Open Access Article
Open Access ArticleDietary exposure to copper sulphate compared to a copper oxide nanomaterial in rainbow trout: bioaccumulation with minimal physiological effects
David
Boyle
 ,
Nathaniel J.
Clark
,
Nathaniel J.
Clark
 ,
Benjamin P.
Eynon
and
Richard D.
Handy†
,
Benjamin P.
Eynon
and
Richard D.
Handy†
 *
*
School of Biological and Marine Sciences, University of Plymouth, Plymouth PL4 8AA, UK. E-mail: R.Handy@plymouth.ac.uk; Tel: +44 (0)1752 584630
First published on 30th June 2021
Abstract
The dietary bioaccumulation potential of engineered nanomaterials (ENMs) remains poorly understood. The aim of the current study was to assess the dietary bioaccumulation of copper from copper oxide (CuO) ENMs or dissolved copper (CuSO4) exposure in fish. Animals were fed a nominal diet of 750 mg kg−1 Cu in the respective forms for 4 weeks, then fed the control diet for a further 2 weeks in a depuration phase. Fish were sampled at weeks 2, 4 and 6 for total Cu analysis. Samples were also taken at week 4 for plasma ions, biochemistry and histology. An in chemico digestibility assay simulating the gut lumen showed the total Cu could be leached (i.e., was bioaccessible) from both diets. Fish from all treatments showed normal growth and survival, and with healthy histology of gills, intestines and liver. At the end of the 4 week exposure, both Cu materials caused an elevated tissue total Cu concentration with the highest in the mid intestine, hind intestine and liver. For example, the week 4 liver total Cu concentrations were 82 ± 7, 259 ± 19 and 281 ± 17 μg g−1 in the control, CuSO4 and CuO ENM treatments, respectively, with no significant differences between the Cu exposures. Compared to the controls, both forms of Cu caused induction of MT in the hind intestine, as well as some depletion of total GSH in the liver. In the post-exposure phase, there was evidence of depuration of total Cu from the mid and hind intestine, and also the carcass, but not the brain or kidney. The liver also maintained similar total Cu concentrations, in keeping with this organ as a central compartment for Cu excretion. There was limited evidence of nanomaterial-specific effects. Overall, the CuO ENMs showed similar patterns of bioaccumulation to CuSO4, with negligible physiology effects.
Environmental significanceUnderstanding the dietary bioaccumulation potential of engineered nanomaterials (ENMs) in fish is an important aspect of environmental risk assessment. Trout fed diets containing excess copper sulphate or copper oxide nanomaterial for 28 days showed no effects on growth, plasma ions or organ histology, despite some total copper accumulation in the internal organs. The animals managed the exposure with some loss of the antioxidant, glutathione, and some metallothionein induction; and showed clearance of copper from the organs in the post-exposure phase. A digestibility assay showed that copper from both forms of exposure was bioaccessible. Crucially, the bioaccumulation hazard from the nano form was similar to the metal salt, implying the existing metal risk assessment would be protective of the nano form. |
1. Introduction
Bioaccumulation potential and toxicity are key considerations in the environmental risk assessment of contaminants in the aquatic environment.1 For poorly soluble contaminants, such as engineered nanomaterials (ENMs), exposure of aquatic animals via the diet is considered more relevant to hazard assessment than aqueous exposure.2 This is because the majority of ENMs released into the aquatic environment will settle out of the water column. Exposure to ENMs may then occur through the food chain via predation of benthic organisms. In addition, it is challenging to maintain dispersions of ENMs in waterborne toxicity tests with fish,3,4 and this has increased interest in using dietary exposure methods instead for ENMs (e.g., ref. 2, 5 and 6).Copper (Cu) is an essential trace metal and dietary Cu requirements in freshwater fish such as rainbow trout (Oncorhynchus mykiss) are in the region of ∼3 mg per kg body weight per day, and aquaculture feeds routinely contain ∼10 mg kg−1 of Cu.7 At concentrations considerably higher than this, Cu has been shown to accumulate in internal tissues and sometimes cause toxicity.8,9 The threshold for the effects of dietary Cu on growth and survival is about 750 mg kg−1 of Cu in the food, or slightly more, depending on the ration size and species of fish (reviews,7,10). At high dietary inclusion rates, CuSO4 have been shown to affect ion homeostasis, cause oxidative stress, and pathologies at sites of Cu accumulation in trout.8,9 At dietary concentrations of 750 mg kg−1 of Cu or less as CuSO4, trout show good maintenance of body system homeostasis, and compensate for the cost of exposure by adjusting the bioenergetics of swimming behaviours.11,12 Only a few studies have considered dietary exposure to Cu ENMs, but total Cu concentrations in tissues have been shown to increase in tissues during dietary exposures.13,14 However, the form(s) of the bioavailable fraction of Cu ENMs in fish tissue are unclear.
The uptake of dissolved Cu at the intestine is well characterised in fish. At the apical membrane of the enterocytes, transport of the Cu ion is via high-affinity Cu uptake proteins (likely the CTR family and DMT1 proteins) with the rate limiting step in Cu assimilation being the ATP-dependent vesicular export of Cu into the blood.15–17 As such, dietary exposures to excess dissolved Cu are characterised by retention of Cu in the intestinal tissues in fish.8,18 The uptake mechanisms for Cu ENMs in the gut of fish remains to be established. Recently, we demonstrated negligible tissue accumulation of total Cu from CuO ENMs using an ex vivo gut sac technique in rainbow trout with a simple physiological saline; CuO ENMs were associated with the mucosa, and unlike dissolved Cu, did not transfer to the blood compartment.19 While CuO ENMs were dispersed in the simple physiological saline, the lack of uptake was unexpected, and further experiments in the same study explored the addition of amino acids that would be present in the chime in vivo. By adding the amino acid, cysteine, Cu was shown to accumulate in the blood compartment of the gut sacs. This was likely caused by increased dissolution of the CuO ENMs in the gut lumen in the presence of cysteine, and transport of Cu-cysteine into the tissue via dissolved Cu uptake pathways.19 Together, these data supported the hypothesis that intact nanoscale particulates of Cu are too large for uptake on solute transporters [see discussion Handy et al.;20 as demonstrated for silver (Ag) ENMs21], but also demonstrate that the chemical composition of gut lumen can drive the transformations of metallic ENMs and subsequent total Cu uptake.19
Notably, the bioaccumulation potential of the CuSO4 and CuO ENMs in fish has been shown using the ex vivo gut sac technique.19 In as little as 4 hours, total Cu from both materials accumulated in the mucosa of the gastrointestinal tract of rainbow trout, with the highest concentrations in the mid and hind intestines.19 To investigate this further, the present study explored the bioaccumulation of CuSO4 and CuO ENMs in juvenile rainbow trout exposed via the diet for 28 days. It also remains unclear whether newly accumulated material can be excreted; therefore, following the exposure period, fish were depurated on the control diet for a further 2 weeks (total 6 weeks) to assess alterations in tissue Cu concentrations. It is also important to link any total Cu accumulation to biological effects; CuSO4 exposure has a well understood aetiology, with the liver as the central organ for metabolism/detoxification, and molecular interactions with reduced glutathione (GSH) and metallothionein (MT). However, the handling process remains unknown for the nano form and so measurements of these biomarkers as well as histological structure of the intestine and liver were made at the end of the exposure. To aid data interpretation, an in chemico digestibility assay was also used to help understand the bioaccessible fraction from the feed in conditions analogous to those found in the gastrointestinal tract.
