Local spin-state tuning of cobalt–iron selenide nanoframes for the boosted oxygen evolution†
Jun-Ye
Zhang‡
a,
Ya
Yan‡
 b,
Bingbao
Mei‡
c,
Ruijuan
Qi
d,
Ting
He
a,
Zhitong
Wang
a,
Wensheng
Fang
a,
Shahid
Zaman
b,
Bingbao
Mei‡
c,
Ruijuan
Qi
d,
Ting
He
a,
Zhitong
Wang
a,
Wensheng
Fang
a,
Shahid
Zaman
 a,
Yaqiong
Su
*e,
Shujiang
Ding
a,
Yaqiong
Su
*e,
Shujiang
Ding
 *e and
Bao Yu
Xia
*e and
Bao Yu
Xia
 *a
*a
aKey Laboratory of Material Chemistry for Energy Conversion and Storage (Ministry of Education), Key Laboratory of Material Chemistry and Service Failure, School of Chemistry and Chemical Engineering, Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan 430074, P. R. China. E-mail: byxia@hust.edu.cn
bSchool of Materials Science and Engineering, University of Shanghai for Science and Technology, 516 Jungong Road, Shanghai 200093, P. R. China
cShanghai Synchrotron Radiation Facility, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201204, P. R. China
dKey Laboratory of Polar Materials and Devices (MOE), Department of Electronics, East China Normal University, 500 Dongchuan Road, Shanghai 200241, P. R. China
eSchool of Chemistry, Xi’an Key Laboratory of Sustainable Energy Materials Chemistry, MOE Key Laboratory for Nonequilibrium Synthesis and Modulation of Condensed Matter, State Key Laboratory of Electrical Insulation and Power Equipment, Xi’an Jiaotong University, Xi’an 710049, P. R. China. E-mail: dingsj@mail.xjtu.edu.cn; yqsu1989@xjtu.edu.cn
First published on 23rd December 2020
Abstract
Hydrogen economy by water splitting is the indispensable cornerstone for sustainable energy yet it is impeded by sluggish anodic water oxidation. Hence, the rational design of highly efficient electrocatalysts for oxygen evolution is the key to unlocking its wider use. Herein, cobalt–iron selenide nanoframes are reported for the efficient water oxidation, which need only 270 mV overpotential to give a 10 mA cm−2 current density and outperforms most cobalt-based catalysts, and even the benchmarked commercial ruthenium oxides (RuO2). More profoundly, iron doping regulates the local spin state of cobalt species, which further accelerates charge transfer and formation of oxygenated intermediates, and consequently contributes to the enhanced oxygen evolution. This work demonstrates a highly efficient oxygen evolution electrocatalyst and may pioneer a promising approach which involves tuning the local electronic structure to achieve the improved electrocatalysis activities in energy conversion technologies.
Broader contextElectrochemical water electrolysis is regarded as a promising approach for sustainable hydrogen production. However, its scalable application is greatly limited by the sluggish kinetics of the anodic oxygen evolution reaction (OER). Consequently, developing highly active electrocatalysts is significant yet challenging for boosting the inferior OER activity. Recently, transition metal compounds with high-valence cobalt active sites have demonstrated their potential in promoting OER. Subsequently, the formation strategies and modulation mechanisms of high-valence cobalt active sites have attracted much attention in developing efficient OER electrocatalysts. Herein, a simple selenization method to prepare hollow cobalt selenides and an effective strategy of utilizing higher spin states of Co species to improve the OER activity of the low-cost CoSe2 are reported. Comprehensive experiment and calculation proofs confirm that the moderate Fe doping can optimize the spin state of adjacent Co atoms, facilitating the formation of high-valence Co sites. This study proposes a novel perspective based on the doping induced spin state tuning to explain the formation mechanism of highly active OER sites and pioneers an interesting method for the modulation of advanced spin-related electrocatalysis. |
With the rapid development of modern society, the present fuel reserves, including coal, petroleum, natural gas, and so on, will be inadequate to meet increasing demand in the future.1 Meanwhile, the use of traditional fossil fuels and carbon-based energy systems has led to irreversible environmental contamination by the effects of greenhouse gases and smog.2 Hence, communities have consciously focused on exploiting and establishing a sustainable energy system.3 Until now, renewable solar, wind, and tidal energy technologies with excellent energy harvesting efficiencies have been successfully invented.4,5 Nevertheless, there are still intractable issues for the stable and efficient conversion and/or storage of these intermittent energy sources.6 Hydrogen as a clean energy carrier is a promising energy source for future development of clean energy worldwide, which can release abundant energy without contaminating the environment.7–9 Water splitting is one of the attractive approaches to scale up the production of hydrogen with high purity, and its large-scale implementation is imperative in sustainable communities.10,11 However, the sluggish anodic oxygen evolution reaction (OER) severely limits the efficiency of hydrogen production at the cathode, which results in a sharp increase in overall cost and energy consumption in water electrolysis.12–15
Noble metal based materials, for example, Ru/Ir compounds, have shown superior capabilities for catalyzing the OER. However, their high price and limited stability are challenging for their use in large-scale applications.16,17 Recently, transition metal based compounds, such as oxides, sulfides and selenides, have demonstrated sufficient activities as well as stability.18–23 Compared to oxides and sulfides, selenides show a better conductivity or metallic property, where a larger atomic radius and smaller ionization energy of selenium atoms contribute to a faster charge transfer. Hence, it would render the metal atom surface more electrophilic and lead to an increased affinity for (oxy)hydroxyl species, and induce the improved OER kinetics.24 Moreover, metal cobalt species with a high valence state (such as tri-valency or even higher) play a key role in OER catalysis due to its optimized affinity for the surface oxygen species.25,26 Great progress has been achieved in engineering the nanostructures of Co-based electrocatalysts for improving their activities in OER catalysis.27,28 However, a rational design for a Co-based electrocatalyst with a high valence state is challenging but it is crucial for the enhanced catalytic capability.29 Cobalt is a 3d transition metal element with seven 3d electrons, its low spin (LS) state and high spin (HS) state correspond to one and three unpaired 3d electrons, respectively.30 Thus, Co can easily form a higher valence state (+3 or even higher) by losing unpaired electrons. Consequently, the structural distortion and lattice orientation control can optimize the electronic structure to achieve the enhanced activity.30–34 Furthermore, Prussian blue analogs (PBAs), comprising transition metal ions and cyanide ligands, have been shown to have excellent potential for transforming metal oxides/sulfides for energy storage and conversion.35,36 Thus, from the PBA platform, designing Co-based selenides with an optimized local electron spin state would be interesting in developing an efficient electrocatalyst for the oxygen involved electrochemical reactions.
