Open Access Article
Open Access ArticleParticle characterisation and bioaccessibility of manganese in particulate matter in silico- and ferromanganese smelters†
Stine Eriksen
Hammer
 a,
Torunn
Ervik
a,
Torunn
Ervik
 *a,
Dag G.
Ellingsen
a,
Yngvar
Thomassen
a,
Stephan
Weinbruch
ab,
Nathalie
Benker
b and
Balazs
Berlinger‡
*a,
Dag G.
Ellingsen
a,
Yngvar
Thomassen
a,
Stephan
Weinbruch
ab,
Nathalie
Benker
b and
Balazs
Berlinger‡
 a
a
aNational Institute of Occupational Health, Gydas vei 8, N-0363 Oslo, Norway. E-mail: torunn.ervik@stami.no
bInstitute of Applied Geosciences, Technical University of Darmstadt, Schnittspahnstrasse 9, D-64287 Darmstadt, Germany
First published on 3rd September 2021
Abstract
The aim of this study was to characterise particulate matter (PM) collected in the furnace area during SiMn and high carbon (HC)–FeMn production in terms of single particle analysis and to determine the bioaccessibility of Mn in the PM in a simulated lung fluid. Airborne PM was collected with Sioutas cascade impactors and respirable cyclones in the breathing zone of tappers and crane operators. Stationary samples were collected from the furnace area with a nanoMOUDI cascade impactor and an ESPnano electrostatic particle collector. Individual particles were characterised by scanning and transmission electron microscopy. Bioaccessibility of Mn was studied in terms of the dissolution of Mn in Gamble solution (24 hours leaching at 37 °C) relative to total Mn. Slag particles, alloy fragments, Mn and Fe oxides as well as carbonaceous particles were observed in the size fraction > 1 μm aerodynamic diameter (dae). Thermally generated condensation particles dominated the dae size range of 0.18–1 μm collected from the tapping fumes, while carbonaceous particles dominated the fraction below 0.18 μm. Condensation generated particles from the furnace area of HC–FeMn production were coated with an amorphous Si–O rich surface layer which seemed to hold primary particles together as aggregates. In the same size range, the particles from the furnace area of SiMn production were dominated by spherical condensation particles rich in Si, Mn and O, but without a Si–O rich surface layer. Instead, the Mn oxides were enclosed in an amorphous Si–O rich matrix. The bioaccessibility of Mn was low to moderate (<30%), but higher for SiMn furnace workers (highest median = 23%) than HC–FeMn furnace workers (highest median = 12%). This difference in bioaccessibility was significant for PM with dae up to 2.5 μm, and most pronounced in the dae size range between 0.25 and 1.0 μm. Also, a significantly higher bioaccessibility of Mn was found for PM larger than dae of 0.5 μm collected among crane operators compared to tappers in the HC–FeMn smelter.
Environmental significanceAdverse health effects on the central nervous system caused by manganese exposure depend on the exposure dose, chemical composition and bioavailability of manganese. To investigate this in an occupational setting, the bioaccessibility of manganese in particulate matter has been investigated in terms of dissolution in Gamble's solution. The particulate matter was collected and size fractionated in the breathing zone of workers in manganese smelters. Particle size is important for deposition in the respiratory tract. In addition, individual particles collected using stationary equipment were characterised. Knowledge of physical and chemical properties of particles combined with information on the bioaccessibility is fundamental to understanding exposure–response associations. |
1 Introduction
Studies on rats have shown that exposure to soluble manganese (Mn) results in higher Mn concentration in the brain than exposure to less soluble Mn.1,2 In humans, exposure to Mn is of health concern. Adverse health effects on the central nervous system associated with Mn exposure have been reported in e.g. welders,3–6 miners,7,8 battery industry workers9,10 as well as workers in the Mn alloy producing industry.11–13 These adverse health effects depend on the exposure dose, chemical composition and bioavailability of Mn.Ferro- and silicomanganese (FeMn and SiMn) are essential additives in steelmaking used to provide specific properties of steel.14 High carbon (HC)–FeMn and SiMn are mainly produced in electric submerged arc furnaces with coke to facilitate the reduction of Mn oxide ores. Other raw materials include fluxes such as dolomite, calcite and quartz in SiMn production and lime in FeMn production. In addition, Mn-rich slag from FeMn production is recycled as feedstock in SiMn production.15 Thermally generated fumes are emitted during tapping, refining and casting of the alloys.16
Previous studies on HC–FeMn and SiMn production showed that the abundance of different particle types strongly depends on the size fraction. HC–FeMn tapping fume particles larger than 0.3 μm were dominated by slag particles, and generally particles were composed of different mineral phases.17 A large number of spherical potassium (K)-rich particles with sizes < 1–2 μm as well as irregular fragments of Mn oxides or mixed oxides > 1 μm were observed in HC–FeMn tapping fume.18 The submicron fraction is dominated by agglomerated Mn oxides, mainly MnO. Similar fume particles were reported by Kero et al. (2015),20 where MnO, MnOx-FeOy, and Mn3O4 were identified depending on the temperature and location during formation, e.g. crucible and filter. According to Ervik et al.19 the predominant phase in the submicron fraction was Mn3O4. In SiMn production, fume from the casting process is composed of slag particles and slag mixed with C-rich particles dominating the size fraction above 0.3 μm.17,18 The submicron fraction was found to be dominated by agglomerated Mn–Si phases.18 Kero et al.20 investigated the size distribution and found that agglomerated spheres dominate the aerodynamic diameter (dae) size range from 0.09 to 0.94 μm. The majority of the SiMn fume particles consisted of Mn, Si and O. Furthermore, SiMn fume thermally generated in a laboratory scale experiment was studied by Ma et al.21 The particle types generated depended on the melt temperature and on the collection position inside their experimental furnace setup. Also, a mixture of Mn– and Mn–Si oxides in both crystalline and amorphous states was observed by X-ray diffraction.
Bioaccessibility is the fraction of a compound released from its matrix becoming available for absorption in the body.22 Bioaccessibility of Mn in the respirable aerosol fraction is of interest since it is a measure of the amount of Mn available for absorption into the systemic circulation via the lungs. In laboratory studies, the bioaccessibility depends on the choice of simulated lung fluid.23 In such studies, different simulated lung fluids have been used, with Gamble solution (pH 7.4) being the most used simulated interstitial lung fluid.24 Gamble solution25 consists of an electrolyte composition simulating the human interstitial fluid,26 but lacks phospholipids which are the most abundant components of the alveolar lining fluid.27
The bioaccessibility of Mn in airborne PM from HC–FeMn or SiMn production has to our knowledge been sparsely investigated. In a study of ambient air in a heavily industrialised area, the bioaccessibility of metals in PM collected near an FeMn smelter and steel making plant was moderate (20–40%) for Mn.28 Bioaccessibility of Mn at work places was studied for welding fume29–31 and in PM from other hot processes.32 It was shown that the bioaccessibility of Mn was generally low (<20%) to moderate, depending on the origin of exposure and the simulated lung fluid used. Even when the bioaccessible fraction of Mn was low in welding fume, a correlation with the Mn concentration in the urine of the welders was found.30
Particle mass size distributions of workplace aerosols in Mn alloy smelters were investigated by Berlinger et al.33 using personal respirable cyclones and a 5-stage cascade impactor. As a follow up study, the chemical composition and bioaccessibility of Mn in terms of dissolution in Gamble solution are investigated in the present paper. Special emphasis is placed on the apparent dissolution of Mn as a function of the production process, particle size distribution and work task. Particles collected at different workplaces in the two smelters were studied in detail by scanning and transmission electron microscopy (SEM and TEM) to better understand the dissolution of Mn in size fractionated PM using Gamble solution.
