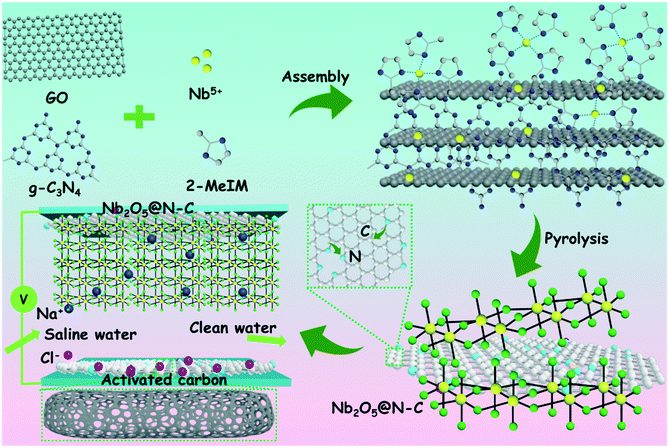
Beneficial synergy of adsorption–intercalation–conversion mechanisms in Nb2O5@nitrogen-doped carbon frameworks for promoted removal of metal ions via hybrid capacitive deionization†
Guizhi
Wang
a,
Tingting
Yan
a,
Junjie
Shen
b,
Jianping
Zhang
a,
Liyi
Shi
a and
Dengsong
Zhang
 *a
*a
aState Key Laboratory of Advanced Special Steel, School of Materials Science and Engineering, International Joint Laboratory of Catalytic Chemistry, Research Center of Nano Science and Technology, Department of Chemistry, College of Sciences, Shanghai University, No. 99 Shangda Road, Shanghai, 200444, China. E-mail: dszhang@shu.edu.cn; Tel: +86 21 66137152
bDepartment of Chemical Engineering, University of Bath, Bath BA2 7AY, UK
First published on 8th December 2020
Abstract
Capacitive deionization (CDI) is an emerging water purification technology, but the ion adsorption capacity of traditional carbon-based CDI electrodes is still unsatisfactory. Herein, a novel faradaic electrode by anchoring Nb2O5 nanoparticles on nitrogen-doped carbon frameworks as anodes and activated carbon (AC) as cathodes in a hybrid capacitive deionization (HCDI) system was originally developed to capture Na+ ions via adsorption–intercalation–conversion mechanisms. The synergistic effects of the nanostructure and carbon coating were beneficial to enhancing electrical conductivity and offering fast Na+ ion diffusion pathways. Impressively, the HCDI system demonstrated an excellent ion adsorption capacity of 35.4 mg g−1 in a 500 mg L−1 NaCl solution at 1.2 V as well as stable regeneration ability. In situ Raman and ex situ XPS measurements unraveled that the mechanism of ion removal from water was the reversible redox reaction of Nb2O5. The new overall understanding of the synergistic effects opens opportunities for the design of HCDI systems for efficient removal of metal ions from saline water.
Environmental significanceThe shortage of clean water has become a global issue as a consequence of population growth and climate change. Less than 3% of all water on earth is fresh water, and only 1.2% of all fresh water is surface water, which can be directly utilized. With this limited amount of usable fresh water, desalination of saline water and brackish water offers a promising solution to the supply of clean water. Capacitive deionization (CDI) has gained increasing attention due to its unique advantages of low energy consumption, low cost, rapid regeneration, and environment friendliness. Unfortunately, the ion adsorption capacity of traditional carbon-based CDI electrode materials is still unsatisfactory. Therefore, a novel faradaic electrode by anchoring Nb2O5 nanoparticles on the nitrogen-doped carbon frameworks (Nb2O5@N–C-1) in a hybrid capacitive deionization (HCDI) system was originally developed to capture Na+ ions via adsorption–intercalation–conversion mechanisms. Benefitting from the synergistic effects of structure optimization (nanostructure) and surface engineering (nitrogen-doped carbon coating), the Nb2O5@N–C-1 electrode demonstrated superior CDI performance in terms of ion adsorption capacity and ion adsorption rates. Furthermore, in situ Raman and ex situ XPS analyses were conducted to verify the adsorption–intercalation–conversion mechanism of ion removal from water during the CDI process. This work presents a new strategy to design highly efficient HCDI systems for efficient removal of metal ions from saline water. |
1 Introduction
In the recent decades, the shortage of clean water has become a global issue as a consequence of population growth and climate change.1–3 Less than 3% of all water on earth is fresh water, and only 1.2% of all fresh water is surface water, which can be directly utilized. With this limited amount of usable fresh water, desalination of saline water and brackish water offers a promising solution to the supply of clean water.4,5 Traditional desalination methods have been examined to remove ions from seawater efficiently. Unfortunately, some of the methods suffer from various drawbacks including massive energy consumption, high cost and significant environmental impacts.6,7 Therefore, it is imperative to develop cost-effective and eco-friendly desalination technologies as promising alternatives. Capacitive deionization (CDI) has gained increasing attention due to its unique advantages of low energy consumption, low cost, rapid regeneration, and environment friendliness.8,9 When applying a low potential on the two electrodes, the ions in solution can be quickly adsorbed and harvested on the oppositely-charged electrodes. Thus, clean water is obtained.10,11The ion removal capacity of CDI is closely dependent on the electrode materials. Carbon-based materials, such as carbon aerogels, carbon nanotubes, activated carbon, mesoporous carbon, and reduced graphene, have been extensively explored as CDI electrode materials due to the advantages of high surface area, porous structure, and electrochemical stability.12–15 Unfortunately, one of the major limitations of carbon-based materials is the unsatisfactory ion adsorption capacity.16,17 In carbon-based electrodes, the ions removed from a solution are reserved on the carbon surface based on the electric double layer (EDL) theory, in which the removal capacity is mainly determined by the pores and surface area. In addition, the co-ion effect would restrain more ions from gathering and cause adsorbed ions to easily return to the solution.18,19 To overcome the drawback, hybrid capacitive deionization (HCDI) has been developed. HCDI systems consist of one carbon electrode and one faradaic electrode.20,21 In general, anions (such as Cl− ions) are electrostatically adsorbed on the carbon-based materials. But cations (such as Na+ ions) are not only removed by surface adsorption, but also captured through a charge transfer reaction.22,23 Compared with those EDL-based electrodes, HCDI systems deliver a higher ion removal capacity and faster ion removal rates.24,25 The selection of faradic materials for HCDI systems usually favored those with substantially improved energy storage domains.26,27 Back in 2012, Pasta28et al. selected Na2−xMn5O10 and Ag as the electrodes and obtained good desalination performance. After that, a series of faradic electrode materials, such as MnO2, TiO2, SnS2, MoS2, Na4Ti9O20, NaTi2(PO4)3, and Prussian blue, have been developed and proved to gain excellent ion removal capacities.29–32 Wang33et al. used the hollow carbon@MnO2 to capture Na+ ions through a redox reaction and obtained a high removal capacity of 30.7 mg g−1. Our former work34 showed that synthesized MoS2–graphene materials reserve/convert Na+ ions via a faradaic reaction. The obtained electrodes demonstrated a high volumetric adsorption capacity of 14.3 mg cm−3 in a 500 mg L−1 NaCl solution at 1.2 V.