Herein, the modified local spin state of Co species by iron (Fe) doping in cobalt selenide nanoframes to achieve an enhanced OER activity is reported. The Fe0.4Co0.6Se2 nanoframes are prepared by a simple solid-state selenization treatment of Co–Fe PBA precursors. With an appropriate Fe doping, the local spin state of Co atoms, corresponding to the t52ge2g electron configuration, is optimized to acquire a higher valence state, which is beneficial for promoting charge transfer and formation of oxygenated species, thereby contributing to the enhanced water oxidation. Specifically, Fe0.4Co0.6Se2 nanoframes only require 270 mV overpotential to give a 10 mA cm−2 current density of oxygen generation, outperforming commercial RuO2 as well as most of the recently reported active OER materials. Interestingly, the evolved surface oxygenated species are found on the Fe0.4Co0.6Se2 nanoframes after the stability test, and these might be the active sites of the oxygen generation. This work provides a cobalt selenide electrocatalyst for water oxidation and clarifies iron doping from the perspective of spin state electron configuration to achieve the improved activity, which has great potential for future design and preparation of highly efficient electrocatalysts.
The Fe0.4Co0.6Se2 nanoframes were obtained by a chemical vapor deposition (CVD) selenization method. The scanning electron microscopy (SEM) image in Fig. 1a shows that the as-prepared Co–Fe PBA exhibits a well-defined nanocubic morphology with a smooth surface and an average size of ∼200 nm. Fe doping is favorable for decreasing the nanocube size, as the Co–Co PBA demonstrates a similar cubic morphology with a size up to ∼1.2 μm (Fig. S1, ESI†). After the selenization process, the solid Co–Fe and Co–Co PBA precursors turn into hollow structures, as shown in Fig. 1b. This formation process of the nanoframe structure may involve different diffusion rates between metal–cyanide breaking and metal–selenium forming (Fig. S1, ESI†).37 The nanoframe surface is conspicuously rough because of its partial collapse (Fig. 1c), and the pores and channels which emerge effectively increase the specific surface area, are beneficial for exposing more active sites and accelerating mass transfer (Fig. S2, ESI†). The hollow structure was further verified by transmission electron microscopy (TEM) observations. As shown in Fig. 1d, the hollow nanoframes retain a rough surface with a partially ruptured wall and a pierced nanocube morphology (Fig. 1e). The uniform lattice fringe in the high-resolution (HR)TEM images is ascribed to the (111) plane of the cobalt selenides with a spacing of 0.258 nm (Fig. 1f). The scanning transmission electron microscopy (STEM) energy dispersive X-ray spectroscopy (EDS) mapping images prove that there is a homogeneous distribution of all the elements including Fe, Co and Se in the Fe0.4Co0.6Se2 nanoframes (Fig. 1g). In addition, the EDS analysis shows that the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio of Fe0.4Co0.6Se2 is 37.8%
Co ratio of Fe0.4Co0.6Se2 is 37.8%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62.2% (Fig. S3, ESI†), which is close to the result obtained from the inductively coupled plasma optical emission spectrometry (ICP-OES) (Co
62.2% (Fig. S3, ESI†), which is close to the result obtained from the inductively coupled plasma optical emission spectrometry (ICP-OES) (Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se = 19.2
Se = 19.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.4
13.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67.4).
67.4).
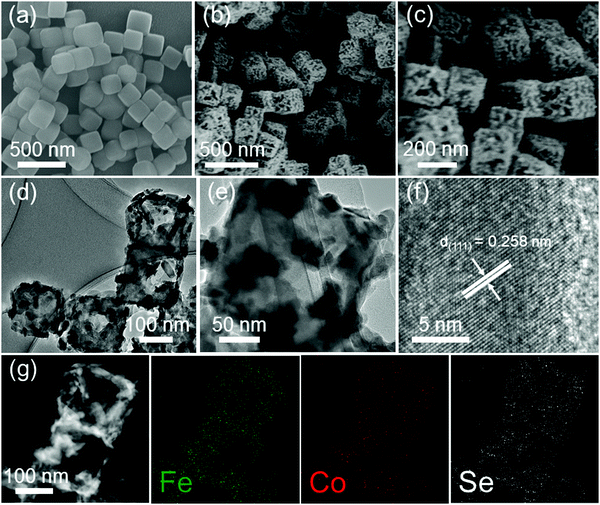 | ||
| Fig. 1 (a) SEM image of a solid nanocube precursor, (b and c) SEM, (d and e) TEM, (f) HRTEM images, and (g) TEM-EDS mapping analysis of the Fe0.4Co0.6Se2 nanoframes. | ||
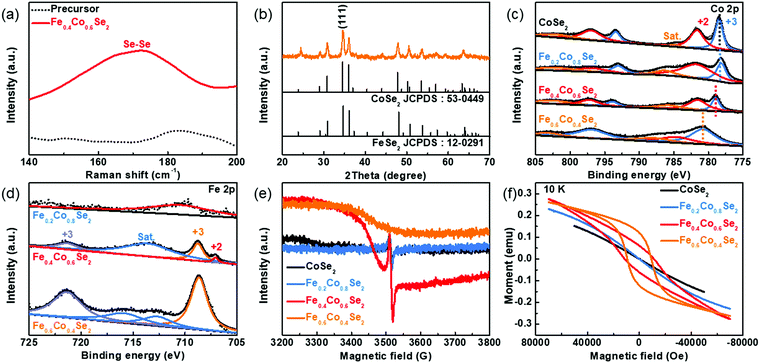
The formation of cobalt selenides was further verified by Raman spectra shown in Fig. 2a. Compared with the initial PBA precursors, a peak which emerged at 182 cm−1 corresponds to the Se–Se bond of Fe0.4Co0.6Se2. Moreover, the crystal information of Fe0.4Co0.6Se2 nanoframes was determined by powder X-ray diffraction (XRD). XRD pattern of the precursor is in accordance with that of the K2CoFe(CN)6 reference (JCPDS: 75-0038), which illustrates the pure Co–Fe PBA nature of the precursor (Fig. S4, ESI†). Similarly, all the characteristic diffraction peaks of Fe0.4Co0.6Se2 match well with the standard CoSe2 (JCPDS 53-0449). Notably, the peaks at 34.5°, 35.9° and 47.7° are corresponding to (111), (120) and (211) crystal