2. Methods
2.1 Experimental design
Juvenile rainbow trout weighing approximately 10 g were obtained from a commercial fish farm, Exmoor Fisheries (Somerset, UK). Upon arrival at the University of Plymouth, trout were kept together in a single tank with flowing dechlorinated Plymouth tap water for a period of two weeks to monitor the health of fish. Fish were fed daily with non-supplemented commercial fish feed (Aller Futura, EX, Kaliningrad, Russia). After the quarantine period, fish were graded and then randomly allocated to nine glass tanks in a flow through system (70 L; 3 tanks per treatment; 32 fish per tank). The total biomass in each tank ranged from 290–310 g (mean ± SD = 299.6 ± 7.2 g). Tanks were randomly allocated to treatments (control, CuSO4 or CuO ENMs) by drawing lots (n = 3 tanks per treatment; no significant difference in tank weights between treatments, one-way ANOVA, p = 0.473). Fish were left overnight before the exposures commenced.The dietary exposure was conducted following the essence of the Organisation for Economic Development and Cooperation (OECD) test guideline, TG 305,1 but with a triplicated study design and additional measurements. Fish were fed one of three diets for 28 days: a control diet (no added Cu), or 750 μg g−1 Cu as CuSO4 or CuO ENMs. Following this, a 14 day depuration period occurred where all treatments were fed the control diet. The total duration of the experiment was 42 days. Fish were fed a 2% body weight ration per day whereby the amount of food was altered each week based on the tank biomass. The ration was carefully fed at approximately 09:00, 13:00 and 17:00 each day and observed to ensure the food was eaten immediately to minimise contact time of the diet with water and any leaching of Cu from the diet into the water. There were no residual food pellets in the water after each feed. Faecal matter in the tanks was removed by careful siphoning after each feed.
The water chemistry was monitored in tanks throughout the experiment. Tanks were supplied continuously with Plymouth tap water with a water turnover rate of (mean ± SD): 0.52 ± 0.12 L min−1 to minimise the build-up of ammonia in the tanks (there was no significant difference in water turnover between treatments, one-way ANOVA, p = 0.919). The measured concentrations of total ammonia [NH3] in the tanks were 13.9 ± 5.2, 14.7 ± 5.6, and 13.8 ± 4.9 μM in control, CuSO4 and CuO ENM tanks, respectively. The water was pH 6.92–7.30, temperature was 17 ± 1 °C, dissolved oxygen was >100% saturation throughout the experiment and the ionic composition of the water, based on weekly measurements using inductively coupled optical emission spectrometry (ICP-OES, Varian 725-ES, Agilent Technologies Inc.), were (in mM): Ca2+, 0.41 ± 0.10; K+, 0.06 ± 0.04; Mg2+, 0.09 ± 0.05; Na+, 0.58 ± 0.23. Concentrations of Cu were also measured in tank water for the duration of the experiment (see section 2.4). The aquatics facility had a set 12 h light and 12 h dark photoperiod.
Fish from the experimental tanks were sampled at days 14 and 28 during the exposure phase and at the end of the depuration phase on clean food (day 42) for trace metal analysis (see section 2.4). Samples for tissue biochemistry and histology were also taken at day 28 (see sections 2.5 and 2.6, respectively). In order to facilitate dissection and to ensure animal welfare during handling, fish were not fed on the morning of the sampling days. This allowed for evacuation of the gut after the final feed the previous day, ∼15 h earlier. To achieve a daily ration of 2% body weight per day throughout the experiment, the half daily ration not fed on sampling days was given to fish on the subsequent days. All procedures with live fish were conducted in accordance with ethical approvals from the Home Office, UK, under the Animals (Scientific Procedures) Act 1986 and in compliance with the EU directive 2010/63/EU.
2.2 Nanomaterial characterisation and diet formulation
Copper oxide ENMs were provided in dry powder form by PlasmaChem GmbH (Berlin, Germany), a partner in the Sustainable Nanotechnologies Project (SUN) which was funded by the EU 7th Framework Programme. The characterisation of the same batch of this material has been reported elsewhere.19,22,23 Briefly, the primary particle sizes of CuO ENMs have been calculated from electron micrographs and were (mean ± SD, n = 100): 18.2 ± 5.6 nm.23 These CuO ENMs show little dissolution in either ultrapure water or Plymouth tap water (<0.1% Cu on a total mass basis after 1 h,23).The diet used throughout the study was the same as used by Clark et al.6 The diet was a commercial fish food (Aller Futura, EX, Kaliningrad, Russia), with a pellet size of 1.5 mm. The intact food pellets were supplemented with CuSO4 or CuO ENMs that was allowed to soak into the pellets and this was then sealed with a topcoat of 10% porcine gelatine (Sigma-Aldrich, UK). A nominal concentration of 750 μg g−1 Cu (as CuSO4 or CuO ENMs) in the diets was chosen based on previous studies of dissolved Cu in trout that have shown tissue Cu accumulation.12,24 To prepare the diets, a stock suspension of the CuO ENMs of 1.064 g 100 mL−1 was prepared first in ultrapure H2O (18.2 MΩ, ELGA, UK) in an acid-washed volumetric flask and dispersed in an ultrasonic bath for 1 h (50/60 Hz, 35 Watts, FB15048, Fisherbrand) before spiking the diets. Nanoparticle tracking analysis has demonstrated this approach gives good dispersions of CuO ENMs, with typical aggregate sizes of around 110 nm depending on concentration and water pH.19,23 In any event, the dispersion effort was mainly to make a uniform sample that could be added to the food, and would not reflect the aggregation state in the final food matrix. A stock solution of CuSO4 was prepared in the same manner but was not sonicated. The diets were prepared in a single batch by adding a total volume of 100 mL of either CuO ENMs or CuSO4 directly to 1 kg of diet and gently mixing the diet with a commercial food mixer for five minutes (model XKM810, Kenwood, UK). The diet was then coated with a solution of 10 g of gelatine in 100 mL of ultrapure water at 60 °C (per kg diet) and mixed into the diet for several additional minutes. Control diets were supplemented with ultrapure water in place of the Cu material and prepared as above. All diets were dried overnight at 40 °C to remove excess water and then stored at 4 °C until fed to fish. Total Cu concentrations of the diets were measured by inductively coupled plasma mass spectrometry (ICP-MS, Thermo Electron Corporation X-Series II quadrupole, see section 2.4). Copper was homogeneously distributed in the exposure diets and were close to the nominal 750 μg Cu g−1 with 9.5 ± 0.3, 685.6 ± 41.5 and 713.3 ± 33.6 μg per g dry weight (means ± SD, n = 5) in the control, CuSO4 and CuO ENMs treatments, respectively. There was no significant difference between the Cu concentrations in the two exposure diets (Student's t-test, p = 0.28).
An in chemico digestibility assay was used to aid data interpretation over the form of Cu in the gut lumen of trout. Two compartments of gastrointestinal tract at different pH values were simulated with artificial solutions: the stomach (0.1 M HCl, 0.9% NaCl, pH 2) and the intestine (0.9% NaCl, pH 7.8). 1 g samples of the experimental diets (n = 3 per treatment) were ground in a ceramic mortar and added to 20 mL of the artificial solutions. Samples were placed on a tube roller (Denley Spiramix 5 Tube Roller Mixer) for 1 h. Afterwards, the samples were centrifuged at 6000 × g for 10 min and the Cu concentration measured in the supernatant by ICP-MS (see section 2.4).
2.3 Tissue collections and blood sampling
Blood sampling and dissection followed Clark et al.6 At days 14, 28 and 42, fish (n = 3 per tank, n = 9 per treatment) were anaesthetised in buffered (NaHCO3, pH 7.0) MS222, pithed to destroy the brain and whole blood removed from the caudal vein into heparinised syringes (Li salt, 1 mg mL−1). The whole blood was then centrifuged, and the plasma removed and stored at −20 °C until analysed for Na+ and K+ concentrations by flame photometry (Sherwood Model 420 Flame Photometer). The fish were then dissected for metal analysis.Concentrations of Cu in tissues were measured at days 14, 28 and 42 of the experiment. Concentrations of other elements were measured in tissues on day 28, i.e. only at the end of the exposure period. Care was taken to avoid cross-contamination between tissues and also between fish. Tissues were dissected and weighed into tubes in the following order: gill, brain, liver (after gall bladder removal), kidney, with the mid- and hind-intestine being dissected last to avoid cross contamination between feed/faeces in the intestine and other tissues. The mid and hind intestine were rinsed in ultrapure water and blotted dry to remove residual feed/faeces before wet weight determination. Tissues and carcasses were freeze dried (Lablyo freeze dryer) for 24 h and stored at room temperature for metal analysis (see section 2.4). Measurements based on tissue dry weight were preferred to eliminate possible artefacts associated with osmotic stress (altered tissue moisture content) during metal exposure.
