Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The impact of binary water–CO2 isotherm models on the optimal performance of sorbent-based direct air capture processes†
John
Young
 *,
Enrique
García-Díez
*,
Enrique
García-Díez
 ,
Susana
Garcia
,
Susana
Garcia
 and
Mijndert
van der Spek
and
Mijndert
van der Spek
 *
*
Research Centre for Carbon Solutions, Heriot-Watt University, Edinburgh, EH14 4AS, UK. E-mail: jpy1@hw.ac.uk; m.van_der_spek@hw.ac.uk
First published on 2nd August 2021
Abstract
Direct air capture (DAC) is an auspicious technology in pursuing negative CO2 emissions. A promising process is temperature vacuum swing adsorption (TVSA) employing amine functionalised adsorbents such as Lewatit® VP OC 1065, which is selected as a benchmark sorbent in this study. To further improve process design, and critically lower costs, detailed modelling of DAC cycles is imperative. However, the multi-component adsorption on these materials, particularly the cooperative adsorption of CO2 and H2O, is crudely understood, and yet to be described in mathematical terms, prohibiting sound modelling efforts. Here, we commit in-depth understanding of the effect of humidity on CO2 adsorption and demonstrate how this impacts modelling of DAC cycles. We present two novel mechanistic co-adsorption isotherm models to describe water's effect on CO2 adsorption and find a good fit to original experimental co-adsorption data. We also show the considerable improvement in predictions of these models when compared to an empirical co-adsorption isotherm model from literature. A detailed TVSA DAC cycle process model is then used elucidating how different co-adsorption models affect the predicted process performance. It is found that the two novel isotherm models generate similar results and Pareto fronts, whilst the minimum work equivalent calculated using the more conservative of the two models is found to be 2.49 MJ kg−1 for the case study considered. These mathematical descriptions laid out will lead to more accurate modelling and optimisation of cyclic DAC adsorption processes, prompting a greater understanding of the material-process combinations ideal for DAC and how costs can be driven down in the years to come. Importantly, they allowed us to independently benchmark a Climeworks type DAC process, providing key DAC performance data to the public domain.
Broader contextCarbon dioxide removal (CDR) technologies are at the centre of scientific and public debate and policymaking. They encompass a suite of processes and systems that actively remove CO2 from the atmosphere, aiming to store the CO2 permanently or to utilise it, for instance, to create jet fuels. Examples of CDR are bioenergy with CO2 capture and storage (BECCS), enhanced weathering and ocean alkalinity, land-based methods, and direct air capture (DAC), discussed here. The key questions for all these systems include: ‘how much CO2 can they remove at what land footprint?’; ‘how much does this cost in terms of energy, physical inputs/outputs and money?’; and ‘how much can we bring the costs down by innovation?’. These numbers are becoming well established and corroborated by independent science for some technologies, e.g., BECCS. For others, this is not yet the case. For solid-sorbent DAC, we still lack detailed and reliable modelling of process performance, which can then be used to identify the window of opportunity for process improvements and cost reductions. We here provide a rigorous, detailed modelling study on adsorbent-based DAC technical performance, where newly developed water–CO2 co-adsorption isotherm models, using a comprehensive set of experimental data, are incorporated. |
1. Introduction
Anthropogenic greenhouse gas (GHG) emissions are causing the dramatically rising temperature of our planet. The level of atmospheric CO2 and other GHGs has become untenable, and drastic action is required to prevent a global temperature rise of 1.5 °C, which has been identified as an important limit by the Intergovernmental Panel on Climate Change (IPCC).1 Negative emissions technologies (NETs) that actively remove CO2 from the atmosphere will be needed to mitigate hard-to-abate and historical emissions and to reach the Paris Agreement targets.2,3 Carbon capture and storage (CCS) has a significant role to play in the development and deployment of NETs. Two promising NETs rely on CCS technology. These are bioenergy with carbon capture and storage (BECCS) and direct air capture (DAC).4 DAC aims to capture CO2 directly from ambient air resulting in net negative emissions if the CO2 is permanently stored.5 Realmonte et al. explored the role DAC can play in a 1.5 °C scenario and emphasise the economic benefit that DAC provides.5 The study finds that policymakers require much lower carbon prices when DAC is available as a technology. However, the authors of this study stress that DAC should be developed alongside other solutions since significant technical challenges exist.1.1 Challenges in sorbent-based DAC process design
Here, we focus on direct air capture of CO2 using amine functionalised adsorbents in temperature vacuum swing adsorption (TVSA) processes. Thus far, much research effort in this space has gone to the development of effective, robust and cheap sorbent materials. More information is available in the ESI,† as well as Table S1 which summarises some of the materials studied for DAC to date. Beyond sorbent development, the main current challenge for adsorbent-based DAC technologies is to further optimise their process design to enhance efficiency and reduce cost. For this, two critical elements are needed: (i) detailed cyclic adsorption process modelling to find optimal process cycles and operating parameters, requiring (ii) accurate mathematical descriptions of the underlying adsorption of each adsorbed component, with a focus on the interaction between CO2 and H2O. Without accurate adsorption descriptions, there is simply little point in undertaking process design studies.The two facets governing adsorption that need to be mathematically described are adsorption equilibrium and dynamics (i.e., mass transfer), and this is done using isotherm and kinetic models, respectively. Casas et al. explain that adsorption process modelling is susceptible to small errors in isotherm models, emphasising the importance of an accurate description, and developing the required isotherm models will be one of the objectives of this work.6 As humidity in air is reported to enhance the equilibrium adsorption of CO2 on amine-functionalised adsorbents, it is pivotal to describe this interaction correctly.7–10 However, a mechanistically consistent mathematical description of this enhancement does not yet exist, impeding accurate modelling of DAC processes, and therefore their further improvement.
Here, we aim to fill the caveats in understanding and modelling of water–CO2 interactions, by deriving mechanistically consistent co-adsorption models, showing how the use of different models influences the modelling of DAC adsorption cycles and thus their technical performance. To this end, we combined adsorption theory with new experiments and modelling studies. The paper is structured as follows: first, the theoretical mechanisms and their implications for CO2 adsorption onto amine-functionalised sorbents are discussed to allow the derivation of sound (co-)adsorption isotherm models. Second, accurate representations of pure component and co-adsorption isotherms were measured experimentally and fit to the co-adsorption isotherm models. Finally, the isotherm models were used in a detailed process model to demonstrate how the different descriptions affect process performance and assert that sound descriptions of the physical processes are indeed critical to the design of efficient sorbent-based DAC plants.
In this work we use Lewatit® VP OC 1065 as an example of a typical primary amine-functionalised adsorbent and we suggest henceforth to use this sorbent as a benchmark for DAC purposes. A key reason to select this sorbent is its commercial availability, and therefore accessibility to any interested party, besides being believed to be (very similar to) the adsorbent that Climeworks uses in their first-generation DAC process.‡
2. Theory of CO2 and H2O adsorption onto amine-functionalised adsorbents
2.1 Pure component adsorption
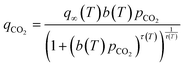 | (1) |
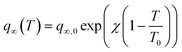 | (2) |
The affinity of the sorbent to CO2 is defined by eqn (3).§
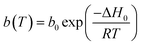 | (3) |
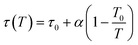 | (4) |
We chose an isotherm model that is commonly used to describe this behaviour, i.e., the Guggenheim–Anderson–de Boer (GAB) model.7,13,20 This model is an extension to the widely utilised Brunauer–Emmett–Teller (BET) equation.61 The derivation of the BET equation assumes that the first layer of adsorption has a heat of adsorption that is different from every subsequent layer, whilst the subsequent layers have a heat of adsorption equivalent to the latent heat of condensation. Meanwhile, the GAB model improves this by assuming that only the 10th layer onwards has a heat of adsorption equal to the latent heat of condensation, whilst the 2nd to 9th layers have a heat of adsorption that is different to the first layer. eqn (5) presents the GAB isotherm model:
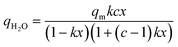 | (5) |
Some studies do not consider temperature dependency beyond its effect on relative humidity with k and c as constant values.13,15 Other studies describe the temperature dependency of k and c according to Anderson's derivation.7,16,20,21 These descriptions as they appear in Anderson's derivation are shown in eqn (6) and (7).21,22 Note that the pre-exponential factors used in the recent studies by Gebald et al.7 and Wurzbacher et al.20 are dropped to present the description according to Anderson's derivation.
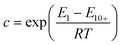 | (6) |
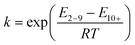 | (7) |
The picture is further complicated when it is considered that the heats of adsorption of the different layers may also be dependent on temperature, as is found by Anderson and Hall.22 Indeed, we know that the heat of condensation for water, or E10+, depends on temperature.
Here, we chose to incorporate the temperature dependency because our experimental results show a temperature-dependency beyond that taken into account by relative humidity, see Fig. S10 in the ESI,† as was also found earlier by Gebald et al. for amine-functionalised cellulose.7 Up to 100 °C, the thermal stability limit of Lewatit® VP OC 1065,23 we fitted the heat of condensation for water to the correlation shown by eqn (8) using experimental data from NIST.24
E10+ = −44.38T + 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220 220 | (8) |
Consequently, to fit an accurate version of the GAB equation, the unknown dependencies of temperature on E1 and E2–9 were empirically fitted to the experimental water isotherms measured in this study.