planes, respectively (Fig. 2b). Considering the similar pattern of FeSe2 (JCPDS 12-0291) to CoSe2 and the homogeneous composition distribution in the Fe0.4Co0.6Se2 nanoframes, it is logical to conclude that there is no evident difference between Fe0.4Co0.6Se2 and CoSe2 in the XRD patterns (Fig. S5, ESI†). Furthermore, the valence states of cobalt selenides with various amounts of Fe doping are further revealed by the X-ray photoelectron spectra (XPS) results (Fig. S6a, ESI†). In the Se 3d spectra, the peak signals of Se 3d3/2 and Se 3d5/2 are marked at 54.0 and 54.9 eV, respectively, (Fig. S6b, ESI†). Additionally, the surface oxidation phenomenon is also confirmed by the signal of Se–O species. The Co 2p spectra are collected to determine the electronic configuration of Co species. The dominant peaks at ∼778.4 eV and ∼793.4 eV for the CoSe2 sample are ascribed to Co(III) 2p3/2 and Co(III) 2p1/2, respectively, where the binding energy of the Co 2p3/2 peak is positively shifted with the increased content of Fe dopants (Fig. 2c).38 Specifically, the Fe0.2Co0.8Se2 shows a similar binding energy of Co 2p3/2 as CoSe2 does. After increasing the Fe content to 40%, the Co 2p3/2 peak shows a positive shift of 0.50 eV to a higher binding energy than that of CoSe2, and a more positive peak shift is further observed in Fe0.6Co0.4Se2, suggesting that the Co species are partly able to form a higher valence state by introducing appropriate Fe dopants. In addition, with the gradual increase in the amount of Fe doping, the peak intensity of Fe 2p XPS spectra also shows a tendency to increase (Fig. 2d). The peak appearance of Fe0.4Co0.6Se2 is observed at 708.6 and 721.3 eV, which is attributed to Fe(III) 2p3/2 and Fe(III) 2p1/2, respectively.39 Considering that the precursor contains Co(II) and Fe(II) only, the solid state selenization is beneficial for the formation of higher valence state Co and Fe (Fig. S7, ESI†). The valence state of 3d metal Co is closely related to its spin state. In order to investigate the unpaired electron signal of the Co atomic orbital, electron paramagnetic resonance (EPR) is used. Compared to the pristine CoSe2 sample, a broad peak related to Fe in the Fe0.4Co0.6Se2, as well as Fe0.6Co0.4Se2, appears at 3500 G in the magnetic field.40 Moreover, a small signal peak is detected at ∼3520 G in the magnetic field (Fig. 2e), which is related to the unpaired electron in the Co 3d orbital. After the Fe doping, the peak signal becomes more evident, confirming the increase in the unpaired electrons with the increase in the amount of Fe doping. However, this signal peak intensity dramatically decreases in Fe0.6Co0.4Se2, indicating that the excessive Fe doping reduces the number of unpaired electrons due to Co atom decrease. In order to further verify the probable variation in the spin states, magnetic behavior experiments are carried out (Fig. 2f). With the gradual increase in the amount of Fe doping, the evident magnetic hysteresis phenomenon revealed by the magnetic field dependence in the Fe0.4Co0.6Se2 and Fe0.6Co0.4Se2 samples suggests that the local spin states of pristine CoSe2 is effectively impacted by Fe doping.41 Due to the LS state of the Co atoms, the 3d electronic configuration of CoSe2 is t62ge1g, with one unpaired electron in the eg orbital.42 Therefore, the partial Fe dopants can enhance the HS states of local Co atoms, resulting in a t52ge2g 3d electronic configuration with three unpaired electrons.43 Consequently, the local spin state modification leads to a more eg orbital electron filling as well as a larger degree of overlap between the eg orbital and OH− adsorbate, which facilitates the formation of a σ bond between the high-valence Co and OH− adsorbate.44
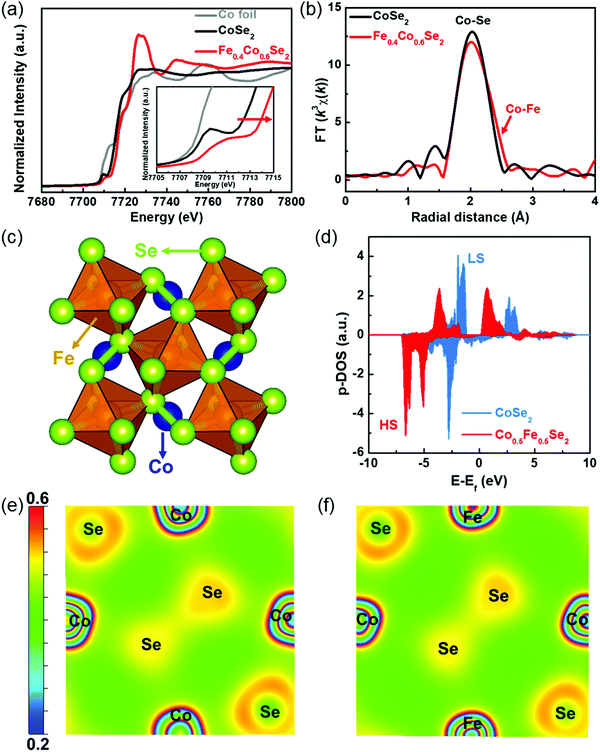
The X-ray absorption fine structure (XAFS) spectra of Fe0.4Co0.6Se2 are collected to accurately elucidate the spin/valence state variation and coordination environment of the Co species. The X-ray absorption near-edge structure (XANES) spectra of Co foil, CoSe2 and Fe0.4Co0.6Se2 samples are compared in Fig. 3a. It is noted that an apparent energy shift towards higher energy (Fig. 3a, inset) corresponds to a higher valence state Co species, and this agrees well with the results of the previously mentioned XPS analysis. It should be emphasized again that Fe effectively enhances the Co valence state in Fe0.4Co0.6Se2. The pre-edge peak at ∼7910 eV is involved in the electric quadrupole transition from the 1s orbital to the 3d orbital, where the peak intensity reflects the unoccupied orbital