One-step synthesis of carbon nitride nanobelts for the enhanced photocatalytic degradation of organic pollutants through peroxydisulfate activation†
Xiaoshan
Zheng
a,
Zhongquan
Wang
a,
Tiansheng
Chen
a,
Jie
Ran
a,
Yuliang
Wu
a,
Cuiwen
Tan
a,
Qianxin
Zhang
b,
Ping
Chen
a,
Fengliang
Wang
c,
Haijin
Liu
d,
Wenying
Lv
 *a and
Guoguang
Liu
*a and
Guoguang
Liu
 *a
*a
aSchool of Environmental Science and Engineering, Guangdong University of Technology, Guangzhou, 510006, China. E-mail: lvwy612@163.com; liugg615@163.com; Fax: +86 13538982812; Fax: +86 2039322548; Tel: +86 20 39322547 Tel: +86 20 39322547
bSchool of Environment, Tsinghua University, Beijing, 100084, China
cState Key Laboratory of Pulp and Paper Engineering, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640, China
dKey Laboratory for Yellow River and Huaihe River Water Environment and Pollution Control, School of Environment, Henan Normal University, Xinxiang 453007, China
First published on 25th November 2020
Abstract
The progress of high-efficiency photocatalysts and the suppression of photoinduced charge recombination remain the primary goals for practical applications. In this study, we demonstrate the convenient synthesis of graphite carbon nitride nanobelts (CNNB) for the enhanced photocatalytic degradation of organic contaminants through peroxydisulfate (PDS) activation. In operation, the PDS serves as an electron acceptor, whereas the organic pollutants acts as hole catchers, thus achieving an external dual transmission mechanism. Consequently, the CNNB/PDS system shows outstanding photocatalytic performance for the removal of sulfamethazine (SMT) under blue-LED light irradiation, which was ∼17- and ∼5 times more efficient than pristine g-C3N4/PDS and CNNB, respectively. Furthermore, a potential SMT degradation pathway was deduced based on the detection of degradation intermediates and theoretical calculations. Finally, reactions looking at several influencing factors indicated that the CNNB/PDS system could be used far more productively for the removal of SMT under ambient aqueous conditions. Hence, this synergistic external dual transmission mechanism process might serve as a promising technology for the removal of organic pollutants.
Environmental significancePharmaceuticals and personal care products (PPCPs) are a class of recalcitrant organic pollutants that have attracted increasing concern, as they can affect humans and aquatic organisms by inducing hormonal disorders, toxicity, and antibiotic resistance. Photocatalysis is considered to be an ideal green and sustainable technology toward addressing global scale environmental pollution. However, developing efficient photocatalysts and suppressing photoinduced charge recombination is still a long-term goal for practical application. Herein, we demonstrate the one-step synthesis of carbon nitride nanobelts (CNNB) for the enhanced photocatalytic degradation of organic pollutants through peroxydisulfate (PDS) activation, in which the PDS acts as an electron acceptor and the organic pollutants as a hole catcher, thus achieving an external dual transmission mechanism. Moreover, our research might extend the promising applications of this organic polymer semiconductor as an efficient photocatalyst for use in water purification and further analyze the mechanism of the photocatalytic degradation of PPCPs. |
1 Introduction
For a few decades, the release of pharmaceutical and personal care products (PPCPs) into the environment has been considered to be an ever-escalating contamination issue, resulting from their inadequate processing by conventional sewage treatment systems.1,2 As a typical representative of PPCPs, sulfonamide antibiotics (SAs) are extensively used in human therapeutics and the farming industry due to their high chemical stability, low prices, and broad antibiotic activity.3 However, due to their poor metabolism, SAs have been detected in manure, which is then transported to the ambient waterway matrix to threaten human and ecosystem health.4 As a typical representative of SAs, sulfamethazine (SMT) is frequently employed in the veterinary industry, where the concentrations detected in surface water range from the μg L−1 to ng L−1.5 Despite these relatively low concentrations, long-term exposure to sulfamethazine antibiotics may induce antibiotic-resistant genes, which pose a considerable menace to human health and surrounding ecosystems.6 Consequently, efficient and economical water remediation technology for the removal of SAs are urgently required.Various technologies have been used to remove such pollution from wastewater and surface water, such as biotechnology, the Fenton reaction, ozone, and photoelectrochemical processes, which are time-consuming, high in cost and require complex operation.7–9 Semiconductor-based photocatalytic techniques toward the establishment of green, sustainable energy, and environmental remediation have garnered widespread attention due to their excellent potential and high efficiency.10–12 Graphite-like carbon nitride (g-C3N4), consisting of earth-abundant and environmentally compatible non-metallic elements, has been developed as a promising photocatalyst for hydrogen production, the reduction of carbon dioxide, and the remediation of aqueous environments, due to its visible light absorption and appropriate band structure.13–15 Nevertheless, the photocatalytic performance of bulk g-C3N4 is extremely limited by its poor electrical conductivity, marginal specific surface area, and rapid recombination of electron–hole pairs.16,17
To address these shortcomings, several strategies, including the construction of heterostructures,18 surface loading of noble metals,19 heteroatom doping,20 and morphology control,21 have been developed. It is well known that morphology design is a good strategy for ameliorating the photocatalytic performance of g-C3N4, due to its excellent structural controllability. The use of hard and soft templates are the most common approaches. However, in the case of the hard template approach, toxic etchants such as NH4HF2 or HF are typically required, which makes the process very hazardous.22,23 For the soft template approach, the synthesized samples are non-uniform and uncontrollable due to a significant amount of gas that is released during the high thermal polymerization process.24 Alternatively, a template-free approach at low-temperature has attracted a lot attention due to its simple operation, cost effectiveness, good controllability, and environmental compatibility.25,26 For instance, Cui et al. synthesized g-C3N4 nanobelts (CNNB)27 through a template-free solvothermal method and found that they exhibited excellent photocatalytic capacity for water splitting. The enhanced photocatalytic performance of CNNB for H2 evolution is due to its high specific surface area and intensive light utilization. In addition, Xia et al. reported a belt-like ordered crystalline carbon nitride, which exhibited excellent ability in photoreducing CO2 to hydrocarbon fuels.28 We therefore propose that the CNNB has strong potential for use in wastewater treatment applications.
In recent decades, hybrid advanced oxidation processes (HAOPs) (where two or more AOPs are used synchronously) have shown great potential for the removal of recalcitrant organic contaminants due to significant synergies.29–31 As one of the most promising HAOPs, the heterogeneous hybrid photocatalysis combined peroxydisulfate (PDS) activation process has been extensively reported in the recent literature.32,33 The integration of PDS into photocatalysts not only generates additional radicals but can also capture photogenerated electrons in their conduction bands, which is conducive to facilitating electron–hole pair separation. As a representative example, Gao et al.34 claimed that persulfate can be effectively activated by MIL-53(Fe) to degrade acid orange 7 in aqueous solution using a visible light emitting diode (LED) light as a light source. However, to the best of our knowledge, research focused on the utilization of CNNB to activate PDS for the photocatalytic degradation of SAs remains limited.
On the basis of these premises, we constructed a facile template-free solvothermal synthesis method to produce carbon nitride nanobelts (CNNB), which exhibit the efficient activation of PDS for SMT degradation under blue-LED light irradiation. The morphological structure, optical and electrochemical properties of the photocatalysts were explored in detail to elucidate the improvement in the photocatalytic properties. The synergistic effects of the CNNB/PDS/blue-LED system are comprehensively investigated and explained. Furthermore, factors that affect the removal of SMT are examined, such as the PDS dosage, catalyst dosage, initial pH, and constituents of the different water samples.
