Open Access Article
Open Access ArticleRisk assessment of soil heavy metal contamination at the census tract level in the city of Santa Ana, CA: implications for health and environmental justice†
Shahir
Masri
 *a,
Alana M. W.
LeBrón
bc,
Michael D.
Logue
a,
Enrique
Valencia
d,
Abel
Ruiz
e,
Abigail
Reyes
f and
Jun
Wu
*a
*a,
Alana M. W.
LeBrón
bc,
Michael D.
Logue
a,
Enrique
Valencia
d,
Abel
Ruiz
e,
Abigail
Reyes
f and
Jun
Wu
*a
aDepartment of Environmental and Occupational Health, Program in Public Health, University of California, Irvine, CA 92697, USA. E-mail: mdlogue@uci.edu; junwu@hs.uci.edu; masris@uci.edu
bDepartment of Health, Society, and Behavior, University of California, Irvine, CA 92697, USA. E-mail: alebron@uci.edu
cDepartment of Chicano/Latino Studies, University of California, Irvine, CA 92697, USA
dOrange County Environmental Justice, Santa Ana, CA 92705, USA. E-mail: enrique@ocej.org
eJóvenes Cultivando Cambios, Santa Ana, CA 92705, USA. E-mail: agruiz@ucdavis.edu
fCommunity Resilience, University of California, Irvine, CA 92697, USA. E-mail: abigail.reyes@uci.edu
First published on 6th May 2021
Abstract
(1) Background: exposure to heavy metals is associated with adverse health effects and disproportionately impacts low-income communities and communities of color. We carried out a community-based participatory research study to examine the distribution of heavy metal concentrations in the soil and social vulnerabilities to soil heavy metal exposures across Census tracts in Santa Ana, CA. (2) Methods: soil samples (n = 1528) of eight heavy metals including lead (Pb), arsenic (As), manganese (Mn), chromium (Cr), nickel (Ni), copper (Cu), cadmium (Cd), and zinc (Zn) were collected in 2018 across Santa Ana, CA, at a high spatial resolution and analyzed using XRF analysis. Metal concentrations were mapped out and American Community Survey data was utilized to assess metals throughout Census tracts in terms of social and economic variables. Risk assessment was conducted to evaluate carcinogenic and non-carcinogenic risk. (3) Results: concentrations of soil metals varied according to landuse type and socioeconomic factors. Census tracts where the median household income was under $50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 had 390%, 92.9%, 56.6%, and 54.3% higher Pb, Zn, Cd, and As concentrations compared to high-income counterparts. All Census tracts in Santa Ana showed hazard index >1, implying the potential for non-carcinogenic health effects, and nearly all Census tracts showed a cancer risk above 10−4, implying a greater than acceptable risk. Risk was predominantly driven by childhood exposure. (4) Conclusions: findings inform initiatives related to environmental justice and highlight subpopulations at elevated risk of heavy metal exposure, in turn underscoring the need for community-driven recommendations for policies and other actions to remediate soil contamination and protect the health of residents.
000 had 390%, 92.9%, 56.6%, and 54.3% higher Pb, Zn, Cd, and As concentrations compared to high-income counterparts. All Census tracts in Santa Ana showed hazard index >1, implying the potential for non-carcinogenic health effects, and nearly all Census tracts showed a cancer risk above 10−4, implying a greater than acceptable risk. Risk was predominantly driven by childhood exposure. (4) Conclusions: findings inform initiatives related to environmental justice and highlight subpopulations at elevated risk of heavy metal exposure, in turn underscoring the need for community-driven recommendations for policies and other actions to remediate soil contamination and protect the health of residents.
Environmental significance statementThis study employed a community-based participatory research approach to collect and analyze a large number of randomly sampled soil measurements so as to yield a high spatially resolved understanding of the distribution of heavy metals in the soil, and in turn reduce exposure misclassification. The assessment examines average metal concentrations at the Census tract level and by landuse type, which facilitates an understanding of potential contributing sources of heavy metals in the soil and the association between soil contamination and socioeconomic characteristics. |
1. Introduction
Heavy metal soil contamination is a global problem due to anthropogenic emissions of metals and their persistence and non-biodegradable nature. Soil-related heavy metal exposure is particularly important in school and residential areas where children play, as well as industrial sites and recreational areas where soil particles can become resuspended into the air. Importantly, low-income communities, communities of color and urban residents incur disproportionately elevated exposures to such metals.1–3Exposure to heavy metals is associated with a range of adverse health outcomes. Evidence of the harmful health effects of lead (Pb) exposure includes asthma4–7 and adverse neurological and cognitive outcomes in children,8–11 as well as pregnancy complications.12–15 Other heavy metals such as nickel (Ni) and chromium (Cr) are also associated with asthma and inflammation,16,17 while cadmium (Cd) has been associated with high blood pressure, hypertension and osteoporosis.18–20
Globally, arsenic (As) is one of the most problematic metals, causing peripheral vascular and cardiovascular diseases in chronically exposed populations and linked with type II diabetes.21–23 Regarding manganese (Mn), Lucchini et al. (2007) found an increased prevalence in Parkinson's disease in communities with chronic environmental exposure,24 and also demonstrated significant deficits in hand dexterity, motor coordination, and odor identification among Mn-exposed adolescents relative to a non-exposed reference group.25,26 Other metals, including Pb and As, are also known to impact the central nervous system.22,23
Kidney damage is associated with Pb, Cd, and (copper) Cu exposures.22,27 At ambient levels, zinc (Zn) and Cu tend to be the least toxic of the metals described, although health effects still include headaches, dizziness, and irritation of the nose, mouth and eyes for elevated Cu exposure, and cytotoxicity for Zn exposure. Importantly, Pb, As, Ni, Cd, and Cr are known human carcinogens, associated with cancers of the lung, kidney, liver, and bladder.16,17,21,28
Sources contributing to soil heavy metal accumulation, and therefore exposure, include lead paint from older homes and structures,29,30 as well as the historic use of lead in on-road vehicles, agricultural products, and smelting process.31 Cr and Ni mostly enter the environment through releases by electroplating processes and the disposal of metal-containing waste, while other heavy metals are emitted through a combination of industrial activities such as mining, agricultural inputs (i.e. pesticides, fertilizers), fossil fuel and waste combustion, and metal processing.32
Evidence of widespread heavy metal exposure and impacts has given rise to policies to bar the sale of certain heavy-metal-containing products (e.g. lead paint) in the U.S. and to the listing of the heavy metals As, Pb, Cd, Cr, Cu, Ni and Zn as priority control pollutants by the United States Environmental Protection Agency (USEPA).33 Despite such measures, households, individuals, and communities continue to incur metal exposures through current and past emissions and products.34,35
Although mostly focused on Pb, evidence has shown that heavy metal exposure is not equally realized across the United States. In Oakland, California, McClintock (2012) reported significantly higher Pb levels in West Oakland, made up predominantly of low-income and African American residents, relative to the affluent, predominantly white Oakland hills area. Similarly, Zhuo et al. (2012) showed the proportion of Latina/o/x residents within Census tracts to correlate with soil Pb levels in Phoenix, Arizona.36 In our prior work investigating soil Pb contamination in Santa Ana, CA, Census tracts with a greater proportion of low-income or Latina/o/x residents showed roughly two- to five-times higher soil Pb levels relative to their higher-income or less-Latina/o/x counterparts.37 Studies have also shown Black1 and Latina/o/x1,2 children, and those from low-income households,1 to consistently show elevated blood lead concentrations compared to non-Latino white children and children from higher income households.
Although evidence suggests that a similar pattern may be evident as it relates to the distribution of other (non-Pb) soil heavy metals in the context of social and economic factors, this area has been less studied compared to Pb.38
Specifically, to our knowledge, few studies to date have carried out a high-resolution assessment of the spatial distribution of multiple heavy metals in an urban environment while also considering the spatial distribution of social and economic characteristics of the resident populations.39,40 Of the studies we identified, none carried out a quantitative health risk assessment using toxicity values to estimate cumulative risk by Census tract.
In this analysis, we built upon our prior investigation of the spatial distribution of soil Pb and exposure-related social vulnerabilities in Santa Ana by considering the concentrations, distributions, and cumulative health risks related to eight heavy metals that are of particular relevance to public health, including Pb, As, Mn, Cr, Ni, Cu, Cd, and Zn.38,41–43 This study centers around the following questions: (1) are residential socioeconomic status and racial/ethnic factors at the Census tract level correlated with concentrations of heavy metals in the soil? (2) Given childhood vulnerability to heavy metal exposure and health impacts, are Census tracts with a higher proportion of children correlated with elevated soil heavy metals? (3) Is Santa Ana at a high risk of non-carcinogenic or carcinogenic health outcomes due to soil contamination? (4) Do economic and social vulnerabilities aggregate so as to create heavy metal-related vulnerability hotspots across certain Census tract?
Based on experiences and concerns that have emerged from discussions with our community-academic partners and affected residents, as well as our review of the literature, it was our hypothesis that Census tracts of lower socioeconomic status, with a higher fraction of residents who identify as Latina/o/x, and those with a higher proportion of children have increased soil heavy metal concentrations; that hotspots exist related to cumulative health risk; and that economic and social vulnerabilities to soil heavy metal exposures correlate with cumulative risk.
2. Materials and methods
This analysis was carried out as part of the ¡Plo-NO Santa Ana! Lead-free Santa Ana! community-academic partnership. Since 2017, our partnership has been working to understand and intervene upon environmental injustices to promote health equity and economic, political, and social well-being in Santa Ana, CA.44 Partners consist of Orange County Environmental Justice; Jóvenes Cultivando Cambios (a youth-led cooperative); and faculty and staff at the University of California, Irvine. Importantly, these community and academic partners were all intimately involved in each step of the research process from visualization and conception to data collection and the guiding of central research questions and analyses. Examples of these collaborative processes in action included various in-person workshops that we held both in Santa Ana and at the university (UC Irvine) during which local residents and volunteers of all ages came together with academic partners in order to discuss issues related to local heavy metal exposure and public health, develop skills related to data collection, and share ideas and questions that would ultimately guide our research. For instance, as a project deliverable, several residents stated an interest in learning whether soil contamination was greater in poor neighborhoods relative to more affluent areas. Based on such input, we then incorporated this research question into our analysis.During the onset of the COVID-19 pandemic, in-person community workshops and meetings turned into weekly virtual meetings, during which community partners were regularly updated as to the most recent findings concerning data analysis. Further details about this partnership and its origin can be found in our previously published work.37,44 Data used in this analysis include soil samples obtained by trained personnel as well as demographic information from the U.S. Census Bureau's American Community Survey.45 This study was classified as exempt by the Institutional Review Board at the University of California, Irvine.