2.2 Effect of humidity on CO2 adsorption
On amine-functionalised adsorbents, it has been shown that CO2 has little impact on H2O equilibrium adsorption up to relative humidities of at least 60%.7,8,25 However, previous studies have shown that H2O enhances CO2 adsorption significantly.7–9,26,27The underlying chemical mechanisms need to be understood in detail to mathematically explain the co-adsorption of CO2 and H2O on amine-functionalised sorbents. Thus far, there has been only one attempt to develop an empirical mathematical description by Stampi-Bombelli et al.,13 further discussed in Section 2.3.3. Meanwhile, Jung and Lee derived an isotherm model from kinetics specifically for ammonium carbamate and bicarbonate formation.28 However, there is no one adsorption mechanism that is valid for all amine-functionalised sorbents, and actually, there may be multiple mechanisms in play on one adsorbent.
Here, we briefly discuss the key mechanisms used to derive mechanistically consistent co-adsorption isotherm models. The mechanisms are threefold: the change of amine efficiency (i.e., CO2 adsorption stoichiometry); a change in the heat of adsorption, also affecting the sorbent's affinity to CO2; and amine site blocking by adsorbed water molecules.
 | ||
| Fig. 1 Species that CO2 adsorbs as on amine-functionalised sorbents in the absence of water. (a) Ammonium carbamate. (b) Paired carbamic acid. | ||
Fig. 1(a) shows an ammonium carbamate ionic pair, which forms from an ammonium carbamate zwitterion precursor requiring two amine groups per adsorbed CO2 molecule.25,27,29–32 Meanwhile, Fig. 1(b) is a pair of carbamic acid species stabilising each other via hydrogen bonding.25,27,31,33,34 The carbamic acid requires stabilisation as it tends to convert back to CO2 and an amine group.35 Yu and Chuang indicated that carbamic acid formation was primarily associated with secondary amines, using in situ FT-IR spectroscopy. However, there is not enough evidence to rule out carbamic acid forming on primary amines.27 Indeed, the molecular modelling study by Buijs and De Flart in 2017 concluded that for the primary-amine based Lewatit® VP OC 1065, the formation of ammonium carbamate is unlikely, with the carbamic acid formation being a more favourable pathway. Although Alesi and Kitchin found, experimentally, that it was inconclusive as to whether carbamic acid or ammonium carbamate formation is the dominant mechanism on Lewatit® VP OC 1065.36,37 In reality, it may be that many different species form on one amine-functionalised sorbent, which is shown to be possible by Yu and Chuang.27
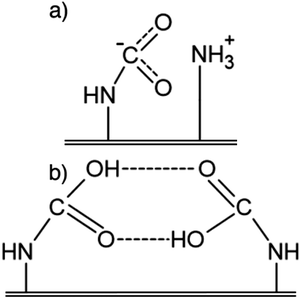
Fig. 2 presents the additional adsorbed species of CO2 that form in the presence of water. Fig. 2(a) shows ammonium bicarbonate, which forms from a paired ammonium carbamate precursor.25,27 Li et al. suggest that, instead of ammonium bicarbonate formation, hydronium carbamate could also be formed from ammonium carbamate.38 The effect on adsorption is very similar for both these pathways, as each species only requires one amine group for CO2 adsorption, as opposed to two for ammonium carbamate. Finally, Fig. 2(b) shows a carbamic acid that is stabilised by a water molecule rather than another carbamic acid.27
 | ||
| Fig. 2 Additional species that CO2 adsorbs as on amine-functionalised sorbents in the presence of water. (a) Ammonium bicarbonate. (b) Water stabilised carbamic acid. | ||
Kinetics may also play a role in the speciation of adsorbed carbon dioxide on amine-functionalised adsorbents. Didas et al. observed that the formation of ammonium bicarbonate is much slower than the formation of ammonium carbamate.25 However, to the best of our knowledge, this has yet to be confirmed for hydronium carbamate. If ammonium bicarbonate does form more slowly from an ammonium carbamate precursor, we would expect an initially fast uptake of CO2 until the amount of ammonium carbamate reaches equilibrium, before the uptake slowly increases further as the ammonium carbamate is converted to ammonium bicarbonate. Aside from chemical kinetics, the presence of water may also inhibit or enhance the diffusion of CO2 to the amine sites affecting the overall kinetics of the adsorption process. This requires further investigation but is beyond the scope of this study.
For independent water adsorption, water molecules hydrogen bond onto the amine groups as the first adsorption layer with multiple layers forming on top also via hydrogen bonding as shown in Fig. 3.27
The above-explained chemistry determines a key concept in CO2 adsorption onto amine sorbents, namely amine efficiency. Amine efficiency has previously been defined as the number of CO2 molecules adsorbed divided by the total number of amine groups available.25,30,39–42 For the formation of ammonium carbamate pairs, the theoretical maximum amine efficiency is 0.5 since this is the stoichiometric ratio of CO2 adsorbed to amine groups required. However, when ammonium bicarbonate or hydronium carbamate forms, the theoretical maximum amine efficiency or stoichiometric ratio increases to 1. For this reason, the amine efficiency can be enhanced in the presence of water.
Besides amine efficiency, humidity has also shown to affect the heat of adsorption of adsorbed species. Yu and Chuang utilised temperature-programmed desorption to calculate each species' binding energy, which is equivalent to the heat of desorption. The calculations find that the binding energy increases in this order: water stabilised carbamic acid < adsorbed water < paired carbamic acid ≈ ammonium carbamate pair.27 Their results show an apparent decrease in the overall heat of desorption in the presence of water occurring through a reduction in the binding energy of ammonium carbamate, and a promotion in the formation of ammonium bicarbonate. However, they also suggest that the presence of water increases the binding of paired carbamic acid and promotes its formation over other species leading to an overall increase in the heat of adsorption.
The heat of adsorption is not only relevant as a standalone quantity, but it also influences the uptake equilibrium, particularly the affinity constant of isotherms (see Section 2.1.1). For example, in the modified temperature-dependent version of the Toth model, shown in eqn (1)–(4), an increase in the magnitude of the heat of adsorption leads a larger affinity constant of the isotherms and vice versa.12 The affinity constant is a measure of the gradient of the isotherm at low partial pressures. As a result, an increase in the heat of adsorption would lead to higher uptakes under DAC conditions because of the very low CO2 concentrations in air.
Finally, we hypothesise there may be a third effect at play. This is water multilayers blocking CO2 access to amine sites, which could affect kinetics and equilibrium uptake alike. Didas et al. observed that water can have a negative impact on CO2 uptake at low partial pressures of CO2 for the amine-functionalised silica they studied with the highest amine coverage.25 We propose that the high amine coverage led to the agglomeration of water multilayers, formed on the amines, preventing CO2 from accessing the amine sites.
2.3 Water–CO2 co-adsorption models
The complexities in determining the exact species formed on adsorption, prevent us from using the classical kinetic approach to deriving an isotherm model. Hence, based on the above discussion, we propose two different models to consistently describe water–CO2 co-adsorption, which we call the mechanistic co-adsorption model and the weighted average dual site Toth (WADST) model. We also discuss a recently published empirical co-adsorption model by Stampi-Bombelli et al.13 Here onwards, we assume that water affects CO2 adsorption, but CO2 does not affect water adsorption. There is previous experimental evidence to support this assumption.7,8,43(1) At high water loadings, amine efficiency may be limited by hydrogen-bonded water structures blocking CO2 access to amine sites.
(2) The presence of water can increase the stoichiometric ratio due to ammonium bicarbonate forming rather than ammonium carbamate.
(3) The presence of water changes the heats of adsorption of adsorbed CO2 species hence the affinity.
Based on these three effects, we propose the mechanistic adjustment of isotherm behaviour described in eqn (9)–(13). First, we postulate a generic equation of CO2 loading including terms for the amine efficiency under actual, ϕ [−], and dry, ϕdry [−] conditions:
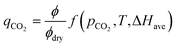 | (9) |
Secondly, effect 1 can be described as the fraction of the sites blocked by hydrogen-bonded water structures deducted from the theoretical maximum available sites, i.e., under zero site blockage, to calculate the fraction of amine sites available for adsorption as in eqn (10).
| ϕavailable = ϕmax − fblocked | (10) |
Thirdly, the fraction of amine sites blocked should be proportional to the size of adsorbed water aggregates. The size of these aggregates adsorbed is further related to the loading of water on the adsorbent. We hypothesise that a parallel can be drawn between these aggregates' growth with increased loading and how crystals grow with time. Crystals nucleate and grow slowly at first. Then when the particles are of sufficient size, they begin to aggregate, speeding up the growth. At small loadings, increasing loading may only slightly increase these structures' size before reaching the critical size needed to start forming aggregates. We propose to use Avrami's equation for this purpose, as shown in eqn (11).44–48
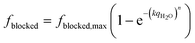 | (11) |
Next, is to describe the increase in the stoichiometric ratio and amine efficiency. As the loading of water increases, the fraction of the sites that exist with a convenient water molecule for stabilised carbamic acid or ammonium bicarbonate formation increases. We suggest that a Maxwell–Boltzmann distribution could describe this.49,50 This distribution is used for many things relating to the probability of two states, including economics.51 Another example is chemical kinetics, where it is used to show how, with an increase in temperature, a higher proportion of molecular collisions have the required energy for a reaction to occur (the well-known Arrhenius’ law). Henceforth, we suggest applying the Maxwell–Boltzmann distribution to our case providing eqn (12).
 | (12) |
Here A [mol kg−1] is a critical water loading value that must be fitted as well as ϕdry [−].
