Open Access Article
Open Access ArticleQuantification of particulate Ag in rainbow trout organs following dietary exposure to silver nitrate, or two forms of engineered silver nanoparticles†
Nathaniel J.
Clark
 *a,
Robert
Clough
*a,
Robert
Clough
 b,
David
Boyle
b,
David
Boyle
 a and
Richard D.
Handy
a and
Richard D.
Handy
 a
a
aSchool of Biological and Marine Sciences, University of Plymouth, Plymouth, UK. E-mail: nathaniel.clark@plymouth.ac.uk
bAnalytical Research Facility, School of Geography, Earth and Environmental Sciences, University of Plymouth, Plymouth, UK
First published on 12th May 2021
Abstract
Understanding the adsorption, distribution, metabolism and excretion (ADME) of engineered nanomaterials (ENMs) in fish has been limited due to analytical constraints in detecting the presence of nanoparticles (NPs) in the tissues. However, single particle inductively coupled plasma mass spectrometry now allows the detection of the particulate silver (Ag) in trout, including the mean particle diameter, mass concentration, and particle number concentration. The aim of this work was to quantify the particulate fraction of Ag in the hind intestine, liver, kidney and carcass following dietary exposure to either no added Ag (control), AgNO3, Ag NPs or Ag2S NPs, and whether this changes following a depuration period. Particulate Ag was found in the hind intestine of all treatments, including the AgNO3 exposure and trace amounts in the controls. At week 4, the particle number concentration (per g dry weight) in the hind intestine was 0.07 ± 0.03 × 109, 318.17 ± 116.71 × 109, 119.51 ± 33.00 × 109 and 0.60 ± 0.22 × 109, for the control, AgNO3, Ag NPs and Ag2S NPs exposures, respectively. In the Ag treatments, the organ particle number concentrations for both the AgNO3 and Ag NPs exposures were significantly higher compared to the Ag2S NP exposure, indicating a lower bioavailability of the latter material. The presence of particles in the AgNO3 exposure indicates that particulate Ag can be made in either the gut lumen or within the intestinal tissue. In conclusion, there was detection of silver-containing particles in the organs following exposure to both dissolved and particulate forms of Ag.
Environmental significanceDetermining the presence of nanoparticles (NPs) in the tissues of fish is an important aspect of the environmental hazard of engineered nanomaterials. Trout were exposed via the diet to either a control (no added silver), silver nitrate (AgNO3), silver nanoparticles (Ag NPs), or silver sulphide nanoparticles (Ag2S NPs). Silver exposures showed the presence of particulates in the intestine, liver and carcass, as measured by the particle mass, or particle number, concentrations. Both the AgNO3 and Ag NP exposures showed similar data, indicating that even dissolved silver may be transformed to a particle hazard in the tissue. The Ag2S NPs were less bioavailable. There was limited clearance of the particles from the organs, suggesting the materials are bioaccumulative. |
1. Introduction
The bioaccumulation potential of dissolved metals, including silver,1 is relatively well known, and is framed around the concept of adsorption, distribution, metabolism and excretion (ADME), with bioaccumulation in key target organs being an important factor in the disposition to the toxicity of metals.2 However, we do not known how these principles apply to engineered nanomaterials (ENMs) because clarity is needed on whether or not the nanomaterials stay in the particulate form or dissolve in the tissues.3 It is also unclear if the target organs for particulate silver inside the organism are the same as those for dissolved silver. Or, indeed, if there is some dissolution or degradation of the particles in the internal organs such that the tissues suffer a dual exposure of both dissolved and particulate metals.Relatively recently, the use of single particle inductively coupled plasma mass spectrometry (spICP-MS) has allowed investigations into the particulate fraction of silver nanoparticle accumulation, with attempts to confirm the presence of particles in the internal tissues of plants4,5 and in whole invertebrates.6,7 However, the little data that exists on fish is in selected tissues (e.g. gill, liver and intestine8), and those studies, so far, have not been intended to identify the main target organs for particles. The only data available on accumulation is based on total metal concentrations, where dietary exposure to equal concentrations of Ag, as AgNO3 or Ag NPs, showed the same target organs for all materials.9 One theoretical explanation for the similarity of total Ag concentrations in the tissues of fish exposed to AgNO3 or Ag NP via the diet is that the Ag NPs have simply dissolved in the acidic conditions of the stomach, and consequently both types of exposure are to the dissolved metal. However, it has been shown that particles are present in the livers of fish after 2 weeks exposure to either AgNO3 or Ag NPs.10 The presence of particles in the tissues from the AgNO3 exposure suggests the dissolved metal is transformed to particulate silver either in the gut lumen of the intestine, and/or inside the internal organs. Such transformative processes from feed to tissue are not typically considered for ADME studies in fish, but it is clear that the notion of particle dissolution then ionic uptake of free metal is an oversimplification. Furthermore, in order to interpret biological effects it would be useful to understand the nature of any particulate fraction in the internal organs. Notably, the mean particle size, the particle number concentration and particle mass concentration have not been well described for a suite of internal organs in fish.
The bioaccumulation potential of ENMs in fish is often measured using the Organisation for Economic Co-operation and Development (OECD) technical guidance (TG) 305 (review,11). This method, when used for metals, calculates bioconcentration factors (BCFs, waterborne exposure) or biomagnification factors (BMFs, dietary exposure) on the basis of the total metal concentrations in the fish tissue. For ENMs such as Ag NPs, it would be pragmatic to continue to make the assessments using total metal in the tissue for regulatory studies. However, in order to understand the hazards due to the nanoparticulate form, the relationship between total metal, dissolved metal and particulate metal in the tissue should be established. Furthermore, it is assumed in subsequent environmental risk assessment that the internal dose is the cause of organ pathologies for ENMs,12 and ultimately the toxicity; but which fraction or form of the ENM is present to cause such effects is not clear. For similar reasons, while the external concentration of Ag as Ag NPs can cause toxicity to fish in a dose-dependent manner,13–15 it is unknown which particle metric defines the tissue concentration, and therefore cause and effect. For waterborne exposures, at least, it has been suggested to be driven by the ion,16 but remains unclear for dietary exposures.
The overall aim of this work was to assess the form of Ag in the organs of rainbow trout following a dietary exposure to either AgNO3, Ag NPs or Ag2S NPs. In a previous study, we reported the target organs and accumulation pattern for total Ag in the organs of trout;9 with most of the total Ag (>97% in all Ag treatments) being in the hind intestine, liver, kidney and carcass. Here, the samples from that study were also analysed by spICP-MS with the specific objective of determining the particle mass concentration, particle number concentration and mean particle size within these organs during a 4 week exposure period. Additional objectives included to determine which particle metric best described the total Ag in the organs to ascertain if the total metal measurements used in the regulatory test remains appropriate for data interpretation with ENMs. Finally, since bioaccumulation might inform on toxic disposition and ADME processes, the ability of the organs to clear particulate Ag was assessed by spICP-MS on the organs following a 2 week depuration period.