2 Materials and methods
2.1 Production and work tasks
Air samples were collected in two different Mn alloy producing smelters in Norway, each operating two furnaces. Smelter 1 produces SiMn and HC–FeMn, whereas Smelter 2 produces only HC–FeMn. Every two to three hours molten alloy and slag are tapped from the furnaces and poured into large ladles. The ladles with molten alloy are transported with cranes to the casting area, similar to what is described in Olsen et al.15 Tapping and casting are open processes which emit thermally generated fumes into the furnace hall. The furnace workers included in this study were tappers and crane operators. Depending on the tapping time, tappers and crane operators worked 45–75 minutes on the tapping floor, three to four times during an eight-hour shift, but the crane operators' tasks were conducted further away from the taphole. The crane operators included in this study operated the cranes near the furnace with remote controllers fixed to their shoulders with straps. They were not expected to have significantly different exposure qualitatively than tappers, however, the level of their exposure proved to be significantly different than in the case of tappers. Details on the work tasks have been published by Berlinger et al.332.2 Sampling
In a first sampling campaign, 6–7 hour air samples were collected by personal sampling in the breathing-zone of 19 tappers and 18 crane operators with five-stage Sioutas cascade impactors (SKC, Eighty Four, PA, USA) loaded with 0.5 μm pore size 25 mm polytetrafluoroethylene (PTFE) substrates and a 2.0 μm pore size PTFE filter as an end filter, as well as respirable cyclones (JS Holdings, Stevenage, UK) loaded with 5.0 μm pore size PVC membrane filters (Millipore Corp., Billerica, MA, USA). Leland Legacy model high flow (SJC, Eighty Four, PA, USA) ensured an air sampling flow of 9 L min−1 for the Sioutas cascade impactors, and in house-built PS103 sampling pumps (National Institute of Occupational Health, Oslo, Norway) were operating at the flow rate of 2.2 L min−1 for the respirable samples. Details have been published by Berlinger et al.33 Additionally, for Smelter 1, particles were collected on copper (Cu) TEM grids with holey carbon film with a hand-held ESPnano model 100 electrostatic deposition sampler (ESPnano Inc., Spokane, WA, USA) (Miller et al., 2010)34 with sampling times between 10 and 200 seconds.In a second sampling campaign conducted only in Smelter 1, stationary air samples were collected for 10 minutes with a 13-stage nanoMOUDI cascade impactor (MSP Corporation, Shoreview, Minnesota, USA) during tapping of both HC–FeMn and SiMn furnaces. The samples were collected around eight meters from the tapping hole. The 50% dae cut-off for each stage was: 10, 5.6, 3.2, 1.8, 1.0, 0.56, 0.32, 0.18, 0.1, 0.056, 0.032, 0.018 and 0.010 μm. Particles with dae < 0.010 μm are collected by filtration on an end-filter. Cu TEM grids with holey carbon films (EMresolution, Sheffield, UK) were affixed to the surface of polyvinyl chloride membrane filters used as substrates in all impactor stages, except for the end-filter. TEM grids were not attached to the end-filter because the predominant mass on this stage is bounce off particles from the previous impaction stages.34 In addition, a TSI Scanning Mobility Particle Sizer (SMPS) instrument (model 3034, TSI Inc., Shoreview, MN, USA) with flow rate 1 L min−1 was running for six hours for each furnace in Smelter 1 during the second sampling campaign. The SMPS was placed next to nanoMOUDI, around eight metres from the tapping hole.
2.3 Transmission and scanning electron microscopy
Particles collected with the electrostatic sampler were characterised by SEM and TEM without coating. Individual particles were investigated with a Hitachi SU6600 field emission SEM (Hitachi, Tokyo, Japan) equipped with a Bruker energy-dispersive X-ray (EDX) detector (Bruker Nano GmbH, Berlin, Germany) and an electron backscatter diffraction (EBSD) detector (NORDIF, Trondheim, Norway). A minimum of 200 particles was investigated on each Cu TEM grid collected with the electrostatic sampler. For the cascade impactor samples, a minimum of 200 particles was investigated in the dae size range 10–1.8 μm (stage 1–4), 1.0–0.18 μm (stage 5–8) and 0.1–0.01 μm (stage 9–13). The phase composition of selected particles was determined by EBSD as described by Ervik et al.36 The samples were also investigated by TEM using a Jeol 2100 F (Tokyo, Japan) instrument (field emission gun) equipped with an EDX silicon drift detector X-Max (Oxford Instruments, Abingdon, UK). The microscope was operated in scanning-TEM (STEM) mode and in TEM modes. The acceleration voltage was 200 kV leading to a point resolution of approximately 0.22 nm. Images were recorded with a charge-coupled device (CCD) camera and treated with Digital Micrograph software (Gatan Inc., Pleasanton, United States) and FIJI image processing software.372.4 Leaching and digestion procedures of air filters and impactor substrates
Gamble solution was prepared as described by Berlinger et al.29 All samples and blanks were added to 10 mL of Gamble solutusted with 0.1 M HCl and 0.1 M NaOH) in 15 mL polypropylene tubes (Sarstedt, Nümbrecht, Germany) and shaken, before leaving for 24 hours in a laboratory oven set to 37 ± 1 °C. The samples were then filtered through 0.45 μm pore size 25 mm mixed cellulose ester membrane filters (Merck Millipore, Billerica, MA, USA) fixed in Disposable Funnel units (Eichrom Technologies, Lisle, USA) sealed with Teflon tape. One mL nitric acid (puriss p.a., Sigma Aldrich – Merck, Darmstadt, Germany) and 100 μL internal standard solution (germanium 25 mg L−1) were added to the filtrate before dilution to 25 mL with deionised water (18.2 MΩ cm, Merck Millipore, Billerica, MA, USA). The membrane filters with the non-dissolved PM were microwave digested (Multiwave PRO, Anton Paar, Graz, Austria) in temperature controlled vessels after adding a mixture of nitric- (1.5 mL), hydrochloric (5 mL) - and hydrofluoric acid (0.6 mL) (puriss p.a., Sigma Aldrich – Merck, Darmstadt, Germany). The digests were diluted to 50 mL with deionised water after adding 200 μL internal standard solution (germanium 25 mg L−1).2.5 Measurement of Mn in PM by mass spectrometry
The element concentrations in the solutions were determined using an Agilent 8800 triple quadrupole inductively coupled plasma mass spectrometer (ICP-MS) (Agilent Technologies, Santa Clara, CA, USA). The instrument was tuned prior to each run, and acid matrix matched standards were prepared for calibration. Details of the ICP-MS setup are given in the ESI (Table S1†).Reproducibility of the leaching procedure was assessed by use of SiMn and HC–FeMn in-house quality control material. This material is dust collected from the furnace emission electrostatic filters and sieved through 45 μm mesh to remove coarse particles. The total Mn concentrations obtained with the current method were comparable to results obtained by a previously validated method.38 The precision of the method is defined as the relative standard deviation of n = 14 SiMn and n = 16 FeMn in-house samples. The precision of Mn was on average 22% for SiMn (∼18% Mndissolved) and 13% for HC–FeMn (∼27% Mndissolved), respectively. All measured Mn concentrations were above the limit of detection. The limit of detection was calculated as three times the standard deviation of ten blank samples, respectively, 40 ng per filter for acid digested and 7.5 ng per filter for Gamble dissolved.
