient data on a wide variety of MNs so that the scientific evidence for replacement is strong.
Conclusions and the way forward
The data analysis for the earthworm tests shows that an apparent steady-state can be observed and a nBAF calculated from the total metal concentration in the media and the organism. The use of total metal concentrations is a pragmatic approach to nBAF that does not rely on knowledge of particle transformations in the tissue. In any case, at this time, it is not technically possible to quantify particle number concentration, particle transformation in different organs, biocorona, etc., in most fish and invertebrates species. However, in the medium term, it would be desirable to at least achieve a standardised method for measuring particle number concentration in fish and/or invertebrate tissues to support bioaccumulation testing protocols. The requirement to minimise vertebrate animal testing under REACH is very clear, and more widely in other regulations for chemicals around the world. A much stronger consideration of the 3Rs is advocated here for the bioaccumulation testing strategy, where it is possible to exit the strategy and have safety information without necessarily needing vertebrate animal testing. The threshold values that enable the testing to move from one tier to the next should be agreed, and it may be that the data in tier 2 on earthworms could be sufficiently robust to waive tier 3 and 4 entirely for some MNs. However, one should also be mindful of the speed of innovation in nanotechnology, and with second and third generation MNs now appearing in the market place. The tiered approach to bioaccumulation testing should be standardised in a way that enables it to be used for a wide variety of MNs. Here we have demonstrated the scheme with some metallic MNs with different properties, and further laboratory work is already underway in the NanoHarmony EU project to expand the data sets to other metallic MNs. It will be for the scientific community to decide on how many different metallic materials are needed to validate the scheme. The scheme has not yet been applied to entirely organic MNs, such as pristine carbon nanotubes or dendrimers, partly because detection methods are not routinely available for the intact MNs in complex matrices such as tissue. Aspect such as dissolution may be less relevant to some organic MNs, although it might be measured in lipophilic media.9 Regardless, any scheme that is agreed should remain precautionary and effective at assessing the chemical safety of MNs, as well as working long into the future for all varieties of new MNs.Author contributions
DB, KT, NJC, and MB conducted the original experiments that provided the data set used here, from the EU projects acknowledged below. JV and NJC conducted the meta-analysis with input from RDH, CG, FN, CS, NvB and TH. Manuscript preparation and writing of the drafts was led by RDH, with JV and NC preparing the data illustrations and statistics. All the co-authors read subsequent drafts.Conflicts of interest
The authors do not declare any conflicts of interest.Acknowledgements
The meta-analysis was supported by grants from Defra UK awarded to RDH (project number 27891; also the ‘project to support a scoping review on the bioaccumulation of nanomaterials.’), and from the European Union's Horizon 2020 research and innovation programme: the NanoHarmony project under grant agreement No 885931. Data on the bioaccumulation from CuO NPs in fish was collected by DB during the FP7 Sustainable Nanotechnologies Project (SUN) grant, contract number 604305. Data on CdTe QDs and CuO materials in earthworms were collected by KT during the FP7 Nanosolutions project, grant agreement 309329. Data on the Ag materials on earthworms and fish were collected by MB and NJC respectively during the H2020 NanoFase project, grant agreement 646002. All the above projects with RDH as the Principal Investigator at the University of Plymouth.References
- A. Besinis, T. De Peralta, C. J. Tredwin and R. D. Handy, Review of nanomaterials in dentistry: interactions with the oral microenvironment, clinical applications, hazards, and benefits, ACS Nano, 2015, 9, 2255–2289 CrossRef CAS PubMed.
- S. F. Hansen, L. R. Heggelund, P. R. Besora, A. Mackevica, A. Boldrin and A. Baun, Nanoproducts–what is actually available to European consumers?, Environ. Sci.: Nano, 2016, 3, 169–180 RSC.
- I. Hincapié, A. Caballero-Guzman, D. Hiltbrunner and B. Nowack, Use of engineered nanomaterials in the construction industry with specific emphasis on paints and their flows in construction and demolition waste in Switzerland, Waste Manage., 2015, 43, 398–406 CrossRef PubMed.
