Open Access Article
Open Access ArticleA critical evaluation of short columns for estimating the attachment efficiency of engineered nanomaterials in natural soils†
Knapp Karin
Norrfors
 a,
Vesna
Micić
b,
Olga
Borovinskaya
c,
Frank
von der Kammer
b,
Thilo
Hofmann
a,
Vesna
Micić
b,
Olga
Borovinskaya
c,
Frank
von der Kammer
b,
Thilo
Hofmann
 b and
Geert
Cornelis
b and
Geert
Cornelis
 *a
*a
aDepartment of Soil and Environment, SLU Swedish University of Agricultural Sciences, PO Box 7014, SE-75007 Uppsala, Sweden. E-mail: geert.cornelis@slu.se
bDepartment of Environmental Geosciences, Centre for Microbiology and Environmental Systems Science, University of Vienna, Althanstrasse 14, 1090 Vienna, Austria
cTOFWERK AG, Schorenstrasse 39, 3645 Thun, Switzerland
First published on 24th May 2021
Abstract
Short, saturated packed columns are used frequently to estimate the attachment efficiency (α) of engineered nanomaterials (ENMs) in relatively homogeneous porous media, but a combined experimental and theoretical approach to obtain α-values for heterogeneous natural soils has not yet been agreed upon. Accurately determined α-values that can be used to study and predict ENM transport in natural soils should vary with ENM and soil properties, but not with experimental settings. We investigated the effect of experimental conditions, and used different methods to obtain soil parameters, essential to calculate α. We applied 150 different approaches to determine α-values from 52 transport experiments using short columns with 5 different natural soils and 20 and 80 nm gold- or 27 nm silver sulphide ENMs. The choice of column end-filter material and pore size appeared critical to avoid overestimating α owing to filter – ENM interactions and/or incomplete saturation of the column. Using a low ionic strength (4.4 × 10–5 mol L−1) artificial rain water as an aqueous medium avoided ENM homo- or heteroaggregation in all soils, as confirmed by single-particle inductively coupled plasma – time of flight mass spectrometry. ENM breakthrough curves could be modelled using colloid filtration theory assuming irreversible attachment only. α-Values calculated from this model, having the grain size represented by a single average size, accounting for dispersivity and effective porosity based on a prior inert tracer test, explained up to 42% of the variance in α as revealed by partial least squares analysis. However, column length and dispersivity remained as important experimental parameters, which calls for further standardisation efforts of column tests with ENMs in natural soils, preferably cross-validated with batch tests.
Environmental significanceWith initial research focus on effects, calculating the exposure of engineered nanomaterials (ENM) in diverse environmental matrices is in most cases not yet possible. A major reason is the lack of agreed upon fate descriptors, i.e. quantifiable parameters that can be used routinely in exposure predictions. The attachment efficiency (α) has frequently been named as the descriptor of choice to describe fate and eventually exposure of ENMs in porous media such as soils, but a validated, generally agreed upon method to determine α for a given ENM – soil combination does not exist. Short column methods are named as the method of choice, but our paper shows that experimental settings of column experiments with heterogeneous natural soils and the chosen theory required to calculate α from the breakthrough curves has a major effect on the final value of α and/or whether there are experimental artefacts. We thus conclude that column tests need to be optimized to reduce experimental artefacts and have formulated several recommendations how strong experimental artefacts can be avoided and which theory is optimal to calculate α. The paper aims to aid the effort of finding a standard column method to determine α-values of natural soils that with some confidence have predictive value beyond the functional column test. |
Introduction
While the last decade has seen much progress in characterizing the possible ecotoxic effects of engineered nanomaterials (ENMs), predicting their fate in different environments often proves elusive. One reason is that many environmental compartments lack generally agreed-upon ENM-specific fate descriptors. Fate descriptors are parameters quantified during functional tests that characterize the behavior of an ENM or other materials in a particular water, soil or other medium. Such parameters can then be used to predict the fate of these materials in a setting other than the functional test, e.g. in the field.1,2There is an intimate interaction between ENMs and surfaces of solids in porous media such as soils, important to quantify for risk assessment, because it determines both bioavailability of the ENMs to soil organisms and potential transport to aquifers sometimes used for drinking water production. Transport prediction of ENMs in soils is also important for ENMs intentionally added to porous media, such as nanopesticides or nanozerovalent iron used in groundwater remediation.
Existing fate descriptors for transport of chemicals in soils such as partitioning coefficients (Kd-values) are deemed inappropriate as ENM fate predictors, because partitioning coefficients are conceptually based on an equilibrium paradigm. Particles are invariantly present in the environment as thermodynamically unstable suspensions and their behavior thus requires kinetic fate descriptors.3,4 First-order interaction rate constants are generally determined by injecting ENM suspensions into packed saturated columns of porous media and applying models to the measured breakthrough curve (BTC) and/or depth profile. These models have to a large extent been built on colloid filtration theory (CFT) where interaction of particles in porous media is seen as a two-stage process, i.e. collision of particles with the surface followed by attachment, a process that is assumed irreversible. The collision frequency of particles with pore surfaces is thought to be determined by measurable, operational parameters such as flow rate, porosity and column dimensions. The attachment efficiency (α), i.e. the chance of a particle attaching following collision with pore walls, is sometimes seen as the best alternative to Kd values.3,4 This parameter is in theory only function of properties of the specific particle and porous medium being studied. In this way, α can serve as a fate descriptor for a particular particle – medium combination and potentially be used beyond the functional (column) test in which it was determined.3
Applying CFT to column tests correctly requires rather precise knowledge of the hydrodynamic conditions in the studied porous medium. The clean filter bed is approximated as grains having a uniform spherical size, to which mass transport of ENMs and their collision frequency is described by the single-collector contact efficiency (η0). While several column experiments with ENMs have been conducted in the last decades, they were mostly done on homogeneous substrates (e.g. standardized sand or glass beads) that indeed have a more or less uniform spherical grain size. The focus of these tests was on changing the chemistry of the elution solution asserting that CFT and general colloidal theories such as the effect of pH and ionic strength are indeed applicable to these substrates.