number. Compared with CoSe2, Fe0.4Co0.6Se2 has a lower pre-edge peak intensity, suggesting a weaker orbital affinity of the 3d orbital for the transition electron, as an empty orbital attracts a transition electron easier than semi-full/full orbital can. Thus, it can be concluded that Co atoms in the Fe0.4Co0.6Se2 have more semi-full/full orbitals and unpaired electrons, which is in accordance with the EPR results of the HS state of Co.45 The Fourier transformations of the Co extended X-ray absorption fine structure (EXAFS) spectra demonstrate the Co–Se bond (∼2.4 Å) in both CoSe2 and Fe0.4Co0.6Se2 (Fig. 3b and Fig. S8, ESI†). The slight peak shift in the Co–Se bond is ascribed to the existence of the Co–Fe bond (∼2.6 Å). The ratio of Co–Se and Co–Fe coordination numbers is 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.4 (Table S1, ESI†). It is possible that the Fe doping induces the formation of Se vacancies, and in turn, this accounts for the observed Co–Fe bonds, which favors charge transfer from active Co centers to coordinate Se atoms for promoting the electrochemical reactions.46 Moreover, density functional theory (DFT) calculations are performed to explain the spin state modification. As shown in Fig. 3c, the Fe doping amount is set as 50% (Co0.5Fe0.5Se2) to construct rational models. The Fe-doped CoSe2 exhibits a more delocalized density of states (DOS) distribution as well as a lower energy level of the d-band center of Co atoms (−3.52 vs. −2.14 eV) with respect to the pristine CoSe2 (Fig. 3d). It is worth noting that the LS state of pristine CoSe2 is 1.52 eV more stable than its HS state, whereas the HS state is 0.28 eV more stable than the LS state for Co0.5Fe0.5Se2. The downshift of the d-band center of Co in the Fe-doped CoSe2 with respect to CoSe2 is helpful for weakening the binding strength of the *OH, *O, and *OOH intermediates on the active centers, and consequently improving the OER activity. Fig. 3e and f show a slight change in charge density distribution of the Fe-doped CoSe2 compared to the pristine CoSe2, demonstrating that Fe doping into the perfect CoSe2 lattice does not influence the valence states of Co atoms very much. The experimentally observed higher valence states of Co may be ascribed to the formation of Se vacancies and local HS state, as shown by the results of XAFS analysis. The Fe species doping can modulate the spin states of Co atoms in the CoSe2, and subsequently improve the catalytic activity of the Co centers. Moreover, theoretical analysis further suggest that Fe doping can evidently enhance the delocalization of electrons, which is convincingly proved as a consequence of the spin state modification of Co atoms.
3.4 (Table S1, ESI†). It is possible that the Fe doping induces the formation of Se vacancies, and in turn, this accounts for the observed Co–Fe bonds, which favors charge transfer from active Co centers to coordinate Se atoms for promoting the electrochemical reactions.46 Moreover, density functional theory (DFT) calculations are performed to explain the spin state modification. As shown in Fig. 3c, the Fe doping amount is set as 50% (Co0.5Fe0.5Se2) to construct rational models. The Fe-doped CoSe2 exhibits a more delocalized density of states (DOS) distribution as well as a lower energy level of the d-band center of Co atoms (−3.52 vs. −2.14 eV) with respect to the pristine CoSe2 (Fig. 3d). It is worth noting that the LS state of pristine CoSe2 is 1.52 eV more stable than its HS state, whereas the HS state is 0.28 eV more stable than the LS state for Co0.5Fe0.5Se2. The downshift of the d-band center of Co in the Fe-doped CoSe2 with respect to CoSe2 is helpful for weakening the binding strength of the *OH, *O, and *OOH intermediates on the active centers, and consequently improving the OER activity. Fig. 3e and f show a slight change in charge density distribution of the Fe-doped CoSe2 compared to the pristine CoSe2, demonstrating that Fe doping into the perfect CoSe2 lattice does not influence the valence states of Co atoms very much. The experimentally observed higher valence states of Co may be ascribed to the formation of Se vacancies and local HS state, as shown by the results of XAFS analysis. The Fe species doping can modulate the spin states of Co atoms in the CoSe2, and subsequently improve the catalytic activity of the Co centers. Moreover, theoretical analysis further suggest that Fe doping can evidently enhance the delocalization of electrons, which is convincingly proved as a consequence of the spin state modification of Co atoms.