- S. Kumar, M. Nehra, N. Dilbaghi, G. Marrazza, A. A. Hassan and K.-H. Kim, Nano-based smart pesticide formulations: Emerging opportunities for agriculture, J. Controlled Release, 2019, 294, 131–153 CrossRef CAS PubMed.
- R. J. B. Peters, H. Bouwmeester, S. Gottardo, V. Amenta, M. Arena, P. Brandhoff, H. J. P. Marvin, A. Mech, F. B. Moniz, L. Q. Pesudo, H. Rauscher, R. Schoonjans, A. K. Undas, M. V. Vettori, S. Weigel and K. Aschberger, Nanomaterials for products and application in agriculture, feed and food, Trends Food Sci. Technol., 2016, 54, 155–164 CrossRef CAS.
- G. Cornelis, K. Hund-Rinke, T. Kuhlbusch, N. van den Brink and C. Nickel, Fate and bioavailability of engineered nanoparticles in soils: a review, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2720–2764 CrossRef CAS.
- F. Gottschalk, E. Kost and B. Nowack, Engineered nanomaterials in water and soils: A risk quantification based on probabilistic exposure and effect modeling, Environ. Toxicol. Chem., 2013, 32, 1278–1287 CrossRef CAS PubMed.
- J. R. Lead, G. E. Batley, P. J. Alvarez, M. N. Croteau, R. D. Handy, M. J. McLaughlin, J. D. Judy and K. Schirmer, Nanomaterials in the environment: behavior, fate, bioavailability, and effects — An updated review, Environ. Toxicol. Chem., 2018, 37, 2029–2063 CrossRef CAS PubMed.
- R. D. Handy, J. Ahtiainen, J. M. Navas, G. Goss, E. A. Bleeker and F. von der Kammer, Proposal for a tiered dietary bioaccumulation testing strategy for engineered nanomaterials using fish, Environ. Sci.: Nano, 2018, 5, 2030–2046 RSC.
- G. D. Veith, D. L. Defoe and B. V. Bergstedt, Measuring and estimating the bioconcentration factor of chemicals in fish, J. Fish. Res. Board Can., 1979, 36, 1040–1048 CrossRef CAS.
- N. W. van den Brink, A. J. Kokalj, P. V. Silva, E. Lahive, K. Norrfors, M. Baccaro, Z. Khodaparast, S. Loureiro, D. Drobne and G. Cornelis, Tools and rules for modelling uptake and bioaccumulation of nanomaterials in invertebrate organisms, Environ. Sci.: Nano, 2019, 6, 1985–2001 RSC.
- D. J. Spurgeon, E. Lahive and C. L. Schultz, Nanomaterial transformations in the environment: effects of changing exposure forms on bioaccumulation and toxicity, Small, 2020, 16, 2000618 CrossRef CAS PubMed.
- OECD, Test No. 317: Bioaccumulation in Terrestrial Oligochaetes, OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris, France, 2010 Search PubMed.
- K. Tatsi, B. J. Shaw, T. H. Hutchinson and R. D. Handy, Copper accumulation and toxicity in earthworms exposed to CuO nanomaterials: Effects of particle coating and soil ageing, Ecotoxicol. Environ. Saf., 2018, 166, 462–473 CrossRef CAS PubMed.
- P. S. Tourinho, C. A. van Gestel, K. Jurkschat, A. M. Soares and S. Loureiro, Effects of soil and dietary exposures to Ag nanoparticles and AgNO3 in the terrestrial isopod Porcellionides pruinosus, Environ. Pollut., 2015, 205, 170–177 CrossRef CAS PubMed.
- H. Ma, P. M. Bertsch, T. C. Glenn, N. J. Kabengi and P. L. Williams, Toxicity of manufactured zinc oxide nanoparticles in the nematode Caenorhabditis elegans, Environ. Toxicol. Chem., 2009, 28, 1324–1330 CrossRef CAS PubMed.
- Y. Deng, E. J. Petersen, K. E. Challis, S. A. Rabb, R. D. Holbrook, J. F. Ranville, B. C. Nelson and B. Xing, Multiple method analysis of TiO2 nanoparticle uptake in rice (Oryza sativa L.) plants, Environ. Sci. Technol., 2017, 51, 10615–10623 CrossRef CAS PubMed.