2.6 Limitations of the leaching method
The bioaccessibility of Mn was studied using Gamble solution. Gamble solution was chosen because it is the most used simulated lung fluid in bioaccessibility studies and facilitates comparison of the results. One alternative to Gamble solution is Hatch solution which additionally contains proteins and enzymes.29,30,32,39 Hatch solution is, however, more analytically challenging in terms of pH stability, formation of precipitates and filtration compared to Gamble solution.Extraction time is important in PM dissolution testing.24 Berlinger et al.29 studied the solubility of Mn and other elements using Gamble and Hatch solutions in different welding fumes with extraction times between 30 minutes and 24 hours. Further dissolution ceased after 8 hours. Caboche et al.40 suggested that 24 hours is sufficient for dissolution testing of Mn based on ambient air PM of different origins. Based on these two studies, a 24 hour extraction time was chosen in this study.
The solid-to-liquid ratio (S/L ratio) should not be too low in order to avoid unnecessary agglomeration of the PM which may result in a smaller surface area.39–41 Pelfrêne et al.41 concluded that the S/L ratio influences the PM dissolution when applying Gamble solution. We used a somewhat higher ratio in our study (average: 0.0003, min: 0.00005 max: 0.005 g/10 mL Gamble solution) than what has been found to give the highest bioaccessibility.39 As the ratio was higher, pronounced agglomeration during the leaching procedure was not expected.
The air samples were collected according to size onto filters. This may lead to increased uncertainty of the estimates of dissolution as the particles are packed onto each other on the filter, and the surface area available for the dissolution is hence reduced. To minimize this effect, shaking of the samples is applied in dissolution testing,24,39 but would most probably not release all particles from the filters as they are incorporated deep into the filter material or closely packed on the filter surface. However, in our study this effect will most probably not result in significant relative differences in Mndissolved between SiMn and HC–FeMn production (Smelter 1) or tapper and crane operators (Smelter 2), as we discuss the same size fractions separately. Also, the respirable samples (Table 3) are compared between plant and occupational groups separately from the cascade impactor samples (Fig. 4). The in-house standards of SiMn and HC–FeMn were directly weighed onto the filters and the PM is not packed as the air samples. These samples may therefore have a larger surface area in contact with the Gamble solution. Even in this case, a similar difference in the percentage of Mndissolved as the collected air samples was observed, 29% for SiMn and 5% for HC–FeMn, respectively.
2.7 Statistical analysis
The percentage of Mndissolved was calculated from the proportion of the concentration of Gamble dissolved Mn to the total concentration of Mn (concentration of Gamble dissolved plus acid digested). Differences in the dissolved Mn fraction between crane operators and tappers as well as between the furnace area of SiMn and HC–FeMn production were tested with the Wilcoxon rank sum test. We define p < 0.05 as statistically significant.All statistical calculations were performed in R studio version 4.0.4.,42 and all figures were produced with the package ‘ggplot2’ version 3.3.2.43 Wilcoxon rank sum test was calculated with the package ‘ggpubr’ version 0.4.0.999.44
3 Results
3.1 Electron microscopy
The results from electron microscopy are divided in two subchapters. The first subchapter includes characterisation of particles collected directly from the taphole by the electrostatic precipitator. The second subchapter regards particles collected further away from the taphole with the cascade impactor. The two particle sampling procedures were separated to elaborate the differences between particle sizes and particle types collected in the surrounding area of the two work tasks, tapping and crane operation. Particles for characterization were only collected in Smelter 1 where both HC–FeMn and SiMn are produced. The average number size distributions from SMPS measurements in Smelter 1 are shown in Fig. S2† and illustrate that a large number of nanosized (∼30 nm) particles exist in the workroom air.| Process | Particle types |
|---|---|
| a Categories of particle abundance: >50% = dominating; >10% = often; <10% = some. | |
| Tapping SiMn furnace | Dominated by condensation particles rich in Mn–Si–O (some particles also have minor amounts of Mg, K and Na) and mixed particles of Mn–O rich crystalline phases surrounded by a Si–O rich amorphous phase. The particles occur as both, individual particles and agglomerates/aggregates. The different particle types were often found in the same agglomerate/aggregate |
| Tapping HC–FeMn furnace | Dominated by spherical condensation particles of crystalline Mn oxides. These occur as single particles or as aggregates/agglomerates, with primary particle sizes between a few nm and 500 nm. All particles were coated with a few nm thick Si–O-rich surface layer (most likely SiO2), which holds the primary particles as aggregates |
 | ||
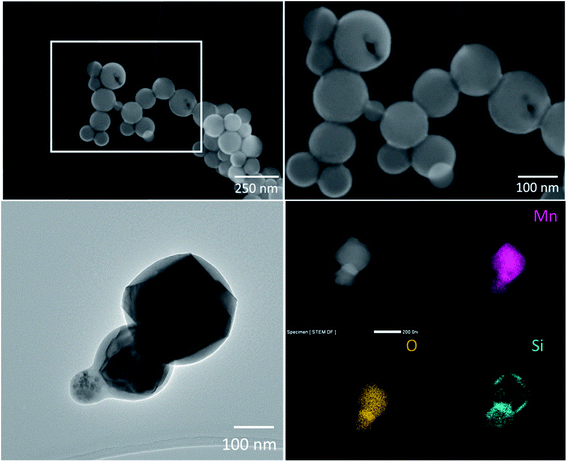
| Fig. 2 TEM image of a particle collected from the SiMn tapping fume in Smelter 1 (left) and STEM-EDX elemental distribution images for Mn, O, Si (right). | ||
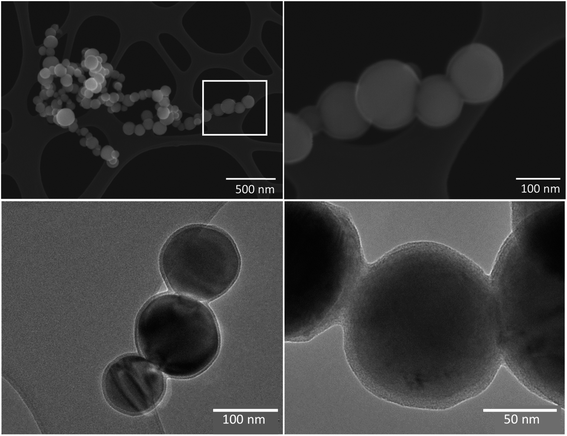
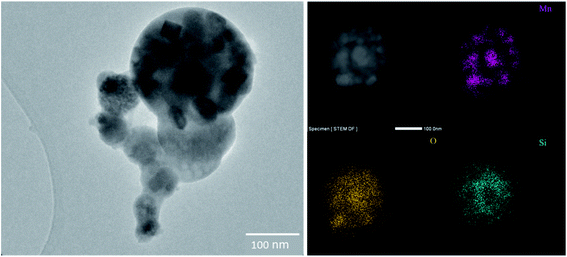