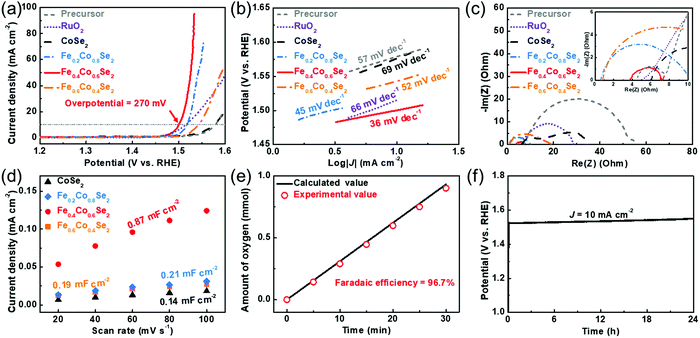
Previous analysis confirms that the cobalt spin state is induced from the LS to the HS by the Fe dopants. This modification could improve the formation and electrophilic properties of adsorbed oxygenated species on the activated Co sites, which contributes to the enhanced oxygen evolution.47–49 Electrochemical evaluation is performed in a three-electrode system (glassy carbon working electrode, graphite counter electrode, and Hg/HgO reference electrode) to illustrate the OER activity of the different active materials. Linear sweep voltammetry (LSV) curves of all catalysts are shown in Fig. 4a. Remarkably, Fe0.4Co0.6Se2 outperforms commercial RuO2, pristine CoSe2, Fe0.2Co0.8Se2, Fe0.6Co0.4Se2 and Co–Fe PBA precursors in the OER measurements. The Fe0.4Co0.6Se2 nanoframes need an overpotential of only 270 mV to give an anodic current density of 10 mA cm−2, outperforming commercial RuO2 (285 mV), precursor (350 mV), CoSe2 (346 mV), Fe0.2Co0.8Se2 (287 mV), Fe0.6Co0.4Se2 (313 mV) and other reported cobalt-based materials (Table S2, ESI†). Apart from the glassy carbon electrode, Fe0.6Co0.4Se2 loaded on the other electrodes still exhibits an exceptional OER activity (Fig. S9, ESI†). To compare the overpotential discrepancy, a volcano plot of the overpotentials is plotted to reveal the impact of different amounts of Fe doping on the activity. As shown in the Fig. S10 (ESI†), the overpotential decreased with the Fe doping, convincingly confirming the increase of OER activity with the increase in the amount of Fe doping. However, this overpotential is dramatically increased in the Fe0.6Co0.4Se2, implicating that excessive Fe doping will lower the OER activity due to the decrease of Co sites. Similarly, the corresponding Tafel slopes also demonstrate the improved reaction kinetics of the Fe-doped CoSe2 nanoframes (Fig. 4b). In particular, the Tafel slope of Fe0.4Co0.6Se2 is 36 mV dec−1, which is much smaller than that of RuO2 (66 mV dec−1), CoSe2 (69 mV dec−1), Fe0.2Co0.8Se2 (45 mV dec−1), Fe0.6Co0.4Se2 (52 mV dec−1) and the Co–Fe PBA precursor (57 mV dec−1). Such a small Tafel slope implies that Fe0.4Co0.6Se2 has a faster reaction kinetics for accelerating the formation of oxygenated species (O*/OOH*) in the OER.13 An enhanced charge transfer ability is further verified from the electrochemical impedance spectra (EIS) (Fig. 4c). As anticipated, Fe0.4Co0.6Se2 has a much lower charge transfer resistance (∼3 Ω) than Fe0.2Co0.8Se2 (∼10 Ω), Fe0.6Co0.4Se2 (∼19 Ω), CoSe2 (∼27 Ω), RuO2 (∼23 Ω) and the Co–Fe precursor (∼51 Ω). Such a low resistance value implies the excellent interfacial interaction, which endows a fast charge transfer between the catalyst and reactants. The LSV curves with different scan rates prove that Fe0.4Co0.6Se2 can support sufficiently fast mass transfer, which is beneficial from the hollow nanoframe morphology (Fig. S11, ESI†). To determine the intrinsic activity, double layer capacitances (Cdl) of different cobalt selenides are obtained by cyclic voltammetry (CV) at different scan rates (Fig. S12, ESI†). The Cdl value of 0.87 mF cm−2 for Fe0.4Co0.6Se2 is much higher than those of Fe0.2Co0.8Se2 (0.21 mF cm−2) and Fe0.6Co0.4Se2 (0.19 mF cm−2), and notably is six times higher than that of CoSe2 (0.14 mF cm−2), revealing that the appropriate Fe dopant is effective for increasing or exposing more active sites (Fig. 4d). Moreover, the capacitance-normalized intrinsic activity further proves Fe doping could significantly improve the intrinsic activity of CoSe2 (Fig. S13, ESI†). The as-prepared Fe0.4Co0.6Se2 nanoframes consistently yield a higher specific OER activity at a 300 mV overpotential than the others (Fig. S14, ESI†). The Fe0.4Co0.6Se2 nanoframes achieve a high turnover frequency (TOF) of 1.23 s−1, which outperforms CoSe2 (0.003 s−1), Fe0.2Co0.8Se2 (0.36 s−1) and Fe0.6Co0.4Se2 (0.015 s−1) (Fig. S15, ESI†). Hence, it can be speculated that moderate Fe doping effectively tunes local electron configuration (spin state) and changes the cobalt valence state which boosts the catalytic activity. In addition, a Faradaic efficiency evaluation demonstrates a closer actual amount of oxygen production to the theoretical value with a superior Faradaic efficiency of 96.7% (Fig. 4e). Furthermore, no evident potential fluctuation during a 24 h test suggests that Fe0.4Co0.6Se2 nanoframes are stable for the alkaline OER (Fig. 4f). The practical cell performance of Fe0.4Co0.6Se2 is also compared with that of RuO2. The LSV curves of the cells reveal that the cathodic Pt/C and anodic Fe0.4Co0.6Se2 couple give a better performance than the Pt/C and RuO2 couple (Fig. S16, ESI†).