Finally, the heat of adsorption can be calculated by taking a weighted average between the wet and dry states. Since 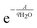 is equal to the fraction of sites that form adsorbed species with water, the weighted average appears as in eqn (13).
is equal to the fraction of sites that form adsorbed species with water, the weighted average appears as in eqn (13).
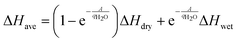 | (13) |
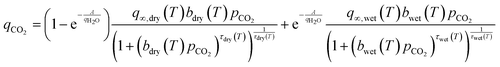 | (14) |
Here the dry site in the isotherm is simply defined by the Toth model shown in eqn (1)–(4). Meanwhile, the wet site is again defined by the same equations and fit, alongside A, to co-adsorption experiments, with the dry site already fixed from pure-component isotherms.
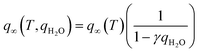 | (15) |
| b(T, qH2O) = b(T)(1 + βqH2O) | (16) |
Here γ [—] and β [—] do not have any specific physical meaning but are simply the parameters that describe co-adsorption and should be fit to wet experiments. The authors of this study also suggest that γ and β should be greater than 0. However, we suggest that γ could have a negative value to take into account the overall CO2 capacity reducing due site blockage. This model is virtually the only co-adsorption model that has been investigated before and we include it in our investigations as a comparison for our models in terms of accuracy of describing the co-adsorption phenomenon and cyclic process performance.
3. Experimental methods
After formulating the mathematical adsorption equilibrium models, these needed to be parametrised with experimental data, which acquisition is described here.The material investigated, Lewatit® VP OC 1065, was obtained from Sigma-Aldrich. It is a divinylbenzene (DVB) crosslinked polymer functionalised with primary amine groups. It has an average pore diameter of 25 nm, a bead size of 0.315–1.25 mm, pore volume of 0.27 cm3 g−1, and a surface area of 50 m2 g−1.23 Meanwhile, the heat capacity is reported as 1.58 kJ kg−1 K−1.52
CO2 and H2O pure component isotherms and co-adsorption isotherms were measured using the DVS Vacuum system, of which a simple schematic is depicted in Fig. 4.54 The DVS uses a gravimetric, magnetic suspension balance to accurately measure any weight changes as a result of adsorption and desorption. It can operate in dynamic mode with gas flowing through the sorption chamber or static mode where gas is pulsed into the chamber. The DVS utilises a turbomolecular pump which ensures extremely thorough outgassing.
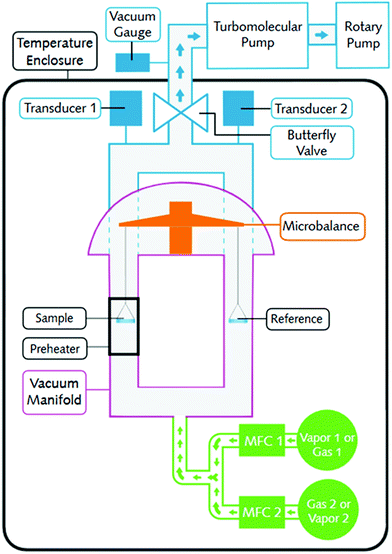 | ||
| Fig. 4 A simple schematic showing the DVS Vacuum. Used with permission of Surface Measurement Systems.53 | ||
The methodology for measuring pure component isotherms first involved an outgassing step where around 50 mg of sample was heated to 100 °C at a pressure of around 1–2 Pa for at least 10 hours. The sample was then cooled to the desired adsorption temperature before the gas and/or vapour was fed to the device by opening the mass flow controllers. The pressure was increased stepwise up to 1 bar allowing the mass of the sample to equilibrate at each step. CO2 sorption was studied under semi-static mode, whilst the dynamic mode was used for H2O sorption. Once 1 bar was reached, the procedure was repeated backwards, i.e., in desorption mode, providing potential hysteresis measurements. A fresh sample of adsorbent was used for every isotherm.
CO2–water co-adsorption isotherm experiments were undertaken using the DVS Vacuum under the explicit assumption that CO2 does not affect H2O equilibrium adsorption. This was demonstrated by Veneman et al. for Lewatit up to a relative humidity of 60%.8 In addition, there is evidence that this assumption also holds for other types of amine-functionalised adsorbents, such as silica and cellulose.7,25 For the co-adsorption experiments, the outgassing step was the same, and semi-static mode was used. Then, the sample was first equilibrated with H2O vapour (for both the 30% and 55% RH experiments). Since CO2 does not affect H2O adsorption, the H2O loading and partial pressure will remain constant for the rest of the experiment, given that no more H2O is added to the chamber. Hence, further pressure increments were implemented using only CO2, and semi-static mode was used (i.e., no outlet from the chamber). An example dynamic profile of the experiments is shown in the ESI,† Fig. S2.
Tests were also conducted to check whether the assumption applied here would hold up to 80% relative humidity. However, a sharp initial drop in the mass was observed in the dynamic profile of the experiment when CO2 was added to the chamber, suggesting the desorption of H2O. We were unable to determine whether the subsequent adsorption was due to CO2, H2O, or both. Further experimental work is currently undertaken to study this phenomenon. An example of this profile is shown in Fig. S4. More information about the methodology is available in the ESI.†
4. DAC process modelling
4.1 Process design
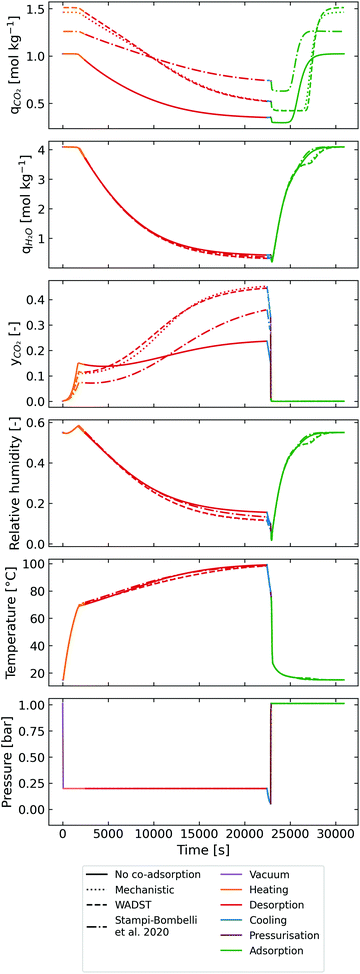
Using the calibrated adsorption isotherms, we moved to cycle modelling to understand the impact of co-adsorption descriptions on process performance and to provide an independent benchmark of a TVSA DAC cycle. The cycle modelled is shown in Fig. 5, where the process is a packed-bed TVSA cycle. The steps of the cycle are as follows: vacuum, heating, a second heating step where the product is extracted (desorption), cooling, pressurisation, and adsorption. The cooling step is important to bring the sorbent temperature to below 90 °C before being exposed to air to avoid unnecessary sorbent degradation through oxidation.52,55,56 This process is similar to the one, without a steam-purge, used by Stampi-Bombelli et al. with an extra addition of the short cooling step to prolong the sorbent's lifetime.13 The process is modelled as a very thin, flat packed bed with length L = 1 cm and diameter D = 10 cm. Although a traditional (column type) packed bed is unlikely to be deployed in a real DAC process, the modelling of a flat packed bed can be a realistic approximation to modelling one of the adsorbent plates described in patents from Climeworks.57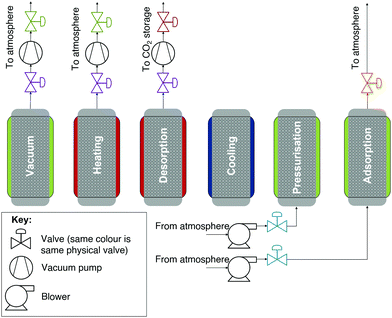 | ||
| Fig. 5 A schematic of the simple DAC TVSA cycle modelled in the case studies. The valves are highlighted in different colours to represent which valves are the same physical valves. | ||
4.2 Model assumptions, mass and energy balances
A physical model is required to study the dynamics of adsorption within a packed bed. This will allow the fitting of mass transfer coefficients to experimental data and the study of DAC process performance. The model used in this study closely follows the work by Casas et al.6 Validation work can be found in the ESI,† Fig. S18, where the model results are compared to an earlier temperature swing adsorption study from Joss et al.58The key assumptions for the model are as follows:
• The fluid is an ideal gas.
• The flow is described by an axially dispersed plug flow model.
• Radial gradients are neglected.
• There is instantaneous thermal equilibrium between the fluid and solid pellets.
• Mass transfer is described by the linear driving force (LDF) model.59 The LDF constants are fitted to dynamic data, shown in Fig. S16 and S17 of the ESI.†
• Mass transfer coefficients, axial dispersion coefficients, solid heat capacities, and heats of adsorption are independent of temperature.