Orthorhombic Nb2O5 (T-Nb2O5), as a representative Na+ ion capturing material with an intercalation–conversion type, is a promising candidate for an HCDI electrode due to the intrinsic structural advantage.35,36 The (001) planes of Nb2O5 have a larger interplanar spacing (3.9 Å) than the size of Na+ ions (2.04 Å), which may be suitable for Na+ ion diffusion.37 Unfortunately, the low electrical conductivity (≈3 × 10−6 S cm−1) and the sluggish diffusion of Na+ ions from aggregation tendency may hinder the further application of Nb2O5. Two effective methods can be adopted to enhance the electrical conductivity and decrease the diffusion length for electrons/ions: (1) nanostructure engineering can make the materials have low dimensions, which offers shorter pathways and faster Na+ ion diffusion;38,39 (2) a composite with carbon can stabilize the materials and form an interconnected network for fast transport of electrons and Na+ ions.40,41 Throughout all sorts of carbon-based supports, graphene gains much attention due to its large surface area, good chemical/physical stability, and superb electrical conductivity.42 Moreover, the heteroatoms (such as nitrogen) doped in the carbon skeleton could boost electrical conductivity, introduce more defects, and improve hydrophilicity.43,44 Graphitic carbon nitride (g-C3N4), as a suitable nitrogen precursor, has a relatively high nitrogen content of 57% and an sp2 hybridized carbon structure. The high level of pyridine type nitrogen can offer abundant lone electron pairs to capture Nb atoms as benign ligands.45 2-Methylimidazole, as another benign N ligand, can coordinate with Nb and prohibit the large aggregation of Nb2O5 nanocrystals during the heating process at elevated temperature.46
Herein, we originally designed Nb2O5 anchored on nitrogen-doped carbon frameworks (denoted Nb2O5@N–C-1) via simple assembly and pyrolysis as anodes and activated carbon (AC) as cathodes. The obtained Nb2O5@N–C-1 has uniform Nb2O5 nanoparticles tightly anchored on the graphene networks. The N-doping improved the electrical conductivity and produced localized highly-reactive regions. Benefitting from the synergistic effects of structure optimization (nanostructure) and surface engineering (nitrogen-doped carbon coating), the Nb2O5@N–C-1 electrode demonstrated superior CDI performance in terms of ion adsorption capacity and ion adsorption rates. Furthermore, in situ Raman and ex situ XPS analyses were conducted to verify the adsorption–intercalation–conversion mechanism of ion removal from water during the CDI process.
2 Materials and methods
First of all, graphene oxide (GO) was prepared by modified Hummer's method,39 and g-C3N4 was obtained by a thermal decomposition/polymerization process.46 Specifically, 0.02 g of GO and 0.1 g of g-C3N4 were mixed in 50 mL of methanol after ultrasonic treatment for 1 h. Next, 0.11 g of NbCl5 was added into the mixture after ultrasonic treatment for 0.5 h to form solution alpha. Meanwhile, 0.17 g of 2-methylimidazole (2-MeIM) was dispersed in 30 mL of methanol after ultrasonic treatment for 0.5 h to form solution beta. Then, solution beta was added dropwise into solution alpha with stirring, and the mixed solution was left at room temperature for 12 h. The Nb/2MeIM/g-C3N4/GO precursors were gained by centrifugation with methanol and drying at 60 °C for 12 h. After that, the dried material was calcined at 800 °C (3 °C min−1) for 2 h under an N2 atmosphere to obtain the Nb2O5@N–C-1 (NbCl5 and 2-MeIM with a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite. Simultaneously, Nb2O5@N–C-2 (0.11 g of NbCl5 and 0.085 g of 2-MeIM with a mole ratio of 2
1) composite. Simultaneously, Nb2O5@N–C-2 (0.11 g of NbCl5 and 0.085 g of 2-MeIM with a mole ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Nb2O5@N–C-0.5 (0.11 g of NbCl5 and 0.34 g of 2-MeIM with a mole ratio of 1
1), Nb2O5@N–C-0.5 (0.11 g of NbCl5 and 0.34 g of 2-MeIM with a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), Nb2O5 (only utilization of NbCl5) and N–C (without utilization of NbCl5) were synthesized through the same procedure.
2), Nb2O5 (only utilization of NbCl5) and N–C (without utilization of NbCl5) were synthesized through the same procedure.