2.1 Study region
Located in the southwestern region of California, in the United States, Santa Ana is a densely populated city with an estimated 337![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 716 residents. With 61 Census tracts, Santa Ana spans 70.6 km2.46 The administrative center of Orange County, Santa Ana is the second biggest city in Orange County and is the eleventh biggest in California.46 The majority of residents in Santa Ana identify as Latina/o/x (77.3%), followed by Asian (11.4%) and white (9.4%), with a high proportion of immigrant residents (45.2%).47 As of 2019, Santa Ana includes 78
716 residents. With 61 Census tracts, Santa Ana spans 70.6 km2.46 The administrative center of Orange County, Santa Ana is the second biggest city in Orange County and is the eleventh biggest in California.46 The majority of residents in Santa Ana identify as Latina/o/x (77.3%), followed by Asian (11.4%) and white (9.4%), with a high proportion of immigrant residents (45.2%).47 As of 2019, Santa Ana includes 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 563 housing units and has a median household income of $65
563 housing units and has a median household income of $65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 (2018 dollars).46
313 (2018 dollars).46
2.2 Field sampling
In Summer–Fall 2018, soil samples were collected across seven landuse types in Santa Ana, CA: arterial roads, parks and gardens, schools, industrial regions, business areas, and residential zones. Since the majority of businesses, schools, and industrial areas were not accessible directly, soil samples were obtained at their boundaries (e.g. roadway near facility). When possible within Census tracts, a minimum of six residential units were sampled. Field teams recorded landuse categories and the location of each sampling site using coordinates identified through global positioning system (GPS) readings. Field teams responsible for soil sampling and the recording of landuse types consisted of local community volunteers, including numerous members of the youth community, who were first trained by a field coordinator.Building upon methodologies by Wu et al.,48 field teams selected sampling locations at each sampling site that were not obstructed by physical barriers. Where possible, field teams marked a three-foot radius, and obtained soil samples from five distinct points (one central point and four other points that were three feet away from the central point) after removing 1 cm of soil (including vegetative matter). At residential units, field teams drew samples from dripline areas around the home and a minimum of two locations throughout the yard (e.g., front yard, back yard). Between four and five samples were collected from each garden site. In preparation for laboratory analysis, all samples were air dried and sieved with brass screen (#50 mesh, twice; #100 mesh once), which yielded fine soil particles. In a Santa Ana pilot soil analysis, we found lead concentrations in soil to be very similar whether sieving for coarse or fine soil particles. Thus, in the present study, we focused on fine soil particles since this size fraction resembled the heavy metal exposures that pose a particular threat to young children.49 In total, 1528 samples across 560 different locations were obtained throughout Santa Ana, CA, resulting in a highly spatially resolved characterization of soil heavy metals. For the purpose of establishing baseline soil heavy metal levels, eight soil samples were collected in areas outside of the Santa Ana, in nearby state and regional parks in the county that were relatively natural and unimpacted by local anthropogenic heavy metal sources such as industry, traffic, and construction.
2.3 Soil analysis
Soil samples were measured using XRF technology (SPECTRO XEPOS HE Benchtop XRF Spectrometer), a well-established procedure for identifying the concentrations of commonly measured heavy metals in different sampling media.50 XRF methods have been used extensively in scientific research39,51,52 and have been shown to give results that are comparable to ICP-MS methods.50 However, compared to ICP-MS and AAS methods, XRF analysis has the advantage of being non-destructive, which allows for flexibility in doing future analyses using the same samples. The instrument employed in this investigation operates optimally at temperatures between 20–25 °C and undergoes regular multi-channel analysis calibration using standard reference materials on a weekly basis, with global calibration conducted twice per year. Each soil sample was analyzed five times to ensure reproducibility and stability of measurements. Absolute measurement errors were in the range of 0.1% and 2.5% for all elements described in this study, with the exception of As and Cd, which had errors of 9.6% and 20.2%, respectively. To reaffirm quality laboratory analysis, a subset of soil samples (n = 18) were subjected to a second round of XRF analysis (five more scans), resulting in a very high correlation (r = 1.0). The limit of detection (LOD) using the XRF instrument was very low and varied depending on the element analyzed. LODs for the elements analyzed in this study were 0.1 ppm (As), 0.2 ppm (Pb, Mn, Cr, Cd, and Zn), 0.5 ppm (Cu), 1 ppm (Ni).532.4 Landuse
Some landuse categories were sufficiently similar such that we consolidated them, resulting in more meaningful sample sizes. For this study, garden and park samples were considered a single landuse category labeled “park,” while business and industrial area samples were consolidated under the label “industrial.” Of note, arterial roadway samples are referred to as “roadways.” Accordingly, there were five landuse types in total in this study including: schools, roadways, parks, residential units, and industrial areas.2.5 Demographics
Population information for Census tracts (n = 61) in Santa Ana, CA, were drawn from the 2010 Census. Demographic factors at the Census tract level, including household income, education, race/ethnicity, insurance coverage, nativity, languages spoken, and age were drawn from the American Community Survey (ACS), which is conducted annually. ACS data averaged over five years from 2012–2016 (henceforth, 2016) was employed in this study since averages represent a more stable estimate of community-level characteristics, and since 2016 was the most recent year for which geo-coded ArcGIS files were available.This study also makes use of a so-called vulnerability index that was developed in our previous work in order to compare social and economic vulnerability with heavy metal concentrations and risk.37 This index took into account six social and economic factors that could result in a community being at an increased health risk due to heavy metal exposure, including: median household income, percent of housing units occupied by renters, percent of population under age five, percent of residents reporting speaking limited or no English, percent of residents without health insurance coverage, and percent of residents with a college education or higher. Values for each factor were calculated based on quartile distribution rankings, and scaled to range from 0 (low risk) to 1 (high risk). Further details on the development of this index can be found in Masri et al. (2020).
2.6 Analysis
Summary statistics for heavy metal concentrations were calculated across all soil samples and for groups of samples based on landuse categories, as well as for one (baseline) group of samples obtained outside of Santa Ana. To visually depict the distribution of heavy metal concentrations and estimate their levels between sampling sites, we employed ArcGIS software to carry out simple kriging for each element using variogram covariances that employed 12 lags and a default lag size, nugget size, and partial sill.To assess the variability in soil metal concentrations and demographic characteristics within Census tracts, we calculated specific demographic indicators including the proportion of residents who identified as Latina/o/x or Hispanic, those who reported speaking no or limited English, residents who identified as immigrant or non-native (henceforth, immigrants), those who did not possess health insurance, residents living in renter-occupied housing, residents under five years old and those with a college education or higher. Once these proportions were calculated, we then matched them with soil samples and sorted them according to their demographic attribute values. This allowed us to separate heavy metal samples into distinct tertiles based on the demographic attributes of the Census tracts from which each sample originated. Details on these methods can be found in our prior work.37 Given a total of n = 1528 samples, the sample size for each tertile was approximately n = 510 ± 20. To determine statistical significance between sample means, we employed a cutoff of p = 0.05.
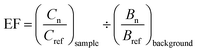 | (1) |
To interpret EF values, we employed a five-category system previously introduced by Sutherland and used in other studies,55,56 which indicates: no or minimal enrichment (EF < 2), moderate enrichment (2 ≤ EF < 5), significant enrichment (5 ≤ EF < 20), very high enrichment (20 ≤ EF < 40), and extremely high enrichment (EF ≥ 40).
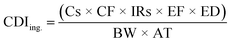 | (2) |
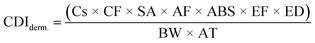 | (3) |
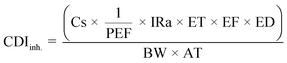 | (4) |
| Factor | Definition | Unit | Value | |
|---|---|---|---|---|
| Child | Adults | |||
| Cs | Soil metal concentration | mg kg−1 | — | — |
| IRs | Soil ingestion rate | mg per day | 200 | 100 |
| SA | Skin surface area available for exposure | cm2 per day | 2373 | 6032 |
| AF | Soil-to-skin adherence factor | mg cm−2 | 0.2 | 0.07 |
| IRa | Inhalation rate | m3 h−1 | 0.53 | 0.83 |
| ED | Exposure duration | Years | 6 | 20 |
| BW | Body weight | kg | 15 | 80 |
| AT | Averaging time | Days | 365 × ED | 365 × ED |
| PEF | Soil-to-air particulate emission factor | m3 kg−1 | 1.36 × 109 | |
| CF | Conversion factor | kg mg−1 | 1 × 10−6 | |
| ET | Exposure time | Hours per day | 24 | |
| EF | Exposure frequency | Days per year | 350 | |
| ABS | Absorption factor | Unitless | 0.03 for As, 0.01 for other metals | |
To derive either child or adult non-carcinogenic risk associated with each exposure route for each metal, the hazard quotient (HQ) for a given metal was calculated by dividing the CDI values for each exposure route by the reference dose (RfD) (mg per kg per day) for that metal, in turn yielding a unitless value according to eqn (5):
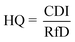 | (5) |
HQ values for each exposure route were then summed together to produce a cumulative HQ value (separate for children and adults). To then characterize the total child or adult non-carcinogenic risk associated with multiple metals exposures, these cumulative HQ values for each metal were summed together to calculate an overall hazard index (HI) expressed as a unitless number, as shown in eqn (6):
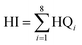 | (6) |
To derive child or adult lifetime cancer risk associated with each exposure route for each metal, the cancer risk for a given metal was calculated by multiplying child or adult CDI values for each exposure route by the cancer slope factor (CSF) for that metal and exposure route, in turn yielding a unitless value according to eqn (7):
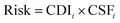 | (7) |
To characterize the total lifetime cancer risk associated with multiple metals exposures, we considered a subset of five heavy metals that are considered carcinogenic according to the California Office of Environmental Health Hazard Assessment (OEHHA). These included Pb, As, Cr, Ni, and Cd. Risk values for each metal were summed together, as shown in eqn (8):
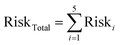 | (8) |
To obtain a unique HI value and cancer risk at the Census tract level, the previously described equations were applied separately for each Census tract using the average heavy metal concentrations measured across all samples collected within a given Census tract. RfDs and CSFs used in this analysis are presented in Table 2 and include those reported in the EPA's Integrated Risk Information System (IRIS).59 For some elements, a range of values were reported. In these cases, we used the lower and upper end of the range of RfDs or CSFs in order to calculate separate lower and upper risk estimates (described below). Where IRIS values differed from California OEHHA values, we included the California-reported values when considering the full range of RfDs and CSFs.60
| Reference dose (mg per kg per day) | Cancer slope factors (mg per kg per day)−1 | |||||
|---|---|---|---|---|---|---|
| RfDing. | RfDinh. | RfDderm. | CSFing. | CSFinh. | CSFderm. | |
| a CalEPA. | ||||||
| Pb | 0.0035 | — | 0.0007 | 0.0085 | 0.042 | 0.425 |
| As | 0.0003 | 0.000004 | 0.00006 | 1.5–9.5a | 12 | 47.5 |
| Mn | 0.024–0.14 | 0.000014 | 0.0048–0.028 | — | — | — |
| Cr(III) | 1.5 | — | 0.3 | — | — | — |
| Cr(VI) | 0.003 | 0.000029 | 0.0006 | 0.42 | 510 | 2.1 |
| Ni | 0.02 | 0.000006 | 0.004 | 0.91 | 0.91 | 4.55 |
| Cu | 0.04 | — | 0.008 | — | — | — |
| Cd | 0.001 | 0.000003 | 0.0002 | — | 1.5–15 | — |
| Zn | 0.3 | — | 0.06 | — | — | — |
To calculate lower-bound HI values and cancer risks for each Census tract, we applied Table 2, making use of the lower values where ranges existed. An additional assumption was that 100% of measured chromium was of the less toxic and non-carcinogenic trivalent form (Cr(III)). In contrast, the calculation of upper-bound estimates made use of Table 2 while applying the upper values where ranges existed. The upper estimate assumed that all chromium was of the more toxic and carcinogenic hexavalent form (Cr(VI)). Our assumption of either 0% or 100% hexavalent chromium for the two scenarios was due to our not knowing the concentrations of these respective forms in the soil of our study area and due to our not being able to identify a common ratio of the these two forms in typical soil, particularly near our region of interest. Thus, we felt this approach was the best way to estimate the potential range of soil contamination and related risk.