The reactive species originating from the photocatalytic processes were identified by electron spin resonance (ESR) measurements and trapping experiments. Subsequently, SMT transformation pathways were deduced from the detection of intermediates via Thermo Scientific Ultimate 3000 RSLC High Resolution liquid chromatography, coupled with Q Exactive Orbitrap accurate mass spectrometry (HRAM LC-MS/MS). Simultaneously, frontier electron density calculations were performed to predict the reactive sites. This work aims to expand the high-performance applications of excellent CNNB as an efficient photocatalyst for environmental water remediation, and the photocatalytic PPCP degradation mechanisms are further analyzed.
2 Experimental methods
2.1 Preparation of the photocatalysts
The g-C3N4 nanobelts (CNNB) were synthesized via a solvothermal approach. Specifically, 8 mmol of melamine and 16 mmol of cyanuric chloride were intensively mixed in 60 mL of acetonitrile over 24 h of magnetic stirring, after which the as-prepared solution was placed into a 100 mL Teflon-lined autoclave. The autoclave was sealed and maintained at 180 °C for 24 h in an electric oven under autogenous pressure. After cooling to room temperature, the obtained products were filtered and sequentially washed with deionized water and absolute ethanol several times and dried at 60 °C overnight. Bulk g-C3N4 was synthesized using a thermal polymerization method. Typically, 3 g of melamine was introduced into a high-quality alumina crucible with a cover, followed by heating in a muffle furnace at a fixed temperature of 550 °C for 4 h at a heating rate of 5 °C min−1. After cooling to room temperature, the obtained bulk sample was ground to a powder. The details of sample characterization are shown in Text S1† (including the instrument models and parameters).2.2 Experimental procedure and analyses
Sulfamethazine (SMT) was chosen to assess the photocatalytic capacity of the catalyst. As shown in Fig. S1,† the photocatalytic experiments were conducted using a custom fabricated reactor. A 9 W LED lamp was utilized as the light source, which emitted light primarily at 450 ± 5 nm. Typically, an aqueous solution of SMT (50 mL, 10 mg L−1) was decanted into a 50 mL beaker, then a certain quantity of PDS was added along with the photocatalyst (0.02 g). Prior to light exposure, the suspensions were magnetically stirred for 30 min in the dark to achieve adsorption and dissociation equilibrium.At specific time intervals, 1 mL volumes of the reaction solution were extracted and immediately added to an excess of sodium thiosulfate, after which the mixed solutions were filtered through 0.45 μm Millipore filters to eliminate the photocatalyst. The SMT concentrations in the solutions were detected via high-performance liquid chromatography (HPLC, LC-20A, Shimadzu, Japan), with the details discussed in Text S2 in the ESI.†
2.3 Determination of the reactive species
Typically, scavenger-quenching experiments were conducted to explore the reactive species (RS) produced during the SMT catalytic degradation process. Na2C2O4, K2Cr2O7, 4-hydroxy-2,2,6,6-tetramethylpiperidinyloxy (TEMPOL), and tertiary butanol (TBA) were employed as photogenerated hole (h+), photogenerated electron (e−), superoxide iron radical (O2˙−), and hydroxyl radical (˙OH) scavengers, respectively. Furthermore, ethanol (EtOH) was employed as a scavenger for both hydroxyl (˙OH) and sulfate (SO4˙−) radicals, whereas NaN3 worked as a scavenger for hydroxyl radicals (˙OH) and singlet oxygen (1O2). ESR signals were recorded using a Bruker JES FA200 spectrometer using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and 4-oxo-2,2,6,6-tetramethylpiperidine (TEMP) as the spin-trap reagents.2.4 Identification of the photocatalytic byproducts
The photocatalytic degradation byproducts of SMT were detected using high-resolution liquid chromatography (Thermo Scientific Ultimate 3000 RSLC), coupled with HRAM LC-MS/MS (Q Exactive Orbitrap), which is detailed in Text S3 of the ESI.†3 Results and discussion
3.1 Characterization of the photocatalysts
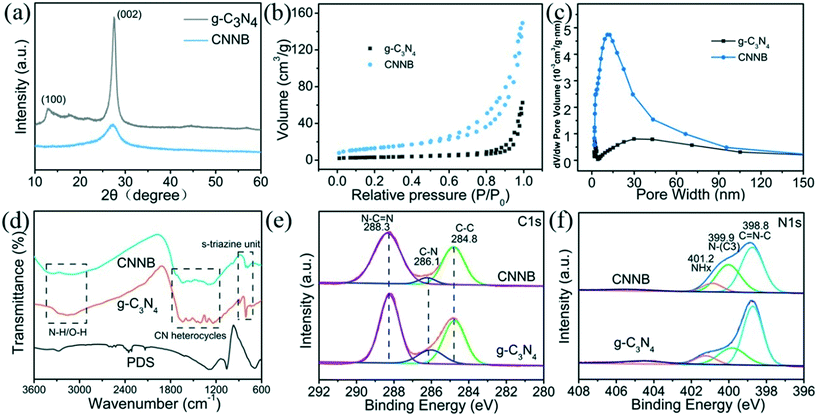
Scanning electron microscopy (SEM) was employed to observe the morphologies of the as-prepared samples. In Fig. 1a, pristine g-C3N4 synthesized using a polycondensation method displays an obvious flake-like structure with a smooth surface. In contrast, the template-free solvothermal synthesized CNNB shows a nanobelt structure with a width of ∼50 nm, as shown in Fig. 1b. The uniform nanobelt structure was further verified by transmission electron microscopy (TEM), as shown in Fig. 1d. Subsequently, the thickness of CNNB was determined by atomic force microscopy (AFM), and the AFM image and the corresponding height distribution profile are presented in Fig. S2.† As shown in Fig. S2,† the average thickness of CNNB is around 2 nm. Besides this, energy-dispersive X-ray spectroscopy (EDS) mapping images (Fig. 1e) reveal the main C, N, and O components and their uniform distribution within the CNNB.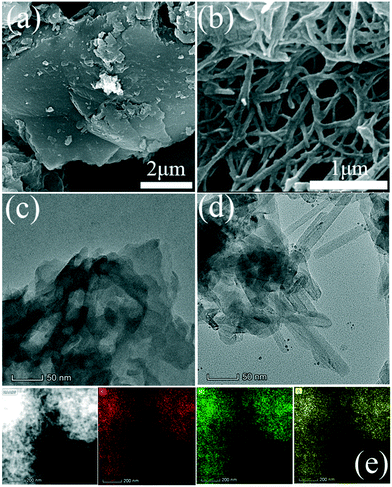 | ||
| Fig. 1 SEM images of (a) pristine g-C3N4 and (b) CNNB. TEM images of (c) pristine g-C3N4 and (d) CNNB. EDS mapping images of (e) CNNB. | ||
The crystal phases of the pristine g-C3N4 and CNNB were verified by X-ray powder diffraction (XRD). As shown in Fig. 2a, both g-C3N4 and CNNB reveal a typical reflection at 27.4°, which were indexed as the (002) planes of g-C3N4, showing the stacking of the conjugated aromatic structures. However, the CNNB peaks are much weaker and broader than those of pristine g-C3N4, which indicates the relatively irregular stacking of the tri-s-triazine units.14 The peak at ∼13.2° can be attributed to the in-plane tri-s-triazine units, the signals of which are too weak to be observed in the CNNB pattern due to the existence of several disordered crystal structures.25
The specific surface areas and porosities of photocatalysts are critical factors for the photocatalytic degradation of organic pollutants. The N2 adsorption–desorption isotherms for g-C3N4 and CNNB exhibit typical type IV with H3-type hysteresis loops, which indicates the presence of mesopores in both photocatalysts (Fig. 2b). The specific surface area of CNNB was measured to be 47.09 m2 g−1, which was higher than that of pristine g-C3N4 (11.05 m2 g−1). The pore size distribution curve (Fig. 2c) verifies the mesoporous structure of CNNB, which is in good agreement with the results obtained from SEM and TEM. These results are consistent with the unique nanobelt structure, which not only provides additional reaction sites, but also facilitates interfacial charge transfer. Based on the above characterization experiments, we successfully synthesized loose porous carbon nitride nanobelts through a facile template-free solvothermal method.