- C. Wang, H. Zhang, L. Ruan, L. Chen, H. Li, X.-L. Chang, X. Zhang and S.-T. Yang, Bioaccumulation of 13C-fullerenol nanomaterials in wheat, Environ. Sci.: Nano, 2016, 3, 799–805 RSC.
- F. Schwab, G. Zhai, M. Kern, A. Turner, J. L. Schnoor and M. R. Wiesner, Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–Critical review, Nanotoxicology, 2016, 10, 257–278 CrossRef CAS PubMed.
- B. P. Colman, C. L. Arnaout, S. Anciaux, C. K. Gunsch, M. F. Hochella, Jr., B. Kim, G. V. Lowry, B. M. McGill, B. C. Reinsch, C. J. Richardson, J. M. Unrine, J. P. Wright, L. Yin and E. S. Bernhardt, Low concentrations of silver nanoparticles in biosolids cause adverse ecosystem responses under realistic field scenario, PLoS One, 2013, 8, e57189 CrossRef CAS PubMed.
- OECD, Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure, OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris, France, 2012, DOI:10.1787/9789264185296-en.
- D. Mackay, Correlation of bioconcentration factors, Environ. Sci. Technol., 1982, 16, 274–278 CrossRef CAS PubMed.
- A. Praetorius, N. Tufenkji, K.-U. Goss, M. Scheringer, F. von der Kammer and M. Elimelech, The road to nowhere: equilibrium partition coefficients for nanoparticles, Environ. Sci.: Nano, 2014, 1, 317–323 RSC.
- E. J. Petersen, S. A. Diamond, A. J. Kennedy, G. G. Goss, K. Ho, J. Lead, S. K. Hanna, N. B. Hartmann, K. Hund-Rinke and B. Mader, Adapting OECD aquatic toxicity tests for use with manufactured nanomaterials: key issues and consensus recommendations, Environ. Sci. Technol., 2015, 49, 9532–9547 CrossRef CAS PubMed.
- ECHA, Guidance for the Implementation of REACH, Helsinki, Finland, 2008 Search PubMed.
- EPA, Framework for Metals Risk Assessment, Report number: EPA 120/R-07/001, U.S. Environmental Protection Agency, Washington, DC, 2007.
- C. Bowman, J. Vorderman, W. Vorderman, C. Hawthorne, M. Camboni, A. Turley and Y. Floredo, Study to assess the impact of possible legislation to increase transparency on nanomaterials on the market: Evaluation report, building blocks report and options assessment report, European Commission, 2014.
- M. B. Nielsen, A. Baun, A. Mackevica, A. Thit, I. Odnevall Wallinder, J. A. Gallego, L. P. Westergaard Clausen, J. Rissler, L. Skjolding, A. Castro Nilsson, T. Cedervall and S. Foss Hansen, Nanomaterials in the European chemicals legislation – methodological challenges for registration and environmental safety assessment, Environ. Sci.: Nano, 2021, 8, 731–747 RSC.
- K. Hund-Rinke, M. Herrchen, K. Schlich, K. Schwirn and D. Völker, Test strategy for assessing the risks of nanomaterials in the environment considering general regulatory procedures, Environ. Sci. Eur., 2015, 27, 24 CrossRef PubMed.
- J. Vassallo, A. Besinis, R. Boden and R. Handy, The minimum inhibitory concentration (MIC) assay with Escherichia coli: an early tier in the environmental hazard assessment of nanomaterials?, Ecotoxicol. Environ. Saf., 2018, 162, 633–646 CrossRef CAS PubMed.
- N. J. Clark, D. Boyle and R. D. Handy, An assessment of the dietary bioavailability of silver nanomaterials in rainbow trout using an ex vivo gut sac technique, Environ. Sci.: Nano, 2019, 6, 646–660 RSC.
- K. Tatsi, T. H. Hutchinson and R. D. Handy, Consequences of surface coatings and soil ageing on the toxicity of cadmium telluride quantum dots to the earthworm Eisenia fetida, Ecotoxicol. Environ. Saf., 2020, 201, 110813 CrossRef CAS PubMed.