2.4 Metal analysis
Concentrations of Cu and other elements were measured in acidified water samples [few drops analytical grade (primer plus, Fisher UK) HNO3; see section 2.1], diets and samples from an in chemico digestibility assay (section 2.2), and tissues sampled from fish (section 2.3). Sub-samples (n = 5) of the diets of approximately 0.1 g were digested in 1 mL HNO3 at 60 °C for 4 h in clean 15 mL Falcon tubes in a water bath. Samples from the digestibility assays were collected as described in section 2.2. Freeze dried tissues were weighed and similarly digested at 60 °C in nitric acid for 4 h. The volume of nitric acid used was 1 mL for livers and 0.5 mL for all other tissues. Due to their greater mass, the freeze dried carcasses were macerated with a stick blender and sub-samples of approximately 0.5 g were digested in 2 mL acid. Samples of a reference material (DORM-3) with known concentrations of Cu were also digested as above to verify the efficacy of the digestion protocol and accuracy of the instrument measurements. With every analysis, procedural blanks, and acid-only samples, were analysed to check for leaching from the test tubes or other incidental contamination from the reagents (not observed, blanks remained below the limit of detection of the instrument). Following digestion, samples were diluted to volume with indium (In) and iridium (Ir) spiked ultrapure water (for use as internal standards). Due to the high background Cu concentrations in livers of trout an additional dilution was performed. Samples from the digestibility assays were also diluted with internal standards prior to Cu measurements. Measured Cu concentrations were compared to matrix matched standards (Fisher, UK) using ICP-MS. Other elements in the tissue digests were measured using ICP-OES.2.5 Tissue biochemistry
Selected tissues were analysed at day 28 for concentrations of proteins, total glutathione (total GSH) and MT, that are involved in Cu metabolism. Following dissection, tissues were snap frozen in liquid nitrogen and stored at −80 °C until required for analysis. For GSH analysis, tissues were homogenised (Cat X520D with a T6 shaft, medium speed, Bennett & Co., Weston-Super-Mare) in ice cold sucrose buffer (in mM: 300 sucrose, 0.1 EDTA, 20 HEPES, pH 7.8). After centrifugation (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 rpm, 2 min) GSH was quantified in supernatant and in buffer with final assay concentrations of (in mM): 76.5 phosphate buffer (pH 7.5), 3.8 EDTA, 0.6 DNTB, 0.2 NADPH and 0.12 U mL−1 glutathione reductase. Data were normalized to the total protein concentration in the supernatant measured with the Pierce BCA Protein Assay Kit according to the manufacturer's instructions (ThermoFisher Scientific, UK).
200 rpm, 2 min) GSH was quantified in supernatant and in buffer with final assay concentrations of (in mM): 76.5 phosphate buffer (pH 7.5), 3.8 EDTA, 0.6 DNTB, 0.2 NADPH and 0.12 U mL−1 glutathione reductase. Data were normalized to the total protein concentration in the supernatant measured with the Pierce BCA Protein Assay Kit according to the manufacturer's instructions (ThermoFisher Scientific, UK).
Concentrations of MT in liver, kidney, mid- and hind-intestines were analysed according to Scheuhammer and Cherian.25 Tissues were homogenised in five volumes of 0.25 M sucrose solution. Homogenates were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min and 100 μL of the supernatant (50 μL of liver) was diluted to 800 μL with glycine buffer (0.5 M, pH 8.5). Five hundred μL of AgNO3 (185.4 μM in glycine buffer) was then added and samples were incubated at room temperature for 5 min to allow the Ag+ to displace other metal ions bound to MT. To remove unbound Ag+ from samples, 100 μL horse haemolysate (Sigma-Aldrich, UK) was added, the samples were boiled for 1.5 min, centrifuged at 1200 × g and the supernatant removed to a second tube. This step was repeated once more and then the supernatant was spun at 15
000 rpm for 2 min and 100 μL of the supernatant (50 μL of liver) was diluted to 800 μL with glycine buffer (0.5 M, pH 8.5). Five hundred μL of AgNO3 (185.4 μM in glycine buffer) was then added and samples were incubated at room temperature for 5 min to allow the Ag+ to displace other metal ions bound to MT. To remove unbound Ag+ from samples, 100 μL horse haemolysate (Sigma-Aldrich, UK) was added, the samples were boiled for 1.5 min, centrifuged at 1200 × g and the supernatant removed to a second tube. This step was repeated once more and then the supernatant was spun at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 15 min. The final supernatant was diluted with ultrapure water and the Ag concentrations in samples were measured with ICP-MS and compared to matrix matched element standards (Fisher, UK). The concentrations of MT in samples were expressed per unit mass of tissue and were calculated by multiplying the measured mass of Ag by 3.55.
000 × g for 15 min. The final supernatant was diluted with ultrapure water and the Ag concentrations in samples were measured with ICP-MS and compared to matrix matched element standards (Fisher, UK). The concentrations of MT in samples were expressed per unit mass of tissue and were calculated by multiplying the measured mass of Ag by 3.55.
2.6 Histology
Fish were sampled at day 28 for histological examination and processed using methods described in Al-Bairuty et al.26 Animals were randomly selected (n = 2 per tank/n = 6 per treatment), humanely killed and the second gill arch, mid intestine, hind intestine and liver collected into 10% buffered formal saline for at least one week for fixation. Tissues were processed using an automated tissue processor (Leica TP1020 semi-enclosed benchtop) where samples were taken from the formal saline into industrial methylated spirit (50–100%), followed by clearing using Histolene and then taken to wax. Tissues were then embedded in wax blocks (Leica EG 1150H), sectioned at 6 μm intervals (Leica RM2235 microtome) and sections dried overnight. Mallory's trichrome was used to stain the gills. Liver, mid- and hind-intestinal morphology was viewed by staining sections with haematoxylin and eosin and using a Leica microscope (DMD108) with a built-in camera.2.7 Data handling and statistical analysis