In calculating health risk, non-carcinogenic toxicity is understood to arise only above a discrete exposure level, or threshold, whereas carcinogenicity is considered to be non-threshold and therefore exhibits effects linearly even at the lowest doses. These distinctions underly the applications of either a reference dose (non-cancer) or cancer slope factor (cancer). For Pb, while no safe threshold of exposure has been identified for non-carcinogenic risk, we assumed an RfD of 0.0035 mg per kg per day, which is commonly applied in the literature.41,61 While it was important to include Pb in our assessment of non-carcinogenic risk, this assumption of a threshold response may yield a conservative estimate for Pb-related health risk.
To convert oral toxicity values to dermal values, since oral values are derived from potential (i.e. administered) doses that don't account for gastrointestinal absorption, we applied conventional methods that adjust for an assumed GI absorption of 20% across inorganic chemicals.62 In the case of inhalation, we converted reference concentrations (RfCs) to RfDs using basic arithmetic that assumed an average inhalation rate of 20 m3 per day and body weight of 80 kg.62 Since converting to child-specific RfDs using child exposure values is often incorrect given the methods used to derive original RfCs,62 we extended our adult-derived RfDs to child exposure scenarios in the present analysis. While this approach can foreseeably create uncertainty for child-related exposure, sensitivity analyses using adult-only conversions versus conversions unique to children and adults confirmed this decision to be of negligible importance as final risk estimates were virtually unchanged.
3. Results
3.1 Spatial patterning of heavy metal soil concentrations
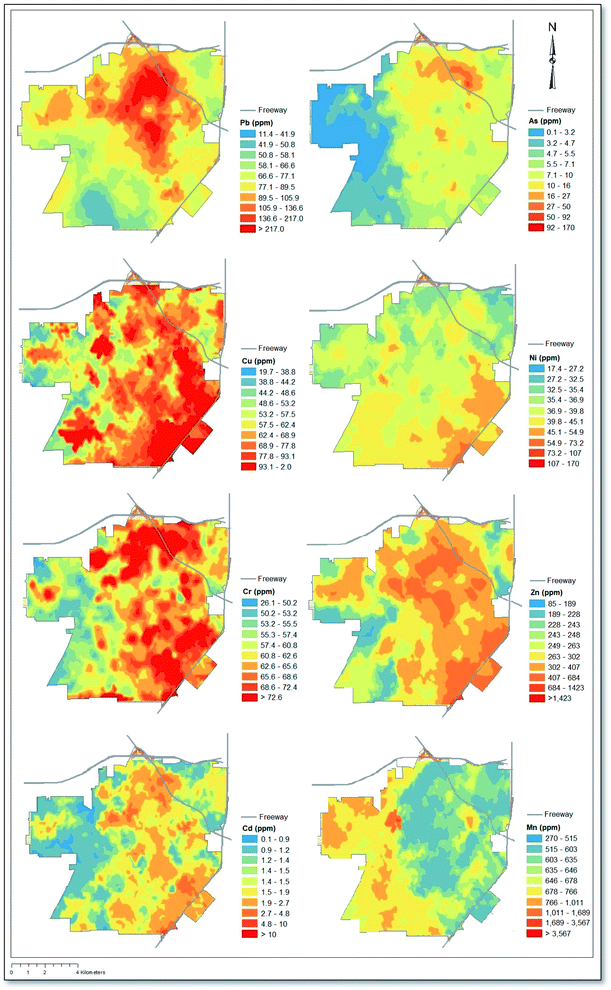
Depicted in Fig. 1 is a map showing interpolated soil concentrations of eight heavy metals derived from kriging. Results relating to Pb concentrations, first published in our original study by Masri et al. (2020), illustrate the highest concentrations in the northeastern quadrant and central region of the city, which corresponds with the downtown area of Santa Ana. By comparison, As and Mn exhibited east–west gradients, with higher As concentrations in the east and the opposite pattern observed for Mn. Cr, Cd, and Zn showed similar patterns as Pb, albeit with high concentrations extending southeast all the way to the state route 55 freeway. Cu exhibited similar patterns as Cr, Cd, and Zn, however Cu concentrations reached their highest in the southeast quadrant. Of note, the southeast quadrant of Santa Ana is also adjacent to the John Wayne Airport (see Fig. S1†).3.2 Descriptive statistics & soil screening levels
Table 3 presents summary statistics for eight soil heavy metal samples and the extent to which either U.S. EPA or California EPA residential soil screening levels (SLs) for the protection of children were exceeded.60,63 Of note, the mean, median, S. D., Min, and Max values presented for Pb in Tables 3 and 4 are excerpted from our first Santa Ana-based soil study by Masri et al. (2020). Where cancer and non-cancer SLs were available, the more protective standard was included in the table. Metals for which at least 1% of samples exceeded federal SLs included Pb (3.9%), As (91.1%), Cr (100%, assuming Cr(VI)), and Ni (100%). CalEPA SLs, which were generally more stringent, were exceeded by Pb (48.2%), As (100%), Cr (100%, assuming Cr(VI)), and Cd (32.6%). For Cr, the proportion of SL exceedances changed drastically depending on whether measured Cr was assumed to be Cr(III) or Cr(VI). Assuming that all measured Cr is the less harmful trivalent form yields 0% exceedance across all samples, compared to 100% exceedance when we assume that all samples are of the hexavalent form.| Pb | As | Mn | Cr | Ni | Cu | Cd | Zn | ||
|---|---|---|---|---|---|---|---|---|---|
| a CalEPA SL values come from CalEPA human-exposure-based screening numbers developed to aid the estimation of cleanup costs for contaminated soil60 while USEPA SL values come from USEPA's updated 2020 table of regional screening levels.63 b Value for carcinogenic outcomes.63 | |||||||||
| Min | 11.4 | 0.1 | 270.1 | 19.3 | 16.0 | 19.7 | 0.1 | 76.8 | |
| 50th | 77.8 | 6.6 | 646.9 | 61.2 | 37.5 | 56.4 | 1.4 | 266.9 | |
| Mean | 123.1 | 8.3 | 663.4 | 64.9 | 38.7 | 67.6 | 1.7 | 328.0 | |
| Max | 2687.0 | 174.8 | 8774.0 | 279.1 | 170.3 | 1950.0 | 23.9 | 3390.0 | |
| S.D. | 181.3 | 9.3 | 236.0 | 20.3 | 9.7 | 79.6 | 1.4 | 233.3 | |
| Screening level (SL) | Cr(III) | Cr(VI) | |||||||
| USEPA | 400 | 0.68b | 1800 | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.3b | 0.76b | 3100b | 71 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| CalEPA | 80 | 0.07 | — | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.3b | 820 | 3000 | 1.7 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| % exceeding SLs | |||||||||
| USEPA | 3.9 | 91.1 | <1 | 0 | 100 | 100 | 0 | 0 | 0 |
| CalEPA | 48.2 | 100.0 | — | 0 | 100 | 0 | 0 | 32.6 | 0 |
| N | Pb | As | Mn | Cr | Ni | Cu | Cd | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| a The landuse type with the highest mean concentration is shown in bold for each column. b N, number of samples. | |||||||||
| Industry | 89 | 122.6 (164.8) | 8.4 (6.2) | 736.0 (866.0) | 72.1 (25.0) | 50.0 (16.0) | 291.8 (221.6) | 2.8 (3.5) | 449.3 (342.7) |
| Park | 161 | 72.5 (75.3) | 7.1 (6.3) | 647.8 (128.0) | 56.0 (10.9) | 37.8 (9.1) | 44.4 (14.7) | 1.2 (0.5) | 201.2 (79.8) |
| Residential | 1173 | 128.4 (187.9) | 8.3 (8.5) | 661.0 (109.5) | 65.7 (20.3) | 37.6 (7.9) | 64.4 (32.2) | 1.7 (1.1) | 338.7 (236.8) |
| Roadway | 76 | 172.9 (251.1) | 11.9 (21.6) | 658.9 (127.3) | 66.0 (25.4) | 43.7 (16.7) | 64.0 (34.6) | 1.6 (0.9) | 313.9 (142.4) |
| School | 10 | 37.9 (12.9) | 9.8 (16.2) | 527.9 (40.3) | 52.8 (5.6) | 34.0 (6.3) | 48.1 (14.2) | 0.88 (0.5) | 279.6 (175.6) |
| All | 1528 | 123.1 (181.3) | 8.3 (9.3) | 663.4 (236.0) | 65.0 (20.3) | 38.7 (9.7) | 67.6 (79.6) | 1.7 (1.4) | 328.0 (233.3) |
As a proportion of Pb samples obtained within a single landuse category, roadway, industry and residential samples each exceeded the 80 ppm California SL at a frequency of approximately 52%, compared to 22% of samples and zero samples exceeding the SL in park and school areas. The 400 ppm federal SL was exceeded most often by samples obtained in the roadway (12%) areas, compared to residential (4%), industrial (3.4%), park (0.6%), and school (0%) landuse types.
For As and Cd, nearly all and roughly one-third of samples, respectively, exceeded the California SL, compared to 91% and 0% exceedances when considering the federal SL. For As, average concentrations were highest near roadways, but also showed relatively high levels near schools (though school samples were limited to n = 10). As a fraction of samples collected within a single landuse type, little variability in As was seen (90–96% of samples exceeding federal SL in each landuse type). Cd showed the highest average concentrations within the industrial landuse type. Samples within this landuse type also exceeded the California SL at the highest frequency (56.2% of samples), compared to residential (33.7%), roadway (32.9%), park (13.7%), and school (0%) samples. Zero Cd samples exceeded the federal SL. For the remaining metals analyzed, the highest average concentrations were measured within industrial landuse types, and the percent exceedance (see Table 3) of state and federal SLs were binary (0% or 100% of samples exceeding the SL) depending on which SL was considered. Table 4 presents the average concentration of each heavy metal according to landuse type.
Although some metals did not exceed state or federal SLs standards, they were nonetheless found to be enriched in the soil relative to background levels. Table 5 summarizes these findings by showing the percent of samples that were enriched in each of eight heavy metals based on the enrichment factor values that were calculated (using eqn (1)) for each of the 1528 soil samples (using Fe as reference element). Of all metals assessed, those that were moderately or more enriched in the greatest proportion of samples included Zn (∼65% of samples) followed by Pb (∼50% of samples), with approximately 10% of samples (in both cases) showing “significant” enrichment. Arsenic showed moderate or greater enrichment across approximately 29% of samples, with roughly 3% showing “significant” enrichment. By comparison, Cu showed <10% enrichment, whereas Mn, Cr, Ni, and Cd showed virtually no enrichment.
| Pb | As | Mn | Cr | Ni | Cu | Cd | Zn | |
|---|---|---|---|---|---|---|---|---|
| a Fe was used as reference element. | ||||||||
| No or minimal enrichment | 50.2 | 71.2 | 99.8 | 99.1 | 99.8 | 92.2 | 99.7 | 34.6 |
| Moderate enrichment | 38.2 | 25.7 | 0.1 | 0.9 | 0.2 | 6.8 | 0.3 | 56.5 |
| Significant enrichment | 10.2 | 3.0 | 0.1 | 0.9 | 8.8 | |||
| Very high enrichment | 1.1 | 0.1 | 0.1 | 0.1 | ||||
| Extremely high enrichment | 0.3 | |||||||
When comparing metal samples in terms of the proportion of SL exceedances and the proportion of enrichment, Pb and As showed both substantial enrichment and SL exceedance. Similarly, Mn and Cu showed agreement in that enrichment and SL exceedances were both found to be minimal. For Cr and Ni, the proportion of SL exceedances changed dramatically depending on the SL used for comparison. Zn was the only metal in which substantial enrichment was found despite not exceeding state or federal SLs. Results were similar when using Al as the reference element instead of Fe, as shown in Table S1.†
Concentration means for each heavy metal were higher than their medians. This, combined with an abundance of outliers above the mean, suggests that the concentration distribution of soil metal was consistently positively skewed. Concentration boxplots for all eight heavy metals in are depicted in Fig. S2† of the ESI.