• No N2 is adsorbed,¶ and the non-CO2/H2O component of air acts as N2.
• Pressure drop is described by the Ergun equation.60
The overall mass balance is:
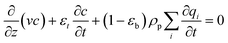 | (17) |
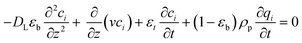 | (18) |
Here DL [m2 s−1] is the axial dispersion coefficient, and ci [mol m−3] is the concentration of component i.
Next, the energy balance is:
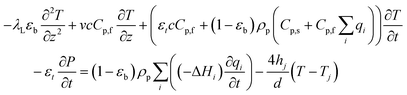 | (19) |
A wall energy balance, like the one presented by Casas et al., is not included in this model.6 Instead, a global heat transfer coefficient is used to predict the heat transfer from the jacket to the inside of the column. We found that this did not significantly affect the model results during validation and substantially improved the computational speed and robustness. However, it does prevent us from calculating the parasitic sensible heat of the contactor. We would like to stress here that the process presented is a hypothetical one to show the effect of co-adsorption, and further detailed design of the contactor would be required to calculate the sensible heat of the contactor accurately.
Other constitutive equations, along with boundary and initial conditions, can be found in the ESI.†
The model calculates the following performance indicators that are relevant to this work:
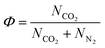 | (20) |
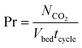 | (21) |
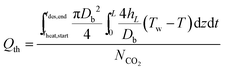 | (22) |
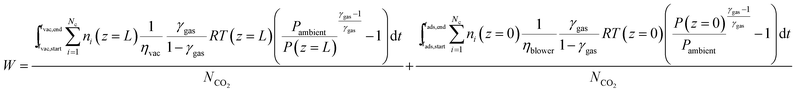 | (23) |
The specific thermal energy is subsequently converted to specific equivalent work via the Carnot efficiency as per the guidance of Danaci et al.61 This allows for both forms of specific energy, thermal and work, to be collected into one term. The equation for this conversion is shown in eqn (24).
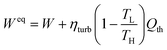 | (24) |
Here Weq [J mol−1] is the specific work equivalent, ηturb [—] is an isentropic turbine efficiency, TL [K] is the lowest temperature that energy can be extracted at, whilst TH [K] is the temperature of the heating medium in the system. TL is assumed to equal to the ambient feed temperature, whilst TH is equal to Tj during the heating step. ηturb is taken as 0.75 since Danaci et al. state that values between 0.7 and 0.8 are appropriate.61
The physical packed-bed column model was implemented in the gPROMS custom modelling suite.62 The partial differential equations were first discretised using a 2nd order central finite differences method. Next, the index of the equations is reduced according to the Pantelides algorithm to create a solvable set of ordinary differential equations.63 Finally, these ordinary differential equations were solved using an implicit Runge–Kutta method and a variable time step. Cyclic steady-state conditions are evaluated at 5 equidistant points along the column. Cyclic steady-state is defined as when the loading, composition, pressure and temperature at each of these points at the end of the cycle is within 0.5% of the values at the end of the previous cycle. There is also the option in the model to monitor the percentage of CO2 saturation reached at the end of the bed during the cycles. This can be used to adjust the adsorption time accordingly, as is done in this work's parametric study.
4.3 Heuristic process optimisation
Finally, a heuristic optimisation was performed in order to compare the performance indicators when using the different co-adsorption isotherm models in the cycle model. The heuristic optimisation approach involves varying many operating variables simultaneously to try and find the optimal design space. To that end, we used gPROMS Global Systems Analysis tool, and more specifically the Monte Carlo-based method function. The baseline case, described fully in Table S9 of the ESI,† is the starting point for this work and is also used to compare the cycle profiles using each co-adsorption isotherm model. Monte Carlo simulations are done varying five main operating variables in the process: vacuum pressure, jacket temperature, heating time, desorption time, and adsorption time. Instead of controlling the adsorption time directly, the percentage of CO2 saturation reached at the end of the bed is controlled. For each variable, a uniform distribution was assumed between the low and high values. These values are detailed in Table 1.| Variable | Unit | Low value | High value |
|---|---|---|---|
| P vac | bar | 0.1 | 0.45 |
| T j,heat/Tj,des | °C | 90 | 100 |
| t heat | s | 100 | 1500 |
| t des | s | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| CO2 bed saturation (z = L, t = tads,end) | % | 95 | 99 |
Sonnleitner et al. found that the stability limit of Lewatit® VP OC 1065 is 90 °C and 110 °C in air and N2, respectively.52 It is assumed that oxygen is the reason why the stability limit is much lower in air. By the time the column reaches these temperatures in our baseline case, the column's gas composition is made up of mostly CO2 and H2O, so 100 °C is chosen as a maximum limit. This is also the operating limit defined by the manufacturer.23 The lower limit of vacuum pressure is chosen to be 0.1 bar, as pressures below this value are not typically achieved on an industrial scale. There is no specific limit on the rest of the parameters, and they were chosen after a preliminary investigation into the operating region of interest.
The actual distribution of the factors can be found in the ESI,† which confirms that they are uniform. The one exception to this being the vacuum pressure distribution of the simulations using the Stampi-Bombelli et al. model. It was observed that the model struggled to converge at less deep vacuum pressures. However, this is not important as, at these vacuum pressures, the purities using this isotherm model are generally very low, hence the results would not be carried forward for further study. For each isotherm model, 3000 samples are simulated using the cycle model to get a thorough design space coverage.
5. Results and discussion
5.1 Pure component equilibria
The measured experimental isotherms for pure CO2 and H2O adsorption are shown in the ESI,† Fig. S6 and S10, respectively, with the desorption branches included. Additionally, the isotherm model fits to the adsorption branches are shown in Fig. S7–S9 and S11 (ESI†), whilst the fitted parameters for CO2 adsorption are shown in Tables 2 and 3. It was decided that taking hysteresis into account in process modelling would make the cycle model solution unnecessarily complex, and there are questions over how the experimental desorption branches, based only on pressure reduction, would extrapolate to a process where temperature is also being increased.| Parameter | Value | Unit |
|---|---|---|
| a Limits were imposed on parameters during the fitting process to ensure that the parameters kept their physical meaning. For example, χ is limited to being greater than or equal to 0 to ensure that the maximum capacity did not increase with increasing temperature and so avoided the isotherms crossing. | ||
| T 0 | 298.15 | K |
| q ∞,0 | 4.86 | mol kg−1 |
| χ | 0.0000 | — |
| b 0 | 2.85 × 10−21 | Pa−1 |
| −ΔH0 | 117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798 798 |
J mol−1 |
| τ 0 | 0.209 | — |
| α | 0.523 | — |
| Parameter | Value | Unit |
|---|---|---|
| q m | 3.63 | mol kg−1 |
| C | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
J mol−1 |
| D | 0.023744 | K−1 |
| F | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 706 |
J mol−1 |
| G | −47.814 | J mol−1 K−1 |
We shall not go into many details on the pure-component isotherms since they have been explored many times before.7,8,11,12,15 However, the hysteresis observed for both CO2 and H2O isotherms is interesting and for CO2 adsorption measurements this is an unexpected insight. Careful attention was applied to ensuring each point of both the adsorption and desorption branches had reached equilibrium, e.g., by increasing the tolerance to move to a next step (mass change per time) from 0.0015% min−1 to 0.0007% min−1. Evidence of this can be seen in the mass change over time gravimetric graph in the ESI,† Fig. S1. Thus, the alternative explanation that equilibrium was not entirely reached before stepping to the next measurement point was tentatively rejected and we believe the hysteresis exhibited is a real phenomenon.
Such hysteresis in CO2 adsorption on amine-functionalised sorbents has been observed before in a study by Zhou et al. in 2014.64 The adsorbent that the authors studied was an amine functionalised SBA-15. We agree with these authors' suggestion that the hysteresis is potentially due to the strongly chemisorbed species formed on adsorption, and an explanation can be found in the fact that the endothermic direction of the reaction (desorption), has a greater activation energy than the exothermic direction. This also explains how the hysteresis becomes less significant as temperature increases as generally, the energy to overcome this barrier is more likely to exist in the system. Previously, a study by Yu and Chuang has shown that some carbamic acid, the species with the highest heat of adsorption in paired form, is only desorbed using temperature-programmed desorption, supporting the explanation provided here.27 Meanwhile, the hysteresis loop exhibited in the H2O isotherms, is common behaviour for multilayer adsorption as a result of metastability of the multilayer in the adsorption branch.19
The fitting of the water isotherms was slightly more complicated due to the unknown nature of the relationship between E1 and E2–9 and temperature. A linear relationship was observed between E2–9 and temperature in the temperature range studied, much like the latent heat of condensation, E10+. Meanwhile, E1 appeared to have a concave downwards relationship with temperature meaning the difference between the heat of adsorption of E1 and E2 − 9 reduces as temperature increases. This is shown in Fig. S12 in the ESI.†Eqn (25) and (26) show the correlations that will fit these two relationships.
| E1 = C − exp(DT) | (25) |
| E2–9 = F + GT | (26) |
Additionally, the average isosteric heats of adsorption as calculated from the Clausius–Clapeyron relation using the experimental isotherm data were −70 kJ mol−1 and −46 kJ mol−1 for CO2 and H2O adsorption, respectively.