Fewer column experiments were done with natural soils often showing much more complex interactions that can rarely be described solely on the basis of irreversible attachment. Correct interpretation of breakthrough curves from soils usually requires consideration of additional processes such as detachment, straining.5–8 Natural soils are also non-uniform and it is not clear whether the single-collector contact efficiency accurately describes the hydrodynamic conditions leading to collision within non-uniform packed columns. If not, the calculated α cannot be expected independent of the experimental conditions of the column test.2
Packed column tests as described by OECD Test no. 312 (ref. 9) have been advised as the functional test of choice to quantify the interaction of ENMs with, amongst others, natural soils during an OECD expert meeting albeit without a recommendation of how these column tests should be performed and how the BTCs should be interpreted.10 Test nr 312 has been developed primarily for predicting transport of dissolved chemicals in soils and effects of experimental design have not yet been investigated when applying this protocol to ENMs.11–18 It is insufficiently known which artefacts may be incurred during ENM column tests and exactly how CFT should be applied to calculate α. Consequently, there is no consensus on how scientifically or regulatory sound estimates of ENM fate in solids can be obtained.2
Previous meta-analyses6,19 collected large amounts of column data from the literature to investigate soil parameters affecting ENM transport. However, experimental column protocols vary widely, not only in terms of column test design, but also how soil parameters essential for calculation of α are determined. Moreover, protocols are sometimes incompletely described and the calculation methods for α vary. There is thus a large amount of hidden variation amongst reported column tests20 with ENM complicating meta-analysis to find trends in ENM behavior in soils.
In this study, experimental artefacts of short column tests are explored and different approaches to calculate α-values are evaluated on the basis of 52 such tests. The columns used in this study are considered short because the length-to-diameter ratio of all soil columns was below 3. This ratio is known to be important and should not be too high to avoid scaling effects. Large length-to-diameter ratios often give rise to a proportionally too large fraction of the total flow occurring preferentially along the boundary between soil grains and column interior. Moreover, transversal dispersivity may become relevant if the diameter is too large. A diameter-to-length ratio of four is therefore often recommended.21,22 ENMs in particular are generally retained strongly during soil column tests. Zero breakthrough is therefore often found in columns as short as 10 cm (e.g. ref. 5). We therefore chose to work with even shorter columns of 6 to 6.5 cm.
The final goal of the current study was not to suggest a standard protocol for α-value determination, but to provide the scientific basis for reaching such a protocol in the future and to offer guidance for a more accurate estimation of α-values for a specific combination of ENM and soil. Applying a uniform, experimentally artefact-free column protocol allows comparing α-values between different soils to rank these in terms of risk following ENM exposure. Accurate α-values allow predicting ENM transport and possibly even bioavailability in the field. Terrestrial nanotechnology, e.g. nano zerovalent iron for decontamination of soils or nanopesticides, could thus be screened more efficiently, better exposing the specific formulation that has the highest mobility in a given soil.
Calculations of attachment rate using CFT are most sensitive to the assumed average grain (also called collector) diameter and assumed porosity of the packed soil column.23 When using homogeneous porous media such as standardized sands, the grain size is relatively monodisperse, which this is not the case for natural soils. Different ways of determining and accounting for grain size and porosity were therefore compared. Most formulae for calculating α include these parameters and should thus in principle remove their effect on α variation. Surface charge has often been mentioned as the single most important parameter in soils to determine interactions with ENMs.19,24 It was therefore investigated whether different measures of the surface potential indeed explained variation in α-values. In addition to grain size and surface charge, parameters such as soil type, column length, flow rate, and input ENM concentration were varied. 150 different approaches were subsequently used to estimate α-values. α-Values should not vary with experimental settings nor with measurable incidental column properties such as porosity, dispersivity or grain size, because these parameters are in principle taken into account by the theoretical approach to calculate α. Combined experimental and theoretical approaches were therefore evaluated positively in this study if the α-values were influenced strongly by ENM and soil properties, and evaluated negatively if α-values are strongly affected by experimental variation.