3.3 Social patterning of heavy metal soil concentrations
Fig. 2 presents average metal concentrations and 95% confidence intervals (CI) by median household income at the Census tract level (tertiles). Average metal concentrations decreased with increasing income bracket for seven of eight metals assessed, the exception being for Mn where the opposite trend was observed as Mn concentrations tended to be higher in the western side of the city. Differences in average concentrations between tertiles were statistically significant (p < 0.05) for each tertile of Pb, Zn, and Cd, and statistically significant for the first and third tertiles of Mn and Cr.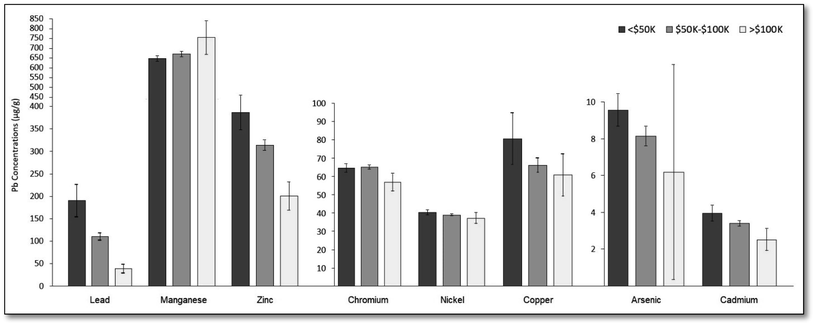 | ||
| Fig. 2 Average heavy metal concentrations and 95% CIs (shown as error bars) of heavy metal soil samples based on the median household income across Census tracts. | ||
The largest disparities existed for Pb, Zn, Cd and As, where on average, soil samples obtained in Census tracts where the median household incomes were below $50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 had 390%, 92.9%, 56.6%, and 54.3% higher concentrations relative to samples obtained in Census tracts where median household incomes were above $100
000 had 390%, 92.9%, 56.6%, and 54.3% higher concentrations relative to samples obtained in Census tracts where median household incomes were above $100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively. Note, the portion of Fig. 2 relating to Pb concentrations was originally published in our first paper on soil lead in Santa Ana.37
000, respectively. Note, the portion of Fig. 2 relating to Pb concentrations was originally published in our first paper on soil lead in Santa Ana.37
Soil samples from Census tracts in the tertile with the lowest proportion of college educated residents had 87.0% and 26.5% higher Pb and Zn concentrations on average relative to those in the highest tertile, respectively. Similarly, soil samples collected in the tertile with the highest proportion of renter occupied housing units had 75.2%, 17.4%, 38.1%, 19.1%, and 33.4% higher Pb, As, Cu, Cd, and Zn concentrations, respectively, compared to samples from the lowest tertile.
Soil samples from the tertile with the highest proportion of residents without health insurance had 96.1%, 17.1%, 14.1%, and 31.9% higher Pb, As, Cd, and Zn concentrations, respectively, and those from the tertile with the highest proportion of children (under age five) had 90.0%, 11.1%, and 27.3% higher Pb, Cd and Zn concentrations, respectively, than to those from the lowest tertiles.
In terms of social patterning, relative to soil samples collected from the lowest tertiles, soil samples collected in tertiles with: the highest proportion of non-English speaking residents had 66.1% and 24.7% higher Pb and Zn concentrations, respectively; the highest proportion of immigrant residents had 96.4%, 18.8%, 20.9%, and 32.4% higher Pb, Cu, Cd, and Zn concentrations, respectively; the highest proportion of Latina/o/x or Hispanic residents had 105.1%, 17.0%, 20.6% and 32.6% higher Pb, As, Cd, and Zn concentrations, respectively. In contrast, the opposite pattern was observed for Mn where the tertile with the lowest proportion of Latina/o/x or Hispanic residents had 11.5% higher Mn concentrations relative to those from the lowest tertile.
3.4 Socio-spatial distribution of heavy metal concentrations and health risks
Correlation coefficients of demographic characteristics plotted against lower- and upper-bound estimates of non-carcinogenic (HI values) and carcinogenic risk at the Census tract level (n = 61) showed moderate (|r| ≥ 0.3), statistically significant (p < 0.05) positive correlations between non-cancer risk (both lower and upper estimates) and the percent of residents under five years of age (r = 0.43 to 0.46), the percent of renter-occupied housing units (r = 0.41 to 0.42), as well as the overall vulnerability index (r = 0.31, lower risk scenario only). Although statistically significant, the correlation between the upper-estimated non-carcinogenic risk and vulnerability index fell just below (r = 0.29) our definition of “moderate” correlation. Statistically significant positive correlations were also found between lower-estimated cancer risk and the percent of residents under five years of age (r = 0.31) and the percent of Hispanic residents (r = 0.30). A statistically significant, near–moderate correlation (r = 0.29) was found between this cancer risk and the percent of renter-occupied housing units. Moderate and significant correlations also existed between average Pb concentrations and the percent of residents under age five (r = 0.51), median household income (r = −0.38), the percent of renter occupied housing units (r = 0.46), and overall vulnerability index (r = 0.42). Although significant, the correlation between Pb and the percent of Hispanic residents was just under moderate (r = 0.29).Moderate and significant negative correlations were found between lower- and upper-estimated cancer risk (r = −0.38 to −0.30) and the percent of immigrants, as well as between the percent of immigrants and As (r = −0.39) and Cr (r = −0.39) concentrations. When divided according to the two most predominant immigrant populations (Latina/o/x and Asian), the percent of Asian residents showed similarly negative (and even stronger) correlations with these variables, while the correlation for Latina/o/x immigrants showed weak and non-significant correlations. Table S2† presents all correlation coefficients relating to average heavy metal concentrations, demographic characteristics, and estimated health risk at the Census tract level.
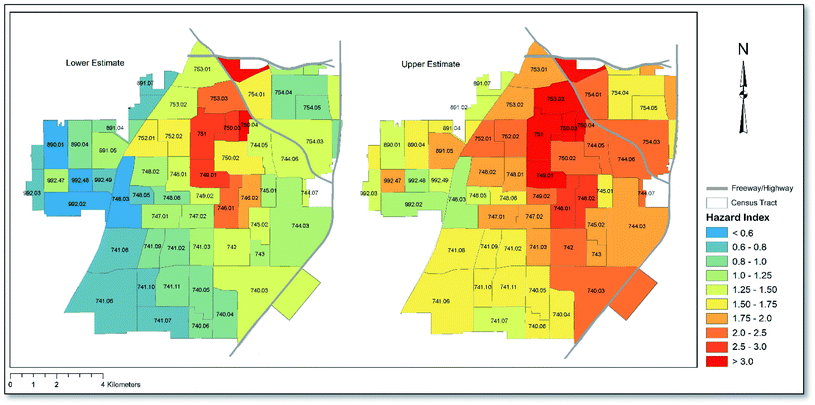
Shown in Fig. 3 is a map depicting Santa Ana Census tracts according to HI scores that were derived using both lower and upper RfD values (see Table 2) where applicable (Mn only). As shown, depending on whether upper or lower RfDs were applied for Mn, between 35 and 61 (57–100%) Census tracts in Santa Ana showed HI ≥ 1, implying the potential for non-carcinogenic health effects across the majority of Census tracts. The pattern of risk was similar across both lower and upper risk scenarios, with the cluster of Census tracts in the central region of the city, just south of the I-5 and SR-22 freeways, showing the highest HI values. When assessing the average concentration of each heavy metal across all sampling points, the cumulative HI for Santa Ana was calculated to be 1.3 under lower risk assumptions. When upper RfD values were assumed, a cumulative HI of 2.0 was calculated, which is twice as high as the threshold (HI = 1) for non-carcinogenic health effects. The HI for the city was driven overwhelmingly by exposure incurred through childhood (∼9 times higher than adults) under both upper and lower RfD scenarios, since children have substantially higher soil ingestion rates and lower body weights. The calculated hazard quotients (child and adult risk combined) associated with each metal decreased in the order Pb > As > Mn > Cr > Ni > Cd > Cu > Zn under the upper RfD scenario and in the order Pb > As > Mn > Ni > Cd > Cu > Zn > Cr under the lower RfD scenario, with ingestion and dermal exposure being the predominant exposure routes under both scenarios, accounting for approximately 80% and 17% of the HI, respectively. A full list of HI and HQ values corresponding to Fig. 3 for children and adults, and according to each exposure route, can be found in Table S3.†
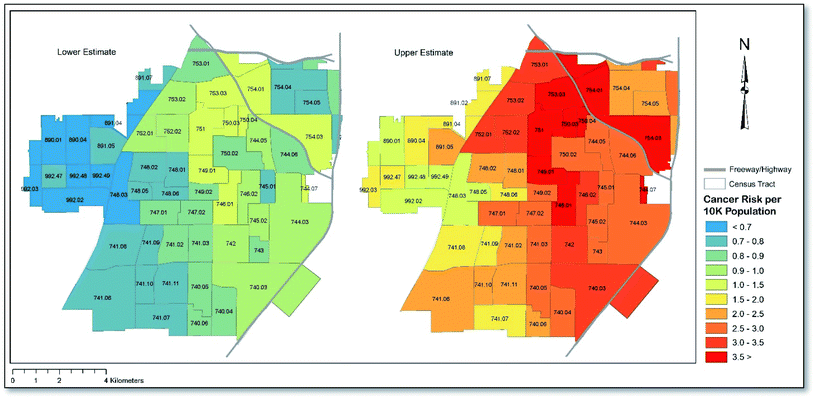
Fig. 4 presents the lower- and upper-estimated cancer risk per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population at the Census tract level. As shown, the application of lower CSFs, including the assumption that all chromium is of the non-carcinogenic form (Cr(III)), yields cumulative cancer risks that are above the upper-end of EPA's generally acceptable risk range (10−6 to 10−4) for 10 of 61 (∼16%) Census tracts. When using upper CSFs, and assuming all Cr is of the carcinogenic form (Cr(VI)), all 61 (100%) Census tracts in the city showed cancer risks in excess of the EPA's generally acceptable risk range. When assessing the average concentration of each carcinogenic metal across all sampling points, the cumulative cancer risk for Santa Ana was calculated to be between 8.5 × 10−5 and 2.7 × 10−4, depending on which set of assumptions were applied. In the case of the latter value, the cancer risk equates to nearly three-times that which the EPA considers generally acceptable. Cancer risk was mostly driven by exposure during childhood, which showed a cumulative risk of approximately three-times that of adults. In terms of each metal, arsenic accounted for the majority of the cancer risk, with a risk that was nearly three-times higher than the next two most high-risk metals (upper CSF scenario). Overall, the calculated risk related to each metal decreased in the order As > Ni > Cr > Pb > Cd under the upper CSF scenario and in the order Ni > As > Pb > Cd under the lower CSF scenario. As with non-carcinogenic risk, ingestion and dermal exposure were the predominant exposure routes under both scenarios, accounting for approximately 80% and 20% of the cumulative cancer risk, respectively. Note that Cr does not show up in the lower scenario ranking since that scenario assumed that all chromium was of the non-carcinogenic form. A full list of cancer risk values corresponding to Fig. 4 for children and adults, and according to each exposure route, can be found in Table S4.†
000 population at the Census tract level. As shown, the application of lower CSFs, including the assumption that all chromium is of the non-carcinogenic form (Cr(III)), yields cumulative cancer risks that are above the upper-end of EPA's generally acceptable risk range (10−6 to 10−4) for 10 of 61 (∼16%) Census tracts. When using upper CSFs, and assuming all Cr is of the carcinogenic form (Cr(VI)), all 61 (100%) Census tracts in the city showed cancer risks in excess of the EPA's generally acceptable risk range. When assessing the average concentration of each carcinogenic metal across all sampling points, the cumulative cancer risk for Santa Ana was calculated to be between 8.5 × 10−5 and 2.7 × 10−4, depending on which set of assumptions were applied. In the case of the latter value, the cancer risk equates to nearly three-times that which the EPA considers generally acceptable. Cancer risk was mostly driven by exposure during childhood, which showed a cumulative risk of approximately three-times that of adults. In terms of each metal, arsenic accounted for the majority of the cancer risk, with a risk that was nearly three-times higher than the next two most high-risk metals (upper CSF scenario). Overall, the calculated risk related to each metal decreased in the order As > Ni > Cr > Pb > Cd under the upper CSF scenario and in the order Ni > As > Pb > Cd under the lower CSF scenario. As with non-carcinogenic risk, ingestion and dermal exposure were the predominant exposure routes under both scenarios, accounting for approximately 80% and 20% of the cumulative cancer risk, respectively. Note that Cr does not show up in the lower scenario ranking since that scenario assumed that all chromium was of the non-carcinogenic form. A full list of cancer risk values corresponding to Fig. 4 for children and adults, and according to each exposure route, can be found in Table S4.†
Cumulative risk, as well as the order of individual metal-related risk, was heavily affected by the specific cancer slope factor used for As. When using an oral CSF of 1.5 (mg per kg per day)−1 listed by the U.S. EPA, as opposed to the more protective value of 9.5 (mg per kg per day)−1 recommended by California Office of Environmental Health Hazard Assessment, overall cancer risk for Santa Ana was calculated to be lower (1.2 × 10−4), albeit still above the upper-end the EPA's generally acceptable risk range. Under this scenario, the relative risk of each metal decreased according to Cr > Ni > As > Pb > Cd. The individual cancer risk remained above the acceptable risk level (10−4) for all but seven Census tracts.