The molecular structures of the as-prepared samples were investigated by Fourier-transform infrared (FTIR) spectroscopy. As shown in Fig. 2d, pristine g-C3N4 and CNNB show similar IR absorption bands, which suggests the conservation of the tri-s-triazine-based structure in the CNNBs. A series of peaks at 3000–3500, 1200–1650, and 807 cm−1 can be attributed to N–H or O–H stretching vibrations, typical CN heterocycle stretching modes, and bending vibrations of the tri-s-triazine units, respectively.35 However, the bands at 1200–1650 cm−1 exhibit a lower absorption intensity in contrast to the FTIR spectrum of pristine g-C3N4, indicating incomplete condensation,26 which was identified from the XRD results.
The surface chemical states and elemental construction of the pristine g-C3N4 and CNNB were probed by X-ray photoelectron spectroscopy (XPS). The high-resolution C 1s spectrum (Fig. 3a) for both pristine g-C3N4 and CNNB exhibits three peaks centered at binding energies of 284.8, 286.1, and 288.3 eV, representing adventitious hydrocarbons (C–C), sp3-coordinnated carbon bonds (C–N), and sp2-bonded carbon (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), respectively.10,13 For the N 1s spectra (Fig. 3b), the peaks at 398.8, 399.9, and 401.2 eV correspond to bicoordinated nitrogen atoms (C
N), respectively.10,13 For the N 1s spectra (Fig. 3b), the peaks at 398.8, 399.9, and 401.2 eV correspond to bicoordinated nitrogen atoms (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C), tricoordinated nitrogen atoms (N–(C)3), and NHx groups in the heptazine framework, respectively.13 According to the FTIR and XPS results, we concluded that the CNNB possesses an aromatic CN framework similar to that of g-C3N4, which is composed of heptazine repeating units.
N–C), tricoordinated nitrogen atoms (N–(C)3), and NHx groups in the heptazine framework, respectively.13 According to the FTIR and XPS results, we concluded that the CNNB possesses an aromatic CN framework similar to that of g-C3N4, which is composed of heptazine repeating units.
3.2 Optical and electrochemical properties
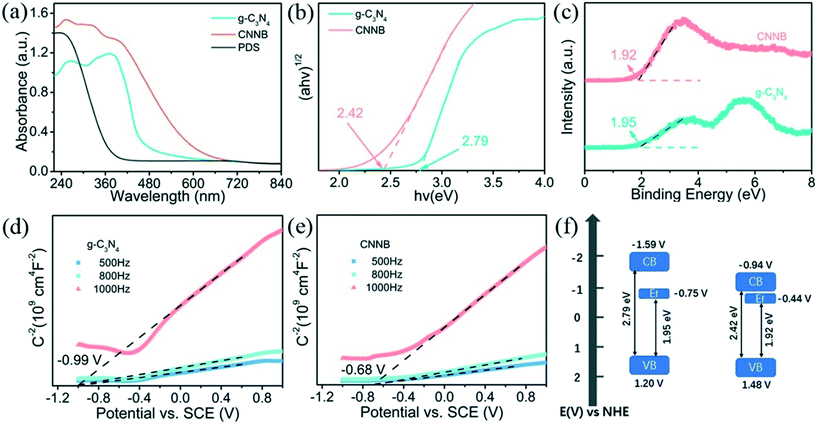
The optical absorption and energy bandgap of the pristine g-C3N4 and CNNB were analyzed by UV-vis diffuse reflectance spectroscopy (Fig. 3a and b). As shown in Fig. 3a, the pristine g-C3N4 exhibits an absorption edge at ∼450 nm, which is consistent with previous reports.36 Compared with pristine g-C3N4, the CNNB exhibits an obvious red-shift from 450 to 600 nm. The unique structure of the CNNB with the multiple-reflection effect of incident light might be responsible for the overall improvement in the light absorption intensity. The bandgaps derived from Tauc plots (Fig. 3b) using the Kubelka–Munk function were 2.79 and 2.42 eV for the pristine g-C3N4 and CNNB, respectively.Valence band X-ray photoelectron spectroscopy (VB-XPS) and Mott–Schottky measurements were used to investigate the energy band structures of the as-prepared samples. The Mott–Schottky experiments were performed in 0.5 M Na2SO4 in the dark at frequencies of 1000, 800, and 500 Hz. Both the pristine g-C3N4 and CNNB exhibit extension lines with positive slopes, which indicates that they are n-type semiconductors. As shown in Fig. 3d and e, the flat band potentials of the pristine g-C3N4 and CNNB were measured to be −0.99 and −0.68 V (vs. SCE), corresponding to −0.75 and −0.44 V (vs. NHE), respectively. As shown in previous reports, the Fermi level (Ef) of n-type semiconductors is approximately equal to the flat band potential.37 Hence, the Ef values of the g-C3N4 and CNNB are −0.75 and −0.44 V (vs. NHE).
According to the VB-XPS spectra (Fig. 3c), the energy gaps between the Ef and valence band (VB) are 1.95 and 1.92 V (vs. NHE) for the pristine g-C3N4 and CNNB, respectively. Hence, the VB positions for the pristine g-C3N4 and CNNB were calculated to be 1.20 and 1.48 V. Furthermore, taking into account the Eg of the samples, the conduction band positions of the pristine g-C3N4 and CNNB could be calculated to be −1.59 and −0.94 V (CB = VB − Eg), respectively. The energy band alignments of the g-C3N4 and CNNB are schematically illustrated in Fig. 3f.
3.3 Photocatalytic activity
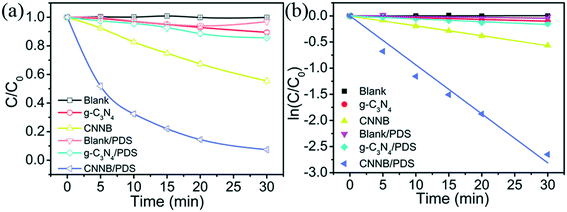
Moreover, the photocatalytic activities of the as-prepared samples were assessed in terms of the removal of SMT antibiotics under 9 W blue-LED light irradiation. Initially, the adsorption experiments were performed under dark conditions, and the pristine g-C3N4 and CNNB showed no obvious adsorption of SMT (Fig. S3†). As seen in Fig. 4a, PDS alone is incapable of oxidizing the SMT under blue-LED irradiation. After 30 min of blue-LED irradiation, the SMT degradation efficiency of the pristine g-C3N4 was 10.5%, whereas the CNNB showed a relatively higher degradation efficiency (44.5%). A degradation efficiency of 14.3% was achieved with the g-C3N4/PDS system, which indicated the moderate catalytic activity of the pristine g-C3N4 for the activation of PDS under blue-LED light irradiation.Conversely, the SMT degradation efficiency was significantly enhanced in the CNNB/PDS system, as demonstrated by a 92.6% degradation efficiency, which was achieved within 30 min of blue-LED exposure. As shown in Fig. 4b, the photocatalytic degradation of SMT follows pseudo first-order kinetics. The degradation kinetic constants of the g-C3N4, CNNB, g-C3N4/PDS, and CNNB/PDS under blue-LED irradiation were determined to be 0.0033, 0.0189, 0.0055, and 0.0937 min−1, respectively.