Material and methods
Overview
Table 1 shows an overview of the experimental variables of the 52 column experiments. Full experimental details of all experiments can be found in (ESI† Table S6). Four agricultural top soils and one reference soil (Lufa 2.2) were used in saturated column tests. The column length was varied, while keeping the column diameter constant. The soils differed in texture, organic carbon content and mineralogy (see ESI† Table S2). The total concentration of injected suspensions of 20 or 80 nm Au or 27 nm Ag2S ENMs and flow rate were varied to investigate the effect of these parameters on α-values.| Parameter | Min | Max |
|---|---|---|
| a Two different methods were used to determine d10 and d50 (see text). | ||
| Column properties | ||
| Column diameter (cm) | 2.5 | 2.5 |
| Column length (cm) | 2.7 | 6.3 |
| Column filter pore size (μm) | 10 | 70 |
| Pump flow rate (mL min−1) | 0.16 | 0.62 |
| Total porosity (%) | 36 | 69 |
| Bulk density (g cm−3) | 1.03 | 1.68 |
| NM properties | ||
| NP core | Au or Ag2S | |
| NP primary TEM diameter (nm) | 20, 27 or 80 | |
| NP stock concentration (mg L−1) | 0.05 | 120 |
| Soil properties | ||
| Soils | 5 different soils | |
| pH | 4.08 | 7.63 |
| Sand (%) | 29.5 | 91.8 |
| Silt (%) | 4.7 | 47 |
| Clay (%) | 3.5 | 14.6 |
| d 10 (μm) | 8.7 | 47.2 |
| d 50 (μm) | 61 | 603 |
| Total carbon (%) | 0.61 | 9.63 |
| CEC (cmol kg−1) | 7.8 | 33.3 |
| ζ-Potential of dispersible soil fraction calculated from the electrophoretic mobility (mV) | −44 | −24 |
| ζ-Potential of soil fraction >25 μm calculated from the streaming potential (mV) | −24 | −3 |
| Oxalate Fe (mg kg−1) | 208 | 2707 |
| Oxalate Al (mg kg−1) | 145 | 3005 |
Practically, the effective or total porosity were used, two single collector diameters (d10 or d50) or an approach using the whole grain size distribution were used, five different correlation equations to calculate the single collector contact efficiency (η0), and finally, five different models to calculate α from experimental or modelled data (Table 3). 150 different approaches were thus compared in their efficiency to account for operational parameters and explain α-variance.
Materials
Both Au NPs and Ag2S NPs were selected since Au and Ag backgrounds in natural soils are low and these ENMs are sparingly soluble. Any total gold or silver measurement will thus conveniently reflect occurrence of ENMs. The stock suspensions were diluted in artificial rainwater (Table 2) immediately before injection of ENMs into the soil column. The initial concentration of Au or Ag in diluted stock suspensions was measured after dilution in 3% HCl (Merck, GR for analysis) followed by inductively coupled plasma-mass spectrometry (ICP-MS) measurements. Preliminary experiments verified that the difference between Au concentrations measured in Au ENM suspensions diluted using 3% HCl or Au concentrations measured after total digestion using aqua regia (and further dilution using 3% HCl) was insignificant.
| Salt | Concentration (M) |
|---|---|
| NaCl | 1.0 × 10−5 |
| (NH4)2SO4 | 5.3 × 10−6 |
| NaNO3 | 5.9 × 10−6 |
| CaCl2 | 3.9 × 10−6 |
Methods
The ζ-potential of the non-dispersible soil fraction (ζn.d.) was calculated from the streaming potential measured with a SurPASS electrokinetic analyzer (Anton Paar, Austria). For each soil sample, the measuring cylinder was rinsed with the respective soil solution and the fraction <25 μm was removed. Soil samples were then mixed with their respective soil solution and wet-packed into the measuring cylinder equipped with a filter membrane of 25 μm mesh size. The streaming potential measurements were subsequently carried out in the respective soil solution as a background electrolyte. The reported ζn.d. values were calculated based on the Fairbrother–Mastin equation29 and are mean results of at least three individual values.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; 1
10; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and 1
100 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) and were agitated for 1 hour. The resulting suspensions were analyzed using single particle ICP-time of flight-MS (sp-ICP-TOF-MS, TOFWERK, Switzerland)32 using an integration time of 3 ms. These ENM concentrations were much higher than those used during the column tests (Table 1) and the natural colloid concentration in saturated soil solution was higher than in column eluates. However, using these more concentrated particle suspensions reduced the probability of detecting false positives. Reducing this probability below 10% requires detection of at least 1538 particles.33 Particle signals were defined based on a threshold that was calculated as average + 3.29 × σ + 2.72 as explained in detail in ref. 34. Heteroaggregates were assumed where Au particle signals co-occurred with Al or Fe particle signals within a single integration time of 3 ms.
1000) and were agitated for 1 hour. The resulting suspensions were analyzed using single particle ICP-time of flight-MS (sp-ICP-TOF-MS, TOFWERK, Switzerland)32 using an integration time of 3 ms. These ENM concentrations were much higher than those used during the column tests (Table 1) and the natural colloid concentration in saturated soil solution was higher than in column eluates. However, using these more concentrated particle suspensions reduced the probability of detecting false positives. Reducing this probability below 10% requires detection of at least 1538 particles.33 Particle signals were defined based on a threshold that was calculated as average + 3.29 × σ + 2.72 as explained in detail in ref. 34. Heteroaggregates were assumed where Au particle signals co-occurred with Al or Fe particle signals within a single integration time of 3 ms.