2. Methodology
2.1. Exposure and tissue collection
A detailed description of the dietary exposure of fish to the Ag materials can be found elsewhere.9 Briefly, a commercially available diet was top dressed with either high purity water (control), AgNO3, Ag NPs or Ag2S NPs, with the materials coated onto the surface of the food pellets using 1% gelatine. The particle size distributions of the Ag NPs and Ag2S NPs can be seen in Fig. S1,† with detailed characterisation performed elsewhere.9 The roughly spherical Ag NP and Ag2S NP primary particle sizes were 55 ± 3 and 37 ± 17 nm, respectively, and the hydrodynamic diameters (Fig. S1†) in ultrapure water were 66 ± 4 and 135 ± 7 nm, respectively.17 The entire experiment was conducted with ethical approval from the UK Home Office via a Project Licence held at the University of Plymouth under the Animals (Scientific Procedures) Act (1986) and in compliance with the Directive 2010/63/EU. Juvenile triploid rainbow trout (∼10 g, n = 96) were fed diets containing no added Ag (control) or nominally 100 mg kg−1 of Ag as AgNO3, Ag NPs or Ag2S NPs for a maximum of 4 weeks. As the aim was to quantify the particulate fraction of Ag in key fish organs, only one exposure concentration was used. Following the exposure, they were all placed on the control diet for a depuration period of 2 weeks (giving a maximum trial duration of 6 weeks). At the appropriate time point, fish were euthanized by an overdose of buffered MS222, with confirmation via pithing (schedule 1 method in accordance with ethical approval), weighed and dissected for their hind intestine, liver and kidney at weeks 2, 4 and 6 (n = 2/tank, n = 6/treatment, total 72 fish). The hind intestine was chosen as a ‘positive control’ for the dietary exposure (i.e., the route of exposure), while the liver was selected as central compartment for internal Ag accumulation. The kidney was also chosen as an organ that does not typically accumulate material from dietary exposures, but is an excretory for some solutes in fish. The carcass was also selected to reflect the potential human health hazard associated with eating contaminated tissue. The tissues were dried and weighed before being cut in half. One half was used for total Ag determination,9 and one half was used for spICP-MS analysis (see section 2.2.). The remaining carcass (with no organs) was dried, homogenised with a hand blender and a ∼25 mg subsample was taken for spICP-MS analysis. Additionally, hind intestine and liver tissues were taken (n = 2/tank, n = 6/treatment, total 24 fish) and fixed for histological imaging (see section 2.3.).2.2. Nano silver extraction protocol and single particle ICP-MS
A validated method to routinely extract and detect nano silver from fish tissues has been documented,10 with the extractions here performed in exactly the same manner. A stock of 25 mmol L−1 CaCl2 (Sigma-Aldrich, CAS number 10035-04-8) was made in ultrapure water and diluted with 25% TMAH (Sigma-Aldrich, CAS number 75-59-2) to make an extraction solution of 20% TMAH containing 5 mmol L−1 CaCl2. Two mL was added to each sample of tissue (21 ± 13 mg, mean ± S.D.). The samples were left overnight at room temperature in a dark, dry storage cupboard and then diluted as appropriate and analysed by spICP-MS the next day.For all sample analysis by spICP-MS, an iCAP RQ ICP-MS instrument (Thermo Fisher), fitted with a Micromist nebuliser and a quartz cyclonic spray chamber cooled to 2 °C was operated in standard mode.10 Prior to use, the spray chamber was removed and washed in 10% nitric acid for 30 min, and the peristaltic tubing was replaced each day. The plasma power was 1550 Watts and the plasma, nebulizer and auxiliary gas flow rates were 14.0, 1.06 and 0.8 L min−1, respectively. A nickel plated sampler and high matrix insert skimmer cones were also used. Before each analytical session, the ICP-MS instrument was tuned for sensitivity and stability, using a solution of 1 μg L−1 Ba, Bi, Ce, Co, In, Li and U so that it performed according to the manufacturer's installation specifications. Sensitivity and stability, with an emphasis on the latter, and an oxide (CeO/Ce) formation rate of below 1% as an indication of the extent of polyatomic interference removal, were the key parameters for instrument tuning. A dwell time of 3 milliseconds was used throughout this work, and a total sampling time was 60 seconds using the 107Ag m/z ratio. The sample wash out time was 60 seconds, using a solution of 4% HCl and 2% HNO3, to ensure no carryover between samples. The sample uptake rate was determined gravimetrically by difference daily by aspirating deionised ultrapure water over 2 min (n = 5) and was between 0.2 and 0.3 mL min−1 for all analysis. The transport efficiency was also calculated daily, in each sample matrix used (n = 5), and according to Pace et al.18 using a 60 nm Au NP standard (BBI Solutions, UK). The instrument was calibrated using a series of dissolved Ag standards ranging from 0 to 4 μg L−1. Quality control measures of procedural blanks (n = 3) and check standards every 10–15 samples were included. All solution/suspension preparation and ICP-MS analyses were undertaken in a laboratory managed under an ISO 9001 certified Quality Management system.
Each sample produced 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 data points which were used to calculate the particle mass concentration, particle number concentration and mean particle size using a bespoke Excel spreadsheet, according to Peters et al.19 and assessed in Clark et al.10 The resulting standardised particle number and particle mass concentrations (per g dry weight of original tissue) were correlated with those of total Ag measurements made on the same organ.9 The limit of detection for particle size was calculated by using the lowest signal possible in a dwell time (333 counts per second [CPS]) and calculating the particle size for this signal. The resulting particle size LOD was 14 nm.
000 data points which were used to calculate the particle mass concentration, particle number concentration and mean particle size using a bespoke Excel spreadsheet, according to Peters et al.19 and assessed in Clark et al.10 The resulting standardised particle number and particle mass concentrations (per g dry weight of original tissue) were correlated with those of total Ag measurements made on the same organ.9 The limit of detection for particle size was calculated by using the lowest signal possible in a dwell time (333 counts per second [CPS]) and calculating the particle size for this signal. The resulting particle size LOD was 14 nm.
2.3. Histology
Samples of the hind intestine and liver were taken to confirm that tissues were intact with normal gross anatomy, and to exclude (for example) diffusive entry of particles through a compromised gut barrier. Histological examination was conducted according to Clark et al.9 At week 4 only, random fish were selected from the tank (n = 2/tank, n = 6/treatment, total 24 fish), euthanized and a cross section of the hind intestine and liver carefully removed. The tissues were immediately fixed in 10% buffered formal saline, and left in this solution for at least one week. The tissues were then processed using an automated tissue processor (Leica TP120 semi-enclosed benchtop) into industrial methylated spirit, and subsequently cleared using histolene, and then taken to wax (approximately 20 h). Liver tissues were then embedded in wax blocks (Leica EG 1150H) and sectioned at 6 μm intervals (Leica RM2235 microtome) and dried overnight. Slides were then stained using haematoxylin and eosin or haematoxylin/alcian blue/van Gieson's stain for the liver and hind intestine, respectively. Haematoxylin and eosin stain the nuclei blue and the cytoplasm pink. The haematoxylin/alcian blue/van Gieson stain the collagen red, mucins blue/turquoise, muscle and red blood cells yellow, the cytoplasm pink, and the nuclei brown/black.2.4. Dissolved silver speciation in different physiological compartments