5.2 Co-adsorption equilibria
Fig. 6 presents the CO2 isotherm results under wet conditions. Here, we present the uptake enhancement as a function of relative humidity and CO2 pressure, where the enhancement factor is defined as the CO2 adsorption under wet conditions divided by the CO2 adsorption under dry conditions at the same temperature and partial pressure of CO2. The co-adsorption enhancement effect primarily manifests itself in the lower pressure region with up to 2.5 times the adsorption capacity as under dry conditions. This is of key importance for DAC processes, as they operate adsorption at partial CO2 pressures of 0.4 mbar. The enhancement seems to asymptote towards unity in the higher-pressure region. Again, this is beneficial for DAC, as desorption commences at significantly higher partial pressures than adsorption (e.g., ∼30 mbar in the baseline case considered in Fig. S12, ESI†).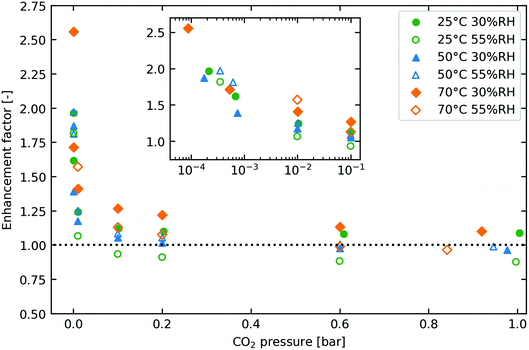 | ||
| Fig. 6 Enhancement factor of co-adsorption experiments plotted against pressure for a range of temperatures and humidities. Here enhancement factor is defined as amount of CO2 adsorbed divided by the amount of CO2 that would be adsorbed under dry conditions at the same temperature and pressure. The isotherm loading values can be found in Fig. 7. Meanwhile, they are converted into amine efficiency in Fig. S13 in the ESI,† using the amine loading of 6.7 mmol g−1 reported by Alesi and Kitchin.37 | ||
The observation that the enhancement of CO2 adsorption occurs mainly at lower CO2 pressures supports the theory that carbamic acid formation is the main adsorption mechanism on Lewatit® VP OC 1065 as earlier suggested by molecular modelling from Buijs and De Flart.36 (See Fig. 1b and 2b in Section 2.2.1) If ammonium bicarbonate or hydronium carbamate was formed from ammonium carbamate, the high enhancement factors observed at low partial pressures would be expected to persist at higher partial pressures as each amine group that is used for adsorbing one CO2 molecule under dry conditions can now adsorb two. (See Fig. 1a and 2a in Section 2.2.1) Indeed, the ϕdry in the mechanistic model is fitted to have a value of 1, suggesting that under dry conditions the same stoichiometry exists as under wet conditions. In addition, Stampi-Bombelli et al. fitted their γ parameter to be 0 on amine-functionalised cellulose, meaning that the maximum adsorption capacity was not affected by water. This is further evidence against an increase in stoichiometry. For these reasons, we believe that paired carbamic acid and water-stabilised carbamic acid are the two main species formed due to CO2 adsorption. However, this does not mean that ammonium carbamate cannot form, and this has been proven to happen on other adsorbents.25,27,29–32 An alternative explanation is that ammonium carbamate forms under dry conditions, but the presence of water promotes carbamic acid formation instead, as suggested by Yu and Chuang.27
The co-adsorption results of Fig. 6 also seem to indicate that the higher-humidity experiments sit slightly below the lower-humidity experiments, even reducing the enhancement to below 1 at higher partial pressures, which seems apparent especially for the 25 °C and 70 °C measurements. This would suggest that water can indeed block some amine sites and thereby lower adsorption capacity, as we hypothesised in Section 2.3.1, but it is difficult to say for sure if this is true as this effect was not visible in the 50 °C experiment. The experiments were repeated to check this, with identical results obtained. Therefore, further experimentation including different experimental methods needs to be undertaken: currently, we are running a campaign of breakthrough experiments that could corroborate or reject the findings here.
Fig. 7 and Fig. S14 (ESI†) shows the fit of the experimental data to the two novel co-adsorption models in this work alongside the empirical model from Stampi-Bombelli et al.13 The parameters found in this fitting process are presented in Table 4. It is noted that no model provides a perfect fit, and there is at least one case for each model where a relatively poor fit is found, indicating the known and unknown complexities of co-adsorption and measuring it. When comparing the three models, it is observed that the WADST and the mechanistic model seems to fit the low-pressure region very well. The low-pressure region is most important for DAC, as the partial pressure of CO2 is always relatively low throughout the cycle, hence this is especially promising. Furthermore, we suggest that there could be changes in the mechanisms at higher CO2 pressures and this explains why the fitting is generally poorer at the higher-pressure regions. The mechanistic model seems to predict the higher-pressure region better than the WADST model, although the predictions are far from perfect.
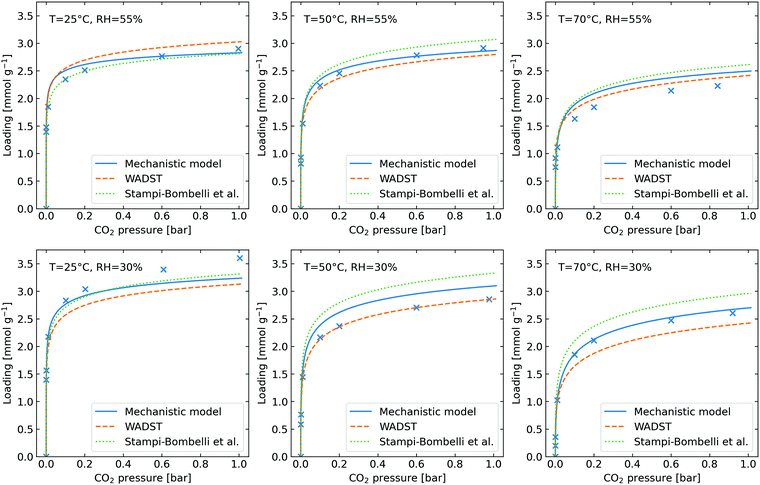 | ||
| Fig. 7 Experimental co-adsorption CO2 isotherms (markers), at various temperatures (T) and relative humidities (RH), fitted to an empirical literature co-adsorption model from Stampi-Bombelli et al. and the two models presented in this work.13 The low pressure range of this graph on a log scale is presented in Fig. S14 in the ESI.† | ||
| Parameter | Value | Unit |
|---|---|---|
| Stampi Bombelli et al. model | ||
| γ | −0.137 | — |
| β | 5.612 | — |
| Mechanistic model | ||
| f blocked.max | 0.433 | — |
| k | 0.795 | kg mol−1 |
| ϕ dry | 1.000 | — |
| A | 1.535 | mol kg−1 |
| −ΔHwet | 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155 155 |
J mol−1 |
| n | 1.425 | — |
| WADST model | ||
| b 0,wet | 1.230 × 10−18 | Pa−1 |
| q ∞,0,wet | 9.035 | mol kg−1 |
| τ 0,wet | 0.053 | — |
| χ wet | 0.000 | — |
| α wet | 0.053 | — |
| −ΔHwet | 203![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 687 687 |
J mol−1 |
| A | 1.532 | mol kg−1 |
Meanwhile, the Stampi-Bombelli et al. consistently overpredicts the capacity at the higher two temperatures. We suggest this is due to the model not considering the effect of temperature on the co-adsorption phenomenon. Mathematically, for a given water loading, it is predicting a constant increase in affinity and decrease in capacity at every temperature.
Another important point to make is that the experimental data has sources of potential error. We are trying to elucidate the effect of three parameters (temperature, pressure, and humidity) at once, and there is a possibility for measurement error in all these as well as in the sample mass. Considering this, we should not expect the models to be able to fit the experimental data perfectly.
Finally, when studying the fitted parameters, the value of the critical water loading parameter, A, is very similar in both the WADST model and the mechanistic model. This is noteworthy as it implies that both models predict the same probability of a CO2 adsorption site having a water molecule available given the same loading of water, which is supportive of our hypotheses on co-adsorption.
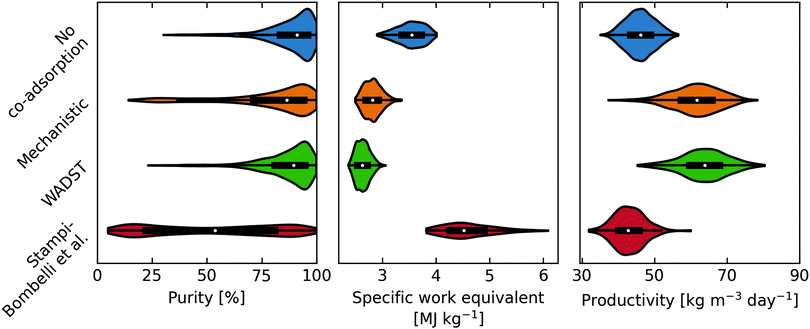
5.3 Effect of co-adsorption model selection on process modelling
In contrast, the Stampi-Bombelli et al. approach leads to a similar working capacity to when no co-adsorption is included. This is due to the model assuming that the effect of water loading on the isotherm parameters varies with water loading but not temperature, meaning that this model also predicts higher loadings at desorption conditions. Meanwhile, the WADST and mechanistic models do take the effect of temperature on co-adsorption into account. This leads to the enhancement predicted by the WADST and mechanistic models being higher at the start of the desorption step yet lower at the end than the empirical model predicts.