- M. Baccaro, A. K. Undas, J. de Vriendt, J. van den Berg, R. Peters and N. W. van den Brink, Ageing, dissolution and biogenic formation of nanoparticles: how do these factors affect the uptake kinetics of silver nanoparticles in earthworms?, Environ. Sci.: Nano, 2018, 5, 1107–1116 RSC.
- R. D. Handy, J. C. McGeer, H. E. Allen, P. E. Drevnick, J. W. Gorsuch, A. S. Green, A.-K. Lundebye-Haldorsen, S. E. Hook, D. R. Mount and W. A. Stubblefield, in Toxicity of Dietborne Metals to Aquatic Organisms, SETAC Press Pensacola, USA, 2005, pp. 59–112 Search PubMed.
- OECD, Test No. 318: Guidance document for the testing of dissolution and dispersion stability of nanomaterials and the use of the data for further environmental testing and assessment strategies, OECD Publishing, Paris, France, 2020, DOI:10.1787/9789264284142-en.
- R. D. Handy, T. B. Henry, T. M. Scown, B. D. Johnston and C. R. Tyler, Manufactured nanoparticles: their uptake and effects on fish-a mechanistic analysis, Ecotoxicology, 2008, 17, 396–409 CrossRef CAS PubMed.
- M. van der Zande, A. Jemec Kokalj, D. J. Spurgeon, S. Loureiro, P. V. Silva, Z. Khodaparast, D. Drobne, N. J. Clark, N. W. van den Brink, M. Baccaro, C. A. M. van Gestel, H. Bouwmeester and R. D. Handy, The gut barrier and the fate of engineered nanomaterials: a view from comparative physiology, Environ. Sci.: Nano, 2020, 7, 1874–1898 RSC.
- R. D. Handy, F. von der Kammer, J. R. Lead, M. Hassellov, R. Owen and M. Crane, The ecotoxicology and chemistry of manufactured nanoparticles, Ecotoxicology, 2008, 17, 287–314 CrossRef CAS PubMed.
- M. R. Mulenos, J. Liu, H. Lujan, B. Guo, E. Lichtfouse, V. K. Sharma and C. M. Sayes, Copper, silver, and titania nanoparticles do not release ions under anoxic conditions and release only minute ion levels under oxic conditions in water: Evidence for the low toxicity of nanoparticles, Environ. Chem. Lett., 2020, 18, 1319–1328 CrossRef CAS.
- J. Dobias and R. Bernier-Latmani, Silver release from silver nanoparticles in natural waters, Environ. Sci. Technol., 2013, 47, 4140–4146 CrossRef CAS PubMed.
- D. M. Mitrano, J. F. Ranville, A. Bednar, K. Kazor, A. S. Hering and C. P. Higgins, Tracking dissolution of silver nanoparticles at environmentally relevant concentrations in laboratory, natural, and processed waters using single particle ICP-MS (spICP-MS), Environ. Sci.: Nano, 2014, 1, 248–259 RSC.
- OECD, Test No. 105: Water solubility, OECD Guidelines for the Testing of Chemicals, Section 1, OECD Publishing, Paris, France, 1995, DOI:10.1787/9789264069589-en.
- OECD, Test No. 318: Dispersion Stability of Nanomaterials in Simulated Environmental Media, OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris, France, 2017, DOI:10.1787/9789264284142-en.
- S. Kuehr, V. Kosfeld and C. Schlechtriem, Bioaccumulation assessment of nanomaterials using freshwater invertebrate species, Environ. Sci. Eur., 2021, 33, 9 CrossRef CAS.
- F. R. Khan, S. K. Misra, J. Garcia-Alonso, B. D. Smith, S. Strekopytov, P. S. Rainbow, S. N. Luoma and E. Valsami-Jones, Bioaccumulation dynamics and modeling in an estuarine invertebrate following aqueous exposure to nanosized and dissolved silver, Environ. Sci. Technol., 2012, 46, 7621–7628 CrossRef CAS PubMed.