To aid data interpretation, the speciation of dissolved silver was modelled using Visual MINTEQ 3.1 by J. P. Gustafsson (https://vminteq.lwr.kth.se/download/). The silver speciation was modelled in three compartments of the gastrointestinal tract, at differing pH values, using a physiologically relevant gut saline: the stomach at pH 2, the mid gut at pH 7.8 and the lower/hind gut at pH 9. The saline used was a physiological saline, containing major electrolytes, which has been used to understand uptake mechanisms into the gut epithelium17 and was (in mmol L−1): NaCl (117.5), KCl, (5.7), CaCl2, (2.5) and MgSO4 (1.2). Additionally, the blood and intracellular compartments were assessed for speciation of ionic silver, with a physiological salines containing NaCl (121.4), KCl (5.1), CaCl2 (1.4) and MgSO4 (1.9) at pH 7.8,17 and KCl (140), Na (10), MgSO4 (1) and CaCl2 (0.1) at pH 7.4, respectively.2.5. Calculations and statistics
The particulate Ag mass body burden in each of the organs (i.e., hind intestine, liver, kidney and carcass) was derived by calculating the total mass in each organ (concentration multiplied by total organ dry weight). The absolute particulate mass of Ag in the fish was calculated by totalling the four organs assessed here. Each of the four organs was then expressed as a percentage of the total particulate Ag mass in the fish. Total Ag measurements9 show these four organs contained over 97% of the Ag, ensuring their use gives a good representation of particulate Ag inside the fish. Statistical analysis were performed using SigmaPlot 13.0. Data were checked for outliers using Grubbs test, following which they were assessed for normality (Shapiro–Wilk test) and equal variance (Brown Forsythe). Statistical differences were assessed using a two-way ANOVA for analysis (time and treatment as factors). The Holm–Sidak post hoc test was used for normally distributed data. Non-normal data was log10 transformed. Where data were non-parametric and could not be transformed, the Kruskal–Wallis test was used and a post hoc test of Tukey's was used. The P values presented correspond to the appropriate post hoc test. The association between organ total Ag concentrations9 with particle mass concentrations or particle number concentration were assessed using the Spearman rank order correlation.3. Results
3.1. Organ particle mass concentration
There were no Ag-containing particles detected in the procedural blanks, the kidney or carcass samples from the unexposed control fish (Table 1). However, in the hind intestine and liver of control fish, traces of Ag-containing particles were found. Throughout the dietary exposure, only the liver of the control fish showed a time dependent change, whereby there was a significant increase in particle mass concentration in samples from fish taken at week 4 compared to week 2 (two-way ANOVA, P = 0.012). Regardless, each organ of the control fish showed a significantly lower particle mass concentration compared to any of the Ag-containing treatments.| Treatment | Organ | Week 2 | Week 4 | Week 6 |
|---|---|---|---|---|
| Data are mean ± standard error of the mean (n = 5/6 fish). Upper case letters denote significant difference between treatments (two-way ANOVA, columns). Lower case letters denotes significant difference between time points (two-way ANOVA, rows). The number of decimal places in the controls are shown for clarity between treatments. The limit of detection (LOD) was 0.001 μg g−1. | ||||
| Control | Hind intestine | 0.027 ± 0.008Aa | 0.018 ± 0.006Aa | 0.015 ± 0.004Aa |
| AgNO3 | 40.7 ± 16.9Ba | 192.9 ± 35.6Ba | 110.7 ± 53.4Ba | |
| Ag NPs | 31.4 ± 10.4Ba | 102.7 ± 18.7Ba | 113.7 ± 25.6Ba | |
| Ag2S NPs | 0.8 ± 0.2Ca | 1.2 ± 0.6Ca | 2.0 ± 1.3Ca | |
| Control | Liver | 0.003 ± 0.001Aa | 0.061 ± 0.039Ab | 0.011 ± 0.003Aab |
| AgNO3 | 120.8 ± 45.6Ba | 267.5 ± 42.5Ba | 174.5 ± 55.0Ba | |
| Ag NPs | 98.4 ± 27.5Bab | 315.5 ± 69.5Ba | 96.9 ± 31.4Bb | |
| Ag2S NPs | 3.2 ± 1.0Ca | 5.3 ± 1.9Ca | 7.6 ± 2.1Ca | |
| Control | Kidney | <LOD | <LOD | <LOD |
| AgNO3 | 10.0 ± 1.3Aa | 30.2 ± 9.6Aa | 34.3 ± 6.9Aa | |
| Ag NPs | 5.2 ± 1.0Aa | 34.5 ± 7.4Aa | 64.0 ± 19.2Aa | |
| Ag2S NPs | 0.5 ± 0.3Aa | 2.6 ± 0.8Aa | 0.3 ± 0.1Ba | |
| Control | Carcass | <LOD | <LOD | <LOD |
| AgNO3 | 3.78 ± 2.01Aa | 4.05 ± 1.79Aa | 0.19 ± 0.04Ab | |
| Ag NPs | 2.16 ± 0.56Aab | 7.86 ± 4.42Aa | 0.47 ± 0.15Ab | |
| Ag2S NPs | 0.11 ± 0.04Ba | 0.61 ± 0.37Ba | <LOD | |
Within fish from the AgNO3 exposure, all organs at each time point had an elevated particle mass concentration compared to the controls (Table 1). The organs of fish exposed to AgNO3 had a particle mass concentration that appeared to increase between weeks 2 and 4 of the exposure phase, but this apparent time trend was not statistically significant for any organ (two-way ANOVA, P > 0.05). For example, the liver particle mass concentration increased from a mean value of 121 ± 46 μg g−1 at week 2 to 268 ± 43 μg g−1 dw at week 4, but the variability between individual animals results in no significant difference between those time points. The organs of fish exposed to the Ag NPs showed a very similar trend compared to those from the AgNO3 exposure, with no statistical difference between the AgNO3 and Ag NP treatments in any organ, or at any time point. Compared to fish from the AgNO3 and Ag NP exposures, the particle mass concentration of fish exposed to Ag2S NPs was lower (10–200 fold) in all organs (Table 1). For example, at week 4 the hind intestine particle mass concentrations were 193 ± 36, 103 ± 19 and 1.2 ± 0.6 μg g−1 dw following exposure to AgNO3, Ag NP and Ag2S NP, respectively (two-way ANOVA, P < 0.001). Regardless, the hind intestine particle mass concentrations of fish from the Ag2S NP exposure did remain significantly elevated compared to the control fish (two-way ANOVA, P < 0.001). Similar to the AgNO3 and Ag NP exposed fish, there was no significant difference over the uptake phase in any of the organs from the Ag2S NP exposure (two-way ANOVA or Kruskal–Wallis, P > 0.05).
After the 4 weeks of exposure, fish were placed on the control diet with no additional Ag for 2 weeks. Generally, the week 6 organ particle mass concentrations were not statistically different from week 4, indicating the organs were not clearing the particles. However, the exception to this was the carcass, where both the AgNO3 and Ag NP exposed fish showed a statistically significant decrease in particle mass concentration after the depuration period. For example, both treatments fell 20-fold from 4.05 ± 1.79 and 7.86 ± 4.42 at week 4 to 0.19 ± 0.04 and 0.47 ± 0.15 μg g−1 in the AgNO3 and Ag NP exposures, respectively. This pattern was also observed in fish exposed to Ag2S NPs, with the particle mass concentration falling from 0.61 ± 0.37 μg g−1 at week 4 to below the limit of detection by week 6.