3 Results and discussion
3.1 Characteristic analysis
These Nb2O5@N–C composites were synthesized via an assembly approach followed by a pyrolysis method at 800 °C under a N2 atmosphere, as shown in Fig. 1. In the assembly process, Nb5+ ions were readily adsorbed on the surface of the negatively charged GO/g-C3N4 complex and further coordinated with 2-methylimidazole (2-MeIM) to form the Nb/2MeIM/g-C3N4/GO precursors (Fig. S1a and b†). After the pyrolysis treatment, the Nb2O5@N–C composites were successfully prepared. The morphology and microstructure of the obtained samples were observed and verified by SEM and TEM (Fig. S2†). These electron microscopy images revealed that the Nb2O5 nanoparticles with an average size of around 50 nm (Fig. S2c, f, and i†) were tightly anchored on the nitrogen-doped graphene sheets. Besides, the density of the Nb2O5 nanocrystals increased with the improvement of Nb5+ utilization, from Nb2O5@N–C-0.5 (Fig. S2a and b†) and Nb2O5@N–C-1 (Fig. S2d and e†) to Nb2O5@N–C-2 (Fig. S2g and h†). The contact between the Nb2O5 nanoparticles and graphene network (Fig. 2a) could supply numerous pathways for ion diffusion and electron transfer, which would improve the electrochemical performance. The HRTEM image of Nb2O5@N–C-1 (Fig. 2b) displays lattice fringes with a spacing of 0.393 nm, which are well attached to the (001) planes of the orthorhombic Nb2O5 phase. In Fig. S3,† prominent diffraction rings were observed in the selected area electron diffraction (SAED) patterns, which confirmed the existence of the (001), (180), and (200) planes of the orthorhombic Nb2O5 phase. Furthermore, the relevant elemental mapping images of Nb2O5@N–C-1 demonstrated the uniform distributions of C, N, O, and Nb elements over the selected area, which proved that the Nb2O5 nanoparticles were homogeneously anchored on the nitrogen-doped graphene networks (Fig. S4†).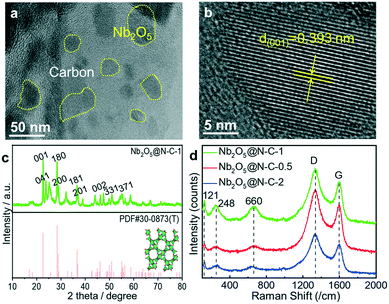 | ||
| Fig. 2 (a) TEM image, (b) HRTEM image, (c) XRD pattern of Nb2O5@N–C-1, and (d) Raman spectra of Nb2O5@N–C-0.5, Nb2O5@N–C-1, and Nb2O5@N–C-2. | ||
The formation and crystalline structure of the Nb2O5 nanocrystals in these Nb2O5@N–C composites were verified by XRD analysis, as shown in Fig. 2c and S5.† These characteristic diffraction peaks located at 22.6°, 25.7°, 28.4°, 28.9°, 36.6°, 37.0°, 46.2°, 50.9°, and 54.9°, could be appropriately indexed to the (001), (041), (180), (200), (181), (201), (002), (331), and (371) planes of the orthorhombic Nb2O5 phase (PDF#30-0873). To figure out the composition and structural properties of the correlative carbon supports, Raman analysis of the obtained samples was conducted (Fig. 2d). Two typical carbon-based characteristic peaks of disordered carbon (D band) and sp2 bonded ordered graphitic carbon (G band) were observed at around 1350 and 1599 cm−1. The value of ID/IG was normally related to the degree of structural disorder and the number of defects. The ID/IG values of Nb2O5@N–C-0.5, Nb2O5@N–C-1, and Nb2O5@N–C-2 were 1.19, 1.16, and 1.11, revealing the abundant vacancies and defects. Moreover, the degree of disorder decreased with the increase of metal contents. Besides, the three small peaks at 121, 248, and 660 cm−1 were the characteristic bands of Nb2O5, which correspond to the vibrations of octahedra, the bending vibration of the Nb–O bond, and the stretching vibration of the Nb–O bond,47,48 respectively. The pore structure characteristics of the Nb2O5@N–C composites were obtained by N2 adsorption/desorption measurements. Both the BET specific surface area and pore volume decreased as the amount of Nb2O5 in the composite increased (Table S1†). Nb2O5@N–C-0.5 exhibited the largest BET specific surface area of 78.6 m2 g−1, in comparison to Nb2O5@N–C-1 (34.1 m2 g−1) and Nb2O5@N–C-2 (29.5 m2 g−1) (Fig. S6a†). Moreover, the pore size distribution plots indicated the mesoporous structures of the three samples, which could supply abundant diffusion channels for ion transportation. Specifically, Nb2O5@N–C-1 showed a similar pore size and volume distribution compared to Nb2O5@N–C-0.5, which are much higher and richer than those of Nb2O5@N–C-2 in the range of 2–10 nm (Fig. S6b†). To further investigate the surface chemical elements and bonding states of the Nb2O5@N–C composites, XPS analysis was conducted, as shown in Fig. S7.† The Nb 3d, C 1s, N 1s, and O 1s signals were obviously seen in the full-scan spectra of Nb2O5@N–C-0.5, Nb2O5@N–C-1, and Nb2O5@N–C-2, proving the successful integration of Nb2O5 in the structure of the carbon frameworks. Moreover, the formation of C–N bonds (Fig. S8†) confirmed the insertion of N atoms into the carbon plane. The N 1s peak consisted of four types of N, namely pyridinic N, pyrrolic N, graphitic N, and oxide N (Fig. S9†). The Nb–O bond (Fig. S10†) verified the presence of Nb2O5. The atomic percentage of the Nb element (Table S2†) increased from Nb2O5@N–C-0.5 to Nb2O5@N–C-2, which was consistent with the Nb precursor utilization in the synthesis process. Since the electrochemical properties and CDI performance were tested in NaCl aqueous solution, the hydrophilicity of the electrode materials was also a vital indicator. We evaluated the hydrophilicity by dynamic water contact angle test (Fig. S11†). The results showed that Nb2O5@N–C-1 has a smaller contact angle (74.29°) than Nb2O5@N–C-0.5 (75.42°) and Nb2O5@N–C-2 (84.14°). The good hydrophilicity of Nb2O5@N–C-1 was due to the porous structure and abundant N content within the carbon frameworks.