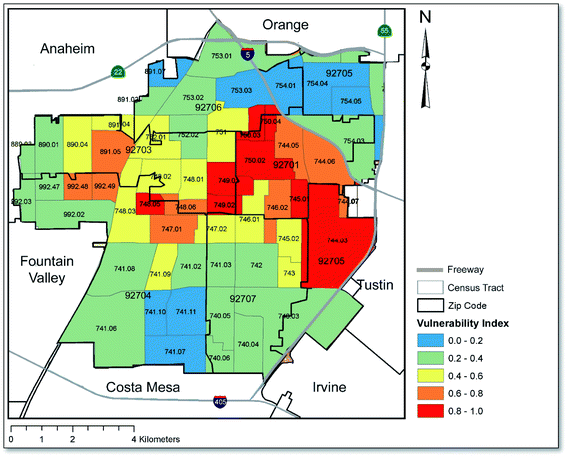
Fig. 5 is excerpted from the supplemental section of our prior published study in order to showcase the Census tracts in Santa Ana that ranked highest in terms of their social and economic vulnerability to soil heavy metal exposure.37 A comparison with Fig. 3 and 4 reveals similar patterning in that the region with the greatest potential health risk based on our risk assessment calculations is also the region where social and economic vulnerability is the greatest; namely, the central region of the map which coincides with downtown Santa Ana. Of note, all freeways and interstate roads surrounding Santa Ana that are described in this paper can be viewed in Fig. 5. Additionally, maps relating to landuse/zoning, total population and household income at the Census tract level are presented in Fig. S3 and S4† of the ESI.
4. Discussion
This study assesses the health risk and spatial distribution associated with soil concentrations of eight heavy metals in the city of Santa Ana (Southern California) in order to calculate a cumulative hazard index score and cancer risk across the region and within Census tracts. Metal concentrations yielded a high spatial variance, with 57% to 100% of Census tracts showing the potential for non-carcinogenic health outcomes (HR > 1) and 16% to 100% showing high risk for carcinogenic outcomes (risk > 10−4), depending on whether lower or upper toxicity values were used.In the case of Pb, nearly half of samples exceeded the California safety recommendation of 80 ppm for soil Pb in play areas. For As, all samples exceeded the state screening level. Other metals such as Cr, Ni, and Cd exceeded at least one screening level, however Cr-related exceedances depended on whether Cr(III) or Cr(VI) was assumed.
Within residential areas, over half of the soil samples had Pb levels above the California EPA safety guideline for Pb in child play areas, and 4% had levels greater than the 400 ppm U.S. EPA standard for play areas. For As and Cd, nearly all and roughly one-third of samples, respectively, exceeded the California SL.
Compared to risk during adulthood, risk during childhood was approximately 9-times higher for non-carcinogenic risk and 3-times higher for carcinogenic risk. This finding is similar to a recent study by Chonokhuu et al. (2019) who assessed similar heavy metals in the soil and found childhood risk to be 2- to 9-times higher than that of adults, and similar to results by Gržetić & Ahmed Ghariani (2008) who calculated childhood risk to be 10-times greater than that of adults.41,42
These findings are relevant to childhood exposure given that children often play in residential areas. For lead, one Census tract housing more than 650 children under age five had mean Pb levels above the 400 ppm U.S. EPA standard. Further details on potential child-related Pb exposure in Santa Ana can be found in our prior work (Masri et al. 2020). Generally speaking, Census tracts that had a greater proportion of children tended to have higher concentrations of Pb, Cd, and Zn and higher HI scores, implying a greater potential for non-carcinogenic risk. These results underscore a critical public health problem since children are particularly vulnerable to the adverse impacts of metals such as Pb.9–11 Contaminated soil and the resuspension of soil Pb have been shown to be important contributors to the burden of blood Pb in children.64–66
For Pb, soil concentrations from this study were similar to recent reports by Johnston et al. (2019) and Wu et al. (2010), as described in our prior work.37,67 Other metals were similarly within the general ranges reported in urban environments across the U.S.38,39,68 Relative to background soil metal concentrations reported across the U.S. and in California, concentrations reported in this study were elevated by a factor of approximately 6- to 18-fold for Pb, 1.6-fold for As, 2-fold for Cu, 2- to 6-fold for Cd, and 5- to 9-fold for Zn, whereas Cr and Ni concentrations were within the approximate background ranges reported in the literature.68,69 These findings are generally consistent with our assessment of heavy metal contamination based on enrichment factors, which found Pb, As, and Zn to be most heavily enriched in the city of Santa Ana, relative to local baseline samples.
Additionally, findings suggest increased exposure-related vulnerability among residents of lower socioeconomic statuses. Moderate and statistically significant positive correlations existed between non-cancer risk and the percent of residents under age five, the percent of renter-occupied housing units, and overall vulnerability index, as well as between cancer risk and the percent of residents under age five and percent Hispanic residents. When considering the percent of immigrant residents, negative correlations existed between this variable and both cancer risk and concentrations of As and Cr. This finding appeared to be driven by the percent of Asian immigrants, rather than Latina/o/x residents, which is consistent with results from our prior work that showed the spatial distribution of these ethnic groups to be oppositely correlated with one another.37 In terms of soil metals, Census tracts of lower median household income had higher mean concentrations than higher income Census tracts (except for Mn). For Pb, this pattern was prominent across all socioeconomic characteristics.
Results were more mixed for other social factors. The exception was for Pb and Zn, which were correlated when comparing each tertile across each socioeconomic factor, thus affirming the existence of a socioeconomic gradient in vulnerability to exposure. Further details on Pb-related tertile correlations can be found in our prior Pb-specific analysis.37 Collectively, these findings demonstrate socioeconomic and environmental inequities in Santa Ana that warrant public awareness, outreach, and intervention to safeguard children and families from heavy metal exposure. Such findings may similarly serve to assist officials in deploying municipal resources to disadvantaged residents and communities.
These results align with geospatial studies that indicate the differential presence of soil heavy metals in low-income communities and communities of color.36,38,70,71 These studies resonate with conceptual frameworks which posit that race and class are social constructs that cause health inequities.72 Notably, social and economic vulnerability to soil heavy metal exposure can compound health effects of heavy metal exposure. For example, greater household-level economic vulnerability, often correlated with a higher likelihood of exposure, is also a risk factor due to limited access to resources that may mitigate the health impacts of heavy metal exposure, such as access to health-promoting foods, the ability to remediate soil, access to health insurance, and having a usual source of care. At the neighborhood level, soil heavy metal exposures may be exacerbated by lower neighborhood socioeconomic position through mechanisms such as limited governmental attention to and remediation of polluted land, and the spatial distribution of health-promoting resources such as quality, affordable foods, education, and health care. Of note, community institutions and community organizing are critical resources that historically address these interconnected social, economic, and environmental injustices.
As it relates to our discussion of socioeconomic factors and environmental pollution, it is important to note that the heavy metal concentrations examined in this study cannot be separated from the history of the land and the legacy and enduring role of structural racism in the United States.73–75 Future studies are warranted that examine the role of structural racism in shaping the spatial distribution of heavy metal concentrations.73 For example, historical redlining practices promulgated by financial and realty industries and implemented by bankers, insurers, and realtors have created a cycle that funnels residents of color and low-income residents to less desirable neighborhoods with poor environmental conditions.35 Additionally, the growing and increasingly interconnected web of restrictive immigrant policies, immigration enforcement practices, and racialized policing increase housing instability and economic vulnerability,76–79 exacerbating processes that concentrate immigrant communities in environmentally disadvantaged areas.80
Beyond the consideration of demographic characteristics, this study also found that heavy metal concentrations varied by landuse category, with samples obtained near major roadways and residential locations showing the greatest concentrations for Pb, whereas for As concentrations were highest near roadways and schools (though limited school samples, n = 10). All other metals were highest for industrial landuse types. These findings are consistent with prior studies.48,81
Potential sources contributing to heavy metal concentrations in the soil of Santa Ana may include both historic and current emissions. For instance, before being phased out in the 1990s, lead-containing gasoline, and therefore vehicle traffic, was a key source of Pb emissions in the U.S.82,83 Traffic is also a known source of Zn emissions since this metal is contained in the rubber tread of vehicle tires as well as the brake lining material.51,84 Because Santa Ana is surrounded by four large roadways, including state routes 22 and 55 as well as the interstate 5 and 405 freeways, the city is particularly vulnerable to pollution from past and present traffic. Additionally, because lead paint was previously applied to many homes and other buildings in the U.S., disruption of painted surfaces through renovation and demolition, as well as weathering, may have contributed to the increased Pb levels in residential areas.29,30
Concentrations of other heavy metals were as expected given their increased concentrations in industrial landuse types. As an industrial center with over 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 432 companies, Santa Ana also consists of many metal-related industries such as metal fabrication, metal cutting, and metal processing.85 Thus, past and present point-source emissions constitute another possible contributor of heavy metals. According to the U.S. EPA Toxic Release Inventory (TRI), industrial facilities located in Santa Ana have emitted approximately 18
432 companies, Santa Ana also consists of many metal-related industries such as metal fabrication, metal cutting, and metal processing.85 Thus, past and present point-source emissions constitute another possible contributor of heavy metals. According to the U.S. EPA Toxic Release Inventory (TRI), industrial facilities located in Santa Ana have emitted approximately 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 lbs (8391 kg) of heavy metal pollution to the surrounding atmosphere in the form of stack or fugitive emissions since 1987.86 What is more, these reported emissions likely represent an underestimate of true emissions given the abundance of potentially minor, albeit important, sources that do not meet TRI reporting criteria. In the case of Pb, for instance, body shops, auto-repair shops, and automobile battery recycling facilities are usually small-scale businesses that do not report to EPA yet may still contribute to emissions. Also, while potentially not meeting TRI-reporting criteria, such sources are more widely dispersed and typically closer to residential units, making them an important source of exposure.