The HC–FeMn tapping fume was dominated by spherical primary condensation particles of Mn oxides, which occur as aggregates/agglomerates with sizes up to several μm, or as single particles with a size range from a few nm up to 500 nm. A selection of about 100 primary Mn oxide particles was investigated by SEM-EBSD. Most particles were identified as Mn3O4 (hausmannite) and some particles were identified as MnO (manganosite). The hausmannite phase was confirmed by applying TEM-SAED on a few selected particles. Typical particles collected during tapping of the HC–FeMn furnace are shown in Fig. 3. An amorphous Si–O rich surface layer (presumably SiO2) seems to hold the primary particles together as aggregates (Fig. 3 lower left and lower right). A STEM-EDX elemental line scan for Mn, Si and O of such a particle is shown in Fig. S3.† Please note that the term aggregate will be used for such particles, as the primary particles are held together by chemical forces. When discussing the findings in general or the particles collected from SiMn furnace area (i.e., without a surface layer covering the primary particles), the term agglomerate/aggregate is used. Agglomerates may consist of primary particles and/or aggregates, and it is not always possible to discriminate between agglomerates and aggregates.
| Stages (dae cut size) | Particle type |
|---|---|
| a d ae = aerodynamic diameter. Categories of particle abundance were: dominating > 50%, often > 10%, some < 10%, rare < 2%. | |
| SiMn | |
| 1–4 (10.0–1.8 μm) | Dominating: irregular mixed slag particles and Fe and Mn oxides |
| Some: slag particles mixed with coke | |
| 5–8 (1.0–0.18 μm) | Dominating: condensation particles rich in Si and Mn oxides (larger condensation particles have minor amounts of Mg), which may co-exist with angular Mn-rich phases identified as hausmannite (Mn3O4) and manganosite (MnO) enclosed in a Si–O rich matrix |
| Often: slag particles mixed with oxides of Mn and Fe in stages 5 and 6 | |
| 9–13 (0.10–0.010 μm) | Dominating: carbonaceous particles (most likely soot) |
| Often: volatile K and/or S rich particles | |
| Some: single particles or small aggregates of particles rich in Si and Mn oxides in stage 9 and 10 | |
| Rare: single particles or small aggregates of Mn–Si oxides in stages 11, 12 and 13 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| HC–FeMn | |
| 1–4 (10.0–1.8 μm) | Dominating: spherical particles with high content of K, Na, chlorine (Cl) and O often with high zinc (Zn) and Mn contents, and irregular slag particles with high MnO content |
| Often: large agglomerates/aggregates of Mn oxides in stage 4 | |
| Some: large agglomerates/aggregates of Mn oxides stage 1–3 | |
| Some: alloy fragments of Mn, Si and Fe | |
| 5–8 (1.0–0.18 μm) | Dominating: condensation particles of Mn oxides occurring as aggregates/agglomerates consisting primarily of Mn3O4 and MnO. The MnO particles show a Si–O-rich surface layer of a few nm thickness |
| Often: carbonaceous particles (most likely soot) | |
| 9–13 (0.10–0.010 μm) | Dominating: carbonaceous particles (most likely soot) |
| Some: single particles or small aggregates of Mn oxides were found in stage 8 and 9 | |
| Rare: single particles or small aggregates of Mn oxides in stage 10–13 | |
In the furnace area of SiMn production, the first four stages (dae cut size: 10.0–1.8 μm) were dominated by irregular mixed slag particles, Mn and Fe oxides and coke particles. The slag particles were rich in Si, calcium (Ca), aluminium (Al) and O, with minor amounts of Mg and Mn. The slag particles in the SiMn furnace area had much lower Mn contents than slag particles in the HC–FeMn furnace area. Most particles are inhomogeneous and consist of several phases. An example of a typical particle is shown in the ESI (Fig. S4†).
The next four impactor stages (dae cut size: 1.0–0.18 μm) are dominated by condensation particles, similar to the particles from the tapping fume (chapter 3.1.1).
The smallest particles (stage 9–13; dae cut size 0.10–0.010 μm) were dominated by carbonaceous particles. In addition, residues of volatile K and S rich particles were observed. The exact composition of these particles was difficult to determine because these particles were volatile under electron bombardment. Some small agglomerates and single particles of Mn–Si oxides were also observed in stage 9 and 10. Mn containing particles were rare in the last four stages (dae cut size: 0.056–0.010 μm).
In the furnace area of HC–FeMn production, the first four stages (dae cut size: 10.0–1.8 μm) were dominated by spherical shaped mixed particles with a high content of K, Na, Cl (often also Zn and Mn) and minor amounts of S and phosphorus (P), as well as irregular shaped particles with a high Mn content. The latter particles often had considerable amounts of Si, Ca and minor amounts of Fe, Al and Mg. The slag in HC–FeMn production is MnO–SiO2–CaO based. An example of a typical particle encountered can be found in the ESI (Fig. S5 and S6†). Some alloy fragments rich in Mn and Si (minor Fe contents) were observed. Large agglomerates/aggregates of Mn oxides were often found in stage 4 (dae cut size 5.6 μm).
The next three impactor stages (dae cut size: 1.0–0.32 μm) were dominated by agglomerates/aggregates of spherical Mn oxides. According to TEM-SAED and SEM-EBSD, these particles primarily consist of hausmannite (Mn3O4) and manganosite (MnO). The MnO particles were observed with a few nm thick Si–O rich surface layer (Fig. 3).
In stage 8 (dae cut size: 0.18 μm) and below, carbonaceous particles dominate and only some Mn-rich particles were found. Below stage 9 (dae cut size: 0.10 μm), Mn-rich particles were rare. In stages 12 and 13 only a few particles were observed (<500).