| Method | CDE | Formula |
|---|---|---|
| C: ENM concentration suspended in pores; t: time; D dispersivity; z: depth in column; U: volumetric flow rate; katt: first order irreversible attachment rate constant; ψ: depth-dependent straining factor (eqn (2)); kstraining: first order straining rate constant, α: attachment efficiency; katt,irrev: attachment rate constant for the irreversible site type; katt,rev: attachment rate constant for the reversible site type; kdet, first order detachment rate constant for the reversible site type; ρ: bulk density; θ porosity; S2: ENM concentration attached to the reversible site type; α: attachment efficiency; dc grain size; L column length; η0: single-collector contact efficiency; R: recovery (eqn (1)); v: linear flow rate; f(θ) a function of the porosity that depends on which correlation equation is being used (see ESI†). | ||
| Continuous |
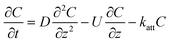
|
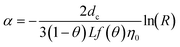
|
| Pulse |
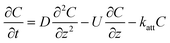
|
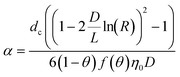
|
| Attachment |
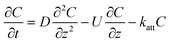
|
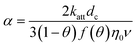
|
| Straining |
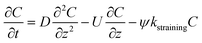
|
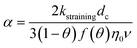
|
| 2-Sites |
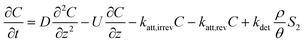
|
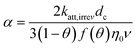
|
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) collector diameter ratio is much smaller than 0.005,37 ENMs might still be strained on rough surfaces and/or because of the occurrence of ENMs homo- or heteroaggregates which increases the colloid
collector diameter ratio is much smaller than 0.005,37 ENMs might still be strained on rough surfaces and/or because of the occurrence of ENMs homo- or heteroaggregates which increases the colloid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) collector diameter ratio.37–39 Finally, the 2-sites model assumes both irreversible and reversible attachment that very often fits BTCs of ENMs well (e.g. ref. 5). Colloids can detach from soil surfaces soil columns when these particles were previously attached in secondary energy minima40 and the probability of detachment increases with particle size because of hydrodynamic torque.41 Previously homo- or heteroaggregated ENMs could thus also experience similar torques. No blocking of sorption sites was assumed while modelling transport of ENMs in the soil columns given that the ENM concentrations were kept purposely low to avoid blocking phenomena. The models in Table 3 have in common that they are relatively simple and only one or in the case of the 2-sites model, three parameters have to be fitted to the BTC.
collector diameter ratio.37–39 Finally, the 2-sites model assumes both irreversible and reversible attachment that very often fits BTCs of ENMs well (e.g. ref. 5). Colloids can detach from soil surfaces soil columns when these particles were previously attached in secondary energy minima40 and the probability of detachment increases with particle size because of hydrodynamic torque.41 Previously homo- or heteroaggregated ENMs could thus also experience similar torques. No blocking of sorption sites was assumed while modelling transport of ENMs in the soil columns given that the ENM concentrations were kept purposely low to avoid blocking phenomena. The models in Table 3 have in common that they are relatively simple and only one or in the case of the 2-sites model, three parameters have to be fitted to the BTC.
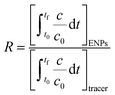 | (1) |
A concentration flux (i.e. Cauchy) boundary condition was set for both column inlet and outlet, both for models for inert tracer and ENM transport, because such conditions are more likely to preserve mass.45 The infiltrating concentration at the column inlet (z = 0) was set at the known concentration of inert tracer or ENM for the duration of the pulse injection (dead time added) and zero at other times, whereas the infiltrating concentration at the column outlet was set at zero.
The depth dependent factor ψ in the “straining” model was calculated as:
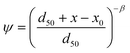 | (2) |
Statistical analysis
Principal component analysis (PCA) was applied to explore covariance between predictor variables and observe clustering of experimental data. Predictor variables were logarithmically transformed if this reduced skewness and, based on a Kolmogorov–Smirnov test, would produce a normally distributed variable. PCA was done on z-scores of transformed predictor variables (see ESI†) using Minitab (version 18). Partial least squares analyses (PLS1) of α-values produced by the different methods was done using Matlab (v2020), which uses the SIMPLS algorithm.55 The ESI† provides background and terminology of PLS1. All α-values were logarithmically (base 10) transformed to reduce skewness and z-scores were calculated. The same transformed z-scores of the predictor variables used for PCA were also used for PLS1. The optimum number of latent variables was found by cross-validation (one-observation out, see ESI†) retaining the number of latent variables that resulted in the lowest squared mean error of prediction. A high number of predictor values tends to spoil PLS analyses reducing their consistency and introducing unnecessary variance.56 The number of predictor variables was therefore reduced by removing intercorrelating variables followed by calculation of the variable importance in projection (VIP) of each variable (see ESI†).Results and discussion
Effect of filters and air-entrapment
Soil properties
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clay content. Mobile soil colloids often consist of 2
1 clay content. Mobile soil colloids often consist of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clays because these minerals have a relatively high surface charge lending them a high stability, especially in the conditions of this study where ionic strength was relatively low.65
1 clays because these minerals have a relatively high surface charge lending them a high stability, especially in the conditions of this study where ionic strength was relatively low.65
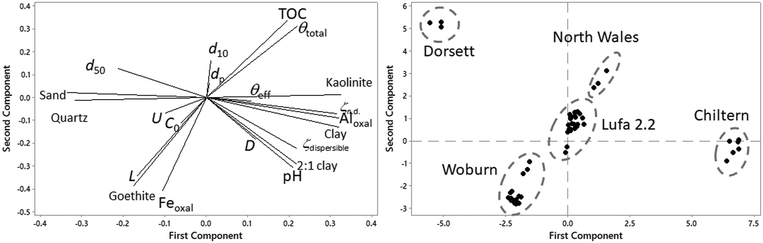 | ||
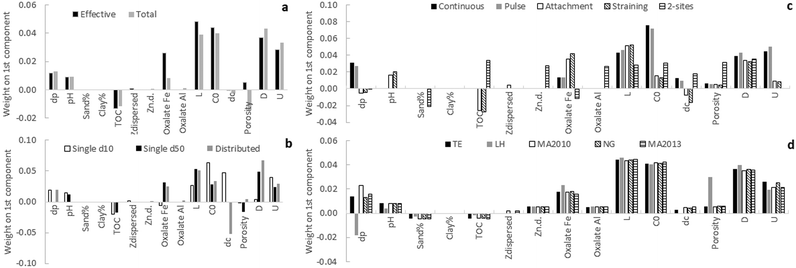
| Fig. 2 Loading and score plot of the principal component analysis of predictors. The core plot indicates also the soils for which the data was obtained. Symbols used in the load plot can be found in ESI† Table S1. Abbreviations: sand: granulometric sand % of the soil; clay: granulometric clay% of the soil; Feoxal: oxalate extractable iron; Aloxal: oxalate extractable aluminum; ζdispersible: ζ-potential of the dispersible fraction; ζn.d.: ζ-potential of the non-dispersible fraction; TOC: total organic carbon. | ||
Mostly oxalate extractable Fe and TOC leverage the second principal component that explains 23% of the total variation. Oxalate extractable Fe and TOC appear not to correlate strongly with the ζ-potentials, possibly because iron oxides are less efficient than amorphous aluminum minerals in increasing the point of zero charge of silica63 and do not affect ζn.d to the same extent than amorphous aluminium minerals. The apparent opposite relation between oxalate extractable Fe and TOC could not be explained. Both parameters were therefore retained for PLS analysis. The goethite concentration was not retained, because oxalate partly extracts goethite.