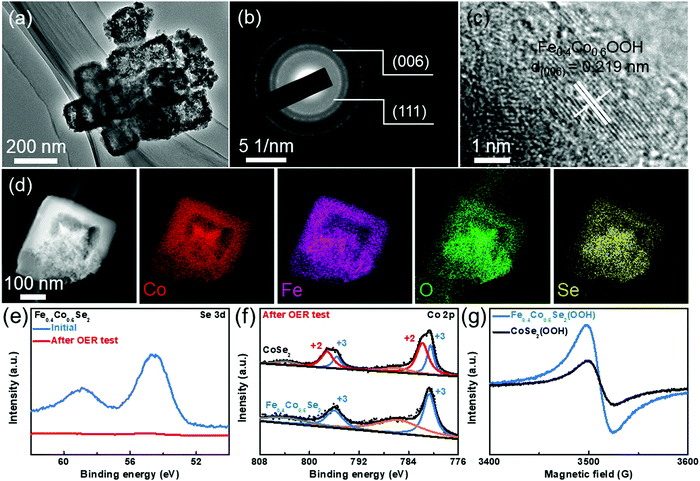
Recent work has proposed that the catalyst surface could be restructured and converted in situ to (oxy)hydroxides during the alkaline electrolysis process.50–52 Based on this perspective, characterization after the OER test is also performed to investigate the surface oxygenated species. Firstly, the XRD pattern after the OER testing shows weaker diffraction peaks than the initial samples before OER tests, implying a possible partial transformation from selenides to (oxy)hydroxides during the OER process (Fig. S17, ESI†). TEM image in Fig. 5a shows the remaining hollow nanoframe structure, suggesting that the Fe0.4Co0.6Se2 catalysts have a robust structure. Moreover, two polycrystalline diffraction rings are observed in the selected area electron diffraction (SAED) pattern of the Fe0.4Co0.6Se2 catalysts after electrolysis (Fig. 5b), which can be indexed to the Co–Fe oxyhydroxide (006) facet and Fe0.4Co0.6Se2(111) facet. HRTEM images verify that the surface oxygenated species layers coexist with Fe0.4Co0.6Se2 after the OER test (Fig. S18, ESI†), which can be indexed as the (006) plane for the Fe0.4Co0.6OOH species and the (111) plane for Fe0.4Co0.6Se2 (Fig. 5c). The STEM-EDS images display uniform dispersions of Fe, Co, O, and Se in the Fe0.4Co0.6Se2 nanoframes (Fig. 5d). More interestingly, the oxygen and selenium elements are well overlapping in the sample, which indicates that the Fe0.4Co0.6Se2 nanoframes are robust in the OER process. Simultaneously, the Fe0.4Co0.6Se2 surface is developed and covered by a thin layer of in situ formed oxyhydroxide Fe0.4Co0.6Se2(OOH). Thus, the cobalt selenide catalysts after OER testing may consist of an active oxyhydroxide surface and an inner conductive selenide core.53 Moreover, the ratio of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co is 39.9%
Co is 39.9%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60.1% in the corresponding EDS spectrum (Fig. S19, ESI†), which is close to that of the initial Fe0.4Co0.6Se2 nanoframes. The weakened Se signal is incorporated in the EDS spectrum, verifying the presence of few remnant selenide species (Table S3, ESI†). Considering that the XPS is a surface-sensitive technique, the Se XPS spectra can show the formation of the surface (oxy)hydroxide layer in the Fe0.4Co0.6Se2(OOH) sample (Fig. 5e). Compared with the initial Fe0.4Co0.6Se2 sample, no conspicuous signal peak is shown after the OER testing. The surface contents of Se and O also show the formation of surface (oxy)hydroxides during the OER testing (Table S4, ESI†). Accompanying the ICP-OES results of the electrolyte, the surface selenides are corroded and dissolved in the electrolyte during the OER process.
60.1% in the corresponding EDS spectrum (Fig. S19, ESI†), which is close to that of the initial Fe0.4Co0.6Se2 nanoframes. The weakened Se signal is incorporated in the EDS spectrum, verifying the presence of few remnant selenide species (Table S3, ESI†). Considering that the XPS is a surface-sensitive technique, the Se XPS spectra can show the formation of the surface (oxy)hydroxide layer in the Fe0.4Co0.6Se2(OOH) sample (Fig. 5e). Compared with the initial Fe0.4Co0.6Se2 sample, no conspicuous signal peak is shown after the OER testing. The surface contents of Se and O also show the formation of surface (oxy)hydroxides during the OER testing (Table S4, ESI†). Accompanying the ICP-OES results of the electrolyte, the surface selenides are corroded and dissolved in the electrolyte during the OER process.
In addition, the Co 2p XPS spectra of Fe0.4Co0.6Se2(OOH) and CoSe2(OOH) are compared to determine the variation of Co valence state (Fig. 5f). After the spectra deconvolution, the Co(II) and Co(III) oxidation states can be discerned in the Co 2p spectrum of CoSe2(OOH). Notably, Co(III) is located at 780.5 eV for Co 2p3/2 and at 795.7 eV for Co 2p1/2. The Co(II) peaks at a low oxidation state including Co 2p3/2 and Co 2p1/2 are fitted around 781.8 and 797.1 eV, respectively.22,54 Nevertheless, only the Co(III) signal peaks are detected in the Fe0.4Co0.6Se2(OOH), indicating that the Co atoms are still prone to form a higher valence state. The Co species at a HS state are still capable of being activated by Fe dopants, even though the selenide catalyst surface is corroded to form the (oxy)hydroxide. Furthermore, the EPR measurements of Fe0.4Co0.6Se2(OOH) and CoSe2(OOH) show that the unpaired electron signal at ∼3520 G for Fe0.4Co0.6Se2(OOH) is evidently stronger than that of the undoped CoSe2(OOH) sample, even though the catalyst surface has already been dominated by the oxyhydroxide layer (Fig. 5g). Only Co(III) is discovered in the Fe0.4Co0.6Se2(OOH), giving the corresponding t42ge2g structure of the 3d electron configuration, which is more prone to having a higher valence state (≥3). In order to further differentiate the surface oxo species of the LS and HS states, Raman spectra analysis is performed before and after the OER testing. No Raman band related to Co (oxy)hydroxide is revealed before the OER testing, whereas after the OER testing, the Raman bands at around 470 and 660 cm−1 can be assigned to the signal bands of the Co (oxy)hydroxide (Fig. S20a and b, ESI†).55 Upon Fe doping, the intensity of the two bands is accentuated and the Raman band intensity achieves the highest in the Fe0.4Co0.6Se2 sample, which shows a similar tendency to the previously mentioned EPR and XPS results. The results of the Raman analysis again verify that the Fe doping makes it easier to oxidize the catalyst surface into (oxy)hydroxide. Thus, due to the local spin state modification by Fe doping, large eg orbital filling and a higher degree of overlapping between the eg orbital and OH− adsorbate are achieved, thereby improving the adsorbed oxygen electrophilicity and the ability to adsorb the hydroxy groups, which would accelerate the formation of (oxy)hydroxide species on the activated Co sites.