3.2 Dissolution of Mn in Gamble solution
Total Mn air concentrations in the respirable aerosol fraction and the percentage of Mndissolved are summarised in Table 3. The air concentrations among tappers were higher compared to crane operators in both Smelter 1 and Smelter 2. The lowest air concentration of total Mn was found among the SiMn production workers.| SiMn | HC–FeMn | |||||
|---|---|---|---|---|---|---|
| Location | Smelter 1 | Smelter 1 | Smelter 2 | |||
| Work task | Crane operators n = 5 | Tappers n = 5 | Crane operators n = 5 | Tappers n = 4 | Crane operators n = 8 | Tappers n = 10 |
| Minimum | 7.2 (15) | 6.8 (12) | 17.0 (6) | 21.4 (8) | 12.2 (4) | 26.0 (2) |
| Lower quartile | 11.3 (20) | 21.5 (17) | 31.7 (7) | 46.3 (10) | 15.6 (7) | 35.0 (3) |
| Median | 14.6 (23) | 23.5 (20) | 39.5 (11) | 55.0 (12) | 24.5 (10) | 40.6 (4) |
| Upper quartile | 14.8 (25) | 26.9 (21) | 43.2 (12) | 56.3 (17) | 29.7 (14) | 53.8 (6) |
| Maximum | 17.0 (27) | 65.0 (44) | 43.5 (18) | 59.5 (30) | 46.9 (18) | 115.0 (7) |
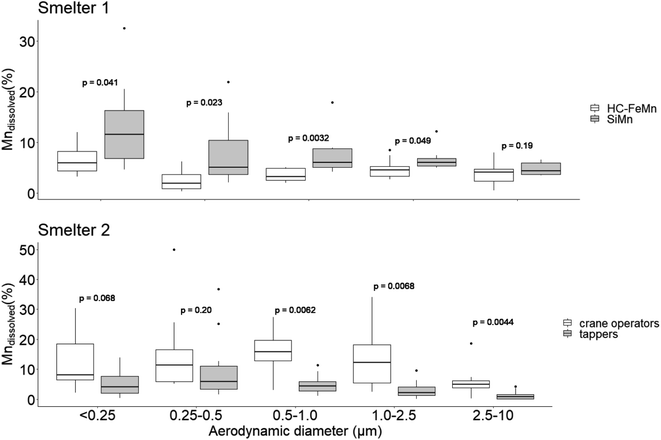
The median percentage of Mndissolved among SiMn furnace workers is somewhat higher than that among HC–FeMn furnace workers (Table 3). The Mndissolved appears to be independent of the work task in Smelter 1. In contrast, the Mndissolved is significantly higher (p = 0.008) for crane operators compared to tappers in HC–FeMn production of Smelter 2.
The percentage of Mndissolved in air samples collected with the Sioutas cascade impactor was determined as a function of particle size among tappers and crane operators in the SiMn and HC–FeMn production (Fig. 4). In air samples collected from HC–FeMn workers in Smelter 1, the percentage of Mndissolved is independent of dae particle size except for a small increase for particles < 0.25 μm. In contrast, in air samples from SiMn furnace workers, Mndissolved increases with decreasing particle size (Fig. 4, Smelter 1). The percentage of Mndissolved is significantly higher for SiMn than in HC–FeMn workers for particle dae sizes up to 2.5 μm. The Mn air concentration in PM collected with the Sioutas cascade impactor is given in the ESI (Fig. S1†).
In the air samples from the HC–FeMn workers in Smelter 2 (Fig. 4, Smelter 2), the percentage of Mndissolved is similar in all size fractions for both crane operators and tappers. However, the percentage of Mndissolved is considerably higher (by a factor of 2–5) with a larger variation within the size fractionated samples among crane operators compared to tappers. In general, the percentage of Mndissolved is low for workers in Smelter 2, and lowest for tappers (Table 3). Tappers have slightly higher air concentrations of Mn in dae size fractions below 2.5 μm compared to crane operators (Fig. S1†).
4 Discussion
There were substantial differences in the morphology, chemical composition and microstructure between particles collected in the furnace area of SiMn production compared to particles collected in the furnace area of HC–FeMn production in Smelter 1. Typically, the dominating Mn oxide particles in the HC–FeMn furnace area were crystalline and had a thin amorphous Si–O surface layer which seemed to hold the particles as aggregates. Both crystalline and amorphous Mn and Si containing oxides were observed in the SiMn tapping fume, but a Si–O surface layer was not observed. The Mn oxide particles observed in the SiMn tapping fume were enclosed in an amorphous Si–O matrix. Nanometre sized particles containing Mn were rarely found in the size bins below 100 nm in any of the smelters. Carbonaceous particles, most probably soot from combustion, dominated the smallest size fraction (<180 nm).In general, the percentage of Mndissolved was low to moderate (<30%) in both type of smelters. A significantly higher percentage of Mndissolved was observed in the SiMn furnace area compared to the HC–FeMn furnace area. In the size fractionated samples, this difference was significant for particles between <0.25 and 2.5 μm.
4.1 Particle characterisation
Collection of particles for characterization was only performed in Smelter 1. The particles collected in the tapping process of HC–FeMn in Smelter 1 are considered to be representative of the fume particles formed in Smelter 2 as the raw materials and the production process are identical. However, the fact that some contamination from the SiMn production process will occur cannot be excluded. Slag particles and spherical particles rich in K, Na, Cl, Zn and O dominated the fraction above dae of 1 μm in fume collected from the HC–FeMn furnace (Table 2). Sources may be raw materials, which always contain Zn, K and Na that partly evaporate in the smelting process. Particles below 2 μm rich in K with various amounts of Na, S, Zn, Cl and O were reported by Gjønnes et al.,18 and high mass concentrations of K in PM were found by Gunst et al.17 In the present study, K rich particles with dae < 1 μm surround the larger spherical K, Na, Cl rich particles (Fig. S5†). Additionally, Mn rich areas were often found inside of these larger particles (Fig. S5†). Larger particles of slag and carbonaceous particles seem to be sintered together in the SiMn furnace area which corresponds to the observations by Gunst et al.17 In agreement with previous observations,17,18 particles with dae > 1 μm were generally inhomogeneous and irregular in shape.Particles collected during tapping of HC–FeMn in the dae size range 0.18–1 μm were dominated by crystalline Mn oxide particles (MnO and Mn3O4). This is in accordance with the results of Gjønnes et al.,18 except that they additionally observed MnO fibers during tapping of HC–FeMn. Furthermore, Ervik et al.19 analysed particles by SEM-EBSD and identified hausmannite (Mn3O4) as the dominating Mn oxide. Agglomerated spheres of Mn oxides dominated the dae size range from 0.09 to 0.94 μm in particles studied by Kero, Slizovskiy,45 which is in good agreement with the results of our study (Table 2). Our SEM and TEM observations indicate that the primary particles collected in HC–FeMn tapping fume are held together by an amorphous Si–O rich surface layer. The presence of such surface layers may have implications for the behaviour and toxicity of the particles, e.g. such as a higher resistance of breakdown or collapse of the aggregate/agglomerate in the lung.46 Additionally, micrometre sized particles are reported to be phagocytized more easily than nanometre sized particles.47,48
In our study, a complex mix of condensation particles was found in the SiMn fume (e.g. mixture of Mn3O4 and Si–O, as well as amorphous and crystalline Si–Mn–O rich particles). Ma et al.21 observed similar particles in SiMn fume from an experimental setup and identified Mn2SiO4 as well as Mn3O4 in the fume at melting temperatures of 1500–1700 °C. In accordance with their findings, we also observed angular Mn3O4 phases, often mixed with an amorphous Si–O phase. The molten metal will react with oxygen in the air and the observation of various MnSi phases in Gjønnes et al.18 (in, e.g., Mn3Si, Mn6Si and Mn5Si2) from SiMn casting is therefore surprising. Some particles collected during tapping of the SiMn furnace consisted of nanometre sized Mn rich phases enclosed in a Si–O rich matrix (Fig. 1 and 2). A silica precursor has been used in previous investigations as an additive in shielding gas in welding. The resulting amorphous silica formed a coating that encapsulated the welding fume particles49 leading to formation of larger particles that may change the deposition efficiency in the lungs. However, amorphous silica particles have earlier shown to have a low retention in rat lungs explained by a high solubility.50 Considering this in our work, a high solubility of the Si–O rich layer may expose the Mn rich phases.