3.2 Electrochemical performance
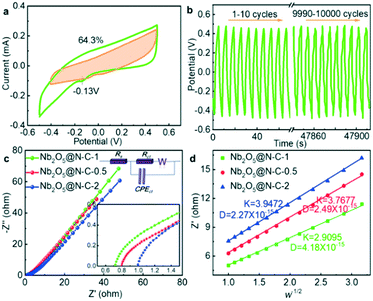
The electrochemical performance of the Nb2O5@N–C composites was firstly evaluated by cyclic voltammetry (CV). The CV plots were obtained at scan rates ranging from 1 to 100 mV s−1 (Fig. S12a–c†). For every composite, the integrated area of the CV curve was gradually enlarged with the increase of scan rates. At the same scan rate, the Nb2O5@N–C-1 curve has the largest integrated area, followed by the Nb2O5@N–C-0.5 and Nb2O5@N–C-2 curves. As a result, Nb2O5@N–C-1 possessed the highest specific capacitance among these composites at any scan rate (Fig. S12d†). To gain a deeper insight into the charge transfer mechanisms in Nb2O5@N–C-1, we quantitatively divided the contributions of the capacitive and diffusion-controlled effects from the CV plots by using a slower scan rate of 0.1 mV s−1. In Fig. 3a, a redox peak appeared at around −0.13 V, demonstrating a faradic reaction of Na+ ions inserting into the Nb2O5@N–C-1 electrode. Therefore, the Na+ ions are removed by the synergistic effects of electro-adsorption of the nitrogen-doped carbon frameworks and the faradaic reaction of Nb2O5 (Fig. S13†). The surface capacitive (shaded areas) and diffusion-controlled contributions of Nb2O5@N–C-1 at 0.1 mV s−1 were 64.3% and 35.7%, respectively.37,49 The results confirmed that Nb2O5 played a leading role in capturing Na+ ions. Fig. S14a–c† show the galvanostatic charge–discharge (GCD) plots of Nb2O5@N–C-0.5, Nb2O5@N–C-1, and Nb2O5@N–C-2 at a current density of 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 A g−1. The GCD plots demonstrated analogous symmetric triangular shapes, exhibiting benign electrochemical reversibility. Besides, when the current density was raised from 0.2 to 1.2 A g−1, the Nb2O5@N–C-1 electrode showed the longest discharge time, which manifested the largest capacitance in accordance with the CV results. Additionally, Nb2O5@N–C-1 showed a lower potential drop (iR) than other materials at the initial discharge process (Fig. S14d†), suggesting a better electrical resistance. Cycling stability was another vital parameter for electrode materials. In Fig. 3b, the GCD plots maintained the original shape after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1, manifesting an outstanding cycle-to-cycle durability. The superior electrochemical property of Nb2O5@N–C-1 was further demonstrated by electrochemical impedance spectroscopy (EIS), as shown in Fig. 3c. The Nyquist plots consisted of a semicircle in the medium frequency region corresponding to charge-transfer resistance (Rct), and a straight segment in the low frequency region corresponding to Warburg diffusion impedance (W). An equivalent circuit (inset of Fig. 3c) was employed to simulate these EIS curves, in which Rs and CPEct represent the solution resistance and constant phase part, respectively.50 Nb2O5@N–C-1 demonstrated the smallest semicircle among the three electrodes, indicating the lowest charge-transfer impedance. According to the fitting results (Table S3†), the Rct values were 14.66, 18.52, and 23.94 Ω for Nb2O5@N–C-1, Nb2O5@N–C-0.5, and Nb2O5@N–C-2, respectively. Furthermore, the diffusion coefficient (DNa+) of Nb2O5@N–C-1 was 4.18 × 10−15 cm2 s−1, much larger than those of Nb2O5@N–C-0.5 (2.49 × 10−15 cm2 s−1) and Nb2O5@N–C-2 (2.27 × 10−15 cm2 s−1), indicating enhanced Na+ ion diffusion and adsorption (Fig. 3d). Therefore, the excellent electrochemical properties may endow Nb2O5@N–C-1 with good CDI performance.
000 cycles at 10 A g−1, manifesting an outstanding cycle-to-cycle durability. The superior electrochemical property of Nb2O5@N–C-1 was further demonstrated by electrochemical impedance spectroscopy (EIS), as shown in Fig. 3c. The Nyquist plots consisted of a semicircle in the medium frequency region corresponding to charge-transfer resistance (Rct), and a straight segment in the low frequency region corresponding to Warburg diffusion impedance (W). An equivalent circuit (inset of Fig. 3c) was employed to simulate these EIS curves, in which Rs and CPEct represent the solution resistance and constant phase part, respectively.50 Nb2O5@N–C-1 demonstrated the smallest semicircle among the three electrodes, indicating the lowest charge-transfer impedance. According to the fitting results (Table S3†), the Rct values were 14.66, 18.52, and 23.94 Ω for Nb2O5@N–C-1, Nb2O5@N–C-0.5, and Nb2O5@N–C-2, respectively. Furthermore, the diffusion coefficient (DNa+) of Nb2O5@N–C-1 was 4.18 × 10−15 cm2 s−1, much larger than those of Nb2O5@N–C-0.5 (2.49 × 10−15 cm2 s−1) and Nb2O5@N–C-2 (2.27 × 10−15 cm2 s−1), indicating enhanced Na+ ion diffusion and adsorption (Fig. 3d). Therefore, the excellent electrochemical properties may endow Nb2O5@N–C-1 with good CDI performance.
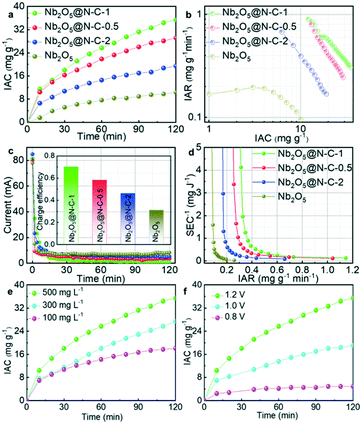
3.3 Capacitive deionization of water
The batch-mode CDI experiments were conducted to evaluate the removal capacity of the electrode pairs. In Fig. 4a, the deionization behaviors of Nb2O5//AC, Nb2O5@N–C-0.5//AC, Nb2O5@N–C-1//AC and Nb2O5@N–C-2//AC were analyzed in a 500 mg L−1 NaCl solution at 1.2 V. It was observed that the ion adsorption capacity (IAC) increased quickly at first and then the trend gradually slowed down within the required time. The Na+ ions were removed through electro-adsorption and electrochemical reactions with Nb2O5@N–C, while the negatively charged Cl− ions were electrically-adsorbed by the AC electrode. After 120 min, the IAC of Nb2O5@N–C-1//AC reached 35.4 mg g−1, much higher than those of Nb2O5@N–C-0.5//AC, Nb2O5@N–C-2//AC, and Nb2O5//AC which were 29.2 mg g−1, 19.4 mg g−1, and 10.4 mg g−1, respectively. The result confirmed that the combinations of the Nb2O5 composite with the nitrogen-doped carbon framework can enhance the removal capacity of pure metal oxides. In addition to IAC, the ion adsorption rate (IAR) is another key indicator of the CDI performance. Fig. 4b shows the Ragone plots of IAR vs. IAC of the four electrodes. IAR was located at a high level firstly and moved downwards with the increase of IAC. The Ragone plot of Nb2O5@N–C-1//AC was located at the top right corner, suggesting that Nb2O5@N–C-1//AC has the fastest ion adsorption rate and the highest removal capacity among the four electrode materials. Additionally, to demonstrate the advantages of Nb2O5@N–C-1//AC over simple carbon substrates and the advantages of the nitrogen-doped carbon framework over commercial AC, the desalination behaviors of Nb2O5@N–C-1//AC along with N–C//AC and AC//AC were tested in a 500 mg L−1 NaCl solution at 1.2 V. Unsurprisingly, the Nb2O5@N–C-1//AC composites exhibited the largest IAC and the quickest IAR (Fig. S15†). The outstanding performance of Nb2O5@N–C-1//AC was further confirmed in Fig. S16† where Nb2O5@N–C-1//AC exhibited the greatest concentration reduction (CR) (0.88 mM) and the fastest average ion adsorption rate (AIAR) (0.50 mg g−1 min−1) among all the electrode materials. The charge utilization and energy loss in the CDI process could be measured by charge efficiency. The charge efficiencies of Nb2O5@N–C-0.5//AC, Nb2O5@N–C-1//AC, Nb2O5@N–C-2//AC, and Nb2O5//AC were calculated using the current transient curves in a 500 mg L−1 NaCl solution at 1.2 V. As shown in Fig. 4c, Nb2O5@N–C-1//AC has a higher charge efficiency (0.70) than Nb2O5@N–C-0.5//AC (0.58), Nb2O5@N–C-2//AC (0.46) and Nb2O5//AC (0.31). A series of trade-off curves between the inverse of specific energy consumption (SEC−1) and IAR were also recorded in a 500 mg L−1 NaCl solution at 1.2 V. In Fig. 4d, the trade-off curve of Nb2O5@N–C-1//AC was always above the curves of Nb2O5@N–C-0.5//AC, Nb2O5@N–C-2//AC, and Nb2O5//AC. This means that Nb2O5@N–C-1//AC reached the fastest IAR in the same SEC−1 compared with other electrodes, demonstrating the most efficient energy utilization. The synergistic effects of the electro-adsorption of the nitrogen-doped carbon framework and the faradaic reaction of Nb2O5 facilitated ion diffusion and ion adsorption. The deionization property of Nb2O5@N–C-1//AC was further analyzed at various initial concentrations of NaCl solution and different applied voltages. In Fig. 4e, it was clear that the IAC of Nb2O5@N–C-1//AC increased from 18.1 and 27.4 to 35.4 mg g−1 as the NaCl concentrations increased from 100 and 300 to 500 mg L−1 at 1.2 V. In general, a higher NaCl concentration reduced the ionic resistance and facilitated fast ion adsorption. Compared with other reported metal-based electrode materials (Table S4†), Nb2O5@N–C-1 exhibited an excellent deionization behavior with a high ion adsorption capacity. Moreover, the Ragone plot moved to the top right with the increase of NaCl concentration, verifying a faster IAR and a higher IAC in a 500 mg L−1 solution (Fig. S17a†). Fig. 4f presents the IAC of Nb2O5@N–C-1//AC at distinct applied voltages of 0.8, 1.0, and 1.2 V. The change of voltages was consistent with the change of NaCl concentrations. Notably, IAC increased from 4.9 and 19.1 to 35.4 mg g−1 when the voltage was raised from 0.8 and 1.0 to 1.2 V. The Ragone plots (Fig. S17b†) also showed a faster IAR and a higher IAC at a higher voltage. The enhanced deionization performance was due to the stronger electrostatic force at a higher applied voltage. The comparison between the CR and AIAR in different combinations of NaCl concentration and voltage also showed that the best performance was achieved when the initial NaCl concentration was 500 mg L−1 and the applied voltage was 1.2 V (Fig. S18†). The regeneration ability was crucial to justifying a deionization material for further applications. The continuous cyclic adsorption–desorption experiments of Nb2O5@N–C-1//AC were conducted in a 100 mg L−1 NaCl solution (Fig. S19†). The adsorption process was started by applying a 1.2 V voltage, and the subsequent desorption process was realized by short circuiting at 0 V. In every cycle, the electrical conductivity dropped to a low level during the adsorption process and recovered to the initial level during the desorption process. The water recovery (WR) was 33.3%, the energy consumption was 100 J g−1 and energy efficiency was 19.58% under the same measurement conditions in one cycle. The deionization rate demonstrated general stability over 20 consecutive adsorption–desorption cycles. After the regeneration test, the Nb2O5 nanoparticles were still tightly anchored on the nitrogen-doped graphene sheets (Fig. S20a and b†). But the size of the Nb2O5 nanoparticles slightly decreased, which could shorten the ion transport pathways and provide rapid diffusion channels in a long-term operation (Fig. S20c†). The above results proved that Nb2O5@N–C-1 possessed outstanding regeneration ability.3.4 Mechanisms of ion removal
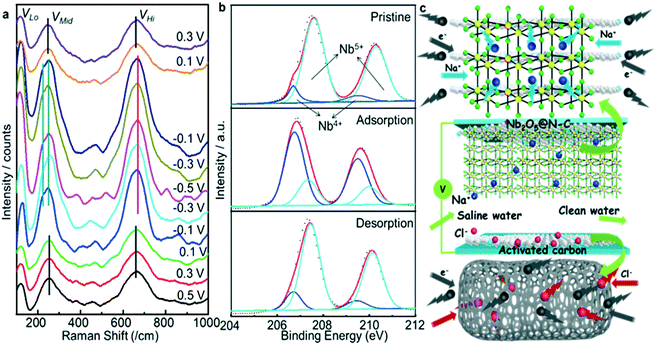
The mechanisms of ion removal from water were investigated by in situ Raman characterization. Fig. 5a shows the in situ Raman spectra when the CV plots (a scan rate of 0.1 mV s−1) reached different stages of ion adsorption and desorption (Fig. S21†). During the ion adsorption process from 0.5 to −0.5 V, the evolution of each band could be summarized as follows. (1) The VHi band kept nearly constant from 0.5 to 0.1 V. When the potential decreased to −0.1 V, the VHi band demonstrated a red shift and the relative intensity increased, which was consistent with the CV curves in which a prominent cathodic peak appeared at this range. (2) The VMid band presented a similar trend with the VHi band. No noticeable change was observed from 0.5 to 0.1 V. After that, the VMid band has split into two small bands from −0.1 to −0.5 V. (3) The VLo band, after being static at the range of 0.5 to 0.1 V, demonstrated an increased relative intensity from −0.1 V. In contrast, the evolution of the three bands in the Na+ ion deintercalation process was the exact opposite of the above-mentioned Na+ ion intercalation. Specifically, during the ion desorption process from −0.5 to 0.5 V, the VHi band was blue-shifted with a reduced intensity; the VMid bands merged into one band, and the VLo band also decreased in intensity. The opposite evolutions of these Raman band groups of Nb2O5 verified the reversible structural conversion via Na+ ion intercalation/deintercalation.51,52 Moreover, ex situ XPS analysis was used to observe the valence changes in the Nb element during the ion adsorption and ion desorption process. As shown in Fig. S22,† there was a clear blue shift from the pristine state to the adsorption state and the subsequent red shift from the adsorption state to the desorption state. The reversible shift of Nb 3d5/2 and Nb 3d3/2 revealed that the valence changes of Nb had a strong correlation with the Na+ ion conversion process. The Nb 3d XPS spectra of the three states were divided into individual peaks to provide more information about the removal mechanisms (Fig. 5b). At the pristine state, Nb5+ played a significant role while Nb4+ played a minor role. After that, most of the Nb5+ ions were reduced into Nb4+ when the electrode adsorbed sufficient Na+ ions. Finally, most of the Nb4+ ions were oxidized to Nb5+ after the applied voltage was removed. The above XPS results indicated that the Na+ ion conversion process arose from the reversible redox reaction of the Nb5+/Nb4+ pair in the Nb2O5@N–C-1 electrode.53–55 The Na+ ion insertion/extraction reaction could be expressed as Nb2O5 + x Na+ + x e− ⇔ NaxNb2O5.38,53 Meanwhile, Cl− ions were adsorbed on the surface of the positively charged AC during the ion adsorption process, and were released back to the salt solution during the ion desorption process. Therefore, the removal mechanisms of Na+ ions and Cl− ions could be distinctly illustrated as shown in Fig. 5c.4 Conclusions
In summary, the exceptional CDI performance of Nb2O5@N–C-1 could be explained by the synergistic effects of the electro-adsorption of the nitrogen-doped carbon framework and the faradaic reaction of Nb2O5. GO, g-C3N4 and 2-MeIM derived nitrogen-doped carbon (N–C) as flexible supports showed superb cycling stability. Besides, the introduction of N–C enhanced the electrical conductivity of Nb2O5 and realized better Na+ ion diffusion. Nb2O5in situ growth on the N–C framework increased the ion diffusion channels and facilitated the fast transport of Na+ ions. Owing to the combination of the electro-adsorption/desorption mechanism of N–C and the insertion/extraction process of Nb2O5, the Nb2O5@N–C-1 electrode displayed an excellent ion removal capacity. Inspiringly, the novel HCDI system equipped with Nb2O5@N–C-1//AC exhibited a superb IAC (35.4 mg g−1 in a 500 mg L−1 NaCl solution at 1.2 V), fast IAR, high charge efficiency, low energy loss, and good regeneration ability. In situ Raman and ex situ XPS analyses verified that the mechanisms of ion removal were reversible intercalation and the faradaic reaction of Nb2O5. This work presents a new strategy to design highly efficient HCDI systems for water purification.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support from the National Natural Science Foundation of China (21906101; 21722704) and the Science and Technology Commission of Shanghai Municipality (19DZ2293100; 18DZ2281400).Notes and references
- T. Oki and S. Kanae, Global Hydrological Cycles and World Water Resources, Science, 2006, 313, 1068 CrossRef CAS PubMed.
- C. J. Vörösmarty, P. B. McIntyre, M. O. Gessner, D. Dudgeon, A. Prusevich, P. Green, S. Glidden, S. E. Bunn, C. A. Sullivan, C. R. Liermann and P. M. Davies, Global threats to human water security and river biodiversity, Nature, 2010, 467, 555–561 CrossRef PubMed.
- G. Wang, T. Yan, J. Zhang, L. Shi and D. Zhang, Trace-Fe-Enhanced Capacitive Deionization of Saline Water by Boosting Electron Transfer of Electro-Adsorption Sites, Environ. Sci. Technol., 2020, 54, 8411–8419 CrossRef CAS PubMed.
- J.-F. Pekel, A. Cottam, N. Gorelick and A. S. Belward, High-resolution mapping of global surface water and its long-term changes, Nature, 2016, 540, 418–422 CrossRef CAS.
- M. Elimelech and W. A. Phillip, The Future of Seawater Desalination: Energy, Technology, and the Environment, Science, 2011, 333, 712 CrossRef CAS PubMed.
- J. E. Dykstra, R. Zhao, P. M. Biesheuvel and A. van der Wal, Resistance identification and rational process design in Capacitive Deionization, Water Res., 2016, 88, 358–370 CrossRef CAS.
- C. Zhang, D. He, J. Ma, W. Tang and T. D. Waite, Faradaic reactions in capacitive deionization (CDI)-problems and possibilities: A review, Water Res., 2018, 128, 314–330 CrossRef CAS PubMed.
- S. P. Surwade, S. N. Smirnov, I. V. Vlassiouk, R. R. Unocic, G. M. Veith, S. Dai and S. M. Mahurin, Water desalination using nanoporous single-layer graphene, Nat. Nanotechnol., 2015, 10, 459–464 CrossRef CAS PubMed.
- C. He, J. Ma, C. Zhang, J. Song and T. D. Waite, Short-Circuited Closed-Cycle Operation of Flow-Electrode CDI for Brackish Water Softening, Environ. Sci. Technol., 2018, 52, 9350–9360 CrossRef CAS PubMed.
- P. Liu, T. Yan, L. Shi, H. S. Park, X. Chen, Z. Zhao and D. Zhang, Graphene-based materials for capacitive deionization, J. Mater. Chem. A, 2017, 5, 13907–13943 RSC.
- T. Wang, C. Zhang, L. Bai, B. Xie, Z. Gan, J. Xing, G. Li and H. Liang, Scaling behavior of iron in capacitive deionization (CDI) system, Water Res., 2020, 171, 115370 CrossRef CAS.
- J. Zhang, T. Yan, J. Fang, J. Shen, L. Shi and D. Zhang, Enhanced capacitive deionization of saline water using N-doped rod-like porous carbon derived from dual-ligand metal-organic frameworks, Environ. Sci.: Nano, 2020, 7, 926–937 RSC.
- Z. U. Khan, T. Yan, J. Han, L. Shi and D. Zhang, Capacitive deionization of saline water using graphene nanosphere decorated N-doped layered mesoporous carbon frameworks, Environ. Sci.: Nano, 2019, 6, 3442–3453 RSC.
- W. Tang, J. Liang, D. He, J. Gong, L. Tang, Z. Liu, D. Wang and G. Zeng, Various cell architectures of capacitive deionization: Recent advances and future trends, Water Res., 2019, 150, 225–251 CrossRef CAS PubMed.
- F. Ji, L. Wang, J. Yang, X. Wu, M. Li, S. Jiang, S. Lin and Z. Chen, Highly compact, free-standing porous electrodes from polymer-derived nanoporous carbons for efficient electrochemical capacitive deionization, J. Mater. Chem. A, 2019, 7, 1768–1778 RSC.
- Y. Chen, J. Chen, C. Lin and C. Hou, Integrating a supercapacitor with capacitive deionization for direct energy recovery from the desalination of brackish water, Appl. Energy, 2019, 252, 113417 CrossRef CAS.
- F. Yu, L. Wang, Y. Wang, X. Shen, Y. Cheng and J. Ma, Faradaic reactions in capacitive deionization for desalination and ion separation, J. Mater. Chem. A, 2019, 7, 15999–16027 RSC.
- J. Cao, Y. Wang, L. Wang, F. Yu and J. Ma, Na3V2(PO4)3@C as Faradaic Electrodes in Capacitive Deionization for High-Performance Desalination, Nano Lett., 2019, 19, 823–828 CrossRef PubMed.
- L. Wang, J. E. Dykstra and S. Lin, Energy Efficiency of Capacitive Deionization, Environ. Sci. Technol., 2019, 53, 3366–3378 CrossRef CAS PubMed.
- M. Li and H. G. Park, Pseudocapacitive Coating for Effective Capacitive Deionization, ACS Appl. Mater. Interfaces, 2018, 10, 2442–2450 CrossRef CAS PubMed.
- F. Zhou, T. Gao, M. Luo and H. Li, Heterostructured graphene@Na4Ti9O20 nanotubes for asymmetrical capacitive deionization with ultrahigh desalination capacity, Chem. Eng. J., 2018, 343, 8–15 CrossRef CAS.
- F. Xing, T. Li, J. Li, H. Zhu, N. Wang and X. Cao, Chemically exfoliated MoS2 for capacitive deionization of saline water, Nano Energy, 2017, 31, 590–595 CrossRef CAS.
- Y. Zhao, B. Liang, X. Wei, K. Li, C. Lv and Y. Zhao, A core-shell heterostructured CuFe@NiFe Prussian blue analogue as a novel electrode material for high-capacity and stable capacitive deionization, J. Mater. Chem. A, 2019, 7, 10464–10474 RSC.
- K. Wang, Y. Liu, Z. Ding, Y. Li, T. Lu and L. Pan, Metal-organic-frameworks-derived NaTi2(PO4)3/carbon composites for efficient hybrid capacitive deionization, J. Mater. Chem. A, 2019, 7, 12126–12133 RSC.
- Z. Yue, T. Gao and H. Li, Robust synthesis of carbon@Na4Ti9O20 core-shell nanotubes for hybrid capacitive deionization with enhanced performance, Desalination, 2019, 449, 69–77 CrossRef CAS.
- B. W. Byles, D. A. Cullen, K. L. More and E. Pomerantseva, Tunnel structured manganese oxide nanowires as redox active electrodes for hybrid capacitive deionization, Nano Energy, 2018, 44, 476–488 CrossRef CAS.
- W. Peng, W. Wang, G. Han, Y. Huang and Y. Zhang, Fabrication of 3D flower-like MoS2/graphene composite as high-performance electrode for capacitive deionization, Desalination, 2020, 473, 114191 CrossRef CAS.
- M. Pasta, C. D. Wessells, Y. Cui and F. La Mantia, A Desalination Battery, Nano Lett., 2012, 12, 839–843 CrossRef CAS PubMed.
- Z. Y. Leong and H. Y. Yang, A Study of MnO2 with Different Crystalline Forms for Pseudocapacitive Desalination, ACS Appl. Mater. Interfaces, 2019, 11, 13176–13184 CrossRef CAS PubMed.
- X. Wen, M. Zhao, M. Zhang, X. Fan and D. Zhang, Efficient Capacitive Deionization of Saline Water by an Integrated Tin disulfide Nanosheet@Graphite Paper Electrode via an in Situ Growth Strategy, ACS Sustainable Chem. Eng., 2019, 8, 1268–1275 CrossRef.
- M. Ding, S. Fan, S. Huang, M. E. Pam, L. Guo, Y. Shi and H. Y. Yang, Tunable Pseudocapacitive Behavior in Metal-Organic-Framework-Derived TiO2@Porous Carbon Enabling High-Performance Membrane Capacitive Deionization, ACS Appl. Energy Mater., 2019, 2, 1812–1822 CrossRef CAS.
- B. Min, J. Choi and K. Jung, Improved capacitive deionization of sulfonated carbon/titania hybrid electrode, Electrochim. Acta, 2018, 270, 543–551 CrossRef CAS.
- S. Wang, G. Wang, T. Wu, C. Li, Y. Wang, X. Pan, F. Zhan, Y. Zhang, S. Wang and J. Qiu, Membrane-Free Hybrid Capacitive Deionization System Based on Redox Reaction for High-Efficiency NaCl Removal, Environ. Sci. Technol., 2019, 53, 6292–6301 CrossRef CAS PubMed.
- J. Han, T. Yan, J. Shen, L. Shi, J. Zhang and D. Zhang, Capacitive Deionization of Saline Water by Using MoS2–Graphene Hybrid Electrodes with High Volumetric Adsorption Capacity, Environ. Sci. Technol., 2019, 53, 12668–12676 CrossRef CAS PubMed.
- B. Deng, T. Lei, W. Zhu, L. Xiao and J. Liu, In-Plane Assembled Orthorhombic Nb2O5 Nanorod Films with High-Rate Li+ Intercalation for High-Performance Flexible Li-Ion Capacitors, Adv. Funct. Mater., 2018, 28, 1704330 CrossRef.
- P. Guo, K. Sun, X. Shang, D. Liu, Y. Wang, Q. Liu, Y. Fu and D. He, Nb2O5/RGO Nanocomposite Modified Separators with Robust Polysulfide Traps and Catalytic Centers for Boosting Performance of Lithium-Sulfur Batteries, Small, 2019, 15, e1902363 CrossRef PubMed.
- X. Wang, Q. Li, L. Zhang, Z. Hu, L. Yu, T. Jiang, C. Lu, C. Yan, J. Sun and Z. Liu, Caging Nb2O5 Nanowires in PECVD-Derived Graphene Capsules toward Bendable Sodium-Ion Hybrid Supercapacitors, Adv. Mater., 2018, 30, e1800963 CrossRef PubMed.
- D. Cao, Z. Yao, J. Liu, J. Zhang and C. Li, H-Nb2O5 wired by tetragonal tungsten bronze related domains as high-rate anode for Li-ion batteries, Energy Storage Mater., 2018, 11, 152–160 CrossRef.
- G. Wang, J. Feng, L. Dong, X. Li and D. Li, Antimony (IV) Oxide Nanorods/Reduced Graphene Oxide as the Anode Material of Sodium-ion Batteries with Excellent Electrochemical Performance, Electrochim. Acta, 2017, 240, 203–214 CrossRef CAS.
- K. Tang, T. Z. X. Hong, L. You and K. Zhou, Carbon-metal compound composite electrodes for capacitive deionization: synthesis, development and applications, J. Mater. Chem. A, 2019, 7, 26693–26743 RSC.
- B. W. Byles, B. Hayes-Oberst and E. Pomerantseva, Ion Removal Performance, Structural/Compositional Dynamics, and Electrochemical Stability of Layered Manganese Oxide Electrodes in Hybrid Capacitive Deionization, ACS Appl. Mater. Interfaces, 2018, 10, 32313–32322 CrossRef CAS PubMed.
- J. Han, L. Shi, T. Yan, J. Zhang and D. Zhang, Removal of ions from saline water using N, P co-doped 3D hierarchical carbon architectures via capacitive deionization, Environ. Sci.: Nano, 2018, 5, 2337–2345 RSC.
- J. Zhang, J. Fang, J. Han, T. Yan, L. Shi and D. Zhang, N, P, S co-doped hollow carbon polyhedra derived from MOF-based core-shell nanocomposites for capacitive deionization, J. Mater. Chem. A, 2018, 6, 15245–15252 RSC.
- P. Liu, H. Wang, T. Yan, J. Zhang, L. Shi and D. Zhang, Grafting sulfonic and amine functional groups on 3D graphene for improved capacitive deionization, J. Mater. Chem. A, 2016, 4, 5303–5313 RSC.
- R. Wang, T. Yan, L. Han, G. Chen, H. Li, J. Zhang, L. Shi and D. Zhang, Tuning the dimensions and structures of nitrogen-doped carbon nanomaterials derived from sacrificial g-C3N4/metal-organic frameworks for enhanced electrocatalytic oxygen reduction, J. Mater. Chem. A, 2018, 6, 5752–5761 RSC.
- G. Wang, J. Deng, T. Yan, J. Zhang, L. Shi and D. Zhang, Turning on electrocatalytic oxygen reduction by creating robust Fe-Nx species in hollow carbon frameworks via in situ growth of Fe doped ZIFs on g-C3N4, Nanoscale, 2020, 12, 5601–5611 RSC.
- Q. Deng, F. Chen, S. Liu, A. Bayaguud, Y. Feng, Z. Zhang, Y. Fu, Y. Yu and C. Zhu, Advantageous Functional Integration of Adsorption-Intercalation-Conversion Hybrid Mechanisms in 3D Flexible Nb2O5@Hard Carbon@MoS2@Soft Carbon Fiber Paper Anodes for Ultrafast and Super-Stable Sodium Storage, Adv. Funct. Mater., 2020, 30, 1908665 CrossRef CAS.
- X. Jiao, Q. Hao, X. Xia, Z. Wu and W. Lei, Metal organic framework derived Nb2O5@C nanoparticles grown on reduced graphene oxide for high-energy lithium ion capacitors, Chem. Commun., 2019, 55, 2692–2695 RSC.
- H. Kim, E. Lim, C. Jo, G. Yoon, J. Hwang, S. Jeong, J. Lee and K. Kang, Ordered-mesoporous Nb2O5/carbon composite as a sodium insertion material, Nano Energy, 2015, 16, 62–70 CrossRef CAS.
- Q. Ji, X. Gao, Q. Zhang, L. Jin, D. Wang, Y. Xia, S. Yin, S. Xia, N. Hohn, X. Zuo, X. Wang, S. Xie, Z. Xu, L. Ma, L. Chen, G. Z. Chen, J. Zhu, B. Hu, P. Müller-Buschbaum, P. G. Bruce and Y. J. Cheng, Dental Resin Monomer Enables Unique NbO2/Carbon Lithium-Ion Battery Negative Electrode with Exceptional Performance, Adv. Funct. Mater., 2019, 29, 1904961 CrossRef CAS.
- D. Chen, J. H. Wang, T. F. Chou, B. Zhao, M. A. El-Sayed and M. Liu, Unraveling the Nature of Anomalously Fast Energy Storage in T-Nb2O5, J. Am. Chem. Soc., 2017, 139, 7071–7081 CrossRef CAS PubMed.
- J. Meng, Q. He, L. Xu, X. Zhang, F. Liu, X. Wang, Q. Li, X. Xu, G. Zhang, C. Niu, Z. Xiao, Z. Liu, Z. Zhu, Y. Zhao and L. Mai, Identification of Phase Control of Carbon-Confined Nb2O5 Nanoparticles toward High-Performance Lithium Storage, Adv. Energy Mater., 2019, 9, 1802695 CrossRef.
- Y. Li, H. Wang, L. Wang, Z. Mao, R. Wang, B. He, Y. Gong and X. Hu, Mesopore-Induced Ultrafast Na+-Storage in T-Nb2O5/Carbon Nanofiber Films toward Flexible High-Power Na-Ion Capacitors, Small, 2019, 15, e1804539 CrossRef.
- F. Liu, X. Cheng, R. Xu, Y. Wu, Y. Jiang and Y. Yu, Binding Sulfur-Doped Nb2O5 Hollow Nanospheres on Sulfur-Doped Graphene Networks for Highly Reversible Sodium Storage, Adv. Funct. Mater., 2018, 28, 1800394 CrossRef.
- Y. Lian, D. Wang, S. Hou, C. Ban, J. Zhao and H. Zhang, Construction of T-Nb2O5 nanoparticles on/in N-doped carbon hollow tubes for Li-ion hybrid supercapacitors, Electrochim. Acta, 2020, 330, 135204 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, structural characterization, electrochemical characterization, CDI test, SEM images, TEM images, particle size distribution, SAED pattern, EDS mappings, XRD patterns, N2 adsorption–desorption isotherms, BJH pore size distribution plots, XPS spectra, dynamic water contact angle analysis, CV curves, specific capacitance, GCD curves, IR drops, plots of IAC vs. deionization time, Ragone plots, comparison between AIAR and CR, regeneration test, elemental quantification, fitted parameters in EIS, and comparison of IAC with other reported materials. See DOI: 10.1039/d0en01003k |
| This journal is © The Royal Society of Chemistry 2021 |