In summary, this study reports a simple solid-state selenization method to prepare Fe0.4Co0.6Se2 nanoframes for the efficient oxygen evolution. The Fe0.4Co0.6Se2 nanoframes require an overpotential of only 270 mV to output a 10 mA cm−2 oxygen production current, which is superior to that of commercial RuO2 as well as most of the excellent reported catalysts. Comprehensive analysis convincingly reveals that the local Co spin state is optimized to a higher valence state by appropriate Fe doping, which accounts for the boosted activity towards the OER. This Fe0.4Co0.6Se2 nanoframe is not only an excellent OER catalyst, but also a cutting-edge example of a rational morphology design and local electron configuration optimization to achieve high activity. This work may form a basis for rationally design of efficient electrocatalysts for future energy conversion technologies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (22075092), the Program for HUST Academic Frontier Youth Team (2018QYTD15) and the National 1000 Young Talents Program of China. The authors also acknowledge the support of the staff of the Analytical and Testing Center of Huazhong University of Science and Technology for their help with the SEM, XRD, Raman, EPR, XPS, ICP-OES, BET and TEM measurements.References
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef
.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC
.
- B. Zhang, X. Zheng, O. Voznyy, R. Comin, M. Bajdich, M. García-Melchor, L. Han, J. Xu, M. Liu, L. Zheng, F. P. García de Arquer, C. T. Dinh, F. Fan, M. Yuan, E. Yassitepe, N. Chen, T. Regier, P. Liu, Y. Li, P. De Luna, A. Janmohamed, H. L. Xin, H. Yang, A. Vojvodic and E. H. Sargent, Science, 2016, 352, 333–337 CrossRef CAS
.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS
.
- J.-Y. Zhang, H. Wang, Y. Tian, Y. Yan, Q. Xue, T. He, H. Liu, C. Wang, Y. Chen and B. Y. Xia, Angew. Chem., Int. Ed., 2018, 57, 7649–7653 CrossRef CAS
.
- Z.-F. Huang, J. Song, Y. Du, S. Xi, S. Dou, J. M. V. Nsanzimana, C. Wang, Z. J. Xu and X. Wang, Nat. Energy, 2019, 4, 329–338 CrossRef CAS
.
- B. Y. Xia, Y. Yan, N. Li, H. B. Wu, X. W. D. Lou and X. Wang, Nat. Energy, 2016, 1, 15006 CrossRef CAS
.
- J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS
.
- J.-Y. Zhang, T. He, M. Wang, R. Qi, Y. Yan, Z. Dong, H. Liu, H. Wang and B. Y. Xia, Nano Energy, 2019, 60, 894–902 CrossRef CAS
.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS
.
- W. Fang, L. Huang, S. Zaman, Z. Wang, Y. Han and B. Y. Xia, Chem. Res. Chin. Univ., 2020, 36, 611–621 CrossRef CAS
.
- C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS
.
- N.-T. Suen, S.-F. Hung, Q. Quan, N. Zhang, Y.-J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC
.
- H. Yang, L. Gong, H. Wang, C. Dong, J. Wang, K. Qi, H. Liu, X. Guo and B. Y. Xia, Nat. Commun., 2020, 11, 5075 CrossRef CAS
.
- J.-Y. Zhang, X. Tian, T. He, S. Zaman, M. Miao, Y. Yan, K. Qi, Z. Dong, H. Liu and B. Y. Xia, J. Mater. Chem. A, 2018, 6, 15653–15658 RSC
.
- R. Zhang, N. Dubouis, M. Ben Osman, W. Yin, M. T. Sougrati, D. A. D. Corte, D. Giaume and A. Grimaud, Angew. Chem., Int. Ed., 2019, 58, 4571–4575 CrossRef CAS
.
- Y. Yao, S. Hu, W. Chen, Z.-Q. Huang, W. Wei, T. Yao, R. Liu, K. Zang, X. Wang, G. Wu, W. Yuan, T. Yuan, B. Zhu, W. Liu, Z. Li, D. He, Z. Xue, Y. Wang, X. Zheng, J. Dong, C.-R. Chang, Y. Chen, X. Hong, J. Luo, S. Wei, W.-X. Li, P. Strasser, Y. Wu and Y. Li, Nat. Catal., 2019, 2, 304–313 CrossRef CAS
.
- P. Cai, J. Huang, J. Chen and Z. Wen, Angew. Chem., Int. Ed., 2017, 56, 4858–4861 CrossRef
.
- X. Lu and C. Zhao, Nat. Commun., 2015, 6, 6616 CrossRef CAS
.
- X.-Y. Yu, Y. Feng, B. Guan, X. W. Lou and U. Paik, Energy Environ. Sci., 2016, 9, 1246–1250 RSC
.
- A. T. Swesi, J. Masud and M. Nath, Energy Environ. Sci., 2016, 9, 1771–1782 RSC
.
- L. Xu, Q. Jiang, Z. Xiao, X. Li, J. Huo, S. Wang and L. Dai, Angew. Chem., Int. Ed., 2016, 55, 5277–5281 CrossRef CAS
.
- J.-Y. Zhang, L. Lv, Y. Tian, Z. Li, X. Ao, Y. Lan, J. Jiang and C. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 33833–33840 CrossRef CAS
.
- S. Niu, W.-J. Jiang, Z. Wei, T. Tang, J. Ma, J.-S. Hu and L.-J. Wan, J. Am. Chem. Soc., 2019, 141, 7005–7013 CrossRef CAS
.
- X. Zheng, B. Zhang, P. De Luna, Y. Liang, R. Comin, O. Voznyy, L. Han, F. P. García de Arquer, M. Liu, C. T. Dinh, T. Regier, J. J. Dynes, S. He, H. L. Xin, H. Peng, D. Prendergast, X. Du and E. H. Sargent, Nat. Chem., 2017, 10, 149 CrossRef
.
- R. G. Hadt, D. Hayes, C. N. Brodsky, A. M. Ullman, D. M. Casa, M. H. Upton, D. G. Nocera and L. X. Chen, J. Am. Chem. Soc., 2016, 138, 11017–11030 CrossRef CAS
.
- Y. Dou, C.-T. He, L. Zhang, H. Yin, M. Al-Mamun, J. Ma and H. Zhao, Nat. Commun., 2020, 11, 1664 CrossRef CAS
.