A good example materialises when comparing the mechanistic and WADST model points, selected as they have identical working capacities, in Table S11 of the ESI.† The mechanistic model predicts that a lower vacuum pressure, longer heating time and longer desorption time are needed to achieve the same working capacities. This is caused by the slight difference in the shape of the isotherm and its dependency on temperature. Essentially, the mechanistic model predicts a higher affinity at higher temperatures. Overall, comparing the mechanistic model to the WADST model (considering the working capacities are identical):
• The lower vacuum pressure leads to a higher electrical energy requirement.
• The overall combined longer heating and desorption time leads to (i) a lower productivity, and (ii) a slightly higher heating requirement, as a slightly higher final temperature is achieved.
This reflects what we see in the distributions with the mechanistic model predicting slightly higher work equivalent and lower productivity. The slight difference in purity is again due to the isotherm shape and can be explained by considering that a longer heating time is required in the mechanistic model case, hence CO2 starts desorbing at higher temperature. So, the lower purity values predicted are explained by the heating time simply being too short to desorb enough CO2 to displace the N2 from the column.
The same arguments can be made when analysing the no co-adsorption, and Stampi-Bombelli et al. cases. The Stampi-Bombelli et al. model predicts a very steep isotherm at low partial pressures of CO2 and regeneration temperatures, leading to the requirement for much lower vacuum pressures to achieve comparable working capacities. So, the electrical work, hence work equivalent, is predicted to be much higher than for the other models, and at the same time, the distribution of purity is larger as only the data points with such low vacuum pressures deliver viable purities. The no co-adsorption case predicts the lowest working capacities at optimal conditions leading to distributions of lower productivities and higher specific work equivalent than for the mechanistic and WADST model cases. However, the isotherm is the least steep at low pressures. So, despite the low working capacities, the work equivalent distribution predicted is better than for the Stampi-Bombelli et al. case since less extreme vacuum pressures are required to achieve the desired purity. Likewise, this is why the no co-adsorption predicts the largest share of high purities of all the models.
To summarise, the mechanistic and WADST model predict that co-adsorption improves process performance, in terms of productivity and specific work equivalent, due to the higher achievable working capacities. However, it does make it slightly harder to achieve the required purity, as the isotherms have a larger gradient in the lower-pressure region. Meanwhile, the Stampi-Bombelli et al. isotherm model predicts that co-adsorption penalises process performance due to requiring deeper vacuum pressures owing to a higher affinity. It is expected that if a lower vacuum pressure limit was chosen, the Stampi-Bombelli et al. model case would achieve the desired purity more easily. This leads to a perhaps obvious but interesting conclusion that there is an optimal gradient in the lower-pressure region. It needs to be steep enough to adsorb CO2 at such low concentrations, without being too steep, at desorption temperatures and partial pressures, to require very deep vacuum pressures. Meanwhile, this gradient is affected by both the affinity constant, b, of the sorbent in dry conditions, and the humidity.
Overall, the findings of the Stampi-Bombelli et al. model are cautiously rejected based on its failure to predict the capacity of the sorbent at higher temperatures, see Section 5.2. However, it does present itself as a valuable option if only the adsorption at one temperature is subject of study.
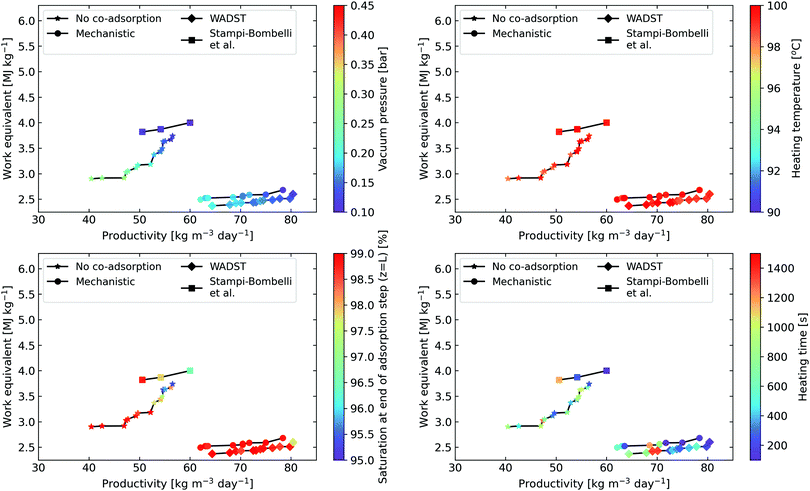 | ||
| Fig. 10 Pareto fronts of specific work equivalent vs productivity for samples that show a purity of greater than 95% as predicted when applying each co-adsorption isotherm model approach. The operating parameter desorption time is left out, but the trends with desorption time are shown in Fig. S23–S26 in the ESI.† Solid lines added as a guide for the eye. The range of the colour bars is the range investigated in the heuristic optimisation. We see here that the heating temperature converges to the upper bound for all values on the pareto front. A breakdown of the work equivalent contributions for the optimal points can be found in Fig. S27 in the ESI.† | ||
Beginning with the common trends, a higher heating temperature always leads to better performance concerning specific work equivalent and productivity until it reaches the chosen degradation temperature limit of ∼100 °C. Furthermore, cycle performance is improved when we run the adsorption step until the sorbent is practically completely saturated, allowing CO2 to breakthrough at the column end (this is contrary to post-combustion capture, where it is vital to achieve high recoveries, as the main goal is to prevent CO2 emissions to the atmosphere). Additionally, vacuum pressure and the heating time present a trade-off between productivity and specific work. Operating at a higher vacuum pressure leads to lower specific equivalent work, as a higher proportion of the desorption energy is supplied via heat which is less valuable in terms of exergy. However, then a longer heating time – reducing productivity – is needed to ensure that the most of the N2 is displaced prior to desorption to achieve the desired purity.
Now, considering which co-adsorption models lead to the best DAC performance predictions, the WADST and mechanistic models clearly predict better performance as a result of co-adsorption (for the constraint of greater than 95% purity). The Pareto front is shorter when these co-adsorption descriptions are applied, implying that there is a diminished trade-off between productivity and energy consumption, demonstrating that the operating conditions must be selected more cautiously to find the optimal point. One notable difference as discussed earlier, is the predicted optimal vacuum pressure which is lower when a co-adsorption description is included. It may be worth investigating whether even lower vacuum pressures can be achieved in actual equipment at an industrial scale. The minimum vacuum pressure was chosen as 0.1 bar since it was assumed that it might be significantly harder to achieve lower pressures: in industrial settings, vacuum pressures are usually higher than 0.1 bar.
Table 5 compares this benchmark case to other DAC technologies in literature. The technologies chosen for comparison are: (i) the Climeworks process using average recorded values recently reported,65 (ii) two cases of a monolithic adsorption process using novel metal–organic frameworks (MOFs),66,67 and (iii) a liquid absorption process. The monolithic adsorption case is believed to be a similar technology to that used by Global Thermostat, albeit they are unlikely to be using such a novel adsorbent, meanwhile the liquid absorption case is similar to that used by Carbon Engineering.69,70
| Process | Packed-bed temperature vacuum swing adsorption | Coated monolith temperature vacuum swing adsorption with steam stripping | Liquid absorption | ||
|---|---|---|---|---|---|
| Material | Lewatit® VP OC 1065 | Unknown – Climeworks process | MIL-101(Cr)-PEI-800 | mmen-Mg2(dobpdc) | Metal hydroxide |
| Working capacity [mol kg−1] | 0.91 | Unknown | 0.75 | 2.55 | N/A |
| Specific heat energy [MJ kg−1] | 9.93 | 11.9 | 9.68 | 4.75 | 5.84 |
| Specific electrical energy [MJ kg−1] | 0.80 | 2.52 | 0.80 | 0.73 | 1.46 |
| Regeneration temperature [°C] | 100 | ∼100 | 100 | 100 | ∼900 |
| Specific work equivalent [MJ kg−1] | 2.49 | 4.55 | 2.45 | 1.54 | 4.76 |
| Specific work equivalent [MJ kg−1] (with additional compression to 150 bar) | 2.93 | 4.99 | 2.89 | 1.98 | 4.76 |
| Ref. | This work | 65 | 66 and 67 | 66 and 67 | 68 |
We believe that using Lewatit® VP OC 1065 in a packed-bed TVSA process represents a realistic benchmark due to the commercial availability of the sorbent and the simple set-up of the process. The calculated heat input into this benchmark is 17% lower than the value reported for Climeworks. This is not unexpected as our case represents a highly ideal and optimised situation. For example, given the Climeworks adsorption bed design (compare, e.g., US patent 2017/0326494 A1),71 it is not expected that they can fully saturate their bed, as our models predict is the optimal case. Also, their electrical energy consumption is higher by 215%. Explanations for this may lie in the performance of blowers and vacuum equipment having significantly lower efficiencies during real operations, and in bed pressure drop being much higher than predicted by our model (note that our model) assumes a thin layer of sorbent where the air flows through in axial direction, while in the Climeworks contactor, the flow is parallel to the adsorbent sheets, then permeates through the sheet, after which it flows parallel along the sheet to the outlet again. As a result, our benchmark specific work equivalent is lower than for the Climeworks process, suggesting that the Climeworks design could be further optimised to use less electricity and to maximise adsorbent use.