Heteroaggregation
Heteroaggregation of ENMs with naturally occurring clays or oxides was limited or inexistent. It is often stressed that heteroaggregation is an important mechanism in environmental media, including soils,2,67 but for the natural soils studied here and the relatively low ionic strength of the aqueous medium used, homo- or heteroaggregation did not occur. sp-ICP-TOF-MS analysis of saturated soil solutions mixed with ENM suspensions resulted in most cases in less than 10% co-occurrences of Au or Ag particle signals with those of Al, Fe or Si (ESI† Table S3). Mixing ENM suspensions with more diluted saturated soil solution did not consistently increase the number of co-occurrences and the fraction of observed co-occurrences was in most cases lower than the probability of random co-occurrences of these particle events (ESI† Fig. S4).The ζ-potential of ENMs in ultrafiltered soil solution varied between −22.8 and −48.1 mV. As discussed earlier, all soils contain a significant concentration of positively charged surface sites with which the negatively charged ENMs interact. However, dispersible natural colloids are most likely 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 clays in these soils as discussed earlier. The highly negative surface potential of these colloids is reflected by ζdispersed that is significantly more negative than ζn.d. that reflects the surface potential of pore walls (ESI† Table S2). The overall electrostatic repulsion of ENMs from dispersed clay particles is thus stronger than from soil pore walls. This explains why ENMs were found to attach to soils as reflected by significant attachment rates, but did not form heteroaggregates with natural colloids. Lack of interaction of gold ENMs with stable natural soil colloids has been observed before66 and contradicts the common assumption that heteroaggregation dominates the fate of ENMs in all environmental media.2,67 Absence of heteroaggregation also confounds support for the straining model (Table 3) given that the individual ENMs are too small to experience significant straining.
1 clays in these soils as discussed earlier. The highly negative surface potential of these colloids is reflected by ζdispersed that is significantly more negative than ζn.d. that reflects the surface potential of pore walls (ESI† Table S2). The overall electrostatic repulsion of ENMs from dispersed clay particles is thus stronger than from soil pore walls. This explains why ENMs were found to attach to soils as reflected by significant attachment rates, but did not form heteroaggregates with natural colloids. Lack of interaction of gold ENMs with stable natural soil colloids has been observed before66 and contradicts the common assumption that heteroaggregation dominates the fate of ENMs in all environmental media.2,67 Absence of heteroaggregation also confounds support for the straining model (Table 3) given that the individual ENMs are too small to experience significant straining.
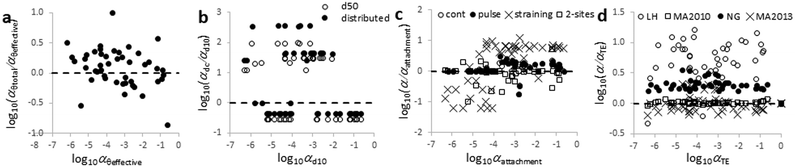
Method comparison to calculate α
The continuous, and pulse models provided similar α values, because relatively low dispersivity values were obtained likely owing to the relatively short columns that were used in this study.69 Moreover, the two-pore volume ENM injections were in many cases long enough to reach plateau values for the BTCs explaining why the continuous or pulse model generated similar results. At the same time, relatively low values calculated with the continuous or pulse models were often negative when recovery was not determined accurately.
Low α-values of the attachment model varied with the subjectively chosen starting katt value of iterative fitting explaining the large differences at relatively low α-values.
α-Values calculated using the straining model were linearly related to those of the attachment model as log10(αstraining) = 1.23 log10(αattachment) + 0.96 (r2 = 0.92). This relation can likely be related mathematically to eqn (2). Straining is an unlikely mechanism in these soil columns as argued before.
The 2-sites model often generated α values similar to the continuous, pulse and attachment models, but kirrev values 10−10 s−1 were sometimes fitted. The 2-sites model requires simultaneous fitting of three parameters, a procedure that may inflate parameters reducing their accuracy. Detachment was negligible in most experiments except for the Woburn soil where BTCs showed significant tailing and the 2-sites model fitted much better (ESI† Fig. S5–S8). However, even in the case of Woburn soil, irreversible attachment rate constants (katt,irrev) were orders of magnitude higher than the detachment rates, which is often the case for ENMs.19
PLS1 analysis
In the ideal case, variations in α should only depend on variations in ENM- and soil properties (dp, pH, sand or clay content, TOC, ζdispersed, ζn.d., oxalate extractable Fe and Al) and the formulae in Table 3 (and ESI†) should remove any residual effect of operational parameters (L, C0, dc, θ, D, U). The methods were therefore evaluated qualitatively in terms of how much variance could be explained by soil or ENM properties and the lack of influence of operational parameters.