- P. V. Silva, C. A. van Gestel, R. A. Verweij, A. G. Papadiamantis, S. F. Gonçalves, I. Lynch and S. Loureiro, Toxicokinetics of pristine and aged silver nanoparticles in Physa acuta, Environ. Sci.: Nano, 2020, 7, 3849–3868 RSC.
- M. N. Croteau, S. K. Misra, S. N. Luoma and E. Valsami-Jones, Bioaccumulation and toxicity of CuO nanoparticles by a freshwater invertebrate after waterborne and dietborne exposures, Environ. Sci. Technol., 2014, 48, 10929–10937 CrossRef CAS PubMed.
- T. Galloway, C. Lewis, I. Dolciotti, B. D. Johnston, J. Moger and F. Regoli, Sublethal toxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete, Environ. Pollut., 2010, 158, 1748–1755 CrossRef CAS PubMed.
- S. Kuehr, B. Meisterjahn, N. Schröder, B. Knopf, D. Völker, K. Schwirn and C. Schlechtriem, Testing the bioaccumulation of manufactured nanomaterials in the freshwater bivalve Corbicula fluminea using a new test method, Environ. Sci.: Nano, 2020, 7, 535–553 RSC.
- S. Kuehr, R. Kaegi, D. Maletzki and C. Schlechtriem, Testing the bioaccumulation potential of manufactured nanomaterials in the freshwater amphipod Hyalella azteca, Chemosphere, 2021, 263, 127961 CrossRef CAS PubMed.
- C. B. Jørgensen, Bivalve filter feeding revisited, Mar. Ecol.: Prog. Ser., 1996, 142, 287–302 CrossRef.
- M. S. Hull, P. Chaurand, J. Rose, M. Auffan, J.-Y. Bottero, J. C. Jones, I. R. Schultz and P. J. Vikesland, Filter-feeding bivalves store and biodeposit colloidally stable gold nanoparticles, Environ. Sci. Technol., 2011, 45, 6592–6599 CrossRef CAS PubMed.
- J. I. Kwak and Y.-J. An, Ecotoxicological effects of nanomaterials on earthworms: a review, Hum. Ecol. Risk Assess., 2015, 21, 1566–1575 CrossRef CAS.
- J. M. Unrine, O. V. Tsyusko, S. E. Hunyadi, J. D. Judy and P. M. Bertsch, Effects of particle size on chemical speciation and bioavailability of copper to earthworms (Eisenia fetida) exposed to copper nanoparticles, J. Environ. Qual., 2010, 39, 1942–1953 CrossRef CAS PubMed.
- W. A. Shoults-Wilson, B. C. Reinsch, O. V. Tsyusko, P. M. Bertsch, G. V. Lowry and J. M. Unrine, Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida), Nanotoxicology, 2011, 5, 432–444 CrossRef CAS PubMed.
- S. Carbone, T. Hertel-Aas, E. J. Joner and D. H. Oughton, Bioavailability of CeO2 and SnO2 nanoparticles evaluated by dietary uptake in the earthworm Eisenia fetida and sequential extraction of soil and feed, Chemosphere, 2016, 162, 16–22 CrossRef CAS PubMed.
- D. T. Stewart, K. Noguera-Oviedo, V. Lee, S. Banerjee, D. F. Watson and D. S. Aga, Quantum dots exhibit less bioaccumulation than free cadmium and selenium in the earthworm Eisenia andrei, Environ. Toxicol. Chem., 2013, 32, 1288–1294 CrossRef CAS PubMed.
- M. G. Vijver, J. P. M. Vink, C. J. H. Miermans and C. A. M. van Gestel, Oral sealing using glue: a new method to distinguish between intestinal and dermal uptake of metals in earthworms, Soil Biol. Biochem., 2003, 35, 125–132 CrossRef CAS.
- M. Diez-Ortiz, E. Lahive, P. Kille, K. Powell, A. J. Morgan, K. Jurkschat, C. A. Van Gestel, J. F. W. Mosselmans, C. Svendsen and D. J. Spurgeon, Uptake routes and toxicokinetics of silver nanoparticles and silver ions in the earthworm Lumbricus rubellus, Environ. Toxicol. Chem., 2015, 34, 2263–2270 CrossRef CAS PubMed.