It is obvious that the SMT removal rate of the CNNB/PDS system is ∼17 fold higher than that of the pristine g-C3N4/PDS system under blue-LED irradiation. These results verify that the unique nanobelt structure of CNNB plays a key role in improving the photocatalytic performance. The high specific surface areas of the CNNB translates to additional reactive sites, while the smaller bandwidth facilitates the migration of photogenerated electrons and holes to the photocatalyst surface to participate in the reaction. This is beneficial for the PDS and the SMT in the capturing of photogenerated electrons and holes, respectively, thus achieving an external dual transmission mechanism. This external dual transmission mechanism, combined with the augmented separation efficiency of photogenerated electron–hole pairs leads to an enhancement in the SMT degradation efficiency.
3.4 Solution matrix effects
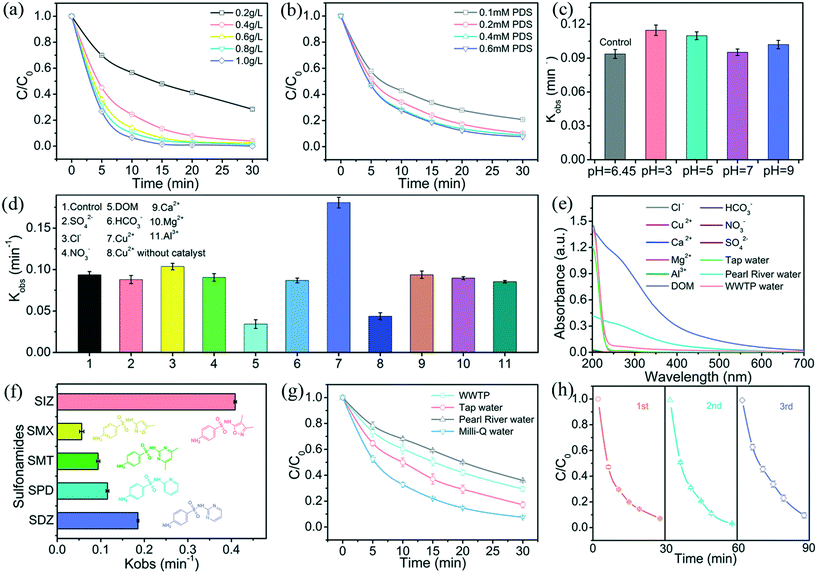
Several experiments were conducted to investigate the effects of the reaction parameters (e.g., catalyst dosage, PDS concentration, initial pH, and water quality constituents) on the removal of SMT via the g-C3N4/PDS system. As shown in Fig. 5a, the SMT degradation efficiency was gradually enhanced with an increase in the catalyst dosage, from 0.2 to 1 g L−1, and its degradation kinetic constant increased from 0.0445 to 0.2523 min−1. This originates from the fact that higher catalyst dosages not only provide additional active sites but also generate extra electrons and holes, resulting in an enhanced SMT degradation efficiency.The effects of PDS concentrations on the removal of SMT were investigated, as illustrated in Fig. 5b. The degradation kinetic constants of SMT are 0.0703, 0.0932, 0.1063, and 0.1152 min−1 when the PDS concentrations are 0.1, 0.2, 0.4, and 0.6 mM, respectively. With increased PDS concentrations (from 0.1 to 0.4 mM) there is a significant enhancement in the degradation of SMT, which is likely due to the higher PDS concentration, which facilitates charge separation and generates additional reactive radicals. However, the degradation kinetic constants appear to attain a plateau when the PDS concentration ranges from 0.4 to 0.6 mM. This might be explained by the fact that photogenerated electrons are stable due to the constant photocatalyst dosage and light intensity, which is not enough to efficiently activate the PDS at a relatively higher concentration.
Generally, pH is considered to be a critical factor in the efficiency of PPCP degradation due to its potential for changing its states. Experiments were carried out to explore the effects of different initial pH levels on SMT degradation via the CNNB/PDS system. The pH of the unadjusted SMT solution was measured to be 6.45. As shown in Fig. 5c, the CNNB/PDS system demonstrates excellent efficacy for SMT degradation over a broad pH range. A significant improvement in the degradation kinetic constant is observed when the initial pH is 3 and 5 (0.1147 min−1 and 0.1098 min−1, respectively), in contrast to pH 6.45, (0.0937 min−1).
Furthermore, at pH 7 and 9, the degradation kinetic constants are 0.0952 and 0.102 min−1, respectively, and still maintain a high level of degradation efficiency. Overall, the effective removal of SMT over a wide pH range confirms the suitability of the CNNB/PDS system to practically alleviate the concerns about the presence of PPCPs under ambient aqueous conditions.
The effects of constituents in the different water samples (inorganic anions, cations, and organic matters) on the degradation efficiency of SMT are shown in Fig. 5d. With the addition of SO42−, NO3−, and HCO3− the degradation kinetic constants for SMT are 0.088, 0.0905, and 0.0868 min−1, respectively, which were not obviously affected. However, the presence of dissolved organic matter (DOM) had a significant effect on degradation, with a low degradation kinetic constant of 0.0342 min−1. The repression of the SMT degradation by DOM might be ascribed to the competition with the reactive species and light-screening effects (see Fig. 5e). As mentioned in a previous report,38 Cl− can be converted to active chlorine species when oxidized by SO4˙−; thus, a slightly improved effect could be observed in the presence of Cl−. As for cations (Mg2+, Ca2+, Al3+), no obvious influence in the degradation of SMT was observed. It is worth noting the significant positive effect that Cu2+ has on the removal of SMT. As illustrated in Fig. 5d, in the presence of Cu2+, the degradation kinetic constant of SMT is 0.1807 min−1, which is much higher than that of the control solution (0.0937 min−1). To further investigate the effects that Cu2+ have on the degradation of SMT, a control system for Cu2+/PDS under blue-LED light irradiation was established, where obvious SMT degradation was observed. Therefore, the synergistic effects between Cu2+ and the CNNB/PDS system initiate an obvious enhancement in the degradation efficiency, and this phenomenon warrants further investigation.
Moreover, to verify the universality and practical feasibility of the CNNB/PDS system, its performance for the removal of other sulfonamides, including sulfisoxazole (SIZ), sulfamethoxazole (SMX), sulfapyridine (SPD) and sulfadiazine (SDZ), was also evaluated. As shown in Fig. 5f, it is clear that all of the selected sulfonamides undergo remarkable degradation. Under the CNNB/PDS/blue-LED system, the degradation kinetic constants of SIZ, SMX, SPD, SDZ, and SMT are 0.4088, 0.0562, 0.1154, 0.1854, and 0.0937 min−1, respectively.
The removal rate of SIZ is the highest and ∼4.3 fold that of SMT, which indicates that 3,4-dimethyl-isoxazole is the most unstable structure among the selected sulfonamides. Moreover, the degradation rate of SDZ is higher than that of SPD, which indicates that the pyrimidine structure is more easily destroyed than that of pyridine, which is similar to the results of a previous study.39 These results indicate that the CNNB/PDS system is a promising technology for the removal of sulfonamides in aqueous media.