The predicted heat and electrical energy consumption of the monolithic adsorption process utilising MIL-101(Cr)-PEI-800 is similar to that predicted by the benchmark case. However utilising mmen-Mg2(dobpdc) in a monolithic adsorption process leads to substantially lower heat and electrical energy consumption. The same research group has since shown that mass transfer limitations and the shape of the isotherm could reduce the effectiveness of mmen-Mg2(dobpdc) for DAC72 although, if these limitations can be overcome, functionalised MOFs could become effective DAC sorbents.
Last, the liquid absorption process uses less heat than our adsorption benchmark. However, this process is penalised in the work equivalent calculation due to the very high-temperature requirement for regeneration (∼900 °C), and as a result, it is the least favourable process using this metric. However it has been noted previously that, currently, the capital costs of this process may be lower than for solid sorbent based DAC.73
6. Conclusions
We have developed two novel approaches to modelling the co-adsorption of water and CO2 onto chemical adsorbents, the ‘mechanistic’ and ‘WADST’ models, and showed, using a detailed DAC model, how the choice of (co-)adsorption isotherm significantly influences DAC process performance, as well as presented an independent benchmark of a TVSA process for direct air capture. To this end, we presented a comprehensive set of new pure-component isotherm data for CO2 and H2O adsorption on Lewatit® VP OC 1065, as well as co-adsorption isotherm data that shows the enhancement, and potential diminution at higher partial pressures, of CO2 adsorption in the presence of water. The pure-component experimental data was fitted to the Toth isotherm model for CO2 adsorption and the GAB isotherm model for water adsorption, the novel mechanistic and WADST co-adsorption models were fitted to the co-adsorption data and a comparison was made with an empirical model presented earlier. The WADST and mechanistic models were especially successful at fitting the co-adsorption data in the crucial lower-pressure region.It was found that the WADST and mechanistic isotherm models both provide relatively similar results. The DAC cycle performance that they predict is improved due to co-adsorption, where the mechanistic model predicts slightly lower productivity, and higher specific energy input than the WADST model. We presented a benchmark DAC process-sorbent combination using the mechanistic co-adsorption isotherm model, which was chosen due to its (i) accuracy, (ii) mechanistic nature, and (iii) conservative predictions compared to the WADST model. Minimising the energy consumption, the specific work equivalent of the process was found to be 2.49 MJ kg−1, achieving a CO2 purity of 95.2%. This compares to the Climeworks process's specific work equivalent of 4.55 MJ kg−1, where the difference can likely be explained by the effects of bed saturation, heat losses and inefficiencies that move the real process away from the modelled scenario. Additionally, vital learnings on how to operate solid sorbent based DAC processes were elucidated from the Pareto fronts that resulted from heuristic optimisation. This demonstrated that it is optimal to use a heating temperature as high as this sorbent's stability allowed (100 °C) and run the column until saturation has just been reached. The vacuum pressure, desorption time, and heating time should then be optimised according to the desired purity and desired placement along the Pareto front, i.e., either favouring higher productivity lowering capital costs, or lower energy consumption lowering operating costs.
In conclusion, this study critically showed the importance of including accurate co-adsorption descriptions in process modelling of solid sorbent DAC systems and the considerable effect co-adsorption has on process performance due to varying working capacity and isotherm shape. Further work needs to be done to properly characterise co-adsorption on amine-functionalised sorbents at higher relative humidities, which are commonly found in the real-world, and study the effect that co-adsorption has on mass transfer, subject of an ongoing investigation. Further to this, a full and independent techno-economic assessment should be performed on this benchmark sorbent-process system to properly benchmark the price of DAC today and to identify opportunities to drive the cost down in the future to support the scale-up of this vital technology.
Author contributions
John Young contributed to the conceptualisation, formal analysis, investigation, methodology, software, validation, visualisation, and writing – original draft. Enrique García-Díez contributed to the investigation, methodology, and writing – review and editing. Susana Garcia contributed to the conceptualisation, formal analysis, funding acquisition, investigation, methodology, and writing – review and editing. Mijndert van der Spek contributed to the conceptualisation, formal analysis, funding acquisition, investigation, methodology, visualisation, and writing – review and editing.Conflicts of interest
There are no conflicts to declare.Note added after first publication
This article replaces the version published on 9th August 2021 to include the value of q∞,0which was missing from Table 2.Acknowledgements
The research in this article was supported in part by the PrISMa Project (299659), funded through the ACT Programme (Accelerating CCS Technologies, Horizon 2020 Project 294766). Financial contributions from the Department for Business, Energy & Industrial Strategy (BEIS) together with extra funding from the NERC and EPSRC Research Councils, United Kingdom, the Research Council of Norway (RCN), the Swiss Federal Office of Energy (SFOE), and the U.S. Department of Energy are gratefully acknowledged. Additional financial support from TOTAL and Equinor is also gratefully acknowledged.References
-
V. Masson-Delmotte, P. Zhai, O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, C. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycokc, M. Tignor and T. Waterfield, Global warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, 2018 Search PubMed
.
- J. C. Minx, W. F. Lamb, M. W. Callaghan, S. Fuss, J. Hilaire, F. Creutzig, T. Amann, T. Beringer, W. de Oliveira Garcia, J. Hartmann, T. Khanna, D. Lenzi, G. Luderer, G. F. Nemet, J. Rogelj, P. Smith, J. L. Vicente Vicente, J. Wilcox and M. del Mar Zamora Dominguez, Environ. Res. Lett., 2018, 13, 063001 CrossRef
.
- S. Fuss, W. F. Lamb, M. W. Callaghan, J. Hilaire, F. Creutzig, T. Amann, T. Beringer, W. de Oliveira Garcia, J. Hartmann, T. Khanna, G. Luderer, G. F. Nemet, J. Rogelj, P. Smith, J. L. V. Vicente, J. Wilcox, M. del Mar Zamora Dominguez and J. C. Minx, Environ. Res. Lett., 2018, 13, 063002 CrossRef
.
- M. Fajardy and N. Mac Dowell, Energy Environ. Sci., 2017, 10, 1389–1426 RSC
.
- G. Realmonte, L. Drouet, A. Gambhir, J. Glynn, A. Hawkes, A. C. Köberle and M. Tavoni, Nat. Commun., 2019, 10, 3277 CrossRef CAS PubMed
.
- N. Casas, J. Schell, R. Pini and M. Mazzotti, Adsorption, 2012, 18, 143–161 CrossRef CAS
.
- C. Gebald, J. A. Wurzbacher, A. Borgschulte, T. Zimmermann and A. Steinfeld, Environ. Sci. Technol., 2014, 48, 2497–2504 CrossRef CAS PubMed
.
- R. Veneman, N. Frigka, W. Zhao, Z. Li, S. Kersten and W. Brilman, Int. J. Greenh. Gas Control, 2015, 41, 268–275 CrossRef CAS
.
- N. R. Stuckert and R. T. Yang, Environ. Sci. Technol., 2011, 45, 10257–10264 CrossRef CAS PubMed
.
- Y. Kuwahara, D. Y. Kang, J. R. Copeland, P. Bollini, C. Sievers, T. Kamegawa, H. Yamashita and C. W. Jones, Chem. – Eur. J., 2012, 18, 16649–16664 CrossRef CAS PubMed
.
- R. Serna-Guerrero, Y. Belmabkhout and A. Sayari, Chem. Eng. J., 2010, 161, 173–181 CrossRef CAS
.
- M. J. Bos, T. Kreuger, S. R. A. Kersten and D. W. F. Brilman, Chem. Eng. J., 2019, 377, 120374 CrossRef CAS
.
- V. Stampi-Bombelli, M. van der Spek and M. Mazzotti, Adsorption, 2020, 26, 1183–1197 CrossRef CAS
.
- J. Toth, Acta Chim. Acad. Sci. Hungaricae, 1971, 69, 311–317 CAS
.
- M. Hefti and M. Mazzotti, Adsorption, 2014, 20, 359–371 CrossRef CAS
.
- M. Hefti and M. Mazzotti, Ind. Eng. Chem. Res., 2018, 57, 15542–15555 CAS
.
- D. Marx, L. Joss, M. Hefti, R. Pini and M. Mazzotti, Energy Procedia, 2013, 37, 107–114 CrossRef CAS
.
- M. Hefti, L. Joss, D. Marx and M. Mazzotti, Ind. Eng. Chem. Res., 2015, 54, 12165–12176 CrossRef CAS
.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CAS
.
- J. A. Wurzbacher, C. Gebald, S. Brunner and A. Steinfeld, Chem. Eng. J., 2016, 283, 1329–1338 CrossRef CAS
.
- R. B. Anderson, J. Am. Chem. Soc., 1946, 68, 686–691 CrossRef CAS
.