500 lbs (8391 kg) of heavy metal pollution to the surrounding atmosphere in the form of stack or fugitive emissions since 1987.86 What is more, these reported emissions likely represent an underestimate of true emissions given the abundance of potentially minor, albeit important, sources that do not meet TRI reporting criteria. In the case of Pb, for instance, body shops, auto-repair shops, and automobile battery recycling facilities are usually small-scale businesses that do not report to EPA yet may still contribute to emissions. Also, while potentially not meeting TRI-reporting criteria, such sources are more widely dispersed and typically closer to residential units, making them an important source of exposure.
The southwest quadrant of Santa Ana is also adjacent to the John Wayne Airport, which may contribute to the accumulation of metals in the soil. Copper in particular was found in greatest concentration in this region of the city. While combustion-related sources (e.g. oil, gas, fuel additives) only account for an estimated 5% of global atmospheric Cu emissions, studies have shown soil concentrations of Cu and other heavy metals to increase near airport runways relative to more distant sampling locations.87–90 Lastly, given the city's agricultural history, the historic use of fertilizers and pesticides constitutes another potentially important source of heavy metals to the environment.32 Phosphate fertilizer, for instance, is known to contain Cd,91 while Zn is contained in a many fertilizers.92 Similarly, lead-arsenate is a pesticide used in treating lumber.31
Future studies should examine the unique and synergistic contributions of contemporary sources of soil heavy metals and the extent to which prior uses of lead in gasoline, paint, and industrial emissions contribute to present-day soil contamination.
Relating to future steps, our partnership is constructing a list of recommendations for community-based actions and policies. These recommendations are grounded in community priorities and focus on remediating soil heavy metals and preventing and addressing metal-related exposures. Through a community organizing strategy, residents have expressed their recommendations about the way our partnership moves forward to support a healthier environment, including opportunities for coalition-building with other local initiatives focused on social, economic, racial, and environmental justice. Thus far, resident recommendations can be categorized into several related approaches: preventing exposure to heavy metals in the environment, remediating contaminated soil, and addressing the effects of metal exposures for residents.
Building on these recommendations, our partnership is developing a public health equity action plan that includes: demanding that governmental agencies with relevant jurisdiction remediate the soil, leveraging education tools to increase community awareness of exposures to heavy metals in soil, investing in community institutions and early life education, improving access to and affordability of healthy foods, and ensuring regular access to quality health care by residents. Additionally, we continue to explore the potential for new systems that are needed to promote community health and health equity, such as building upon local food autonomy initiatives, developing a soil remediation cooperative, and developing new and inclusive forms of communication across generations and social identities in Santa Ana.
A notable strength of this analysis is its foundation, which stems from principles of community-academic partnerships and community priorities.93–96 Accordingly, the research questions, study design, analysis, and ongoing development of recommendations were individually guided by our collaboration. Community-academic partnerships hold promise for translating research into action in order to improve community health and health equity.94,96 Another strength of this study is the large number of randomly collected soil samples (n = 1528), which enables a high-resolution understanding of the distribution of soil heavy metals and reduces exposure misclassification. High-density sampling also facilitates an examination of mean metal concentrations within Census tracts, which extends prior studies that only examined associations at the zip code level.97 Additionally, the characterization of soil metals across landuse categories is an important strength that enhances knowledge of potential source of contamination. Lastly, the consideration of multiple heavy metals across multiple exposure routes and life stages in order to produce risk estimates for both carcinogenic and non-carcinogenic health outcomes for each Census tract across a city represents an important strength as it relates to understanding spatial risk variability throughout an urban environment and the intersection of such risk with population vulnerability characteristics.
This study also has limitations that are important to discuss. While this analysis includes a sizable number of sampling sites, one limitation is the uncertainty of metal concentrations between sampling sites, which can lead to exposure misclassification in instances where samples are less concentrated. A second limitation is that correlations between soil heavy metals and Census tract-level social and economic characteristics do not necessarily reflect associations at the individual level. Also worth noting, and related to these prior limitations, is the characterization of risk at the Census tract level, which does not take into account the distributions of different landuse types within each Census tract and the types of activities likely to occur within such landuse types, which may then influence exposure (e.g. child interaction with soil likely differs between parks and industrial areas). Additionally, this analysis can only provide a general assessment of risk since risk assessment involves several assumptions that do not account for individual differences in risk and vulnerability. For instance, risk estimates produced in this study do not reflect certain behaviors such as “pica” (generally defined as the tendency to ingest non-food substances), which in some people (particularly children) manifests as the ingestion of soil.98 The EPA-recommended soil ingestion rate to estimate exposure among such children is 5-times higher (1000 mg per day) than that used in this study.98 Having said that, the ingestion rate for children used in our analysis was nonetheless an “upper percentile” ingestion rate, as recommended by the EPA to ensure the assessment and protection of the most vulnerable subgroups,99 which means that our ingestion-related exposure and risk estimates are likely higher than what would be expected for the average child. Since other exposure factors used in our analysis (e.g. adult soil ingestion rate, adult and child body weight, adult and child skin surface area, etc.) are based on average population-based exposure factors, we do not anticipate the overestimation of risk due to these other assumptions.
Also of note when interpreting our results is that this study did not take into consideration the ingestion of heavy metals through the dietary route. Had we considered this additional exposure pathway, our calculated chronic daily intake levels of heavy metals would have been greater, resulting in higher estimated risk (particularly for metals such as Pb, As, and Cd which have been widely documented in various foods100–102). In this regard, while our analysis likely overestimates some aspects of heavy-metal-related health risk through the soil (e.g. use of “upper percentile” ingestion for children), the absence of dietary considerations likely results in bias in the opposite direction (i.e. underestimating risk).
To further discuss potential limitations of our study, the vulnerability index is composed of U.S. Census estimates, which may underestimate the population in sub-locales of Santa Ana. For instance, in Santa Ana a sizable proportion of youth and adults of color have engaged with the criminal legal system and thus may not be represented in Census estimates of the population.103,104 Any systematic undercounts of the population may contribute to conservative estimates of the cumulative burden of exposure to metals. Further, Santa Ana is experiencing a gentrification process that has augmented housing instability, homelessness, and housing quality concerns that may also contribute to population undercounts.
Lastly, for school and industrial landuse types, it is important to note that our sampling protocol (not entering restricted properties) resulted in the collection of some soil samples near the property perimeters of such sites rather than inside, which might have influenced our results. While it is difficult to predict in which direction such bias may have occurred, it is nonetheless worth discussing the possibilities. If we assume vehicle-related traffic to be the predominant source of heavy metal contamination, then it is reasonable to expect results to bias to the “high” side for school sites (since sampling was restricted to school boundaries which are closer to roads). However, if we consider building paint to be a dominant source (e.g. historic Pb paint), then results could bias to the low side. For industrial sites, we believe our results are more likely to exhibit either non-differential bias or bias to the low side, since heavy metal emissions can conceivably be greater within industrial property boundaries, as opposed to outside (depending on the specific industry). Future investigations focused on assessing both the sources and concentrations of soil heavy metals in the environment, as well as their correlation with health outcomes, and the effectiveness of interventions to remediate the soil, are needed to better understand these issues and to help prevent community exposures.
5. Conclusions
This spatial assessment of soil heavy metal levels across Census tracts in Santa Ana, CA, showed that Census tracts with a greater proportion of children, lower percentage of college educated residents, lower median household income, higher fraction of residents lacking health insurance coverage, and higher percentage of renters, had higher average Pb and Zn levels relative to other Census tracts. Additionally, Census tracts with a greater proportion of residents who were non-native, spoke limited English, and identified as Latina/o/x or Hispanic had much higher concentrations of these metals than other Census tracts. A similar pattern was evident for As and Cd. Overall, the majority of Census tracts showed the potential for non-carcinogenic health outcomes, with a lower, albeit still relevant, number of Census tracts showing high risk for carcinogenic outcomes. Both cancer and non-cancer risk at the Census tract level exhibited positive correlations with indicators of social as well as physiological vulnerability. These results highlight important areas of vulnerability and environmental inequity regarding heavy metal exposure, and suggest the need for community organizing and interventions to reduce and ultimately eliminate inequities in soil-related heavy metal exposure.Author contributions
Conceptualization, S. M., A. L., E. V., A. R., J. W.; methodology, S. M., A. L., J. W.; software, S. M.; validation, S. M., M. L.; formal analysis, S. M.; investigation, E. R.; resources, E. V.; data curation, S. M., M. L.; writing—original draft preparation, S. M.; writing—review and editing, S. M., A. L., E. V., M. L., J. W; visualization, S. M., A. L., J. W.; supervision, J. W.; project administration, J. W.; funding acquisition, A. L., J. W. All authors have read and agreed to the published version of the manuscript.Funding
This study stems from work supported by Orange County Environmental Justice, the California Endowment Building Healthy Communities, the University of California, Irvine, Program in Public Health and Department of Chicano/Latino Studies, the National Institute of Environmental Health Sciences (ES030353), the Regents of the University of California, and the National Institutes of Health (UL1 TR001414). Any findings, opinions, and conclusions as well as recommendations that are expressed in this publication are those of the author(s) and do not necessarily reflect the views of the NIH, the UCI Institute for Clinical and Translational Science, or The Regents of the University of California.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the following organizations and individuals for their support: Jóvenes Cultivando Cambios, Orange County Environmental Justice, UCI Community Resilience, Yenni Diaz, Oladele Ogunseitan, the Ying Lab, the numerous individuals who have joined community discussions and collectively organized to understand and address concerns about environmental inequities and visions for a future city of Santa Ana, CA, and the UCI students who assisted in research related to this study.References
- R. L. Jones, D. M. Homa, P. A. Meyer, D. J. Brody, K. L. Caldwell, J. L. Pirkle and M. J. Brown, Trends in Blood Lead Levels and Blood Lead Testing Among US Children Aged 1 to 5 Years, 1988-2004, Pediatrics, 2009, 3, 376–385, DOI:10.1542/peds.2007-3608.
- S. J. Rothenberg, F. A. Williams, S. Delrahim, M. Kraft, M. H. Lu, M. Manalo, M. Sanchez and D. J. Wooten, Blood Lead Levels in Children in South Central Los Angeles, Arch. Environ. Health, 1996, 51, 383–388, DOI:10.1080/00039896.1996.9934426.
- H. W. Mielke, J. C. Anderson, K. J. Berry, P. W. Mielke, R. L. Chaney and M. Leech, Lead Concentrations in Inner-City Soils as a Factor in the Child Lead Problem, Am. J. Publ. Health, 1983, 73, 1366–1369, DOI:10.2105/ajph.73.12.1366.
- K. G. Wu, C. Y. Chang, C. Y. Yen and C. C. Lai, Associations between environmental heavy metal exposure and childhood asthma: A population-based study, J. Microbiol. Immunol. Infect., 2019, 52, 352–362, DOI:10.1016/j.jmii.2018.08.001.
- M. Boskabady, N. Marefati, T. Farkhondeh, F. Shakeri, A. Farshbaf and M. H. Boskabady, The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review, Environ. Int., 2018, 120, 404–420, DOI:10.1016/j.envint.2018.08.013.
- I. J. Wang, W. J. Karmaus and C. C. Yang, Lead exposure, IgE, and the risk of asthma in children, J. Expo. Sci. Environ. Epidemiol., 2017, 27, 478–483, DOI:10.1038/jes.2017.5.
- P. Pugh Smith and J. O. Nriagu, Lead poisoning and asthma among low-income and African American children in Saginaw, Michigan, Environ. Res., 2011, 111, 81–86, DOI:10.1016/j.envres.2010.11.007.