- A. Laycock, M. Diez-Ortiz, F. Larner, A. Dybowska, D. Spurgeon, E. Valsami-Jones, M. Rehkämper and C. Svendsen, Earthworm uptake routes and rates of ionic Zn and ZnO nanoparticles at realistic concentrations, traced using stable isotope labeling, Environ. Sci. Technol., 2016, 50, 412–419 CrossRef CAS PubMed.
- OECD, Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei), OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, France, 2016, DOI:10.1787/9789264264496-en.
- W. K. Al-Murrani, General, Biological and Biomedical Statistics, Troubador, Leicester, 2021 Search PubMed.
- W. M. Meylan, P. H. Howard, R. S. Boethling, D. Aronson, H. Printup and S. Gouchie, Improved method for estimating bioconcentration/bioaccumulation factor from octanol/water partition coefficient, Environ. Toxicol. Chem., 1999, 18, 664–672 CrossRef CAS.
- M. van der Zande, R. J. Vandebriel, E. Van Doren, E. Kramer, Z. Herrera Rivera, C. S. Serrano-Rojero, E. R. Gremmer, J. Mast, R. J. Peters and P. C. Hollman, Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure, ACS Nano, 2012, 6, 7427–7442 CrossRef CAS PubMed.
- A. R. Al-Jubory and R. D. Handy, Uptake of titanium from TiO2 nanoparticle exposure in the isolated perfused intestine of rainbow trout: nystatin, vanadate and novel CO2-sensitive components, Nanotoxicology, 2013, 7, 1282–1301 CrossRef CAS PubMed.
- D. Boyle, N. J. Clark, T. L. Botha and R. D. Handy, Comparison of the dietary bioavailability of copper sulphate and copper oxide nanomaterials in ex vivo gut sacs of rainbow trout: effects of low pH and amino acids in the lumen, Environ. Sci.: Nano, 2020, 7, 1967–1979 RSC.
- A. Gupta and S. Lutsenko, Evolution of copper transporting ATPases in eukaryotic organisms, Curr. Genomics, 2012, 13, 124–133 CrossRef CAS PubMed.
- R. D. Handy and F. B. Eddy, in Physicochemical Kinetics and Transport at Chemical-Biological Interphases, John Wiley, Chichester, 2004, pp. 337–356 Search PubMed.
- R. D. Handy, M. M. Musonda, C. Phillips and S. J. Falla, Mechanisms of gastrointestinal copper absorption in the African walking catfish: Copper dose-effects and a novel anion-dependent pathway in the intestine, J. Exp. Biol., 2000, 203, 2365–2377 CrossRef CAS PubMed.
- J. J. Powell, N. Faria, E. Thomas-McKay and L. C. Pele, Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract, J. Autoimmun., 2010, 34, J226–J233 CrossRef CAS PubMed.
- S. Xu, B. Z. Olenyuk, C. T. Okamoto and S. F. Hamm-Alvarez, Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances, Adv. Drug Delivery Rev., 2013, 65, 121–138 CrossRef CAS PubMed.
- J. E. Scott, Ion binding: patterns of 'affinity' depending on types of acid groups, Symp. Soc. Exp. Biol., 1989, 43, 111–115 CAS.
- OECD, Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei), OECD Publishing, Paris, France, 2004, DOI:10.1787/9789264070325-en.
- J. R. Velicogna, D. M. Schwertfeger, A. H. Jesmer, R. P. Scroggins and J. I. Princz, The bioaccumulation of silver in Eisenia andrei exposed to silver nanoparticles and silver nitrate in soil, NanoImpact, 2017, 6, 11–18 CrossRef.
- N. Garcia-Velasco, M. Gandariasbeitia, A. Irizar and M. Soto, Uptake route and resulting toxicity of silver nanoparticles in Eisenia fetida earthworm exposed through standard OECD tests, Ecotoxicology, 2016, 25, 1543–1555 CrossRef CAS PubMed.
- ISO, ISO 11268-2:1998, Soil quality — Effects of Pollutants on Earthworms (Eisenia fetida) — Part 2: Determination of effects on reproduction, International Organization for Standardization, Geneva, Switzerland, 1998 Search PubMed.
