Inducing non-stoichiometry in nano-hydroxyapatite for ultra-fast sequestration of uranyl ions in water: mechanism delineation using XAS†
Abstract
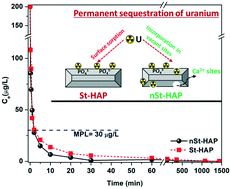
The presence of radionuclides in water bodies imposes severe environmental concerns, and thus their permanent sequestration is of immense importance. In this study, the sorption of uranyl ions was comparatively evaluated using as-synthesized stoichiometric and non-stoichiometric hydroxyapatite (St-HAP and nSt-HAP, respectively) nanomaterials. Results showed the successful synthesis of both St-HAP (Ca/P = 1.67) and nSt-HAP (Ca/P = 1.35), which possessed a nanorod-like structure. The sorption studies confirmed the efficient and ultrafast removal of uranium. The induction of non-stoichiometry resulted in comparatively faster removal, i.e., below the drinking water permissible limit within 2 min of interaction, with an elevated sorption capacity of 175 mg g−1 compared to 97 mg g−1 of St-HAP. The impact of all the studied environmental parameters such as pH, ionic strength, bicarbonate and humic acid was found to be minimal for nSt-HAP, suggesting the selective removal of uranium with good environmental applicability of nSt-HAP. Due to the higher uranium sorption capacity of the Ca-deficient nSt-HAP, the mechanism of sorption was investigated in terms of the bonding state using the XAS technique. This study revealed that specific sites are available where U(VI) is preferentially trapped in the case of nSt-HAP, which led to the enhanced and ultrafast sequestration of uranium from water.


 Please wait while we load your content...
Please wait while we load your content...