- P. Grandjean and P. J. Landrigan, Neurobehavioural effects of developmental toxicity, Lancet Neurol., 2014, 13, 330–338, DOI:10.1016/S1474-4422(13)70278-3.
- A. Reuben, A. Caspi, D. W. Belsky, J. Broadbent, H. Harrington, K. Sugden, R. M. Houts, S. Ramrakha, R. Poulton and T. E. Moffitt, Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood, JAMA, J. Am. Med. Assoc., 2017, 317, 1244–1251, DOI:10.1001/jama.2017.1712.
- R. L. Canfield, C. R. Henderson, D. A. Cory-Slechta, C. Cox, T. A. Jusko and B. P. Lanphear, Intellectual Impairment in Children with Blood Lead Concentrations below 10 ug per Deciliter, N. Engl. J. Med., 2003, 348, 1517–1526, DOI:10.1056/NEJMoa022848.
- B. P. Lanphear, R. Hornung, J. Khoury, K. Yolton, P. Baghurst, D. C. Bellinger, R. L. Canfield, K. N. Dietrich, R. Bornschein and T. Greene, et al. Low-level environmental lead exposure and children's intellectual function: An international pooled analysis, Environ. Health Perspect., 2005, 113, 894–899, DOI:10.1289/ehp.7688.
- D. A. Kennedy, C. Woodland and G. Koren, Lead exposure, gestational hypertension and pre-eclampsia: A systematic review of cause and effect, J. Obstet. Gynaecol., 2012, 32, 512–517, DOI:10.3109/01443615.2012.693987.
- A. E. Poropat, M. A. S. Laidlaw, B. Lanphear, A. Ball and H. W. Mielke, Blood lead and preeclampsia: A meta-analysis and review of implications, Environ. Res., 2018, 160, 12–19, DOI:10.1016/j.envres.2017.09.014.
- C. M. Taylor, J. Golding and A. M. Emond, Adverse effects of maternal lead levels on birth outcomes in the ALSPAC study: A prospective birth cohort study, Br. J. Obstet. Gynaecol., 2015, 22, 322–328, DOI:10.1111/1471-0528.12756.
- X. Xie, G. Ding, C. Cui, L. Chen, Y. Gao, Y. Zhou, R. Shi and Y. Tian, The effects of low-level prenatal lead exposure on birth outcomes, Environ. Pollut., 2013, 175C, 30–34, DOI:10.1016/j.envpol.2012.12.013.
- M. Cempel and G. N. Nikel, A review of its sources and environmental toxicology, Pol. J. Environ. Stud., 2006, 15, 375–382 CAS.
- M. S. T. Mugadza, Chromium, an essential nutrient and pollutant: A review, Afr. J. Pure Appl. Chem., 2013, 7, 310–317 Search PubMed 10.5897/AJPAC2013.0517.
- A. H. Wu, J. Wu, C. Tseng, J. Yang, S. Shariff-Marco, S. Fruin, T. Larson, V. W. Setiawan, S. Masri and J. Porcel, et al. Association Between Outdoor Air Pollution and Risk of Malignant and Benign Brain Tumors: The Multiethnic Cohort Study, JNCI Cancer Spectr., 2020, 4, 1–8, DOI:10.1093/jncics/pkz107.
- J. K. Nduka, H. I. Kelle and J. O. Amuka, Health risk assessment of cadmium, chromium and nickel from car paint dust from used automobiles at auto-panel workshops in Nigeria, Toxicol. Rep., 2019, 6, 449–456, DOI:10.1016/j.toxrep.2019.05.007.
- L. Järup, M. Berglund, C. G. Elinder, G. Nordberg and M. Vahter, Health effects of cadmium exposure - A review of the literature and a risk estimate, Scand. J. Work. Environ. Health, 1998, 24, 1–51 CrossRef.
- J. I. Anetor, H. Wanibuchi and S. Fukushima, Arsenic exposure and its health effects and risk of cancer in developing countries: Micronutrients as host defence, Asian Pac. J. Cancer Prev., 2007, 8, 13–23 Search PubMed.
- M. Hutton, Human Health Concerns of Lead, Mercury, Cadmium and Arsenic. in Lead, Mercury, Cadmium and Arsenic in the Environment, ed. T. C. Hutchinson and K. M. Meema, John Wiley & Sons Ltd: Hoboken, NJ, 1987; pp. 53–68, ISBN 0471911267 Search PubMed.
- Y.-C. Jang, Y. Somanna and H. Kim, Source, Distribution, Toxicity and Remediation of Arsenic in the Environment – A review, Int. J. Appl. Environ. Sci., 2016, 11, 973–6077 Search PubMed.
- R. G. Lucchini, E. Albini, L. Benedetti, S. Borghesi, R. Coccaglio, E. C. Malara, G. Parrinello, S. Garattini, S. Resola and L. Alessio, High prevalence of Parkinsonian disorders associated to manganese exposure in the vicinities of ferroalloy industries, Am. J. Ind. Med., 2007, 50, 788–800, DOI:10.1002/ajim.20494.
- R. G. Lucchini, S. Guazzetti, S. Zoni, F. Donna, S. Peter, A. Zacco, M. Salmistraro, E. Bontempi, N. J. Zimmerman and D. R. T. Smith, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission, Neurotoxicology, 2012, 33, 687–696, DOI:10.1016/j.neuro.2012.01.005.
- J. A. Menezes-Filho, C. de O. Novaes, J. C. Moreira, P. N. Sarcinelli and D. Mergler, Elevated manganese and cognitive performance in school-aged children and their mothers, Environ. Res., 2011, 111, 156–163, DOI:10.1016/j.envres.2010.09.006.
- Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Copper; Atlanta, 1999 Search PubMed.
- California Office of Environmental Health Hazard Assessment Chemicals Known to the State to Cause Cancer or Reproductive Toxicity; Sacramento, 2021.
- D. E. Jacobs, R. P. Clickner, J. Y. Zhou, S. M. Viet, D. A. Marker, J. W. Rogers, D. C. Zeldin, P. Broene and W. Friedman, The Prevalence of Lead-Based Paint Hazards in US Housing, Environ. Health Perspect., 2002, 110(10), 599–606 CrossRef.
- M. Rabinowitz, A. Leviton and D. Bellinger, Home refinishing, lead paint, and infant blood lead levels, Am. J. Publ. Health, 1985, 75, 403–404, DOI:10.2105/AJPH.75.4.403.
- Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Lead; Atlanta, 2002 Search PubMed.
- R. A. Wuana and F. E. Okieimen, Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation, ISRN Ecol., 2011, 2011, 1–20, DOI:10.5402/2011/402647.
- U.S. Environmental Protection Agency (EPA), Priority Pollutant List, Washington D.C., 2014 Search PubMed.
- G. Markowitz and D. RosnerLead Wars: The Politics of Science and the Fate of America's Children; University of California Press: Berkeley, 2013; ISBN 9780520283930 Search PubMed.
- M. Hanna-Attisha What the Eyes Don't See: A Story of Crisis, Resistance, and Hope in an American City; Penguin Random House: New York, 2018; ISBN 9780399590856 Search PubMed.
- X. Zhuo, C. G. Boone and E. L. Shock, Soil lead distribution and environmental justice in the phoenix metropolitan region, Environ. Justice, 2012, 5, 206–213, DOI:10.1089/env.2011.0041.
- S. Masri, A. LeBrón, M. Logue, E. Valencia, A. Ruiz, A. Reyes, J. M. Lawrence and J. Wu, Social and spatial distribution of soil lead concentrations in the City of Santa Ana, California: Implications for health inequities, Sci. Total Environ., 2020, 743, 1–11, DOI:10.1016/j.scitotenv.2020.140764.
- D. M. Diawara, J. S. Litt, D. Unis, N. Alfonso, L. A. Martinez, J. G. Crock, D. B. Smith and J. Carsella, Arsenic, cadmium, lead, and mercury in surface soils, Pueblo, Colorado: Implications for population health risk, Environ. Geochem. Health, 2006, 28, 297–315, DOI:10.1007/s10653-005-9000-6.
- D. A. Griffith, D. L. Johnson and A. Hunt, The geographic distribution of metals in urban soils: The case of Syracuse, NY, Geojournal, 2009, 74, 275–291, DOI:10.1007/s10708-008-9233-x.
- H. W. Mielke, C. R. Gonzales, M. K. Smith and P. W. Mielke, The urban environment and children's health: Soils as an integrator of lead, zinc, and cadmium in New Orleans, Louisiana, U.S.A., Environ. Res., 1999, 81, 117–129, DOI:10.1006/enrs.1999.3966.
- S. Chonokhuu, C. Batbold, B. Chuluunpurev, E. Battsengel, B. Dorjsuren and B. Byambaa, Contamination and health risk assessment of heavy metals in the soil of major cities in Mongolia, Int. J. Environ. Res. Publ. Health, 2019, 16, 1–15, DOI:10.3390/ijerph16142552.
- I. Gržetić and R. H. Ahmed Ghariani, Potential health risk assessment for soil heavy metal contamination in the central zone of Belgrade (Serbia), J. Serb. Chem. Soc., 2008, 73, 923–934, DOI:10.2298/JSC0809923G.
- N. S. Yuswir, S. M. Praveena, A. Z. Aris, S. N. S. Ismail and Z. Hashim, Health risk assessment of heavy metal in urban surface soil (Klang District, Malaysia), Bull. Environ. Contam. Toxicol., 2015, 95, 80–89, DOI:10.1007/s00128-015-1544-2.
- A. M. W. Lebrón, I. R. Torres, E. Valencia, M. L. Dominguez, D. G. Garcia-Sanchez, M. D. Logue and J. Wu, The state of public health lead policies: Implications for urban health inequities and recommendations for health equity, Int. J. Environ. Res. Publ. Health, 2019, 16, 1–28, DOI:10.3390/ijerph16061064.
- United States Census Bureau American Community Survey Available online: https://www.census.gov/programs-surveys/acs.
- The City of Santa Ana Santa Ana Facts and Figures Available online: https://www.santa-ana.org/library/services/facts-and-figures.
- CA.gov Census 2020 California Hard-to-Count Fact Sheet, Santa Ana City in Orange County; Washington D.C., 2020.
- J. Wu, R. Edwards, X. E. He, Z. Liu and M. Kleinman, Spatial analysis of bioavailable soil lead concentrations in Los Angeles, California, Environ. Res., 2010, 110, 309–317, DOI:10.1016/j.envres.2010.02.004.
- D. Stalcup Memorandum: Recommendations for Sieving Soil and Dust Samples at Lead Sites for Assessment of Incidental Ingestion; Washington, D.C., 2016.
- A. Al Maliki, A. K. Al-lami and H. M. Hussain, Nadhir Al-Ansari Comparison Between Inductively Coupled Plasma and X-Ray Flourescence Performance for Pb Analysis in Environmental Soil Samples, Environ. Earth Sci., 2017, 76, 2–7, DOI:10.1007/s12665-017-6753-z.
- S. Masri, C.-M. Kang and P. Koutrakis, Composition and sources of fine and coarse particles collected during 2002–2010 in Boston, MA, J. Air Waste Manage. Assoc., 2015, 65(3), 287–297 CrossRef CAS.
- R. Carr, C. S. Zhang, N. Moles and M. Harder, Identification and mapping of heavy metal pollution in soils of a sports ground in Galway City, Ireland, using a portable XRF analyser and GIS, Environ. Geochem. Health, 2008, 30, 45–52, DOI:10.1007/s10653-007-9106-0.
- S. A. Instruments, Analyzing Trace Elements in Pressed Pellets of Geological Materials Using ED-XRF, 2016, available online: https://www.spectro.com/landingpages/xrf-xepos-application-analyzing-trace-elements-in-pressed-pellets-of-geological-materials Search PubMed.