To further investigate the feasibility of the use of the CNNB/PDS system in practical applications, the elimination of SMT was evaluated in various ambient water matrices (ultrapure water, tap water, wastewater effluent water, and Pearl River water). As shown in Fig. 5g, the removal rates for SMT in ultrapure water, tap water, wastewater effluent water, and Pearl River water are 92.45%, 82.79%, 70.65%, and 64.01%, respectively. Not surprisingly, ultrapure water shows the highest removal rate, as there is no interference from inorganic anions, cations, and organic species.40 A slight inhibition was observed in tap water, which might be ascribed to co-existing anions that cause some scavenging effects. However, the degradation of SMT is significantly disrupted in wastewater effluent and Pearl River water, where the removal rate of SMT decreases by 21.8% and 28.44%, respectively.
The presence of complex ions and organic species in wastewater effluent and Pearl River water might cause antagonistic effects and a light screening effect (see Fig. 5e) on the degradation of SMT. In spite of the SMT removal rates exhibited due to the differences between the various ambient water matrices, the CNNB/PDS system still exhibits strong potential for use in practical applications.
The stability and reusability of the CNNB in the degradation of SMT were examined, which is extremely important for photocatalysts from the perspective of practical applications. Typically, for each recycling run, the photocatalyst was recovered through centrifugation at 4000 rpm, repeatedly washing with absolute ethanol and deionized water, after that drying at 60 °C overnight. As shown in Fig. 5h, there is no distinct inactivation of the photocatalyst after three cycles. Moreover, the XRD patterns, FTIR spectra, XPS spectra and SEM images of the CNNB prior to and following the photocatalytic reaction show almost no changes (Fig. S4†). These results verify the good recyclability and outstanding structural stability of the photocatalyst, which indicates its strong potential for use in practical applications for environmental remediation.
3.5 Photoelectrochemical and optical properties
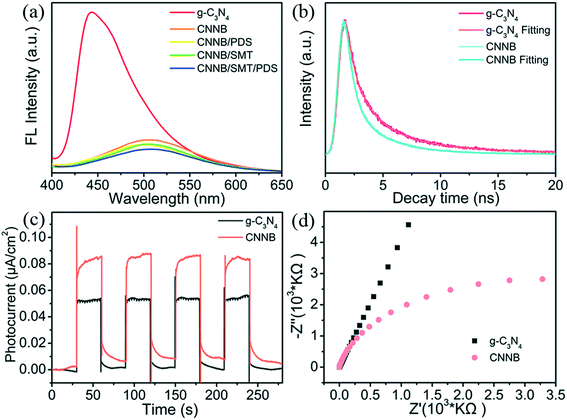
Several characterization methods were performed to confirm the outstanding PDS activation of CNNB. The PL spectra reveal the recombination and separation behavior of photoinduced charge carriers in the as-prepared samples.39 As illustrated in Fig. 6a, all of the PL spectra were recorded at an excitation wavelength of 380 nm. Substantial PL quenching was detected in the CNNB spectrum compared with the spectrum of pristine g-C3N4, which indicates suppressed photoinduced charge recombination and enhanced photocatalytic activity. Moreover, obvious red-shifts in the PL maximum between the CNNB and pristine g-C3N4 were correlated with reduced bandgap energies, which are evident in the UV-vis diffuse reflectance spectra.41Furthermore, the PL intensities of the CNNB/PDS, CNNB/SMT, and CNNB/PDS/SMT systems are all lower than that of CNNB, particularly the CNNB/PDS/SMT system, which possesses the weakest emission peak. These results confirm that both the PDS and the pollutants exhibit robust synergistic promotional effects. Moreover, to further reveal the photophysical properties of the radiative charge pairs, time-resolved fluorescence decay spectroscopy was carried out (Fig. 6b). The fluorescence decay curves of the solid samples were matched using the triple-exponential function:42
R(t) = B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−1/τ1) + B2 exp(−1/τ1) + B2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−1/τ2) + B3 exp(−1/τ2) + B3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−1/τ3) exp(−1/τ3) | (1) |
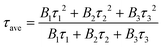 | (2) |
The τave values for pristine g-C3N4 and CNNB are 3.70 and 2.29 ns, respectively. The remarkably shortened singlet exciton lifetime might be due to the markedly accelerated photoexciton dissociation in CNNB, which indicates its high photocatalytic activity.
To further illustrate the charge carrier behavior, transient photocurrent response curves and electrochemical impedance spectroscopy (EIS) were measured and recorded and the results are shown in Fig. 6c and d. As anticipated, CNNB exhibits remarkably strengthened photocurrent intensity compared to pristine g-C3N4 under blue-LED irradiation, manifesting the higher separation and transfer efficiencies of the photoinduced charges. Meanwhile, EIS tests were performed to obtain excess data on the charge transfer process. As shown in Fig. 6d, a noticeable reduction in the arc radius of the CNNB was detected, which indicates that the charge transfer resistance of the CNNB is much lower than that of pristine g-C3N4. All of the results reveal that the uniform structure CNNB promotes the transport of photogenerated charge carriers, while suppressing the recombination of radiative charges, which enhances its photocatalytic activity.
3.6 Potential photocatalytic mechanism
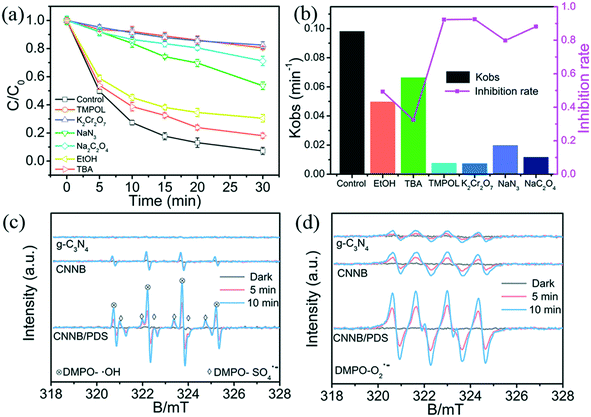
To explore the photocatalytic mechanism of the CNNB/PDS system, the reactive species (RSs) were identified, mainly by means of scavenger-quenching experiments using Na2C2O4 (for h+), EtOH (for ˙OH and SO4˙−), TBA (for ˙OH), NaN3 (for 1O2 and ˙OH), TMPOL (for O2˙−), and K2Cr2O7 (for e−).31,40Fig. 7a and S5a† exhibit the different influences on the removal of SMT under blue LED-driven CNNB/PDS and the CNNB system using various trapping agents, respectively. As illustrated in Fig. 7a, the degradation rate constant of SMT removal via the CNNB/PDS system for the blank control and samples containing EtOH, TBA, TMPOL, K2Cr2O7, NaN3, and Na2C2O4 are 0.0981, 0.0497,0.0663, 0.0075, 0.0073, 0.0198, and 0.0116 min−1, respectively.Furthermore, according to eqn (3)–(7), the inhibition rates of SO4˙−, ˙OH, O2˙−, e−, 1O2, and h+ are calculated as 16.94%, 32.42%, 92.36%, 92.58%, 47.44%, and 88.19% respectively. The experimental results show that O2˙−, e−, and h+ play key roles in the removal of SMT using the CNNB/PDS system under blue LED light illumination.
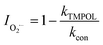 | (3) |
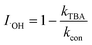 | (4) |
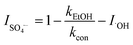 | (5) |
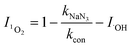 | (6) |
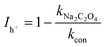 | (7) |
In eqn (3)–(7), I is the inhibition rate of a specified reactive species (e.g., SO4˙−, ˙OH, O2˙−, 1O2, and h+) to the blank control rate constant (kcon) and ki is the rate constant for the removal of SMT by a certain scavenger-quenching agent.