To assess growth performance in each tank, the average body weight of the fish in each tank was calculated each week as the total biomass (g)/number of fish per tank. Data analysis was performed in SigmaPlot v. 14.0 (Systat Software Inc.). Data were checked for outliers using Grubb's test, after which data were tested for normality (Shapiro–Wilk test) and equality of variances (Brown–Forsythe test), and if not normally distributed were log10 transformed prior to statistical analysis. If data transformation failed, data were analysed using appropriate non-parametric statistical tests on untransformed data. There were 3 tanks per treatment and Cu accumulation and other endpoints of exposures to CuSO4 and CuO ENMs were assessed by pooling n = 3 fish from each replicate tank to give n = 9 fish per treatment, overall. To confirm the validity of this approach, tank effects were assessed in replicate tanks within each treatment by comparing growth performance and Cu accumulation in tissues. Analyses indicated that there were no significant differences in these metrics between replicate tanks and pooling fish for further analyses was a justified statistical approach. Statistical differences were assessed using either a one-way ANCOVA (growth and cumulative feed intake) or one-way or two-way ANOVA with Holm–Sidak test a posteriori, or, were data were not normally distributed and transformation failed, by Kruskal-Wallis followed by Dunn's test to identify differences between treatments. Data are presented as means ± standard error (SEM) except where stated.3. Results
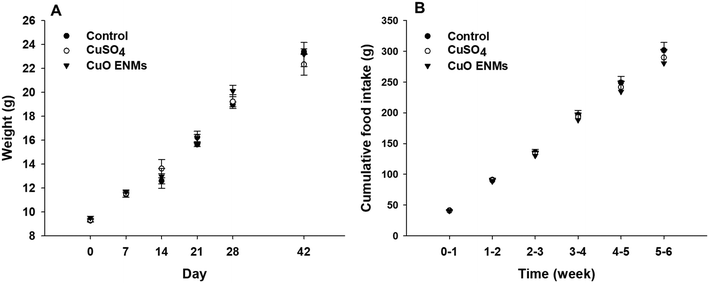
3.1 Growth, food intake and mortality of rainbow trout
Rainbow trout grew steadily throughout the experiment, from an initial mass of approximately 9.5 g to approximately 23 g after 42 days (Fig. 1). Growth was highly comparable between replicate tanks in each treatment (one-way ANCOVAs, p > 0.05) and there were also no statistically significant differences in growth over time between the treatments (one-way ANCOVA, p = 0.564). Fish in all treatments consumed all food of a ration of 2% body mass day−1 and there were no treatment dependent differences in cumulative food intake during the experiment (one-way ANCOVA, p = 0.890; Fig. 1). Over the course of the study there was a total loss of 18 fish, with all of these fish lost in the first 11 days of the experiment as fish hierarchies were established. Of these, the majority were euthanized due to injuries caused through aggressive acts of other fish e.g. fin damage. Losses occurred in all treatments and were not related to Cu exposure.3.2 In chemico digestibility assay
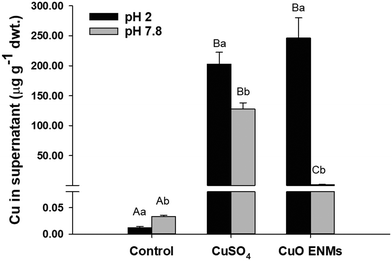
The in chemico digestibility assay was used to inform on labile or dissolved fractions of Cu in the experimental diets. Copper was released from all diets but there were significant differences between diets and between pH 2.0 and pH 7.8 (two-way ANOVA, p < 0.001; Fig. 2). Copper release from control diets was low and equated to <1% of total Cu at both pH 2.0 and pH 7.8. In comparison, Cu release from diets spiked with either CuSO4 or CuO ENMs was greater both in mass concentration measured in the supernatant and also as a percentage of total Cu in the diets. At pH 2.0, release of Cu from diets containing CuSO4 or CuO ENMs was not significantly different and was 202 ± 20 and 246 ± 34 μg total Cu g−1 (30 ± 3 and 35 ± 5%), respectively. In contrast, at pH 7.8, there was significantly greater release of Cu from diets containing CuSO4 than CuO ENMs and was 127.8 ± 10.3 and 1.5 ± 0.2 μg total Cu g−1 (18.65 ± 1.50 and 0.21 ± 0.03%), respectively.3.3 Metal analyses in water and tissues
Concentrations of total Cu were measured in water in the tanks throughout the experiment and the samples were gathered at intervals throughout the day, including immediately after feeding and before faeces were siphoned from tanks. Measured Cu concentrations were 0.2 ± 0.1 μg L−1 (n = 18) in control tanks but were significantly higher but still low in tanks fed CuSO4, 3.3 ± 0.5 μg L−1 or CuO ENMs, 3.5 ± 0.4 μg L−1.During the 28 day exposure, trout fed CuSO4 and CuO ENMs showed significant increases in concentrations of Cu compared to controls in all tissues examined (one-way ANOVAs or Kruskal-Wallis tests, p < 0.05; Table 1). With the exception of the kidney at day 28, there were no significant differences in Cu accumulation between the Cu treatments. Overall, the increases in Cu concentrations were highest in the mid and hind intestines, consistent with exposure via the diet, and also in the liver, which is the central tissue for metal metabolism. Smaller but significant increases were evident in the kidney, gill, brain and the remaining carcass. In all tissues except the liver, which showed significant increases in Cu accumulation between days 14 and 28 days, there were no further increases in Cu accumulation after the first 14 days of exposure.
| Tissue | 14 days (exposure) | 28 days (exposure) | 42 days (depuration) |
|---|---|---|---|
| Data are means ± SEM (n = 8/9 samples). Different uppercase letters indicate significant differences between time-points within treatment; different lowercase letters indicate significant differences between treatments within time-point (one-way ANOVA or Kruskal-Wallis, p < 0.05). | |||
| Mid intestine | |||
| Control | 5.52 ± 0.77Aa | 6.03 ± 0.60Aa | 6.58 ± 0.99Aa |
| CuSO4 | 29.84 ± 2.76Ab | 32.12 ± 7.36Ab | 8.09 ± 0.97Ba |
| CuO ENMs | 27.09 ± 6.93Ab | 28.48 ± 6.06Ab | 6.70 ± 0.65Ba |
| Hind intestine | |||
| Control | 9.10 ± 1.25Aa | 11.00 ± 0.92Aa | 16.67 ± 1.99Aa |
| CuSO4 | 210.75 ± 28.03Ab | 226.77 ± 28.32Ab | 12.87 ± 1.17Ba |
| CuO ENMs | 140.42 ± 34.06Ab | 200.15 ± 16.18Ab | 9.08 ± 0.73Ba |
| Liver | |||
| Control | 87.38 ± 10.43Aa | 82.01 ± 6.97Aa | 102.62 ± 13.48Aa |
| CuSO4 | 170.98 ± 11.15Ab | 259.30 ± 19.22Bb | 236.25 ± 19.37Bb |
| CuO ENMs | 184.65 ± 7.33Ab | 281.14 ± 16.73Bb | 255.71 ± 21.84Bb |
| Kidney | |||
| Control | 5.55 ± 0.42Aa | 4.51 ± 0.24Aa | 5.35 ± 1.07Aa |
| CuSO4 | 10.65 ± 1.39Ab | 7.21 ± 1.04Ab | 8.23 ± 1.86Ab |
| CuO ENMs | 9.15 ± 0.98Ab | 12.01 ± 1.99Ac | 7.54 ± 0.71Aab |
| Gill | |||
| Control | 4.08 ± 0.40Aa | 3.88 ± 0.15Aa | 3.77 ± 0.16Aa |
| CuSO4 | 7.83 ± 1.16Ab | 5.39 ± 0.43Aab | 4.61 ± 0.30Aa |
| CuO ENMs | 6.27 ± 0.37Aab | 8.36 ± 0.52Ab | 3.64 ± 0.08Ba |
| Brain | |||
| Control | 4.48 ± 0.23Aa | 4.18 ± 0.20Aa | 4.94 ± 0.19Aa |
| CuSO4 | 5.66 ± 0.34Ab | 5.35 ± 0.26Ab | 7.14 ± 0.28Bb |
| CuO ENMs | 5.45 ± 0.31Ab | 5.98 ± 0.25ABb | 6.62 ± 0.33Bb |
| Carcass | |||
| Control | 1.31 ± 0.12Aa | 0.99 ± 0.04Aa | 1.34 ± 0.10Aa |
| CuSO4 | 3.38 ± 0.73ABab | 3.39 ± 0.27Ab | 1.37 ± 0.07Ba |
| CuO ENMs | 3.83 ± 0.19Ab | 2.78 ± 0.51Ab | 1.53 ± 0.04Aa |
After the 14 day depuration phase (day 42 of the experiment), Cu concentrations in the mid and hind intestine, gill and carcass had decreased to control levels (one-way ANOVAs or Kruskal-Wallis tests, p > 0.05; Table 1). The same trend was not evident in other tissues. In the kidney and especially the liver and brain, Cu concentrations were elevated compared to controls and there was no change compared to day 28 of the exposure.