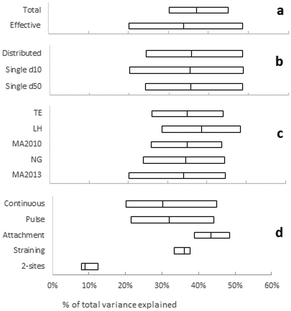
Fig. 4 shows that the fraction of variation explained varies between 8% for most approaches that are based on a 2-sites model and maximum 49% for an approach using an effective porosity, a single d10, the LH correlation equation in combination with the attachment model.
2-sites models were in many cases only marginally influenced by operational parameters (Fig. 5), but approaches using this model consistently explained a low fraction of the total variance (Fig. 4). This model fitted BTCs often best (ESI† Fig. S5–S8), but the simultaneous fitting of three parameters may reduce accuracy of the fitted parameters relatively to more simple models with only one parameter. 2-Sites models were therefore no longer considered in further analyses.
Conclusions
Our study can contribute to developing a standard protocol to obtain fate descriptors of ENMs for natural soils, because we identified several potential artefacts and how to avoid these. A standard column assay should always verify whether saturated conditions have been attained and whether the filter material at the column inlet and outlet does not retain ENMs. We also recommend to represent the soil grain size by its average diameter (d50) during α calculations. This value should be obtained from the grain size distribution measured in aqueous suspensions of the soil. Using a low ionic strength medium during column tests minimizes homo- and heteroaggregation. This is important to support the assumptions of relatively simple models to calculate α, models that are based on CFT where attachment to pore walls is the only assumed mechanism.We were not able to recommend a final experimental and theoretical approach to calculate α-values that are not influenced by operational parameters. An approach that used effective porosity (rather than total porosity), d50 to represent grain size, and the attachment model produced, in the context of this study, α-values that were least affected by operational parameters. This approach requires determination of the effective porosity and dispersivity as well as numerical fitting of a katt reaction rate constant. α determinations using effective porosity and dispersivity require a prior inert tracer test to ascertain the hydrodynamic properties of the saturated column test. Adopting these recommendations when exploring systematic effects of natural soils and ENM properties will minimize experimental artefacts thus increasing the likelihood that significant trends between α-values and soil characteristics are observed. However, the low fraction of total variance explained and the residual effect of column length and dispersivity calls for further standardization efforts, preferably cross-validating α-values with a recently developed batch method for determining α-values in natural soils.72
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
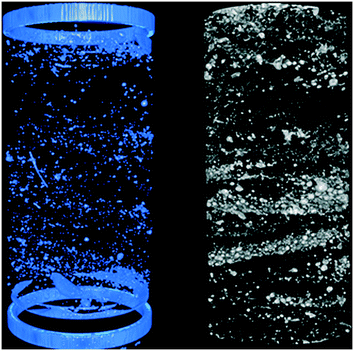
This research was funded by the EU Horizon2020 project NanoFASE (Nanomaterial Fate and Speciation in the Environment; grant no. 646002) and the EU's 7th Framework Programme project GuideNANO (FP7/2007–2013; grant no. 604387), as well as The Swedish Research Council (grant no. 2012-03937). The authors thank Dr. Johannes Koestel for the X-ray tomography measurements and corresponding image processing.References
- V. Micić, N. Bossa, D. Schmid, M. R. Wiesner and T. Hofmann, Environ. Sci. Technol., 2020, 54, 1250–1257 CrossRef PubMed.
- C. Svendsen, L. A. Walker, M. Matzke, E. Lahive, S. Harrison, A. Crossley, B. Park, S. Lofts, I. Lynch, S. Vázquez-Campos, R. Kaegi, A. Gogos, C. Asbach, G. Cornelis, F. von der Kammer, N. W. van den Brink, C. Mays and D. J. Spurgeon, Nat. Nanotechnol., 2020, 15, 731–742 CrossRef CAS PubMed.
- A. Praetorius, N. Tufenkji, K.-U. Goss, M. Scheringer and F. Von der Kammer, Environ. Sci.: Nano, 2014, 1, 317–323 RSC.
- G. Cornelis, Environ. Sci.: Nano, 2015, 2, 19–26 RSC.
- G. Cornelis, L. Pang, C. Doolette, J. K. Kirby and M. J. McLaughlin, Sci. Total Environ., 2013, 463–464, 120–130 CrossRef CAS PubMed.
- E. Goldberg, M. Scheringer, T. D. Bucheli and K. Hungerbuhler, Environ. Sci. Technol., 2014, 48, 12732–12741 CrossRef CAS PubMed.
- M. Baalousha, G. Cornelis, T. Kuhlbusch, I. Lynch, C. Nickel, W. Peijnenburg and N. van den Brink, Environ. Sci.: Nano, 2016, 3, 323–345 RSC.
- P. Babakhani, J. Bridge, R.-A. Doong and T. Phenrat, Adv. Colloid Interface Sci., 2017, 246, 75–104 CrossRef CAS PubMed.
- OECD, Test No. 312: Leaching in Soil Columns, OECD Publishing, 2021 Search PubMed.
- D. Kühnel and C. Nickel, Sci. Total Environ., 2014, 472, 347–353 CrossRef PubMed.