Typically, a sequence of ESR measurements were conducted using DMPO and TEMP as radical spin trapping agents to gain insights into the reactive species. As shown in Fig. 7c–d, there were no ESR signals detected in the dark for the pristine g-C3N4, CNNB, and CNNB/PDS, which suggests that all of the active species were generated during the photocatalytic process. Upon exposure to blue light, a series of signals were observed and increased with prolonged irradiation times. As shown in Fig. 7c, the ESR signals of DMPO–˙OH were observed for CNNB and CNNB/PDS; however, they were not observed for pristine g-C3N4, which may be attributed to its low concentration.
For the CNNB/PDS/blue LED system, the ESR signal of DMPO–˙OH was more intense than that of CNNB, and the ESR signal of DMPO–SO4˙− was observed, indicating that the presence of PDS not only promotes the yield of ˙OH but also generates additional SO4˙−. Furthermore, ESR signals for DMPO–˙O2˙− (Fig. 7d) and TEMP–1O2 (Fig. S6†) were observed for all of the samples, revealing the generation of O2˙ and 1O2. Moreover, CNNB/PDS exhibits more intense ESR signals for both DMPO–O2˙− and TEMP–1O2 than those of pristine g-C3N4 and the CNNB, which testifies that the addition of PDS improves the generation of O2˙− and 1O2.
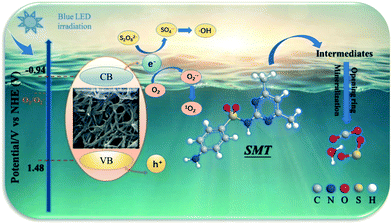
Overall, the enhanced mechanisms of the hybrid advanced oxidation processes can be ascribed to the external dual transmission mechanism. According to the above results, a probable photocatalytic mechanism for the CNNB/PDS/blue-LED system was proposed (Scheme 1). Benefiting from the unique structure of CNNB, its extensive specific surface area provides a significant population of reactive sites, which are beneficial for PDS and SMT to capture photogenerated electrons and holes, respectively, thereby effectively achieving an external dual transmission mechanism.
Under blue LED light exposure, photogenerated electron–holes pairs were formed (eqn (8)). Since the CB potential of CNNB is −0.94 V, which is lower than the standard reduction potential of O2/O2˙− (−0.33 V vs. NHE), this indicates that photogenerated electrons (e−) can be captured by dissolved oxygen to form O2˙− (eqn (9)).43 Moreover, e− can also be captured by PDS to generate SO4˙−, which further enhances the separating efficiency of the photogenerated electron–hole pairs (eqn (10)).
As the valance band potential of CNNB (+1.48 V) is more negative than the potential of ˙OH/OH− (+1.99 V vs. NHE), the h+ from CNNB cannot oxidize OH− to form ˙OH.44 Therefore, the ˙OH detected by ESR likely originates from O2˙− and SO4˙− according to the following eqn (11)–(14).39,40,45 Meanwhile, 1O2 is produced because of the reaction that occurs between O2˙− and h+.17 In summary, the following reactive species contribute to the removal of SMT under the CNNB/PDS/blue LED system.
| CNNB + hv → h+ + e− | (8) |
| O2 + e− → O2˙− | (9) |
| S2O82− + e− → 2SO4˙− | (10) |
| 2H+ + O2˙− → HO2˙ | (11) |
| 2HO2˙ → H2O2 + 1O2 | (12) |
| H2O2 + e− → ˙OH | (13) |
| SO4˙− + OH− → ˙OH + SO42− | (14) |
| O2˙− + h+ → 1O2 | (15) |
| O2˙−/h+/1O2/˙OH/SO4˙− + SMT → products | (16) |
3.7 Proposed SMT degradation pathway of the CNNB/PDS system
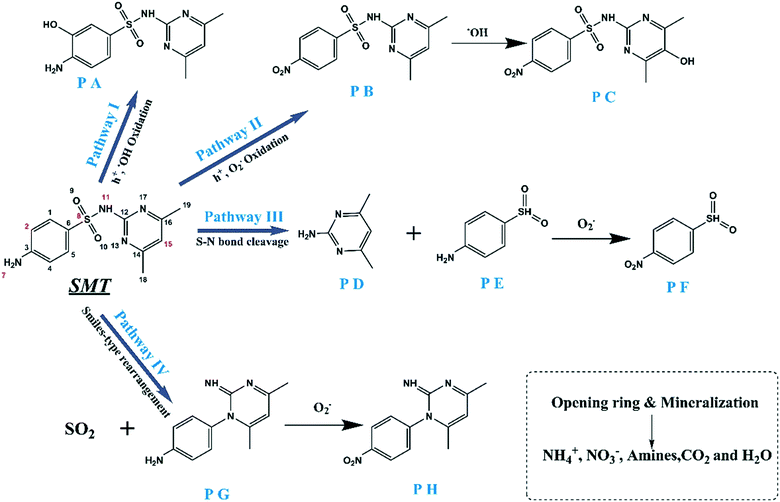
To further investigate the SMT degradation pathway in the CNNB/PDS system, the generated intermediate products were identified using HRAM LC-MS/MS. The detected intermediate products are summarized in Table S1,† which are assigned as products A–H. As reported in our preliminary work,39 the frontier molecular orbital theory (FMOT) of SMT, including the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), was adapted to predict the primary reactive sites for RS attack. Based on these identified intermediate products and FMOT,39 a potential pathway of SMT degradation using the CNNB/PDS system was proposed, as shown in Fig. 8. In general, the pathway of the photocatalytic degradation of SMT can be summarized according to the following four main pathways.In pathway I, degradation product PA is formed through the addition of a hydroxyl radical to the aniline ring, in response to the high-density 2∑(CHOMOri)2 value of C (2), meaning that it is inclined to react with ˙OH. Then, in pathway II, N (7) with a high-density 2∑(CHOMOri)2 value is attacked by h+, which results in the consecutive adduction of amino groups in the aniline ring, to form nitro-substituted SMT (PB).46 In addition, the PB was further attacked by ˙OH to form PC. In the case of pathway III, the cleavage of sulfonamide bonds proceeds to produce 2-amino-4,6 dimethylpyrimidine (PD) and PE commonly found in the oxidation process of SMT.47,48 Furthermore, the PE was further oxidized by RS to form PF. Finally, in pathway IV, a typical Smiles-type rearrangement of SMT proceeds to generate 4-(2-imino-4,6-dimethylpyrimidin-1(2H)-yl)aniline (PG) with an accompanying outflow of SO2.6,49 Meanwhile, the PG is further oxidized to form PH. All of these intermediate products and SMT are then further oxidized by RS, and finally mineralized to NH4+, CO2, H2O, and other small molecules.
4 Conclusion
In summary, we successfully synthesized carbon nitride nanobelts (CNNB) though a one-step solvothermal approach. The CNNB/PDS system shows excellent photocatalytic activity for the degradation of SMT owing to an external dual transmission mechanism, which captures electrons and holes through PDS and organic pollutants, respectively. The degradation rates for SMT using this system are ∼17 and ∼5 times higher than those of pristine g-C3N4/PDS and the CNNB, respectively. The identification of reactive species reveals that extra SO4˙− is generated upon the addition of PDS, whereas O2˙ and h+ play key roles in the CNNB/PDS system under blue LED light exposure. Possible SMT transformation pathways were proposed using HRAM-MS/MS and FMOT. In addition, the versatility and practicability of the CNNB/PDS system were evaluated though various influencing factors. This work details a novel hybrid advanced oxidation process with an external dual transmission mechanism for the efficient treatment of SA-contaminated water.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21677040 and 22076029) and the Guangzhou Municipal Science and Technology Project (No. 201903010080).References
- D. N. Bulloch, E. D. Nelson, S. A. Carr, C. R. Wissman, J. L. Armstrong, D. Schlenk and C. K. Larive, Occurrence of halogenated transformation products of selected pharmaceuticals and personal care products in secondary and tertiary treated wastewaters from southern California, Environ. Sci. Technol., 2015, 49, 2044–2051 CrossRef CAS PubMed.
- O. M. Rodriguez-Narvaez, J. M. Peralta-Hernandez, A. Goonetilleke and E. R. Bandala, Treatment technologies for emerging contaminants in water: a review, Chem. Eng. J., 2017, 323, 361–380 CrossRef CAS.
- K. M. Doretto, L. M. Peruchi and S. Rath, Sorption and desorption of sulfadimethoxine, sulfaquinoxaline and sulfamethazine antimicrobials in Brazilian soils, Sci. Total Environ., 2014, 476, 406–414 CrossRef.
- C. Zhou, C. Lai, D. Huang, G. Zeng, C. Zhang, M. Cheng, L. Hu, J. Wan, W. Xiong, M. Wen, X. Wen and L. Qin, Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven, Appl. Catal., B, 2018, 220, 202–210 CrossRef CAS.
- W. Baran, E. Adamek, J. Ziemiańska and A. Sobczak, Effects of the presence of sulfonamides in the environment and their influence on human health, J. Hazard. Mater., 2011, 196, 1–15 CrossRef CAS PubMed.
- R. Yin, W. Guo, N. Ren, L. Zeng and M. J. W. R. Zhu, New insight into the substituents affecting the peroxydisulfate nonradical oxidation of sulfonamides in water, Water Res., 2020, 171, 115374 CrossRef CAS PubMed.
- H. Dong, G. Zeng, L. Tang, C. Fan, C. Zhang, X. He and Y. He, An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures, Water Res., 2015, 79, 128–146 CrossRef CAS.
- M. Li, M. Sun, H. Dong, J. Zhang, Y. Su and Z. Qiang, Enhancement of micropollutant degradation in UV/H2O2 process via iron-containing coagulants, Water Res., 2020, 172, 115497 CrossRef CAS PubMed.
- M. Jia, Z. Yang, H. Xu, P. Song, W. Xiong, J. Cao, Y. Zhang, Y. Xiang, J. Hu, C. Zhou, Y. Yang and W. Wang, Integrating N and F co-doped TiO2 nanotubes with ZIF-8 as photoelectrode for enhanced photo-electrocatalytic degradation of sulfamethazine, Chem. Eng. J., 2020, 388, 124388 CrossRef CAS.
- M. Zhu, S. Kim, L. Mao, M. Fujitsuka, J. Zhang, X. Wang and T. Majima, Metal-Free Photocatalyst for H2 Evolution in Visible to Near-Infrared Region: Black Phosphorus/Graphitic Carbon Nitride, J. Am. Chem. Soc., 2017, 139, 13234–13242 CrossRef CAS PubMed.
- S. Wang, B. Y. Guan and X. W. D. Lou, Construction of ZnIn2S4-In2O3 Hierarchical Tubular Heterostructures for Efficient CO2 Photoreduction, J. Am. Chem. Soc., 2018, 140, 5037–5040 CrossRef CAS PubMed.
- F. Wang, Y. Feng, P. Chen, Y. Wang, Y. Su, Q. Zhang, Y. Zeng, Z. Xie, H. Liu, Y. Liu, W. Lv and G. Liu, Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C 3 N 4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination, Appl. Catal., B, 2018, 227, 114–122 CrossRef CAS.
- H. Yu, R. Shi, Y. Zhao, T. Bian, Y. Zhao, C. Zhou, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Alkali-Assisted Synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution, Adv. Mater., 2017, 29, 1605148 CrossRef PubMed.
- J. Fu, B. Zhu, C. Jiang, B. Cheng, W. You and J. Yu, Hierarchical Porous O-Doped g-C3 N4 with Enhanced Photocatalytic CO2 Reduction Activity, Small, 2017, 13, 1603938 CrossRef PubMed.
- Q. Zhang, C. Tan, X. Zheng, P. Chen, M. Zhuo, T. Chen, Z. Xie, F. Wang, H. Liu, Y. Liu, X. Zhang, W. Lv and G. Liu, Dual metal-free polymer reactive sites for the efficient degradation of diclofenac by visible light-driven oxygen reduction to superoxide radical and hydrogen peroxide, Environ. Sci.: Nano, 2019, 6, 2577–2590 RSC.
- T. Chen, Q. Zhang, Z. Xie, C. Tan, P. Chen, Y. Zeng, F. Wang, H. Liu, Y. Liu, G. Liu and W. Lv, Carbon nitride modified hexagonal boron nitride interface as highly efficient blue LED light-driven photocatalyst, Appl. Catal., B, 2018, 238, 410–421 CrossRef CAS.
- X. Zheng, Q. Zhang, T. Chen, Y. Wu, J. Hao, C. Tan, P. Chen, F. Wang, H. Liu, W. Lv and G. Liu, A novel synthetic carbon and oxygen doped stalactite-like g-C3N4 for broad-spectrum-driven indometacin degradation, J. Hazard. Mater., 2020, 386, 121961 CrossRef CAS PubMed.
- S. Hua, D. Qu, L. An, W. Jiang, Y. Wen, X. Wang and Z. Sun, Highly efficient p-type Cu3P/n-type g-C3N4 photocatalyst through Z-scheme charge transfer route, Appl. Catal., B, 2019, 240, 253–261 CrossRef CAS.
- F. Wang, Y. Wang, Y. Feng, Y. Zeng, Z. Xie, Q. Zhang, Y. Su, P. Chen, Y. Liu, K. Yao, W. Lv and G. Liu, Novel ternary photocatalyst of single atom-dispersed silver and carbon quantum dots co-loaded with ultrathin g-C3N4 for broad spectrum photocatalytic degradation of naproxen, Appl. Catal., B, 2018, 221, 510–520 CrossRef CAS.
- G. Zhang, M. Zhang, X. Ye, X. Qiu, S. Lin and X. Wang, Iodine Modified Carbon Nitride Semiconductors as Visible Light Photocatalysts for Hydrogen Evolution, Adv. Mater., 2014, 26, 805–809 CrossRef CAS PubMed.
- Y. Wang, H. Wang, F. Chen, F. Cao, X. Zhao, S. Meng and Y. Cui, Facile synthesis of oxygen doped carbon nitride hollow microsphere for photocatalysis, Appl. Catal., B, 2017, 206, 417–425 CrossRef CAS.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for Friedel-Crafts reaction of benzene, Angew. Chem., Int. Ed., 2006, 45, 4467–4471 CrossRef CAS PubMed.
- A. Vinu, K. Ariga, T. Mori, T. Nakanishi, S. Hishita, D. Golberg and Y. Bando, Preparation and Characterization of Well-Ordered Hexagonal Mesoporous Carbon Nitride, Adv. Mater., 2005, 17, 1648–1652 CrossRef CAS.
- F. Yang, D. Liu, Y. Li, L. Cheng and J. Ye, Salt-template-assisted construction of honeycomb-like structured g-C3N4 with tunable band structure for enhanced photocatalytic H2 production, Appl. Catal., B, 2019, 240, 64–71 CrossRef CAS.
- S. Wang, F. He, X. Zhao, J. Zhang, Z. Ao, H. Wu, Y. Yin, L. Shi, X. Xu, C. Zhao, S. Wang and H. Sun, Phosphorous doped carbon nitride nanobelts for photodegradation of emerging contaminants and hydrogen evolution, Appl. Catal., B, 2019, 257, 117931 CrossRef CAS.