Concentrations of Na+ and K+ were measured in blood plasma at days 14, 28 and 42 of the experiment (Table 2) and in tissues at day 28 of the exposure. Blood plasma ion concentrations showed some deviations during the experiment but these appeared to be unrelated to treatment. There were also a few significant effects of the Cu exposures on tissue Na+ and K+ concentrations, with the exception of the hind intestine and the kidney. In the kidney, concentrations of Na+ were significantly decreased in fish treated with CuSO4 or CuO ENMs, but there was no nano-effect (Kruskal-Wallis test, p = 0.004). Measured Na+ concentrations were 248 ± 14, 194 ± 10 and 197 ± 6 μmol g−1 in fish fed control, CuSO4 and CuO ENMs. In the hind intestine, concentrations of both Na+ and K+ were decreased by both Cu treatments compared to controls and there was also a small nano-effect with K+ significantly lower in the hind intestine of fish fed CuO ENMs compared to CuSO4 (one-way ANOVAs, p < 0.05). Concentrations of Na+ in the hind intestine were 107 ± 8, 72 ± 4 and 70 ± 5 μmol g−1 in fish fed control, CuSO4 and CuO ENMs. Concentrations of K+ were 265 ± 10, 213 ± 11 and 182 ± 10 μmol g−1 in fish fed control, CuSO4 and CuO ENMs.
| Treatment | Day 14 | Day 28 | Day 42 | |
|---|---|---|---|---|
| Data are means ± SEM of n = 4–9 samples. There were no treatment or time related effects of dietary Cu exposure on plasma Na+ or K+ concentrations (Kruskal-Wallis tests, p > 0.05). | ||||
| Na+ | Control | 160.4 ± 1.2 | 145.7 ± 2.6 | 157.6 ± 6.1 |
| CuSO4 | 149.8 ± 2.7 | 138.7 ± 2.2 | 142.7 ± 3.3 | |
| CuO ENMs | 148.5 ± 5.1 | 139.1 ± 2.8 | 149.2 ± 2.0 | |
| K+ | Control | 1.93 ± 0.51 | 1.10 ± 0.17 | 1.46 ± 0.23 |
| CuSO4 | 2.74 ± 0.69 | 1.98 ± 0.35 | 1.43 ± 0.33 | |
| CuO ENMs | 1.87 ± 0.32 | 1.48 ± 0.26 | 1.38 ± 0.31 | |
3.4 Tissue biochemistry
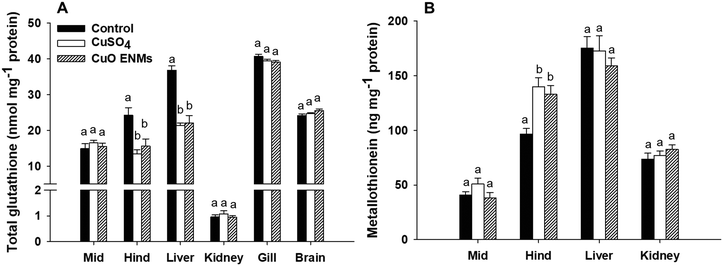
There were treatment related effects of dietary Cu exposure on concentrations of both GSH and MT, but only in some tissues (Fig. 3). In trout fed either CuSO4 or CuO ENMs, total GSH was significantly lower in the hind intestine compared to control fish, but there was no significant difference between the Cu treatments (one-way ANOVA, p < 0.001). This pattern of effects was also evident in the liver where exposure to either CuSO4 or CuO ENMs caused significantly lower total GSH concentrations compared to controls (one-way ANOVA, p < 0.001) and total GSH was depleted by approximately 40%. In all other tissues examined (mid-intestine, kidney, gill or brains), there were no significant effects of the Cu exposures on total GSH concentrations in trout. Exposure to Cu also affected concentrations of MT, but only in the hind intestine. Compared to controls, concentrations of MT were significantly elevated in hind intestine of fish fed CuSO4 and CuO ENMs (one-way ANOVA, p < 0.001). However, there was no difference in MT concentrations between fish fed CuSO4 and CuO ENMs.3.5 Histological examination
All the organs examined showed normal histology with no evidence of Cu-dependent pathology or loss of anatomical integrity (Fig. 4). The gut barrier remained intact with no evidence of oedema, erosion, atrophy or hyperplasia of the mucous epithelium or the underlying muscularis (Fig. 4A–C). The gills showed normal, healthy, histology in all treatments, without any Cu-dependent oedema of the secondary lamellae, hyperplasia of the gill epithelium, lamellar fusion, aneurisms or congestion of the vasculature (Fig. 4D–F). The livers showed normal sinusoids and parenchyma, with no evidence of Cu-dependent fatty change or lipidosis in any treatment (Fig. 4H–J). There was no evidence of peri-venule bleeding or reactive hyperplasia in the liver tissue. One fish from the CuSO4 treatment showed foci of vacuole formation in one area of the liver, but this was without fatty change or hyperplasia in the surrounding parenchyma (i.e., likely a random artefact), and not observed in any of the other fish.4. Discussion
This study shows that fish will consume a diet containing CuSO4 or CuO ENMs, with total Cu transferring to the organs, predominantly the mid intestine, hind intestine and liver. Both of the Cu exposures resulted in tissue total Cu burdens significantly elevated compared to the controls, with no significant differences between the Cu forms at any time point in any organ, except a transient elevation in the kidney of fish in the CuO ENM exposure. The presence of either Cu form in the diet did not impact the growth of the fish, plasma ions or histological integrity. However, both Cu exposures depleted the hind intestine total GSH, as well as increasing its MT expression at the end of the exposure. In the post-exposure phase where all fish were fed the control diet, depuration of total Cu was observed, where most of the elevated tissue concentrations returned to those similar to the controls. The exception to this was the liver in both the Cu treatments, which remained markedly elevated, and consistent with the organs role in Cu metabolism and excretion. The brain and kidney also did not completely clear the total Cu to control levels.4.1 Dietary exposure and total Cu accumulation
Fish are known to consume diets contaminated with additional Cu as CuSO4 (e.g., trout;11,24 tilapia;27 African catfish9). The threshold for dietary effects on growth and survival is above 750 mg Cu kg−1 food for juvenile trout, with exposures as high as 2000 mg kg−1 often causing little mortality.7 In the present study, a background incidence of mortality was observed (18 fish in total, ∼2% per treatment), and these were distributed randomly throughout the treatments, including the controls (no added Cu), and therefore could not be attributed to the presence of CuSO4 or CuO ENMs in the diets. There was some detectable Cu in the aquarium water, but this was at a trace level (<3 μg l−1), and the total Cu concentrations in the gill remained low (Table 1). There was also no evidence of pathology in the gills, which is typically associated with waterborne CuSO4 or Cu ENM exposures (e.g., Al-Bairuty et al.28). Taken together, these observations confirm dietary, rather than waterborne, exposure in the present study.Copper is an essential nutrient for fish, and as expected, there was a detectable background of total Cu present in the control tissues (Table 1). The control values for Cu ranging from around 3–11 μg g−1 dw in most organs, and around 100 dw μg g−1 dw in the liver (Table 1), being consistent with previous reports (e.g., ref. 26 and 29). The dietary exposure to the CuSO4 resulted in significant elevations of Cu in the mid intestine, hind intestine and liver in keeping with the route of exposure, and some small increases in the kidney and brain (Table 1), which is broadly similar to other reports for dietary copper exposure in trout.11,24,29 For instance, following a five week exposure to around 1 g Cu kg−1 food as CuSO4, the liver total Cu concentration reached 224 μg g−1 (∼3.5 μmol g−1) compared to 52 μg g−1 (∼0.8 μmol g−1) in controls for juvenile trout.24 The liver of fish exposed to dietary CuSO4 showed a similar trend in the present study, with livers from exposed fish reaching concentrations roughly three times those of the controls (Table 1). This is in keeping with the notion of the liver being a central compartment in Cu metabolism.30
There is less data on dietary exposure to CuO ENMs in fish. In the present study, the target organs and accumulation of total Cu from the dietary CuO exposure was the same as that for CuSO4, although the kidney accumulated more total Cu from exposure to the nano form (Table 1). There have been only a few other studies on dietary exposure to Cu ENMs or CuO ENMs in fish (sea bream;13 Russian sturgeon;14 snow trout31); and these studies can be regarded as preliminary because the material characterisation was often not reported and/or metal salt controls were not included in the study designs. Nonetheless, at least one study showed that fish fed diets containing Cu ENMs had elevated total Cu in the carcass and the liver compared to unexposed controls,14 in keeping with the findings here. In terms of accumulation in different regions of the gut (Table 1), the total Cu concentration of the hind intestine was higher than the mid intestine, indicating the former is the site of accumulation from CuO ENM exposures. This is generally consistent with finding that the distal regions of the gut are involved in the uptake of metal (form unknown) from exposures to ENMs in trout (TiO2,;32 Ag,6).
A crucial question for hazard assessment is whether the hazard from nano forms of Cu are different from the metal salt. The present study shows there were no significant differences between the total Cu concentrations in the organs following exposure to the CuSO4 or CuO ENM treatments, except for the kidney which had a higher total Cu concentration in the latter (Table 1). Similarities in organ concentrations of total Cu were found in red sea bream fed equal mass concentrations (4 mg kg−1) of CuSO4 or CuO ENMs. After 60 days of exposure, there was no significant difference in the total Cu concentration of the muscle, liver or gills.13 There appear to be no other reports of Cu accumulation in the brain or kidney following dietary exposure to Cu ENMs. Both Cu treatments caused increases in total Cu in the brain, but there was no additional elevation in total Cu associated with the nano form (Table 1). Nonetheless, with the brain as a target organ for Cu from CuO ENM exposures, the neurological deficits observed with excess dietary Cu salts, such as changes in circadian rhythms,12 could also be a similar concern for the nano form. For the kidney, more total Cu was accumulated in the exposure to the nano form, perhaps suggesting some additional hazard over the metal salt for this organ. The elevated total Cu might arise from melanomacrophage activity trapping particulate Cu in the parenchyma as observed in aqueous exposures to Cu ENMs in trout.28 However, Cu excretion via the urine is usually minimal in trout, with the liver being the main excretory organ,30 and with normal plasma ions (Table 2), it suggests that renal function was not lost.
However, any bioaccumulation hazard might be transient since the intestines and the carcass showed decreases in total Cu concentrations back to control levels in the post-exposure phase (Table 1), in keeping with dissolved Cu being an essential nutrient that is homeostatically controlled. Notably, total Cu from the CuO ENM exposure was also eliminated by the intestines (Table 1). Copper uptake and excretion is regulated by vesicular trafficking systems involving Cu-ATPase(s) that load Cu into the Golgi apparatus with subsequent vesicle formation.15,33 Increased turnover of the Cu trafficking in the gut epithelial cells by this mechanism might contribute to an apparent excretion of total Cu from the intestines. However, more likely, the normal process of sloughing of the intestinal epithelial cells would contribute to the decrease in total Cu in the intestines in the post-exposure phase. In contrast to the intestines and carcass, neither the liver, kidney nor brain showed decreases of total Cu in the post-exposure phase, regardless of the type of Cu exposure (Table 1). While there was no difference between CuSO4 and CuO ENMs in that regard, it highlights that the clearance of Cu from some organs is usually slower than uptake, as expected.27 For the kidney, as outlined above, the melanomacrophage activity might prolong the apparent retention of Cu in the organ. In the case of the brain, Cu concentrations increased in the post-exposure phase following exposure to CuSO4, but not for CuO ENMs (Table 1). Some redistribution of the Cu body burden is expected post-exposure.27 However, fluxes of metals across the blood brain barrier of fishes is poorly understood, although retention of Cu by metal binding ligands such as melatonin is probable.30 Whether or not intact CuO particles are taken up by the brain of fishes and retained requires investigation.
4.2 Digestibility and bioaccessible fractions in vivo
The almost identical accumulation of total metal from either dietary CuO ENM or CuSO4 exposures (Table 1) argues that the bioaccessibility of the nano form should be similar to that of CuSO4. One theoretical argument is that the CuO ENMs simply dissolve in the gut lumen, so that ultimately, both exposures are to dissolved metals. The in chemico digestibility assay at pH 2 (i.e., stomach pH of a carnivorous fish) showed an equal amount of total Cu was released from the diet (Fig. 2), indicating similar bioaccessibility of CuO ENMs and CuSO4. Similarly, in our previous gut sac studies, when the CuO ENMs (same batch as used here) were incubated in gut physiological saline at pH 2 to mimic behaviour in the stomach, 94% of the CuO ENMs became dissolved after 4 h with a dissolution rate of 1.4 mg h−1;19 and with gastric emptying at the temperature and ration used likely to take more than 24 h,34 in the stomach at least, the animals would be exposed to dissolved metal regardless of the original form. Albeit with a small fraction of the original CuO ENMs remaining. However, there is likely another transformation(s) further along in the gut lumen. Boyle et al.19 argued that Cu would be predominantly complexed with carbonate [68.15% CuCO3 (aq) and 29.38% Cu(CO3)22−] with only 0.71% as Cu2+ in the gut lumen at neutral pH values. The high ionic strength of the gut saline would also promote the formation of agglomerates, with precipitation of particulates observed at pH 7.8.19 It is therefore very likely that secondary Cu particles would form in the intestine and any existing CuO ENMs would aggregate onto the gut surface. The exposure in the intestine might therefore be to a mix of particulate and dissolved species of copper.The total Cu from the CuO diet was much less digestible than that of CuSO4 in the simulated intestine region, as measured by the appearance of total Cu in the supernatant at pH 7.8 (Fig. 2). However, this apparent lower bioaccessibility of the nano form in the intestinal lumen conditions could be offset by other factors in vivo. For example, the dissolution of the CuO ENMs at circumneutral pH was accelerated by the presence of essential amino acids in the diet, cysteine and histidine.19 Thus overall, in vivo, the total metal accumulated from either CuO ENMs and CuSO4 are similar (Table 1). Interestingly, the gut sac studies of Boyle et al.19 also showed that the initial rates of total Cu uptake to the serosal compartment was slower for CuO ENMs compared to CuSO4. In the same study on gut sacs, the dissolution of the ENMs was also influenced by amino acids in the gut lumen. Clearly, the particle transformations in the gut lumen in the presence of complex mixtures of electrolytes, amino acids, gut enzymes and other colloids, and how this influences the uptake rate, requires further investigation. Transformations are also possible in the internal organs, since trout fed excess Cu as metal salts, show metal granules in the liver.35
4.3 Growth and sub-lethal biochemical effects
Fish are well-known for ingesting metal-contaminated food and will continue to eat food containing more than 2000 mg Cu kg−1 dw.7,10 In the present study, the presence of nominally 750 mg kg−1 had no effect on cumulative food intake or growth, throughout the experiment. Notably, there was also no statistical difference in growth (Fig. 1), food intake, or Cu accumulation with the triplicates of each treatment, suggesting that the single replicate (pseudoreplication) approach for the regulatory use of the dietary TG 305 method is sufficient with respect to the use of animals (3Rs). Also the one concentration approach can minimise the number of animals tested,36 and was used here. The lack of effect of dietary CuSO4 on growth at the inclusion levels (Fig. 1) was expected and consistent with previous reports on trout (e.g., ref. 24 and 37). In the case of the diet containing CuO ENMs, growth and cumulative food intake were also unaffected (Fig. 1). In other studies, the Cu ENM inclusions in the diets of red sea bream were low (2–8 mg kg−1), and perhaps unsurprisingly, did not alter growth or nutritional performance.13 Russian sturgeon fed CuO ENMs at an inclusion of 16.25 mg kg−1 showed normal weight gain compared to unexposed controls. For other ENMs, Clark et al.6 showed that trout fed diets containing Ag ENMs at 100 mg Ag kg−1 of food, also had no effect on food intake or growth. Fish fed up to 1000 mg kg−1 of Zn as ZnO ENMs, also showed normal body weight compared to unexposed controls.5 Taken together, so far, this suggest fish may be nutritionally tolerant of diets containing metallic ENMs in a similar way to that of dissolved metals.Copper is an oxidising metal that also binds to –SH groups and so there are concerns for oxidative stress during exposures to dissolved Cu.9 In this regard, fish have biochemical defences; including the total GSH pool and MT (Fig. 3). The total GSH pool is a first line of defence against oxidative stress, with the reduced form of GSH as the anti-oxidant, although it is also a Cu carrier in the cytoplasm. The total GSH was unaffected in the gill, kidney and brain (Fig. 3A). The highest tissue total Cu concentrations in the present study were the hind intestine and the liver, in keeping with the route of exposure, and the two tissues showed some decreases in total GSH (∼40% in the liver, Fig. 3A). However, the pool was not depleted and there was no evidence of inflammation or oxidative damage in the organ histology (Fig. 4), indicating that the fish were moderating the effects of the exposure. Crucially, there were no differences in the total GSH response between the CuSO4 and the CuO ENMs exposures (Fig. 3), so no additional hazard from the nano form. El Basuini et al.13 also found that exposure to either 4 mg kg−1 dietary Cu as CuSO4 or Cu ENMs increased reactive oxygen metabolites in the blood plasma compared to unexposed controls, but there was no material-type effect. Wang et al.14 made similar observations for total antioxidant capacity in the livers of Russian sturgeon.