- R. B. Anderson and W. Keith Hall, J. Am. Chem. Soc., 1948, 70, 1727–1734 CrossRef CAS PubMed
.
- Lanxess, 2021.
- P. J. Linstrom and W. G. Mallard, J. Chem. Eng. Data, 2001, 46, 1059–1063 CrossRef CAS
.
- S. A. Didas, M. A. Sakwa-Novak, G. S. Foo, C. Sievers and C. W. Jones, J. Phys. Chem. Lett., 2014, 5, 4194–4200 CrossRef CAS PubMed
.
- Y. Kuwahara, D. Y. Kang, J. R. Copeland, N. A. Brunelli, S. A. Didas, P. Bollini, C. Sievers, T. Kamegawa, H. Yamashita and C. W. Jones, J. Am. Chem. Soc., 2012, 134, 10757–10760 CrossRef CAS PubMed
.
- J. Yu and S. S. C. Chuang, Energy Fuels, 2016, 30, 7579–7587 CrossRef CAS
.
- W. Jung and K. S. Lee, J. Nat. Gas Sci. Eng., 2020, 84, 103489 CrossRef CAS
.
- U. Tumuluri, M. Isenberg, C. S. Tan and S. S. C. Chuang, Langmuir, 2014, 30, 7405–7413 CrossRef CAS PubMed
.
- W. C. Wilfong, C. S. Srikanth and S. S. C. Chuang, ACS Appl. Mater. Interfaces, 2014, 6, 13617–13626 CrossRef CAS PubMed
.
- C. S. Srikanth and S. S. C. Chuang, J. Phys. Chem. C, 2013, 117, 9196–9205 CrossRef CAS
.
- X. Wang, V. Schwartz, J. C. Clark, X. Ma, S. H. Overbury, X. Xu and C. Song, J. Phys. Chem. C, 2009, 113, 7260–7268 CrossRef CAS
.
- A. Danon, P. C. Stair and E. Weitz, J. Phys. Chem. C, 2011, 115, 11540–11549 CrossRef CAS
.
- Z. Bacsik, N. Ahlsten, A. Ziadi, G. Zhao, A. E. Garcia-Bennett, B. Martín-Matute and N. Hedin, Langmuir, 2011, 27, 11118–11128 CrossRef CAS PubMed
.
-
P. Jäger, C. N. Rentzea and H. Kieczka, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000, pp. 553–560 Search PubMed
.
- W. Buijs and S. De Flart, Ind. Eng. Chem. Res., 2017, 56, 12297–12304 CrossRef CAS PubMed
.
- W. R. Alesi and J. R. Kitchin, Ind. Eng. Chem. Res., 2012, 51, 6907–6915 CrossRef CAS
.
- K. Li, J. D. Kress and D. S. Mebane, J. Phys. Chem. C, 2016, 120, 23683–23691 CrossRef CAS
.
- L. A. Darunte, A. D. Oetomo, K. S. Walton, D. S. Sholl and C. W. Jones, ACS Sustainable Chem. Eng., 2016, 4, 5761–5768 CrossRef CAS
.
- W. Chaikittisilp, J. D. Lunn, D. F. Shantz and C. W. Jones, Chem. – Eur. J., 2011, 17, 10556–10561 CrossRef CAS PubMed
.
- M. A. Sakwa-Novak and C. W. Jones, ACS Appl. Mater. Interfaces, 2014, 6, 9245–9255 CrossRef CAS
.
- S. A. Didas, A. R. Kulkarni, D. S. Sholl and C. W. Jones, ChemSusChem, 2012, 5, 2058–2064 CrossRef CAS
.
- S. A. Didas, R. Zhu, N. A. Brunelli, D. S. Sholl and C. W. Jones, J. Phys. Chem. C, 2014, 118, 12302–12311 CrossRef CAS
.
- A. Kolmogorov, Izv. Akad. Nauk USSR Ser. Math., 1937, 1, 355–359 Search PubMed
.
- C. W. Price, Acta Metall, Mater., 1990, 38, 727–738 CAS
.
- M. Avrami, J. Chem. Phys., 1939, 7, 1103–1112 CrossRef CAS
.
- M. Avrami, J. Chem. Phys., 1940, 8, 212–224 CrossRef CAS
.
- M. Avrami, J. Chem. Phys., 1941, 9, 177–184 CrossRef CAS
.
- J. Maxwell, London, Edinburgh, Dublin Philos. Mag. J. Sci. 4th Ser., 1860, 19, 19–32 CrossRef
.
- J. Maxwell, London, Edinburgh, Dublin Philos. Mag. J. Sci. 4th Ser., 1860, 20, 21–37 CrossRef
.
- J. W. Park, C. U. Kim and W. Isard, Phys. A, 2012, 391, 4883–4890 CrossRef
.
- E. Sonnleitner, G. Schöny and H. Hofbauer, Biomass Convers, Biorefinery, 2018, 8, 379–395 CAS
.
- J. P. Young, V. Martis, S. Garcia and M. van der Spek, 11th Trondheim Conf. CO2 Capture, Transp. Storage., in press.
- Surface Measurement Systems, 2021.
- P. Bollini, S. Choi, J. H. Drese and C. W. Jones, Energy Fuels, 2011, 25, 2416–2425 CrossRef CAS
.
- G. Calleja, R. Sanz, A. Arencibia and E. S. Sanz-Pérez, Top. Catal., 2011, 54, 135–145 CrossRef CAS
.
-
US 2020/0001224 A9, 2020 Search PubMed
.
- L. Joss, M. Gazzani, M. Hefti, D. Marx and M. Mazzotti, Ind. Eng. Chem. Res., 2015, 54, 3027–3038 CrossRef CAS
.
- E. Glueckauf, Trans. Faraday Soc., 1955, 51, 1540–1551 RSC
.
- S. Ergun, Chem. Eng. Prog., 1952, 48, 89–94 CAS
.
- D. Danaci, P. A. Webley and C. Petit, Front. Chem. Eng., 2021, 2, 1–11 Search PubMed
.
- Process Systems Enterprise, gPROMS, http://www.psenterprise.com/products/gproms, 1997-2021.
- C. C. Pantelides, SIAM J. Sci. Stat. Comput., 1988, 9, 213–231 CrossRef
.
- L. Zhou, J. Fan, G. Cui, X. Shang, Q. Tang, J. Wang and M. Fan, Green Chem., 2014, 16, 4009–4016 RSC
.
- S. Deutz and A. Bardow, Nat. Energy, 2021, 6, 203–213 CrossRef CAS
.
- A. Sinha, L. A. Darunte, C. W. Jones, M. J. Realff and Y. Kawajiri, Ind. Eng. Chem. Res., 2017, 56, 750–764 CrossRef CAS
.
- A. Sinha, L. A. Darunte, C. W. Jones, M. J. Realff and Y. Kawajiri, Ind. Eng. Chem. Res., 2020, 59, 503–505 CrossRef CAS
.
- N. McQueen, M. J. Desmond, R. H. Socolow, P. Psarras and J. Wilcox, Front. Clim, 2021, 2, 38 Search PubMed
.
-
E. Ping, M. Sakwa-Novak and P. Eisenberger, Int. Conf. Negat. CO2 Emiss. May 22–24, 2018, Göteborg, Sweden, 2018, pp. 1–9 Search PubMed
.
- D. W. Keith, G. Holmes, D. Angelo and K. Heidel, Joule, 2018, 2, 1573–1594 CrossRef CAS
.
-
US 2017/0326494 A1, 2017 Search PubMed
.
- L. A. Darunte, T. Sen, C. Bhawanani, K. S. Walton, D. S. Sholl, M. J. Realff and C. W. Jones, Ind. Eng. Chem. Res., 2019, 58, 366–377 CrossRef CAS
.
- R. Hanna, A. Abdulla, Y. Xu and D. G. Victor, Nat. Commun., 2021, 12, 368 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ee01272j |
| ‡ Climeworks’ patent describing the kind of material that may be used in their process and simultaneously supplying an accurate description of Lewatit® VP OC 1065: “polystyrene matrix material modified with amine groups, specifically primary amine groups”.57 |
| § The equation more commonly used to describe this parameter13 has been re-arranged in this work, which also changes the meaning of b0. The original equation causes the relationship between ΔH0 and b to be dependent on the arbitrarily assigned T0. For this reason, the equation has been changed to remove this dependence, and the motivation becomes apparent in Section 2.3.1, where ΔH0 is a parameter that varies due to co-adsorption. |
| ¶ It is experimentally confirmed that the amount of N2 adsorbed is very low under ambient conditions (∼0.01 mmol g−1). Three isotherms are showing this are found in the ESI,† Fig. S5. Henceforth, we assume no N2 is adsorbed. |
| || The authors believe that the heuristic optimisation represents a realistic DAC case when a co-adsorption description is included. The only parameter which is still uncertain is the heat transfer coefficient, and experimental data from a scaled-up unit is required to estimate this accurately. In any case, the sensitivity analysis in Fig. S28 of the ESI,† shows that the heat transfer coefficient does not have a large effect on the work equivalent or purity of the process. It does have a significant impact on productivity, however. |
| This journal is © The Royal Society of Chemistry 2021 |