- N. Saleh, H. J. Kim, T. Phenrat, K. Matyjaszewski, R. D. Tilton and G. V. Lowry, Environ. Sci. Technol., 2008, 42, 3349–3355 CrossRef CAS PubMed.
- X. Liu, M. Wazne, C. Christodoulatos and K. L. Jasinkiewicz, J. Colloid Interface Sci., 2009, 330, 90–96 CrossRef CAS PubMed.
- Y. G. Wang, Y. S. Li and K. D. Pennell, Environ. Toxicol. Chem., 2008, 27, 1860–1867 CrossRef CAS PubMed.
- X. Liu, D. M. Carroll, E. J. Petersen, Q. Huang and C. L. Anderson, Environ. Sci. Technol., 2009, 43, 8153–8158 CrossRef CAS PubMed.
- R. L. Johnson, G. O. B. Johnson, J. T. Nurmi and P. G. Tratnyek, Environ. Sci. Technol., 2009, 43, 5455–5460 CrossRef CAS PubMed.
- D. P. Jaisi and M. Elimelech, Environ. Sci. Technol., 2009, 43, 9161–9166 CrossRef CAS PubMed.
- R. Doshi, W. Braida, C. Christodoulatos, M. Wazne and G. O'Connor, Environ. Res., 2008, 106, 296–303 CrossRef CAS PubMed.
- A. J. Pelley and N. Tufenkji, J. Colloid Interface Sci., 2008, 321, 74–83 CrossRef CAS PubMed.
- P. Babakhani, J. Bridge, R.-A. Doong and T. Phenrat, Water Resour. Res., 2017, 53, 4564–4585 CrossRef.
- J. Lewis and J. Sjöstrom, J. Contam. Hydrol., 2010, 115, 1–13 CrossRef CAS PubMed.
- J. Lewis and J. Sjöstrom, J. Contam. Hydrol., 2010, 115, 1–13 CrossRef CAS PubMed.
- S. Banzhaf and K. H. Hebig, Hydrol. Earth Syst. Sci., 2016, 20, 3719–3737 CrossRef CAS.
- S. Loureiro, P. S. Tourinho, G. Cornelis, N. W. Van Den Brink, M. Díez-Ortiz, S. Vázquez-Campos, V. Pomar-Portillo, C. Svendsen and C. A. Van Gestel, in Soil Pollution, Academic Press, 2018, pp. 161–190 Search PubMed.
- J. Fang, X. Q. Shan, B. Wen, J. M. Lin and G. Owens, Environ. Pollut., 2009, 157, 1101–1109 CrossRef CAS PubMed.
- J. Ren, A. I. Packman and C. Welty, Colloids Surf., A, 2001, 191, 133–144 CrossRef CAS.
- M. Baccaro, S. Harrison, H. van den Berg, L. Sloot, D. Hermans, G. Cornelis, C. A. M. van Gestel and N. W. van den Brink, Environ. Pollut., 2019, 252, 155–162 CrossRef CAS PubMed.
- N. Geitner, C. Hendren, G. Cornelis, R. Kaegi, J. Lead, G. Lowry, I. Lynch, B. Nowack, E. Petersen, E. Bernhardt, S. Brown, W. Chen, C. de Garidel-Thoron, J. Hanson, S. Harper, K. Jones, F. von der Kammer, A. Kennedy, J. Kidd, C. Matson, C. Metcalfe, J. Pedersen, W. Peijnenburg, J. Quik, S. M. Rodrigues, J. Rose, P. Sayre, M. Simonin, C. Svendsen, R. Tanguay, N. Tufenkji, T. van Teunenbroek, G. Thies, Y. Tian, J. Rice, A. Turner, J. Liu, J. Unrine, M. Vance, J. White and M. Wiesner, Environ. Sci.: Nano, 2019, 7, 13–36 RSC.
- R. J. Hunter, Zeta Potential in Colloid Science, Principles and Applications, Academic Press, London, 1988 Search PubMed.
- F. Fairbrother and H. Mastin, J. Chem. Soc., Trans., 1924, 125, 2319–2330 RSC.
- O. Omotoso, D. K. McCarty, S. Hillier and R. Kleeberg, Clays Clay Miner., 2006, 54, 748–760 CrossRef CAS.
- S. Hillier, in Clay Mineral Cements in Sandstones, ed. R. H. Worden and S. Morad, International Association of Sedimentologists, 2003, Special Publication, vol. 34, pp. 213–251 Search PubMed.
- O. Borovinskaya, B. Hattendorf, M. Tanner, S. Gschwind and D. Günther, J. Anal. At. Spectrom., 2013, 28, 226–233 RSC.
- J. Tuoriniemi, G. Cornelis and M. Hassellöv, Anal. Chem., 2012, 29, 743–752 Search PubMed.
- F. Loosli, J. Wang, S. Rothenberg, M. Bizimis, C. Winkler, O. Borovinskaya, L. Flamigni and M. Baalousha, Environ. Sci.: Nano, 2019, 6, 763–777 RSC.
- I. B. Oliveira, A. H. Demond and A. Salehzadeh, Soil Sci. Soc. Am. J., 1996, 60, 49–53 CrossRef.
- J. Koestel, Vadose Zone J., 2018, 17, 170062 CrossRef.
- S. A. Bradford, J. Simunek, M. Bettahar, M. T. Van Genuchten and S. R. Yates, Environ. Sci. Technol., 2003, 37, 2242–2250 CrossRef CAS PubMed.
- Y. Fujita and M. Kobayashi, Chemosphere, 2016, 154, 179–186 CrossRef CAS PubMed.