There were no effects on the MT concentrations in the organs, except for some induction of MT in the hind intestine compared to unexposed controls for both the CuSO4 and CuO ENM treatments (Fig. 3B). Again, there was no difference between the latter, implying no additional hazard from the nano form. The increase in MT is consistent with its role as a metal chelator, and in the hind intestine where higher total Cu concentrations were observed (Table 1). Some MT induction is expected in the intestine and liver of trout during dietary exposure to CuSO4.11 In the present study, the latter organ showed no exposure-dependent increases in MT, perhaps because the liver also has the ability to chelate Cu in copper–sulphur rich particles,35 or a sufficient threshold of intracellular dissolved Cu was not reached in the liver cells. Regardless, it is curious that CuO ENMs also induce MT, albeit only in the hind intestine (Fig. 3B). This implies that some dissolved Cu is generated inside the intestinal tissue by dissolution of the particles, or from uptake of dissolved Cu via particle dissolution in the lumen. This aspect that requires further investigation, but at least one study showed increased expression of the genes for the epithelial Cu channel, Ctr1, and Cu-ATPase in Caco-2 cells during exposures to CuO materials.38
During aqueous exposures, dissolved Cu interferes with osmoregulation and Na+ homeostasis via the gill.39 This is not observed in dietary exposures to CuSO4, because the gills are not directly exposed to dissolved Cu and so plasma electrolytes are largely unaffected.11 Similarly, in the present study, there were no effects of either CuSO4 or CuO ENM exposures on plasma ions (Table 2). There were some transient decreases on Na+ and/or K+ in the kidney and hind intestine, but these are likely not of physiological importance because electrolyte homeostasis of the plasma was maintained. Similar observation were made for dietary Ag and Ag ENMs in trout.6 So it seems that, like CuSO4, dietary exposure to the nano form of Cu is not an osmoregulatory hazard to trout.
4.4 Conclusions and perspective on environmental risk assessment
This study demonstrates that trout will ingest diets containing CuO ENMs with subsequent accumulation of total Cu in the internal organs. This raises a trophic transfer concern for the nano form of the metal. However, the bioaccumulation of total Cu was similar in fish fed diets containing CuSO4 or CuO ENMs at the concentration and conditions used here. Both treatments also showed evidence of post-exposure reductions in the Cu concentrations in the intestines and carcass, but not in the liver, kidney and brain. Again, the pattern was broadly similar in both CuSO4 and CuO ENM treatments, with the exception of the kidney. Consequently, existing biomagnification factors (BMFs), considerations for uptake and excretion, and weight of evidence used in metals risk assessments for dissolved Cu may also be protective of the CuO ENMs used here. More studies on different sizes, shapes, and compositions of Cu-containing ENMs would be needed to build a consensus on whether the existing dissolved Cu risk assessment could be applied to all nano forms of Cu. With respect to toxicity, there were no effects on growth, survival, histology or plasma ions. There was some evidence of partial total GSH depletion in the liver and MT induction in the gut, but this was in the context of maintaining normal growth and health of the animals. Taken together, the dose used here might therefore be applied in context of estimating oral probable no effect concentration (PNECoral) in a Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) risk assessment for both the dissolved and the nano form.Finally, a tiered approach to bioaccumulation testing has been proposed,2 with an ‘in vitro’ tier applying the gut sac technique19 in combination with the in chemico digestibility assay used here. The digestibility assay showed that both forms were bioaccessible at stomach pH, less so for the CuO EMNs at the neutral pH of the intestine (i.e., CuSO4 ≥ CuO ENMs). The gut sac study on the same material used here19 showed marginally less total Cu accumulation in the muscularis from the nano form (i.e., CuSO4 > CuO ENMs). Both those data would predict that the dietary hazard of the nano form is not greater than the metal salt. This is indeed the case, with total Cu bioaccumulation in vivo being largely the same in the present study (i.e., CuSO4 = CuO ENMs). This tiered approach has also been applied to Ag ENMs, where the metal salt is also protective of the nano forms (AgNO3 = Ag ENMs > Ag2S ENMs,6,21). Together, with the data here, this adds to the weight of evidence in support of a tiered approach to bioaccumulation testing that also seeks to minimise the use of vertebrate animals in vivo.2
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Sustainable Nanotechnologies Project (SUN) grant, contract number 604305 funded under the EU FP7 research programme. RDH was the PI at the University of Plymouth. The final draft of this manuscript was supported by the NanoHarmony project funded by the EU H2020 research programme; grant agreement no. 885931, with RDH as the PI at Plymouth. The authors thank Andrew Atfield for technical support in biology and Dr Andrew Fisher and Dr Robert Clough for support on trace metal analysis.References
- OECD, Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure, Organisation for Economic Cooperation and Development, Paris, 2012 Search PubMed.
- R. D. Handy, J. Ahtiainen, J. M. Navas, G. Goss, E. A. J. Bleeker and F. von der Kammer, Proposal for a tiered dietary bioaccumulation testing strategy for engineered nanomaterials using fish, Environ. Sci.: Nano, 2018, 5, 2030–2046 RSC.
- D. Boyle, H. Boran, A. J. Atfield and T. B. Henry, Use of an exposure chamber to maintain aqueous phase nanoparticle dispersions for improved toxicity testing in fish, Environ. Toxicol. Chem., 2015, 34, 583–588 CrossRef CAS PubMed.
- B. J. Shaw, C. C. Liddle, K. M. Windeatt and R. D. Handy, A critical evaluation of the fish early-life stage toxicity test for engineered nanomaterials: experimental modifications and recommendations, Arch. Toxicol., 2016, 90, 2077–2107 CrossRef CAS PubMed.
- M. Connolly, M. Fernández, E. Conde, F. Torrent, J. M. Navas and M. L. Fernández-Cruz, Tissue distribution of zinc and subtle oxidative stress effects after dietary administration of ZnO nanoparticles to rainbow trout, Sci. Total Environ., 2016, 551, 334–343 CrossRef PubMed.
- N. J. Clark, D. Boyle, B. P. Eynon and R. D. Handy, Dietary exposure to silver nitrate compared to two forms of silver nanoparticles in rainbow trout: bioaccumulation potential with minimal physiological effects, Environ. Sci.: Nano, 2019, 6, 1393–1405 RSC.
- S. J. Clearwater, A. M. Farag and J. Meyer, Bioavailability and toxicity of dietborne copper and zinc to fish, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2002, 132, 269–313 CrossRef.
- M. H. Berntssen, K. Hylland, S. E. W. Bonga and A. Maage, Toxic levels of dietary copper in Atlantic salmon (Salmo salar L.) parr, Aquat. Toxicol., 1999, 46, 87–99 CrossRef CAS.
- I. Hoyle, B. Shaw and R. Handy, Dietary copper exposure in the African walking catfish, Clarias gariepinus: Transient osmoregulatory disturbances and oxidative stress, Aquat. Toxicol., 2007, 83, 62–72 CrossRef CAS PubMed.
- R. Handy, J. McGeer, H. Allen, P. Drevnick, J. Gorsuch, A. Green, A. Lundebye-Haldorsen, S. Hook, D. Mount and W. Stubblefield, in Toxicity of Dietborne Metals to Aquatic Organisms, ed. J. Meyer, W. Adams, K. Brix, S. Luoma, D. Mount, W. Stubblefield and C. Wood, SETAC Press, Pensacola, USA, 2005, pp. 59–112 Search PubMed.