3.2. Organ particle number concentration
There were no Ag-containing particles found in the kidney or the carcass samples from control (unexposed) fish (Table 2). However, a small number of Ag-containing particles were found within the hind intestine and liver of the control fish. These concentrations equate to 25–40 particles in a time scan, which was above the LOD of ∼15 particles per scan ([3 × S.D. of the blank] + the blank). At each week during the dietary exposure, the control samples had particle number concentrations that were at least one order of magnitude lower than that of all of the fish exposed to Ag-containing treatments within the same organ.| Treatment | Organ | Week 2 | Week 4 | Week 6 |
|---|---|---|---|---|
| Data are mean ± standard error of the mean (n = 5/6 fish). Upper case letters denote significant difference between treatments (two-way ANOVA, columns). Lower case letters denotes significant difference between time points (two-way ANOVA, rows). The number of decimal places in the controls are shown for clarity between treatments. The limit of detection (LOD) was 0.0007 × 109 g−1 dw. | ||||
| Control | Hind intestine | 0.022 ± 0.006Ab | 0.070 ± 0.031Aa | 0.009 ± 0.004Ab |
| AgNO3 | 135.5 ± 45.2Bab | 318.2 ± 116.7Ba | 72.1 ± 31.5Bb | |
| Ag NPs | 172.1 ± 58.7Ba | 119.5 ± 33.0Ba | 81.8 ± 16.8Ba | |
| Ag2S NPs | 0.6 ± 0.2Ca | 0.6 ± 0.2Ca | 0.4 ± 0.2Ca | |
| Control | Liver | 0.007 ± 0.001Aa | 0.006 ± 0.003Aa | 0.002 ± 0.001Aa |
| AgNO3 | 68.3 ± 13.5Ba | 83.4 ± 20.2Ba | 54.5 ± 11.3Ba | |
| Ag NPs | 76.9 ± 21.0Ba | 72.6 ± 17.0Ba | 51.7 ± 8.9Ba | |
| Ag2S NPs | 6.6 ± 1.4Ba | 5.4 ± 1.6Ba | 6.9 ± 2.2Ba | |
| Control | Kidney | <LOD | <LOD | <LOD |
| AgNO3 | 27.3 ± 8.6Aa | 67.1 ± 20.4Aa | 47.2 ± 15.7Aa | |
| Ag NPs | 26.6 ± 5.2Aa | 41.6 ± 14.2Aa | 85.8 ± 19.3Aa | |
| Ag2S NPs | 0.1 ± 0.1Ba | 0.5 ± 0.1Bb | 0.1 ± 0.01Ba | |
| Control | Carcass | <LOD | <LOD | <LOD |
| AgNO3 | 1.47 ± 0.32Aa | 1.53 ± 0.10ABa | 0.50 ± 0.13Aa | |
| Ag NPs | 1.51 ± 0.33ABa | 1.77 ± 0.18Aa | 0.84 ± 0.20Aa | |
| Ag2S NPs | 0.09 ± 0.03Ba | 0.16 ± 0.05Ba | <LOD | |
Within fish from the AgNO3 treatment, the organs at 4 week of the exposure showed a significantly elevated Ag-containing particle number concentration compared to the controls (Table 2). Generally, the particle number concentration in all the organs from fish exposed to AgNO3 appeared to increase from week 2 to 4, but not in a statistically significant manner (two-way ANOVA, P > 0.05). The organs from Ag NP exposed fish showed a very similar pattern of particle number concentration compared to the AgNO3 exposed fish, and there was no significant difference between the Ag NP and AgNO3 exposed fish in any organs at any time point (Table 2). For example, the hind intestines from fish at week 2 have particle number concentrations of around 135 ± 45 and 172 ± 59 × 109 particles per g dw following exposure to AgNO3 or Ag NPs, respectively (Kruskal–Wallis, P > 0.05). Compared to the hind intestines of fish exposed to AgNO3 or Ag NPs, the Ag2S NP exposed fish had significantly lower particle number concentrations (200- to 300-fold) at each time point during the exposure (two-way ANOVA, P < 0.001). Regardless, the hind intestine from fish exposed to the Ag2S NPs contained a significantly higher particle mass concentration compared to the controls (two-way ANOVA, P < 0.001). This pattern of Ag2S NP exposure resulting in a lower particle number concentration compared to the AgNO3 and Ag NP exposures was also observed to a lesser extent in the kidney (80- to 200-fold) and carcass (10- to 20-fold).
Between week 4 and 6, all treatments were placed on the control diet with no added Ag. During this depuration phase on normal food, all the fish that had been fed the Ag-containing treatments showed a tendency for the particle number concentration to fall up to 8-fold at week 6 compared to week 4 (Table 2), but there was no significant difference between the weeks. For example, in the kidney from fish exposed to Ag2S NP, the particle number concentration was 0.5 ± 0.1 and 0.1 ± 0.01 × 109 particles per g dw at week 4 and 6, respectively. However, this downward trend was not significantly lower at week 6 compared to week 4 in the kidney or other organs (two-way ANOVA, P > 0.05), indicating that particles were not cleared from the organs post-exposure. The only organ to return to pre-exposure tissue concentrations (i.e., that of the controls) was the Ag2S NP carcass, where the particle number concentration fell from 0.16 ± 0.05 to below the limit of detection of the instrument.
3.3. Organ mean particle size
The mean particle size of the Ag-containing particles in the hind intestine, liver, kidney and carcass are shown in Table 3. No particles were observed in the kidney or carcass of control fish. The mean size of the particles in the hind intestine of control fish was 27 ± 5 nm and the liver was 47 ± 2 nm at week 4 of the exposure. There was no change in the mean size of particles detected in the hind intestine or liver over the 6 week experiment (Table 3).| Organ | Treatment | Week 2 | Week 4 | Week 6 |
|---|---|---|---|---|
| Data are mean ± standard error of the mean (n = 5/6 fish). Upper case letters denote significant difference between treatments (within columns). Lower case letters denotes significant difference between time points (within rows). Note the original mean primary particle sizes were 55 ± 3 and 35 ± 17 nm for the Ag NPs and Ag2S NPs, respectively. The limit of detection (LOD) was 14 nm. | ||||
| Hind intestine | Control | 32.4 ± 1.7Aa | 26.8 ± 5.3Aa | 33.4 ± 3.9Aa |
| AgNO3 | 41.5 ± 5.8Aa | 41.3 ± 6.3ABa | 46.7 ± 7.4Aa | |
| Ag NPs | 42.0 ± 3.7Aa | 42.7 ± 2.6Ba | 46.8 ± 4.9Aa | |
| Ag2S NPs | 38.8 ± 3.3Aa | 31.3 ± 4.0ABa | 33.7 ± 2.6Aa | |
| Liver | Control | 45.8 ± 3.2Aa | 46.9 ± 2.0Aa | 59.1 ± 7.7Aa |
| AgNO3 | 62.9 ± 7.8Aa | 76.8 ± 6.7BCa | 73.7 ± 10.2Aa | |
| Ag NPs | 52.7 ± 6.8Aa | 82.2 ± 10.9Cb | 60.3 ± 5.1Aab | |
| Ag2S NPs | 41.9 ± 1.6Aa | 53.2 ± 0.9ABa | 57.3 ± 4.4Aa | |
| Kidney | Control | N/A | N/A | N/A |
| AgNO3 | 36.2 ± 2.6Aa | 43.1 ± 9.4Aa | 44.9 ± 5.7Aa | |
| Ag NPs | 31.0 ± 2.1Aa | 46.8 ± 7.2Aa | 39.2 ± 2.3Aa | |
| Ag2S NPs | 33.1 ± 2.4Aa | 39.7 ± 4.0Aa | 35.1 ± 3.2Aa | |
| Carcass | Control | N/A | N/A | N/A |
| AgNO3 | 60.2 ± 8.8Aa | 62.8 ± 9.3Aa | 38.7 ± 2.9Aa | |
| Ag NPs | 56.8 ± 6.7Aab | 71.3 ± 12.5Aa | 43.8 ± 4.7Ab | |
| Ag2S NPs | 50.0 ± 4.7Aa | 59.4 ± 8.5Aa | 55.3 ± 5.5Aa | |
Within fish from the AgNO3 treatment, there were no statistically significant time-related changes in mean particle size in any organ (P > 0.05). However, the livers from fish exposed to AgNO3 at week 4 had a mean particle size (two-way ANOVA, P < 0.001) that was significantly higher compared to the control fish (P = 0.049). The same pattern was observed in the livers from fish exposed to Ag NPs, with a significant increase in mean particle size compared to the control fish (two way ANOVA, P = 0.022). Some transient changes were observed within fish exposed to Ag NPs. For example, there was a significant increase in mean size at week 4 compared to week 2 (two-way ANOVA, P = 0.015). Within fish exposed to Ag2S NPs, there was no time-dependent change in the mean particle size, and mean particle size was not significantly different from the controls in any organ at any time point. At week 4, the mean particle size in the liver of Ag2S NP exposed fish was significantly reduced compared to fish exposed to Ag NPs (P = 0.029; two-way ANOVA).
Following the depuration period where all fish were fed the control diet, there were no major changes in mean particle size. The mean particle sizes in the carcasses of fish exposed to AgNO3 and Ag NP decreased from 62.8 ± 9.3 and 71.3 ± 12.5 at week 4 to 38.7 ± 2.9 and 43.8 ± 4.7 at week 6, respectively, but only the Ag NP exposure was significantly lower over time (two-way ANOVA, P = 0.042).