- N. Tufenkji, G. F. Miller, J. N. Ryan, R. W. Harvey and M. Elimelech, Environ. Sci. Technol., 2004, 38, 5932–5938 CrossRef CAS PubMed.
- M. W. Hahn and C. R. O'Melia, Environ. Sci. Technol., 2004, 38, 210–220 CrossRef CAS PubMed.
- J. Bergendahl and D. Grasso, Chem. Eng. Sci., 2000, 55, 1523–1532 CrossRef CAS.
- A. J. Valocchi, Water Resour. Res., 1985, 21, 808–820 CrossRef CAS.
- J. Šimůnek, M. T. Van Genuchten and M. Šejna, Vadose Zone J., 2016, 15, 1–25 CrossRef.
- J. Šimůnek and J. W. Hopmans, in Methods of Soil Analysis, 2002, pp. 139–157, DOI:10.2136/sssabookser5.4.c7.
- F. J. Leij, T. H. Skaggs and M. T. Van Genuchten, Water Resour. Res., 1991, 27, 2719–2733 CrossRef CAS.
- N. Tufenkji and M. Elimelech, Environ. Sci. Technol., 2004, 38, 529–536 CrossRef CAS PubMed.
- W. Long and M. Hilpert, Environ. Sci. Technol., 2009, 43, 4419–4424 CrossRef CAS PubMed.
- H. Ma, J. Pedel, P. Fife and W. P. Johnson, Environ. Sci. Technol., 2010, 44, 4383 CrossRef CAS.
- K. E. Nelson and T. R. Ginn, Water Resour. Res., 2011, 47(5), 1–17 CrossRef.
- H. Ma, M. Hradisky and W. P. Johnson, Environ. Sci. Technol., 2013, 47, 2272–2278 CrossRef CAS PubMed.
- M. Götzinger and W. Peukert, Langmuir, 2004, 20, 5298–5303 CrossRef PubMed.
- J. M. Bennett, J. L. Stanford and E. J. Ashley, J. Opt. Soc. Am., 1970, 60, 224–232 CrossRef CAS.
- M. J. Martin, B. E. Logan, W. P. Johnson, D. G. Jewett and R. G. Arnold, J. Environ. Eng., 1996, 122, 407–415 CrossRef CAS.
- A. A. Porubcan and S. Xu, Water Res., 2011, 45, 1796–1806 CrossRef CAS PubMed.
- S. de Jong, Chemom. Intell. Lab. Syst., 1993, 18, 251–263 CrossRef CAS.
- A. Hoskuldsson, Chemom. Intell. Lab. Syst., 2001, 55, 23–38 CrossRef CAS.
- G. Cornelis, A. M. Forsberg-Grivogiannis, N. P. Skold, S. Rauch and J. Perez-Holmberg, Environ. Sci.: Nano, 2017, 4, 2225–2234 RSC.
- L. J. Gimbert, P. M. Haygarth, R. Beckett and P. J. Worsfold, Environ. Sci. Technol., 2005, 39, 1731–1735 CrossRef CAS PubMed.
- M. Snehota, V. Jelinkova, M. Sobotkova, J. Sacha, P. Vontobel and J. Hovind, Water Resour. Res., 2015, 51, 1359–1371 CrossRef.
- W. Ho and K. Sirkar, Membrane Handbook, Springer, US, 2012 Search PubMed.
- S. K. Kumahor, P. Hron, G. Metreveli, G. E. Schaumann and H.-J. Vogel, Sci. Total Environ., 2015, 535, 113–121 CrossRef CAS PubMed.
- N. M. DeNovio, J. E. Saiers and J. N. Ryan, Vadose Zone J., 2004, 3, 338–351 CrossRef CAS.
- M. Arias, M. T. Barral and F. Diaz-Fierros, Clays Clay Miner., 1995, 43, 406–416 CrossRef CAS.
- G. Cornelis, C. Doolette, M. Thomas, M. J. McLaughlin, J. K. Kirby, D. Beak and D. Chittleborough, Soil Sci. Soc. Am. J., 2012, 76, 891–902 CrossRef CAS.
- J. N. Ryan and M. Elimelech, Colloids Surf., A, 1996, 107, 1–56 CrossRef CAS.
- B. Meisterjahn, E. Neubauer, F. Von der Kammer, D. Hennecke and T. Hofmann, J. Chromatogr. A, 2014, 1372, 204–211 CrossRef CAS PubMed.
- G. Cornelis, K. M. Hund-Rinke, T. Kuhlbusch, N. Van den Brink and C. Nickel, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2720–2764 CrossRef CAS.
- I. L. Molnar, W. P. Johnson, J. I. Gerhard, C. S. Willson and D. M. O'Carroll, Water Resour. Res., 2015, 51, 6804–6845 CrossRef.
- A. U.-H. Khan and W. A. Jury, J. Contam. Hydrol., 1990, 5, 119–131 CrossRef CAS.
- M. Bromly, C. Hinz and L. A. G. Aylmore, Eur. J. Soil Sci., 2007, 58, 293–301 CrossRef.
- J. N. Ryan, M. Elimelech, R. A. Ard, R. W. Harvey and P. R. Johnson, Environ. Sci. Technol., 1999, 33, 63–73 CrossRef CAS.
- A. A. Turner, N. M. K. Rogers, N. K. Geitner and M. R. Wiesner, Environ. Sci.: Nano, 2020, 7, 1719–1729 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0en01089h |
| This journal is © The Royal Society of Chemistry 2021 |