- R. Handy, D. Sims, A. Giles, H. Campbell and M. Musonda, Metabolic trade-off between locomotion and detoxification for maintenance of blood chemistry and growth parameters by rainbow trout (Oncorhynchus mykiss) during chronic dietary exposure to copper, Aquat. Toxicol., 1999, 47, 23–41 CrossRef CAS.
- H. Campbell, R. Handy and D. Sims, Increased metabolic cost of swimming and consequent alterations to circadian activity in rainbow trout (Oncorhynchus mykiss) exposed to dietary copper, Can. J. Fish. Aquat. Sci., 2002, 59, 768–777 CrossRef CAS.
- M. F. El Basuini, A. M. El-Hais, M. A. Dawood, A. E.-S. Abou-Zeid, S. Z. EL-Damrawy, M. M. E.-S. Khalafalla, S. Koshio, M. Ishikawa and S. Dossou, Effect of different levels of dietary copper nanoparticles and copper sulfate on growth performance, blood biochemical profiles, antioxidant status and immune response of red sea bream (Pagrus major), Aquaculture, 2016, 455, 32–40 CrossRef CAS.
- H. Wang, H. Zhu, X. Wang, E. Li, Z. Du, J. Qin and L. Chen, Comparison of copper bioavailability in copper-methionine, nano-copper oxide and copper sulfate additives in the diet of Russian sturgeon Acipenser gueldenstaedtii, Aquaculture, 2018, 482, 146–154 CrossRef CAS.
- R. Handy, M. Musonda, C. Phillips and S. Falla, Mechanisms of gastrointestinal copper absorption in the African walking catfish: copper dose-effects and a novel anion-dependent pathway in the intestine, J. Exp. Biol., 2000, 203, 2365–2377 CrossRef CAS PubMed.
- J. Burke and R. Handy, Sodium-sensitive and-insensitive copper accumulation by isolated intestinal cells of rainbow trout Oncorhynchus mykiss, J. Exp. Biol., 2005, 208, 391–407 CrossRef CAS PubMed.
- S. R. Nadella, M. Grosell and C. M. Wood, Physical characterization of high-affinity gastrointestinal Cu transport in vitro in freshwater rainbow trout Oncorhynchus mykiss, J. Comp. Physiol., B, 2006, 176, 793–806 CrossRef CAS PubMed.
- D. Boyle, K. V. Brix, H. Amlund, A.-K. Lundebye, C. Hogstrand and N. R. Bury, Natural arsenic contaminated diets perturb reproduction in fish, Environ. Sci. Technol., 2008, 42, 5354–5360 CrossRef CAS PubMed.
- D. Boyle, N. J. Clark, T. L. Botha and R. D. Handy, Comparison of the dietary bioavailability of copper sulphate and copper oxide nanomaterials in ex vivo gut sacs of rainbow trout: effects of low pH and amino acids in the lumen, Environ. Sci.: Nano, 2020, 7, 1967–1979 RSC.
- R. D. Handy, T. B. Henry, T. M. Scown, B. D. Johnston and C. R. Tyler, Manufactured nanoparticles: their uptake and effects on fish—a mechanistic analysis, Ecotoxicology, 2008, 17, 396–409 CrossRef CAS PubMed.
- N. J. Clark, D. Boyle and R. D. Handy, An assessment of the dietary bioavailability of silver nanomaterials in rainbow trout using an ex vivo gut sac technique, Environ. Sci.: Nano, 2019, 6, 646–660 RSC.
- R. C. Bicho, F. C. Santos, J. J. Scott-Fordsmand and M. J. Amorim, Effects of copper oxide nanomaterials (CuO NMs) are life stage dependent–full life cycle in Enchytraeus crypticus, Environ. Pollut., 2017, 224, 117–124 CrossRef CAS PubMed.
- D. Boyle, N. J. Clark and R. D. Handy, Toxicities of copper oxide nanomaterial and copper sulphate in early life stage zebrafish: effects of pH and intermittent pulse exposure, Ecotoxicol. Environ. Saf., 2020, 190, 109985 CrossRef CAS PubMed.
- C. Kamunde and C. M. Wood, The influence of ration size on copper homeostasis during sublethal dietary copper exposure in juvenile rainbow trout, Oncorhynchus mykiss, Aquat. Toxicol., 2003, 62, 235–254 CrossRef CAS PubMed.
- A. Scheuhammer and M. G. Cherian, Quantification of metallothionein by silver saturation, Methods Enzymol., 1991, 205, 78–83 CAS.
- G. A. Al-Bairuty, D. Boyle, T. B. Henry and R. D. Handy, Sublethal effects of copper sulphate compared to copper nanoparticles in rainbow trout (Oncorhynchus mykiss) at low pH: physiology and metal accumulation, Aquat. Toxicol., 2016, 174, 188–198 CrossRef CAS PubMed.
- B. J. Shaw and R. D. Handy, Dietary copper exposure and recovery in Nile tilapia, Oreochromis niloticus, Aquat. Toxicol., 2006, 76, 111–121 CrossRef CAS PubMed.
- G. A. Al-Bairuty, B. J. Shaw, R. D. Handy and T. B. Henry, Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss), Aquat. Toxicol., 2013, 126, 104–115 CrossRef CAS PubMed.
- S. Clearwater, S. Baskin, C. Wood and D. McDonald, Gastrointestinal uptake and distribution of copper in rainbow trout, J. Exp. Biol., 2000, 203, 2455–2466 CrossRef CAS PubMed.
- R. D. Handy, Chronic effects of copper exposure versus endocrine toxicity: two sides of the same toxicological process?, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2003, 135, 25–38 CrossRef.
- A. Afshari, I. Sourinejad, A. Gharaei, S. A. Johari and Z. Ghasemi, The effects of diet supplementation with inorganic and nanoparticulate iron and copper on growth performance, blood biochemical parameters, antioxidant response and immune function of snow trout Schizothorax zarudnyi (Nikolskii, 1897), Aquaculture, 2021, 736638 CrossRef.
- A. R. Al-Jubory and R. D. Handy, Uptake of titanium from TiO2 nanoparticle exposure in the isolated perfused intestine of rainbow trout: nystatin, vanadate and novel CO2-sensitive components, Nanotoxicology, 2013, 7, 1282–1301 CrossRef CAS PubMed.
- M. D. Harrison and C. T. Dameron, Molecular mechanisms of copper metabolism and the role of the Menkes disease protein, J. Biochem. Mol. Toxicol., 1999, 13, 93–106 CrossRef CAS PubMed.
- J. From and G. Rasmussen, A growth model, gastric evacuation, and body composition in rainbow trout, Salmo gairdneri Richardson, 1836, Dana, 1984, 3, 61–139 Search PubMed.
- R. P. Lanno, B. Hicks and J. W. Hilton, Histological observations on intrahepatocytic copper-containing granules in rainbow trout reared on diets containing elevated levels of copper, Aquat. Toxicol., 1987, 10, 251–263 CrossRef CAS.
- N. Burden, S. Creton, L. Weltje, S. K. Maynard and J. R. Wheeler, Reducing the number of fish in bioconcentration studies with general chemicals by reducing the number of test concentrations, Regul. Toxicol. Pharmacol., 2014, 70, 442–445 CrossRef CAS PubMed.
- H. Campbell, R. Handy and D. Sims, Shifts in a fish's resource holding power during a contact paired interaction: the influence of a copper-contaminated diet in rainbow trout, Physiol. Biochem. Zool., 2005, 78, 706–714 CrossRef CAS PubMed.
- C. Gao, L. Zhu, F. Zhu, J. Sun and Z. Zhu, Effects of different sources of copper on Ctr1, ATP7A, ATP7B, MT and DMT1 protein and gene expression in Caco-2 cells, J. Trace Elem. Med. Biol., 2014, 28, 344–350 CrossRef CAS PubMed.
- M. Grosell, C. Nielsen and A. Bianchini, Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2002, 133, 287–303 Search PubMed.
Footnote |
| † Visiting Professor, Department of Nutrition, Cihan University-Erbil, Erbil, Kurdistan Region, Iraq. |
| This journal is © The Royal Society of Chemistry 2021 |