Carbonaceous particles dominate the dae size fraction below 0.18 μm, both in HC–FeMn and SiMn furnace area, with some single particles and small agglomerates of Mn oxides and Mn–Si oxides. The fact that few nanometre sized Mn containing particles were observed, and instead seem to be bound in aggregates/agglomerates, is an important finding. This is because a change in particle size affects the deposition fate upon inhalation. In addition, translocation of particles to other organs is only relevant for the smallest particles. It should also be noted that even though few particles were observed in the last two stages of the cascade impactor, it is likely that particles of this size exist in large numbers as is shown in the particle size distribution (Fig. S2†) and observed in this industry previously.20,33 Residues of secondary organic particles have been found from combustion in high temperature industrial processes, as was found, for example, in an Al plant.51 Such small secondary particles evaporate under electron bombardment in EM. Secondary organic particles might also exist in the workroom air of Mn smelters. In addition, small soot particles below dae of 30 nm are not easily detected in SEM.
4.2 Bioaccessibility
Our study shows that the percentage of Mndissolved is low to moderate (<30%), which is comparable to what was reported close to an FeMn production plant and steel making industry.28 However, the percentage of Mndissolved is higher in our samples than in PM collected from different welding fumes dissolved in Gamble solution29 indicating a lower bioaccessibility of the latter. A higher Mn dissolution in Hatch than Gambles has been reported for Mn in welding fumes.29The percentage of Mndissolved was higher in PM collected among SiMn furnace workers compared to HC–FeMn furnace workers. The largest difference in the percentage of Mndissolved was observed for PM with dae between 0.25 and 1.0 μm. This size range is dominated by agglomerates and agglomerates/aggregates of primary particles. Gjønnes et al.18 suggested a higher health risk for workers exposed to Mn in HC–FeMn compared to SiMn production. Their conclusion was based on the assumption that Mn oxides are more soluble than SiMn, as was shown by Thomassen et al.52 We observed a surface layer of amorphous Si–O on MnO-containing particles in the HC–FeMn furnace area holding the primary particles together into agglomerates/aggregates. Such an amorphous layer was not observed for particles in the SiMn fume. Instead, Mn rich crystalline phases were encapsulated in a Si rich amorphous matrix (Fig. 3). Amorphous and crystalline Si–Mn–O rich particles without a surface layer or an additional matrix were also observed. Amorphous SiO2 has been shown to dissolve substantially in Gamble solution.53 The higher percentage of Mndissolved in SiMn fume PM may, thus, be a result of the amorphous Si–O matrix dissolving in Gamble solution and releasing small Mn rich phases.
The air concentration of Mn [μg m−3] in PM was higher in the breathing zone of tappers compared to that of the crane operators (Table 3). This result was not unexpected, as it has been previously shown that the tappers were exposed to a statistically significant higher mass concentration of PM than the crane operators.33 The difference in Mndissolved is, however, only statistically significant between crane operators and tappers in Smelter 2, which only included a HC–FeMn furnace. The largest differences in Mndissolved between tappers and crane operators are observed for PM with dae > 500 nm, representing most of the mass.33 In this size fraction, we found irregular slag particles with high MnO content and particles with higher amount of Mn and Zn, as well as minor amounts of K, Na and Cl. The particle composition in different working areas of the HC–FeMn smelter was also studied by Gunst et al.,17 and a different chemical composition of PM collected among tappers and crane operators was found. Particulate matter collected among tappers contained higher mass fractions of K, Na and Zn than among crane operators. In our study, a dominating fraction of the particles observed in the first stages of the cascade impactor contained K, Na, Cl and Zn together with Mn, but such particles did not dominate in samples collected directly from the tapping fume with the electrostatic sampler. The amount of such particles may explain the difference in the percentage of Mndissolved between tappers and crane operators. The difference from Gunst's17 study may be a result of the composition of the raw material used in the production during sampling. Further investigations are needed to conclude on the observed difference in bioaccessible Mn between tappers and crane operators in the HC–FeMn industry.
4.3 Conclusion
Detailed SEM and TEM analysis of samples collected in the furnace hall of HC–FeMn and SiMn production showed different particle types related to work area and task. The results of this study indicate that the different physicochemical characteristics of the particles might explain the difference in Mndissolved seen in PM samples collected by the workers in this industry. Still, further investigations are needed to conclude if the difference in Mndissolved is related to the dissolution of the Si-matrix observed in particles collected from the SiMn tapping fume.Our results show that the concentration of Mn in PM in air was significantly higher for tappers and crane operators, but the Mndissolved was significantly lower for tappers than crane operators. Further research is needed to investigate if this difference in Mndissolved between crane operators and tappers is related to the physicochemical properties of the particles.
In conclusion, the results have shown that connecting the knowledge of particle characteristics and bioaccessibility is important to gain more information on how particles may behave in contact with lung fluids. The physicochemical properties of single particles and Mn bioaccessibility are key factors to be considered in risk assessment. With regards to this, our size resolved data on particle characteristics and bioaccessibility give additional information which can be combined with what is already known about the particle size dependent deposition efficiency in lungs. Furthermore, the results of this study can be applied in future epidemiological studies and toxicological assays.