- S. Makama, J. Piella, A. Undas, W. J. Dimmers, R. Peters, V. F. Puntes and N. W. van den Brink, Properties of silver nanoparticles influencing their uptake in and toxicity to the earthworm Lumbricus rubellus following exposure in soil, Environ. Pollut., 2016, 218, 870–878 CrossRef CAS PubMed.
- M. J. van der Ploeg, R. D. Handy, P. L. Waalewijn-Kool, J. H. van den Berg, Z. E. Herrera Rivera, J. Bovenschen, B. Molleman, J. M. Baveco, P. Tromp and R. J. Peters, Effects of silver nanoparticles (NM-300K) on Lumbricus rubellus earthworms and particle characterization in relevant test matrices including soil, Environ. Toxicol. Chem., 2014, 33, 743–752 CrossRef CAS PubMed.
- K. Schlich, T. Klawonn, K. Terytze and K. Hund-Rinke, Effects of silver nanoparticles and silver nitrate in the earthworm reproduction test, Environ. Toxicol. Chem., 2013, 32, 181–188 CrossRef CAS PubMed.
- ASTM, Standard guide for conducting laboratory soil toxicity or bioaccumulation tests with the lumbricid earthworm Eisenia fetida and the enchytraeid potworm Enchytraeus albidus, E 1676-04, American Society for Testing and Materials, Philadelphia, USA, 2004 Search PubMed.
- J. G. Coleman, D. R. Johnson, J. K. Stanley, A. J. Bednar, C. A. Weiss Jr, R. E. Boyd and J. A. Steevens, Assessing the fate and effects of nano aluminum oxide in the terrestrial earthworm, Eisenia fetida, Environ. Toxicol. Chem., 2010, 29, 1575–1580 CrossRef CAS PubMed.
- ASTM, Standard guide for conducting laboratory soil toxicity or bioaccumulation test with the Lumbricid earthworm Eisenia fetida, E1676–97, American Society for Testing and Materials, Philadelphia, USA, 1998 Search PubMed.
- S. Li, F. Irin, F. O. Atore, M. J. Green and J. E. Cañas-Carrell, Determination of multi-walled carbon nanotube bioaccumulation in earthworms measured by a microwave-based detection technique, Sci. Total Environ., 2013, 445, 9–13 CrossRef PubMed.
- E. J. Petersen, Q. Huang and J. W. J. Weber, Bioaccumulation of radio-labeled carbon nanotubes by Eisenia foetida, Environ. Sci. Technol., 2008, 42, 3090–3095 CrossRef CAS PubMed.
- I. Jośko, M. Kusiak and P. Oleszczuk, The chronic effects of CuO and ZnO nanoparticles on Eisenia fetida in relation to the bioavailability in aged soils, Chemosphere, 2020, 128982 Search PubMed.
- L. R. Heggelund, M. Diez-Ortiz, S. Lofts, E. Lahive, K. Jurkschat, J. Wojnarowicz, N. Cedergreen, D. Spurgeon and C. Svendsen, Soil pH effects on the comparative toxicity of dissolved zinc, non-nano and nano ZnO to the earthworm Eisenia fetida, Nanotoxicology, 2014, 8, 559–572 CrossRef CAS PubMed.
- A. Romero-Freire, S. Lofts, F. J. Martín Peinado and C. A. van Gestel, Effects of aging and soil properties on zinc oxide nanoparticle availability and its ecotoxicological effects to the earthworm Eisenia andrei, Environ. Toxicol. Chem., 2017, 36, 137–146 CrossRef CAS PubMed.
- OECD, Test No. 207: Earthworm, Acute Toxicity Tests, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, France, 1984, DOI:10.1787/9789264070042-en.
- C. García-Gómez, M. Babin, A. Obrador, J. Álvarez and M. Fernández, Toxicity of ZnO nanoparticles, ZnO bulk, and ZnCl2 on earthworms in a spiked natural soil and toxicological effects of leachates on aquatic organisms, Arch. Environ. Contam. Toxicol., 2014, 67, 465–473 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1en00444a |
| This journal is © The Royal Society of Chemistry 2021 |