- K. Huang, X. Luo and Z. Zheng, Application of a combined approach including contamination indexes, geographic information system and multivariate statistical models in levels, distribution and sources study of metals in soils in Northern China, PLoS One, 2018, 13, 1–18, DOI:10.1371/journal.pone.0190906.
- K. Loska, J. Cebula, J. Pelczar, D. Wiechuła and J. Kwapuliński, Use of enrichment, and contamination factors together with geoaccumulation indexes to evaluate the content of Cd, Cu, and Ni in the Rybnik water Reservoir in Poland, Water, Air, Soil Pollut., 1997, 93, 347–365, DOI:10.1023/A:1022121615949.
- R. A. Sutherland, Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii, Environ. Geol., 2000, 39, 611–627, DOI:10.1007/s002540050473.
- United States Environmental Protection Agency Update of Standard Default Exposure Factors, 2014, available online: https://www.epa.gov/risk/update-standard-default-exposure-factors Search PubMed.
- United States Environmental Protection Agency, National Contingency Plan §300.430, 2011, pp. 76–87 Search PubMed.
- USEPA, Integrated Risk Information System (IRIS): IRIS Chemicals Available online: https://comptox.epa.gov/dashboard/chemical_lists/IRIS, accessed on Sep 9, 2020.
- CalEPA Human-Exposure-Based Screening Numbers Developed to Aid Estimation of Cleanup Costs for Contaminated Soil; Sacramento, 2005.
- M. Harmanescu, L. M. Alda, D. M. Bordean, I. Gogoasa and I. Gergen, Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania, Chem. Cent. J., 2011, 64, 3–10, DOI:10.1186/1752-153X-5-64.
- RAIS Risk Assessment Information System (RAIS). Toxicity Values. 2.3 Derivation of Dermal Toxicity Values, Available online: https://rais.ornl.gov/tutorials/toxvals.html#2.3DerivationofDermalToxicityValues, accessed on Sep 9, 2020.
- USEPA Regional Screening Levels (RSLs) - Generic Tables as of May 2020 Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables, accessed on Jan 9, 2020.
- M. Maisonet, F. J. Bove and W. E. Kaye, A Case-Control Study to Determine Risk Factors for Elevated Blood Lead Levels in Children, Idaho, Toxicol. Ind. Health, 1997, 13, 67–72, DOI:10.1177/074823379701300106.
- M. Weitzman, A. Aschengrau, D. Bellinger, R. Jones, J. S. Hamlin and A. Beiser, Lead-contaminated soil abatement and urban children's blood lead levels, JAMA, J. Am. Med. Assoc., 1993, 269, 1647–1654 CrossRef CAS PubMed.
- S. Zahran, M. A. S. Laidlaw, S. P. McElmurry, G. M. Filippelli and M. Taylor, Linking Source and Effect: Resuspended Soil Lead, Air Lead, and Children's Blood Lead Levels in Detroit, Michigan, Environ. Sci. Technol., 2013, 47, 2839–2845 CrossRef CAS.
- J. E. Johnston, M. Franklin, H. Roh, C. Austin and M. Arora, Lead and Arsenic in Shed Deciduous Teeth of Children Living Near a Lead-Acid Battery Smelter, Environ. Sci. Technol., 2019, 53, 6000–6006, DOI:10.1021/acs.est.9b00429.
- K. Sharma, N. T. Basta and P. S. Grewal, Soil heavy metal contamination in residential neighborhoods in post-industrial cities and its potential human exposure risk, Urban Ecosyst., 2015, 18, 115–132, DOI:10.1007/s11252-014-0395-7.
- D. Diamond; D. Baskin; D. Brown; L. Lund; J. NajitaAnalysis of Background Distributions of Metals in the Soil at Lawrence Berkeley National Laboratory; Berkeley, CA, 2009.
- H. W. Mielke, C. R. Gonzales, E. Powell, M. Jartun and P. W. Mielke, Nonlinear association between soil lead and blood lead of children in metropolitan New Orleans, Louisiana: 2000-2005, Sci. Total Environ., 2007, 388, 43–53, DOI:10.1016/j.scitotenv.2007.08.012.
- N. McClintock, A critical physical geography of urban soil contamination, Geoforum, 2015, 65, 69–85, DOI:10.1016/j.geoforum.2015.07.010.
- J. C. Phelan, B. G. Link and P. Tehranifar, Social Conditions as Fundamental Causes of Health Inequalities: Theory, Evidence, and Policy Implications, J. Health Soc. Behav., 2010, 51, 28–40, DOI:10.1177/0022146510383498.
- G. C. Gee and D. C. Payne-Sturges, Environmental health disparities: A framework integrating psychosocial and environmental concepts, Environ. Health Perspect., 2004, 112(17), 1645–1653 CrossRef PubMed.
- A. Schulz and M. Northridge, Social determinants of health: implications for environmental health promotion, Health Educ. Behav., 2004, 31, 455–471 CrossRef.
- D. R. Williams and C. Collins, Racial residential segregation: A fundamental cause of racial disparities in health, Publ. Health Rep., 2001, 116, 404–416 CrossRef CAS.
- J. Rugh and M. Hall, Deporting the American Dream: Immigration Enforcement and Latino Foreclosures, Sociol. Sci., 2016, 3, 1077–1102, DOI:10.15195/v3.a46.
- A. M. W. LeBron, A. J. Schulz, C. Gamboa, A. Reyes, E. A. Viruell-Fuentes and B. A. Israel, They Are Clipping Our Wings: Health Implications of Restrictive Immigrant Policies for Mexican-Origin Women in a Northern Border Community, Race Soc. Probl., 2018, 10, 174–192 CrossRef.
- D. William. Lopez Separated: Family and Community in the Aftermath of an Immigration Raid; Johns Hopkins University Press: Baltimore, 2019 Search PubMed.
- V. C. Nichols, A. M. W. LeBrón and I. Francisco, Pedraza Policing Us Sick: The Health of Latinos in an Era of Heightened Deportations and Racialized Policing, Polit. Symp., 2018, 293–297 Search PubMed.
- M. Hall and E. Greenman, Housing and neighborhood quality among undocumented Mexican and Central American immigrants, Soc. Sci. Res., 2013, 42, 1712–1725 CrossRef PubMed.
- M. Y. Hanfi and I. V. Yarmoshenko, Health risk assessment quantification from heavy metals contamination in the urban soil and urban surface deposited sediment, J. Taibah Univ. Sci., 2020, 14, 285–293, DOI:10.1080/16583655.2020.1735735.
- R. Newell and K. RogersThe US Experience with the Phasedown of Lead in Gasoline; Washington, DC, 2003 Search PubMed.
- J. O. Nriagu, The Rise and Fall of Lead in Gasoline, Sci. Total Environ., 1990, 92, 13–28 CrossRef CAS.
- A. Thorpe and R. M. Harrison, Sources and properties of non-exhaust particulate matter from road traffic: A review, Sci. Total Environ., 2008, 400, 270–282, DOI:10.1016/j.scitotenv.2008.06.007.
- U.S. Census Bureau, Quick Facts: Santa Ana City, California, 2010–2018 Available online: https://www.census.gov/quickfacts/fact/map/santaanacitycalifornia/INC110218 Search PubMed.
- United States Environmental Protection Agency Toxic Release Inventory (TRI) Explorer, Available online: https://enviro.epa.gov/triexplorer/tri_release.geography.
- M. Radomska, S. Madzhd, L. Cherniak and O. Mikhyeyev, Environmental Pollution in the Airport Impact Area–Case Study of the Boryspil International Airport, Environ. Probl., 2020, 5, 76–82, DOI:10.23939/ep2020.02.076.
- G. E. Collins, R. E. Morris, J. F. Wei, M. Smith, M. H. Hammond, V. Michelet, J. D. Winkler, P. M. Serino and Y. Guo, Spectrophotometric detection of trace copper levels in jet fuel, Energy Fuels, 2002, 16, 1054–1058, DOI:10.1021/ef010271a.
- Agency for Toxic Substances and Disease Registry Toxicological Profile for Copper; Atlanta, 2004 Search PubMed.
- S. Ray, P. S. Khillare and K. H. Kim, The effect of aircraft traffic emissions on the soil surface contamination analysis around the International Airport in Delhi, India, Asian J. Atmos. Environ., 2012, 6, 118–126, DOI:10.5572/ajae.2012.6.2.118.
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Cadmium; Atlanta, 2002 Search PubMed.
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Zinc; Atlanta, 2002 Search PubMed.
- B. A. Israel, A. J. Schulz, E. A. Parker and A. B. Becker, Review of community-based research: assessing partnership approaches to improve public health, Annu. Rev. Publ. Health, 1998, 19, 173–202, DOI:10.1146/annurev.publhealth.19.1.173.
- T. Wolff, M. Minkler, S. M. Wolfe, B. Berkowitz, L. Bowen, F. D. Butterfoss, B. D. Christens, V. T. Francisco, A. T. Himmelman, K. S. Lee, Collaborating for Equity and Justice: Moving Beyond Collective Impact, The Nonprofit Quarterly, Winter, 2016, pp. 42–53 Search PubMed.
- A. M. W. LeBrón, K. Cowan, W. D. Lopez, N. L. Novak, M. Ibarra-Frayre and J. Delva, The Washtenaw ID Project: A Government-Issued ID Coalition Working Toward Social, Economic, and Racial Justice and Health Equity, Health Educ. Behav., 2019, 46, 53S–61S, DOI:10.1177/1090198119864078.
- R. González, The Spectrum of Community Engagement to Ownership; Oakland, CA, 2019.
- N. Krieger, J. T. Chen, P. D. Waterman, M.-J. Soobader, S. V. Subramanian and R. Carson, Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US), J. Epidemiol. Community Health, 2003, 57, 186–199, DOI:10.1136/jech.57.3.186.
- United States Environmental Protection Agency, National Center for Environmental Assessment Update for Chapter 5 of the Exposure Factors Handbook: Soil and Dust Ingestion, Washington, DC, 2017 Search PubMed.
- U.S. EPA (U.S. Environmental Protection Agency), Guidelines for Human Exposure Assessment. (EPA/100/B-19/001), Risk Assessment Forum, EPA, Washington, D.C., U.S., 2019 Search PubMed.
- D. Jureša and M. Blanuša, Mercury, arsenic, lead and cadmium in fish and shellfish from the Adriatic Sea, Food Addit. Contam., 2003, 20, 241–246, DOI:10.1080/0265203021000055379.
- U.S. Food and Drug Administration, Total Diet Study: Elements Results Summary Statistics (Market Baskets 2006 through 2011); College Park, MD, 2017 Search PubMed.
- A. Oskarsson, A. Widell, I. M. Olsson and K. P. Grawé, Cadmium in food chain and health effects in sensitive population groups, BioMetals, 2004, 17, 531–534, DOI:10.1023/B:BIOM.0000045733.38583.8e.
- V. Anderson, J. Buatti, T. Martin, J. Chin, L. Gasser-Ordaz, J. R. Graham, A. Kantor, J. Barack, G. Kristie, E. Aguilasocho, A. Feathers, and D. Koch, Second Chances for All: Why Orange County Probation Should Stop Choosing Deportation over Rehabilitation for Immigrant Youth, Irvine, CA, 2013 Search PubMed.
- K. Avila; B. E. Helzer and A. LaiThe State of Orange County: An Analysis of Orange County's Policies on Immigration and a Blueprint for an Immigrant Inclusive Future; Santa Ana, CA, 2019 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1em00007a |
| This journal is © The Royal Society of Chemistry 2021 |