- Q. Gu, Y. Liao, L. Yin, J. Long, X. Wang and C. Xue, Template-free synthesis of porous graphitic carbon nitride microspheres for enhanced photocatalytic hydrogen generation with high stability, Appl. Catal., B, 2015, 165, 503–510 CrossRef CAS.
- Y. Cui, Z. Ding, X. Fu and X. Wang, Construction of conjugated carbon nitride nanoarchitectures in solution at low temperatures for photoredox catalysis, Angew. Chem., Int. Ed., 2012, 51, 11814–11818 CrossRef CAS PubMed.
- P. Xia, M. Antonietti, B. Zhu, T. Heil, J. Yu and S. Cao, Designing Defective Crystalline Carbon Nitride to Enable Selective CO2 Photoreduction in the Gas Phase, Adv. Funct. Mater., 2019, 29(15), 1900093 CrossRef.
- F. Mendez-Arriaga, R. A. Torres-Palma, C. Petrier, S. Esplugas, J. Gimenez and C. Pulgarin, Mineralization enhancement of a recalcitrant pharmaceutical pollutant in water by advanced oxidation hybrid processes, Water Res., 2009, 43, 3984–3991 CrossRef CAS PubMed.
- S. Chakma and V. S. Moholkar, Investigations in Synergism of Hybrid Advanced Oxidation Processes with Combinations of Sonolysis + Fenton Process + UV for Degradation of Bisphenol A, Ind. Eng. Chem. Res., 2014, 53, 6855–6865 CrossRef CAS.
- R. Li, M. Cai, Z. Xie, Q. Zhang, Y. Zeng, H. Liu, G. Liu and W. Lv, Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation, Appl. Catal., B, 2019, 244, 974–982 CrossRef CAS.
- P. Chen, Q. Zhang, L. Shen, R. Li, C. Tan, T. Chen, H. Liu, Y. Liu, Z. Cai, G. Liu and W. Lv, Insights into the synergetic mechanism of a combined vis-RGO/TiO2/peroxodisulfate system for the degradation of PPCPs: Kinetics, environmental factors and products, Chemosphere, 2019, 216, 341–351 CrossRef CAS PubMed.
- L. Zeng, S. Li, X. Li, J. Li, S. Fan, X. Chen, Z. Yin, M. Tadé and S. Liu, Visible-light-driven sonophotocatalysis and peroxymonosulfate activation over 3D urchin-like MoS2/C nanoparticles for accelerating levofloxacin elimination: Optimization and kinetic study, Chem. Eng. J., 2019, 378, 122039 CrossRef CAS.
- Y. Gao, S. Li, Y. Li, L. Yao and H. Zhang, Accelerated photocatalytic degradation of organic pollutant over metal-organic framework MIL-53(Fe) under visible LED light mediated by persulfate, Appl. Catal., B, 2017, 202, 165–174 CrossRef CAS.
- F. Yu, Z. Wang, S. Zhang, H. Ye, K. Kong, X. Gong, J. Hua and H. Tian, Molecular Engineering of Donor–Acceptor Conjugated Polymer/g-C3N4 Heterostructures for Significantly Enhanced Hydrogen Evolution Under Visible-Light Irradiation, Adv. Funct. Mater., 2018, 28, 1804512 CrossRef.
- F. Wang, P. Chen, Y. Feng, Z. Xie, Y. Liu, Y. Su, Q. Zhang, Y. Wang, K. Yao, W. Lv and G. Liu, Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin, Appl. Catal., B, 2017, 207, 103–113 CrossRef CAS.
- H. Huang, K. Xiao, N. Tian, F. Dong, T. Zhang, X. Du and Y. Zhang, Template-free precursor-surface-etching route to porous, thin g-C3N4 nanosheets for enhancing photocatalytic reduction and oxidation activity, J. Mater. Chem. A, 2017, 5, 17452–17463 RSC.
- H. V. Lutze, N. Kerlin and T. C. Schmidt, Sulfate radical-based water treatment in presence of chloride: formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate, Water Res., 2015, 72, 349–360 CrossRef CAS PubMed.
- P. Chen, Q. Zhang, X. Zheng, C. Tan, M. Zhuo, T. Chen, F. Wang, H. Liu, Y. Liu, Y. Feng, W. Lv and G. Liu, Phosphate-modified m-Bi2O4 enhances the absorption and photocatalytic activities of sulfonamide: Mechanism, reactive species, and reactive sites, J. Hazard. Mater., 2020, 384, 121443 CrossRef CAS PubMed.
- P. Chen, L. Blaney, G. Cagnetta, J. Huang, B. Wang, Y. Wang, S. Deng and G. Yu, Degradation of Ofloxacin by Perylene Diimide Supramolecular Nanofiber Sunlight-Driven Photocatalysis, Environ. Sci. Technol., 2019, 53, 1564–1575 CrossRef CAS PubMed.
- W. Iqbal, B. Yang, X. Zhao, M. Waqas, M. Rauf, C. Guo, J. Zhang and Y. Mao, Gaseous bubble-assisted in-situ construction of worm-like porous g-C3N4 with superior visible light photocatalytic performance, Appl. Catal., A, 2019, 573, 13–21 CrossRef CAS.
- T. Chen, Q. Zhang, X. Zheng, Z. Xie, Y. Zeng, P. Chen, H. Liu, Y. Liu, W. Lv and G. Liu, Photocatalyst with a metal-free electron–hole pair double transfer mechanism for pharmaceutical and personal care product degradation, Environ. Sci.: Nano, 2019, 6(11), 3292–3306 RSC.
- Y. Wang, B. Jing, F. Wang, S. Wang, X. Liu, Z. Ao and C. Li, Mechanism Insight into enhanced photodegradation of pharmaceuticals and personal care products in natural water matrix over crystalline graphitic carbon nitrides, Water Res., 2020, 180, 115925 CrossRef CAS PubMed.
- Y. Yang, C. Zhang, D. Huang, G. Zeng, J. Huang, C. Lai, C. Zhou, W. Wang, H. Guo, W. Xue, R. Deng, M. Cheng and W. Xiong, Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation, Appl. Catal., B, 2019, 245, 87–99 CrossRef CAS.
- J. Zhang, X. Zhao, Y. Wang, Y. Gong, D. Cao and M. Qiao, Peroxymonosulfate-enhanced visible light photocatalytic degradation of bisphenol A by perylene imide-modified g-C3N4, Appl. Catal., B, 2018, 237, 976–985 CrossRef CAS.
- S. Cao, Y. Zhang, N. He, J. Wang, H. Chen and F. Jiang, Metal-free 2D/2D heterojunction of covalent triazine-based frameworks/graphitic carbon nitride with enhanced interfacial charge separation for highly efficient photocatalytic elimination of antibiotic pollutants, J. Hazard. Mater., 2020, 391, 122204 CrossRef CAS PubMed.
- G. Li, K. Zhang, C. Li, R. Gao, Y. Cheng, L. Hou and Y. Wang, Solvent-free method to encapsulate polyoxometalate into metal-organic frameworks as efficient and recyclable photocatalyst for harmful sulfamethazine degrading in water, Appl. Catal., B, 2019, 245, 753–759 CrossRef CAS.
- Q. Yang, D. Chen, L. Chu and J. Wang, Enhancement of ionizing radiation-induced catalytic degradation of antibiotics using Fe/C nanomaterials derived from Fe-based MOFs, J. Hazard. Mater., 2020, 389, 122148 CrossRef CAS PubMed.
- J. Gao, C. Hedman, C. Liu, T. Guo and J. A. Pedersen, Transformation of sulfamethazine by manganese oxide in aqueous solution, Environ. Sci. Technol., 2012, 46, 2642–2651 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0en00985g |
| This journal is © The Royal Society of Chemistry 2021 |