Funding
This study was partly funded by the Sickness Absence, Work and Health programme of the Norwegian Research Council under grant 218350 and partly by the Confederation of Norwegian Enterprise Working Environment Fund.Author contributions
SEH conducted the dissolution tests, analysed the samples by ICP-MS, performed data analysis and wrote the manuscript. TE performed sampling and SEM analysis, and participated in the discussion of the results and manuscript writing. NB carried out the TEM analyses. SW helped with statistical analysis, participated in discussion of the results and manuscript writing. YT and DGE took part in the study initiation, participated in the discussion of results as well as preparation and reviewing of the manuscript. BBE performed sampling and was engaged in the study design, discussion of the results as well as manuscript preparation and reviewing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financially support from the funders. SEH would like to thank Stephen Dorn and Jon Hovik for their skilled laboratory work.References
- H. Roels, G. Meiers, M. Delos, I. Ortega, R. Lauwerys and J. P. Buchet, et al., Influence of the route of administration and the chemical form (MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats, Arch. Toxicol., 1997, 71(4), 223–230 CrossRef CAS PubMed.
- D. C. Dorman, M. F. Struve, R. A. James, M. W. Marshall, C. U. Parkinson and B. A. Wong, Influence of Particle Solubility on the Delivery of Inhaled Manganese to the Rat Brain: Manganese Sulfate and Manganese Tetroxide Pharmacokinetics Following Repeated (14-Day) Exposure, Toxicol. Appl. Pharmacol., 2001, 170(2), 79–87 CrossRef CAS PubMed.
- D. G. Ellingsen, R. Konstantinov, R. Bast-Pettersen, L. Merkurjeva, M. Chashchin and Y. Thomassen, et al., A neurobehavioral study of current and former welders exposed to manganese, Neurotoxicology, 2008, 29(1), 48–59 CrossRef CAS PubMed.
- F. Taube, Manganese in occupational arc welding fumes—aspects on physiochemical properties, with focus on solubility, Ann. Occup. Hyg., 2013, 57(1), 6–25 CAS.
- Z. Long, Y.-M. Jiang, X.-R. Li, W. Fadel, J. Xu and C.-L. Yeh, et al., Vulnerability of welders to manganese exposure–A neuroimaging study, Neurotoxicology, 2014, 45, 285–292 CrossRef CAS PubMed.
- S. R. Criswell, S. S. Nielsen, M. N. Warden, H. P. Flores, J. Lenox-Krug and S. Racette, et al., MRI Signal Intensity and Parkinsonism in Manganese-Exposed Workers, Occup. Environ. Med., 2019, 61(8), 641–645 CrossRef CAS PubMed.
- J. Rodier, Manganese poisoning in Moroccan miners, Br. J. Ind. Med., 1955, 12(1), 21 CAS.
- W. W. Dlamini, G. Nelson, S. S. Nielsen and B. A. Racette, Manganese exposure, parkinsonian signs, and quality of life in South African mine workers, Am. J. Ind. Med., 2020, 63(1), 36–43 CrossRef CAS PubMed.
- A. M. Emara, S. H. El-Ghawabi, O. I. Madkour and G. H. El-Samra, Chronic manganese poisoning in the dry battery industry, Br. J. Ind. Med., 1971, 28(1), 78 CAS.
- H. A. Roels, P. Ghyselen, J. P. Buchet, E. Ceulemans and R. R. Lauwerys, Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust, Br. J. Ind. Med., 1992, 49(1), 25–34 CAS.
- J.-D. Wang, C. Huang, Y. Hwang, J. Chiang, J. Lin and J. Chen, Manganese induced parkinsonism: an outbreak due to an unrepaired ventilation control system in a ferromanganese smelter, Occup. Environ. Med., 1989, 46(12), 856–859 CAS.
- R. Bast-Pettersen, D. G. Ellingsen, S. M. Hetland and Y. Thomassen, Neuropsychological function in manganese alloy plant workers, Int. Arch. Occup. Environ. Health, 2004, 77(4), 277–287 CrossRef CAS PubMed.
- M. Bouchard, D. Mergler, M. E. Baldwin and M. Panisset, Manganese cumulative exposure and symptoms: a follow-up study of alloy workers, Neurotoxicology, 2008, 29(4), 577–583 CrossRef CAS PubMed.
- L. Holappa. Handbook of Ferroalloys: Chapter 2. Basics of Ferroalloys, Elsevier Science, 2013 Search PubMed.
- S. E. Olsen, S. Olsen, M. Tangstad and T. Lindstad, Production of Manganese Ferroalloys: Tapir academic press, 2007 Search PubMed.
- I. T. Kero, P. A. Eidem, Y. Ma, H. Indresand, T. A. Aarhaug and S. Grådahl, Airborne Emissions from Mn Ferroalloy Production, JOM, 2019, 71(1), 349–365 CrossRef CAS.
- S. Gunst, S. Weinbruch, M. Wentzel, H. M. Ortner, A. Skogstad and S. Hetland, et al., Chemical composition of individual aerosol particles in workplace air during production of manganese alloys, J. Environ. Monit., 2000, 2(1), 65–71 RSC.
- K. Gjønnes, A. Skogstad, S. Hetland, D. G. Ellingsen, Y. Thomassen and S. Weinbruch, Characterisation of workplace aerosols in the manganese alloy production industry by electron microscopy, Anal. Bioanal. Chem., 2011, 399(3), 1011–1020 CrossRef PubMed.
- T. K. Ervik, N. Benker, S. Weinbruch, A. Skogstad, Y. Thomassen and D. G. Ellingsen, et al., Phase identification of individual crystalline particles by combining EDX and EBSD: application to workplace aerosols, Anal. Bioanal. Chem., 2018, 410(11), 2711–2721 CrossRef CAS PubMed.
- I. Kero, M. K. Naess and G. Tranell, Particle size distributions of particulate emissions from the ferroalloy industry evaluated by electrical low pressure impactor (ELPI), J. Occup. Environ. Hyg., 2015, 12(1), 37–44 CrossRef CAS PubMed.
- Y. Ma, I. Kero and G. Tranell, Fume formation from oxidation of liquid SiMn alloy, Oxid. Met., 2018, 89(1–2), 211–231 CrossRef CAS.
- J. C. Ng, A. Juhasz, E. Smith and R. Naidu, Assessing the bioavailability and bioaccessibility of metals and metalloids, Environ. Sci. Pollut. Res., 2015, 22(12), 8802–8825 CrossRef PubMed.
- F. Kastury, E. Smith and A. L. Juhasz, A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal (loid) s from ambient particulate matter or dust, Sci. Total Environ., 2017, 574, 1054–1074 CrossRef CAS PubMed.
- C. L. Wiseman, Analytical methods for assessing metal bioaccessibility in airborne particulate matter: a scoping review, Anal. Chim. Acta, 2015, 877, 9–18 CrossRef CAS PubMed.
- O. Moss, Simulants of lung interstitial fluid, Health Phys., 1979, 36(3), 447–448 CAS.
- H. Ren, Y. Yu and T. An, Bioaccessibilities of metal(loid)s and organic contaminants in particulates measured in simulated human lung fluids: A critical review, Environ. Pollut., 2020, 265, 115070 CrossRef CAS PubMed.
- M. Kendall, Fine airborne urban particles (PM2.5) sequester lung surfactant and amino acids from human lung lavage, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2007, 293(4), L1053–L1058 CrossRef CAS PubMed.
- S. Mbengue, L. Y. Alleman and P. Flament, Bioaccessibility of trace elements in fine and ultrafine atmospheric particles in an industrial environment, Environ. Geochem. Health, 2015, 37(5), 875–889 CrossRef CAS PubMed.
- B. Berlinger, D. G. Ellingsen, M. Náray, G. Záray and Y. Thomassen, A study of the bio-accessibility of welding fumes, J. Environ. Monit., 2008, 10(12), 1448–1453 RSC.
- D. G. Ellingsen, E. Zibarev, Z. Kusraeva, B. Berlinger, M. Chashchin and R. Bast-Pettersen, et al., The bioavailability of manganese in welders in relation to its solubility in welding fumes, Environ. Sci: Process. Impacts, 2013, 15(2), 357–365 CAS.
- B. Berlinger, S. Weinbruch, D. G. Ellingsen, E. Zibarev, V. Chashchin and M. Chashchin, et al., On the bio-accessibility of 14 elements in welding fumes, Environ. Sci.: Processes Impacts, 2019, 21(3), 497–505 RSC.
- B. Berlinger, U. Skogen, C. Meijer and Y. Thomassen, Workplace exposure to particulate matter, bio-accessible, and non-soluble metal compounds during hot work processes, J. Occup. Environ. Hyg., 2019, 16(6), 378–386 CrossRef CAS PubMed.
- B. Berlinger, M. D. Bugge, B. Ulvestad, H. Kjuus, K. Kandler and D. G. Ellingsen, Particle size distribution of workplace aerosols in manganese alloy smelters applying a personal sampling strategy, Environ. Sci: Process. Impacts, 2015, 17(12), 2066–2073 CAS.
- A. Miller, G. Frey, G. King and C. A. Sunderman, Handheld Electrostatic Precipitator for Sampling Airborne Particles and Nanoparticles, Aerosol Sci. Technol., 2010, 44, 417–427 CrossRef CAS.
- Y. Fujitani, S. Hasegawa, A. Fushimi, Y. Kondo, K. Tanabe and S. Kobayashi, et al., Collection characteristics of low-pressure impactors with various impaction substrate materials, Atmos. Environ., 2006, 40(18), 3221–3229 CrossRef CAS.
- T. K. Ervik, N. Benker, S. Weinbruch, Y. Thomassen, D. G. Ellingsen and B. Berlinger, Size distribution and single particle characterization of airborne particulate matter collected in a silicon carbide plant, Environ. Sci: Process. Impacts, 2019, 21(3), 564–574 CAS.
- J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair and T. Pietzsch, et al., Fiji: an open-source platform for biological-image analysis, Nat. methods, 2012, 9(7), 676–682 CrossRef CAS PubMed.
- Y. Thomassen, E. Nieboer, D. Ellingsen, S. Hetland, T. Norseth and J. Øyvind Odland, et al., Characterisation of workers' exposure in a Russian nickel refinery, J. Environ. Monit., 1999, 1(1), 15–22 RSC.
- F. Kastury, E. Smith, R. R. Karna, K. G. Scheckel and A. Juhasz, An inhalation-ingestion bioaccessibility assay (IIBA) for the assessment of exposure to metal (loid) s in PM10, Sci. Total Environ., 2018, 631, 92–104 CrossRef PubMed.
- J. Caboche, E. Perdrix, B. Malet and L. Y. Alleman, Development of an in vitro method to estimate lung bioaccessibility of metals from atmospheric particles, J. Environ. Monit., 2011, 13(3), 621–630 RSC.
- A. Pelfrêne, M. R. Cave, J. Wragg and F. Douay, In vitro investigations of human bioaccessibility from reference materials using simulated lung fluids, Int. J. Environ. Res. Public Health, 2017, 14(2), 112 CrossRef PubMed.
- RStudio, RStudio: Integrated Development for R. RStudio, Inc, Boston, MA, 2015, http://www.rstudio.com Search PubMed.
- H. Wickham, ggplot2: Elegant Graphics for Data Analysis. Version 3.3.2, Springer-Verlag, New York; 2016, available at: https://ggplot2.tidyverse.org Search PubMed.
- A. A. Kassambara ggpubr: 'ggplot2' Based Publication Ready Plots. Version: 0.4.0, 2020, available at: https://rpkgs.datanovia.com/ggpubr/ Search PubMed.
- I. Kero, D. Slizovskiy, B. Wittgens and G. TranellFume Formation from Liquid Ferromanganese. Sustainable Industrial Processing Summit, Takano Intl. Symp. On Metals and Alloys, vol. 3. 2015 Search PubMed.
- J. Pauluhn. Common Denominators of Carbon Nanotubes, in Nanomaterials, ed. (DFG) DF, Wiley-VCH Verlag GmbH & Co., Bonn, Germany, 2013, pp. 68–83 Search PubMed.
- H. M. Braakhuis, M. V. D. Z. Park, I. Gosens, W. H. De Jong and F. R. Cassee, Physicochemical characteristics of nanomaterials that affect pulmonary inflammation, Part. Fibre Toxicol., 2014, 11(1), 18 CrossRef PubMed.
- M. Riediker, D. Zink, W. Kreyling, G. Oberdörster, A. Elder and U. Graham, et al., Particle toxicology and health - where are we? Part, Fibre Toxicol, 2019, 16(1), 19 CrossRef PubMed.
- N. Topham, J. Wang, M. Kalivoda, J. Huang, K.-M. Yu and Y.-M. Hsu, et al., Control of Cr6+ emissions from gas metal arc welding using a silica precursor as a shielding gas additive, Ann. Occup. Hyg., 2012, 56(2), 233–241 CAS.
- M. P. Sutunkova, S. N. Solovyeva, B. A. Katsnelson, V. B. Gurvich, L. I. Privalova and I. A. Minigalieva, et al., A paradoxical response of the rat organism to long-term inhalation of silica-containing submicron (predominantly nanoscale) particles of a collected industrial aerosol at realistic exposure levels, Toxicology, 2017, 384, 59–68 CrossRef CAS PubMed.
- Y. Thomassen, W. Koch, W. Dunkhorst, D. G. Ellingsen, N. P. Skaugset and L. Jordbekken, et al., Ultrafine particles at workplaces of a primary aluminium smelter, J. Environ. Monit., 2006, 8(1), 127–133 RSC.
- Y. Thomassen, D. G. Ellingsen, S. Hetland and G. Sand, Chemical speciation and sequential extraction of Mn in workroom aerosols: analytical methodology and results from a field study in Mn alloy plants, J. Environ. Monit., 2001, 3(6), 555–559 RSC.
- R. R. Larson, S. G. Story and K. T. Hegmann, Assessing the solubility of silicon dioxide particles using simulated lung fluid, The Open Toxicol. J., 2010, 4(1), 51–55 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1em00243k |
| ‡ Current address: Soos Research and Development Center, University of Pannonia, Zrinyi Miklos str. 18, H-8800 Nagykanizsa, Hungary. |
| This journal is © The Royal Society of Chemistry 2021 |