3.4. Particle metrics versus total Ag concentration
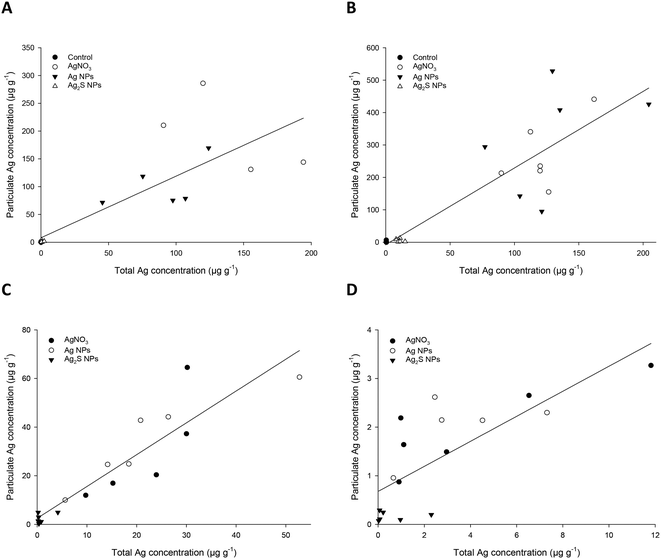
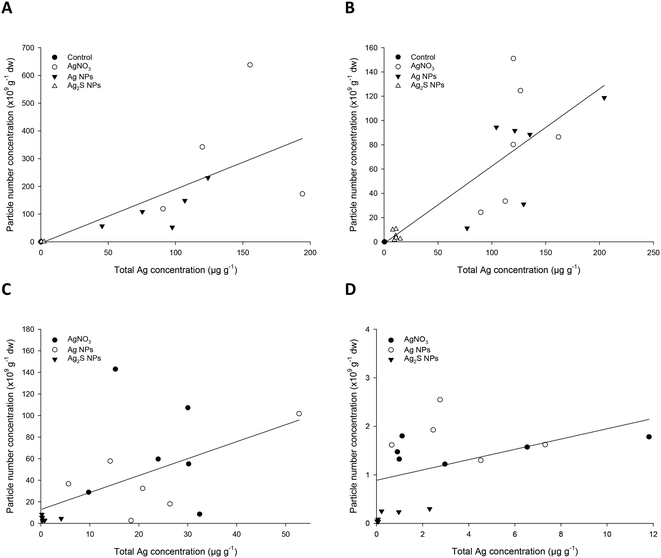
One question that arises for the practical application of spICP-MS in ecotoxicology is whether or not the total Ag measurements are predictive of the particle mass concentration or particle number concentration, especially where it is expected that a particle will not appreciably dissolve inside a tissue. Fig. 1 shows the correlations between the total Ag in the tissues (taken from our previous study of total metal in the same fish9) compared to the particle mass concentration measured here for the control, AgNO3, Ag NP and Ag2S NP exposed fish. In each organ, there was a positive correlation between the amount of total Ag and the mass of particles present, with the best correlation, with an r2 of 0.838, being found for the kidney. There was a significant relationship between the mass of particles measured and that of the total Ag in the hind intestine, liver, kidney and carcass (all P < 0.001, Spearman correlation).Similarly for the particle mass concentration, there was a positive relationship between the number of particles present in an organ, and the total Ag concentration (Fig. 2). The r2 values of these ranged from 0.23 to 0.72, with the equivalent values lower than those in the particle mass concentration measurements (Fig. 1). Regardless, there was a significant relationship between the particle number concentration and the total Ag present in the organs (P = 0.01 or below, Spearman correlation).
3.5. Burden of particle mass in each organ
Calculation of the organ burden as the proportion of the total particulate Ag mass in the fish is useful for identifying the main target organs in the exposure (Table 4). Following exposure to AgNO3, the fish organ burden was ranked in the order of carcass > liver > hind intestine > kidney, with around 51, 36, 6.7 and 5.5% of the body burden, respectively. Fish exposed to Ag NPs and Ag2S NPs showed this trend, typically with no significant difference between treatments for the same organ type. The only exception to this was the hind intestine at week 4, where the AgNO3 exposed fish had a significantly elevated (8-fold) organ burden compared to theAg2S NP exposed fish (two-way ANOVA, P = 0.016), but neither was significantly different when compared to the Ag NP exposed fish (P = 0.234 and 0.156 for the AgNO3 and Ag2S NP treatments, respectively).| Organ | Treatment | Week 2 | Week 4 | Week 6 |
|---|---|---|---|---|
| Data are mean ± standard error of mean (n = 5/6 fish). Upper case letters denote significant difference between treatments (within columns). Lower case letters denotes significant difference between time points (within rows). Control values were omitted for clarity due to small signals in the hind intestine and liver only. | ||||
| Hind intestine | AgNO3 | 6.7 ± 3.0Aa | 13.8 ± 5.9Aa | 15.9 ± 4.9Aa |
| Ag NPs | 7.7 ± 3.1Aa | 4.4 ± 2.1ABa | 19.5 ± 3.3Aa | |
| Ag2S NPs | 7.4 ± 5.6Aa | 1.7 ± 1.2Ba | 9.7 ± 6.8Aa | |
| Liver | AgNO3 | 36.4 ± 7.5Aa | 46.1 ± 6.1Aab | 64.7 ± 8.4ABb |
| Ag NPs | 38.3 ± 34.7Aa | 42.1 ± 7.4Aa | 42.5 ± 9.1Aa | |
| Ag2S NPs | 34.7 ± 10.5Aa | 20.7 ± 5.0Aa | 88.2 ± 7.5Bb | |
| Kidney | AgNO3 | 5.5 ± 2.9Aa | 3.8 ± 1.1Aa | 9.5 ± 2.3Aa |
| Ag NPs | 1.1 ± 0.1Aa | 3.7 ± 0.8Aa | 19.5 ± 3.3Ab | |
| Ag2S NPs | 2.2 ± 1.3Aa | 9.1 ± 3.4Aa | 2.0 ± 0.8Ba | |
| Carcass | AgNO3 | 51.4 ± 9.1Aa | 40.9 ± 8.0Aa | 10.0 ± 3.4Ab |
| Ag NPs | 52.9 ± 10.6Aa | 50.6 ± 8.0Aa | 19.0 ± 3.6Bb | |
| Ag2S NPs | 55.6 ± 12.7Aa | 68.4 ± 7.0Aa | <LOD | |
Following the depuration period, fish from all treatments showed a change in the order of organ burden to liver > hind intestine = carcass = kidney (Table 4); confirming the liver as the main target organ. This general observation was reflected in some statistically significant changes in the depuration period (Table 4). Within the fish exposed to AgNO3, the only significant change was to the burden associated with the carcass, decreasing from 41% of the total particulate Ag in the fish at week 4 to 10% at week 6 (two-way ANOVA, P < 0.001). This change in the carcass burden was also in fish exposed to Ag NPs (51 and 19% at week 4 and 6, respectively; two-way ANOVA, P = 0.006). Notably, in fish exposed to Ag2S NPs, all the Ag was cleared from the carcass to below the limit of detection (Table 4). There were also some treatment related differences after 6 weeks. For example, the organ burden of the carcass from AgNO3 exposed fish was nearly twice that of fish exposed to Ag NPs (two-way ANOVA, P = 0.009). However, there was no significant difference between the AgNO3 and Ag NP hind intestine, liver or kidney burden (two-way ANOVA, P = 0.566, 0.057 and 0.330, respectively). Additionally, there was some differences in the organ burden between the type of ENM exposure following the depuration period (Table 4). The liver burden of Ag NP exposed fish was around half that from those in the Ag2S NP exposure (43% versus 88%, respectively; two-way ANOVA, P < 0.001). Similar observations were made for the kidney of the Ag NP exposed fish, which had 20% of the total body burden, compared to the 2% in the Ag2S NPs exposed fish (two-way ANOVA, P = 0.005).
3.6. Dissolved Ag speciation within physiological compartments
The speciation of dissolved silver was considered in a range of physiological compartments (Table S1†). The calculations were referenced against 0.93 mmol L−1 Ag+ which is synonymous with the exposure concentration of 100 mg kg−1. Regardless of the compartment (stomach, intestinal regions, intracellular or blood), the dominant Ag species was the sparingly soluble AgCl2− complex, consisting of 74–75% of the total Ag species. Free Ag+ was <0.1% of the total, and the remainder comprised other poorly soluble chloride complexes (AgCl (aq) AgCl2− or AgCl32−). Together, these speciation calculations show that silver would be mainly insoluble (i.e., particulate) chloride complexes in physiological conditions.3.7. Histological examination of tissues
The hind intestine and liver were examined at the end of the exposure to establish if any unexpected changes in gross anatomy occurred that might influence the interpretations of the particle counts in the animals. Both the hind intestine and the liver showed normal morphology (Fig. S2†), with those from the controls similar to those from any Ag treatments. The hind intestine was intact in all treatments, with no evidence of erosion of the microvilli tips or necrosis, mucocyte proliferation, or reactive hyperplasia in the epithelium. The underlying muscularis was normal with no evidence of inflammation. For the liver, there was no loss of sinusoid space, no foci of necrosis in the parenchyma, no peri-venule haemorrhage, and no evidence of lipidosis or significant fatty change.4. Discussion
This is the first study to assess the body distribution of Ag-containing particles in fish. The key new findings include the presence of trace amounts of particulate Ag material in the control fish, suggesting a natural background of biogenic or environmental particles. In all Ag-exposed fish during the four week exposure phase, Ag-containing particles were found in all organs analysed with the key organs containing particulate Ag being the liver and carcass. There was no appreciable difference between the AgNO3 and Ag NP exposures, but Ag2S NPs were less bioavailable. In all organs where particles were found, regardless of the treatment (including the controls), the mean particle sizes were similar, ranging from 30 to 80 nm, suggesting some transformation of the materials. However, there was little evidence of particle clearance from the organs in the post-exposure phase.4.1. Particulate Ag in the organs
Silver-containing particles were found in the hind intestine and liver of the control fish. These were not attributed to contamination from the reagents, or artefacts arising from the extraction protocol, as the procedural blanks did not contain this signal. The particles were therefore from naturally occurring Ag that has been incorporated into the tissue, either as part of a metal sequestration strategy in the case of trout liver [e.g., as evidenced for Cu (ref. 20)], or formed from sparingly soluble Ag complexes [e.g., AgCl;21] during the lifetime of the fish. Interestingly, particles were not found in the kidney or carcass of control fish, and this is consistent with no detectable total Ag in those compartments.9 The absence of appreciable Ag in the kidney is also consistent with the protective role of the liver in preventing Ag absorbed via the gut from distributing to the rest of the body. The presence of background Ag particles in control samples have been reported in other organisms, such as tomato plants (Solanum lycoperisicum; Au NPs22).Dietary exposure to AgNO3 resulted in a significantly higher particle mass concentration and particle number concentration in all of the organs measured compared to the controls (Tables 1 and 2). The particle mass concentration and particle number concentrations in the organs at weeks 2 and 4 of the exposure were similar; suggesting the organs had reached an apparent dynamic ‘steady state’ during the exposure. There was no statistically significant clearance of particle number concentration from the organs during the two week depuration phase (Table 1), consistent with the concern that Ag bioaccumulates in fish.23 The presence of particles in the hind intestine is consistent with route of exposure and adsorption for AgNO3. It is probable that the particles were initially formed in the gut lumen from AgNO3, where the millimolar concentrations of chloride could drive AgCl-containing particle formation.24 This would imply that Ag from AgNO3 was taken up in the particulate form, although formation of AgCl-containing particles in the tissues cannot be excluded (Table S1†). The particle number concentration in the livers from the AgNO3 treatment were generally lower than the hind intestine, indicating the gut barrier offered some protection from the exposure. Nonetheless, the particle number concentration in the liver was readily detected from the AgNO3 exposure, and consistent with the liver as a central compartment in metabolism. However, the liver could not sequester all of the Ag particles internalised, as some were distributed in the kidney and carcass that were similar in size to those in the hind intestine (Table 3).
To assess for the bioaccumulation potential of ENMs, the dietary route of exposure has been recommended,11 and seems to work for Ag NP exposure. In order to detect the ENMs, there needs to be a reasonable chance that Ag will stay in the particulate form during adsorption from the gut. The present data shows the particulate profile for the organs in terms of particle mass concentration (Table 1) and particle number concentration (Table 2). The profile is the same as for the AgNO3 exposure, and for each target organ also consistent with total Ag measurements from the animals.9 Kleiven et al.25 made similar observations from Ag accumulation in Atlantic salmon exposed to ∼60 mg kg−1 Ag as either 110AgNO3 or 110Ag NPs (the latter either citrate coated or uncoated) using a slurry for administration via oral gavage. After 2 days, the measured radioactivity in the intestine was similar for both the metal salt and the ENM exposed fish,25 although the form of Ag was not determined. Baccaro et al.6 used ENMs from the same source and of the same types as used in this study, and also found no significant differences in particle mass concentrations in earthworms from AgNO3 and Ag NP exposure.
Fish exposed to Ag2S NPs had a significantly higher particle mass concentration and particle number concentration in each organ compared to the control (Tables 1 and 2), indicating there was some uptake of this material. However, each organ also contained significantly fewer particles than fish exposed to all the other Ag treatments (Table 2); suggesting the particles in the Ag2S form were the least bioavailable. For example, the particle number concentration in the livers of fish from the Ag2S NP treatment was an order of magnitude less than that in the equivalent AgNO3 and Ag NP treatments (Table 2). Similar observations were made with earthworms exposed to the same ENMs.6 Despite the lower accumulation of Ag2S NPs here, the distribution to target organs in trout were the same as the other silver treatments, and there was no evidence of particle clearance from the organs in the post-exposure phase, except in the kidney (Table 2).
While total Ag accumulation is typically reported, the elimination of silver is less often studied. In one study using radiolabelled AgNO3 in the water,26 the elimination of dissolved silver from trout was highly dependent on the Ag speciation in the original exposure medium (a low chloride solution where Ag+ was present, and a higher chloride concentration where AgCl complexes could form). Notably, there was faster elimination of Ag from the AgCl rather than Ag+ exposures; which was attributed to simple diffusion out of the gills or sloughing of the mucous.26 In the present study, the dominant Ag species from the AgNO3 exposure, in both the gut lumen and blood was calculated to be sparingly soluble AgCl2 (Table S1†). The latter would form insoluble particulates that could not be excreted on any solute transport pathway. Hence, the retention of Ag particles in the organs here following dietary AgNO3 exposure.
To aid data interpretation, the histology of the hind intestine and liver were examined as the known main target organs for the different Ag exposures (Table 1). There was no gross change or loss of integrity of the hind intestine (Fig. S2†) as a gut barrier, as we previously observed in the mid-intestine,9 indicating that any particles in the internal organs had not simply entered the animal by diffusion through a damaged gut. Similarly, the absence of pathology in the liver and the presence of bile in the gall bladder during dissection, argues that the liver was likely functional. Therefore, loss of integrity of the liver is not an explanation for the particle retention by internal organs in the post-exposure phase.
There appear to be no other reports on the elimination of particulate Ag following dietary Ag NP or Ag2S NP exposures in fish. Here, there was no elimination of the particles in the hind intestine or liver during the post-exposure phase. Trout are known to deposit insoluble metal granules in the liver when metals are presented in excess in the diet (e.g., Cu20). Other animals can also deposit ENMs taken up across the gut, or make biogenic particles inside the tissues27 as part of a metal sequestration strategy. The absence of pathology in the hind intestine and liver (Fig. 1), despite measurable particle number concentrations in those organs is also consistent with that notion. Our previous study on the same fish used here showed the physiological integrity of the animals, despite total Ag accumulation.9
4.2. Origin and transformation of particles in the internal organs
The formation of sparingly soluble silver complexes, either in the gut lumen, or in the high ionic strength of the intracellular space or blood (Table S1†) is a likely explanation for the appearance of Ag-containing particles in the trout tissue following exposure to AgNO3 (discussed above). Dissolved Ag as AgNO3 has also shown to produce 20–40 nm particles in simulated intestinal juices of mammals (pH 8.2), although not at the acidity of the stomach (pH 1.3) where the Ag remains dissolved.28 This phenomenon of particle formation from AgNO3 might also explain the background of Ag-containing particles in the control fish, given that the trace amounts of dissolved silver in the natural environment are bioaccumulative.23 However, for the Ag NP exposures (primary size 55 nm) and Ag2S NP exposures (primary size 35 nm), the resulting particle sizes in the tissues were not the same, approximately 30–80 nm, regardless of ENM treatment. This implies that either the ENMs were modified inside the tissue directly, and/or the particles undertook some transformations on their journey from the gut lumen to the internal organs.For the same Ag NPs used here, dissolution experiments showed only a tiny fraction (<1%, low μg L−1 amounts) of Ag release from the particles in gut saline at pH 2 representing the stomach, or at pH 7.8 for trout intestine.17 Consequently, the Ag NPs were likely taken up as particles, and the mean particle sizes in the tissue encompass the original primary particle size ranges. The smaller particles might arise from trace Ag release and/or degradation of the particles inside the tissue (e.g., in the lysosomal compartment of the liver), while slightly larger ones might arise from AgCl formation on the surface of the particles in the blood, or more likely, reactions with sulphur compounds in the tissue to form a biogenic Ag2S coating on the Ag NPs.19 At any one time, the burden of particles in the organ will be in different compartments (the sinusoidal space, blood, sub-cellular locations, etc.) and therefore one might expect a range of sizes due to that local chemistry. Similar arguments apply to the Ag2S NPs, except those show no dissolution in gut saline10 and they are already sulfidated.
4.3. Regulatory implications and particle metrics for bioaccumulation
The OECD TG 305 requires that the total concentration of the test substance be determined in the whole body (carcass) of the fish or the liver. In the case of metals, the total metal concentration in the tissue is determined. Clearly, it would be beneficial in the regulatory context to continue to measure total metal by routine ICP-MS, or similar methods, rather than using more complex spICP-MS. However, for total metal measurements to be valid for the bioaccumulation potential of ENMs, one must be able to correlate the total metal concentrations in the fish with the particle metrics. The particle mass concentration, rather than particle number concentration generally correlated better with the total metal concentration in the fish (Fig. 1 and 2). For the former, the r2 values for linear fits were around 0.7 for the individual organs, but only 0.58 for the carcass. The correlation coefficients for particle mass concentration for the liver (0.904) or carcass (0.804) with total Ag were much better. The residual ‘error’ in the correlation coefficients is around 10% for the individual organs and 20% for the carcass. Some of this error is explained by the spICP-MS technique which sets a threshold to define a particle from the dissolved signal. Therefore, the degree of correlation is partly dependent on the instrument sensitivity, and the amount of dissolved Ag present in the sample, which limits the smallest size particle that can be detected. Nonetheless, the correlations with total metal are a reasonable prospect to enable the continued use of total metal as the metric for the endpoints in regulatory bioaccumulation tests.The tendency for the exposure treatments to cluster together in Fig. 1 and 2 indicate some substance specificity in tissue total and particulate metal concentrations. This substance specific tissue accumulation is also observed with organic chemicals.29 One approach to resolving substance specific differences is to use threshold values to trigger bioaccumulation testing, as is used for organic chemicals (e.g., an octanol–water partition coefficient, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow > 3, used to trigger the OECD TG 305 fish bioaccumulation test11). A similar approach could be adopted for metallic ENMs, such as existing evidence of total metal uptake or trophic transfer.30 Poor correlations between total and particulate metal may result in use of the total metal for any regulatory decision making; but should also recognise that the latter does not necessarily inform on the presence of particles in the organism. Alternatively, if the correlations between the two measurements are reasonable, as is the case here, then the total metal also indicates the presence of particles in the tissues. The precise thresholds for any correlation coefficients used in such an approach would need to be agreed by consensus building in the scientific community.
Kow > 3, used to trigger the OECD TG 305 fish bioaccumulation test11). A similar approach could be adopted for metallic ENMs, such as existing evidence of total metal uptake or trophic transfer.30 Poor correlations between total and particulate metal may result in use of the total metal for any regulatory decision making; but should also recognise that the latter does not necessarily inform on the presence of particles in the organism. Alternatively, if the correlations between the two measurements are reasonable, as is the case here, then the total metal also indicates the presence of particles in the tissues. The precise thresholds for any correlation coefficients used in such an approach would need to be agreed by consensus building in the scientific community.
5. Conclusions
In conclusion, the dietary exposure of rainbow trout to either AgNO3, Ag NPs or Ag2S NPs resulted in the appearance of Ag-containing particles in the internal organs and carcass. Unexposed control fish also had an incidental background of particulates in some organs. In the case of AgNO3 exposure, speciation calculations show the typical ionic composition of the gut lumen and/or blood plasma will result in sparingly soluble silver complexes; and these may act as nuclei for particle formation, with the subsequent detection of nanoparticles by spICP-MS in the organs. Some transformations of Ag NPs inside the tissues must also be occurring, as the sizes of the particles detected in the organs spanned the mean particle size of the starting materials. These effects could include some degradation or dissolution resulting in shrinkage of the Ag NPs, or indeed sulfidation of the surface to increase the size of the materials. The mechanisms involved need further investigation. Crucially, we show that particulate materials inside the organs are primarily responsible for the total Ag bioaccumulation previously observed.9 The bioaccumulation hazard is therefore from the particulates, not dissolved Ag per se, regardless of the form of Ag administered. Fortunately, the bioaccumulation of Ag-containing particles following dietary exposure to AgNO3 and Ag NPs were similar, and with the Ag2S NPs being slightly less accumulative, and thus the existing bioaccumulation potential data for the metal salt may protect for the nano form in the environmental risk assessment. However, the presence of intact ENMs in the carcass and negligible clearance of the particles post-exposure raises concerns about the human health risks associated with the edible flesh, and the use of Ag NPs as biocides in aquaculture.Author contributions
NC: study design, sampling, data generation, data analysis, manuscript preparation. RC: data generation, data analysis, manuscript preparation. DB: study design, sampling, data analysis, manuscript preparation. RH: study design, manuscript preparation and funding.Conflicts of interest
The authors do not declare any conflicts of interest.Acknowledgements
The authors would like to thank Dr Andrew Fisher for ICP-MS assistance and Dr Jo Triner for histological training. This work was funded by the EU H2020 NanoFASE project; grant agreement no. 646002, with the final draft of this manuscript supported by the NanoHarmony project; grant agreement no. 885931. Both projects were with RDH as the PI at the University of Plymouth.References
- C. M. Wood, 1—silver, Fish physiology, 2011, vol. 31, pp. 1–65 Search PubMed.
- D. Schlenk and W. H. Benson, Target organ toxicity in marine and freshwater teleosts: Organs, CRC press, 2017 Search PubMed.
- B. J. Shaw and R. D. Handy, Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions, Environ. Int., 2011, 37, 1083–1097 CrossRef CAS PubMed.
- P. Wang, E. Lombi, S. K. Sun, K. G. Scheckel, A. Malysheva, B. A. McKenna, N. W. Menzies, F. J. Zhao and P. M. Kopittke, Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants, Environ. Sci.: Nano, 2017, 4, 448–460 RSC.
- S. Wagener, H. Jungnickel, N. Dommershausen, T. Fischer, P. Laux and A. Luch, Determination of nanoparticle uptake, distribution, and characterization in plant root tissue after realistic long-term exposure to sewage sludge using information from mass spectrometry, Environ. Sci. Technol., 2019, 53, 5416–5426 CrossRef CAS PubMed.
- M. Baccaro, A. K. Undas, J. de Vriendt, J. Van Den Berg, R. Peters and N. W. van den Brink, Ageing, dissolution and biogenic formation of nanoparticles: how do these factors affect the uptake kinetics of silver nanoparticles in earthworms?, Environ. Sci.: Nano, 2018, 5, 1107–1116 RSC.
- S. Makama, J. Piella, A. Undas, W. J. Dimmers, R. Peters, V. F. Puntes and N. W. van den Brink, Properties of silver nanoparticles influencing their uptake in and toxicity to the earthworm Lumbricus rubellus following exposure in soil, Environ. Pollut., 2016, 218, 870–878 CrossRef CAS PubMed.
- H. K. Sung, E. Jo, E. Kim, S. K. Yoo, J. W. Lee, P. J. Kim, Y. Kim and I. C. Eom, Analysis of gold and silver nanoparticles internalized by zebrafish (Danio rerio) using single particle-inductively coupled plasma-mass spectrometry, Chemosphere, 2018, 209, 815–822 CrossRef CAS PubMed.
- N. J. Clark, D. Boyle, B. P. Eynon and R. D. Handy, Dietary exposure to silver nitrate compared to two forms of silver nanoparticles in rainbow trout: bioaccumulation potential with minimal physiological effects, Environ. Sci.: Nano, 2019, 6, 1393–1405 RSC.
- N. J. Clark, R. Clough, D. Boyle and R. D. Handy, Development of a suitable detection method for silver nanoparticles in fish tissue using single particle ICP-MS, Environ. Sci.: Nano, 2019, 6, 3388–3400 RSC.
- R. D. Handy, J. Ahtiainen, J. M. Navas, G. Goss, E. A. J. Bleeker and F. von der Kammer, Proposal for a tiered dietary bioaccumulation testing strategy for engineered nanomaterials using fish, Environ. Sci.: Nano, 2018, 5, 2030–2046 RSC.
- R. D. Handy and G. Al-Bairuty, Effects of Nanomaterials on the Body Systems of Fishes, Ecotoxicology of Nanoparticles in Aquatic Systems, 2019, vol. 156 Search PubMed.
- K. Bilberg, M. B. Hovgaard, F. Besenbacher and E. Baatrup, In vivo toxicity of silver nanoparticles and silver ions in zebrafish (Danio rerio), J. Toxicol., 2012, 2012 DOI:10.1155/2012/293784.
- S. George, H. Gardner, E. K. Seng, H. Chang, C. Wang, C. H. Y. Fang, M. Richards, S. Valiyaveettil and W. K. Chan, Differential effect of solar light in increasing the toxicity of silver and titanium dioxide nanoparticles to a fish cell line and zebrafish embryos, Environ. Sci. Technol., 2014, 48, 6374–6382 CrossRef CAS PubMed.
- K. Rajkumar, N. Kanipandian and R. Thirumurugan, Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita, Appl. Nanosci., 2016, 6, 19–29 CrossRef CAS.
- D. Boyle and G. G. Goss, Effects of silver nanoparticles in early life-stage zebrafish are associated with particle dissolution and the toxicity of soluble silver, NanoImpact, 2018, 12, 1–8 CrossRef.
- N. J. Clark, D. Boyle and R. D. Handy, An assessment of the dietary bioavailability of silver nanomaterials in rainbow trout using an ex vivo gut sac technique, Environ. Sci.: Nano, 2019, 6, 646–660 RSC.
- H. E. Pace, N. J. Rogers, C. Jarolimek, V. A. Coleman, C. P. Higgins and J. F. Ranville, Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry, Anal. Chem., 2011, 83, 9361–9369 CrossRef CAS PubMed.
- R. J. Peters, Z. H. Rivera, G. van Bemmel, H. J. Marvin, S. Weigel and H. Bouwmeester, Development and validation of single particle ICP-MS for sizing and quantitative determination of nano-silver in chicken meat, Anal. Bioanal. Chem., 2014, 406, 3875–3885 CAS.
- R. P. Lanno, B. Hicks and J. W. Hilton, Histological observations on intrahepatocytic copper-containing granules in rainbow trout reared on diets containing elevated levels of copper, Aquat. Toxicol., 1987, 10, 251–263 CrossRef CAS.
- M. Nocchetti, A. Donnadio, V. Ambrogi, P. Andreani, M. Bastianini, D. Pietrella and L. Latterini, Ag/AgCl nanoparticle decorated layered double hydroxides: synthesis, characterization and antimicrobial properties, J. Mater. Chem. B, 2013, 1, 2383–2393 RSC.
- Y. Dan, W. Zhang, R. Xue, X. Ma, C. Stephan and H. Shi, Characterization of gold nanoparticle uptake by tomato plants using enzymatic extraction followed by single-particle inductively coupled plasma–mass spectrometry analysis, Environ. Sci. Technol., 2015, 49, 3007–3014 CrossRef CAS PubMed.
- H. T. Ratte, Bioaccumulation and toxicity of silver compounds: a review, Environ. Toxicol. Chem., 1999, 18, 89–108 CrossRef CAS.
- L.-G. Wu, T. Wang and Z. Jiang, Formation of AgCl nanoparticle in reverse microemulsion using polymerizable surfactant and the resulting copolymer hybrid membranes, J. Membr. Sci., 2013, 429, 95–102 CrossRef CAS.
- M. Kleiven, B. O. Rosseland, H. C. Teien, E. J. Joner and D. Helen Oughton, Route of exposure has a major impact on uptake of silver nanoparticles in Atlantic salmon (Salmo salar), Environ. Toxicol. Chem., 2018, 37, 2895–2903 CrossRef CAS PubMed.
- C. M. Wood, M. Grosell, C. Hogstrand and H. Hansen, Kinetics of radiolabelled silver uptake and depuration in the gills of rainbow trout (Oncorhynchus mykiss) and European eel (Anguilla anguilla): the influence of silver speciation, Aquat. Toxicol., 2002, 56, 197–213 CrossRef CAS PubMed.
- M. van der Zande, A. J. Kokalj, D. J. Spurgeon, S. Loureiro, P. V. Silva, Z. Khodaparast, D. Drobne, N. J. Clark, N. W. van den Brink and M. Baccaro, The gut barrier and the fate of engineered nanomaterials: a view from comparative physiology, Environ. Sci.: Nano, 2020, 7, 1874–1898 RSC.
- A. P. Walczak, R. Fokkink, R. Peters, P. Tromp, Z. E. Herrera Rivera, I. M. Rietjens, P. J. Hendriksen and H. Bouwmeester, Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model, Nanotoxicology, 2012, 7, 1198–1210 CrossRef PubMed.
- G. D. Veith, D. L. DeFoe and B. V. Bergstedt, Measuring and estimating the bioconcentration factor of chemicals in fish, J. Fish. Res. Board Can., 1979, 36, 1040–1048 CrossRef CAS.
- United States Environment Protection Agency, Framework for Metals Risk Assessment, Report number, EPA 120/R-07/001 [March 2007], Office of the Science Advisor, Risk Assessment Forum, Environmental Protection Agency, United States of America, 2007, Available at: https://www.epa.gov/sites/production/files/2013-09/documents/metals-risk-assessment-final.pdf Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1en00188d |
| This journal is © The Royal Society of Chemistry 2021 |