- S. Wan, W. Jin, X. Guo, J. Mao, L. Zheng, J. Zhao, J. Zhang, H. Liu and C. Tang, ACS Sustainable Chem. Eng., 2018, 6, 15374–15382 CrossRef CAS
.
- L. J. Enman, M. B. Stevens, M. H. Dahan, M. R. Nellist, M. C. Toroker and S. W. Boettcher, Angew. Chem., Int. Ed., 2018, 57, 12840–12844 CrossRef CAS
.
- S. Zhou, X. Miao, X. Zhao, C. Ma, Y. Qiu, Z. Hu, J. Zhao, L. Shi and J. Zeng, Nat. Commun., 2016, 7, 11510 CrossRef CAS
.
- S. Chen, Z. Kang, X. Hu, X. Zhang, H. Wang, J. Xie, X. Zheng, W. Yan, B. Pan and Y. Xie, Adv. Mater., 2017, 29, 1701687 CrossRef
.
- Y. Tong, Y. Guo, P. Chen, H. Liu, M. Zhang, L. Zhang, W. Yan, W. Chu, C. Wu and Y. Xie, Chem, 2017, 3, 812–821 CAS
.
- Y. Liu, C. Xiao, M. Lyu, Y. Lin, W. Cai, P. Huang, W. Tong, Y. Zou and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 11231–11235 CrossRef CAS
.
- J. Jiang, F. Sun, S. Zhou, W. Hu, H. Zhang, J. Dong, Z. Jiang, J. Zhao, J. Li, W. Yan and M. Wang, Nat. Commun., 2018, 9, 2885 CrossRef
.
- L. Han, X.-Y. Yu and X. W. Lou, Adv. Mater., 2016, 28, 4601–4605 CrossRef CAS
.
- X.-Y. Yu, L. Yu, H. B. Wu and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 5331–5335 CrossRef CAS
.
- J. Nai, Y. Lu, L. Yu, X. Wang and X. W. Lou, Adv. Mater., 2017, 29, 1703870 CrossRef
.
- H. van der Heide, R. Hemmel, C. F. van Bruggen and C. Haas, J. Solid State Chem., 1980, 33, 17–25 CrossRef CAS
.
- D. Telesca, Y. Nie, J. I. Budnick, B. O. Wells and B. Sinkovic, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 214517 CrossRef
.
- Y. Yang, L. Dang, M. J. Shearer, H. Sheng, W. Li, J. Chen, P. Xiao, Y. Zhang, R. J. Hamers and S. Jin, Adv. Energy Mater., 2018, 8, 1703189 CrossRef
.
- G. Shen, R. Zhang, L. Pan, F. Hou, Y. Zhao, Z. Shen, W. Mi, C. Shi, Q. Wang, X. Zhang and J.-J. Zou, Angew. Chem., Int. Ed., 2020, 59, 2313–2317 CrossRef CAS
.
- K. Adachi, M. Matsui and Y. Omata, J. Phys. Soc. Jpn., 1981, 50, 83–89 CrossRef CAS
.
- S. F. Jafri, E. S. Koumousi, M.-A. Arrio, A. Juhin, D. Mitcov, M. Rouzières, P. Dechambenoit, D. Li, E. Otero, F. Wilhelm, A. Rogalev, L. Joly, J.-P. Kappler, C. Cartier dit Moulin, C. Mathonière, R. Clérac and P. Sainctavit, J. Am. Chem. Soc., 2019, 141, 3470–3479 CrossRef CAS
.
- G. Li, Q. Xu, W. Shi, C. Fu, L. Jiao, M. E. Kamminga, M. Yu, H. Tüysüz, N. Kumar, V. Süβ, R. Saha, A. K. Srivastava, S. Wirth, G. Auffermann, J. Gooth, S. Parkin, Y. Sun, E. Liu and C. Felser, Sci. Adv., 2019, 5, eaaw9867 CrossRef CAS
.
- B. E. Van Kuiken and M. Khalil, J. Phys. Chem. A, 2011, 115, 10749–10761 CrossRef CAS
.
- S.-F. Hung, Y.-Y. Hsu, C.-J. Chang, C.-S. Hsu, N.-T. Suen, T.-S. Chan and H. M. Chen, Adv. Energy Mater., 2018, 8, 1701686 CrossRef
.
- Z. Lu, H. Wang, D. Kong, K. Yan, P.-C. Hsu, G. Zheng, H. Yao, Z. Liang, X. Sun and Y. Cui, Nat. Commun., 2014, 5, 4345 CrossRef CAS
.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS
.
- J. Huang, J. Chen, T. Yao, J. He, S. Jiang, Z. Sun, Q. Liu, W. Cheng, F. Hu, Y. Jiang, Z. Pan and S. Wei, Angew. Chem., Int. Ed., 2015, 54, 8722–8727 CrossRef CAS
.
- P. Chen, K. Xu, Z. Fang, Y. Tong, J. Wu, X. Lu, X. Peng, H. Ding, C. Wu and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 14710–14714 CrossRef CAS
.
- X. Xu, F. Song and X. Hu, Nat. Commun., 2016, 7, 12324 CrossRef CAS
.
- Z. Yang, J.-Y. Zhang, Z. Liu, Z. Li, L. Lv, X. Ao, Y. Tian, Y. Zhang, J. Jiang and C. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 40351–40359 CrossRef CAS
.
- X. Zou, Y. Liu, G.-D. Li, Y. Wu, D.-P. Liu, W. Li, H.-W. Li, D. Wang, Y. Zhang and X. Zou, Adv. Mater., 2017, 29, 1700404 CrossRef
.
- S. C. Petitto, E. M. Marsh, G. A. Carson and M. A. Langell, J. Mol. Catal. A: Chem., 2008, 281, 49–58 CrossRef CAS
.
- K. Jayaramulu, J. Masa, D. M. Morales, O. Tomanec, V. Ranc, M. Petr, P. Wilde, Y.-T. Chen, R. Zboril, W. Schuhmann and R. A. Fischer, Adv. Sci., 2018, 5, 1801029 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, SEM, TEM, EDX, XRD, XPS, EPR, BET, Raman, magnetic tests and other electrochemical measurements. See DOI: 10.1039/d0ee03500a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |