Microaerobic conditions in anaerobic sludge promote changes in bacterial composition favouring biodegradation of polymeric siloxanes†
Abstract
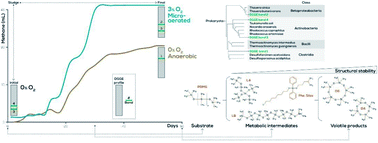
Volatile organic silicon compounds (VOSiC) are harmful pollutants to the biota and ecological dynamics as well as biogas-based energy conversion systems. However, there is a lack of understanding regarding the source of VOSiCs in biogas, especially arising from the biochemical conversion of siloxane polymers such as polydimethylsiloxanes (PDMS). The biodegradation of PDMS was evaluated under anaerobic/microaerobic conditions (PO2 = 0, 1, 3, 5%), using wastewater treatment plant (WWTP) sludge as an inoculum and PDMS as a co-substrate (0, 50, 100, 500 ppm). On average, strictly anaerobic treatments produced significantly less methane than the 3 and 5% microaerated ones, which show the highest PMDS biodegradation at 50 ppm. Thauera sp. and Rhodococcus sp. related phylotypes were identified as the most abundant bacterial groups in microaerated treatments, and siloxane-related molecules were identified as remnants of PDMS catabolism. Our study demonstrates that microaeration promotes changes to the native bacterial community which favour the biological degradation of PDMS. This confirms that the presence of VOSiC (e.g., D4–D6) in biogas is not only due to its direct input in wastewaters, but also to the PDMS microbial catabolism. Microaerobic conditions enhance both PDMS and (subsequent) VOSiC degradation in the liquid phase, increasing the concentrations of D4 and D5 in biogas, and the production of less toxic siloxane-based derivatives in the liquid phase. This study suggests that microaeration of the anaerobic sludge can significantly decrease the concentration of PDMSs in the WWTP effluent. However, for WWTPs to become effective barriers for the emission of these ecotoxic contaminants to the environment, such a strategy needs to be coupled with an efficient biodegradation of VOSiCs from the biogas.
- This article is part of the themed collections: Celebrating Latin American Chemistry and Polymers in liquid formulations


 Please wait while we load your content...
Please wait while we load your content...